1. Introduction
Worldwide, breast cancer caused 670,000 deaths in 2022, making it the leading cause of cancer-related deaths among women. Additionally, there were 8.2 million people alive who had been diagnosed with breast cancer in the past five years [
1,
2]. Approximately 70% of early breast cancer (eBC) cases are hormone receptor-positive (HR+) and human epidermal growth factor receptor 2 negative (HER2-) [
3,
4,
5]. Current therapy guidelines recommend, that all patients with HR+ eBC receive either tamoxifen or aromatase inhibitors (AI), with or without ovarian suppression depending on individual risk and menopausal status, for at least five years in the adjuvant setting [
6,
7,
8]. However, the risk of local or distant recurrence remains elevated particularly in patients with large node-positive tumors or an unfavourable tumor biology [
9,
10,
11,
12]. For risk-adapted (post-neo)adjuvant treatment, combining endocrine therapy with poly(adenosine diphosphate–ribose) polymerase- (PARP-) or cyclin-dependent kinase 4 and 6- (CDK4/6-)inhibitors has been shown to significantly improve outcomes in intermediate to high-risk patients with HR+ HER2- disease [
3,
4,
9,
13,
14,
15,
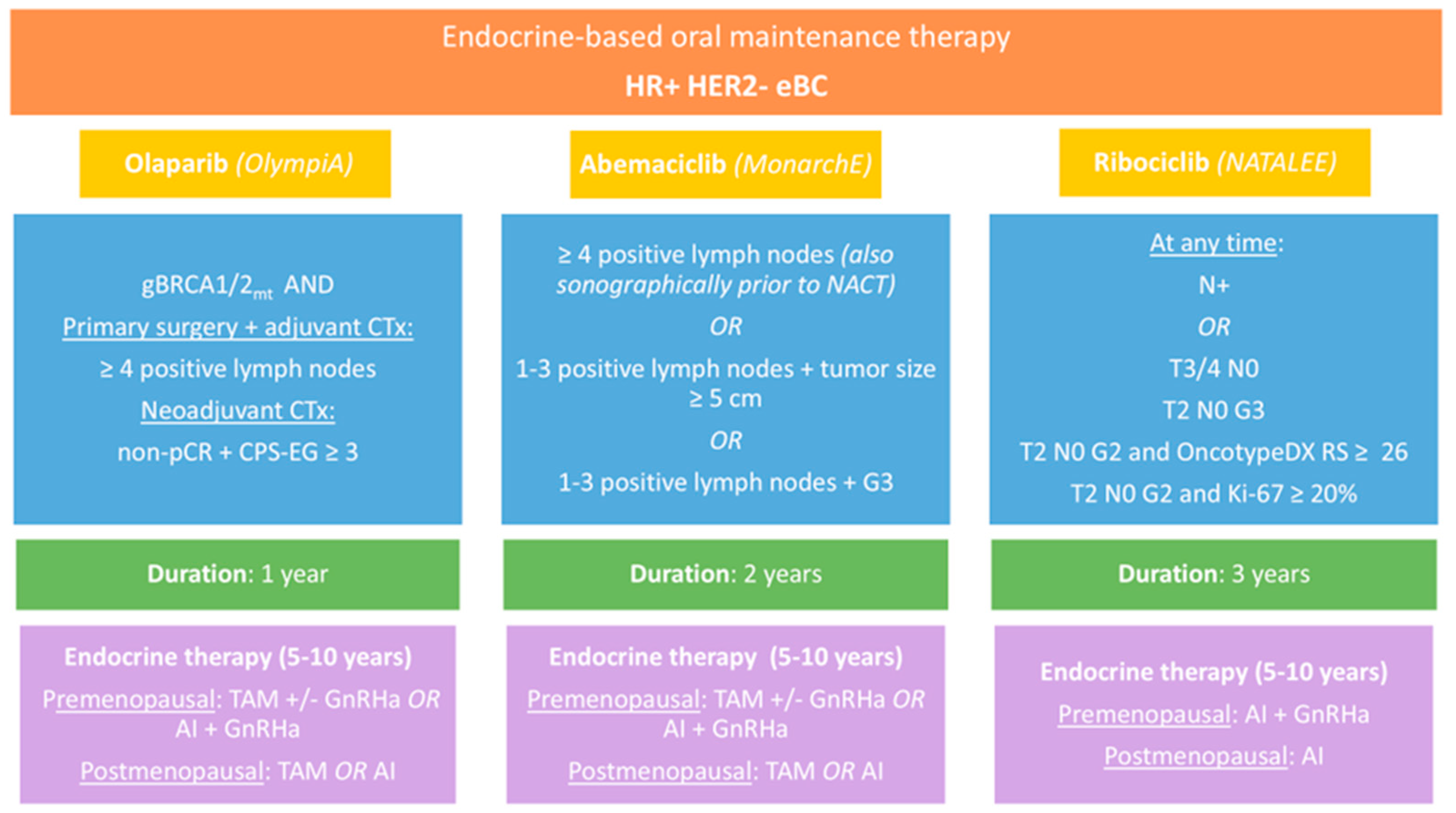
16]. Currently approved agents for endocrine-based maintenance therapy are olaparib, abemaciclib and ribociclib. All three agents have been examined in randomized phase III trials, however, the inclusion criteria in each trial differed, resulting in different patient populations eligible for treatment (
Table 1,
Figure 1).
While CDK4/6 inhibitors abemaciclib and ribociclib are indicated based on tumor characteristics, the PARP inhibitor olaparib is approved for high-risk patients with germline BRCA1/2 mutations. The randomized OlympiA trial [
9,
13] was originally designed for triple negative eBC but amended for HR+ HER2- tumors with high risk of recurrence. Eligible patients were required to have completed definitive local treatment, including radiotherapy, as well as (neo-)adjuvant chemotherapy, containing anthracyclines, taxanes or the combination of both for at least six cycles. The use of platinum agents was allowed. To meet high-risk criteria, patients with HR+ HER2- eBC receiving primary surgery and adjuvant chemotherapy were required to have at least four pathologically confirmed metastatic lymph nodes. Patients receiving neoadjuvant chemotherapy (NACT) were eligible in case of residual disease at the time of surgery (no pathologic complete response = non-pCR) and a CPS+EG-Score ≥ 3. The CPS+EG score is calculated based on the clinical and pathological stage as well as the estrogen receptor status and nuclear grade and correlated with relapse probability after neoadjuvant chemotherapy [
13,
17]. Olaparib was administered for one year and combined with adjuvant endocrine therapy.
Unlike PARP inhibitors, CDK4/6 inhibitors provide therapeutic potential for a broader patient population, as their effectiveness is independent of BRCA mutation status. Three CDK4/6 inhibitors are approved for use in the metastatic setting. However, only two have demonstrated a significant benefit in eBC, whereas palbociclib failed to do so in the PENELOPE-B and PALLAS trials [
14,
18,
19]. Abemaciclib was approved for eBC following the randomized monarchE study [
3,
14]. The trial focused on patients with high-risk disease characteristics, such as high nodal burden (≥ 4 positive nodes) or 1-3 positive nodes and at least one of the following: (cohort 1) tumor size ≥ 5cm, histologic grade 3 or (cohort 2) centrally assessed Ki-67 ≥ 20%. However, the approval label does not include cohort 2. In contrast to the OlympiA and monarchE trials, the NATALEE study investigating ribociclib also included patients with intermediate risk of relapse [
4,
15,
16]. Eligibility required patients to be either node-positive or to have stage IIB/III eBC at the worst point since diagnosis. Additionally, patients with stage IIA node-negative eBC were included if they met one of the following: histologic grade G3, or G2 with either a high proliferation index (Ki-67 ≥ 20%) or classification in a genomic high-risk group. 28% of study participants had node-negative disease at time of diagnosis.
While all three agents improved outcomes in selected subgroups of HR+ HER2- eBC patients, real-world experience is still limited. Notably, the application of the NATALEE inclusion criteria is expected to significantly increase patient volume [
20]. The aim of the present analysis was to evaluate how many patients can be considered potential candidates for one or more maintenance therapy options at a large university breast cancer center.
2. Materials and Methods
The single-center retrospective analysis was conducted at the certified Breast Cancer Center of the University Hospital Schleswig-Holstein, Campus Lübeck, and included all patients who were treated for eBC between January 2014 and December 2023. This analysis was carried out in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethical Committee of the University of Luebeck (file number: 2024-496). The analysis included both men and women with non-metastatic disease. The evaluation of HR and HER2 receptor expression was performed by certified pathologists in accordance with established local standards. Tumors were classified as HR+ if they exhibited positive ER and/or PR expression via immunohistochemistry, with a minimum of 1% for ER and 1% for PR. HER2 immunoreactivity was assessed on a scale of 0 to 3+ using the approved test. Tumors with immunohistochemical score of 2+ were analyzed by in situ hybridization. For the assessment of HER2 status, ASCO/CAP guidelines at the time of tissue examination were followed.
In order to identify the potential candidates for the respective substances olaparib, abemaciclib and ribociclib, the approval criteria for olaparib, abemaciclib and ribociclib were applied. In patients who met the OlympiA inclusion criteria, the gBRCA1/2 mutation status was not systematically recorded due to the retrospective nature of the analysis. Therefore, these are potential candidates for PARP inhibitor therapy with olaparib. In line with the monarchE and NATALEE studies, the indication for abemaciclib and ribociclib for patients undergoing neoadjuvant chemotherapy was based on the pretherapeutic or postoperative TNM stage, whichever was prognostically worse.
The data analysis was conducted using Excel 2311 and Statistical Package for Social Sciences (IBM SPSS Statistics, Version 29.0.2.0, Armonk, NY: IBM Corp). The results were used to create a Venn diagram using Python 3.10.12 with the following libraries: math, matplotlib.pyplot, and matplotlib_venn.
3. Results
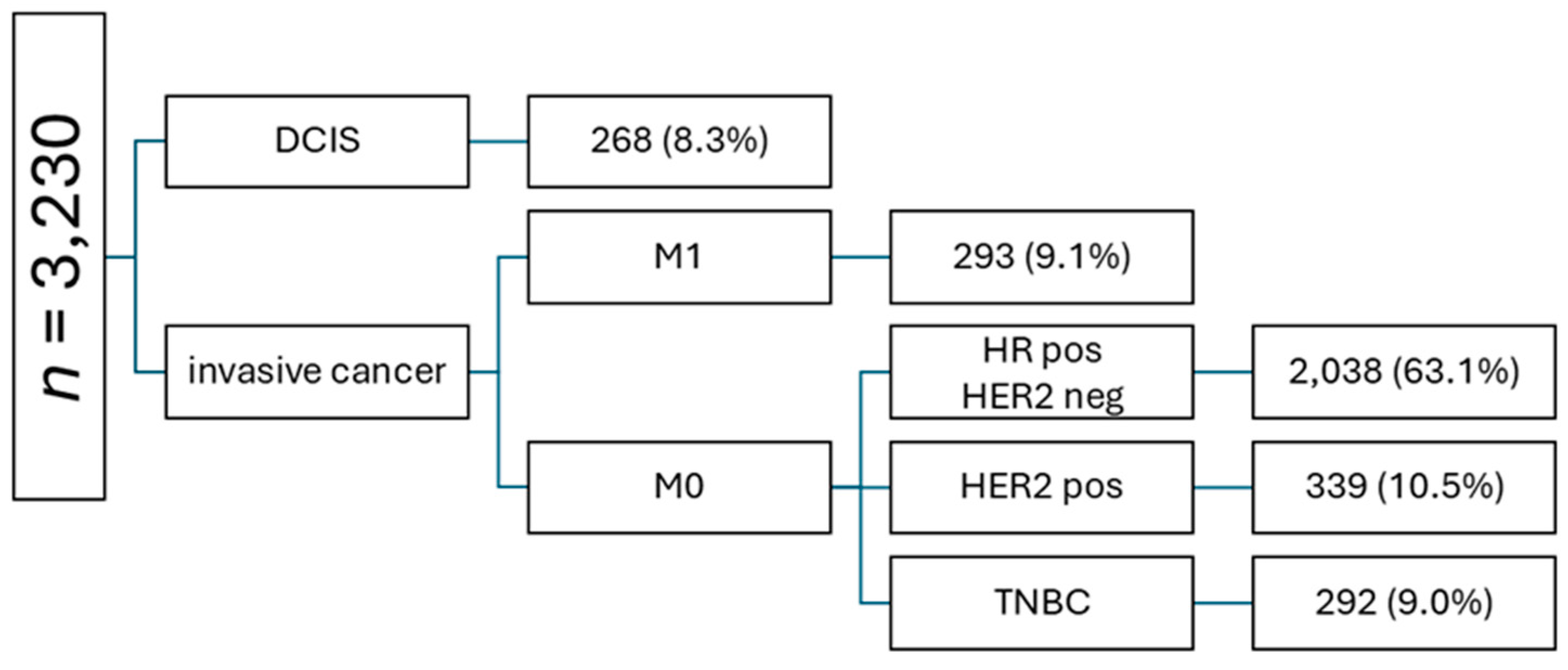
A total of 3,230 patients with newly diagnosed breast cancer were identified (
Figure 2). 268 patients with ductal carcinoma in situ (DCIS) and 293 with de novo metastatic disease were excluded. The most prevalent tumor subtype among patients with invasive eBC was HR+ HER2− (2,038, 63.1%), followed by HER2+ (10.5%) and TNBC (9.0%). 2,038 patients with invasive HR+ HER2- eBC were included in further analyses. Out of these patients, 244 (12.0%) received NACT and 1,794 (88.0%) underwent primary surgical treatment.
In total, 146 out of 2,038 patients (7.2%) would have met the criteria for a potential use of olaparib according to the OlympiA trial, provided they had a germline BRCA1 or BRCA2 mutation. Due to the retrospective nature of our study, the data on the prevalence of (likely) pathogenic mutations in this patient population were not available. Among the 1,794 patients undergoing primary surgery, 60 (3.3%) had a pN2 stage and 35 (2.0%) pN3 stage. Out of the 244 patients who received NACT, 51 (20.9%) had non-pCR and a CPS-EG score ≥ 3 (39 [16.0%] CPS-EG score 3 and 12 [4.91%] score 4). In the case of a gBRCA mutation, these patients would have been eligible for olaparib therapy.
The criteria for abemaciclib according to the approval label (i.e., cohort 1 of the monarchE study) were met by a total of 312 (15.3%) out of the 2,038 patients. Among these, 95 (30.4%) had ≥ 4 positive lymph nodes after primary surgery, and 36 (11.5%) had ≥ 4 positive/suspicious axillary lymph nodes before and/or after NACT (≥ cN2 and/or ≥ ypN2). 48 (15.4%) had tumor size of ≥ 5 cm with 1-3 positive lymph nodes after primary surgery, while 25 (8.0%) had tumors ≥ 5 cm and 1-3 positive/suspicious axillary lymph nodes before and/or after NACT (cN1 and/or ypN1). 68 (21.8%) patients had grade 3 tumors < 5 cm and 1-3 positive lymph nodes after primary surgery, while 40 (12.8%) had grade 3 tumors < 5 cm and 1-3 positive/suspicious nodes before and/or after NACT (cN1/ypN1). As mentioned before, the approval criteria do not include cohort 2 (i.e., patients with 1-3 positive nodes and Ki67 ≥ 20%). Were these criteria included in the approval label, additional 46 patients (12,9%) would have been eligible for abemaciclib, while for 63 additional patients with 1-3 positive/suspicious axillary lymph nodes, no Ki-67 value was available.
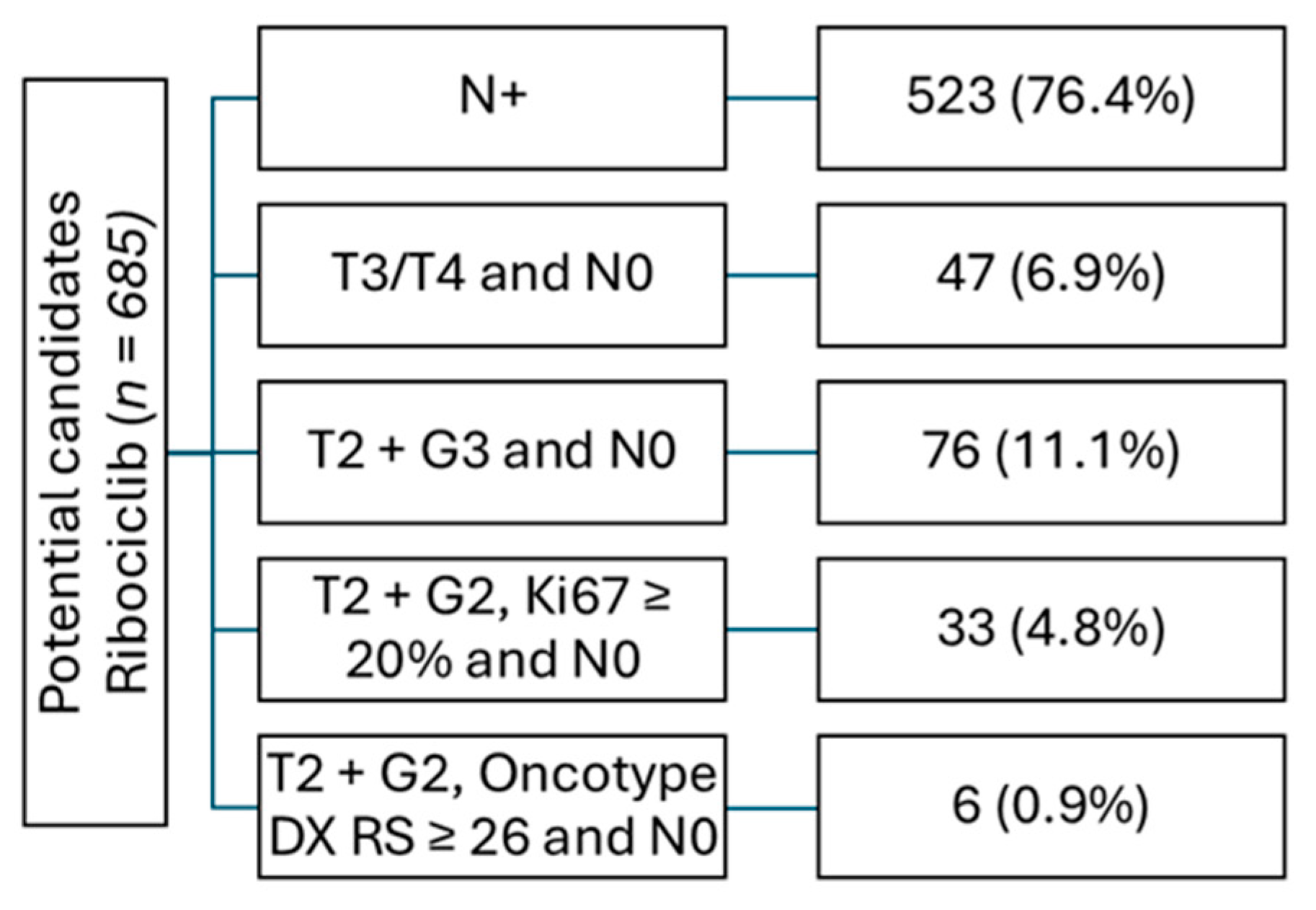
A total of 685 patients (33.6%) met the indication criteria for ribociclib according to the NATALEE study. Within this cohort, 523 (76.4%) had node-positive disease, thereby meeting the indication for ribociclib based solely on nodal status. Among the 162 node-negative patients, 47 (29.0%) had a T3/T4 stage, and 76 (46.9%) had a T3/G2 stage. In addition, 33 (20.4%) patients met NATALEE study criteria due to a T2/G2 stage with a Ki67 index of ≥ 20%, and only 6 (3.7%) due to a T2/G2 stage with an additional high genomic risk (e.g., Oncotype DX with a recurrence score ≥ 26 or high-risk profile in Prosigna/PAM50, Mammaprint, or Endopredict). It should be noted, however, that 47 patients with a T2/G2 stage and low Ki67 index did not undergo genomic testing and therefore it remains unclead whether they would have been eligible for ribociclib treatment in accordance with the NATALEE criteria (
Figure 3).
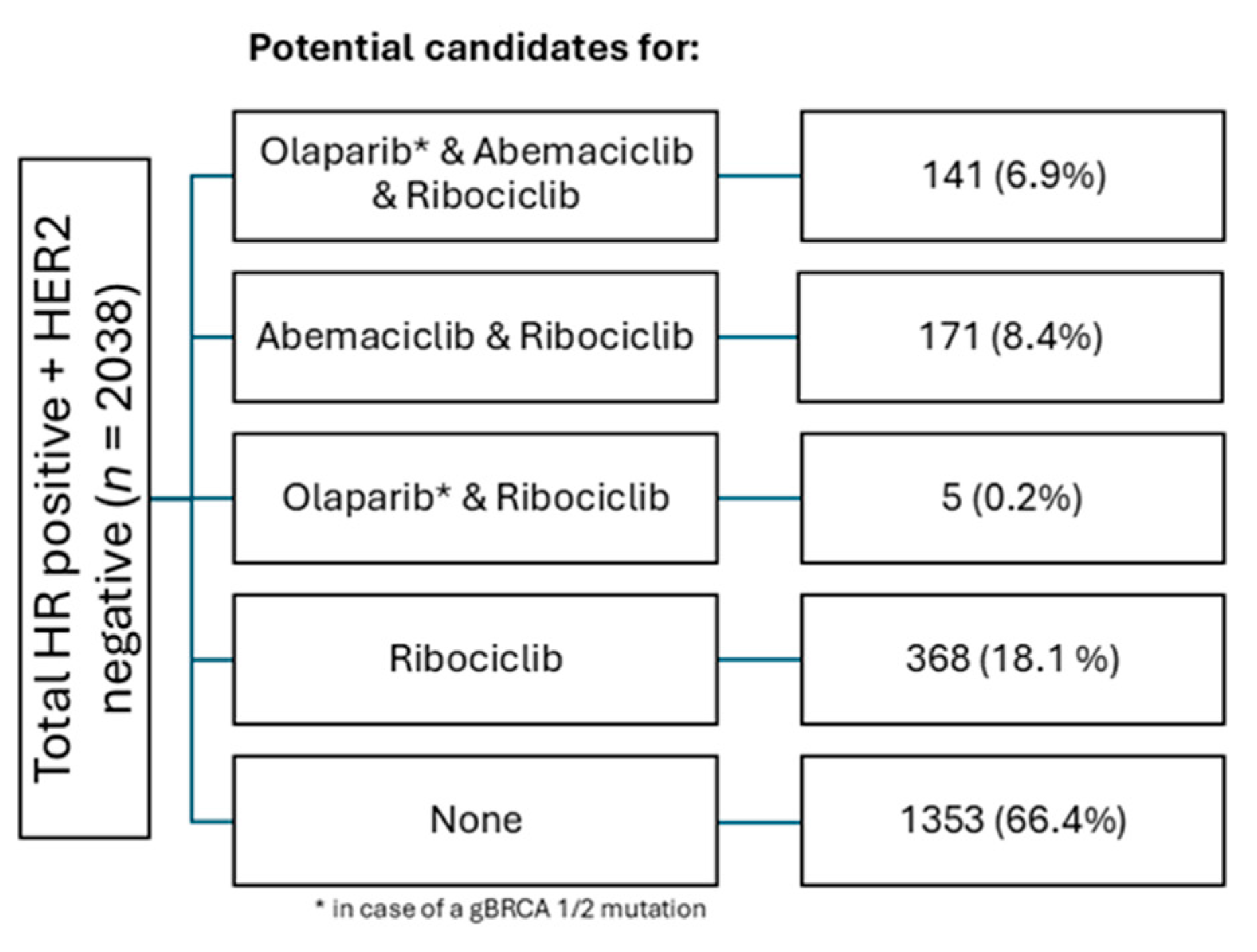
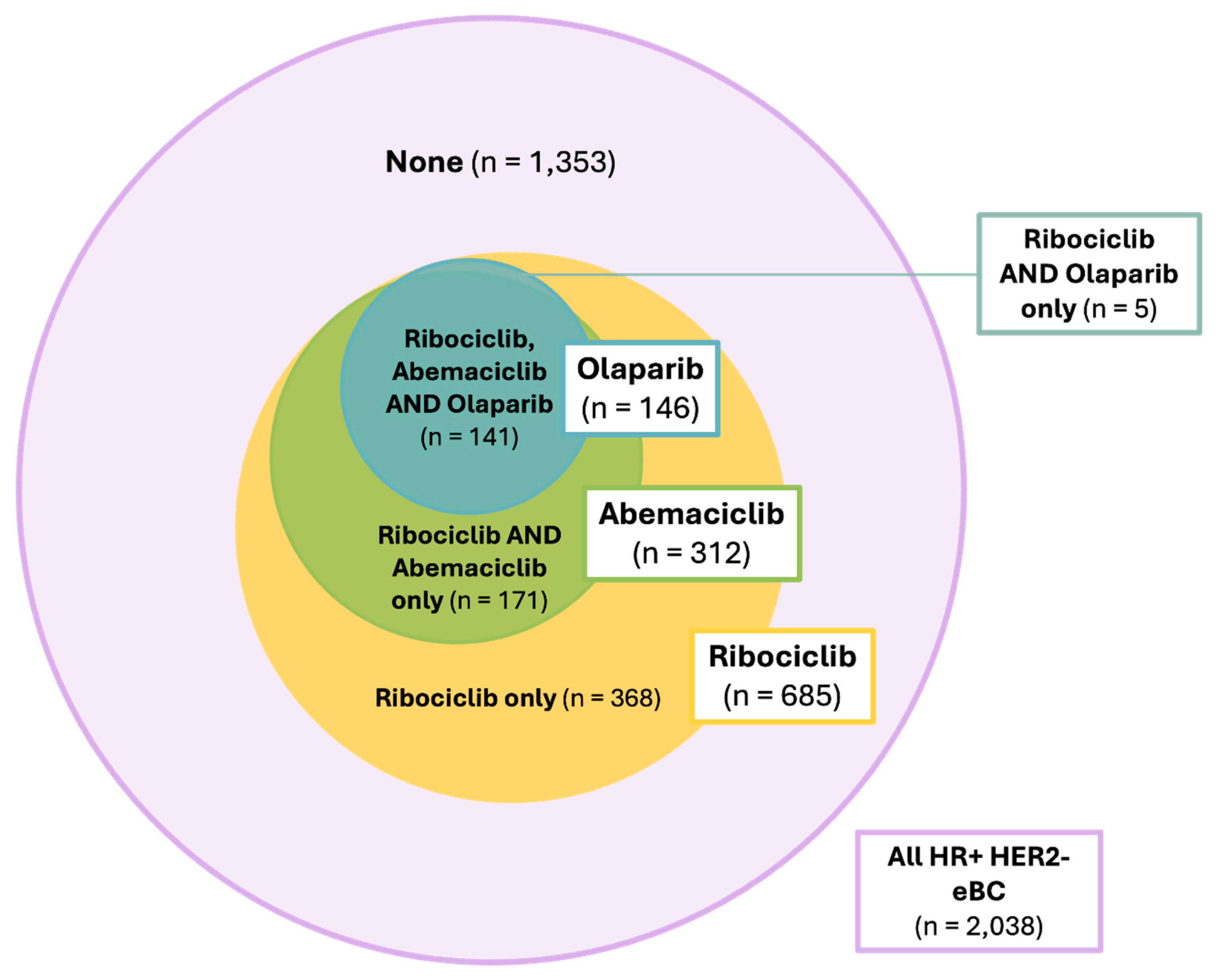
Out of 2,038 patients with HR+ HER2- eBC, 141 (6.9%) of patients met the indication for all three agents (
Figure 4). 171 (8.4%) of patients met the criteria for abemaciclib and ribociclib, and 5 (0.2%) could have been treated with olaparib or ribociclib. The largest subgroup, however, consisted of patients with an indication for ribociclib only (368, 18.1%). In total, 1,353 (66.4%) of all patients with HR+ HER2- eBC did not meet the criteria for any form of combined oral maintenance therapy (
Figure 5).
Therapy monitoring of oral endocrine-based maintenance therapies in early breast cancer is performed in regular intervals. The approval of ribociclib led to increased therapeutic options. However, therapy duration of ribociclib is one year longer compared to abemaciclib, which leads to a higher demand of health care providers’ personnel capacity (
Figure 1).
Table 2 describes the estimated duration of patient contacts during therapy management. Time requirement per patient contact will be reduced in the course of therapy. Approximately 31 patients per year will be eligible for abemaciclib treatment, whereas 69 patients per year will be eligible for abemaciclib or ribociclib treatment (
Table 3 and
Table 4). Provided 100% therapy adherence, health care providers will spend 165 hours for therapy management of abemaciclib per year and 506 hours for therapy management of ribociclib per year. Thus, the approval of ribociclib leads to a significantly higher demand of health care providers’ personnel capacity. Assuming a full-time position with 8 hours/day for a year with 220 working days (weekdays minus public holidays and statutory vacation days in Germany), the 506 hours for doctor-patient contacts in the context of potential ribociclib prescriptions represent a part-time position at 29% (0.29).
4. Discussion
To the best of our knowledge, this is the largest analysis on the potential indication for all three agents used for endocrine-based maintenance therapy in early breast cancer. While olaparib and abemaciclib are only recommended for patients with node-positive HR+ HER2- disease, the NATALEE study included node-negative patients with intermediate risk of relapse as well. In accordance, we show that the number of potential candidates for combined adjuvant treatment will more than double due to ribociclib approval in 2024. We could furthermore demonstrate that the approval of ribociclib leads to three times higher demand of health care providers’ personnel capacity.
While 7% of all evaluated patients met the indications for all three agents, 18% were eligible for ribociclib only. It remains to be discussed how prescribing ribociclib for this large group of patients may impact the workload of outpatient oncologic clinics. Particularly before starting ribociclib therapy and during the first two cycles, blood sampling is recommended every 2 weeks, which may be reduced to monthly from the 3rd cycle. From then on, laboratory examinations are only required if clinically indicated. In September 2024, the recommended number of electrocardiograms was reduced to two (before therapy initiation and 2 weeks afterwards) instead of three [
21]. Despite this reduction in diagnostic ECG screenings, there remains a significantly increased number of additional patient appointments in clinics due to consultations, counseling sessions, and follow-up visits. Socioeconomic adjustments, such as expanding staffing capacity, seem both reasonable and necessary to manage this increased workload resulting from the higher frequency of doctor-patient interactions.
When comparing the results presented here with those from other real-world analyses, there are some minor differences in the percentage distribution of the respective patient cohorts for each substance. In contrast to another real-world analysis which found that a total of 43% of all HR+/HER2- eBC cases met the NATALEE inclusion criteria [
20], only 34% of patients in our study met those criteria. However, among the T2 N0 G2 cohort, there remain 47 patients (2%) who did not receive genomic testing due to low risk clinical-pathological characteristics and may also have been considered potential candidates for ribociclib therapy depending on their genomic risk levels.
The determination of the required ER and PR expression for a positive HR status at a minimum of 1% was based on the results of a large retrospective data analysis from the National Cancer Database (NCDB), which demonstrated an OS benefit in patients with low ER expression (1–10%) who received endocrine therapy compared to those who did not [
22]. In this respect, the present analysis differs from the previous study by Schäffler et al., which defined a positive HR status as 10% or higher [
20].
OlympiA and monarchE trials enrolled patients with high risk tumors, however, inclusion criteria in the NATALEE study were much broader, thus allowing for inclusion of patients with intermediate risk profiles. As a result, the question arose whether all patients fulfilling the inclusion criteria should be offered combined therapy in the clinical routine. At the ESMO Congress 2024, Fasching et al. reported that ribociclib improved iDFS in patients with node-negative tumors from 87.0% to 92.1% after 4 years (absolute benefit 5.1%, HR 0.666) [
15]. While 88% of patients enrolled in the NATALEE study had node-positive tumors [
4,
15], 75% of potential candidates for ribociclib therapy in our study were node-positive. Thus, our real-world analysis revealed a lower percentage of node-negative patients who are eligible for therapy compared to the NATALEE study cohort.
In our study, 15% of patients were identified as potential candidates for adjuvant abemaciclib. This is in accordance with other global real-world analyses showing that this population may comprise between 14% and 18% of all patients with HR+ HER2- tumors [
20,
23,
24]. An important principle in the context of planned neoadjuvant treatment is the precise documentation of the number of sonographically suspicious axillary lymph nodes before start of therapy. This is crucial because the number of metastatic nodes after treatment can be lower due to treatment effects, and it is worth noting that the indication for abemaciclib therapy can be based on both pretherapeutic and post-surgery tumor stage, whichever meets the monarchE inclusion criteria [
25,
26].
When evaluating patients eligible for adjuvant olaparib, Dannehl et al. identified a total of 7.5% of all patients with HR+ HER2- eBC as potential candidates [
27], which closely aligns with the 7.2% found in our study. However, it is important to note that literature indicates that only 2.7% to 7.8% of all patients with HR+ HER2- eBC have a gBRCA mutation, with varying prevalence rates for gBRCA1 and gBRCA2 mutations [
28,
29]. Thus, a maximum of 12 out of 146 potential candidates for olaparib would actually be able to receive olaparib. Interestingly, some mutational carriers may be more likely to be eligible for olaparib if they receive primary surgery, since patients with ≥ 4 positive lymph nodes at time of diagnosis not always reach a CPS-EG score of 3 or higher.
It remains to be discussed whether all patients with an indication for at least one of the three substances for oral maintenance therapy would actually begin treatment in clinical practice, and whether they would complete the entire course of therapy. The latest study data indicate that 35.5% of patients discontinued ribociclib early, with a median time to discontinuation of 4 months. In the ribociclib plus NSAI arm, the primary reason for early discontinuation was adverse events (AEs), which occurred in 19.5% of cases [
4,
30,
31]. Special situations, such as those involving older patients, as well as patient-related factors like therapy adherence and compliance, could reduce the actual number of patients undergoing oral maintenance therapy in everyday clinical settings, even though the prescribing information generally allows for dose adjustments or temporary therapy pauses in the case of severe adverse drug reactions [
32]. Since combined endocrine therapy with ribociclib is prescribed for a fixed duration of 3 years, it is essential to consider the health-related quality of life (HR-QoL) in these patients. For ribociclib in eBC, there are only a few studies assessing HR-QoL [
33] and overall, there is a general lack of real-world data on HR-QoL in patients treated with CDK 4/6 inhibitors [
34,
35]. Another aspect to consider are the potential forensic implications that could arise from the approval of ribociclib for HR2+ HER2- eBC, particularly if genomic testing is not conducted in patients with T2 N0 G2 disease. This could hypothetically lead to undertreatment and subsequent worsening of prognosis for these patients, potentially resulting in legal disputes.
5. Conclusions
This single-center analysis examined the number of potential candidates for combined endocrine therapy in a real-world context. While just under 7% of the cohort had an indication for all three agents (olaparib, abemaciclib, and ribociclib), over 18% met the approval criteria for ribociclib only, whereas over 66% of patients had no indication for combined endocrine therapy at all. To the best of our knowledge, this is the largest analysis addressing all three therapy strategies. It remains to be discussed to what extent the broad indication criteria for the NATALEE study will increase the workload in clinics through more frequent physician-patient interactions. It also remains open how therapy recommendations will impact the actual treatments administered, as more frequent patient visits and potential side effects may impact both compliance and therapy adherence.
Author Contributions
Conceptualization, N.T., L.H., K.M., A.R., M.BP.; methodology, N.T., L.H., A.R., M.BP.; software, N.T. T.ML.; validation, format analysis, investigation, resources, data curation N.T., L.H., M.BP.; writing—original draft preparation, N.T., L.H., D.D., A.H., A.R., M.BP.; writing—review and editing, all authors.; visualization, N.T., L.H., T.ML, A.R., M.BP.; supervision, A.R., M.BP.; project administration, N.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University of Lübeck and was evaluated favourably (Date of approval 16.09.2024. Reference number 2024-496).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
NT received honoraria for lectures and participation in advisory boards: Novartis, ExactSciences, Georg Thieme Verlag. Support for attending meetings from Astra Zeneca. DD received honoraria for lectures and participation in advisory boards: Astra Zeneca, Gilead, Novartis, Daichii Sankyo, Onkowissen, Oncologics. Travel expenses: Gilead and Daichi Sankyo. FF received lecture honoraria from Novartis and travel expenses from Astra Zeneca. FH received honoraria for lectures and participation in advisory boards: Pfizer, Novartis, Daiichi Sankyo, Eisei, AstraZeneca, GSK, ViforPharma, MSD, Roche, Clovis Oncology. Travel expenses: MSD, AstraZeneca, Intuitive, Olympus, Gynesonics. HP received honoraria for lectures and participation in advisory boards: Roche, Novartis, Pfizer, Eli Lilly, AstraZeneca, MSD, GSK, Daiichi Sankyo, Gilead, Pierre Fabre, Viatris. Travel expenses: Novartis, Gilead, Daiichi Sankyo. AH received honoraria for lectures and participation in advisory boards: AstraZeneca, Agendia, Amgen, Clovis, DaichiiSankyo, Eisai, ExactScience, Gilead, GSK, Hexal, Lilly, MSD, Novartis, Onkowissen, Pfizer, Roche, Pierre-Fabre, Seagen. AR has received lecture and consulting honoraria from: Roche, Pfizer, Novartis, Celgen, Novartis, ExactSciences, Pierre Fabre, Lilly, Seagen, Astra Zeneca, Eisai, MSD, Hexal, Amgen. MBP is Associate Editor of Archives of Gynecology and Obstetrics and European Journal of Surgical Oncology. Honoraria for lectures and participation in advisory boards: Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, Canon, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Medical, Syantra, resitu, Pierre Fabre, ExactSciences. Study support: Damp Stiftung, AWOgyn, AGO-B, Claudia von Schilling Breast Cancer Research Foundation, Ehmann Stiftung, EndoMag, Mammotome, MeritMedical, Sirius Medical, Gilead, Hologic, ExactSciences. Travel expenses: Eli Lilly, ExactSciences, Pierre Fabre, Pfizer, Daiichi Sankyo, Roche. Other authors declare they have no financial interests.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ferlay J, E.M.; Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf (accessed on 26 November 2024).
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Lipatov, O.; Nowecki, Z.; McAndrew, N.; Kukielka-Budny, B.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.S.; Fasching, P.A.; Crown, J.; et al. Ribociclib plus Endocrine Therapy in Early Breast Cancer. N Engl J Med 2024, 390, 1080–1091. [Google Scholar] [CrossRef]
- Institute, N.C. Cancer Stat Facts: Female Breast Cancer Subtypes. Surveillance, Epidemiology and End Results Program 2024.
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2024, 35, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Diagnostik und Therapie früher und fortgeschrittener Mammakarzinome. Herausgegeben von der Kommission Mamma (vertreten durch: Wolfgang Janni) der Arbeitsgemeinschaft Gynäkologische Onkologie e.V. in der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe e.V. sowie in der Deutschen Krebsgesellschaft e.V. 2024.
- Park-Simon, T.W.; Muller, V.; Albert, U.S.; Banys Paluchowski, M.; Bauerfeind, I.; Blohmer, J.U.; Budach, W.; Dall, P.; Ditsch, N.; Fallenberg, E.M.; et al. Arbeitsgemeinschaft Gynakologische Onkologie Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2024. Breast Care (Basel) 2024, 19, 165–182. [Google Scholar] [CrossRef]
- Geyer, C.E., Jr.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol 2022, 33, 1250–1268. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.S.; Hennigs, A.; Feisst, M.; Moderow, M.; Heublein, S.; Deutsch, T.M.; Togawa, R.; Schafgen, B.; Wallwiener, M.; Golatta, M.; et al. Impact of age on indication for chemotherapy in early breast cancer patients: results from 104 German institutions from 2008 to 2017. Arch Gynecol Obstet 2023, 308, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Heusinger, K.; Haeberle, L.; Niklos, M.; Hein, A.; Bayer, C.M.; Rauh, C.; Schulz-Wendtland, R.; Bani, M.R.; Schrauder, M.; et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 2011, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Loehberg, C.R.; Almstedt, K.; Jud, S.M.; Haeberle, L.; Fasching, P.A.; Hack, C.C.; Lux, M.P.; Thiel, F.C.; Schrauder, M.G.; Brunner, M.; et al. Prognostic relevance of Ki-67 in the primary tumor for survival after a diagnosis of distant metastasis. Breast Cancer Res Treat 2013, 138, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; O’Shaughnessy, J.; Martin, M.; Boyle, F.; Cortes, J.; Rugo, H.S.; Goetz, M.P.; Hamilton, E.P.; Huang, C.S.; Senkus, E.; et al. Adjuvant Abemaciclib Plus Endocrine Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, High-Risk Early Breast Cancer: Results From a Preplanned monarchE Overall Survival Interim Analysis, Including 5-Year Efficacy Outcomes. J Clin Oncol 2024, 42, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Stroyakovskiy, D.; Yardley, D.; Huang, C.S.; Crown, J.P.; Bardia, A.; Chia, S.; Im, S.A.; Martin Jimenez, M.; Xu, B.; et al. LBA13 Adjuvant ribociclib (RIB) plus nonsteroidal aromatase inhibitor (NSAI) in patients (Pts) with HR+/HER2− early breast cancer (EBC): 4-year outcomes from the NATALEE trial. Annals of Oncology 2024, 35, S1207. [Google Scholar] [CrossRef]
- Slamon, D.J.; Fasching, P.A.; Hurvitz, S.; Chia, S.; Crown, J.; Martín, M.; Barrios, C.H.; Bardia, A.; Im, S.A.; Yardley, D.A.; et al. Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breast cancer. Ther Adv Med Oncol 2023, 15, 17588359231178125. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Jeruss, J.S.; Tucker, S.L.; Kolli, A.; Newman, L.A.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U.; et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol 2011, 29, 1956–1962. [Google Scholar] [CrossRef]
- Loibl, S.; Marme, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.B.; Bear, H.; McCarthy, N.; Mele Olive, M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J Clin Oncol 2021, 39, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Dueck, A.C.; Frantal, S.; Martin, M.; Burstein, H.J.; Greil, R.; Fox, P.; Wolff, A.C.; Chan, A.; Winer, E.P.; et al. Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J Clin Oncol 2022, 40, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, H.; Mergel, F.; Pfister, K.; Lukac, S.; Fink, A.; Veselinovic, K.; Rack, B.; Fink, V.; Leinert, E.; Dimpfl, M.; et al. The Clinical Relevance of the NATALEE Study: Application of the NATALEE Criteria to a Real-World Cohort from Two Large German Breast Cancer Centers. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Novartis Pharma. Fachinformation Kisqali 200mg Filmtablette; 2024.
- Choong, G.M.Y.; Hoskin, T.L.; Boughey, J.C.; Ingle, J.N.; Goetz, M.P. The impact of adjuvant endocrine therapy (AET) omission in ER-low (1-10%) early-stage breast cancer. Journal of Clinical Oncology 2024, 42, 513–513. [Google Scholar] [CrossRef]
- Sheffield, K.M.; Peachey, J.R.; Method, M.; Grimes, B.R.; Brown, J.; Saverno, K.; Sugihara, T.; Cui, Z.L.; Lee, K.T. A Real-World US Study of Recurrence Risks using Combined Clinicopathological Features in HR-Positive, HER2-Negative Early Breast Cancer. Future Oncology 2022, 18, 2667–2682. [Google Scholar] [CrossRef]
- Dannehl, D.; Volmer, L.L.; Weiss, M.; Matovina, S.; Grischke, E.M.; Oberlechner, E.; Seller, A.; Walter, C.B.; Hahn, M.; Engler, T.; et al. Feasibility of Adjuvant Treatment with Abemaciclib-Real-World Data from a Large German Breast Center. J Pers Med 2022, 12. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Gruber, I.V.; Hartkopf, A.; Paluchowski, P.; Krawczyk, N.; Marx, M.; Brucker, S.; Hahn, M. Axillary ultrasound for prediction of response to neoadjuvant therapy in the context of surgical strategies to axillary dissection in primary breast cancer: a systematic review of the current literature. Arch Gynecol Obstet 2020, 301, 341–353. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Untch, M.; Krawczyk, N.; Thurmann, M.; Kuhn, T.; Sehouli, J.; Gasparri, M.L.; de Boniface, J.; Gentilini, O.D.; Stickeler, E.; et al. Current trends in diagnostic and therapeutic management of the axilla in breast cancer patients receiving neoadjuvant therapy: results of the German-wide NOGGO MONITOR 24 survey. Arch Gynecol Obstet 2022. [CrossRef]
- Dannehl, D.; Engler, T.; Volmer, L.L.; Tegeler, C.M.; Fusshoeller, J.; Gabrysch, E.; Eissler, K.; Seller, A.; Grischke, E.M.; Hahn, M.; et al. Which Patients Do We Need to Test for BRCA1/2 Mutation? Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Armstrong, N.; Ryder, S.; Forbes, C.; Ross, J.; Quek, R.G. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol 2019, 11, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wan, J.; Lam, F.C.; Tang, H.; Marley, A.R.; Song, Y.; Miller, C.; Brown, M.; Han, J.; Adeboyeje, G. A comprehensive literature review and meta-analysis of the prevalence of pan-cancer BRCA mutations, homologous recombination repair gene mutations, and homologous recombination deficiencies. Environ Mol Mutagen 2022, 63, 308–316. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Lacko, A.; Sohn, J.; Cruz, F.; Ruiz Borrego, M.; Manikhas, A.; Hee Park, Y.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.S.; et al. A phase III trial of adjuvant ribociclib plus endocrine therapy versus endocrine therapy alone in patients with HR-positive/HER2-negative early breast cancer: final invasive disease-free survival results from the NATALEE trial. Annals of Oncology. [CrossRef]
- Barrios, C.H.; Harbeck, N.; Hortobagyi, G.N.; O’Shaughnessy, J.; Huang, C.S.; Jimenez, M.M.; Juric, D.; Pistilli, B.; Xu, B.; De Laurentiis, M.; et al. 113MO NATALEE update: Safety and treatment (tx) duration of ribociclib (RIB) + nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+/HER2− early breast cancer (EBC). ESMO Open 2024, 9. [Google Scholar] [CrossRef]
- Spring, L.; Griffin, C.; Isakoff, S.J.; Moy, B.; Wander, S.A.; Shin, J.; Abraham, E.; Habin, K.; Patel, J.M.; Comander, A.H.; et al. Phase II study of adjuvant endocrine therapy with CDK 4/6 inhibitor, ribociclib, for localized ER+/HER2- breast cancer (LEADER). Journal of Clinical Oncology 38, 531-531. [CrossRef]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Muñoz, M.; Paré, L.; González Farré, B.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. The Lancet Oncology 2020, 21, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Oswald, L.B.; Arredondo, B.; Kadono, M.; Martinez-Tyson, D.; Meade, C.D.; Penedo, F.; Antoni, M.H.; Soliman, H.; Costa, R.L.B.; Jim, H.S.L. A mixed-methods study of cyclin-dependent kinase 4 and 6 inhibitor symptom burden and quality of life among metastatic breast cancer patients and providers. Cancer Med 2021, 10, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, V.; Barchiesi, G.; Martorana, F.; Zucchini, G.; Muratore, M.; Fontanella, C.; Arpino, G.; Del Mastro, L.; Giuliano, M.; Puglisi, F.; et al. Health-related quality of life in breast cancer patients treated with CDK4/6 inhibitors: a systematic review. ESMO Open 2022, 7, 100629. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).