1. Introduction
Soil microorganisms are momentous components of both natural and managed ecosystems [
1]. Each gram of soil may contain thousands of microorganisms, including bacteria and some small prokaryotes, fungi, and viruses. Soil microbes, especially those found in wetlands, drive the cycling and transformation of soil organic carbon (SOC) and other nutrients, promote the flow of chemical energy and information [
2], and also participate in processes such as pollutant degradation and environmental remediation [
3], playing a significant role in maintaining the balance and stability of wetland ecosystems [
4]. Changes in wetland environmental conditions, such as nutrient content, water content, pH, vegetation, etc., can affect the structure and function of wetland microorganisms [
5,
6].
Metagenomic next-generation sequencing (mNGS) refers to a metagenomic research method that uses next-generation sequencing technology to directly study the composition of microorganisms in samples through high-throughput sequencing and data analysis techniques [
7]. mNGS breaks through the limitations of traditional microbiology based on culture and identification and can obtain almost all DNA or RNA sequences in the sample. Based on conventional research methods such as specific target molecule amplification, restriction enzyme digestion, and electrophoresis, researchers have expanded the Sanger sequencing method and established a metagenomic research method for 16S rRNA clone library sequencing [
8]. The emergence of mNGS has made metagenomic methods a popular tool in microbial research. For the advantages of independent cultivation, relatively simple operation, and theoretically unbiased detection of all microorganisms in the sample. Although there are problems such as the large amount of data obtained, complex analysis methods and processes, and difficulty in unifying and standardizing experimental and data analysis processes [
9,
10], the genetic material of microbial communities can be identified through subsequent optimization analysis methods, which can fully explore their genetic diversity and biochemical reactions, and even further combine with environmental variables to explore the response mechanism between microorganisms and the environment.
Wetlands are one of the most productive and valuable ecosystems in the world, providing 40% of the global ecosystem service value [
11,
12,
13,
14]. Poyang Lake is located in the middle and lower reaches of the Yangtze River and is the most typical large-scale shallow-water lake in China [
15]. The habitat types and structures of the intertidal wetlands are diverse, providing a vast and diverse habitat for organisms. Poyang Lake has also become an important biological resource reservoir in China’s subtropical regions [
16,
17]. The unique hydrological rhythm of alternating flood and dry seasons causes periodic inundation and exposure of intertidal habitats, which is an important driving force affecting the species composition and microbial diversity of plant communities in floodplain lakes and intertidal wetlands [
18]. In recent years, against the backdrop of increased human activity interference, the water level of Poyang Lake has become low and withered [
19,
20,
21,
22], the duration of flooding has become shorter, and the beaches have been exposed earlier, leading to changes in the microbial community in the soil [
23]. Exploring the changes in biodiversity and functional gene abundance of communities can effectively evaluate the ecological effects caused by changes in hydrological conditions, which is of great significance for maintaining and protecting biodiversity in Poyang Lake, and predicting the distribution pattern evolution of microbial communities under future water level fluctuations. As of now, it is unclear how changes in soil moisture content in Poyang Lake wetland affect the functionality of microbial communities. Therefore, it is necessary to conduct in-depth analysis of the main functions driven by microorganisms in different soil moisture contents.
This study investigated three soil samples with different moisture contents in the Poyang Lake wetland. Metagenome technology was used to analyze the microbial composition and abundance of the soil in the Poyang Lake wetland and its impact on regional functional distribution. The purpose of the study is to (1) Investigate the differences in soil physicochemical factors and microbial community structure with different water contents. (2) Describe the distribution profile of carbon, nitrogen, and antibiotics resistance genes under different soil moisture contents. (3) Explain the profound connection between microbial community structure and underground ecological function driving processes. The analysis of these factors will provide important insights into microbial community structure as a worthwhile indicator of ecological functional changes.
2. Materials and Methods
2.1. Soil sampling
In October 2022, soil samples were collected from the Poyang Lake region in the range of E116°25’and N 28°58’. Three different sampling points (S1 of E116°25’20.45’’and N 28°58’32.91’’, S2 of E116°25’21.72’’and N 28°58’35.49’’, S3 of E116°23’43.94’’and N 28°58’15.74’’) of the Poyang Lake wetland were selected for soil sampling, S1 was submerged by lake water (sedimentary state) and had the highest water content, S2 had a high-water content (semi-submerged state), S3 has the lowest water content and is covered with plants (drought). Soil samples were taken at a depth of 10 cm from the surface with an aseptic spatula and plant material and detritus were removed during sampling, and 3 samples were taken from each sampling point. The sample is named according to its source, for example, the sample from the S1 sampling point is named the S1, and 3 samples taken from sampling point S1 were denoted as S1-1, S1-2, S1-3 respectively, and so on. 9 samples were collected in total. Each sample was separated into two groups: one was dried at room temperature for soil physicochemical property measurement and the other was stored at -20oC for DNA extraction.
2.2. Measurement of soil physicochemical properties
The water content (WC) of the soil samples was identified by dividing the mass difference between fresh and dry soil of each sample by the mass of the dry soil, which was obtained by heating the soil sample at 105oC for 24 h. Soil pH was measured using a pH meter (Leici PHSJ-4F, Shanghai City, China) in a 1:5 soil/water suspension after shaking at 25°C for 1 h in an incubator shaker at 5000 r/min. Soil total carbon (TC) was determined using an element analyzer (Sercon Integra 2, UK), and organic carbon (SOC) was determined by the potassium dichromate volume-external heating method. Total nitrogen (TN) was determined by an automatic nitrogen analyzer. Ammonium nitrogen and nitrate nitrogen were extracted by potassium chloride solution-spectrophotometry. Nitrate nitrogen was calculated by ultraviolet spectrophotometry. Three soil replicates from each sampling site were used for physicochemical analysis, and the average and standard deviation of three replicate determinations were calculated.
2.3. DNA extraction, sequencing, and data processing
The macrogenomic DNA was extracted from 9 soil samples respectively using the DNeasy powersoil kit (QIAGEN) following the manufacturer’s protocol, and the quality and concentration of DNA were monitored by Nanodrop spectrophotometer, Qbuit fluorometer, and agarose gel electrophoresis.
Prepared DNA samples were sent to BENAGEN (Wuhan, China) for shotgun metagenomics sequencing. 9 samples were sequenced respectively by second-generation Illumina to obtain raw reads. Fastp software was employed to remove low-quality tags and to obtain high-quality sequencing data (Clean Tags). MEGAHIT software was used for metagenomic assembly, and contig sequences shorter than 300 bp were filtered. Using MetaGeneMark (
http://exon.gatech.edu/meta_gmhmmp.cgi, Version 3.26) software default parameters to identify the coding regions of the genome, acquired with the results of single sample assembly gene prediction, and obtain the translated protein sequence. Using MMseqs2 (
https://github.com/soedinglab/mmseqs2, Version 12-113 e3) software to remove redundancy, similarity threshold set to 95%, coverage threshold set to 90%, build the redundant data sets.
2.4. Bioinformatics analysis
Non-redundant gene sets analysis was performed on the BMKCloud (
http://www.biocloud.net/), which is a biological information cloud platform. The abundance of biotaxa and species in each sample was obtained by comparing protein sequences of non-redundant genes with the Nr database using BLAST. The functional annotation information was obtained by comparing the protein sequences of non-redundant genes with KEGG, eggNOG, GO, and CAZy carb enzyme databases using BLAST.
2.5. Statistical analysis
The above results revealed the different samples’ overall functional profiles. Using functional gene information from relevant literature, we extracted all annotated genes and drew a histogram of gene abundance for carbon and nitrogen cycling processes in different pathways using ORIGIN mapping software. Analysis of significant differences between and inter-groups was performed with SPSS software. The R language was used to create a heatmap showing the correlation between microbial functional genes involved in carbon and nitrogen cycling and soil physicochemical factors.
3. Results
3.1. Physicochemical properties of soil samples
The physicochemical properties measured in the study showed that the three samples reflected significantly different soil structures (
Table 1). The water content is the major factor studied in this article, and it gradually decreases from sample S1 to sample S3. Among the various chemical indicators measured, the contents of NH
4+- N represented the highest abundance in S1, and then in order S3 and S2, but there is no significant difference between them. All other indicators, such as NO
3-N, TC, TN, and SOC, exhibited significant differences between different samples and displayed similar trends of change, that as the moisture content decreases, it first increases and then decreases, all showing the highest abundance in S2, and there are significant differences among the three samples. However, soil C/N of S1 is similar to S3 and there is no difference, with S2 being the highest sample.
The correlation heat map between physical and chemical properties (Supporting information Figure S1) indicates that the soil moisture content is negatively correlated with ammonium nitrogen, nitrite, and pH, and positively correlated with other physicochemical factors.
3.2. Metagenomic sequencing
The basic information about metagenomic next-generation sequencing of soil samples from Poyang Lake Wetland ware shown in
Table 2. Short reads were obtained through Illumina sequencing platforms. After filtering the connectors, short fragments, and low-quality data were obtained, a total of 599558464 clean reads, and 89397499433 bp clean data. After assembly, an average of 361194 contigs were obtained from each sample, with an average metagenome size of 298872220 bp.
3.3. Taxonomic composition of microbial communities
The analysis results of the soil microbial diversity index at each sampling point in the Poyang Lake wetland were shown in
Table 3. The Observed Species, ACE, and Chao1 index, which represents the species richness in soil, revealed that the microbial diversity in three samples showed the same trend, with S2 having the highest species richness and S1 having the lowest. The Pielou index was used in the Alpha index to measure the evenness of species diversity distribution, with species distribution in S1 being the most uniform. Shannon and Simpson reflect the diversity of a community, which is influenced by the richness and evenness of species in the sample community. The larger the Shannon index, the higher the diversity of the sample community, with S1 having the highest value.
The composition and abundance of soil microorganisms in different samples were shown in
Supporting information Figure S2, indicated that a total of 4 domains, 7 kingdoms, 199 phyla, 832 families, 3470 genera, and 23813 species were identified by annotating the total microbial species of three collected soil samples. The prokaryotic microbial communities are mainly composed of bacteria (76.05-89.88%), with a relatively small proportion of archaea (1.32-9.60%). The eukaryotic microbial community is mainly composed of fungi (0.01-0.14%). In addition, the annotation results of the metagenome contain a large number of high-quality sequences (7.23-20.00%) that have not been classified, indicating that there is still a large amount of unknown biological information for exploration.
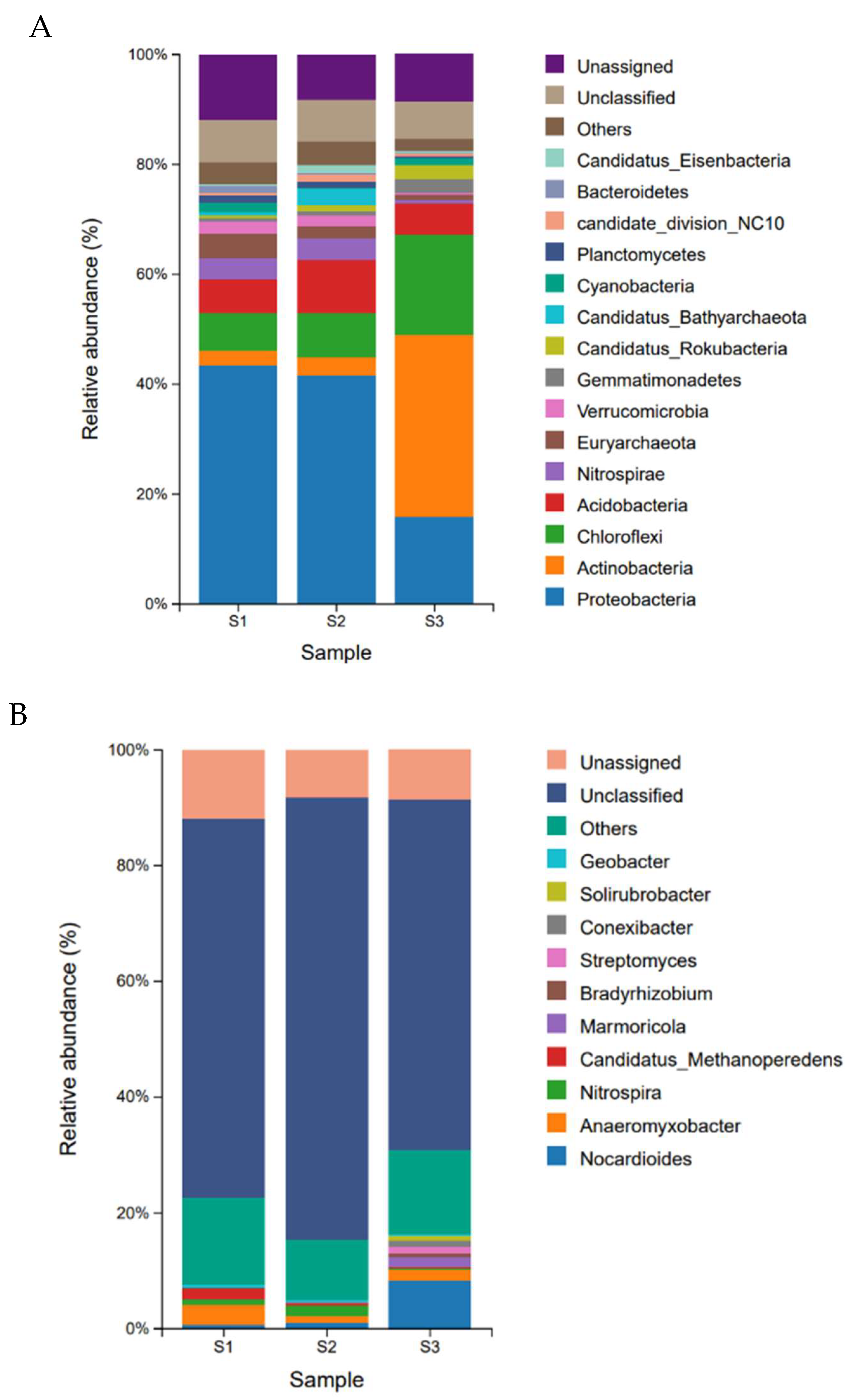
Microbial communities vary significantly among soil samples with different water contents, displaying similar classifications but varying abundances among species. At the phylum level (
Figure 1A), bacteria are divided into 199 phyla, mainly including Proteobacteria, Actinobacteria, Chloroflexi, Acidobacteria, Nitrospirae, Verrucomicrobia, Gemmatimonadetes, Candidatus RoKubacteria, Cyanobacteria, etc. Among them, Proteobacteria is the dominant phylum in S1 and S2 samples, accounting for 43.49% and 41.65% respectively. Actinobacteria is the dominant phylum in S3, accounting for 33.24% which is 12.8 and 10.4 times higher than the abundance of S1 and S2, respectively.
At the genus level (
Figure 1B), there are a total of 3470 genera in the annotated classification of bacteria, mainly including
Nocardioides,
Anaeromyxobacter,
Nitrospira,
Candidatus Methanoperedens,
Marmoricola,
Bradyrhizobium, etc. The dominant genus in S1 is
Anaeromyxobacter (3.46%), while
Nitrospira in S2 (1.85%), and Nocardia-like (8.25%) in S3. The relative abundance of
Candidatus Methanoperedens with the second abundance of S1 is 1.77%, which is 7.1 and 8.4 times higher than that of S2 and S3, respectively. The microbial diversity of archaea communities is significantly lower than that of bacterial communities. It was found that the most abundant archaea is
Euryarchaeota, which accounts for 2.58% of the total microbial taxonomic level, and
Bathyrachaeota accounts for 1.26% of the total microbial taxonomic level.
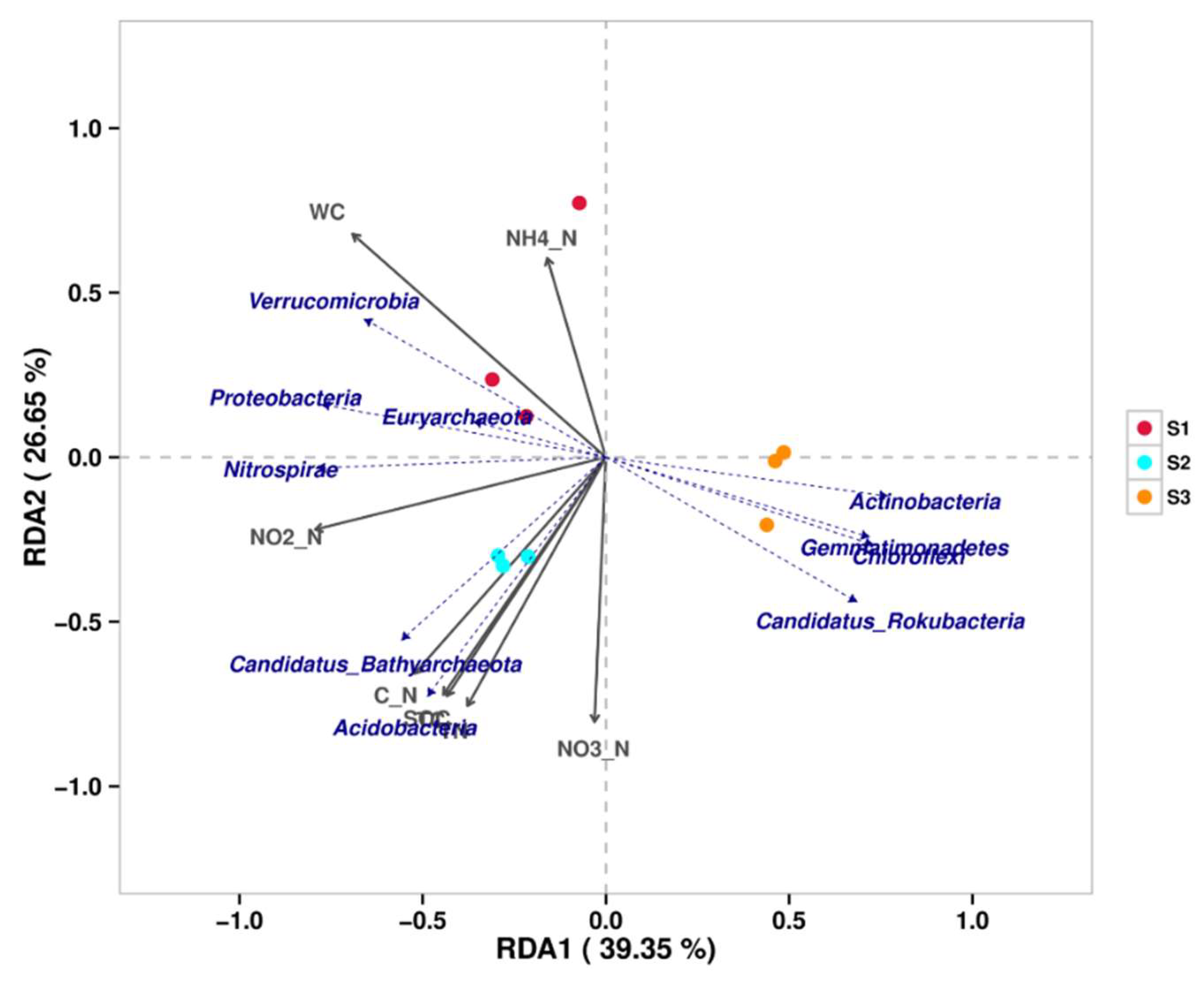
In the correlation analysis between microbial phylum level and physicochemical factors, the cumulative interpretation rates of axis one and axis two in the RDA graph are 66% (
Figure 2). Three samples are clustered separately and there are significant differences among them. The soil moisture content (WC) is positively correlated with Verrucomicrobia, Proteobacteria, and Euryarchaeota, and negatively correlated with Actinobacteria, Gemmatimonadetes, Chloroflexi, and Candidatus Rokubacteria. Nitrospirae is positively correlated with nitrite in soil. The content of soil C/N, organic carbon, total carbon, and total nitrogen is positively correlated with Candidatus Bathyachiaeota and Acidobacteria.
3.4. Microbial carbon fixation genes
The assimilation of CO
2 into organic material is quantitatively the most important biosynthetic process on Earth [
24,
25,
26]. Six autotrophic CO
2 fixation pathways have been found in various environments to date: including aerobic pathways, such as the Calvin cycle (CBB), 3-hydroxy propionic acid dual cycle (3-HP), and 3-hydroxy propionic acid cycle/4-hydroxybutyric acid cycle (3HP/4HB), and in the anaerobic pathways, such as the reducing tricarboxylic acid cycle (rTCA), reducing acetyl CoA pathway (WL), and dicarboxylic acid/4-hydroxybutyric acid cycle (DC/HB). The enzymes catalyzing limiting steps in a given pathway are usually conserved and act as key enzymes, and the corresponding coding genes, often named marker genes [
27], are commonly used in microbial ecological studies. In this study,
cbbL was used for ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO) of the CBB pathway,
aclA for the ATP citrate lyase in the rTCA pathway,
acsA for the carbon-monoxide dehydrogenase catalytic subunit in the WL pathway,
accA for the acetyl-CoA carboxylase carboxyl transferase subunit alpha in the 3HP/4HB pathway.
pcc for the Malonyl-CoA Reductase in the 3-HP pathway.
hcd for the 4-hydroxybutyryl-CoA dehydratase in the DC/HB pathway.
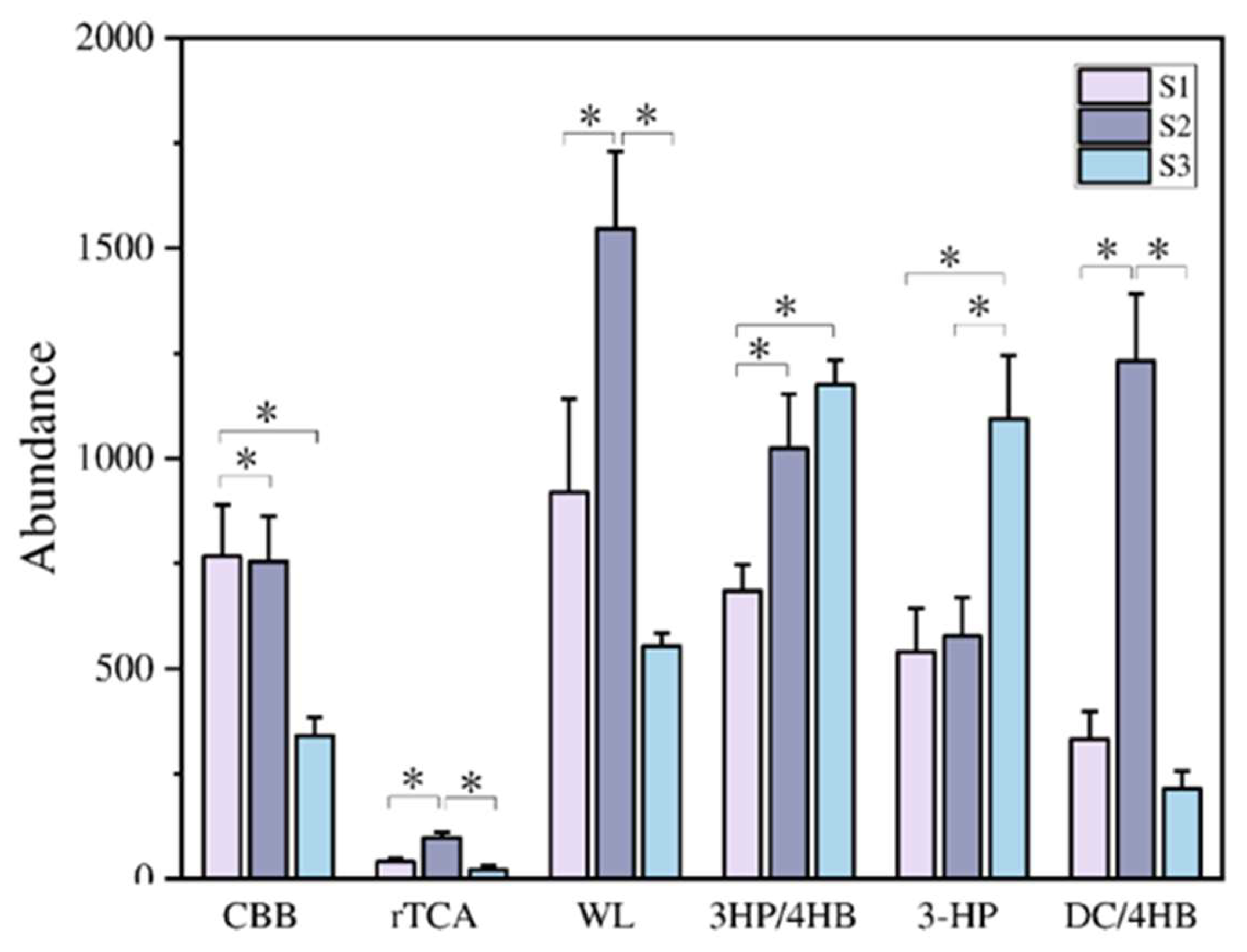
Six marker genes for carbon fixation pathways were detected in three samples with different soil moisture contents (
Figure 3). Among them, the abundance of the marker gene
aclA in the rTCA pathway was the lowest in all three samples. The
acsA in the WL pathway had the highest abundance in samples S1 and S2, while the
accA gene in the 3HP/4HB pathway had the highest abundance in sample S3.
3.5. Microbial methane cycling genes
Methane metabolism, driven by methanogenic and methanotrophic microorganisms, plays a pivotal role in the carbon cycle [
28,
29,
30]. The key enzyme corresponding coding genes in the natural methane cycle pathway driven by microorganisms are as follows [
31,
32]:
mcr, methyl coenzyme-M reductase gene;
mtr, tetrahydromethanopterin S-methyltransferase gene;
mer, F
420-dependent methylenetetrahydromethano pterin dehydrogenase gene;
mtd, methylene– tetrahydromethanopterin reductase gene;
mch, methenyltetrahydromethanopterin cyclohydrolase gene;
ftr, formylmethanofuran-tetrahydromethanopterin N-formyltransferase gene;
fwd, formylmethanofuran dehydrogenase gene;
fdo, formate dehydrogenase iron-sulfur subunit gene;
fdh, formate dehydrogenase gene;
mdh, malate dehydrogenase gene;
pmo, methane/ammonia monooxygenase.
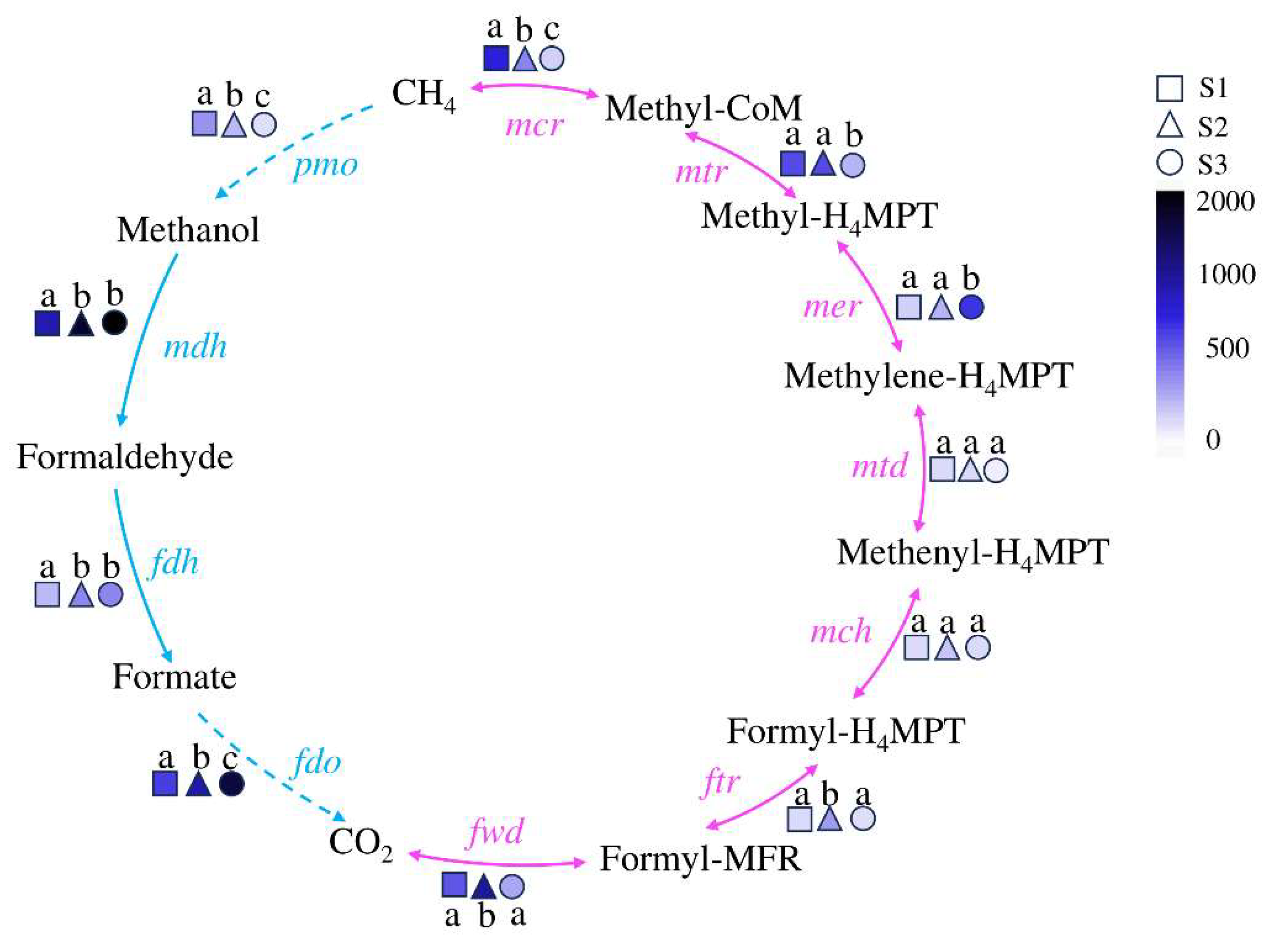
The distribution of methane metabolism functional genes in soils with different water contents was shown in
Figure 4. The KEGG gene functional annotation indicates that the functional genes of each step in the methane cycle are distributed in all three samples, forming a complete cycle. The genes related to methane metabolism in S1 are concentrated in the anaerobic process, and their abundance is higher than that in S3, except for the
mer gene. The genes related to methane metabolism in S3 are concentrated in the aerobic pathway, and their abundance is higher than that in S1, (except for the
pmo gene). and S2, whether in aerobic or anaerobic conditions, has a relatively average distribution of functional genes.
3.6. Microbial N cycling genes
Microbial nitrogen cycling mainly involves six pathways [
33,
34,
35], each of which has corresponding key enzymes, the marker genes coding by microorganisms for each key enzyme are as follows [
36,
37]: nitrogenase gene
nifH in nitrogen fixation; ammonia monooxygenase gene
amoA/amoB, hydroxylamine oxidoreductase gene
hao and nitrite oxidoreductase gene
nxrA/nor in nitrification; nitrate reductase gene
narG, nitrite reductase gene
nirK/nirS, nitric oxide reductase gene
norB and nitrous oxide reductase gene
nosZ in denitrification; N
2H
4 synthase gene
hzsA and N
2H
4 oxidoreductase
hzo in anaerobic ammonia oxidation; nitrate assimilation reductase gene
nasA/narB and nitrite assimilation reductase gene
nirA/nirB in assimilation nitrogen reduction; nitrate dissimilatory reductase gene
napA, nitrite dissimilatory reductase gene
nrfa in dissimilar nitrogen reduction.
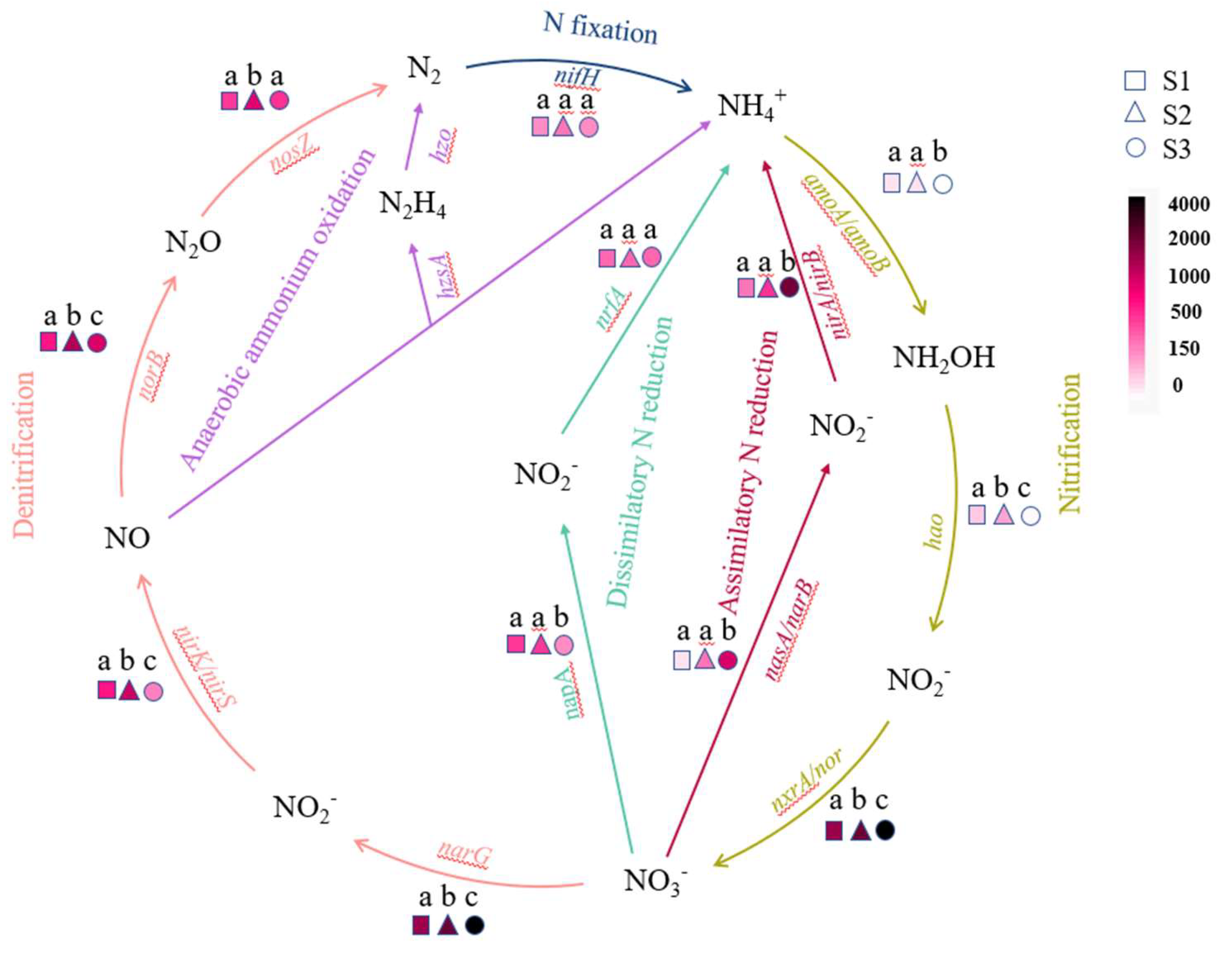
The presence of key enzyme genes related to the nitrogen cycling pathway in three soil samples was shown in
Figure 5. Except for the key enzyme genes in anaerobic ammonia oxidation that have not been discovered yet, other pathways exist in all three samples. It can be seen that the nitrogen cycling profiles of three different soil states varied with changes in soil moisture content, the abundance of key enzyme genes related to anaerobic ammonia oxidation in S3 is extremely low and can be ignored. The functional genes driving nitrogen cycling by microorganisms in S1 and S2 are significantly enriched in Denitrification and Dissimilatory N reduction processes, while S3 is enriched in Nitrification and Assimilatory N reduction processes.
3.7. Microbial antibiotic resistance genes
Antibiotic resistance genes are a new type of environmental pollutant widely present in environmental microorganisms and media [
38]. Antibiotic resistance genes in the environment can not only replicate and increase with the proliferation of microorganisms but also migrate and spread between different microorganisms, directly or indirectly affecting ecological security and human health [
39,
40].
We analyzed the top eight antibiotic-resistance genes in the abundance of soil microorganisms in Poyang Lake wetland (
Figure 6). The most abundant resistance gene type among the three samples is multidrug. Except for tetracycline-resistance genes, which have the highest abundance in S2, the abundance of other antibiotic-resistance genes is highest in S3.
3.8. The correlation between C、N-cycle functional genes and physicochemical factors
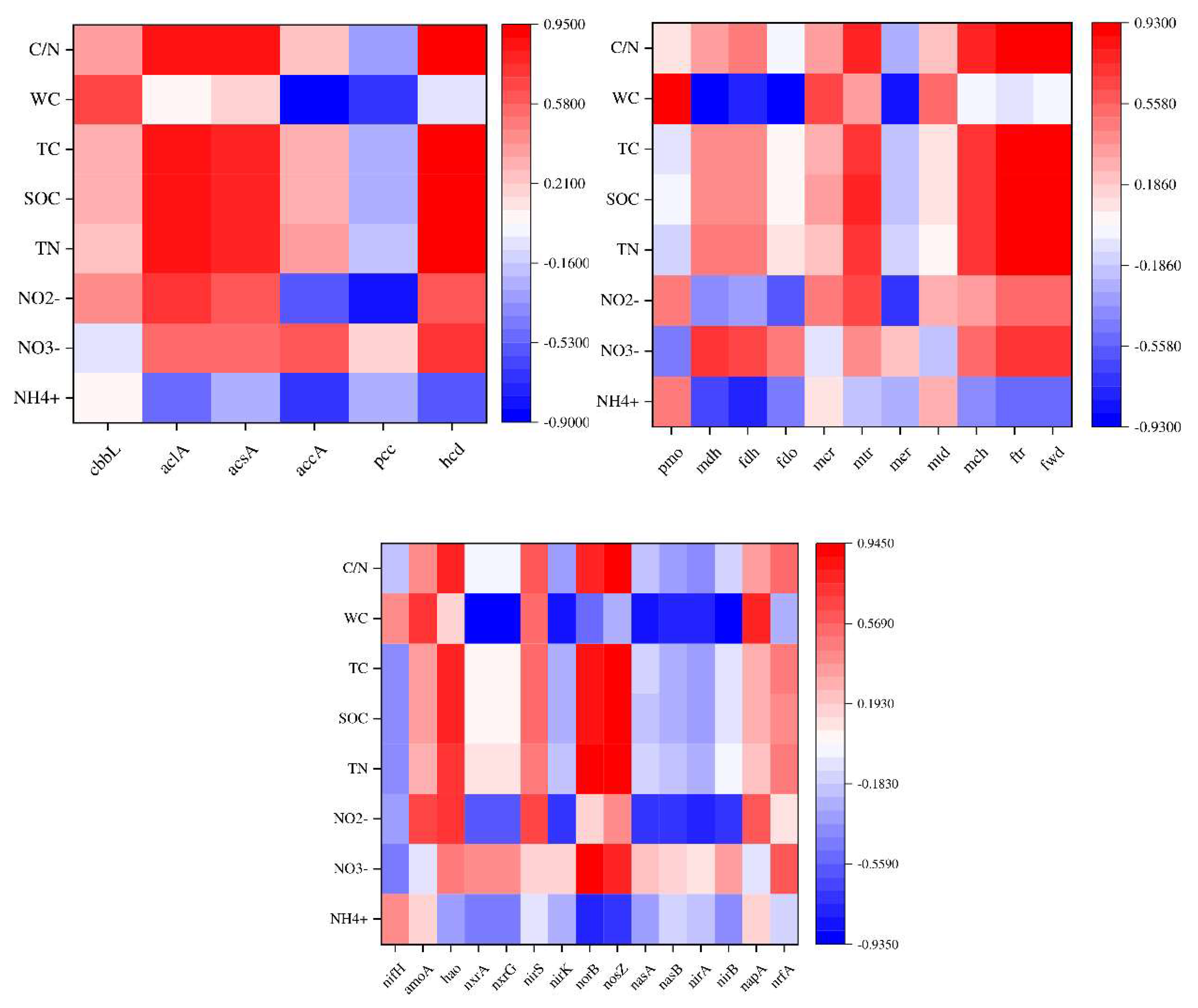
Correlation analysis was conducted between carbon, nitrogen, and methane cycling relating functional genes driven by microorganisms in three samples with different moisture contents soil and soil physicochemical properties (
Figure 7). The abundance of soil functional genes in different states was significantly or extremely significantly correlated with environmental factors. Among them, water content is a key factor affecting soil carbon and nitrogen ecological functions driven by microorganisms.
In the carbon fixation pathway, the abundance of cbbl is significantly positively correlated with water content, while the abundance of aclA, acsA, and hcd is highly significantly positively correlated with C/N, total carbon, total nitrogen, and organic carbon content, and negatively correlated with ammonia nitrogen content. The abundance of accA and pcc is significantly negatively correlated with water content and nitrite nitrogen content, and accA is also significantly negatively correlated with ammonium nitrogen content.
During the methane cycle, there is a highly significant positive correlation between pmo abundance and water content. In anaerobic processes, the mdh, fdh, and fdo are significantly negatively correlated with water content, negatively correlated with nitrite and ammonium nitrogen, and positively correlated with nitrate. During aerobic processes, mcr and mtd are positively correlated with water content. mtr, mch, ftr, fwd are significantly positively correlated with C/N, total carbon, total nitrogen, and organic carbon content, positively correlated with nitrite and nitrate content, and negatively correlated with ammonium nitrogen content.
During the nitrogen cycling, there are a significant positive correlation between amoA during ammonia oxidation and water content and nitrite, while hao is significantly positively correlated with C/N, total carbon, total nitrogen, organic carbon, and nitrite content; During denitrification, norB and nasZ are significantly positively correlated with C/N, total carbon, total nitrogen, organic carbon, and nitrate content, and significantly negatively correlated with ammonium nitrogen; The assimilation and nitrogen reduction processes of nasA, narB, nirA, nirB are significantly negatively correlated with water content and nitrite.
4. Discussion
Wetlands, as an important factor of ecosystems, play a critical role in regulating climate change as a carbon sink and a carbon source, provide a unique habitat, and support biodiversity [
41]. The Poyang Lake is the largest freshwater lake in China, and one of the largest freshwater lake/wetland complexes in Asia, which plays a momentous role in regional and global biodiversity conservation [
42,
43]. In this study, high-throughput sequencing was performed to analyze the microbial characteristics in 3 soil samples with different water content and to dissect the interactions between the microbial community functional structure, ecological responses, and biogeochemical processes.
4.1. The difference in moisture content results in differences in the physicochemical properties of wetland soil
Wetlands serve as a medium for receiving water and drainage, and hydrological conditions are the fundamental attributes of wetland ecosystems [
44]. Water conditions determine the physical properties of wetland soil, the type, the structure of surface plant communities, and soil microbial community, affecting ecosystem productivity [
45].
The physicochemical properties of 3 soil samples, with different moisture from the Poyang Lake wetland, were determined in the study (
Table 1). It was found that the soil from all three sampling points was weakly acidic, therefore, pH value is not a key influencing factor on the physicochemical properties of the Poyang Lake wetland. Among them, except for ammonium nitrogen, all other forms of carbon and nitrogen content, soil C/N ratio are highest in S2. The higher C/N ratio in S2 means better soil quality under this moisture content condition, indicating that excessive or insufficient soil moisture in wetlands is not conducive to the accumulation of their carbon and nitrogen content.
Similar to our research findings, previous studies have also demonstrated that water conditions are a key factor affecting wetland soil properties. The study on soils from the East Dongting Lake wetland, China showed that the water content can control the structure and function of wetlands, and changes in water content can alter the archaeal distribution patterns [
46]; The different water conditions in East Dongting Lake wetlands jointly affect the soil microbial biomass carbon, nitrogen, and enzyme activities [
47].
4.2. The difference in soil physicochemical properties results in differences in soil microbial community structure
Thanks to the diverse environmental conditions, the soil contains the most diverse microbial community on earth [
48,
49]. The spatiotemporal specificity of soil physicochemical properties could prompt microorganisms to evolve rich strategies to cope with extreme environments. Therefore, even slight differences in soil environment can lead to changes in the composition, quantity, and function of microbial populations [
50,
51,
52].
From the relative abundance of soil microorganisms in the Poyang Lake wetland (
Figure 1), it can be seen that at the phylum level, S1 and S2, which are dominated by Proteobacteria, have similar microbial compositions, but there are significant differences compared to S3, which is dominated by Actinobacteria. At the genus level, there are significant differences among the three samples. S1 forms a specific community of dominant genus of
Anaeromyxobacter under strict anaerobic conditions, and the second abundance genus
Candidatus Methanoperedens, which can generate methane. S2 forms a specific community dominated by
Nitrospira, which has nitrification in the nitrogen cycle, oxidizing nitrite to nitrate to provide proton power. S3 grows a specific community of aerobic actinomycetes with
Nocardiodies as the primary bacterial genus, capable of producing multiple antibiotics. The RDA analysis of the correlation between microbial communities and soil physicochemical properties (
Figure 2) manifested that among the microbial community composition of the three soils in Poyang Lake, water content, TC, TN, SOC, and soil C/N ratio are the key influencing factors of the microbial community in Poyang Lake wetland.
In the early research on the structure and function of the soil microbial community in the Poyang Lake wetland, correlation analysis, and RDA analysis showed that the composition of the soil bacterial community in Poyang Lake wetland was mainly influenced by soil organic matter and nutrient elements (TOC, TN), and soil moisture content was also one of the influencing factors [
53,
54]. This is roughly consistent with our research results. According to Liu et al.’s study, the planktonic bacteria in the Poyang Lake wetland were most abundant in the Bacteroidetes, Actinobacteria, and Proteobacteria, and their diversity was significantly affected by hydrological rhythms [
55].
In the context of extreme drought and prolonged dry seasons, the microbial community in the Poyang Lake wetland is significantly influenced by element content and water conditions, gradually forming a relatively stable and specific composition structure.
4.3. The differences in soil microbial community structure result in differences in ecological function distribution
Microorganisms, as an important component of lake wetland ecosystems, drive the cycling of nutrients and the migration and transformation of pollutants such as heavy metals in lake wetlands [
56,
57,
58]. At the same time, the community composition and function of wetland soil microorganisms are also significantly influenced by element content, water conditions, and lake wetland management methods.
The main carbon fixation pathways of soil under different flooding conditions are different. According to results shown in
Figure 3, under long-term flooding conditions like S1, the carbon fixation pathways are mainly WL and CBB pathways. Under S2 is mainly WL and DC/4HB through anaerobic pathways, while under S3 conditions, 3HP/4HB and 3-HP through aerobic pathways are mainly involved. The CBB cycle is the most common CO
2 fixation method in organisms [
59]. The key enzyme type I rubisco enzyme (CbbL) in the CBB pathway is commonly found in green-like bacterial communities (plants, cyanobacteria, green algae, alpha proteobacteria, beta proteobacteria, and gamma proteobacteria, etc.) and red-like bacterial communities (red algae, brown algae, alpha-proteobacteria, and beta-proteobacteria, etc.) [
60]. The S1 and S2 carbon fixed functional genes
cbbL have relatively high abundance, which is speculated to be due to their high relative abundance of Proteobacteria (43.49% 41.65%). According to results shown in
Figure 7, water content, TC, TN, SOC, and C/N are key factors affecting the distribution of carbon fixation functional genes.
The anaerobic degradation of methane in nature is mainly achieved through the reverse reaction process of the Methane production pathway, which is usually mediated by a type of anaerobic methanotrophic archaea (ANME) [
61,
62]. Previous studies have found that methyl coenzyme M reductase (Mcr) is a key enzyme for methane production and activation of alkane molecules [
63]. Among them,
mcrA, which is the coding gene for one of the two subunits of Mcr, is often used to detect the abundance and population of methane-metabolizing archaea in the environment. From the methane cycle diagram (
Figure 4), it can be seen that the abundance of the
mcrA gene varies significantly in soils under different flooding conditions, with S1 having the highest abundance and S3 having the lowest abundance. The ANME group with the highest abundance is found in S1 and
Figure 1B shows the typical ANME archaea
Candidatus Menthanoperedens with high abundance in S1. In addition, in the heat map of the correlation between methane cycle functional genes and soil physicochemical factors (
Figure 7), there is a positive correlation between
mcr and water content, nitrite, indicating a certain relationship between the anaerobic methane oxidation process involved in
mcr and nitrite content. Therefore, the soil of Poyang Lake wetland under flooded conditions plays a prominent role in the anaerobic methane oxidation process.
The nitrification process in the nitrogen cycle is catalyzed by ammonia-oxidizing bacteria and nitrifying bacteria, respectively [
64]. In 2015, both ammonia oxidation and nitrite reduction were actualized by some lineages of
Nitrospira, known as complete ammonia oxidation (Comammox), have been found [
65]. The abundance of
Nitrospira, the dominant bacterial genus in S2, is significantly higher than that in other samples. Meanwhile, S2 has a relatively high abundance of functional genes (
amoA, hao, nxrA) during the nitrification process. However, the abundance of
nxrA in S3 is significantly higher than that in S2, and we speculate that this is due to the high abundance of
Chloroflexi in S3, which also has nitrite-reducing ability. In addition, studies have shown a significant negative correlation between the abundance of
Nitrospira and the concentration of ammonia nitrogen. In this study, S2 with the lowest ammonia nitrogen concentration (
Table 1) contained the highest abundance of
Nitrospira, which also confirms this.
4.4. The differences in soil microbial community structure result in differences in antibiotic resistance distribution
Antibiotic resistance is a global health challenge, involving the transfer of bacteria and genes between humans, animals and the environment [
66,
67]. On the map of antibiotic resistance gene abundance distribution, S3 has the highest abundance except for the tetracycline-resistance gene, which is reasonable as the wetland soil closest to human activities. In addition, the most abundant genus of
Nocardioides in S3 has strong adaptability to relatively harsh environments and can be widely distributed by regulating intracellular metabolism, synthesizing secondary metabolites, and secreting special enzymes. Linking a high abundance of resistance genes with a high abundance of
Nocardioides genus can confirm the special function of S3 soil microorganisms on resistance genes.
5. Conclusion
The microbial community composition and soil physicochemical properties of the three soil samples with different moisture contents in the Poyang Lake wetland exhibit distinct characteristics, and their mutual influence leads to their different ecological functions. This study analyzed the microbial community composition characteristics and functional gene abundance in different soil samples of wetlands, in order to gain a deeper understanding of the relationship between underground microbial communities and field ecological features. The abundance of Candidatus menthanoperedons and the high abundance of mcrA in S1 mutually confirm the prominent role of S1 in the anaerobic oxidation pathway of methane in the methane cycle process. The dominant bacterial genera Nitrospira in S2 is mutually confirmed with a large number of nitrification functional genes (amoA, hao, nxrA), indicating the prominent role of S2 in nitrification during the nitrogen cycle. The dominant bacterial genera Nocardia in S3 is mutually confirmed with a large number of discovered antibiotic resistance genes, indicating the important function of S3 in resistance genes and its outstanding research value for microbial resistance issues. The above study has preliminarily confirmed the indicator role of soil microbial communities in predicting wetland ecological functions, which will help us better formulate plans for restoring ecological balance and regulating climate change. Although it is unclear whether these conclusions can be extended to other wetland ecosystem types due to increased environmental heterogeneity and climate uncertainty, understanding the reactions of soil microorganisms and their potential impacts is crucial for a deeper understanding of the functions of underground wetland ecosystems.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org; Figure S1. Correlation heatmap among various soil physicochemical properties in Poyang Lake wetland. The correlation range between physical and chemical properties is from -1 to 1, with blue representing positive values and red representing negative values. A p-value greater than 0.5 between pairwise properties is significant. The size of the circle represents the strength of the correlation; Figure S2. Histogram of soil microbial classification and their relative abundance in soil samples with different water content from Poyang Lake wetland. (a) Kingdom level (b) Class level (c) Order level (d) Family level; Figure S3. The β diversity PCA diagram of soil microbial communities in different water content samples from the Poyang Lake wetland. Different colors represent three soil samples with different moisture contents. (a) Diversity of microbial composition at the phylum level. The interpretation rate of PC1 and PC2 is 91.36%. (b) Diversity of microbial composition at the class level. The interpretation rate of PC1 and PC2 is 95.56%.; Figure S4. Cluster diagram of functional genes related to carbon cycling in the soil with different water contents from the Poyang Lake wetland. A p-value greater than 1 between pairwise properties is significant.; Figure S5. Heat map of the correlation between nitrogen cycling genes and physicochemical properties with red representing positive values and blue representing negative values. A p-value greater than 0.5 between pairwise properties is significant. (a) The correlation between carbon fixation pathway genes and physicochemical properties. (b) The correlation between carbon decomposition pathway genes and physicochemical properties; Figure S6. Heat map of correlation between soil nitrogen cycling functional genes and physicochemical factors in Poyang Lake wetland. The correlation range is from -1 to 1, with red representing positive values and blue representing negative values. P<0.5 between pairwise properties is significant.
Author Contributions
Methodology, Y.L.; Software, X.Z.; Validation, X.P.; Formal analysis, Y.L.; Investigation, Z.L., L.Z., H.N.; Resources, H.Y., H.N., L.Z., Z.L.; Data curation, Y.L.; Writing-original draft, Y.L.; Writing-review&editing, Z.L.; Visualization, X.Z., X.P.; Supervision, L.Z., Z.L.; Project administration, Z.L.; Funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
The National Natural Science Foundation of China (31960015), and Natural Science Foundation of Jiangxi Province, China (20192BAB204001).
Data Availability Statement
The sequencing dataset for this study was uploaded to the National Microbial Data Center of China (
https://www.nmdc.cn/), and the BioProject number is NMDC20147401.
Acknowledgments
Thank the all authors for their joint efforts.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nybo, S.E.; Khan, N.E.; Woolston, B.M.; Curtis, W.R. Metabolic engineering in chemolithoautotrophic hosts for the production of fuels and chemicals. Metab Eng. 2015, 30, 105–120. [Google Scholar] [CrossRef]
- De, S.L.; Cabezas, A.; Marzorati, M.; Friedrich, M.W.; Boon, N.; Verstraete, W. Microbial community analysis of anodes from sediment microbial fuel cells powered by rhizodeposits of living rice plants. Appl Environ Microbiol. 2010, 76, 2002–2008. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci Total Environ. 2017, 15, 697–703. [Google Scholar] [CrossRef]
- Li, T, T.; Hu, H.; Wang, J, S.; Li, Z, Y.; L, X, I. Progress in research methods of soil microbial structure and diversity in wetlands. Chinese Journal of Soil Science. 2016, 47, 758–762. [CrossRef]
- Zhang, K.; Li, M.; Yan, Z.; Li, M.; Kang, E.; Yan, L.; Zhang, X.; Li, Y.; Wang, J.; Yang, A.; Niu, Y.; Kang, X. Changes in precipitation regime lead to acceleration of the N cycle and dramatic N2O emission. Sci Total Environ. 2022, 20, 152140. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Zhang, J.; Zhu, B.; Zhao, W.; Wang, Z.; Yang, T.; Yu, C. A review of factors affecting the soil microbial community structure in wetlands. Environ Sci Pollut Res Int. 2024, 31, 46760–46768. [Google Scholar] [CrossRef]
- Taş, N.; De, J, A, E. ; Li, Y.; Trubl, G.; Xue, Y.; Dove, N, C. Metagenomic tools in microbial ecology research. Curr Opin Biotechnol. 2021, 67, 184–191. [Google Scholar] [CrossRef]
- Fukuda, K.; Ogawa, M.; Taniguchi, H.; Saito, M. Molecular approaches to studying microbial communities: targeting the 16S ribosomal RNA gene. J UOEH. 2016, 38, 223–232. [Google Scholar] [CrossRef]
- Chiu, CY.; Miller, S, A. Clinical metagenomics. Nat Rev Genet. 2019, 20, 341–355. [Google Scholar] [CrossRef]
- Han, D.; Li, Z.; Li, R.; Tan, P.; Zhang, R.; Li, J. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019, 45, 668–685. [Google Scholar] [CrossRef]
- Gougoulias, C.; Clark, JM.; Shaw, L, J. The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J Sci Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef]
- Salimi, S.; Almuktar, SAAAN. ; Scholz, M. Impact of climate change on wetland ecosystems: A critical review of experimental wetlands. J Environ Manage. 2021, 15, 112160. [Google Scholar] [CrossRef]
- Kirwan, M, L. ; Megonigal, J, P. Tidal wetland stability in the face of human impacts and sea-level rise. Nature. 2013, 504, 53–60. [Google Scholar] [CrossRef]
- Bridgham, S, D. ; Cadillo-Quiroz, H.; Keller, J, K.; Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Chang Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Jiang, Y, M. ; Zhang, C.; Huang, X, L.; Ni, C, Y.; Wang, J, F.; Song, P, F.; Zhang, Z, B. Effect of heavy metals in the sediment of Poyang Lake estuary on microbial communities structure base on Mi-seq sequencing. China Environmental Science. 2016, 36, 3475–3486. [Google Scholar]
- Kou, W.; Zhang, J.; Lu, X.; Ma, Y.; Mou, X.; Wu, L. Identification of bacterial communities in sediments of Poyang Lake, the largest freshwater lake in China. Springerplus. 2016, 1, 401. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, L.; Chen, H.; Deng, S.; Ji, H.; Liang, F. Assessing habitat quality at Poyang Lake based on InVEST and Geodetector modeling. Ecol Evol. 2023, 13, e10759. [Google Scholar] [CrossRef]
- Ren, Q.; Yuan, J.; Wang, J.; Liu, X.; Ma, S.; Zhou, L.; Miao, L.; Zhang, J. Water level has higher influence on soil organic carbon and microbial community in Poyang Lake Wetland than vegetation type. Microorganisms. 2022, 10, 131. [Google Scholar] [CrossRef]
- You, Q.; Yang, W.; Jian, M.; Hu, Q. A comparison of metric scoring and health status classification methods to evaluate benthic macroinvertebrate-based index of biotic integrity performance in Poyang Lake wetland. Sci Total Environ. 2021, 761, 144112. [Google Scholar] [CrossRef]
- Dai, X.; Yu, Z.; Yang, G.; Wan, R. Role of flooding patterns in the biomass production of vegetation in a typical herbaceous wetland, Poyang Lake Wetland, China. Front Plant Sci. 2020, 11, 521358. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, Y, T.; He, S, Y.; Mou, X.; Wu, L. Dynamics of bacterioplankton community structure in response to seasonal hydrological disturbances in Poyang Lake, the largest wetland in China. [CrossRef]
- Li, B.; Yang, G.; Wan, R.; Lai, X.; Wagner, P.D. Impacts of hydrological alteration on ecosystem services changes of a large river-connected lake (Poyang Lake), China. J Environ Manage. 2022, 310, 114750. [Google Scholar] [CrossRef]
- Yu, Y, W.; Jorge, G, M.; Lin, L, S.; Min, Z.; Zhao, S, W.; Huan, Z.; Jun, X. Drivers and changes of the Poyang Lake wetland ecosystem. Wetlands. 2019, 39, 35–44. [CrossRef]
- Xu, M.; Li, X.; Kuyper, T.W; Xu, M.; Li, X.; Zhang, J. High microbial diversity stabilizes the responses of soil organic carbon decomposition to warming in the subsoil on the Tibetan Plateau. Glob Chang Biol. 2021, 27, 2061–2075. [Google Scholar] [CrossRef]
- Moomaw, W, R.; Chmura, G, L.; Davies, G, T.; Finlayson, C, M.; Middleton, B, A.; Natali, S, M.; Perry, J, E.; Roulet, N.; Sutton-Grier, A, E. Wetlands in a changing climate: Science, Policy and Management. Wetlands. 2018, 38, 183–205. [CrossRef]
- Zhang, N.; Liu, W.; Yang, H.; Yu, X.; Gutknecht, J, L.; Zhang, Z.; Wan, S.; Ma, K. Soil microbial responses to warming and increased precipitation and their implications for ecosystem C cycling. Oecologia. 2013, 173, 1125–1142. [CrossRef]
- Li, Y.; Xiong, L.; Zeng, K.; Wei, Y.; Li, H.; Ji, X. Microbial-driven carbon fixation in natural wetland. J Basic Microbiol. 2023, 63, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Lennon, J, T.; Nguyễn-Thùy, D.; Phạm, T, M.; Drobniak, A.; Tạ, P, H.; Phạm, N, D.; Streil, T.; Webster, K, D.; Schimmelmann, A. Microbial contributions to subterranean methane sinks. Geobiology. 2017, 15, 254–258. [CrossRef]
- Chamberlain, S, D.; Anthony, T, L.; Silver, W, L.; Eichelmann, E.; Hemes, K, S.; Oikawa, P, Y.; Sturtevant, C.; Szutu, D, J.; Verfaillie, J, G.; Baldocchi, D, D. Soil properties and sediment accretion modulate methane fluxes from restored wetlands. Glob Chang Biol. 2018, 24, 4107–4121. [CrossRef]
- Bridgham, S, D.; Cadillo-Quiroz, H.; Keller, J, K.; Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Chang Biol. 2013, 19, 1325–1346. [CrossRef]
- Qu, Y.; Zhao, Y.; Yao, X.; Wang, J.; Liu, Z.; Hong, Y.; Zheng, P.; Wang, L.; Hu, B. Salinity causes differences in stratigraphic methane sources and sinks. Environ Sci Ecotechnol. 2023, 19, 100334. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Malfatti, S, A.; McFarland, J, W.; Anderson, F, E.; Pati, A.; Huntemann, M.; Tremblay, J.; Glavina, D, R, T.; Waldrop, M, P.; Windham-Myers, L.; Tringe, S, G. Patterns in wetland microbial community composition and functional gene repertoire associated with methane emissions. MBio. 2015, 19, 6, e00066-15. [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat Rev Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Ramond, J, B.; Jordaan, K.; Díez, B.; Heinzelmann, S, M.; Cowan, D, A. Microbial biogeochemical cycling of nitrogen in arid ecosystems. Microbiol Mol Biol Rev. 2022, 86, e0010921. [CrossRef]
- Ortiz, M.; Bosch, J.; Coclet, C.; Johnson, J.; Lebre, P.; Salawu-Rotimi, A.; Vikram, S.; Makhalanyane, T.; Cowan, D. Microbial nitrogen cycling in antarctic soils. Microorganisms. 2020, 8, 1442. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Yu, K. Application of microbial gene databases in the annotation of nitrogen cycle functional genes. Microbiology China. 2020, 47, 3021–3038. [Google Scholar] [CrossRef]
- Ma, X, Y.; Jiang, L.; Song, Y, Y.; Sun, L.; Song, C, C.; Hou, A, X.; Gao, J, L.; Du, Y. Effects of temperature and moisture changes on functional gene abundance of soil nitrogen cycle in permafrost peatland. Acta Ecologica Sinica. 2021, 41, 6707–6717.
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of antibiotic resistance genes in the environment. Environ Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Qu, J, H.; Wang, Y, K.; Mai, W, N.; Wan, D, J.; Lu, X, Y. Responses of microbial community and antibiotic resistance genes to co-existence of chloramphenicol and salinity. Appl Microbiol Biotechnol. 2022, 106, 7683–7697. [CrossRef]
- Erwin, K.L. Wetlands and global climate change: the role of wetland restoration in a changing world. Wetlands Ecology & Management. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Chen, M.; Wei, X.; Huang, H.; Lü, T. Poyang Lake basin: a successful, large-scale integrated basin management model for developing countries. Water Sci Technol. 2011, 63, 1899–1905. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Y.; Zhang, C.; Shen, H.; Wang, D. Water level fluctuations influence microbial communities and mercury methylation in soils in the Three Gorges Reservoir, China. J Environ Sci (China). 2018, 68, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.L.; Silver, W.L. Mineralogical associations with soil carbon in managed wetland soils. Glob Chang Biol. 2020, 26, 6555–6567. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M, O.; Xue, W.; Tang, M.; Du, D, L.; Yu, F, H. Synergistic effects of soil microbes on solidago canadensis depend on water and nutrient availability. Microb Ecol. 2020, 80, 837–845. [CrossRef]
- Li, W.; Feng, D.; Yang, G.; Deng, Z.; Rui, J.; Chen, H. Soil water content and pH drive archaeal distribution patterns in sediment and soils of water-level-fluctuating zones in the East Dongting Lake wetland, China. Environ Sci Pollut Res Int. 2019, 26, 29127–29137. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Z, G.; Xiao, H, X.; Li, Y, F.; Peng, W, X. Changes of soil microbial biomass carbon, nitrogen, and enzyme activities in East Dongting Lake wetlands at different water levels. Ying Yong Sheng Tai Xue Bao. 2021, 32, 2958–2966. [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat Rev Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, D, A.; Jansson, J, K.; Remais, J, V.; Rich, V, I.; Singh, B, K.; Trivedi, P. Climate change microbiology - problems and perspectives. Nat Rev Microbiol. 2019, 17, 391–396. [CrossRef]
- Zhang, H.; Zheng, S.; Ding, J.; Wang, O.; Liu, F. Spatial variation in bacterial community in natural wetland-river-sea ecosystems. J Basic Microbiol. 2017, 57, 536–546. [Google Scholar] [CrossRef]
- Cheung, M, K.; Wong, C, K.; Chu, K, H.; Kwan, H, S. Community structure, dynamics and interactions of bacteria, archaea and fungi in subtropical coastal wetland sediments. Sci Rep. 2018, 8, 14397. [CrossRef]
- Zhou, Z.; Meng, H.; Liu, Y.; Gu, J, D.; Li, M. Stratified bacterial and archaeal community in mangrove and intertidal wetland mudflats revealed by high throughput 16S rRNA gene sequencing. Front Microbiol. 2017, 8, 2148. [CrossRef]
- Guo, J.; Wang, X.; Cao, X.; Qi, W.; Peng, J.; Liu, H.; Qu, J. The influence of wet-to-dry season shifts on the microbial community stability and nitrogen cycle in the Poyang Lake sediment. Sci Total Environ. 2023, 903, 166036. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Wu, J.; Kong, Z.; Feinstein, L, M.; Ding, X.; Ge, G.; Wu, L. Bacterial and fungal community composition and functional activity associated with lake wetland water level gradients. Sci Rep. 2018, 8, 760. [CrossRef]
- Liu, Y, J.; Liu, X.; Mou, X, Z.; Wu, L. Research status of microorganisms in a large, shallow lake Poyang Lake wetland. Microbiology China, 2019, 46, 3453–3460. [CrossRef]
- Sims, A.; Zhang, Y.; Gajaraj, S.; Brown, P.B.; Hu, Z. Toward the development of microbial indicators for wetland assessment. Water Res. 2013, 47, 1711–1725. [Google Scholar] [CrossRef]
- Mellado, M.; Vera, J. Microorganisms that participate in biochemical cycles in wetlands. Can J Microbiol. 2021, 67, 771–788. [Google Scholar] [CrossRef]
- Sánchez, O. Constructed wetlands revisited: microbial diversity in the -omics era. Microb Ecol. 2017, 73, 722–733. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Milo, R. The global mass and average rate of rubisco. Proc Natl Acad Sci USA. 2019, 116, 4738–4743. [Google Scholar] [CrossRef]
- Uchino, Y.; Yokota, A. "Green-like" and "red-like" RubisCO cbbL genes in Rhodobacter azotoformans. Mol Biol Evol. 2003, 20, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Leu, A, O.; Xie, G, J.; Guo, J.; Feng, Y.; Zhao, J, X.; Tyson, G, W.; Yuan, Z.; Hu, S. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J. 2018, 12, 1929–1939. [CrossRef]
- Leu, A, O.; Cai, C.; McIlroy, S, J.; Southam, G.; Orphan, V, J.; Yuan, Z.; Hu, S.; Tyson, G, W. Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae. ISME J. 2020, 14, 1030–1041. [CrossRef]
- Venturini, A, M.; Dias, N, M, S.; Gontijo, J, B.; Yoshiura, C, A.; Paula, F, S.; Meyer, K, M.; Nakamura, F, M.; França, A, G, D.; Borges, C, D.; Barlow, J.; et al. Increased soil moisture intensifies the impacts of forest-to-pasture conversion on methane emissions and methane-cycling communities in the Eastern Amazon. Environ Res. 2022, 212, 113139. [CrossRef]
- Galloway, J, N.; Townsend, A, R.; Erisman, J, W.; Bekunda, M.; Cai, Z.; Freney, J, R.; Martinelli, L, A.; Seitzinger, S, P.; Sutton, M, A. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science. 2008, 320, 889–892. [CrossRef]
- Wang, B.; Zhao, J.; Guo, Z.; Ma, J.; Xu, H.; Jia, Z. Differential contributions of ammonia oxidizers and nitrite oxidizers to nitrification in four paddy soils. ISME J. 2015, 9, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wen, D. Characterization of antibiotic resistance across Earth’s microbial genomes. Sci Total Environ. 2022, 816, 151613. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Microbial community composition. (A) Relative abundance of the major phyla based on metagenomic sequences in the Poyang Lake wetland, (B) Relative abundance of the major genus. Unassigned: A sequence that has not been accurately identified or classified into known biological species. Unclassified: Although DNA sequences or genes have been identified to a certain level, they cannot be clearly localized to specific biological taxonomic units at more specific taxonomic levels. Other: Species with relatively low abundance.
Figure 1.
Microbial community composition. (A) Relative abundance of the major phyla based on metagenomic sequences in the Poyang Lake wetland, (B) Relative abundance of the major genus. Unassigned: A sequence that has not been accurately identified or classified into known biological species. Unclassified: Although DNA sequences or genes have been identified to a certain level, they cannot be clearly localized to specific biological taxonomic units at more specific taxonomic levels. Other: Species with relatively low abundance.
Figure 2.
RDA analysis is used to reflect the correlation between physicochemical factors and microbial species (phylum level) in the soil of the Poyang Lake wetland. The dashed arrow in the figure represents the level of microbial phylum, while the solid arrow represents physical and chemical factors. The arrow representing microbial species is closer to a certain physicochemical factor, indicating that the physicochemical factor has the greatest impact on that species’s abundance. C_N represents the carbon-to-nitrogen ratio of the soil.
Figure 2.
RDA analysis is used to reflect the correlation between physicochemical factors and microbial species (phylum level) in the soil of the Poyang Lake wetland. The dashed arrow in the figure represents the level of microbial phylum, while the solid arrow represents physical and chemical factors. The arrow representing microbial species is closer to a certain physicochemical factor, indicating that the physicochemical factor has the greatest impact on that species’s abundance. C_N represents the carbon-to-nitrogen ratio of the soil.
Figure 3.
The abundance of key enzyme involved in six carbon fixation pathways in three samples. * mark the significance of differences (p<0.05).
Figure 3.
The abundance of key enzyme involved in six carbon fixation pathways in three samples. * mark the significance of differences (p<0.05).
Figure 4.
The abundance of methane cycling and functional genes driven by soil microorganisms in Poyang Lake wetland. The pink line represents the process of methane generation and the blue line represents the process of methane oxidation. The genes on the arrows are the functional genes of the key enzymes in this turnover process. The different shapes represent different samples, and the filling colors from light to deep represent the abundance of the gene from low to high, while the letters “a”, “b”, and “c” indicate significant differences between samples.
Figure 4.
The abundance of methane cycling and functional genes driven by soil microorganisms in Poyang Lake wetland. The pink line represents the process of methane generation and the blue line represents the process of methane oxidation. The genes on the arrows are the functional genes of the key enzymes in this turnover process. The different shapes represent different samples, and the filling colors from light to deep represent the abundance of the gene from low to high, while the letters “a”, “b”, and “c” indicate significant differences between samples.
Figure 5.
The nitrogen cycling driven by soil microorganisms and the abundance of its functional genes in the Poyang Lake wetland. Different colored arrows represent different nitrogen cycling processes in soil, and the genes on the arrows are the functional genes of the key enzymes in this turnover process. The different shapes represent different samples, and the filling colors from light to deep represent the abundance of the gene from low to high, while the letters “a”, “b”, and “c” indicate significant differences between samples.
Figure 5.
The nitrogen cycling driven by soil microorganisms and the abundance of its functional genes in the Poyang Lake wetland. Different colored arrows represent different nitrogen cycling processes in soil, and the genes on the arrows are the functional genes of the key enzymes in this turnover process. The different shapes represent different samples, and the filling colors from light to deep represent the abundance of the gene from low to high, while the letters “a”, “b”, and “c” indicate significant differences between samples.
Figure 6.
The abundance of resistance genes secreted by microorganisms in soils with different moisture contents in Poyang Lake wetland. “*” means significant differences (p<0.05) and “**” means extremely significant differences (p<0.01).
Figure 6.
The abundance of resistance genes secreted by microorganisms in soils with different moisture contents in Poyang Lake wetland. “*” means significant differences (p<0.05) and “**” means extremely significant differences (p<0.01).
Figure 7.
Correlation heatmap between soil physicochemical factors and genes related to carbon decomposition, methane cycle, and nitrogen cycle. Red represents positive correlation, blue represents negative correlation. The darker the color, the stronger the correlation.
Figure 7.
Correlation heatmap between soil physicochemical factors and genes related to carbon decomposition, methane cycle, and nitrogen cycle. Red represents positive correlation, blue represents negative correlation. The darker the color, the stronger the correlation.
Table 1.
Physicochemical characteristics of soil samples from Poyang Lake wetland.
Table 1.
Physicochemical characteristics of soil samples from Poyang Lake wetland.
|
Sample |
S1 |
S2 |
S3 |
| Physicochemical property |
|
| WC (%) |
25.25±1.05 (c) |
11.05±1.35 (b) |
3.75±0.65 (a) |
| pH |
6.34±0.1 (ab) |
6.55±0.23 (b) |
6.01±0.27 (a) |
| NH4+-N (mg/kg) |
5.64±0.99 (a) |
2.27±0.69 (a) |
3.42±1.22 (a) |
| NO3-N (mg/kg) |
1.46±0.12 (a) |
10.55±1.52 (c) |
6.01±1.15 (b) |
| NO2-N (mg/kg) |
0.07±0.01 (ab) |
0.09±0.01 (b) |
0.05 (a) |
| TN (g/kg) |
0.19±0.06 (a) |
1.64±0.02 (c) |
0.49±0.09 (b) |
| TC (g/kg) |
1.73±0.57 (a) |
21.38±0.34 (c) |
4.77±1.2 (b) |
| SOC (g/kg) |
1.59±0.52 (a) |
20.56±0.84 (c) |
4.08±0.98 (b) |
| EC (us/cm) |
23.80±7.17 (a) |
163.75±35.65 (b) |
70.42±11.10 (a) |
| C/N |
9.16±0.49 (a) |
13.10±0.28 (b) |
9.17±1.00 (a) |
Table 2.
Basic information about metagenomic next-generation sequencing of soil samples from Poyang Lake Wetland.
Table 2.
Basic information about metagenomic next-generation sequencing of soil samples from Poyang Lake Wetland.
| Scheme 50. |
Raw reads |
Clean reads |
N50 |
GC content (%) |
| S1 |
66,361,603 |
66,359,298 |
653 |
56.37 |
| S2 |
65,391,661 |
65,387,372 |
860.6 |
63.84 |
| S3 |
68,294,339 |
68,293,247 |
770 |
58.3 |
Table 3.
Alpha-diversity AVERAGE (standard deviation) of the soil microorganism in the Poyang Lake Wetland, showing observed species, ACE, Chao1, Shannon, and Simpson indices.
Table 3.
Alpha-diversity AVERAGE (standard deviation) of the soil microorganism in the Poyang Lake Wetland, showing observed species, ACE, Chao1, Shannon, and Simpson indices.
| Sample |
Observed species |
ACE |
Chao1 |
Shannon |
Simpson |
Pielou |
| S1 |
10755
(687.89) |
10759.98
(687.44) |
10757.22
(687.65) |
6.28
(0.17) |
0.99
(0.002) |
0.68
(0.016) |
| S2 |
13403.33
(458.68) |
13413.72
(457.42) |
13407.63
(458.28) |
6.23
(0.01) |
0.99 |
0.64
(0.002) |
| S3 |
13279
(83.48) |
13284.83
(83.59) |
13281.72
(83.05) |
5.99
(0.02) |
0.98
(0.001) |
0.63
(0.002) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).