Introduction
Silver-Russell Syndrome (SRS), also known as Russell-Silver Syndrome, is a rare genetic disorder characterized by significant prenatal and postnatal growth retardation, distinctive facial features, and body asymmetry. First described in the early 1950s, SRS presents a complex clinical picture that often complicates its diagnosis, particularly in the antenatal setting [
1,
2]. The syndrome is primarily associated with intrauterine growth restriction (IUGR), where affected infants typically exhibit low birth weight and may present with relative macrocephaly at birth [
3,
4].
Antenatal diagnosis of SRS is challenging due to the subtlety of its phenotypic manifestations, which may not be fully apparent until later in gestation. However, early suspicion can arise from routine ultrasound examinations that reveal growth discrepancies, such as asymmetric limb development or facial dysmorphisms [
5,
6]. The Netchine-Harbison clinical scoring system (NH-CSS) has been proposed as a valuable tool for clinical diagnosis, requiring the identification of at least four out of six specific criteria [
2,
7]. This scoring system may help aslo clinicians in recognizing the syndrome during the first trimester, allowing for timely intervention and management strategies.
Genetic testing plays a pivotal role in confirming the diagnosis of SRS, with approximately 60% of cases attributable to epigenetic alterations, particularly hypomethylation at the imprinting control region (ICR) on chromosome 11p15 [
3,
4,
8]. Maternal uniparental disomy of chromosome 7 (UPD(7)mat) is another significant genetic cause, accounting for a smaller subset of cases [
3,
9]. The integration of genetic testing with clinical assessments can enhance diagnostic accuracy and facilitate appropriate prenatal counseling for affected families.
In this case report, we present a detailed account of a first-trimester suspicion of Silver-Russell Syndrome, highlighting the diagnostic approach and the importance of early recognition in optimizing outcomes for affected infants.
Case Presentation
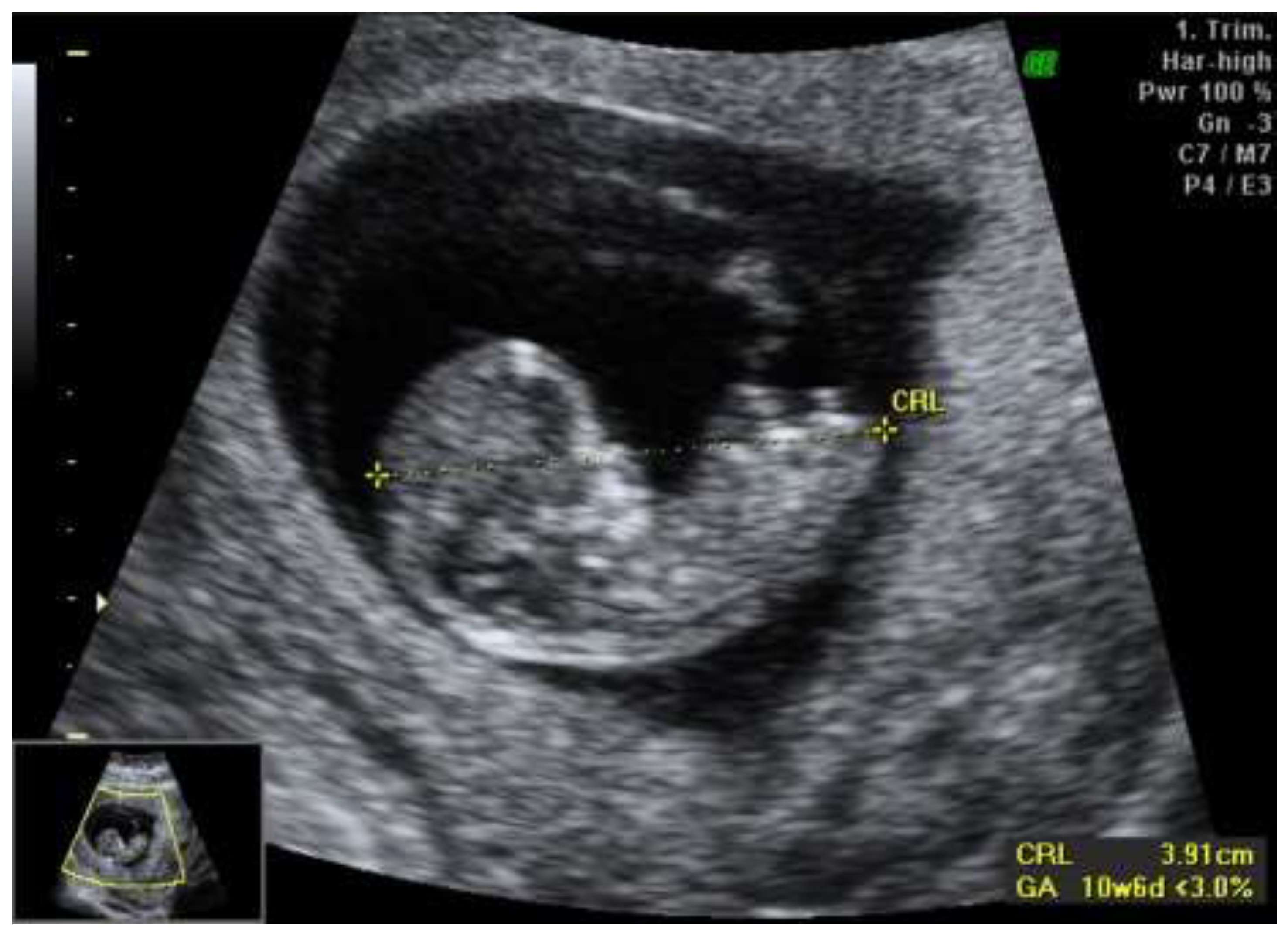
We report a case of a 28-year-old primigravida who presented for routine prenatal care at 11 weeks of gestation. Initial ultrasound examination revealed asymmetric growth restriction, raising suspicion for potential fetal anomalies. The anatomy appeared normal, with no overt structural defects noted. The first-trimester combined screening test for chromosomal anomalies indicated a low risk, with a PAPP-A of 0.7 MoM and a beta-hCG of 0.73 MoM, suggesting a favorable outcome regarding common aneuploidies (
Figure 1).
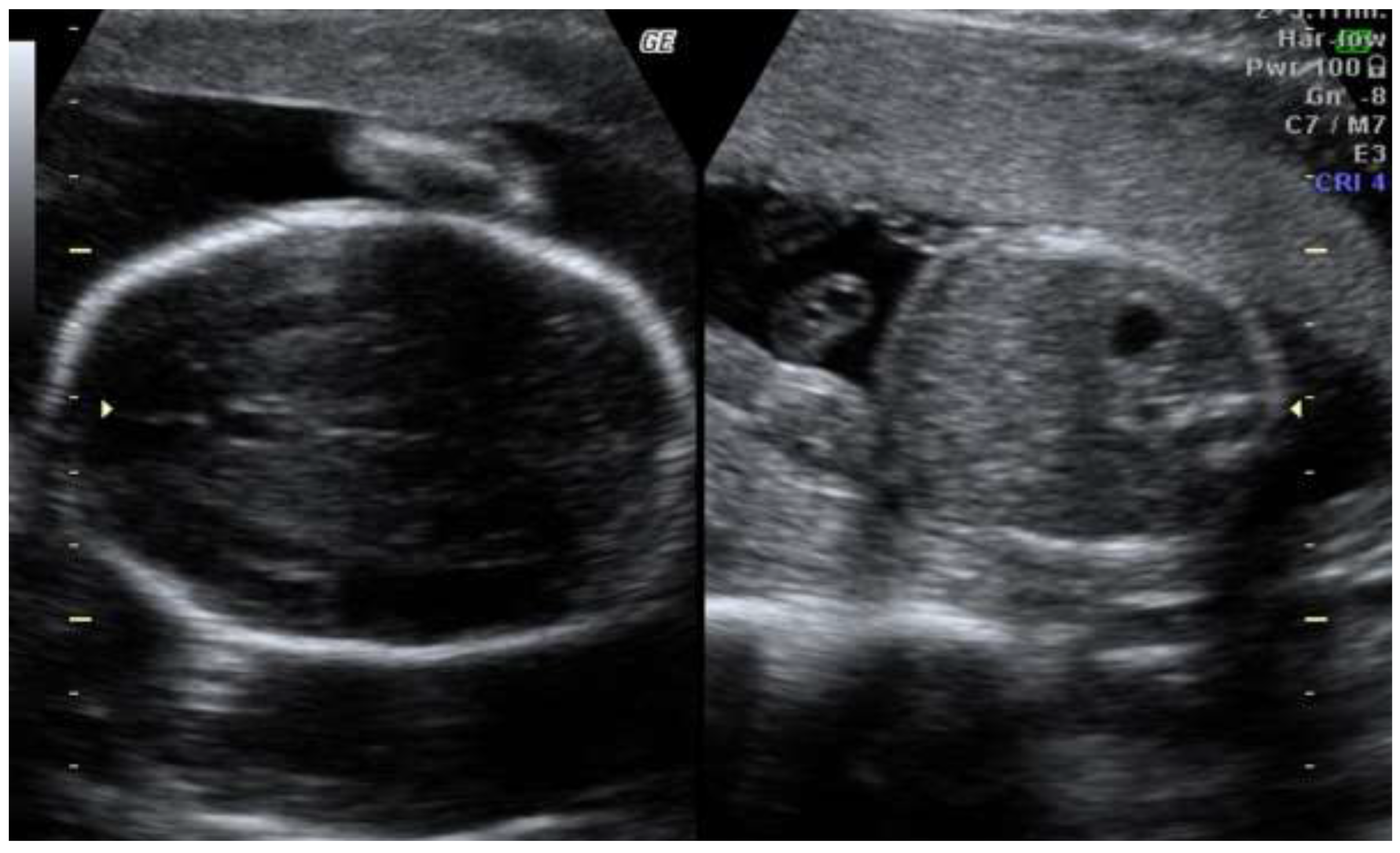
The second trimester scan revealed progression of asymmetric fetal growth restriction (
Figure 2)
Given the findings of asymmetric growth restriction and the mother's age, further diagnostic evaluation was warranted. Amniocentesis was performed at 20 weeks of gestation to obtain fetal cells for genetic analysis. The amniotic fluid analysis included both ChromoSeq and targeted testing for Silver-Russell Syndrome (SRS). The results returned positive for SRS, confirming the prenatal diagnosis.
To further investigate the genetic basis of the condition, both parents underwent testing to exclude maternal uniparental disomy (UPD), a common etiology in SRS cases. Genetic testing results for both parents were negative for UPD, indicating that the SRS diagnosis was likely due to other genetic mechanisms, such as hypomethylation at the imprinting control region on chromosome 11p15, which is known to account for a significant proportion of SRS cases.
This case highlights the importance of early detection and comprehensive genetic evaluation in cases of suspected Silver-Russell Syndrome, particularly when faced with asymmetric growth restriction in the first trimester. The findings underscore the need for a multidisciplinary approach to prenatal care, enabling timely interventions and informed parental counseling.
Discussion
The case presented highlights the complexities involved in the antenatal diagnosis of Silver-Russell Syndrome (SRS), particularly in the context of first-trimester asymmetric growth restriction. SRS is a genetically heterogeneous disorder characterized by prenatal and postnatal growth failure, relative macrocephaly, and distinctive facial features [
8,
10]. The early identification of growth discrepancies, such as those observed in this case, is crucial for timely intervention and management.
The combination of normal anatomical findings and low-risk results from the first-trimester combined screening test for chromosomal anomalies in our patient is consistent with findings in the literature, which suggest that first-trimester markers such as PAPP-A and beta-hCG can provide valuable insights into fetal health [
11,
12]. In this case, the PAPP-A level of 0.7 MoM and beta-hCG of 0.73 MoM indicated a low risk for common aneuploidies, yet the presence of asymmetric growth restriction necessitated further investigation. This aligns with studies indicating that first-trimester growth patterns can be predictive of later fetal growth outcomes [
13].
The decision to perform amniocentesis was supported by the need for definitive genetic diagnosis, particularly given the suspicion of SRS. The positive results for SRS from targeted testing underscore the importance of genetic evaluation in cases of suspected growth restriction. Previous research has shown that genetic testing can identify the underlying causes in a significant proportion of SRS cases, including hypomethylation at the imprinting control region on chromosome 11p15 and maternal uniparental disomy of chromosome 7 [
8,
10]. In our case, the negative results for UPD in both parents suggest that the SRS diagnosis may be attributed to other genetic mechanisms, which is consistent with the heterogeneous nature of the syndrome [
14].
The findings from this case also emphasize the need for a multidisciplinary approach in managing pregnancies complicated by growth restriction. Early recognition of SRS can facilitate appropriate prenatal counseling and prepare healthcare providers for potential postnatal challenges, including feeding difficulties and growth monitoring [
5]. Furthermore, understanding the genetic basis of SRS can inform management strategies and potential interventions that may improve outcomes for affected infants.
In conclusion, this case illustrates the critical role of early detection and comprehensive genetic assessment in the antenatal diagnosis of Silver-Russell Syndrome. The integration of clinical findings, genetic testing, and a thorough understanding of the syndrome's complexities can enhance diagnostic accuracy and ultimately improve care for affected families.
References
- Raso M, Chiarelli F. Recent insights in Silver-Russell syndrome. Pediatr Dimens. 2020, 5. [Google Scholar] [CrossRef]
- Azzi S, Salem J, Thibaud N, Chantot-Bastaraud S, Lieber E, Netchine I, et al. A prospective study validating a clinical scoring system and demonstrating phenotypical-genotypical correlations in Silver-Russell syndrome. J Med Genet. 2015, 52, 446–53. [Google Scholar] [CrossRef]
- Xatzipsalti, M. A variant of uncertain significance in HMGA2 gene, in a child with Silver-Russell syndrome-like phenotype: A case report. J Clin Images Med Case Rep. 2023, 4. [Google Scholar] [CrossRef]
- Spiteri B, Stafrace Y, Calleja-Agius J. Silver-Russell syndrome: A review. Neonatal Netw. 2017, 36, 206–12. [Google Scholar] [CrossRef] [PubMed]
- Wikiera B, Nocoń-Bohusz J, Noczyńska A. Silver-Russell syndrome and Turner syndrome in a girl with short stature treated with growth hormone – Case report. Pediatr Endocrinol Diabetes Metab. 2022, 28, 301–4. [Google Scholar] [CrossRef] [PubMed]
- Lahmamssi F, Saadaoui L, Aynaou H, Salhi H, Ouahabi H. An unusual association: Silver-Russell syndrome and ectopic thyroid. Cureus. 2022. [CrossRef]
- Mahindrakar N, Jeeragi M, Hebbal K. Congenital growth disorder: Silver-Russell syndrome - A case report. J Clin Images Med Case Rep. 2022, 3. [Google Scholar] [CrossRef]
- Hashemian S, Vakili R, Sadr-Nabavi A, Esfehani R, Jaripour M. Molecular evaluation of children with clinical Russell-Silver phenotypes: The first report from Iran. Iran J Pediatr. 2019. [CrossRef]
- Stark Z, Ryan M, Bruno D, Burgess T, Savarirayan R. Atypical Silver–Russell phenotype resulting from maternal uniparental disomy of chromosome 7. Am J Med Genet A. 2010, 152A, 2342–5. [Google Scholar] [CrossRef]
- Su J, Wang J, Fan X, Fu C, Zhang S, Zhang Y, et al. Mosaic upd(7q)mat in a patient with Silver-Russell syndrome. Mol Cytogenet. 2017, 10. [Google Scholar] [CrossRef]
- Javadzadeh M, Saneifard H, Hosseini A. Torticollis as the main presentation in a child with Russell-Silver syndrome: A case report. Case Rep Pediatr. 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Kirkegaard I, Henriksen T, Uldbjerg N. Early fetal growth, PAPP-A and free β-HCG in relation to risk of delivering a small-for-gestational age infant. Ultrasound Obstet Gynecol. 2011, 37, 341–7. [Google Scholar] [CrossRef] [PubMed]
- Prior T, Mullins E, Bennett P, Kumar S. Are 1st-trimester β-human chorionic gonadotrophin and pregnancy-associated plasma protein A levels predictive of intrapartum fetal compromise in a selected normal population? Aust N Z J Obstet Gynaecol. 2014, 54, 418–23. [Google Scholar] [CrossRef]
- Sotiriadis A, Figueras F, Eleftheriades M, Papaioannou G, Chorozoglou G, Dinas K, et al. First-trimester and combined first- and second-trimester prediction of small-for-gestational age and late fetal growth restriction. Ultrasound Obstet Gynecol. 2018, 53, 55–61. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).