Submitted:
11 December 2024
Posted:
11 December 2024
You are already at the latest version
Abstract
It is well-known that individual pea (Pisum sativum L.) cultivars differ in their symbiotic responsivity. This trait is typically manifested with an increase in seed weights due to inoculation with rhizobial bacteria and arbuscular mycorrhizal fungi. The aim of this work was to characterize the alterations in root proteome of highly responsive pea genotype k-8274 and low-responsive genotype k-3358 grown in non-sterile soil, which were associated with root colonization with rhizobial bacteria and arbuscular mycorrhiza fungi in comparison to proteome shifts caused by soil supplementation with mineral nitrogen salts. Our results clearly indicate that supplementation of the soil with mineral nitrogen-containing salts switched the root proteome of both genotypes to assimilation of the available nitrogen, whereas the processes associated with nitrogen fixation were suppressed. Surprisingly, inoculation with rhizobial bacteria had only a minor effect on root proteomes of the both genotypes. The most pronounced response was observed for highly responsive k-8274 genotype inoculated simultaneously with rhizobial bacteria and arbuscular mycorrhizal fungi. This response involved activation of the proteins related to redox metabolism and suppression of excessive nodule formation. In turn, the low-responsive genotype k-3358 demonstrated a pronounced inoculation-induced suppression of protein metabolism and enhanced diverse defense reactions in pea roots under the same soil conditions. The results of the study shed light on the molecular basis of differential symbiotic responsivity in different pea cultivars.
Keywords:
1. Introduction
2. Results
2.1. Protein Isolation and Tryptic Digestion
2.2. Protein Annotation
2.3. Label-Free Relative Quantification of the Annotated Proteins
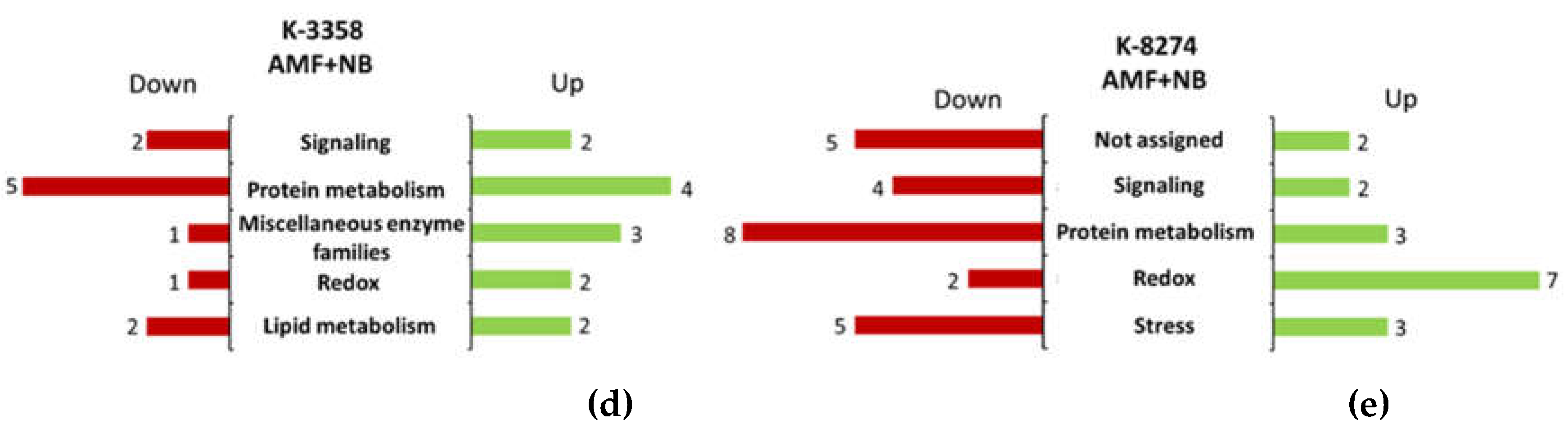
2.4. Functional Annotation of Differentially Expressed Proteins
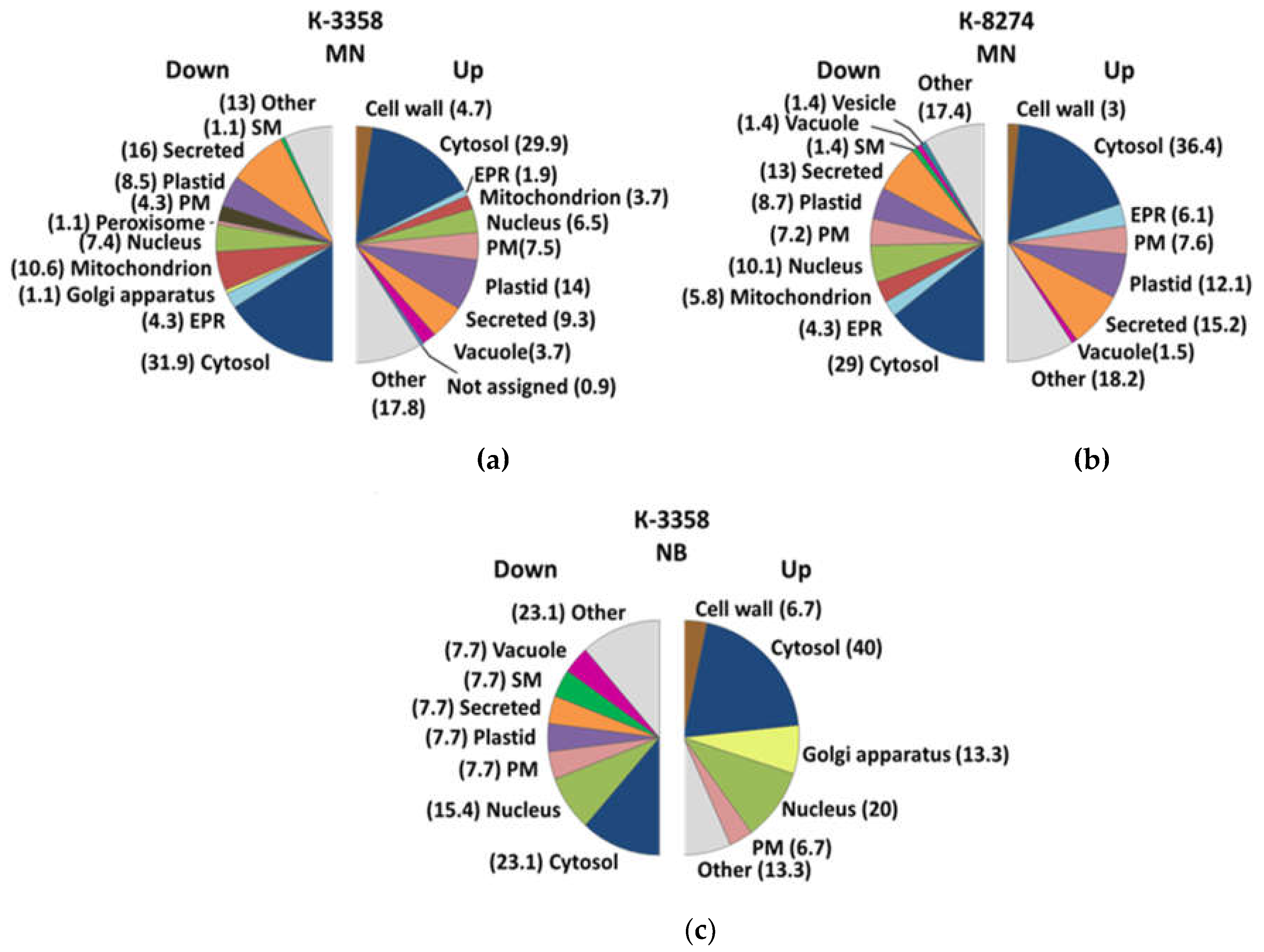
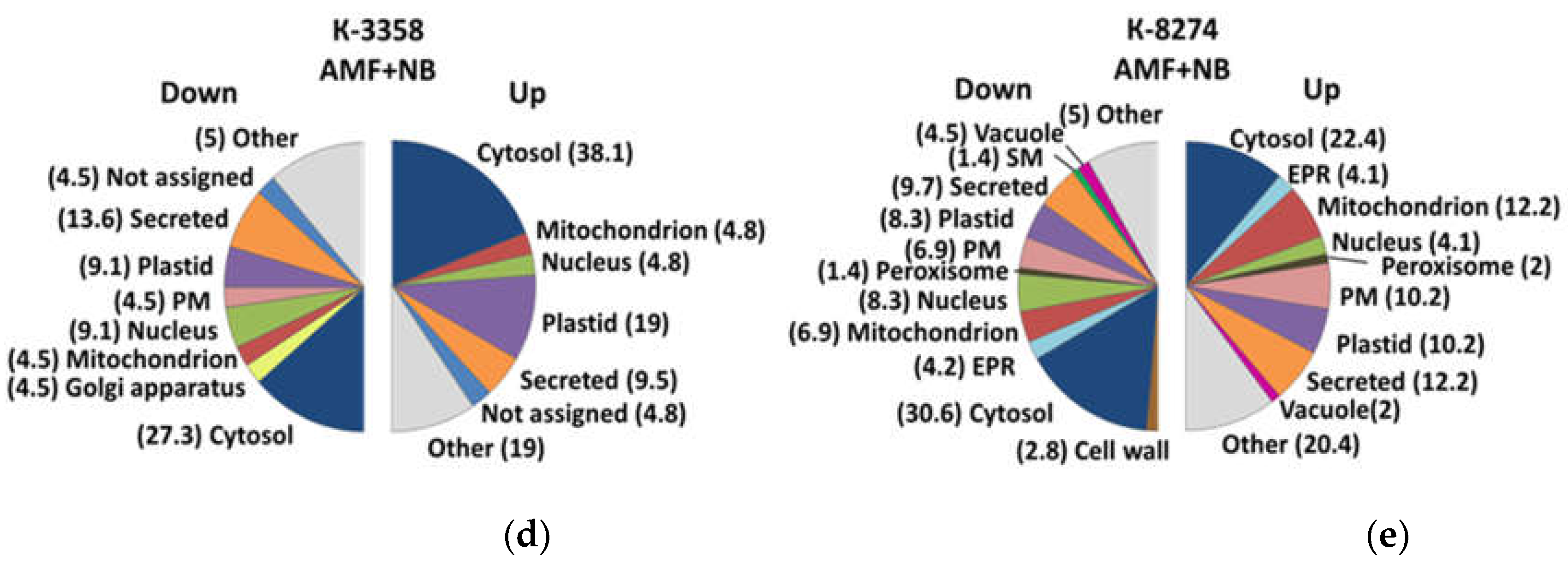
2.5. Subcellular Localization
3. Discussion
3.1. Inter-Genotype Differences in Proteome Signatures
3.2. Subcellular Root Proteome Response of the k-3358 and k-8274 Plants to Soil Complementation with Mineral Nitrogen Localization
3.3. Root Proteome Response of the k-3358 and k-8274 Plants to Inoculation with Symbiotic Rhizobia
3.4. Root Proteome Response of the k-3358 and k-8274 Plants to Combined Inoculation with Nodule Bacteria and AM Fungi
4. Materials and Methods
4.1. Reagents
4.2. Plant Experiment
4.3. Protein Extraction
4.4. Determination of Protein Concentration
4.5. Tryptic Digestion
4.6. Solid Phase Extraction
4.7. Nano LC-MS/MS
4.8. Data Processing and Post-Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, S.; Riaz, U.; Murtaza, G.; Jamil, M.; Ahmed, M.; Hussain, A.; Abbas, Z. Chemical Fertilizers, Formulation, and Their Influence on Soil Health. In Microbiota and Biofertilizers; Springer International Publishing: Cham, 2021; pp. 1–15. ISBN 9783030487706. [Google Scholar]
- Yu, X.; Li, H.; Doluschitz, R. Towards Sustainable Management of Mineral Fertilizers in China: An Integrativ Analysis and Review. Sustainability 2020, 12, 7028. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global Inputs of Biological Nitrogen Fixation in Agricultural Systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Liu, A.; Ku, Y.-S.; Contador, C.A.; Lam, H.-M. The Impacts of Domestication and Agricultural Practices on Legume Nutrient Acquisition through Symbiosis with Rhizobia and Arbuscular Mycorrhizal Fungi. Front. Genet. 2020, 11, 583954. [Google Scholar] [CrossRef] [PubMed]
- Noceto, P.-A.; Bettenfeld, P.; Boussageon, R.; Hériché, M.; Sportes, A.; van Tuinen, D.; Courty, P.-E.; Wipf, D. Arbuscular Mycorrhizal Fungi, a Key Symbiosis in the Development of Quality Traits in Crop Production, Alone or Combined with Plant Growth-Promoting Bacteria. Mycorrhiza 2021, 31, 655–669. [Google Scholar] [CrossRef]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to Plant Health and Productivity from Enhancing Plant Microbial Symbionts. Front. Plant Sci. 2020, 11, 610065. [Google Scholar] [CrossRef]
- Allito, B.B.; Ewusi-Mensah. , N.; Alemneh, A.A. Rhizobia Strain and Host-Legume Interaction Effects on Nitrogen Fixation and Yield of Grain Legume: A Review. Mol. Soil Biol. 2015, 6. [Google Scholar]
- Bourion, V.; Heulin-Gotty, K.; Aubert, V.; Tisseyre, P.; Chabert-Martinello, M.; Pervent, M.; Delaitre, C.; Vile, D.; Siol, M.; Duc, G.; et al. Co-Inoculation of a Pea Core-Collection with Diverse Rhizobial Strains Shows Competitiveness for Nodulation and Efficiency of Nitrogen Fixation Are Distinct Traits in the Interaction. Front. Plant Sci. 2017, 8, 2249. [Google Scholar] [CrossRef]
- Tawaraya, K. Arbuscular Mycorrhizal Dependency of Different Plant Species and Cultivars. Soil Sci. Plant Nutr. 2003, 49, 655–668. [Google Scholar] [CrossRef]
- Shtark O., Y. , Zhukov V. A., Sulima A. S., Singh R., Naumkina T. S., Akhtemova G. A., Borisov A.Y. Prospeсts for the Use of Multi-Component Symbiotic Systems of the Legumes. Ekol. Genet. 2015, 13. [Google Scholar]
- Shtark, O.Y.; Borisov, A.Y.; Zhukov, V.A.; Tikhonovich, I.A. Mutually Beneficial Legume Symbioses with Soil Microbes and Their Potential for Plant Production. Symbiosis 2012, 58, 51–62. [Google Scholar] [CrossRef]
- Zhukov, V.A.; Zhernakov, A.I.; Sulima, A.S.; Kulaeva, O.A.; Kliukova, M.S.; Afonin, A.M.; Shtark, O.Y.; Tikhonovich, I.A. Association Study of Symbiotic Genes in Pea (Pisum Sativum L.) Cultivars Grown in Symbiotic Conditions. Agronomy (Basel) 2021, 11, 2368. [Google Scholar] [CrossRef]
- Zorin, E.A.; Sulima, A.S.; Zhernakov, A.I.; Kuzmina, D.O.; Rakova, V.A.; Kliukova, M.S.; Romanyuk, D.A.; Kulaeva, O.A.; Akhtemova, G.A.; Shtark, O.Y.; et al. Genomic and Transcriptomic Analysis of Pea (Pisum Sativum L.) Breeding Line “Triumph” with High Symbiotic Responsivity. Plants 2023, 13, 78. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms Underlying Legume-Rhizobium Symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Shumilina, J.; Soboleva, A.; Abakumov, E.; Shtark, O.Y.; Zhukov, V.A.; Frolov, A. Signaling in Legume-Rhizobia Symbiosis. Int. J. Mol. Sci. 2023, 24, 17397. [Google Scholar] [CrossRef]
- Pandey, A.K.; Rubiales, D.; Wang, Y.; Fang, P.; Sun, T.; Liu, N.; Xu, P. Omics Resources and Omics-Enabled Approaches for Achieving High Productivity and Improved Quality in Pea (Pisum Sativum L.). Züchter Genet. Breed. Res. 2021, 134, 755–776. [Google Scholar] [CrossRef]
- Mamontova, T.; Afonin, A.M.; Ihling, C.; Soboleva, A.; Lukasheva, E.; Sulima, A.S.; Shtark, O.Y.; Akhtemova, G.A.; Povydysh, M.N.; Sinz, A.; et al. Profiling of Seed Proteome in Pea (Pisum Sativum L.) Lines Characterized with High and Low Responsivity to Combined Inoculation with Nodule Bacteria and Arbuscular Mycorrhizal Fungi. Molecules 2019, 24, 1603. [Google Scholar] [CrossRef] [PubMed]
- Shtark, O.Y.; Danilova, T.N.; Naumkina, T.S.; Vasilchikov, A.G.; Chebotar, V.K.; Kazakov, A.E.; Zhernakov, A.I.; Nemankin, T.A.; Prilepskaya, N.A.; Borisov, A.U.; et al. Analysis of pea (Pisum sativum L.) source material for breeding of cultivars with high symbiotic potential and choice of criteria for its evaluation. Ekol. Genet. 2006, 4, 22–28. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Deng, X.; Wang, P.; Geng, S.; Gao, W.; Guo, P.; Chen, Q.; Li, C.; Qu, Y. Genome-Wide Analysis of Serine Carboxypeptidase-like Protein (SCPL) Family and Functional Validation of Gh_SCPL42 Unchromosome Conferring Cotton Verticillium Der Verticillium Wilt Stress in Gossypium Hirsutum. BMC Plant Biol. 2022, 22, 421. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Cambra, I.; González-Melendi, P.; Santamaría, M.E.; Díaz, I. C1A Cysteine-Proteases and Their Inhibitors in Plants. Physiol. Plant. 2012, 145, 85–94. [Google Scholar] [CrossRef]
- Barlier, I.; Kowalczyk, M.; Marchant, A.; Ljung, K.; Bhalerao, R.; Bennett, M.; Sandberg, G.; Bellini, C. The SUR2 Gene of Arabidopsis Thaliana Encodes the Cytochrome P450 CYP83B1, a Modulator of Auxin Homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 14819–14824. [Google Scholar] [CrossRef]
- Trujillo, D.I.; Silverstein, K.A.T.; Young, N.D. Nodule-Specific PLAT Domain Proteins Are Expanded in the Medicago Lineage and Required for Nodulation. New Phytol. 2019, 222, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Kim, S.-D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus Licheniformis K11 in Pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Kessler, F.; Schnell, D.; Blobel, G. Identification of Proteins Associated with Plastoglobules Isolated from Pea (Pisum Sativum L.) Chloroplasts. Planta 1999, 208, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Chai, L.; Zhang, S.; Yu, R.; Zhang, X.; Xu, C.; Hu, Y. Catabolism of Strigolactones by a Carboxylesterase. Nat. Plants 2021, 7, 1495–1504. [Google Scholar] [CrossRef]
- Zhukov, V.A. , Akhtemova G.A., Zhernakov A.I., Sulima A.S., Shtark O.Yu., Tikhonovich I.A. Evaluation of the Symbiotic Effectiveness of Pea (Pisum Sativum L.) Genotypes in Pot Experiment. Sel’skokhozyaistvennaya Biologiya 2017, 52, 607–614. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.-S. Protein Disorder in Plant Stress Adaptation: From Late Embryogenesis Abundant to Other Intrinsically Disordered Proteins. Int. J. Mol. Sci. 2024, 25, 1178. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Albacete, A.; van der Graaff, E.; Eom, S.H.; Großkinsky, D.K.; Böhm, H.; Janschek, U.; Rim, Y.; Ali, W.W.; Kim, S.Y.; et al. The Arabidopsis PLAT Domain Protein1 Promotes Abiotic Stress Tolerance and Growth in Tobacco. Transgenic Res. 2015, 24, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Function of MYB Domain Transcription Factors in Abiotic Stress and Epigenetic Control of Stress Response in Plant Genome. Plant Signal. Behav. 2016, 11, e1117723. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Zhao, W.; Fu, L.; Han, Y.; Wang, K.; Yan, L.; Li, Y.; Zhang, X.-H.; Min, D.-H. Genome-Wide Analysis of the Serine Carboxypeptidase-like Protein Family in Triticum Aestivum Reveals TaSCPL184-6D Is Involved in Abiotic Stress Response. BMC Genomics 2021, 22, 350. [Google Scholar] [CrossRef]
- Reinprecht, Y.; Schram, L.; Marsolais, F.; Smith, T.H.; Hill, B.; Pauls, K.P. Effects of Nitrogen Application on Nitrogen Fixation in Common Bean Production. Front. Plant Sci. 2020, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of Nitrate on Nodule and Root Growth of Soybean (Glycine Max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M. The Influence of Nitrate, Water Potential and Oxygen Tension on Nitrogen Fixation in Detached Pea Roots. Plant Food Hum Nutr 1978, 28, 65–69. [Google Scholar] [CrossRef]
- da-Silva, J.R.; Alexandre, A.; Brígido, C.; Oliveira, S. Can Stress Response Genes Be Used to Improve the Symbiotic Performance of Rhizobia? AIMS Microbiol. 2017, 3, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Kozik, A.; Matvienko, M.; Scheres, B.; Paruvangada, V.G.; Bisseling, T.; van Kammen, A.; Ellis, T.H.; LaRue, T.; Weeden, N. The Pea Early Nodulin Gene PsENOD7 Maps in the Region of Linkage Group I Containing Sym2 and Leghaemoglobin. Plant Mol. Biol. 1996, 31, 149–156. [Google Scholar] [CrossRef]
- Denancé, N.; Szurek, B.; Noël, L.D. Emerging Functions of Nodulin-like Proteins in Non-Nodulating Plant Species. Plant Cell Physiol. 2014, 55, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Paladi, R.K.; Srivastava, A.K.; Suprasanna, P. Thiourea and Hydrogen Peroxide Priming Improved K+ Retention and Source-Sink Relationship for Mitigating Salt Stress in Rice. Sci. Rep. 2021, 11, 3000. [Google Scholar] [CrossRef]
- Kosmacz, M.; Weits, D.A. Oxygen Perception in Plants. In Low-Oxygen Stress in Plants; Plant cell monographs; Springer Vienna: Vienna, 2014; pp. 3–17. ISBN 9783709112533. [Google Scholar]
- De Hoff, P.L.; Brill, L.M.; Hirsch, A.M. Plant Lectins: The Ties That Bind in Root Symbiosis and Plant Defense. Mol. Genet. Genomics 2009, 282, 1–15. [Google Scholar] [CrossRef]
- Coba de la Peña, T.; Frugier, F.; McKhann, H.I.; Bauer, P.; Brown, S.; Kondorosi, A.; Crespi, M. A Carbonic Anhydrase Gene Is Induced in the Nodule Primordium and Its Cell-Specific Expression Is Controlled by the Presence of Rhizobium during Development. Plant J. 1997, 11, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.K.; Schorderet, M.; Reinhardt, D. The Role of the Cell Wall Compartment in Mutualistic Symbioses of Plants. Front. Plant Sci. 2014, 5, 238. [Google Scholar] [CrossRef] [PubMed]
- Mouriz, A.; López-González, L.; Jarillo, J.A.; Piñeiro, M. PHDs Govern Plant Development. Plant Signal. Behav. 2015, 10, e993253. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Bilkova, A.; Jackson, P.; Dabravolski, S.; Riber, W.; Didi, V.; Houser, J.; Gigli-Bisceglia, N.; Wimmerova, M.; Budínská, E.; et al. Dirigent Proteins in Plants: Modulating Cell Wall Metabolism during Abiotic and Biotic Stress Exposure. J. Exp. Bot. 2017, 68, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty Acids in Arbuscular Mycorrhizal Fungi Are Synthesized by the Host Plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef]
- Marino, D.; González, E.M.; Frendo, P.; Puppo, A.; Arrese-Igor, C. NADPH Recycling Systems in Oxidative Stressed Pea Nodules: A Key Role for the NADP+ -Dependent Isocitrate Dehydrogenase. Planta 2007, 225, 413–421. [Google Scholar] [CrossRef]
- Frolov, A.; Didio, A.; Ihling, C.; Chantzeva, V.; Grishina, T.; Hoehenwarter, W.; Sinz, A.; Smolikova, G.; Bilova, T.; Medvedev, S. The Effect of Simulated Microgravity on the Brassica Napus Seedling Proteome. Funct. Plant Biol. 2018, 45, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Greifenhagen, U.; Frolov, A.; Blüher, M.; Hoffmann, R. Plasma Proteins Modified by Advanced Glycation End Products (AGEs) Reveal Site-Specific Susceptibilities to Glycemic Control in Patients with Type 2 Diabetes. J. Biol. Chem. 2016, 291, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Spiller, S.; Frolov, A.; Hoffmann, R. Quantification of Specific Glycation Sites in Human Serum Albumin as Prospective Type 2 Diabetes Mellitus Biomarkers. Protein Pept. Lett. 2017, 24, 887–896. [Google Scholar] [CrossRef]
- Lohse, M.; Nagel, A.; Herter, T.; May, P.; Schroda, M.; Zrenner, R.; Tohge, T.; Fernie, A.R.; Stitt, M.; Usadel, B. Mercator: A Fast and Simple Web Server for Genome Scale Functional Annotation of Plant Sequence Data: Mercator: Sequence Functional Annotation Server. Plant Cell Environ. 2014, 37, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Schwacke, R.; Ponce-Soto, G.Y.; Krause, K.; Bolger, A.M.; Arsova, B.; Hallab, A.; Gruden, K.; Stitt, M.; Bolger, M.E.; Usadel, B. MapMan4: A Refined Protein Classification and Annotation Framework Applicable to Multi-Omics Data Analysis. Mol. Plant 2019, 12, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Wheeler, G.L. The Ascorbate Biosynthesis Pathway in Plants Is Known, but There Is a Way to Go with Understanding Control and Functions. J. Exp. Bot. 2024, 75, 2604–2630. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.; Truffault, V.; Baldet, P.; Gautier, H. Ascorbate Oxidase in Plant Growth, Development, and Stress Tolerance. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Springer International Publishing: Cham, 2017; pp. 273–295. ISBN 9783319740560. [Google Scholar]
- Chungopast, S.; Hirakawa, H.; Sato, S.; Handa, Y.; Saito, K.; Kawaguchi, M.; Tajima, S.; Nomura, M. Transcriptomic Profiles of Nodule Senescence in Lotus Japonicus and Mesorhizobium Loti Symbiosis. Plant Biotechnol. (Tsukuba) 2014, 31, 345–349. [Google Scholar] [CrossRef]
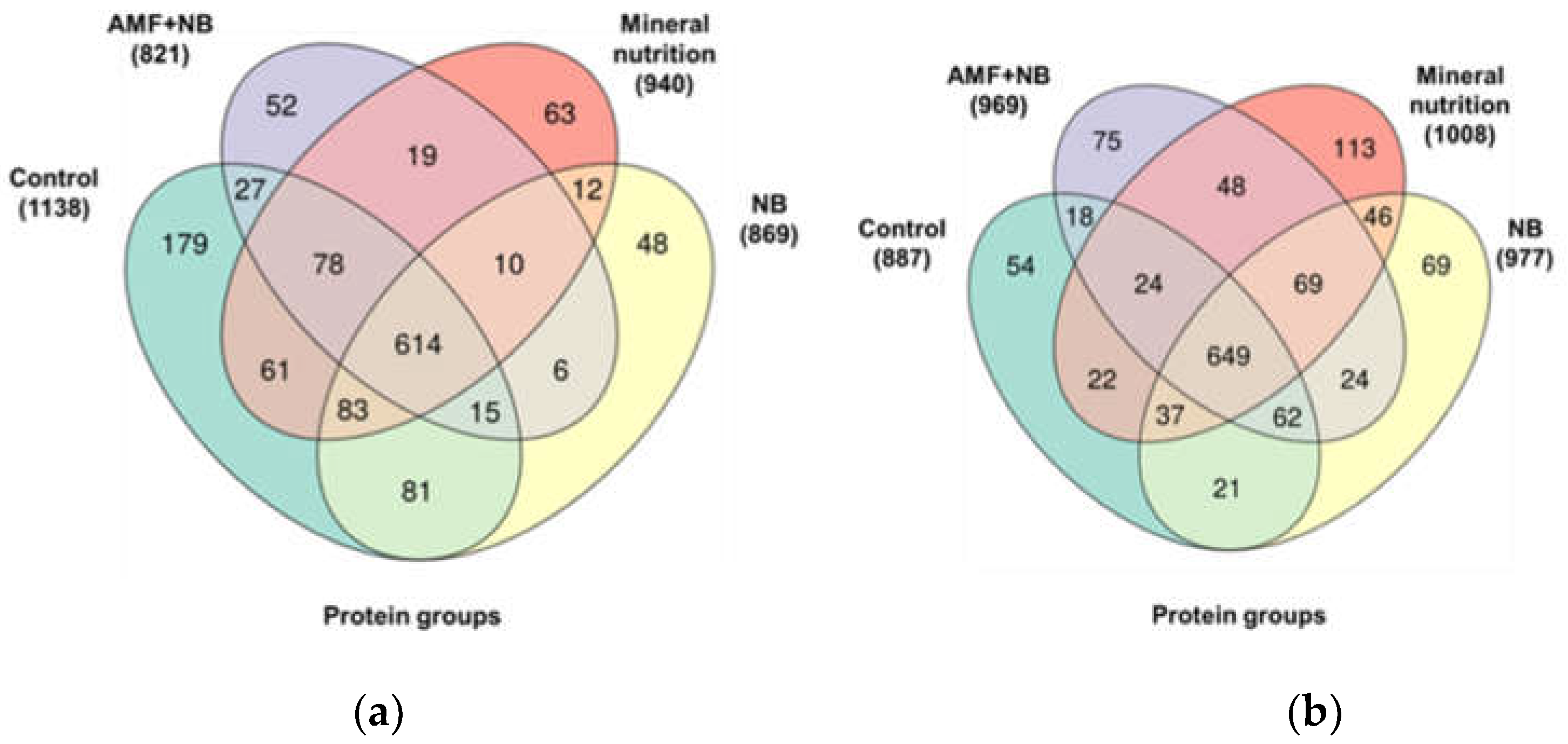
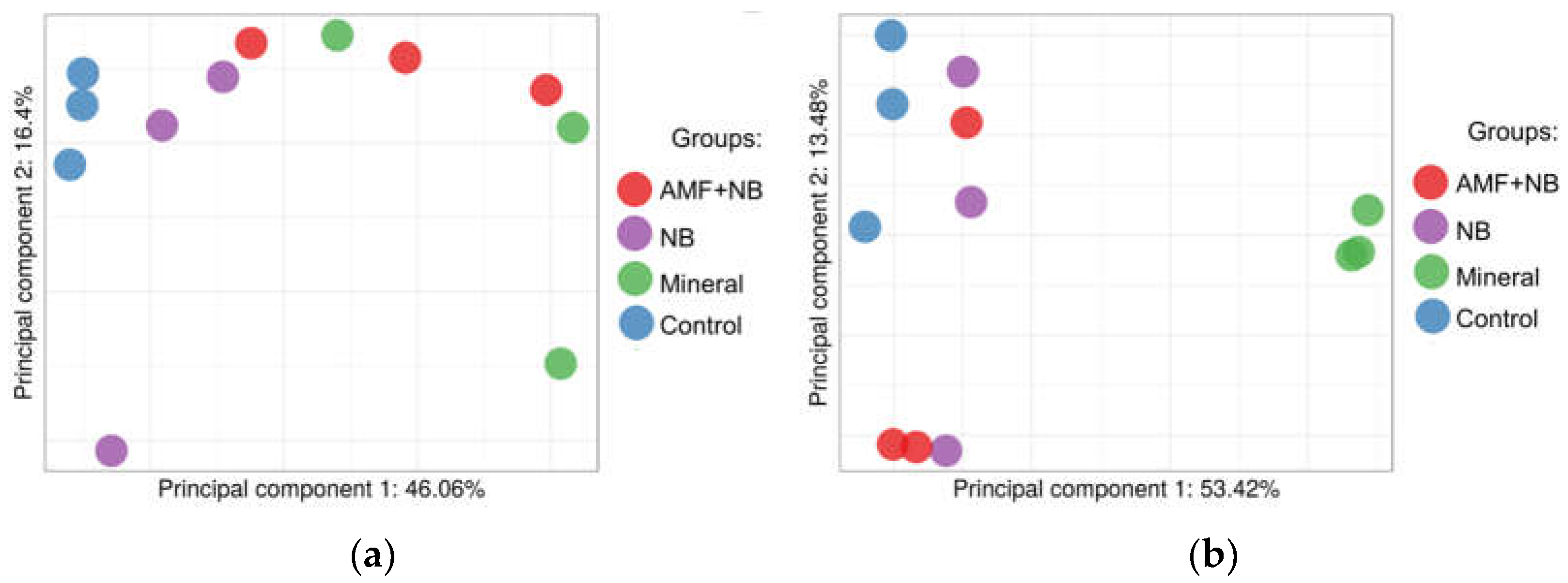
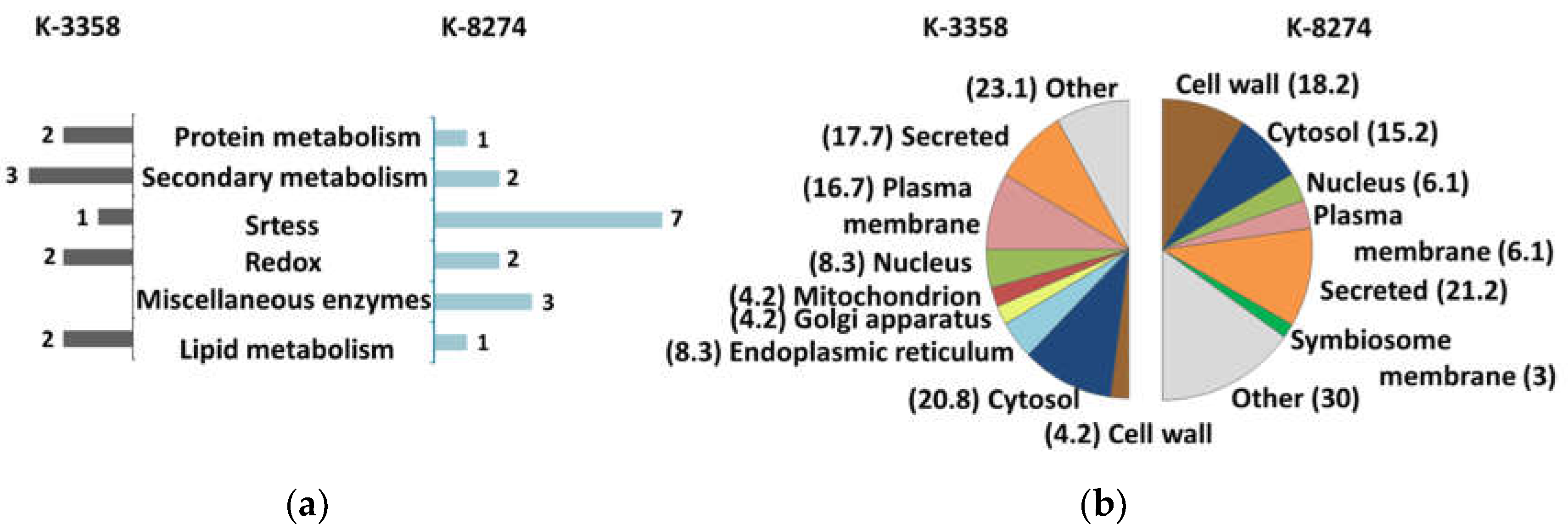
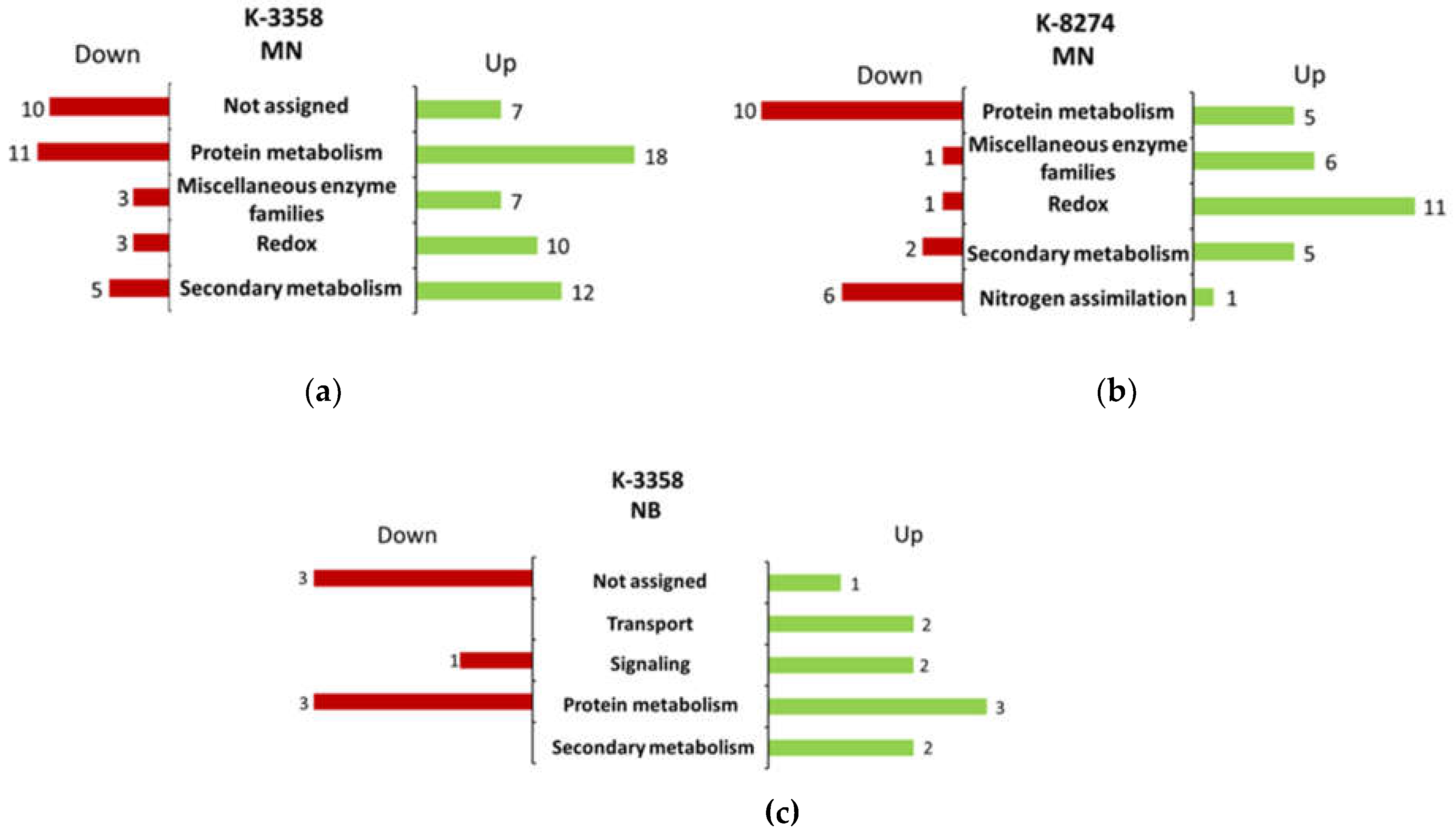
| № | Description | Accessiona | Directionof alterationsb | FCс | padj d | Prediction of localization | Functional annotation |
|---|---|---|---|---|---|---|---|
| Control of K-8274 in comparison with control of K-3358 | |||||||
| 1 | Dirigent protein | Psat7g248720.1 | up | 5.3 | 1.1E-02 | Sc | Secondary metabolism |
| 2 | data Histone H2B | Psat0s3083g0040.1 | up | 3.7 | 3.8E-03 | 3.8E-03 | DNA metabolism |
| 3 | Membrane steroid-binding protein 2-like | Psat6g199920.1 | up | 3.6 | 5.7E-03 | PM | Redox |
| 4 | HTH myb-type domain-containing protein | Psat5g257320.1 | down | 16 | 2.8E-02 | Nc | RNA metabolism |
| 5 | Phospholipase D | Psat5g302040.1 | down | 6.4 | 2.5E-03 | Ct, PM, Pl, EPR, Mt, Nc, Pd, Vc | Lipid metabolism |
| 6 | Carboxypeptidase | Psat1g016320.1 | down | 4.1 | 3.9E-03 | Sc | Protein metabolism |
| K-3358: Control in comparison with mineral nutrition | |||||||
| 7 | Histone H2B | Psat0s3083g0040.1 | up | 11.4 | 2.5E-05 | Nc | DNA metabolism |
| 8 | Non-symbiotic hemoglobin | Psat7g205800.1 | up | 4.3 | 1.8E-02 | Ct, CW, Pd, PM | Redox |
| 9 | Ferredoxin-nitrite reductase | Psat7g123960.1 | up | 4.2 | 2.0E-03 | Pl | Nitrogen assimilation |
| 10 | Heat shock protein DnaJ | Psat5g156720.1 | down | 1390.8 | 2.5E-05 | Pl | Stress |
| 11 | Leghemoglobin K | Psat7g013000.1 | down | 150.9 | 5.4E-05 | Ct | Nitrogen assimilation |
| 12 | Carbonic anhydrase | Psat0s2720g0080.1 | down | 129.2 | 7.8E-04 | Mt | Minor carbohydrate metabolism |
| K-8274: Control in comparison with mineral nutrition | |||||||
| 13 | Putative L-ascorbate oxidase | Psat4g070480.1 | up | 6.4 | 3.7E-02 | Sc, CW, Vc, PM, Pd | Development |
| 14 | Lipoxygenase | Psat0s1212g0080.1 | up | 4.1 | 2.5E-02 | Ct | Hormones |
| 15 | Ferredoxin-nitrite reductase | Psat7g123960.1 | up | 3.3 | 1.6E-02 | Pl | N-metabolism |
| 16 | Beta-fructofuranosidase cell wall isozyme 2-like protein | Psat4g188080.1 | down | 246.3 | 2.1E-03 | Sc | Major carbohydrate metabolism, Minor carbohydrate metabolism |
| 17 | Putative chromatin regulator PHD family | Psat5g278840.1 | down | 121.1 | 9.6E-05 | Nc | Protein metabolism |
| 18 | Early noduline 7 | Psat0s240g0040.1 | down | 85.5 | 6.3E-04 | SM | Nitrogen assimalation |
| K-3358: Control in comparison with inoculation by nodule bacteria | |||||||
| 19 | 60S ribosomal protein L5-2 | Psat0s1164g0040.1 | up | 3.9 | 2.5E-02 | Ct | Protein metabolism |
| 20 | Glutelin type-B-like protein | Psat7g061840.2 | up | 2.5 | 2.1E-02 | Ct | Protein metabolism |
| 21 | Phenylalanine ammonia-lyase | Psat6g072360.1 | up | 2.5 | 1.7E-02 | Ct | Secondary metabolism |
| 22 | CBS/octicosapeptide/phox/Bemp1 (PB1) domain protein | Psat5g058880.1 | down | 16.4 | 2.1E-02 | Pl, Mt | Amino acid metabolism, Protein metabolism |
| 23 | Putative serine/threonine-protein kinase | Psat1g036280.1 | down | 2.4 | 2.5E-02 | PM | Protein metabolism |
| 24 | Leghemoglobin | Psat0s241g0080.1 | down | 2.3 | 2.0E-02 | Ct | Nitrogen assimalation |
| K-3358: Control in comparison with inoculation by arbuscular micorrhiza and nodule bacteria | |||||||
| 25 | Caffeic acid O-methyltransferase | Psat3g198600.1 | up | 2.8 | 1.6E-02 | Ct, Nc, Pd, Pl | Secondary metabolism |
| 26 | Histone H2B | Psat0s3083g0040.1 | up | 2.5 | 8.2E-03 | Nc | DNA metabolism |
| 27 | Leucine-tRNA ligase, cytoplasmic isoform X1 | Psat5g170080.1 | up | 2.4 | 4.0E-02 | Ct | Protein metabolism |
| 28 | Putative serine/threonine-protein kinase | Psat1g036280.1 | down | 3.6 | 8.2E-03 | PM | Protein metabolism |
| 29 | Carboxypeptidase | Psat1g016320.1 | down | 3.3 | 8.2E-03 | Sc | Protein metabolism |
| 30 | Fatty acyl-CoA synthetase family protein | Psat7g253640.1 | down | 2.9 | 8.2E-03 | Ct, Nc | Lipid metabolism |
| K-8274: Control in comparison with mineral nutrition | |||||||
| 31 | Monocopper oxidase SKS1-like protein | Psat4g070480.1 | up | 5.5 | 1.0E-02 | Sc, CW | Development |
| 32 | HTH myb-type domain-containing protein | Psat5g257320.1 | up | 4.2 | 1.4E-02 | Nc | RNA metabolism |
| 33 | Arginase 1 | Psat0s13993g0040.1 | up | 3.4 | 2.7E-02 | Mt | Amino acid metabolism, Stress |
| 34 | Heat shock protein DnaJ | Psat5g156720.1 | down | 67.8 | 4.4E-03 | Mt | Stress |
| 35 | Early nodulin ENOD18 | Psat3g134480.1 | down | 44.9 | 9.5E-03 | Ct | Nitrogen assimalation |
| 36 | Putative serine/threonine-protein kinase | Psat1g036280.1 | down | 26.3 | 2.9E-03 | PM | Protein metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





