Submitted:
05 December 2024
Posted:
05 December 2024
You are already at the latest version
Abstract
The present study aimed to describe the reproductive cycle of the black-footed limpet (Patella depressa Pennant, 1777) from an intertidal rocky shore in the Algarve coast (southern Portugal). Samples were collected monthly between January 2017 and December 2018, being the species gametogenic cycle described based on gonad histology and the mean gonadal index. The presence of both transitional and mosaic hermaphrodites, indicate that some individuals are able to change sex (sequential hermaphroditism). Despite the occurrence of hermaphroditism, sex proportions were approximately equal, suggesting the absence of protandric sex change in this species. The population exhibited an extensive occurrence of ripe and spawning gonads, throughout almost all study period, probably related to consecutive processes of gonadal re-ripening and partial spawning events. The reproductive dynamics of P. depressa displayed clear inter-annual differences, with a short resting period recorded in 2017 (June – August) and the absence of resting gonads in 2018. Continued monitoring of this population and collection of environmental data is required to further improve the knowledge on the reproductive dynamics of this species. Such information is crucial for proposing additional management measures for the sustainable recreational harvesting of limpets in southern Portugal.
Keywords:
1. Introduction
2. Materials and Methods
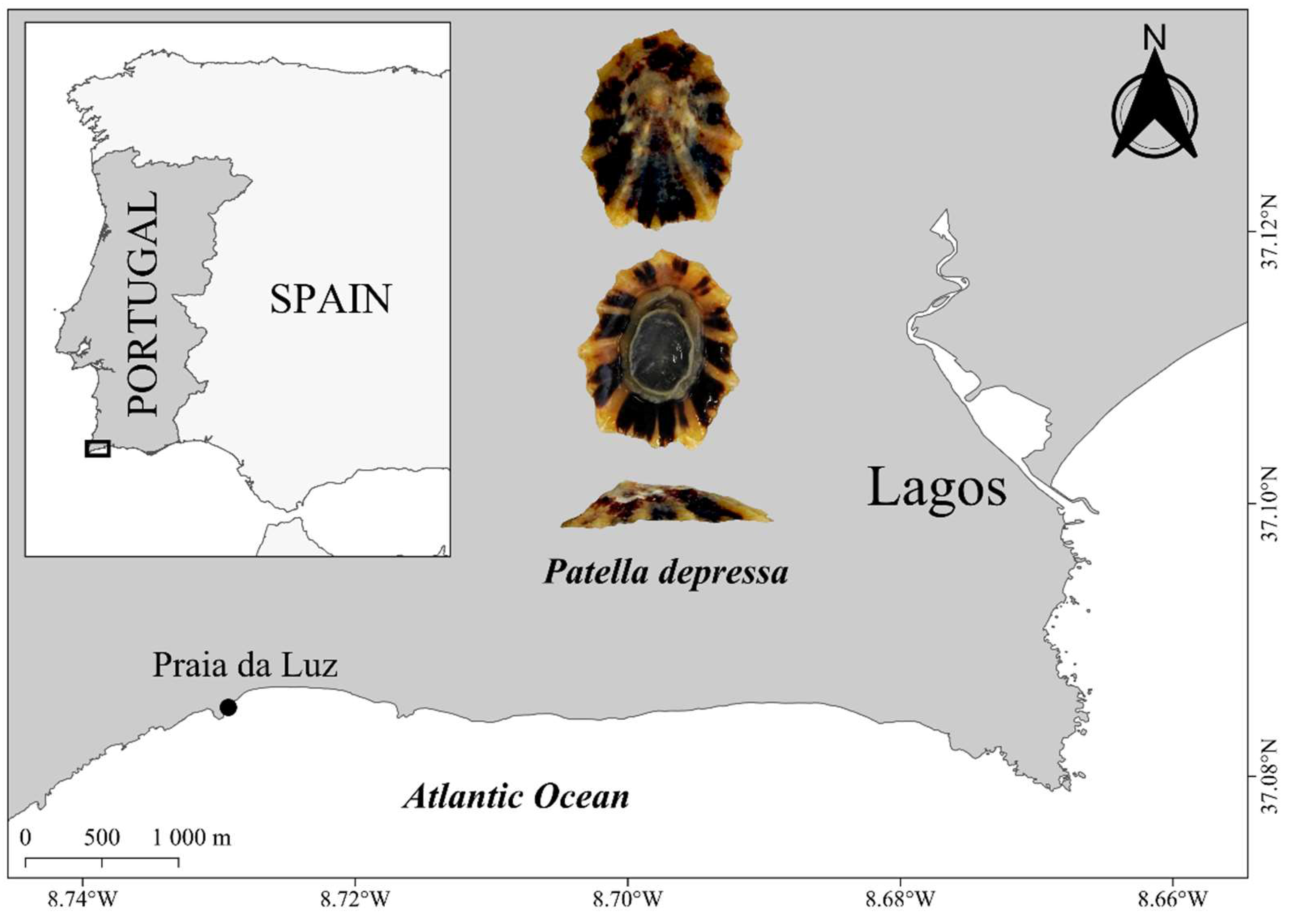
2.1. Study Area and Field Sampling
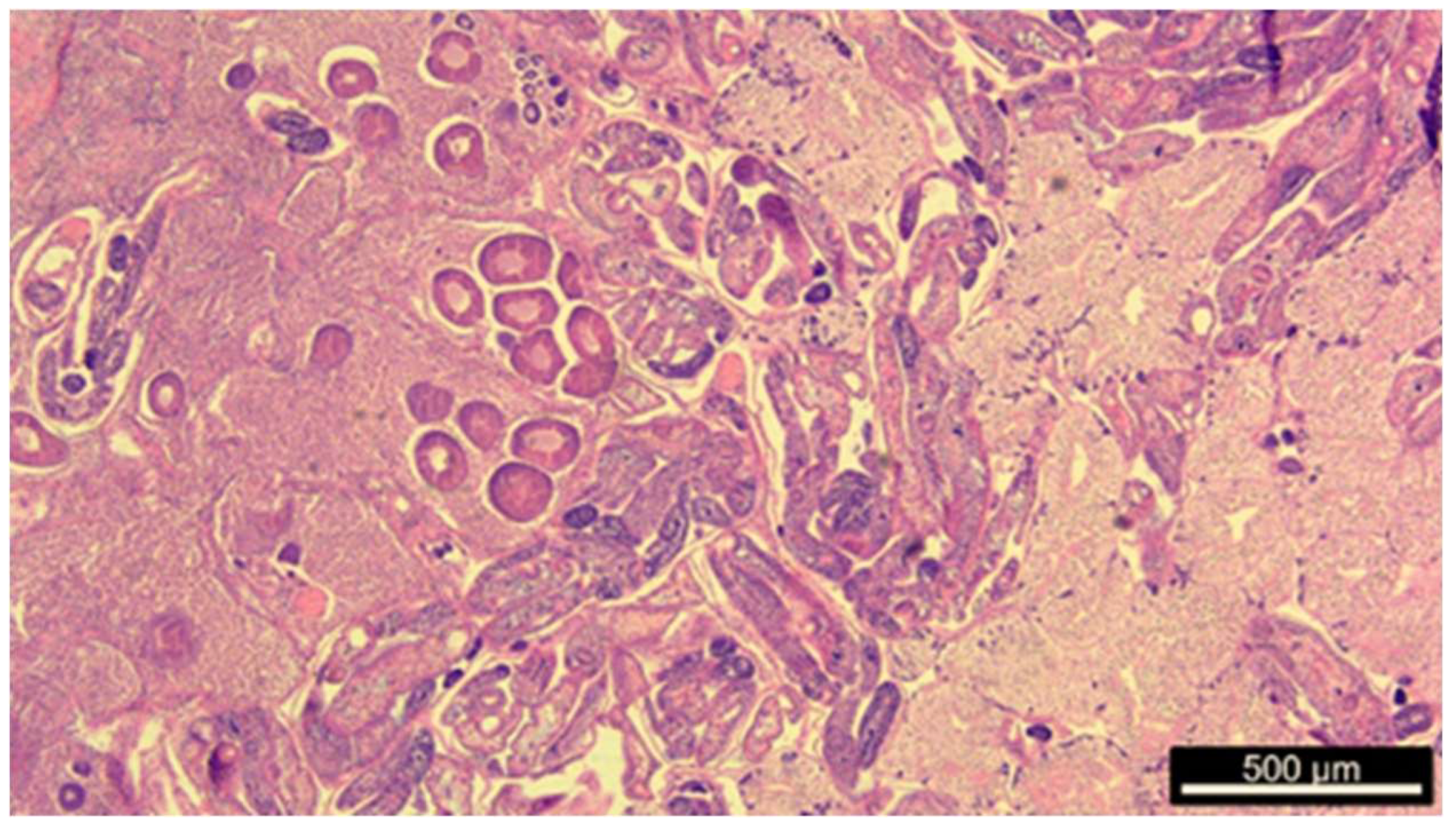
2.2. Gonad Histology and Mean Gonadal Index
2.3. Data Treatment and Statistical Analyses
3. Results
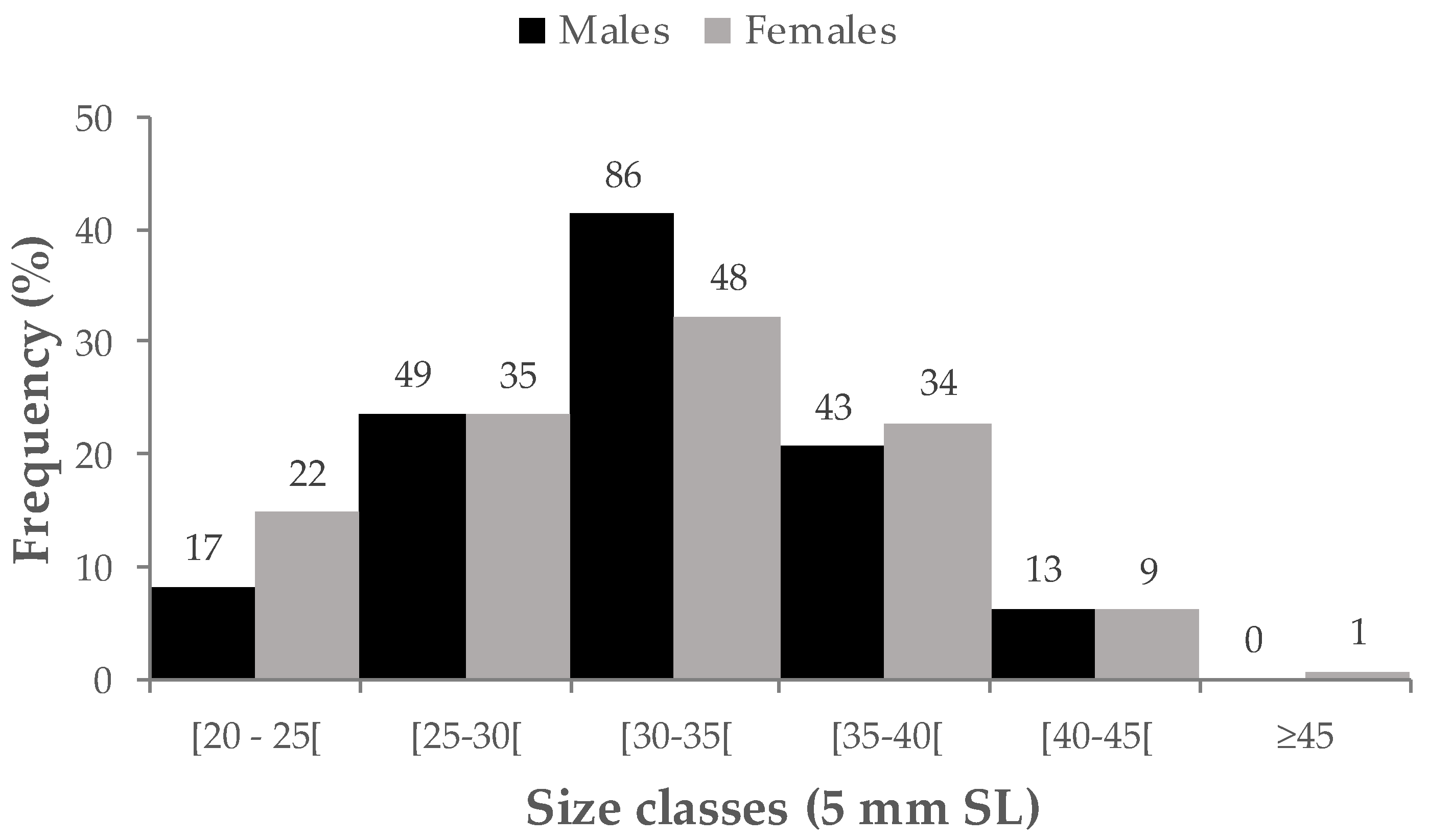
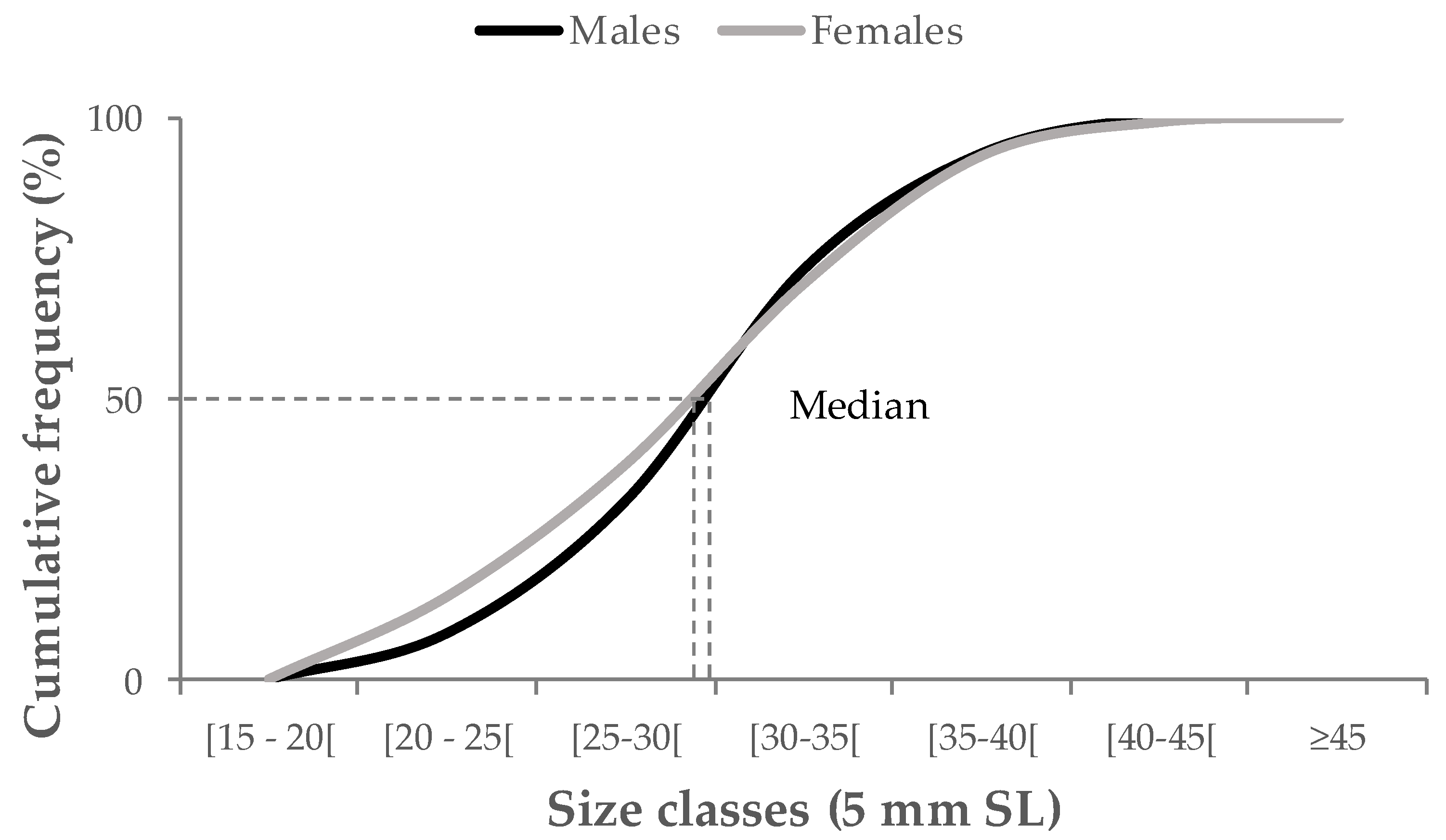
3.1. Population composition and sex ratios
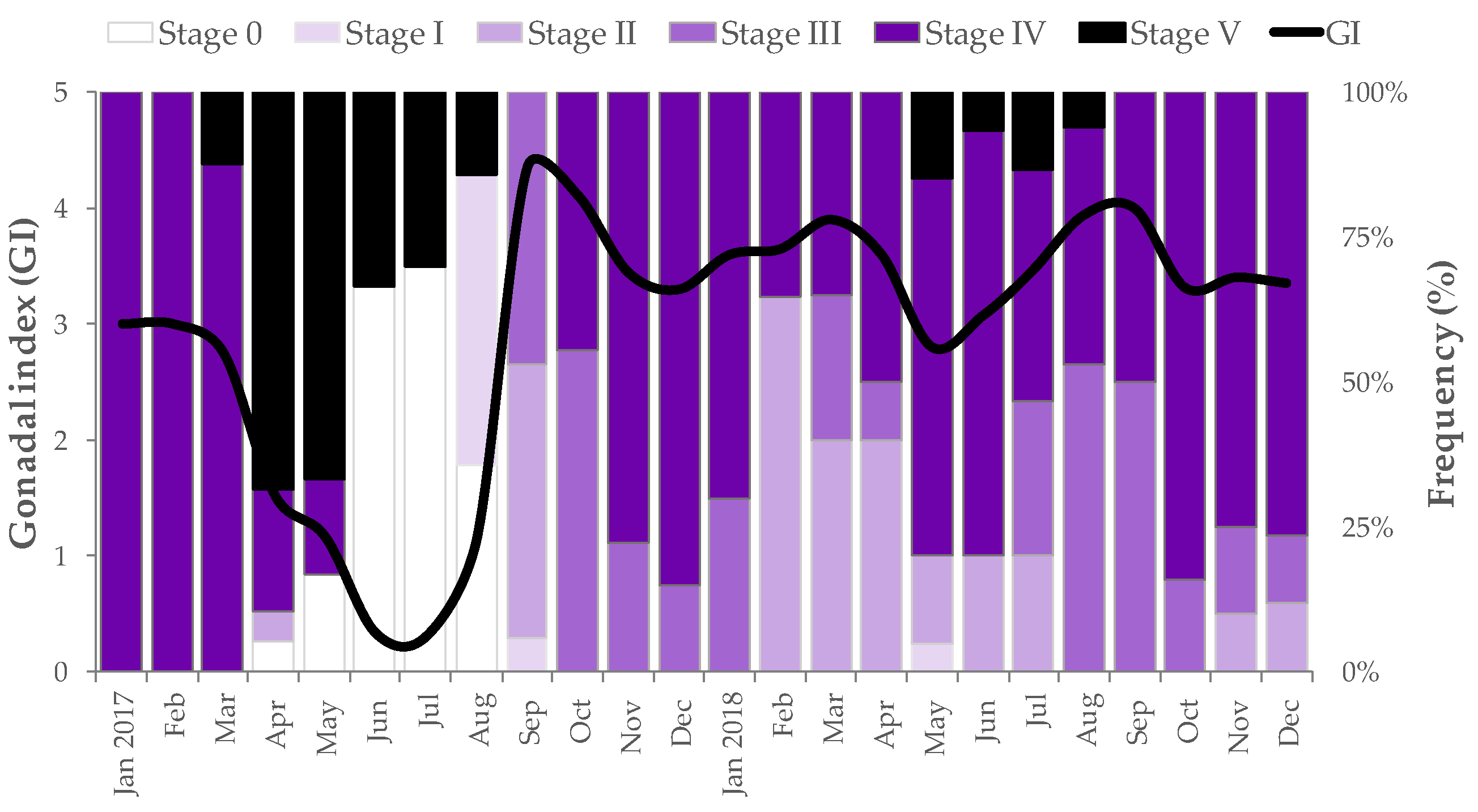
3.2. Reproductive Cycle, Gonadal Index and Environmental Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, S.R.; Coleman, R.A.; Della Santina, P.; Hawkins, S.J.; Burrows, M.T.; Hartnoll, R.G. Regional scale differences in the determinism of grazing effects in the rocky intertidal. Mar. Ecol. Prog. Ser. 2005, 287, 77–86. [Google Scholar] [CrossRef]
- Coleman, R.A.; Underwood, A.J.; Benedetti-Cecchi, L.; et al. A continental scale evaluation of the role of limpet grazing on rocky shores. Oecologia. 2006, 147, 556–564. [Google Scholar] [CrossRef]
- Brazão, S.E. Biochemical studies of the limpet Patella depressa Pennant, 1777 from marinas and rocky shores of the Portuguese coast: a marker approach. Master Thesis, Universidade do Algarve, Faro, Portugal, 2009. http://hdl.handle.net/10400.1/652.
- Burgos-Rubio, V.; De la Rosa, J.; Altamirano, M.; Espinosa, F. The role of patellid limpets as omnivorous grazers: a new insight into intertidal ecology. Mar Biol. 2015, 162, 2093–2106. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Denny, M.W.; Mach, K.J.; Miller, L.P. Thermal stress and morphological adaptations in limpets. Funct. Ecol. 2009, 23, 292–301. [Google Scholar] [CrossRef]
- Davies, P.S. Effect of environment on metabolic activity and morphology of Mediterranean and British species of Patella. Pubbl. Stn. Zool. Napoli. 1969, 37, 641–656. [Google Scholar]
- Branch, G.M. The biology of limpets: physical factors, energy flow and ecological interactions. Oceanogr. Mar. Biol., an Annual Review 1981, 19, 235–380. [Google Scholar]
- Guallart, J.; Calvo, M.; Acevedo, I.; Templado, J. Two-way sex change in the endangered limpet Patella ferruginea (Mollusca, Gastropoda). Invertebr. Reprod. Dev. 2013, 57, 247–253. [Google Scholar] [CrossRef]
- Orton, J.H. Observations on Patella vulgata. Part I. Sex phenomena, breeding and shell-growth. J. Mar. Biol. Assoc. UK 1928, 15, 851–862. [Google Scholar] [CrossRef]
- Ghiselin, M.T. Evolutionary aspects of marine invertebrate reproduction. In Reproduction of Marine Invertebrates, Giese, A.C., Pearse, J.S., Pearse, V.B., Eds.; Blackwell Scientific Publications, Palo Alto, California, 1987; Volume 9, pp. 609–666.
- Borges, C.D.; Hawkins, S.J.; Crowe, T.P.; Doncaster, C.P. The influence of simulated exploitation on Patella vulgata populations: protandric sex change is size-dependent. Ecol. Evol. 2016, 6(2), 514–531. [Google Scholar] [CrossRef]
- Rivera-Ingraham, G.A.; Espinosa, F.; García-Gómez, J.C. Environmentally mediated sex change in the endangered limpet Patella ferruginea (Gastropoda: Patellidae). J. Mollus. Stud. 2011, 77, 226–231. [Google Scholar] [CrossRef]
- Orton, J.H.; Southward, A.J.; Dodd, J.M. Studies on the biology of limpets II. The breeding of Patella vulgata L. in Britain. J. Mar. Biol. Assoc. UK 1956, 35, 149–176. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Umapathy, U.; Moura, P.; Pereira, F.; Carvalho, A.N.; Gaspar, M.B. Size at sex change and reproductive cycle of the limpets Patella vulgata and Patella ulyssiponensis (Mollusca: Patellogastropoda) from intertidal rocky shores of the Algarve coast (southern Portugal). Invertebr. Reprod. Dev. 2019, 63, 294–308. [Google Scholar] [CrossRef]
- Thompson, G.B. Distribution and population – dynamics of the limpet Patella aspera (Lamarck) in Bantry Bay. J. Exp. Mar. Bio. Ecol. 1979, 40, 115–135. [Google Scholar] [CrossRef]
- Martins, G.M. , Borges, C.D.G.; Vale, M.; Ribeiro, P.A.; Ferraz, R.R.; Martins, H.R.; Santos, R.S.; Hawkins, S.J. Exploitation promotes earlier sex changes in a protandrous patellid limpet, Patella aspera Röding, 1798. Ecol. Evol. 2017, 7, 3616–3622. [Google Scholar] [CrossRef]
- Espinosa, F.; Guerra-García, J.M.; Fa, D.; García-Gómez, J.C. Gonochorism or protandrous hermaphroditism? Evidence of sex change in the endangered limpet Patella ferruginea. Mar. Biodivers. Rec. 2009, 2, e153. [Google Scholar] [CrossRef]
- Prusina, I.; Ezgeta-Balic, D.; Ljubimir, S.; Dobroslavić, T.; Glamuzina, B. On the reproduction of the Mediterranean keystone limpet Patella rustica: histological overview. J. Mar. Biol. Assoc. UK 2014, 94, 1651–1660. [Google Scholar] [CrossRef]
- Borges, C.D.G.; Doncaster, C.P.; MacLean, M.A.; Hawkins, S.J. Broad-scale patterns of sex ratios in Patella spp.: a comparison of range edge and central range populations in the British Isles and Portugal. J. Mar. Biol. Assoc. UK 2015, 95, 1141–1153. [Google Scholar] [CrossRef]
- Guerra, M.T.; Gaudêncio, M.J. Aspects of the ecology of Patella spp. on the Portuguese coast. Hydrobiologia 1986, 142, 57–69. [Google Scholar] [CrossRef]
- Boaventura, D.; Alexander, M.; Della Santina, P.; Smith, N.D.; Ré, P.; Cancela da Fonseca, L.; Hawkins, S.J. The effects of grazing on the distribution and composition of low-shore algal communities on the central coast of Portugal and on the southern coast of Britain. J. Exp. Mar. Bio. Ecol. 2002, 267, 185–206. [Google Scholar] [CrossRef]
- Brazão, S.; Boaventura, D.; Morais, S.; Narciso, L.; Ré, P. Reproduction of Patella depressa Pennant, 1777 on the central Portuguese coast. Bol. Inst. Esp. Oceanogr. 2003, 19, 453–460. [Google Scholar]
- Orton, J.H.; Southward, A.J. Studies on the biology of limpets. IV. The breeding of Patella depressa Pennant on the North Cornish coast. J. Mar. Biol. Assoc. UK 1961, 41, 653–662. [Google Scholar] [CrossRef]
- Fretter, V.; Graham, A. The prosobranch molluscs of Britain and Denmark. Part 1. Pleurotomariacea, Fissurellacea and Patellacea. J. Mollus. Stud. 1976; 1, 1–38. [Google Scholar]
- McCoy, M.D. Hawaiian limpet harvesting in historical perspective: A review of modern and archaeological data on Cellana spp. from the Kalaupapa Peninsula, Moloka’i Island. Pac. Sci. 2008, 62, 21–38. [Google Scholar] [CrossRef]
- Mau, A.; Jha, R. Aquaculture of two commercially important molluscs (abalone and limpet): existing knowledge and future prospects. Rev Aquac. 2018, 10, 611–625. [Google Scholar] [CrossRef]
- Pombo, O.A.; Escofet, A. Effect of exploitation on the limpet Lottia gigantea: a field study in Baja California (Mexico) and California (U.S.A.). Pac. Sci. 1996, 50, 393–403. [Google Scholar]
- Olivares-Paz, A.; Quinteiro, J.; Rey-Méndez, M. Autentificación de lapas del género Fissurella (Mollusca: Vetigastropoda) en la costa chilena, mediante PCR-RFLP. Invest. Mar. 2006, 34, 113–118. [Google Scholar] [CrossRef]
- Moro, L.; Herrera, R. Las lapas: un recurso en extinción. Medio Ambiente Canarias 2000, 16, 20–22. [Google Scholar]
- Casal, G.; Aceña-Matarranz, S.; Fernández-Márquez, D.; Fernández, N. Distribution and abundance patterns of three coexisting species of Patella (Mollusca Gastropoda) in the intertidal areas of the NW Iberian Peninsula: Implications for management. Fish. Res. 2018, 198, 86–98. [Google Scholar] [CrossRef]
- Henriques, P.; Sousa, R. , Pinto, A.R.; Delgado, J.; Faria, G.; Alves, A.; Khadem, M. Life history traits of the exploited limpet Patella candei (Mollusca: patellogastropoda) of the north-eastern Atlantic. J. Mar. Biol. Assoc. UK 2012, 92, 1379–1387. [Google Scholar] [CrossRef]
- Sousa, R.; Delgado, J.; Pinto, A.R.; Henriques, P. Growth and reproduction of the north-eastern Atlantic keystone species Patella aspera (Mollusca: Patellogastropoda). Helgol. Mar. Res. 2017, 71, 8. [Google Scholar] [CrossRef]
- Sousa, R.; Vasconcelos. J.; Henriques, P.; Pinto, A.R.; Delgado, J.; Riera, R. Long-term population status of two harvested intertidal grazers (Patella aspera and Patella candei), before (1996–2006) and after (2007–2017) the implementation of management measures. J. Sea. Res. 2019, 144, 33–38. [CrossRef]
- Santos, R.R.; Hawkins, S.; Monteiro, L.R.; Alves, M.; Isidro, E.J. Marine research, resources and conservations in the Azores. Aquat. Conserv. Mar. Freshw. Ecosyst. 1995, 5, 311–354. [Google Scholar] [CrossRef]
- Côrte-Real, H.B.S.M.; Hawkins, S.J.; Thorpe, J.P. Population differentiation and taxonomic status of the exploited limpet Patella candei in the Macaronesian islands (Azores, Madeira, Canaries). Mar. Biol. 1996, 125, 141–152. [Google Scholar] [CrossRef]
- Martins, G.M.; Jenkins, S.R.; Hawkins, S.J.; Neto, A.I.; Medeiros, A.R.; Thompson, R.C. Illegal harvesting affects the success of fishing closure areas. J. Mar. Biol. Assoc. UK 2011, 91, 929–937. [Google Scholar] [CrossRef]
- Diogo H, Pereira JG, Schmiing M. 2016. Catch me if you can: non-compliance of limpet protection in the Azores. Mar Pol. 63:92–99. [CrossRef]
- Bowman, R.S.; Lewis, J.R. Geographical variation in the breeding cycles and recruitment of Patella spp. Hydrobiologia 1986, 142, 41–56. [Google Scholar] [CrossRef]
- Moore, P.; Thompson, R.C.; Hawkins, S.J. Effects of grazer identity on the probability of escapes by a canopy-forming macroalga. J. Exp. Mar. Biol. Ecol. 2007, 344, 170–180. [Google Scholar] [CrossRef]
- Ibanez, M.; Pena, J.; Feliu, J. Reproduction of Patella spp. on the Basque coast of Spain. Hydrobiologia 1986, 142, 327. [Google Scholar] [CrossRef]
- Ribeiro, P.A.; Xavier, R.; Santos, A.M.; Hawkins, S.J. Reproductive cycles of four species of Patella (Mollusca: Gastropoda) on the northern and central Portuguese coast. J. Mar. Biol. Assoc. UK 2009, 89, 1215–1221. [Google Scholar] [CrossRef]
- Fernández, N.; Alborés, I.; Aceña-Matarranz, S. Spatial variability of the reproductive cycle and physiological condition of Patella spp. (Mollusca Gastropoda) in the NW of the Iberian Peninsula: implications for exploitation. Fish. Res. 2016 179, 76–85. [CrossRef]
- Morais, S.; Boaventura, D.; Narciso, L.; Ré, P.; Hawkins, S.J. Gonad development and fatty acid composition of Patella depressa Pennant (Gastropoda: Prosobranchia) populations with different patterns of spatial distribution, in exposed and sheltered sites. J. Exp. Mar. Bio. Ecol. 2003, 294, 61–80. [Google Scholar] [CrossRef]
- Rebouta, R. Reprodução, desenvolvimento larvar e crescimento juvenil de Patella depressa em laboratório. Master Thesis, Escola superior de turismo e tecnologia do mar, Politécnico de Leiria, Peniche, Portugal, 2022. [Google Scholar]
- I.H. Dados de temperatura da água e altura da onda registados na boia oceanográfica costeira de Faro (2016–2018). Lisboa, Instituto Hidrográfico, 2020.
- Weather Underground. Weather History for Faro Airport Station, Faro, Portugal, for Daily observations between January 2017 and December 2018. Last modified April 15, 2024. https://www.wunderground.com/history/monthly/pt/montenegro/LPFR/date/2017-1.
- Martoja, R.; Martoja-Pierson, M. Initiation aux techniques del’histologie animale. Masson et Cie, Paris, 1967, 345p.
- Seed, R. Ecology. In: Marine mussels: their ecology and physiology. Bayne, B.L. Ed.; Cambridge University Press, Cambridge, 1976, pp. 13–65.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2024. URL https://www.R-project.org/.
- Castro, J. J. Predação humana no litoral rochoso alentejano: caracterização, impacte ecológico e conservação. Doctoral Thesis, Universidade de Évora, Évora, Portugal, 2004. [Google Scholar]
- Dodd, J.M. Studies on the biology of limpets: III. Hermaphroditism in the three British species of Patella. J. Mar. Biol. Assoc. UK 1956, 35, 327–340. [Google Scholar] [CrossRef]
- Branch, G.M. The ecology of Patella Linnaeus from the Cape Peninsula, South Africa. 2. Reproductive cycles. Trans. R. Soc. S. Aft. 1974, 41, 111–160. [Google Scholar] [CrossRef]
- Creese, R.G.; Schiel, D.R.; Kingsford, M.J. Sex change in a giant endemic limpet, Patella kermadecensis, from the Kermadec Islands. Mar. Biol. 1990, 104, 419–426. [Google Scholar] [CrossRef]
- Garwood, P. R. The cycle of gonad development in Patella vulgata (Mollusca; Gastropoda): The use of oocyte diameter measurements and gravimetric estimates of sexual maturity. Sarsia 1987, 72, 29–35. [Google Scholar] [CrossRef]
- Little, C.; Kitching, J.A. The biology of rocky shores. University of Oxford Press, Oxford, 1996, 240p.
- Charnov, E.L. Natural selection and sex change in Pandalid shrimp: test of a life history theory. Am. Nat. 1979, 113, 715–734. [Google Scholar] [CrossRef]
- Munday, P.L.; Buston, P.M.; Warner, R.R. Diversity and flexibility of sex-change strategies in animals. Trends Ecol. Evol. 2006, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Allsop, D.J.; West, S.A. Constant relative age and size at sex change in sequentially hermaphroditic fish. J. Evol. Biol. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Wright, W.G. Intraspecific density mediates sex-change in the territorial patellacean limpet Lottia gigantea. Mar. Biol. 1989, 100, 353–364. [Google Scholar] [CrossRef]
- Fretter, V.; Graham, A.; Ponder, W.F.; Lindberg, D.R. Prosobranchia Introduction. In Mollusca, the Southern Synthesis. Part B. Fauna of Australia, Beesley, P.L., Ross, G.J.B., Wells, A., Eds.; Melbourne: CSIRO, 1998; Volume 5, pp. 605–638. [Google Scholar]
- Lewis, J.R. Latitudinal trends in reproduction, recruitment and population characteristics of some rocky littoral molluscs and cirripedes. Hydrobiologia 1986, 142, 1–13. [Google Scholar] [CrossRef]
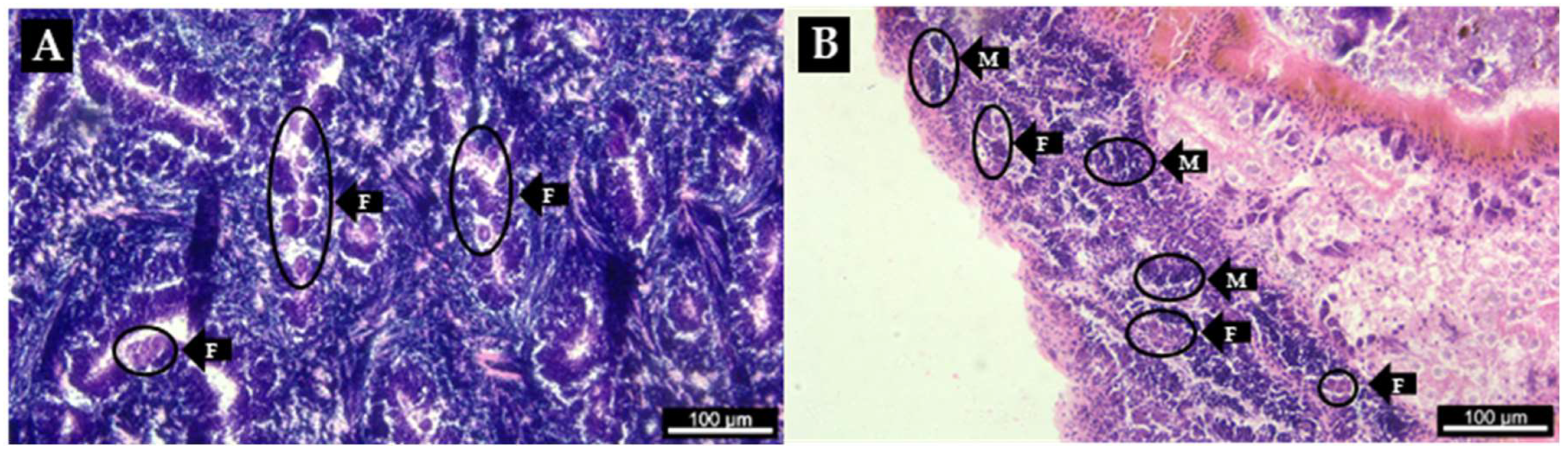
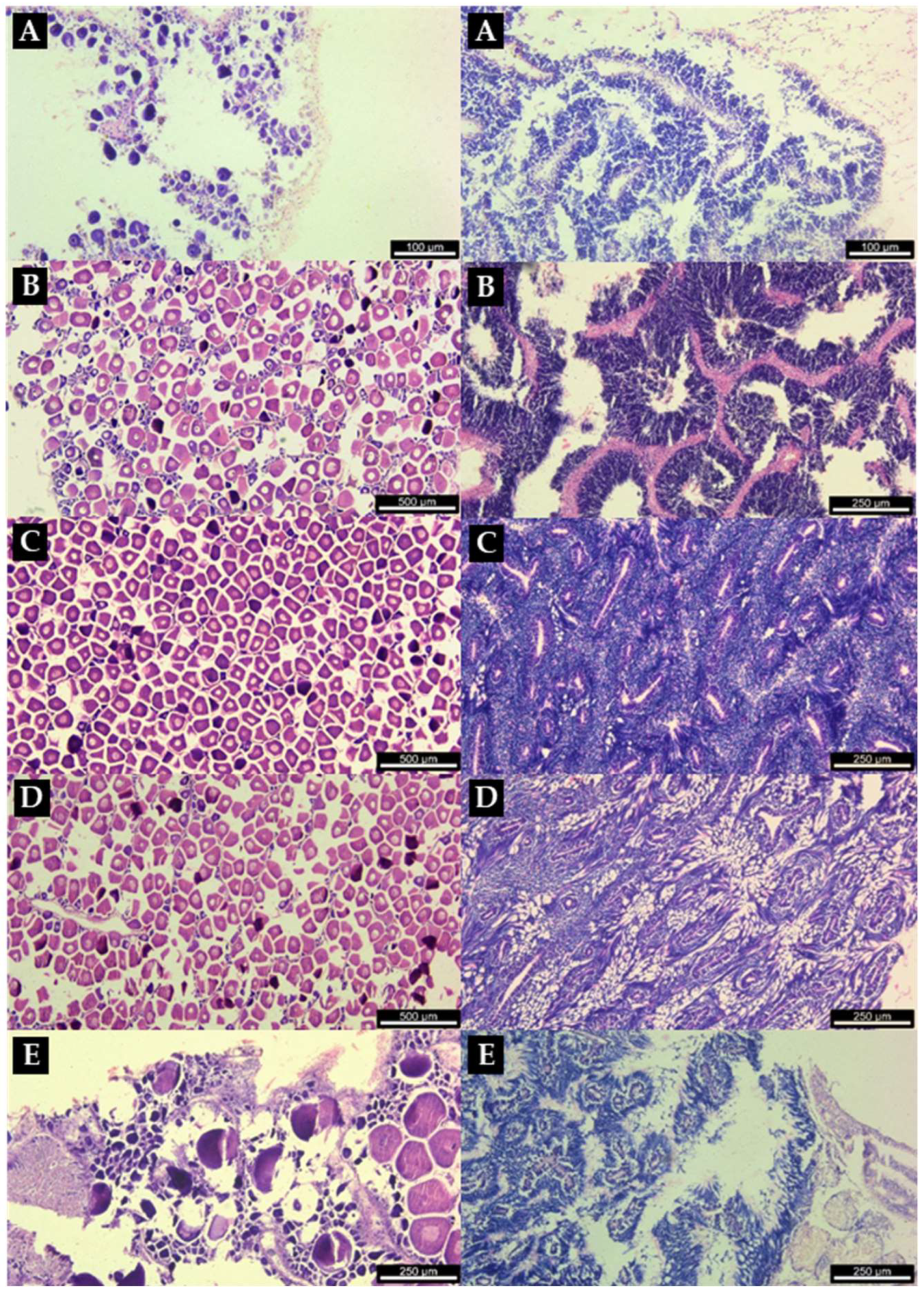
| Patella depressa | N | Sex ratio (M: F) | Mean SL±SD (min–max) |
Mean TW±SD (min–max) |
|---|---|---|---|---|
| Males | 208 | 1M: 0.72F | 32.0 ± 5.0 (17.2–43.6) | 4.3 ± 2.2 (0.6–11.7) |
| Females | 149 | 31.6 ± 5.8 (16.2–45.8) | 4.3 ± 2.8 (0.4–13.6) | |
| Undifferentiated | 27 | 22.3 ± 3.6 (11.7–30.0) | 1.2 ± 0.6 (0.1–2.9) | |
| Parasites/Pathologies | 6 | 28.4 ± 5.8 (23.5–39.3) | 3.6 ± 2.9 (1.4–8.9) | |
| Hermaphrodites (transitional) | 11 | 32.4 ± 7.5 (22.2–43.2) | 5.1 ± 4.2 (0.7–13.4) | |
| Hermaphrodites (mosaic) | 6 | 24.4 ± 2.2 (21.3–27.7) | 1.6 ± 0.5 (0.9–2.3) | |
| Total | 407 | 31.0±5.8 (11.7–45.8) | 4.1±2.6 (0.1–13.6) |
| AT | SST | WS | WH | |
|---|---|---|---|---|
| Patella depressa GI | ρ = 0.031 | ρ = 0.077 | ρ = −0.528 | ρ = −0.461 |
| p = 0.888 | p = 0.722 | p = 0.008 | p = 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





