1. Introduction
Malus hupehensis, classified as a spontaneous apomixis polyploid species, possesses distinct advantages such as robust graft compatibility, minimal coefficient of variation, elevated germination survival rates, enhanced resistance to waterlogging, and negligible variation among offspring. It is esteemed as an exceptional rootstock for apple cultivation and has gained extensive application in the apple production sector [
1,
2].
Roots are indispensable for plants, facilitating the absorption of soil moisture and nutrients, as well as their subsequent transportation to other parts of the plant. The root system of plants is predominantly composed of primary roots, adventitious roots, and lateral roots. Adventitious roots and lateral roots form a larger surface area of the root system, which determines the lateral expansion of the root system and the soil volume available for nutrient acquisition [
3,
4]. The morphogenesis of lateral roots encompasses a series of pivotal processes: initially, the lateral root primordium is triggered at an appropriate location on the parent root, followed by the formation of the lateral root primordium through cell division and differentiation; subsequently, these lateral root primordia continue to evolve and ultimately penetrate the epidermal layer to emerge as lateral roots [
5].
Plant hormones represent a suite of naturally occurring organic compounds within plants that regulate gene expression, individual development and development, organ differentiation, and responses to environmental stress [
6]. Presently, the acknowledged plant hormones encompass auxin, cytokinin (CK), gibberellin (GA), ethylene, abscisic acid (ABA), brassinolactone (BR), strigolactone, among others. These hormones are instrumental in the formation and development of lateral roots. For instance, auxin promotes differentiation of lateral root primordium cells and regulates root morphogenesis; CTK and ABA serve to suppress lateral root formation; while the interplay among ethylene, BR, and auxin, in conjunction with strigolactone, influences the lateral root formation in
Arabidopsis [
7,
8,
9].
In this experiment, Malus hupehensis was employed as the experimental subject to investigate the impact of diverse hormonal treatments on the lateral root development of its seedlings. This was achieved by administering a variety of hormones to the nutrient solution, thereby aiming to furnish a theoretical framework for the enhancement of its cultivation and utilization.
2. Materials and Methods
2.1. Test Materials
A total of 810 full-grown 60-day-old Malus hupehensis seedlings, each with 8 to 10 leaves and free from pests and diseases, were selected for the study. These seedlings were cultivated in the scientific research greenhouse of the College of Horticulture at Northwest A&F University, under controlled indoor temperatures of (25 ± 1) ℃/ (15 ± 1) ℃ (light/dark). The photoperiod was set at 12 hours, with an average humidity of approximately 70~80%. During the light phase, the photosynthetic photon flux density (PPFD) was maintained at around 800 μmolm-2s-1.
2.2. Treatment Method
2.2.1. Different Concentrations of IAA, IBA, GA, 6-BA, BR and MeJA Were Used to Treat M. hupehensis Seedlings
M. hupehensis seedlings were transferred to a 1/2 Hoagland hydroponic nutrient solution for a period of two weeks. Throughout this period, the 1/2 Hoagland nutrient solution was replaced once a week. The hydroponic apparatus comprised a square, non-porous plastic basin (50cm × 35cm × 15cm), with the plastic basin being enveloped in black plastic sheeting. Polystyrene foam boards were utilized as planting platforms, with each board containing 30 pre-drilled holes. The seedlings were inserted into these holes, with their stems secured by sponges to ensure that the roots were fully submerged within the basin. A total of 30 plants were accommodated in each container, and air was continuously infused into each plastic basin via an air pump. Treatments included the application of exogenous hormones at concentrations of 0.1mg L-1 IAA, 0.5mg L-1 IBA, 0.02mg L-1 GA, 0.02mg L-1 6-BA, 0.1mg L-1 6-BA, 0.05mg L-1 BR, 0.1mg L-1 BR, 0.1mg L-1 MeJA along with a control treatment. Ninety seedlings were subjected to each treatment, amounting to a total of 810 seedlings. After one week of treatment, routine maintenance was performed, with the nutrient solution being replaced weekly for an additional month of cultivation. In addition, a further 90 seedlings were cultivated in 1/2 Hoagland solution without any exogenous hormones as controls. Following one month of culture, the same parameters were assessed in both the treated and untreated seedlings. A random selection of 15 seedlings from each of the 8 treated groups and 1 control group was conducted, resulting in a total of 135 seedlings for evaluation. The harvested samples were promptly immersed in liquid nitrogen and preserved at -80 °C in preparation for subsequent hormone level analysis.
2.2.2. Morphological Measurement of Root System of M. hupehensis Seedlings
The root images were captured using the EPSON EXPRESSION 10000XL scanner (model LA1600, Canada), configured to a resolution of 400 dpi. The WinRHIZO Pro root analysis software (version 2003 b, Canada) was employed to process the scanned images, measuring the root length, surface area, and volume, calculating the number of lateral roots. Furthermore, the plant height was determined by measuring the distance from the top of the foam board to the stem tip with a ruler. The stem diameter was gauged in both orientations using a vernier caliper, with the average value taken to represent the stem thickness. Each group had a subset of 15 plants randomly chosen for the purpose of measurement.
2.2.3. Methods of Endogenous Hormone Measurement
The enzyme-linked immunosorbent assay (ELISA) was employed to quantify the levels of each hormone. Sampling points were consistently chosen at identical locations, and the experiment was conducted with three biological replicates. The hormone standard utilized in the assay was procured from Sigma Company.
2.2.4. Data Analysis
The SPSS11.5 software was employed to conduct statistical analysis on the acquired dataset. The significant disparities between the control and treatment groups were assessed utilizing the T-test methodology. Furthermore, SigmaPlot 12 was utilized for the generation of the resultant data.
3. Results
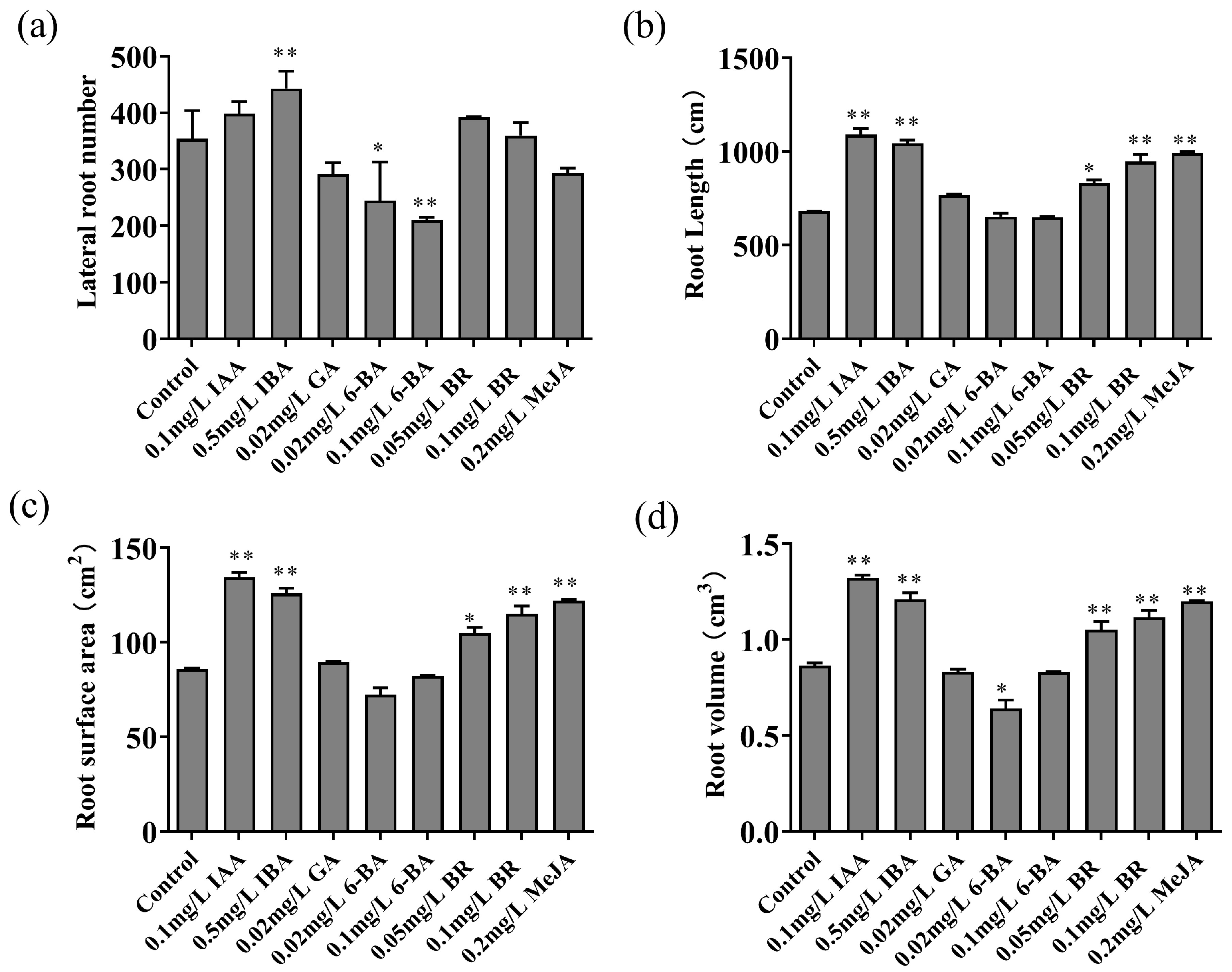
3.1. Effects of Different Plant Hormone Treatments on Lateral Root Number, Root Length, Root Surface Area and Root Volume
Comparing the root morphology diagrams of different treatment groups with the control group, it was observed that
M. hupehensis seedlings treated with IAA, IBA, and BR exhibited a greater number of lateral roots compared to the control. Conversely, seedlings subjected to GA, 6-BA, and MeJA treatments displayed a reduced number of lateral roots relative to the control. Notably, the IBA treatment at a concentration of 0.5mg L
-1 yielded the highest number of lateral roots, showcasing a significant discrepancy from the control group. The 6-BA treatment at a concentration of 0.1mg L
-1 yielded the least number of lateral roots, with the 0.02mg L
-1 6-BA treatment also demonstrating a significant decrement compared to the control group (
Figure 1a). Moreover, the root length and surface area of seedlings treated with IAA, IBA, BR, and MeJA were markedly superior to those of the control group (
Figure 1b,c). The root volume of seedlings treated with 0.02mg L
-1 6-BA was notably reduced compared to the control, whereas the root volume of seedlings treated with IAA, IBA, BR, and MeJA was substantially greater than that of the control group (
Figure 1d).
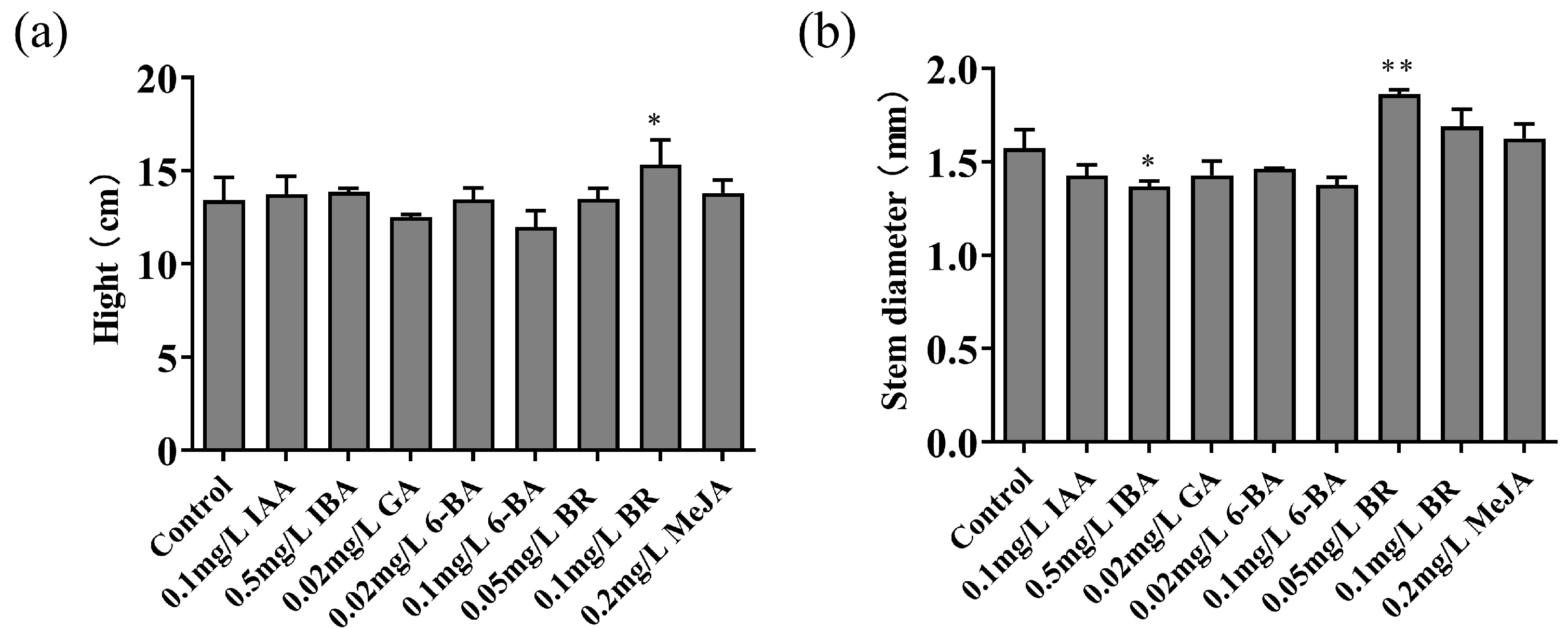
3.2. Effects of Different Plant Hormone Treatments on Seedling Height and Stem Diameter
The height of
M. hupehensis seedlings treated with a concentration of 0.1mg L
-1 BR was observed to significantly exceed that of the ontrol group. Furthermore, no discernible discrepancies in development were observed between the various treatment groups and the control group (
Figure 2a). Moreover, the diameter of the stems in the subset of
M. hupehensis that received 0.05mg L
-1 of BR was significantly greater than that of the control group. Conversely, the stem diameter of the
M. hupehensis exposed to 0.5mg L
-1 of IBA was notably reduced in comparison to the control group (
Figure 2b).
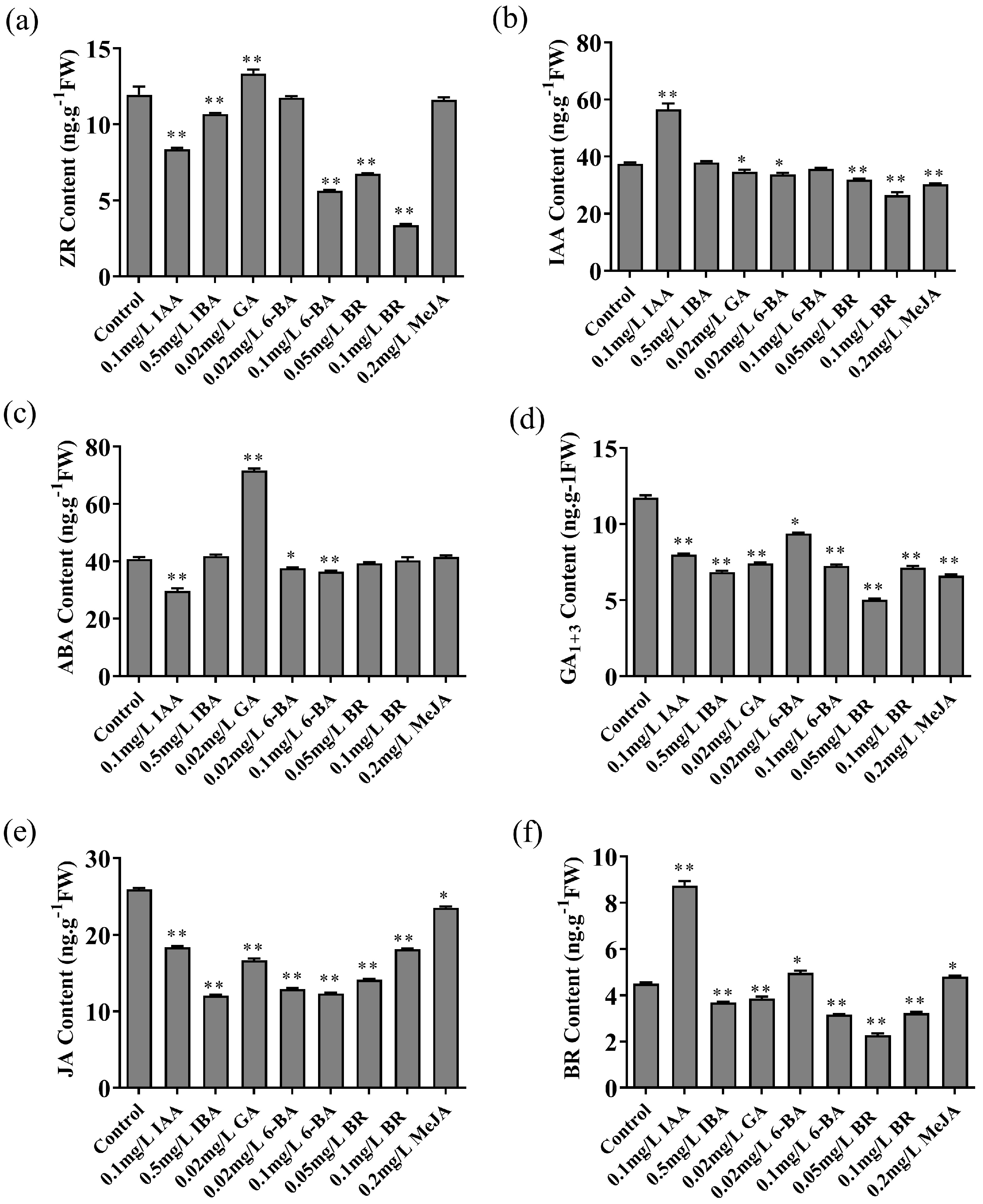
3.3. Effects of Different Plant Hormone Treatments on the Concentrations of ZR, IAA, ABA, GA1+3, JA and BR of M. hupehensis
M. hupehensis seedlings were subjected to various exogenous hormonal treatments, and the concentrations of endogenous hormones ZR, IAA, ABA, GA, JA, and BR were quantified in both the experimental and control groups (
Figure 3). The ZR concentration in seedlings treated with 0.02mg L
-1 GA was remarkably elevated compared to the control group, whereas the ZR concentration in seedlings exposed to 0.1mg L
-1 IAA, 0.5mg L
-1 IBA, 0.1mg L
-1 6-BA, 0.05mg L
-1 BR and 0.1mg L
-1 BR was substantially lower than that of the control group (
Figure 3a). The IAA concentration in
M. hupehensis seedlings treated with 0.1mg L
-1 IAA was significantly greater than that of the control group. Conversely, the IAA concentration in seedlings treated with 0.02mg L
-1 GA, 0.02mg L
-1 6-BA, 0.05mg L
-1 BR, 0.1mg L
-1 BR and 0.2mg L
-1 MeJA was significantly reduced compared to the control group (
Figure 3b). Following treatment with 0.02mg L
-1 GA, the
M. hupehensis exhibited a marked elevation in ABA concentrations compared to the control group. Conversely, the application of 0.1mg L
-1 IAA and 0.1mg L
-1 6-BA to the
M. hupehensis resulted in a significant reduction of ABA levels (
Figure 3c).The concentrations of GA
1+3 and JA in the
M. hupehensis treated with 0.1mg L
-1 IAA, 0.5mg L
-1 IBA, 0.02mg L
-1 GA, 0.02mg L
-1 6-BA, 0.1mg L
-1 6-BA, 0.05mg L
-1 BR, 0.1mg L
-1 BR and 0.2mg L
-1 MeJA were all markedly diminished when compared to the control group (
Figure 3d,e). The BR concentration in
M. hupehensis seedlings treated with all hormones displayed significant deviations from the control group. Specifically, the BR concentration in the 0.1mg L
-1 IAA treatment was extremely significantly elevated compared to the control group. In contrast, the BR concentrations in treatments involving 0.5mg L
-1 IBA、0.02mg L
-1 GA、0.1mg L
-1 6-BA、0.05mg L
-1 BR and 0.1mg L
-1 BR were all significantly diminished in comparison to the control group (
Figure 3f).
4. Discussion
Auxin is a kind of plant hormone containing an unsaturated aromatic ring and a carboxyl side chain. As the first hormone to be identified in plants, auxin plays a pivotal role in nearly every facet of plant development and developmental regulation. Its functions encompass not only the regulation of cell division and the promotion of cell elongation but also the influence on the morphogenesis of individual plants and their organs through the establishment of concentration gradient distributions [
10]. The transport of auxin within plants can be categorized into long-range and short-range conveyance based on the traverse distance. Long-range auxin transport relies on the phloem for rapid conveyance from the stem apex to the roots, which is classified as non-polar transport. On the other hand, short-range transport is characterized by unidirectional movement between cells and is considered polar transport. Auxin polar transport is synergically mediated by influx and efflux transporters. Common auxin transporters include influx transporters AUX/LAX protein family, including AUX1, LAX1, LAX2, LAX3; The efflux transporters PIN protein family and the ABCB/PGP protein family with influx and efflux transporter functions [
11,
12].
Auxin is the regulatory center of lateral root development in plants, intricately participating in and orchestrating this development by modulating the expression of diverse genes involved in synthesis and transport, as well as by manipulating a variety of signaling element complexes [
13]. In Arabidopsis, the transcription factors IDD14, IDD15, and IDD16 facilitate auxin biosynthesis through the regulation of
YUC5 and
TAA1 expression, thereby exerting a positive influence on lateral root development [
14]. Furthermore, the application of melatonin modulates auxin transport by impacting the expression of genes pertinent to auxin transport, including
PIN5,
TT4,
TT5, etc., thus fostering lateral root development [
15]. In this experiment, exogenous auxin extremely significantly reduced the level of endogenous auxin, increased the level of endogenous auxin, offset the effects of cytokines on initiation and elongation of lateral roots, and significantly increased the number of lateral roots, root length, root surface area and root volume of
M. hupehensis seedlings, thus promoting lateral root development.
6-BA is the first generation of artificially synthesized cytokinin. Cytokinin is generally considered to be a negative regulator of lateral root formation, and the number of lateral roots increases significantly in plants with reduced cytokinin content [
16]. The administration of exogenous cytokinins impedes lateral root formation by obstructing the polar transport of auxin, disrupting the distribution pattern of auxin, and interfering with the auxin signaling transduction pathway, thereby precluding the pericycle founder cells into the cell cycle [
17,
18]. Subsequent investigations revealed that the exogenous application of cytokinin augmented the consumption of PIN1 auxin transporter at specific polar domains and suppressed PIN1 gene expression, consequently inhibiting auxin transport towards the lateral root primordia and thereby stifling lateral root formation [
19]. In this experiment, exogenous 6-BA significantly reduced endogenous auxin levels, significantly reduced lateral root number and inhibited lateral root development in
M. hupehensis seedlings.
Brassinosteroids represent the collective appellation for a class of polyhydroxylated sterols. In 1970, researchers isolated a series of plant development-promoting compounds from rape pollen, subsequently denominating them as Brassins [
20]. Following this discovery, researchers elucidated the chemical structure of brassin through single-crystal X-ray diffraction, and accordingly designated it as Brassinolide (BL) [
21]. To date, researchers have characterized at least 70 brassinolide analogues [
22], collectively referred to as Brassinosteroids (BR). In soybeans and tomatoes, the absence of BR, mutations in the BR signaling pathway, or mutations in the BR receptor have been associated with reduced plant height [
23]. In rice, mutation of the receptor kinase XIAO leads to decrease of BRs content in the whole plant, inhibition of cell division, and ultimately diminutive plant height [
24]. In this experiment, exogenous application of BR significantly increased the plant height and stem diameter of
M. hupehensis seedlings.
5. Conclusions
Hydroponically cultivated M. hupehensis seedlings exhibit differential responses to various exogenous hormonal treatments in terms of lateral root development. When compared to the untreated control, the application of exogenous auxin led to an increase in the number of lateral roots, root length, root surface area, and root volume in M. hupehensis seedlings, thereby fostering lateral root development. Conversely, the 6-BA exerted an inhibitory effect on lateral root development, contrasting with the action of auxin. Treatment with brassinosteroids was found to stimulate an increase in plant height and stem diameter in M. hupehensis. Simultaneously, the application of these exogenous hormones also exerted influence on the levels of endogenous hormones. Following treatment with 0.1mg L-1 BR, the levels of ZR, IAA, and GA1+3 in the lateral roots of M. hupehensis seedlings were significantly reduced in comparison to the control. On the other hand, after the seedlings were treated with 0.1mg L-1 IAA, there was a significant elevation in IAA and BR levels within the roots, whereas the levels of ABA and GA1+3 were notably decreased.
Author Contributions
Conceptualization, J.L. and J.M.; formal analysis, J.L., Y.M. and S.S.; investigation, J.L., Y.M. and S.S.; resources, J.M.; data curation, Y.J., Y.D., K.X., M.G., J.M., and X.W.; writing—original draft preparation, J.L.; writing—review and editing, J.M.; supervision, J.M.; project administration, J.M.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32372675, 32372657, 32102359), the Young Talent fund of University Association for Science and Technology in Shaanxi, China (20240218), the Science and Technology Major Project of Xinjiang Production and Construction Corps, China (2023AB077), the Chinese Universities Scientific Fund (2452023005), the China Apple Research System (CARS-27), the Cyrus Tang Foundation, and the Fundamental Research Funds for the Central Universities, China.
Data Availability Statement
The original contributions presented in the study are included in the article: further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, T.C.; Guo, J.H.; Qiao, Y.R.; Yang, H.Q.; Fan, W.G. Effects of methyl jasmonate on photosynthetic characteristics, root configuration and nitrogen uptake in Malus hupehensis. J. Anhui Agric. Univ. 2024, 51, 239–243. [Google Scholar] [CrossRef]
- Yang, H.Q.; Fan, W.G. Advances in research of apple root system architecture and it’s regulation. Yuan Yi Xue Bao. 2012, 39, 1673–1678. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Shu, J.Q.; Li, W.M.; Wang, G.Z. Role of auxin andnitrate signaling in the development of root systemarchitecture. FRONT PLANT SCI. 2021, 12, 690363. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wirén, N. Local auxin biosynthesis acts downstream of brassinosteroids to trigger root foraging for nitrogen. Nat Commun. 2021, 12, 5437. [Google Scholar] [CrossRef] [PubMed]
- Vilches-Barro, A.; Maizel, A. Talking through walls: mechanisms of lateral root emergence in Arabidopsis thaliana. Curr Opin Plant Biol. 2015, 23, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.F.; Feng, W.J.; Niu, X.L.; Zhang, C.L.; Ma, J.H.; Guo, P.Y. Physiological Mechanisms in Phytohormone Regulation of Plant Lateral Root Development. Plant Physiol. 2015, 51, 2101–2108. [Google Scholar] [CrossRef]
- Koltai, H. Cellular events of strigolactone signaling and their crosstalk with auxin in roots. J Exp Bot. 2015, 66, 4855–4861. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Yun, J.; Likhacheva, A.V.; Alonso, J.M. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007, 19, 2169–2185. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Shen, J.; Brady, S.R.; Muday, G.K.; Asami, T.; Yang, Z. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol. 2004, 134, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Herud-Sikimić, O.; Stiel, A.C.; Kolb, M.; Shanmugaratnam, S.; Berendzen, K.W.; Feldhaus, C.; Höcker, B.; Jürgens, G. A biosensor for the direct visualization of auxin. Nature. 2021, 592, 768–772. [Google Scholar] [CrossRef]
- Harrison, C.J. Auxin transport in the evolution of branching forms. New Phytol. 2017, 215, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Berman, A.; Shani, E. Plant Hormone Transport and Localization: Signaling Molecules on the Move. Annu Rev Plant Biol. 2023, 74, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Santos Teixeira, J.A.; ten Tusscher, K.H. The systems biology of lateral root formation: connecting the dots. Mol Plant 2019, 12, 784–803. [Google Scholar] [CrossRef]
- Cui, D.Y.; Zhao, J.B.; Jing, Y.J.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The arabidopsis IDD14, IDD15, and IDD16 cooperatively regulatelateral organ morphogenesis and gravitropism by promoting auxin biosynthesis andtransport. PLoS Genetics. 2013, 9, e1003759. [Google Scholar] [CrossRef]
- Ren, S.; Rutto, L.; Katuuramu, D. Melatonin acts synergistically with auxin to promote lateral rootdevelopment through fine tuning auxin transport in Arabidopsis thaliana. PLoS One. 2019, 14, e0221687. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2019, 15, 2532–2550. [Google Scholar] [CrossRef]
- Laplaze, L.; Benkova, E.; Casimiro, I.; Maes, L.; Vanneste, S.; Swarup, R.; Weijers, D.; Calvo, V.; Parizot, B.; Herrera-Rodriguez, M.B.; Offringa, R.; Graham, N.; Doumas, P.; Friml, J.; Bogusz, D.; Beeckman, T.; Bennett, M. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell. 2007, 19, 3889–3900. [Google Scholar] [CrossRef]
- Li, X.; Mo, X.; Shou, H.; Wu, P. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant Cell Physio. 2006, 47, 1112–1123. [Google Scholar] [CrossRef]
- Marhavý, P.; Duclercq, J.; Weller, B.; Feraru, E.; Bielach, A.; Offringa, R.; Friml, J.; Schwechheimer, C.; Murphy, A.; Benková, E. Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr Biol. 2014, 24, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins--a new family of plant hormones from rape pollen. Nature. 1970, 225, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.; Steffens, G.L.; Flippen-Anderson, J.L.; Cook, J. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Bajguz, A. Metabolism of brassinosteroids in plants. Plant Physiol. Biochem. 2007, 45, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.J.; Koncz, C. Brassinosteroids and plant steroid hormonesignaling. Plant Cell. 2002, 14, S97–110. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Bao, L.; Jeong, S.Y.; Kim, S.K.; Xu, C.; Li, X.; Zhang, Q. XIAO is involved in the control of organ size by contributing to the regulation of signaling and homeostasis ofbrassinosteroids and cell cycling in rice. Plant J. 2012, 70, 398–408. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).