1. Introduction
The biological response to hernia prosthetics remains a topic of ongoing debate among herniologists. One key issue is mesh shrinkage, caused by the retraction of fibrotic tissue ingrown into the prosthetic material. [1 – 4] This phenomenon is often cited as a contributing factor to the reduction of mesh surface area, potentially leading to inadequate coverage of the hernia defect and an increased risk of recurrence. [5 – 7] Another critical concern is the stiff fibrotic incorporation that occurs shortly after implantation. This characteristic of conventional hernia meshes is believed to be a primary cause of the discomfort and chronic pain frequently reported by patients following prosthetic abdominal hernia repair. [2, 4, 8 – 10] The formation of a hard, fibrotic plaque sharply contrasts with the highly mobile nature of the abdominal wall, sometimes resulting in painful restrictions of natural muscular movements.
Literature suggests that this issue may be linked to the static nature of flat meshes, which, when encased by immobile and rigid tissue, can hinder normal muscle function in the repaired groin. [
11] Additionally, recent studies on hernia genesis, particularly focused on inguinal protrusions, have highlighted the degenerative origins of the disease. [12 – 16] This renewed interest in developing more physiological and pathogenetically aligned approaches to abdominal wall hernia repair has led to the creation of a new category of hernia repair devices based on dynamic 3D scaffolds with regenerative features. [17 - 24] Building on this innovative line of research, the Stenting & Shielding (S&S) Hernia System—a dynamic, responsive device—has been developed. Constructed from TPE material, these devices consist of a rayed element assembled around a central mast connected to an oval shield. Delivered laparoscopically, they expand within the hernia defect to achieve permanent obliteration. Their compliance with the natural movements of the abdominal wall, combined with their ability to elicit a probiotic biological response, sets them apart from traditional flat and static meshes. The dynamic responsivity of the S&S device likely promotes the incorporation of viable tissue, including newly developed arteries, veins, nerves, and notably, myocytes resembling the typical components of the abdominal wall. The present study aims to demonstrate the development and maturation of muscle elements within the S&S Hernia System at various postoperative stages, using a cohort of experimental pigs that underwent repair of induced abdominal wall defects with the S&S Hernia System.
Material and Methods
The experimental trial was conducted in accordance with the Animal Care Protocol for Experimental Surgery, as stipulated by the Italian Ministry of Health. The protocol was officially approved on June 1st, 2021 with the Decree No. 379/2021-PR.
Between February 2022 and July 2024, ten female pigs, each with bilateral muscular defects previously purchased in the lower abdomen, were selected for the trial. Each animal underwent laparoscopic placement of two Stenting & Shielding (S&S) Hernia Devices. The S&S device is specifically engineered to provide a dissection-free, minimally invasive repair for a range of abdominal wall hernias, including inguinal, incisional, femoral, Spigelian, and obturator hernias. The pigs, aged 4 to 6 months and weighing between 40 and 60 kg, were chosen for this study. All laparoscopic implantations were carried out under general anesthesia. The anesthesia protocol included premedication with zolazepam and tiletamine (6.3 mg/kg) and xylazine (2.3 mg/kg), induction with propofol (0.5 mg/kg), and maintenance with isoflurane in combination with pancuronium (0.07 mg/kg). Postoperative care involved antibiotic prophylaxis with oxytetracycline (20 mg/kg/day) for three days.
Stenting & Shielding Hernia System: the Structure
The S&S Hernia System used in this study is constructed from medical-grade polypropylene-based Thermo-Polymer Elastomer (TPE), with its mechanical properties detailed in
Table 1.
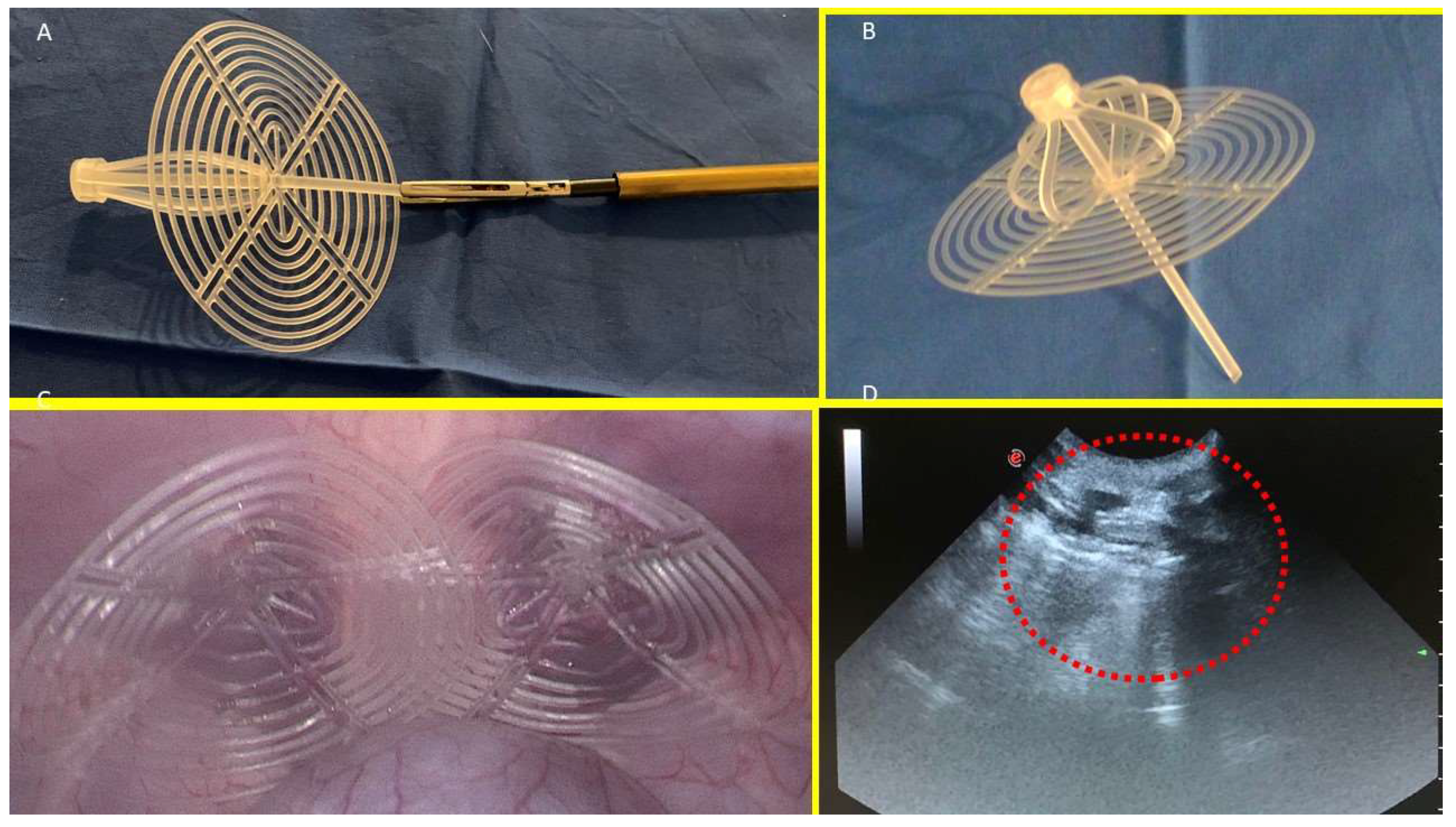
The S&S device consists of two primary components: an eight-rayed structure assembled around a central mast, and a 3D oval shield dimensioned 10x8 cm. The oval shield has a central ring intended to be connected with the mast. The mast has a button-like arrangement at its distal end, along with two conical enlargements (stops) near the button. Initially, the device is configured as a compact cylindrical unit, with the oval shield threaded onto the mast via its central ring (
Figure 1A & B).
This design allows the device to be delivered into the abdomen of the pig through a 12 mm trocar channel. Once positioned at the hernia defect, a metallic tube is used to push the oval shield, advancing the device into the pre-existing muscular defect. When the cylindrical structure is inside the defect the shield is pressed beyond the first or the second conic enlargements on the mast, that practically act as stops: his allows the cylindrical structure to modify its shape into a 3D scaffold that permanently occupies the hernia opening. This final configuration blocks the shield preventing any backward movement and firmly fastening the 3D scaffold within the defect. In this configuration the shield covers and overlaps the muscular defect remaining in direct contact with the abdominal content (Fig. 1C). The final diameter of the 3D scaffold utilized for the experiment is approximately 4.5 cm.
Follow up protocol
The follow-up protocol involved the sacrifice of 2 pigs between 4 and 6 weeks postoperatively (short-term period), 2 between 3 and 4 months (mid-term), 5 between 6 and 8 months (long-term), and 1 at 18 months (extra-long term). Scheduled ultrasound (Fig. 1D) and laparoscopic controls were carried out at defined postoperative intervals to ensure the 3D scaffold remained properly positioned and to visually determine if adhesions between abdominal viscera and the shield occurred.
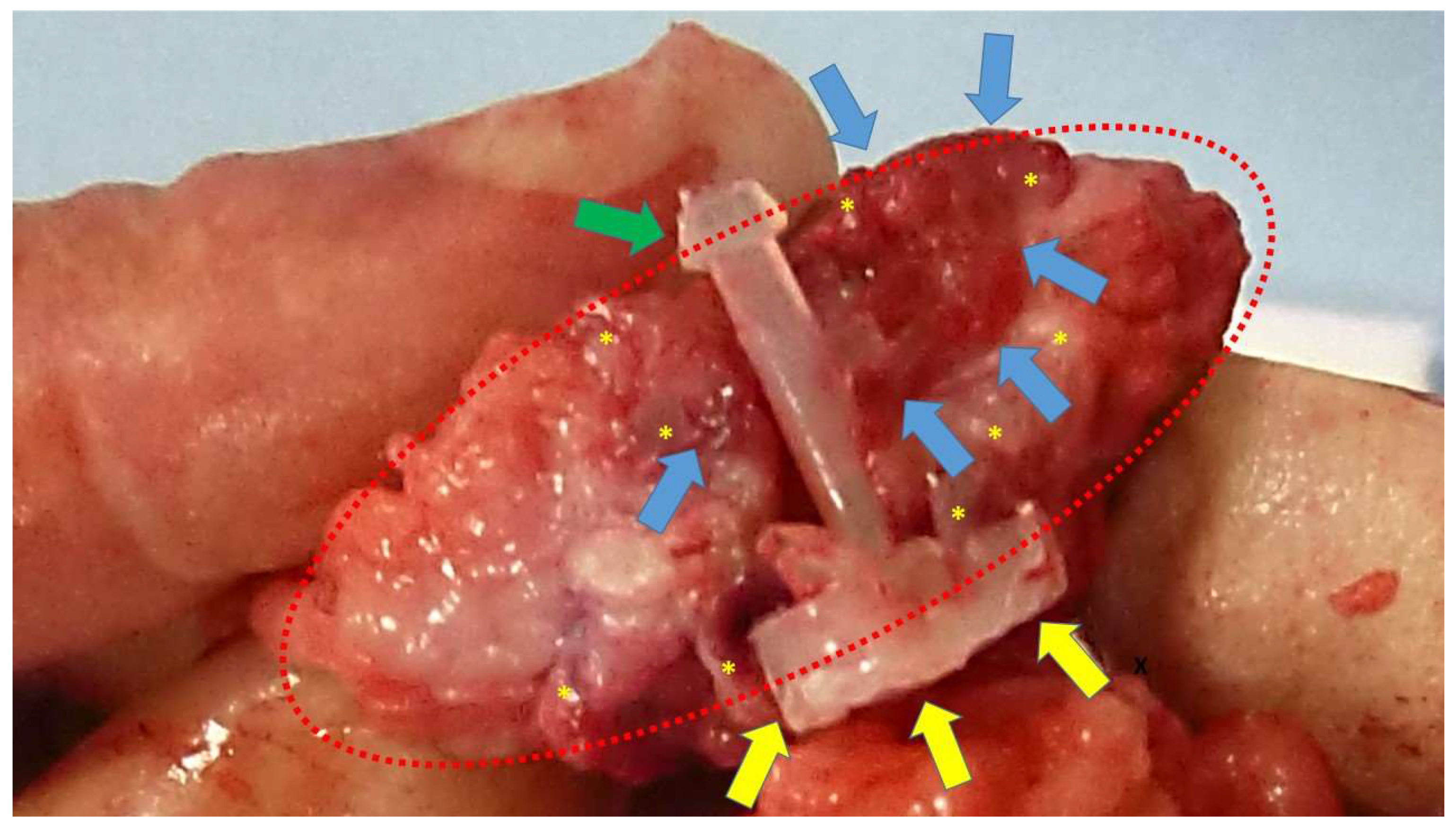
At animal sacrifice the S&S devices were excised en bloc through an abdominal incision in the lower midline. Then the host’s native tissue around the devices were carefully excised from the device that was subsequently bisected to allow for macroscopic evaluation of the tissue ingrown into the 3D scaffold. (
Figure 2)
The explanted S&S devices were subsequently sent to a pathologist for detailed histological examination.
Histology and Histochemical Methods
The tissue specimens excised from the core of the 3D scaffold of the S&S device were fixed in 10% phosphate-buffered formalin for a minimum of 12 hours before being embedded in paraffin. Sections were cut to a thickness of 4 μm and stored at room temperature until analysis. Basic histology using Haematoxylin and Eosin (H&E) staining was performed to evaluate the histomorphological characteristics. Sections were also analyzed using Azan–Mallory staining, which employs two acid dyes: azocarmine and aniline blue. This staining technique allows for the differentiation of various tissue components, staining collagen blue, muscle tissue reddish, and chromatin and erythrocytes red.
Histopathological Assessment
Two pathologists evaluated the histologic sections in a blinded manner respect to timing of device placement. Samples were examined under high-power light microscopy to observe the device/tissue interface and perform a semi-quantitative histological assessment. The analysis focused on assessing the width of the muscle surface as revealed by Azan–Mallory staining. The morphological features of myocytes were acknowledged and measured using digital images of stained sections. Measurements of the muscle area were taken from four non-overlapping fields at 50x magnification. Images were acquired using a bright-light microscope, equipped with a digital camera, and analyzed using image capture software (Leica DMLB microscope, Nikon DS-Fi-1 digital camera, NIS Basic Research Nikon software).
Statistical Analysis
Muscle area was evaluated in 20 biopsies excised from the 3D scaffold of the S&S device excised at different times post implantation: 4 samples 3-5 weeks postop, 5 samples 3-4 months postop, 10 samples 6-8 months postop and 2 at 18 months post-surgery. A one-way ANOVA between 20 biopsies was conducted to compare the muscular ingrowth in 3D scaffold of the S&S Hernia System respect to time in the short, mid, long and extra-long term. Moreover, the Tukey–Kramer multiple comparison test was used to analyse significance of differences between stages. SAS software (version 9.3. SAS) was used for the analyses.
3. Results
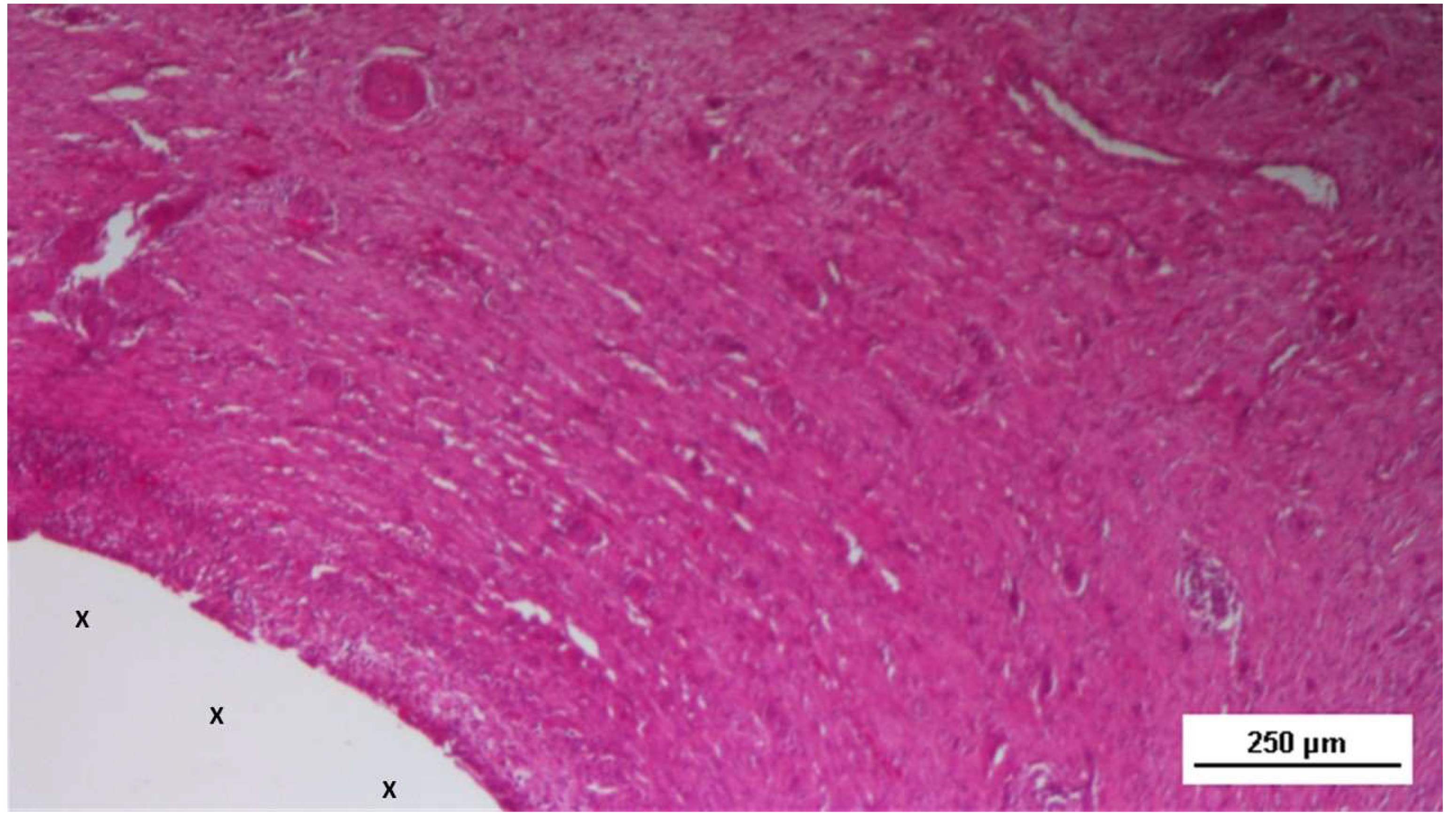
At animal euthanasia none of the pigs exhibited adhesions to the abdominal viscera. In the biopsies taken from the 3D implant excised 4-6 weeks after placement (short term), the presence of lax and well perfused connective tissue was observed. Already in this phase, multiple spots of muscular elements and several strips of muscle bundles in progressive maturation could be detected in the unstructured connective tissue and close to prosthetic tissue. (
Figure 3,
Figure 4,
Figure 5,
Figure 6)
S&S fabric (X). - AM 50X
Actually, the typical features of the early phase of muscle development, characterized by vesiculated nuclei, prominent nucleoli and moderate basophilia, were markedly evidenced in the newly ingrown tissue.
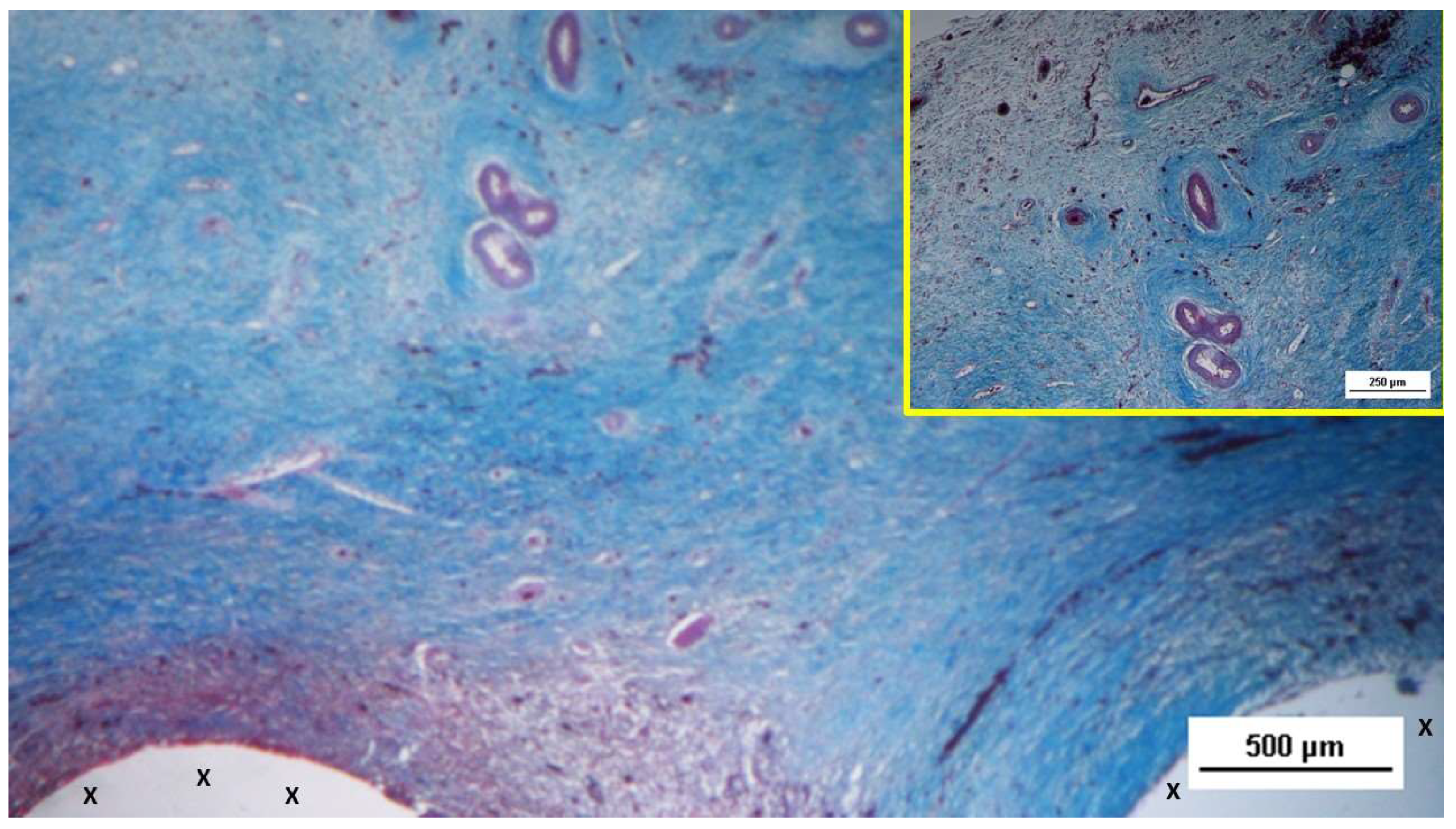
The development of muscle elements in biopsy samples taken from the 3D scaffold of the S&S device 3 to 4 months after placement (mid-term) showed a noticeable increase in both quantity and quality. At this stage, a greater number of muscle element clusters, along with extensive areas of muscle bundles, were observed adjacent to the device fabric and interspersed within well-organized connective tissue (
Figure 7,
Figure 8,
Figure 9,
Figure 10).
The muscle bundles showed in a stage of progressive development, with myocytes displaying spindle-formed contours, small nuclei, and eosinophilic cytoplasm. These features are characteristic of the evolving phase of muscle structure formation. [
26]
In the long-term period, 6 to 8 months post-surgery, the muscular presence within the structure of the S&S device further improved, assuming the outline of mature muscular elements grouped in the characteristic bundles typical of normal muscle structure. These muscular components were disseminated within a network of interlaced connective tissue ordered along lines of force, and accompanied by numerous mature arteries and veins (
Figure 11,
Figure 12,
Figure 13,
Figure 14).
Highly magnified images also highlighted the typical spindle-formed shape of the myocytes, with striated elements, hyperchromatic small nuclei and eosinophilic cytoplasm. This structure closely resembles that of normal human muscle bundles. [
25]
In the tissue specimens removed from the 3D scaffold of the S&S device in the extra-long term, the newly formed muscular elements, embedded within a viable connective matrix, appeared as well-organized clusters of muscle bundles that exhibited a consolidated stage of structural maturation. (
Figure 15,
Figure 16,
Figure 17,
Figure 18)
Statistical analysis
Average area of muscle tissue of 20 biopsies excised from the 3D scaffold of the S&S device at different stages post implantation were compared with one-way Anova and post hoc Tukey test. Results showed statistically significant differences between stages (
Figure 19) and, in particular, suggest that muscular ingrowth in 3D scaffold of the S&S Hernia System progressively increases over time.
Specifically, our results suggest that in the long term and extra long-term muscle area media are is significantly greater than in the short term. However, there is no statistical difference between short tem and midterm values. (
Table 2)
4. Discussion
Prosthetic implants are currently regarded as the most effective tools for abdominal hernia repair. [
26] The introduction of biologically compatible flat meshes in hernia treatment has significantly improved outcomes. [
27] However, despite the wide range of available repair techniques and implant types, no definitive gold standard has been established. Nevertheless, notwithstanding notable advancements, recent concerns have emerged within the scientific community regarding the high incidence of complications specifically associated with conventional static implants. [2, 28]
A substantial body of research addresses the biological response to these traditional implants. [29 –32] The term "scar plate" commonly refers to the rigid, fibrotic plaque formed by conventional flat meshes. These meshes are dynamically passive and unable to accommodate abdominal movements. During motion, the rigid, uneven surface of the fibrotic, motionless implants can cause friction against the surrounding muscular structure, leading to discomfort and pain. [2, 4, 8] This issue appears to be linked to the suboptimal tissue development within the implants, where the formation of poor quality scar tissue results in the well-documented phenomenon of mesh shrinkage. Shrinkage of the implant, leading to a reduction in surface area (up to 30%), has been identified as a significant factor contributing to hernia recurrence [5, 6]. Furthermore, mesh fixation presents another challenge, as it restricts the natural movement of the abdominal wall and can lead to complications such as tissue tears, bleeding, and hematoma. [1, 2, 4, 7] These issues underscore the limitations of current approaches and highlight the need for new solutions in hernia treatment. The scientific community is increasingly focused on exploring new strategies that move beyond the traditional concept of reinforcing the abdominal wall through the incorporation of fibrotic tissue in mesh implants.
Building on the latest findings in the pathophysiology of herniated abdominal walls, particularly with regard to inguinal hernias, a new perspective has recently gained traction. A series of scientific studies have demonstrated that the degenerative damage observed in herniated groins typically presents the histological characteristics of chronic compressive injury. [11- 16] Notably, there is no other significant source of chronic compression in this region aside from the constant pressure exerted by the abdominal viscera against the abdominal wall. Consequently, this steady visceral impact may be recognized as a key factor in the development of hernia disease. [33, 34]
These evidences strongly suggest that an effective approach to hernia repair should prioritize the regeneration of degenerated abdominal wall components, a strategy that markedly differs from the traditional method of reinforcing the abdominal wall with a fibrotic plaque, typical of conventional meshes. This noteworthy paradigm shift has catalyzed the development of a new device for dissection-free laparoscopic hernia repair: the Stenting & Shielding (S&S) Hernia System.
The S&S device, constructed from a polypropylene-based Thermo-Polymer Elastomer (TPE), features a cylindrical, multi-rayed structure connected to an oval shield made from the same material. Once laparoscopically inserted into the abdomen, the device is placed within the hernia defect, where a proprietary mechanism transforms the cylindrical structure into a 3D scaffold that fully occupies the defect. The shield then covers and overlaps the hernia opening, with its posterior surface facing the parietal peritoneum. The S&S device, which requires no fixation, remains compliant with the natural movements of the abdominal wall, providing permanent obliteration of the defect. In experimental porcine models, this innovative device demonstrated a probiotic biological response, characterized by the ingrowth of newly developed tissue resembling the typical components of the abdominal wall, including arteries, veins, nerves, and notably, newly formed muscle structures.
The dynamic and responsive nature of the S&S device stands in stark contrast to the static behaviour of conventional flat meshes, suggesting that the difference in biological response is directly tied to the distinct physical properties of the implants. A static, regressive biological response is observed in traditional meshes, while a dynamic probiotic biological response is evident in the S&S device.
From a pathogenetic viewpoint, the regenerative effect of the S&S Hernia System aligns with the goal of the treatment: to halt degeneration and promote the regeneration of the compromised components of the herniated abdominal wall. To validate the regenerative capabilities of the S&S device positioned within the muscular environment of the abdominal wall, it is crucial to emphasize the ingrowth of myocytes—key components of the abdominal barrier—into the 3D scaffold of the device. Therefore, the primary objective of this study was to confirm the existence and amount of these specialized connective structures within the device. A secondary goal was to document the progression of muscle ingrowth by time. The results of this study confirmed that, even in the short term post-implantation, the development of connective tissue in the device was composed of viable tissue with several spots of emerging muscle elements in early developmental stages. In this phase, the nuclei of the myocytes were already formed, though the striated muscle structure was not yet fully developed. The muscle elements exhibited moderate basophilia with vesiculated nuclei and prominent nucleoli.
As the study progressed to the mid-term phase (approximately 3-4 months), there was a notable increase in both the quantity and quality of muscle elements within the device. These elements progressively evolved, eventually displaying characteristics of mature muscle fibers: spindle-shaped myocytes with hyperchromic small nuclei and eosinophilic cytoplasm. In the long-term phase (6-8 months post-implantation), the structure of the myocytes closely resembled that of regular human muscle tissue, with widespread muscle ingrowth observed within the implant fibers. This pattern of maturation was further corroborated by the histological assessment of biopsies taken in the extra-long term post-implantation, where mature myocytes were found in large, healthy muscular elements in the stage of consolidated maturation within the 3D scaffold of the S&S device. Overall, the histological analysis of the specimens excised in the long and extra-long stage revealed that the evidenced myocytes exhibited the typical structure of organized muscle tissue, comparable to normal human muscle bundles. [35 – 38]
These distinctive histological outcomes were further validated by statistical analysis conducted across the four stages of the investigation, reinforcing the concept of a probiotic biological response induced by the S&S Hernia System.
5. Conclusion
The results of this study underscore the importance of device design in achieving high-quality tissue ingrowth as biological response in hernia repair devices. The proprietary design of the S&S Hernia System appears to confer a dynamic responsiveness to the cyclical loads of the abdominal wall, which is essential for inducing an enhanced biological response. This ensures the ingrowth of mature cellular elements that closely resemble the typical tissue components of the abdominal wall. However, the probiotic biological response observed in this newly designed hernia device is not entirely unprecedented, as similar features have been noted in another dynamic responsive scaffold for inguinal hernia repair. [17, 24] These kind of devices can thus be categorized within a new class of hernia repair systems: the dynamic regenerative scaffolds. Nevertheless, this investigation has a notable limitation: it does not fully explain the mechanisms underlying the observed neomyogenesis. Preliminary hypotheses suggest that specific growth factors may play a role in stimulating the development of new myocytes and muscle bundles. Ongoing research is focused on clarifying these potential mechanisms. However, the promising results obtained from the experimental animal model provide a strong foundation for further clinical studies. These studies will determine whether the excellent biological responses observed in this investigation can be replicated in human patients. If confirmed, the S&S Hernia System could revolutionize hernia repair, simplifying surgical procedures and improving outcomes for patients.
Author Contributions
AG Conceptualization PR Investigation AA Formal Analysis RV SupervisionCL Data Curation CG Resources DBG Software BE Validation NC Methodology RG2nd Original Draft Preparation RW Methodology RG: Review & editing.
Funding
This research received no external funding.
Data Availability Statement
All data supporting the reported results are available upon request from the corresponding author.
Conflicts of Interest
The corresponding author is the developer of the device described in the report. The other authors declare no conflict of interest.
References
- Amid, PK. Causes, prevention, and surgical treatment of postherniorrhaphy neuropathic inguinodynia: Triple neurectomy with proximal end implantation. Hernia 2004, 8, 343–349. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer PJ, Kingsnorth AN, Mohillo RG, Small PK, Lammers B, Horeysee G. Randomized clinical trial assessing impact of a lightweight or heavyweight on chronic pain after inguinal hernia repair. Br J Surg 2005, 92, 166–70. [CrossRef] [PubMed]
- Rutkow IM, Robbins AW. Mesh plug hernia repair: a follow-up report. Surgery 1995, 117, 597–598. [CrossRef]
- Aasvang E, Kehlet H. Surgical management of chronic pain after inguinal hernia repair. Br J Surg 2005, 92, 795–801. [CrossRef]
- Klinge U, Klosterehalfen B, Muller M, Ottinger AP, Schumpelick V. Shrinking of polypropylene mesh in vivo: An experimental study in dogs. Eur J Surg 1998, 164, 96.
- Amid, PK. Shrinkage: fake or fact? In: Schumpelick V, Nyhus LM, editors. Meshes: benefits and risks. Springer Berlin 2004.
- G. Zanghì, M. Arena, R. Vecchio, G. Benfatto, G. Di Stefano. Dynamic self-regulating prosthesis in inguinal hernia repair. Il Giornale di chirurgia 2011, 32, 495–497.
- Kim-Fuchs C, Angst E, Vorburger S, Heibling C, Candinas D, Schlumpf R. Prospective randomized trial comparing sutured with sutureless mesh fixation for Lichtenstein hernia repair: long-term results. Hernia. 2012, 16, 21–7. [CrossRef]
- Open tension-free Lichtenstein repair of inguinal hernia: use of fibrin glue versus sutures for mesh fixation. P. Negro, F. Basile, A. Brescia, M. Buonanno, G. Campanelli, S. Canonico, G. Corrado, G. Cascarella, N. Di Lorenzo, E. Falletto, L. Fei, M. Francucci, C. Stabilini, C. Fronticelli, A. Gaspari, G. Zanghì. Hernia. 2011; 15, 7–14.
- La colla di fibrina nelle ernioplastiche tension-free: nostra Esperienza. G.Benfatto, S.M. Benfatto, A. Strazzanti, R.M.C. Giovinetto A. Jirys, G.M.Salina, F. Mugavero, G. Zanghì, A. Giovinetto. Giornale di Chirurgia. 2006; 27, 392–394.
- Amato G, Romano G, Agrusa A, Marasa S, Cocorullo G, Gulotta G, Goetze T, Puleio R.
- Biologic response of inguinal hernia prosthetics: a comparative study of conventional static meshes versus 3D dynamic implants. Artif Organs. 2015, 39.
- Amato G, Marasa L, Sciacchitano T, Bell SG, Romano G, Gioviale MC, Lo Monte AI, Romano M. Histological findings of the internal inguinal ring in patients having indirect inguinal hernia. Hernia 2009, 13, 259–62. [CrossRef]
- Amato G, Ober E, Romano G, Salamone G, Agrusa A, Gulotta G, Bussani R. Nerve degeneration in inguinal hernia specimens. Hernia 2011, 15, 53–58.
- Amato G, Romano G, Salamone G, Agrusa A, Saladino VA, Silvestri F, Bussani R. Damage to the vascular structures in inguinal hernia specimens. Hernia. 2012, 16, 63–67. [CrossRef] [PubMed]
- Amato G, Agrusa A, Romano G, Salamone G, Cocorullo G, Mularo SA, Marasa S, Gulotta G. Histological findings in direct inguinal hernia. Hernia. 2013, 17, 757–63.
- Amato G, Agrusa A, Romano G, Salamone G, Gulotta G, Silvestri F, Bussani R. Muscle degeneration in inguinal hernia specimens. Hernia. 2012, 16, 327–31.
- Amato G, Agrusa A, Romano G. Fixation-free inguinal hernia repair using a dynamic self-retaining implant. Surg Technol Int 2012, 30, XXII:22/17.
- Amato G, Romano G, Agrusa A, Cocorullo G, Gulotta G, Goetze T. Dynamic inguinal hernia repair with a 3d fixation-free and motion-compliant implant: a clinical study. Surg Technol Int. 2014, 24, 155–65.
- Amato G, Lo Monte AI, Cassata, Damiano G, Romano G, Bussani R. A new prosthetic implant for inguinal hernia repair: its features in a porcine experimental model. Artificial Organs 2011, 35, E181–E190.
- Amato G, Romano G, Puleio R, Agrusa A, Goetze T, Gulotta E, Gordini L, Erdas E, Calò P. Neomyogenesis in 3D Dynamic Responsive Prosthesis for Inguinal Hernia Repair. Artif Organs. 2018, 42, 1216–1223. [CrossRef]
- Amato G, Agrusa A, Puleio R, Calò PG, Goetze T, Romano G Neo-nervegenesis in 3D dynamic responsive implant for inguinal hernia repair. Qualitative study. International Journal of Surgery 2020, 76, 114–119.
- Amato G, Puleio R, Rodolico V, Agrusa A, Calò PG, Di Buono G. Romano G, Goetze T. Enhanced angiogenesis in the 3D dynamic responsive implant for inguinal hernia repair ProFlor®. Artif Organs. 2021, 00, 1–10.
- Amato G, Agrusa A, Di Buono G, Calò PG, Cassata G, Cicero L, Romano G. Inguinal Hernia: Defect Obliteration with the 3D Dynamic Regenerative Scaffold ProflorTM. Surg Technol Int. 2021, 38, 199–205.
- Amato, G.; Puleio, R.; Romano, G.; Calò, P.G.; Di Buono, G.; Cicero, L.; Cassata, G.; Goetze, T.; Buscemi, S.; Agrusa, A.; Rodolico, V. Physiologic Cyclical Load on Inguinal Hernia Scaffold ProFlor Turns Biological Response into Tissue Regeneration. Biology 2023, 12, 434. [Google Scholar] [CrossRef]
- Diogo, R. , Ziermann J. M. Development, metamorphosis, morphology, and diversity: The evolution of chordate muscles and the origin of vertebrates. Dev Dyn. 2015, 244, 1046–1057. [Google Scholar] [CrossRef]
- Read R., C. Recent advances in the repair of groin herniation. Curr Probl Surg 2003, 40, 13–79. [Google Scholar] [CrossRef]
- Amid PK, Shulman AG, Lichtenstein IL, Hakakha M. Biomaterials for abdominal wall hernia surgery and principles of their applications. Langenbecks Arch Chir 1994, 379, 168–71. [CrossRef]
- EU Hernia Trialist Collaboration Mesh compared with nonmesh methods on open groin hernia repair. Systematic review of randomized controlled trial. Br J Surg 2000, 87, 854–859. [CrossRef] [PubMed]
- Debord, JR. The historical development of prosthetics in hernia surgery. Surg Clin North Am 1998, 78, 973–1006. [Google Scholar] [CrossRef] [PubMed]
- Klinge U, Klosterehalfen B, Muller M, Ottinger AP, Schumpelick V. Shrinking of polypropylene mesh in vivo: An experimental study in dogs. Eur J Surg 1998, 164, 96.
- Amid, P.K. Lichtenstein tension-free hernioplasty: Its inception, evolution, and principles Hernia. 2004; 8, 1–7. [Google Scholar]
- Klosterhalfen B, Junge K, Klinge U. The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Devices 2005, 2, 103–117. [CrossRef]
- Amato G, Agrusa A., Rodolico V., Puleio R., Di Buono G., Amodeo S., Gulotta E., Romano G. Combined inguinal hernia in the elderly. Portraying the progression of hernia disease. Int J Surg. 2016, 33 (Suppl 1), S20–S29. [CrossRef]
- Amato G, Romano G, Erdas E, Medas F, Gordini L, Podda F, Calò P. External hernia of the supravesical fossa: Rare or simply misidentified? Int J Surg. 2017, 41, 119–126. [CrossRef] [PubMed]
- Kinali M, Arechavala-Gomeza V, Cirak S, Glover A, Guglieri M, Feng L, Hollingsworth KG, Hunt D, Jungbluth H, Roper HP, Quinlivan RM, Gosalakkal JA, Jayawant S, Nadeau A, Hughes-Carre L, Manzur AY, Mercuri E, Morgan JE, Straub V, Bushby K, Sewry C, Rutherford M, Muntoni F. Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology 2011, 76, 346–353.
- Husnain Kh Haider, Syed Ali Akbar, Muhammad Ashraf, Angiomyogenesis for Myocardial Repair. Antioxidants & Redox Signaling 2009, 11, 1929–44.
- Golpanian, S. , El-Khorazaty J. , Mendizabal A., DiFede D. L., Suncion V., Karantalis V., Fishman J. E., Ghersin E., Balkan W., M. Hare J. M., Effect of Aging on Human Mesenchymal Stem Cell Therapy in Ischemic Cardiomyopathy Patients. J Am Coll Cardiol. 2015, 6, 125–132. [Google Scholar] [CrossRef]
- Carlson B. M. The biology of long-term denervated skeletal muscle. Eur J Trans Myol - Basic Appl Myol. 2014, 24, 5–11.
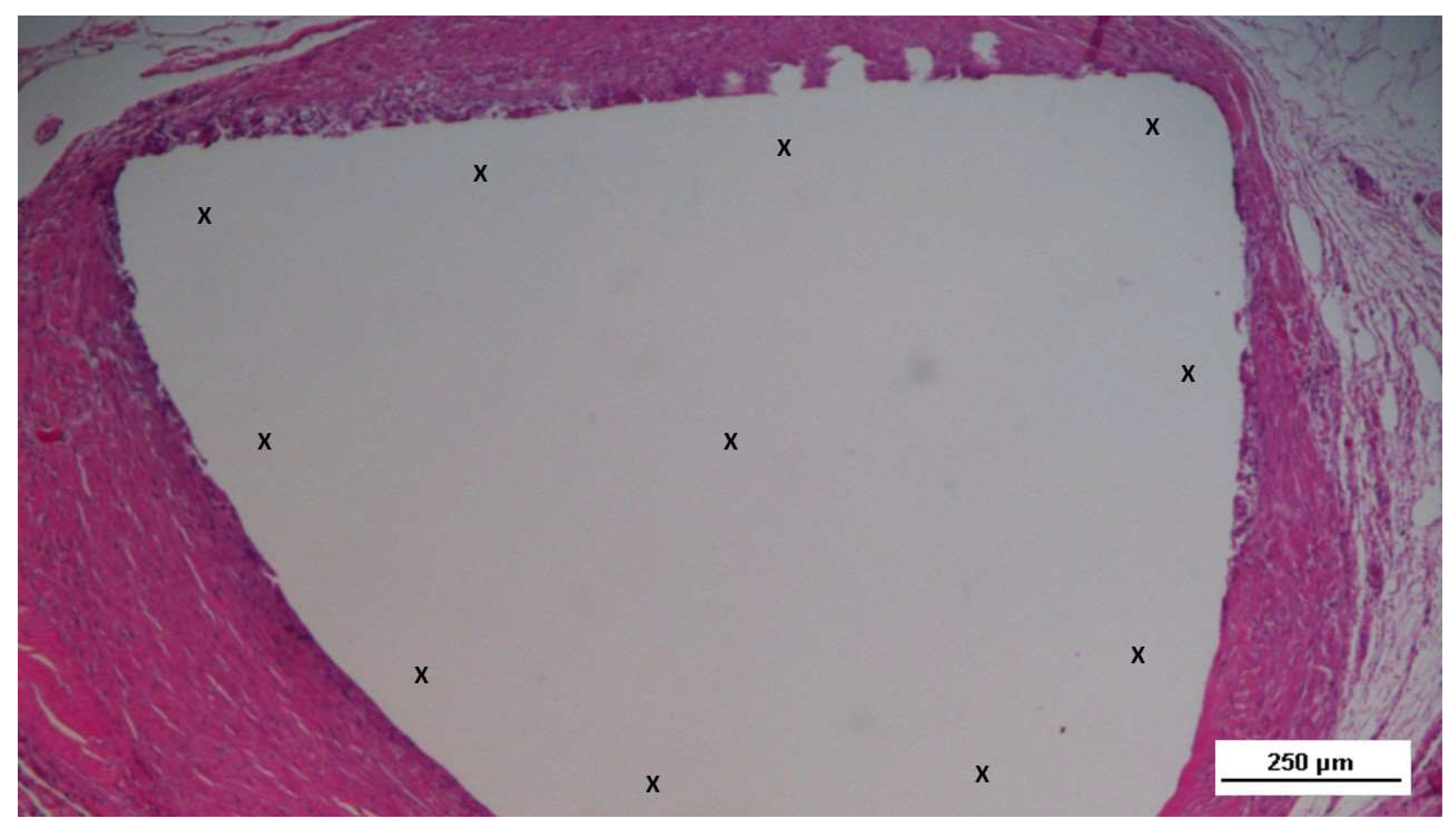
Figure 1.
A: The Stenting & Shielding (S&S) Hernia System in its pre-implantation state, ready for insertion into the abdominal cavity of the experimental pig. B: The S&S device in its deployed configuration. C: The two shields of the S&S Hernia System laparoscopically positioned in the lower abdominal wall, facing the abdominal viscera. D: Ultrasound scan of the S&S device three months post-surgery, showing the 3D scaffold occupied by newly ingrown tissue (red circle).
Figure 1.
A: The Stenting & Shielding (S&S) Hernia System in its pre-implantation state, ready for insertion into the abdominal cavity of the experimental pig. B: The S&S device in its deployed configuration. C: The two shields of the S&S Hernia System laparoscopically positioned in the lower abdominal wall, facing the abdominal viscera. D: Ultrasound scan of the S&S device three months post-surgery, showing the 3D scaffold occupied by newly ingrown tissue (red circle).
Figure 2.
Scaffold of a bisected S&S device (red circle) removed six months post-surgery. The 3D chamber formed by the bent rays of the device (*) is clearly filled with newly developed viable muscle structures (blue arrows). The image also highlights the distal portion of the mast with the conic stop (green arrow) and the button-like enlargement on the opposite side (yellow arrows).
Figure 2.
Scaffold of a bisected S&S device (red circle) removed six months post-surgery. The 3D chamber formed by the bent rays of the device (*) is clearly filled with newly developed viable muscle structures (blue arrows). The image also highlights the distal portion of the mast with the conic stop (green arrow) and the button-like enlargement on the opposite side (yellow arrows).
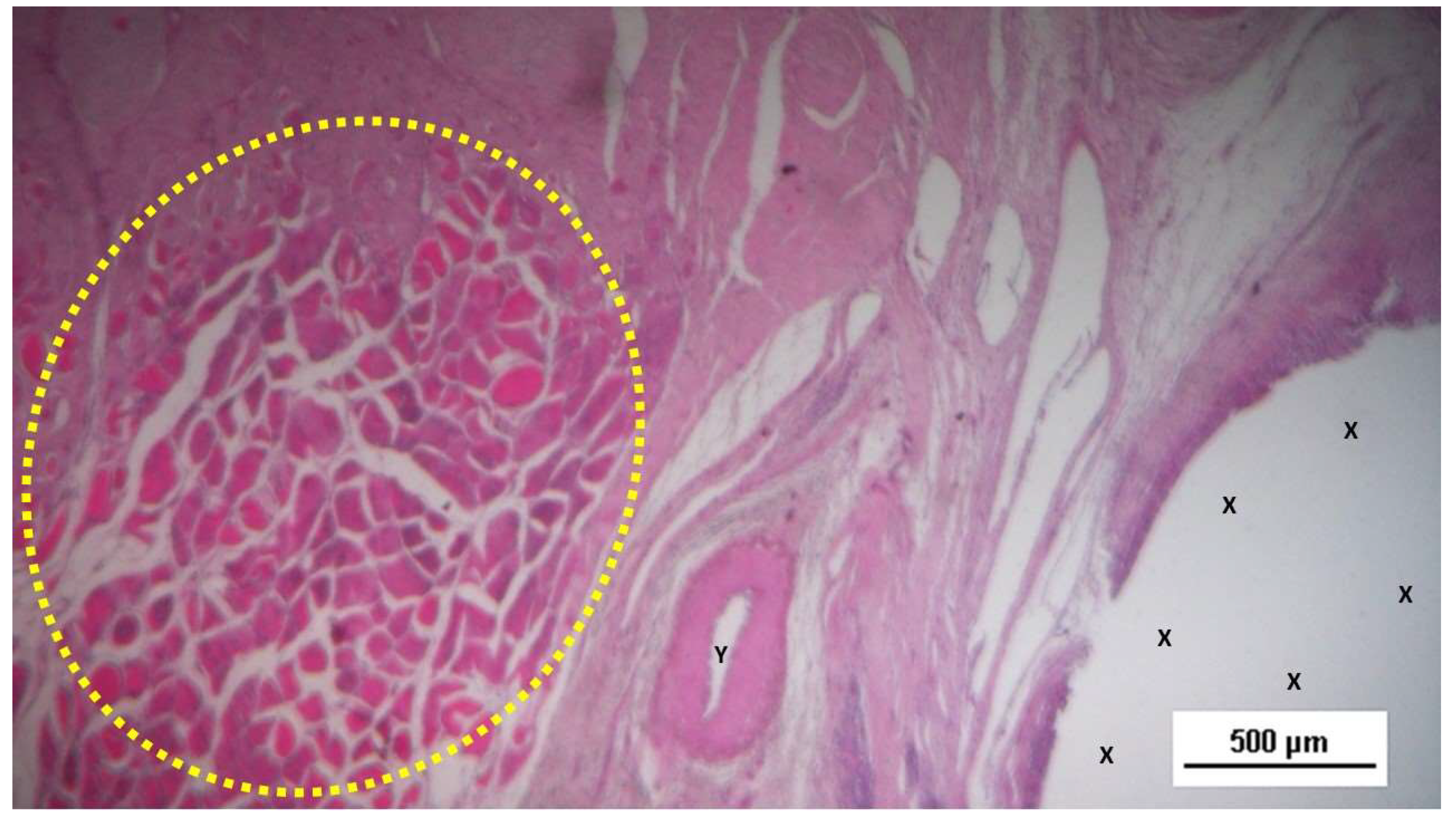
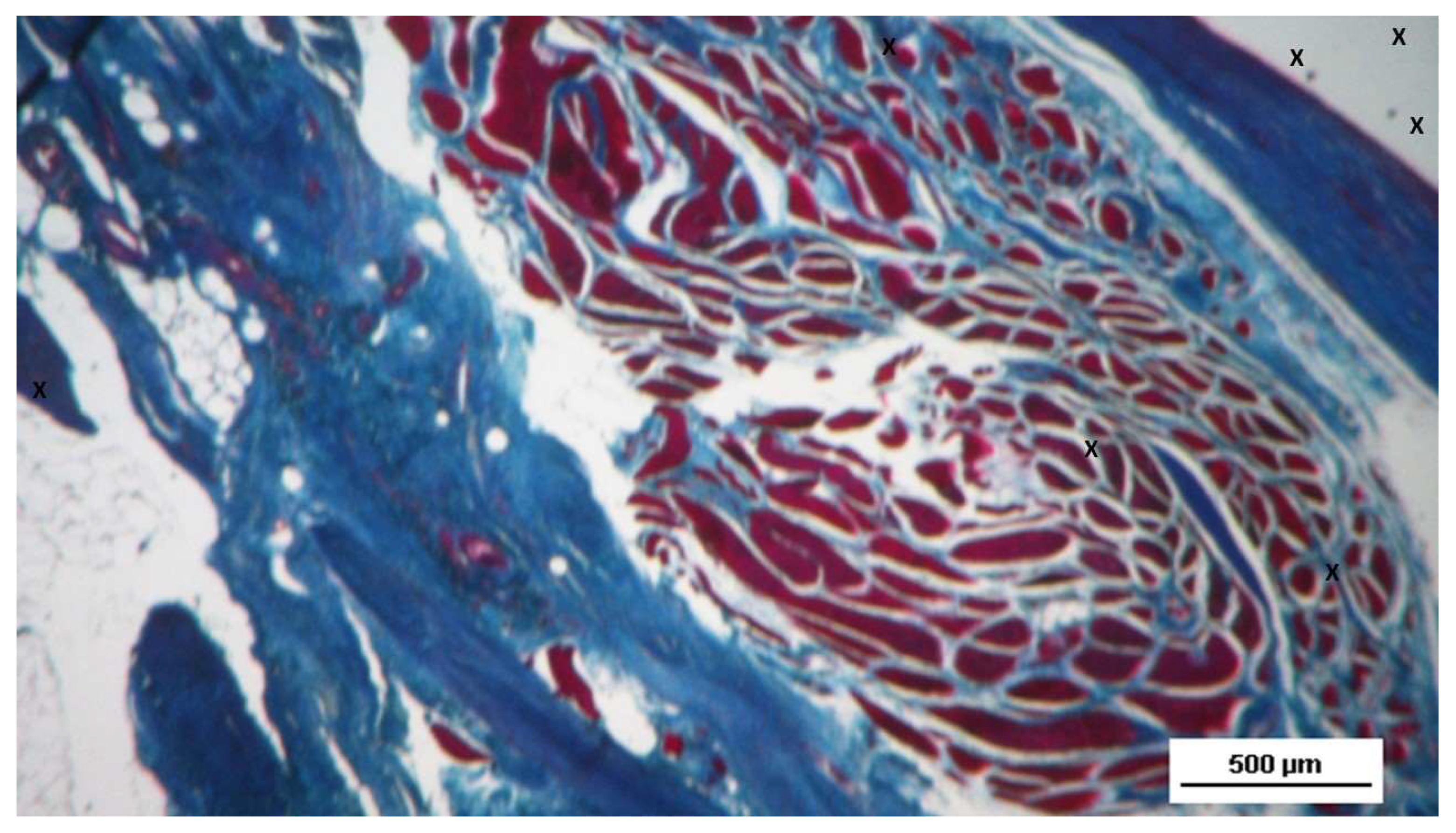
Figure 3.
Biopsy sample from the 3D scaffold of the S&S device excised 4 weeks post-implantation (short-term): microphotograph showing myocytes in the early stages of development surrounded by well-perfused connective tissue and numerous vascular structures in early development, adjacent to the S&S device fabric (X). HE 50X.
Figure 3.
Biopsy sample from the 3D scaffold of the S&S device excised 4 weeks post-implantation (short-term): microphotograph showing myocytes in the early stages of development surrounded by well-perfused connective tissue and numerous vascular structures in early development, adjacent to the S&S device fabric (X). HE 50X.
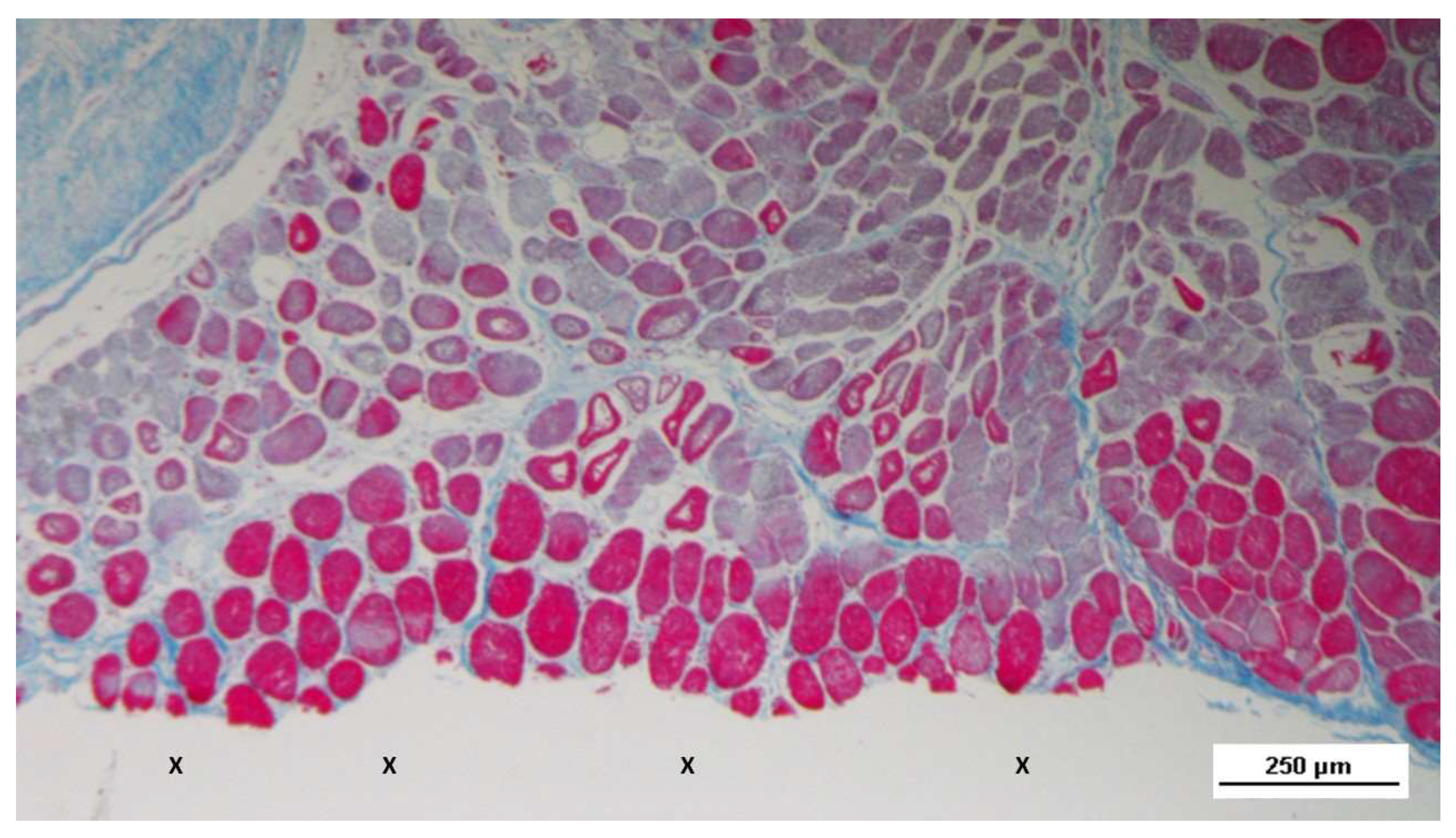
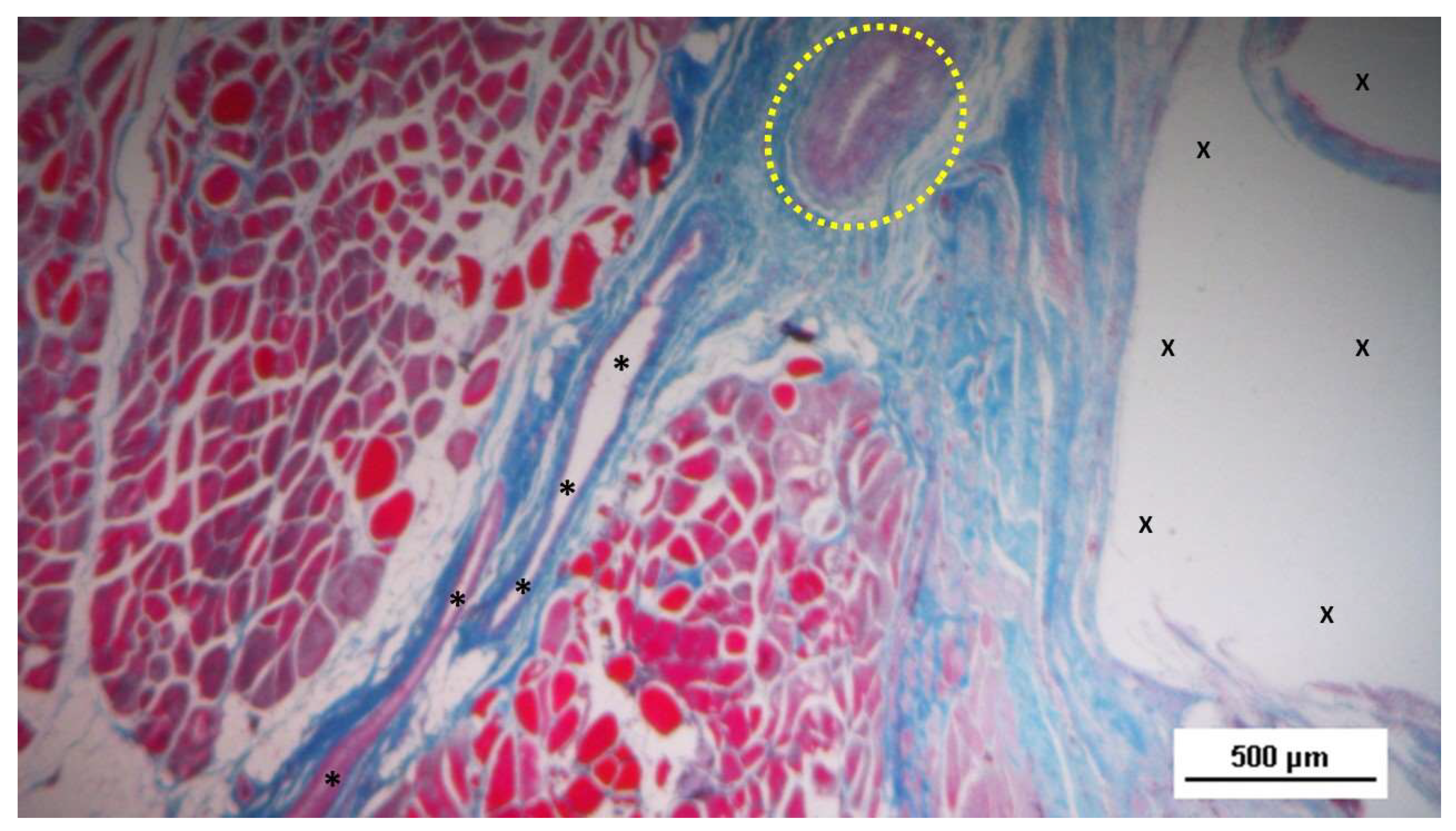
Figure 4.
- Biopsy sample excised 5 weeks post-op: in a context of unstructured connective tissue, multiple spots of muscular elements and several strips of muscle bundles in an initial phase of development are highlighted, particularly close to the S&S structure (X). The target structures in the upper margin of the photomicrographs and in the inset show some clusters of vascular elements in the early developmental stage. AM 25X (main image) - and AM 50X (inset).
Figure 4.
- Biopsy sample excised 5 weeks post-op: in a context of unstructured connective tissue, multiple spots of muscular elements and several strips of muscle bundles in an initial phase of development are highlighted, particularly close to the S&S structure (X). The target structures in the upper margin of the photomicrographs and in the inset show some clusters of vascular elements in the early developmental stage. AM 25X (main image) - and AM 50X (inset).
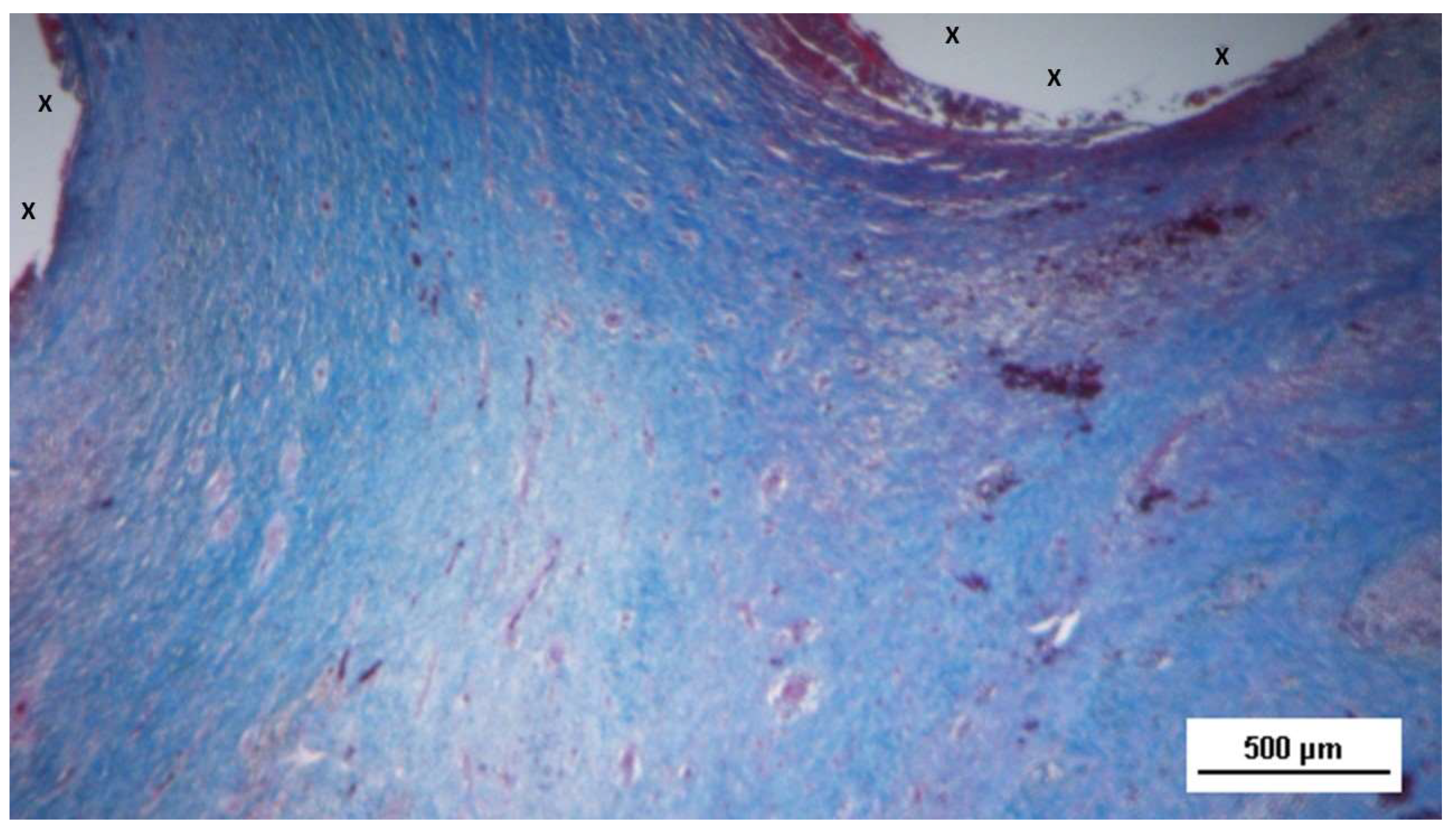
Figure 5.
Biopsy specimen excised 4 weeks post-op: In a context of unstructured connective tissue, multiple spots of muscular elements and several strips of muscle bundles are highlighted, particularly in the peri-prosthetic area, in an initial phase of development.
Figure 5.
Biopsy specimen excised 4 weeks post-op: In a context of unstructured connective tissue, multiple spots of muscular elements and several strips of muscle bundles are highlighted, particularly in the peri-prosthetic area, in an initial phase of development.
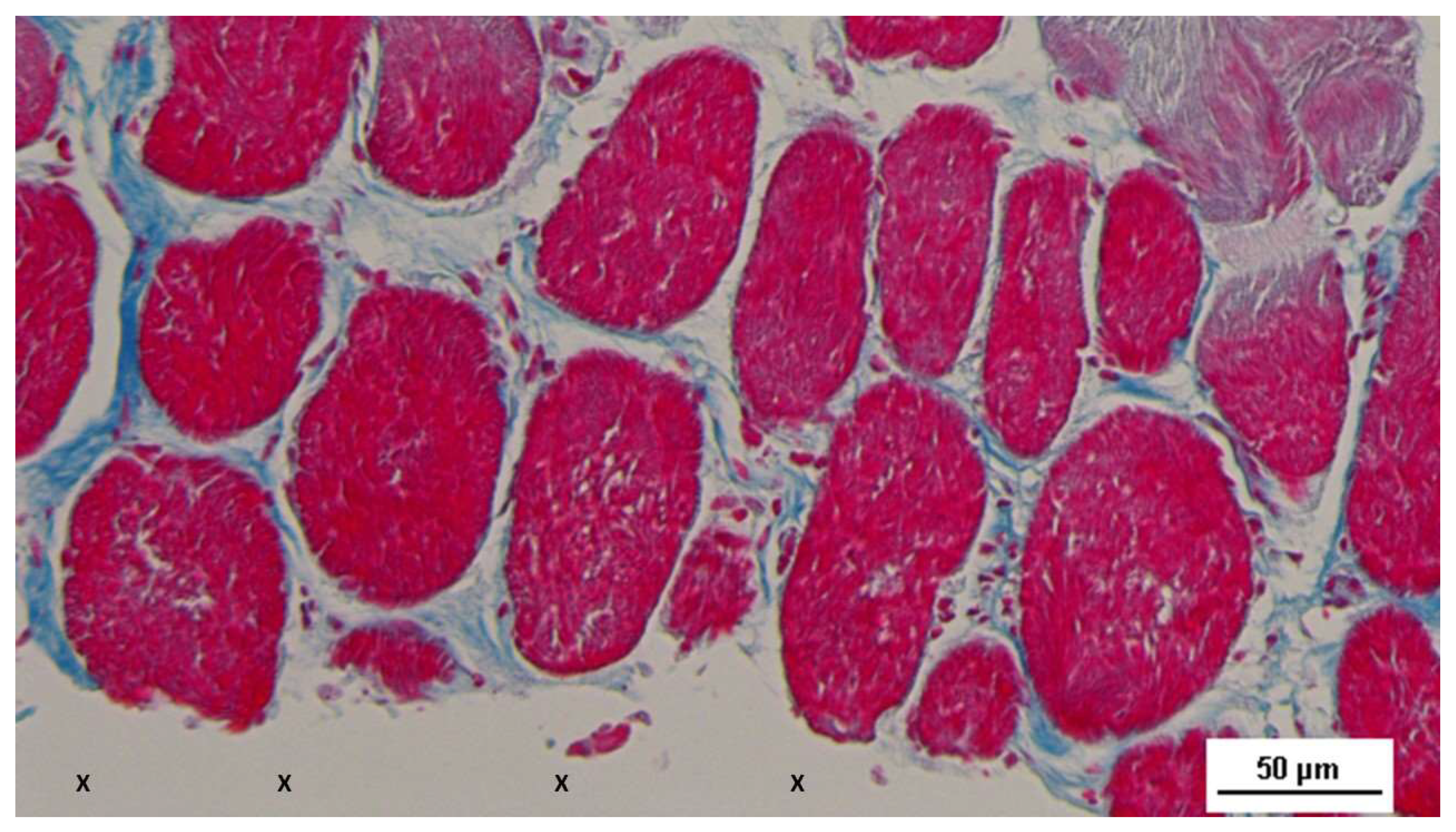
Figure 6.
- Biopsy specimen excised 5 weeks post-op: in a surround of well-organized connective tissue, numerous clusters of muscle elements, together with large areas of structured muscle fascicles, are evident adjacent to the 3D scaffold of the device (X). AM 50X.
Figure 6.
- Biopsy specimen excised 5 weeks post-op: in a surround of well-organized connective tissue, numerous clusters of muscle elements, together with large areas of structured muscle fascicles, are evident adjacent to the 3D scaffold of the device (X). AM 50X.
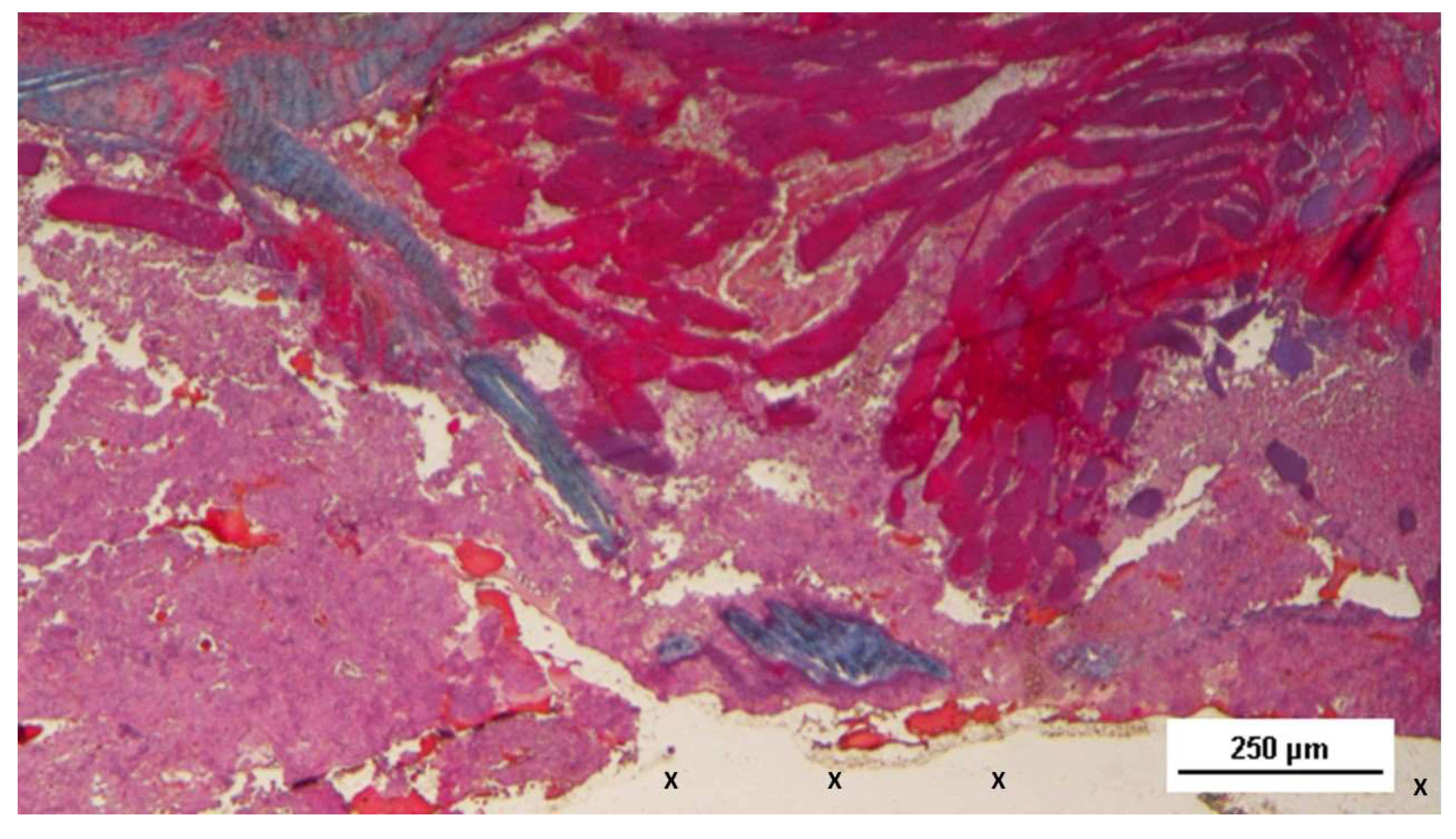
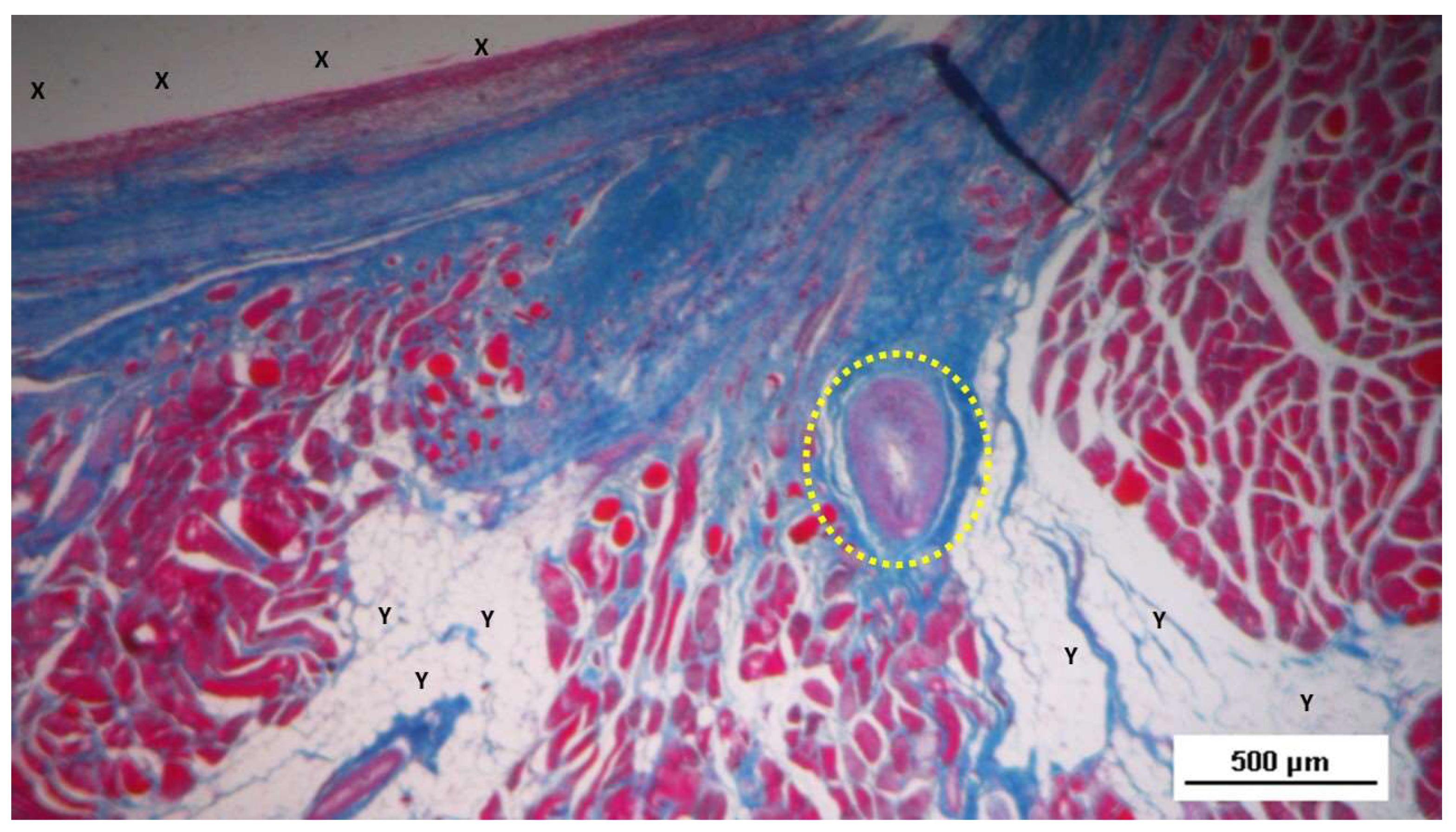
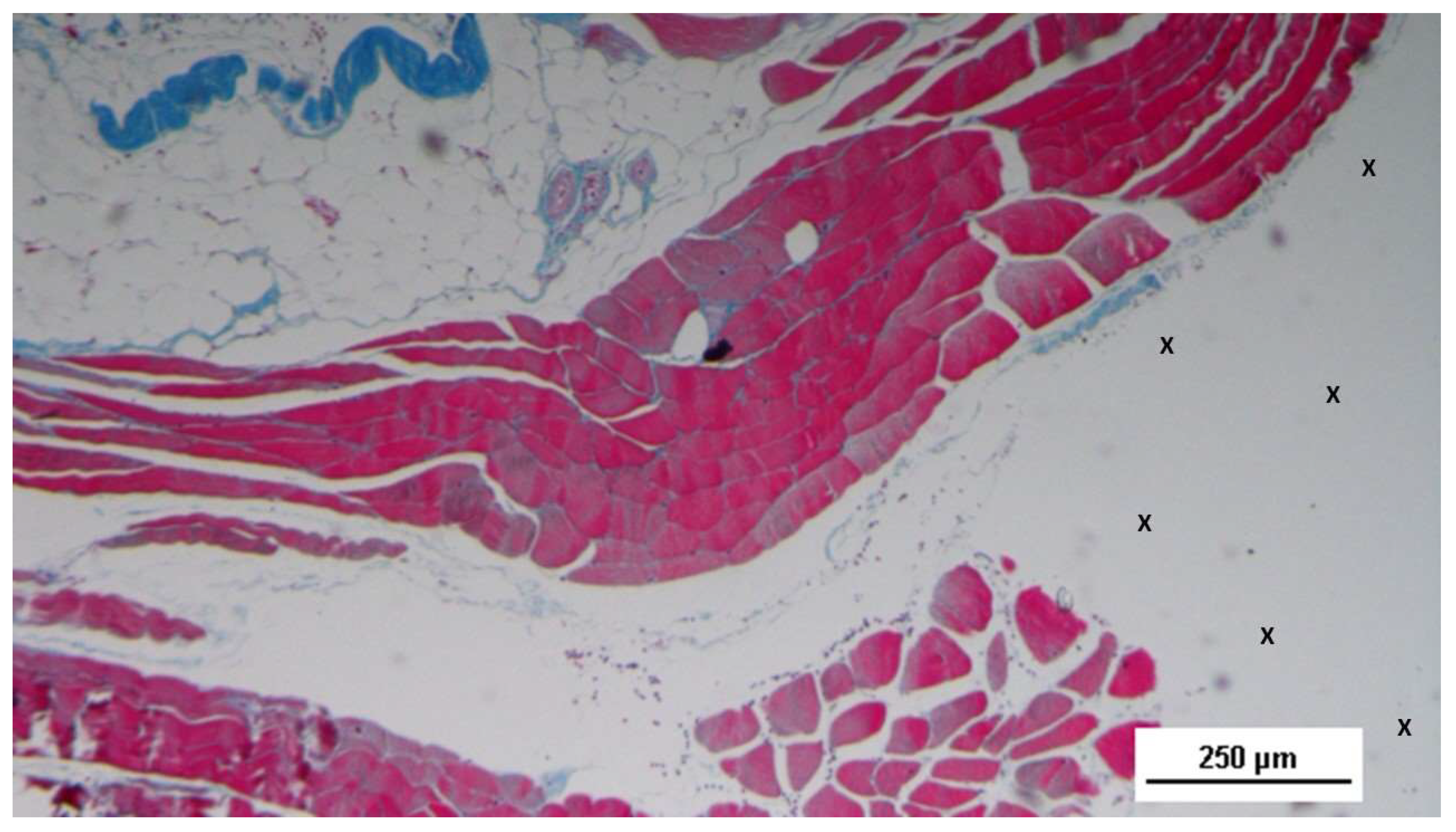
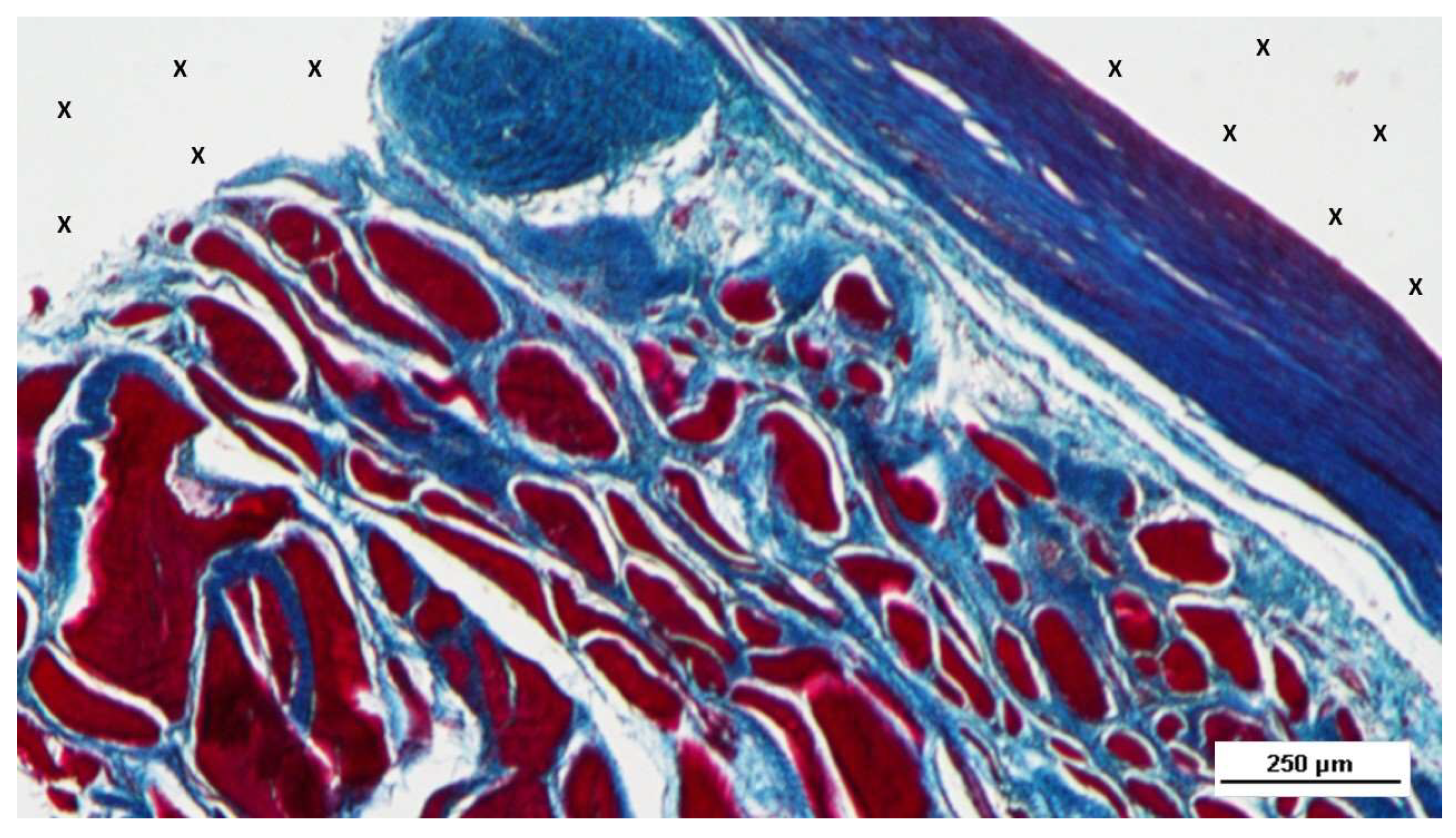
Figure 7.
Biopsy sample excised 3 months post-operation revealing a significant amount of muscle structures in advanced development, arranged in bundles (red elongated structures) near a large vascular element (yellow circle), in close proximity to the S&S fabric (X). HE 25X.
Figure 7.
Biopsy sample excised 3 months post-operation revealing a significant amount of muscle structures in advanced development, arranged in bundles (red elongated structures) near a large vascular element (yellow circle), in close proximity to the S&S fabric (X). HE 25X.
Figure 8.
Biopsy specimen excised 3 months post-surgery: microphotograph showing several bundles of muscular elements (red spots) in advanced stages of development near the S&S scaffold fabric (X), within a matrix of loose and viable connective tissue (stained in blue). AM 50X.
Figure 8.
Biopsy specimen excised 3 months post-surgery: microphotograph showing several bundles of muscular elements (red spots) in advanced stages of development near the S&S scaffold fabric (X), within a matrix of loose and viable connective tissue (stained in blue). AM 50X.
Figure 9.
Biopsy excised from the 3D scaffold of the S&S Hernia System 3 months post-surgery: numerous muscle structures grouped in bundles stained in red and near the 3D scaffold of the S&S fabric (X). The muscle elements close to the fabric show advanced development, while those farther away appear less mature. 50X.
Figure 9.
Biopsy excised from the 3D scaffold of the S&S Hernia System 3 months post-surgery: numerous muscle structures grouped in bundles stained in red and near the 3D scaffold of the S&S fabric (X). The muscle elements close to the fabric show advanced development, while those farther away appear less mature. 50X.
Figure 10.
High magnification microphotograph of a tissue sample excised 4 months post-surgery (detail from
Figure 9): showing muscle bundles in advanced stages of development (colored in red) in proximity of the 3D scaffold of the S&S fabric (X). AM 200X.
Figure 10.
High magnification microphotograph of a tissue sample excised 4 months post-surgery (detail from
Figure 9): showing muscle bundles in advanced stages of development (colored in red) in proximity of the 3D scaffold of the S&S fabric (X). AM 200X.
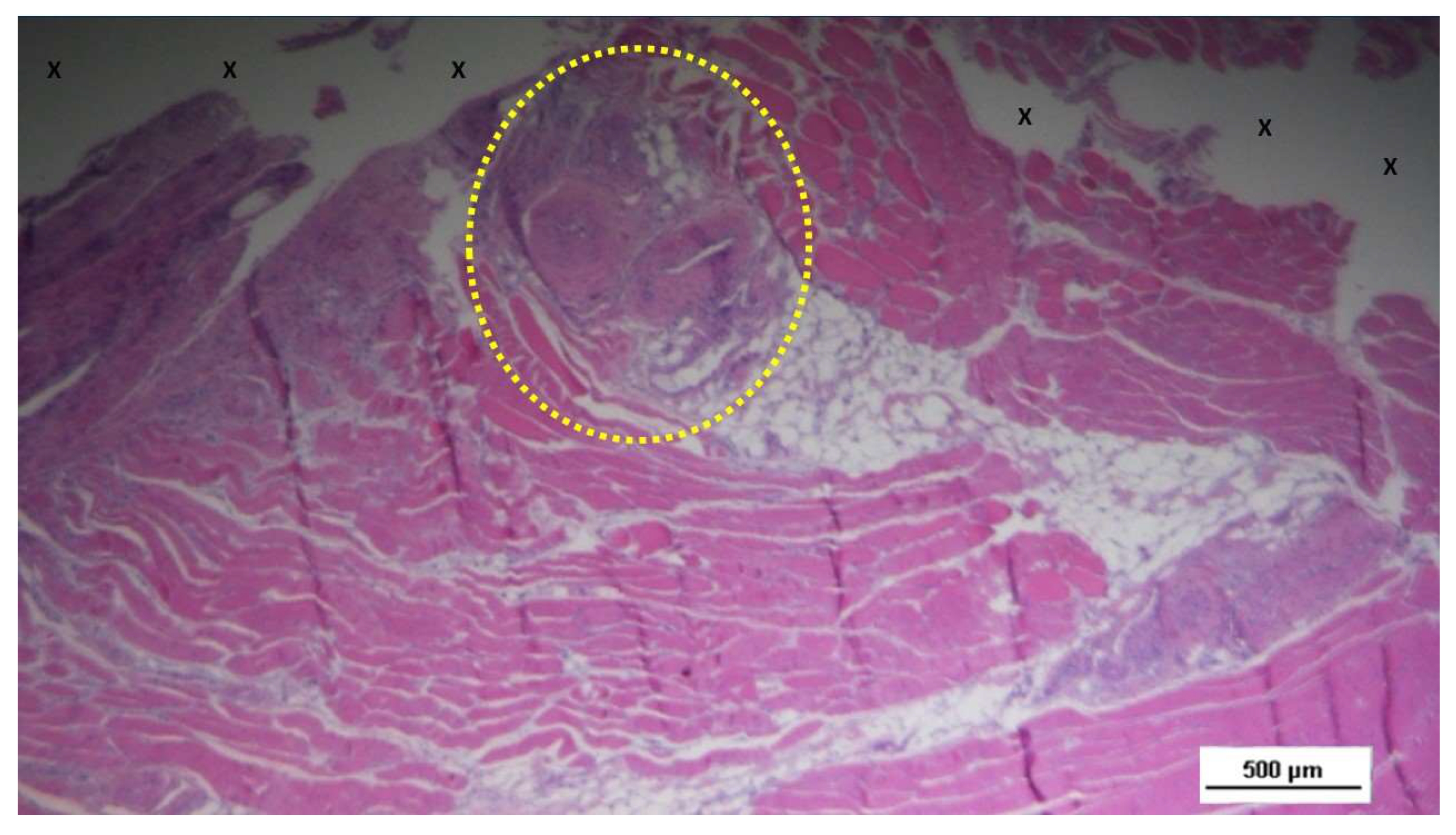
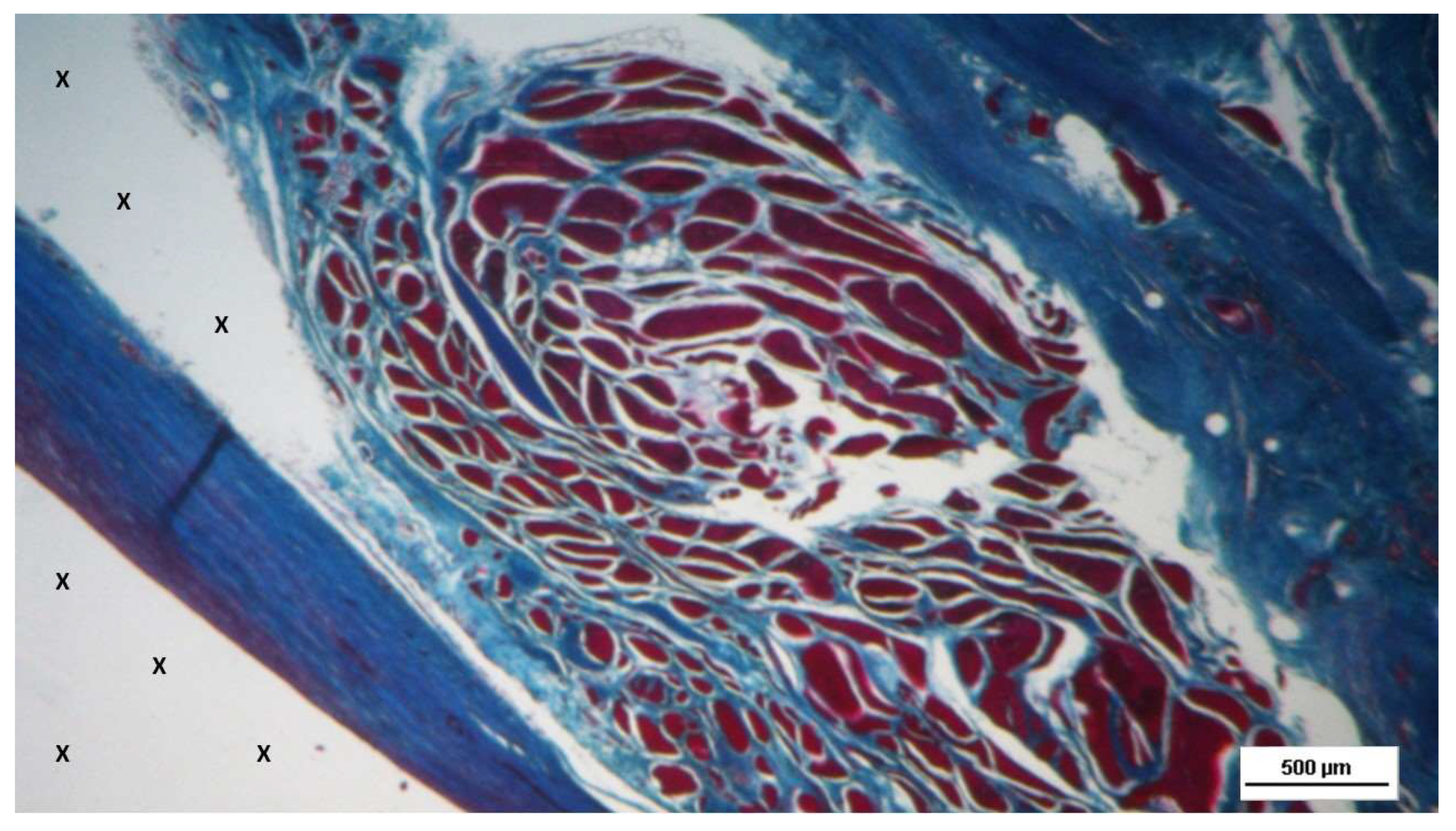
Figure 11.
Biopsy sample excised from the 3D scaffold of the S&S device 6 months post-implantation: microphotograph showing a large area of mature muscle elements grouped in bundles (red spots within the yellow circle) near a large arterial structure (Y) and adjacent to the S&S scaffold fabric (X). HE 25X.
Figure 11.
Biopsy sample excised from the 3D scaffold of the S&S device 6 months post-implantation: microphotograph showing a large area of mature muscle elements grouped in bundles (red spots within the yellow circle) near a large arterial structure (Y) and adjacent to the S&S scaffold fabric (X). HE 25X.
Figure 12.
Biopsy specimen excised from the 3D scaffold of the S&S device 6 months post-surgery: microphotograph showing three clusters of fully developed muscle bundles (stained in red) interspersed between two areas of adipocytes (Y) and close to a large arterial structure (yellow circle). The blue-stained tissue adjacent to the device fabric corresponds to well-perfused connective tissue. AM 25X.
Figure 12.
Biopsy specimen excised from the 3D scaffold of the S&S device 6 months post-surgery: microphotograph showing three clusters of fully developed muscle bundles (stained in red) interspersed between two areas of adipocytes (Y) and close to a large arterial structure (yellow circle). The blue-stained tissue adjacent to the device fabric corresponds to well-perfused connective tissue. AM 25X.
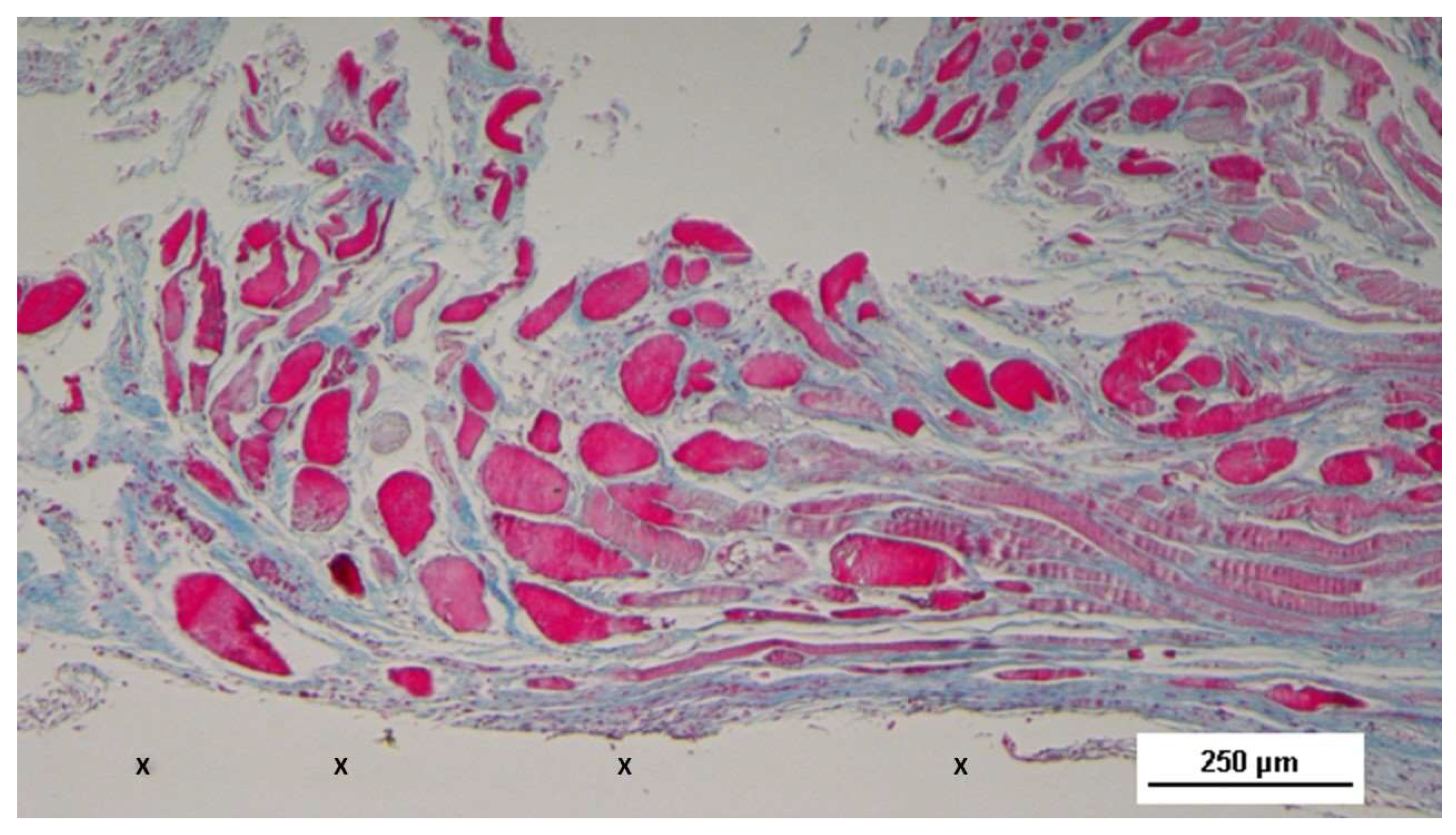
Figure 13.
Biopsy sample excised from the 3D scaffold of the S&S device 6 months post-implantation. The image shows the S&S scaffold fabric (X) surrounded by well-hydrated, viable connective tissue (stained in blue), close to a large arterial structure (yellow circle) and two elongated veins (*) between two large areas of muscle bundles in advanced development (stained in red). AM 25X.
Figure 13.
Biopsy sample excised from the 3D scaffold of the S&S device 6 months post-implantation. The image shows the S&S scaffold fabric (X) surrounded by well-hydrated, viable connective tissue (stained in blue), close to a large arterial structure (yellow circle) and two elongated veins (*) between two large areas of muscle bundles in advanced development (stained in red). AM 25X.
Figure 14.
Tissue specimen taken from the 3D scaffold of the S&S device 7 months post-operation: highlighting elongated bundles composed of mature muscle elements (colored in red) near the S&S scaffold fabric (X). The blue convoluted structure in the upper left margin corresponds to a nerve AM 50X.
Figure 14.
Tissue specimen taken from the 3D scaffold of the S&S device 7 months post-operation: highlighting elongated bundles composed of mature muscle elements (colored in red) near the S&S scaffold fabric (X). The blue convoluted structure in the upper left margin corresponds to a nerve AM 50X.
Figure 15.
Biopsy sample excised from the 3D scaffold of the S&S device 18 months post-implantation showing a thick layer of muscular structures (stained in red) in a stage of consolidated maturation encircling the fabric of the S&S Hernia System (X). HE 50X.
Figure 15.
Biopsy sample excised from the 3D scaffold of the S&S device 18 months post-implantation showing a thick layer of muscular structures (stained in red) in a stage of consolidated maturation encircling the fabric of the S&S Hernia System (X). HE 50X.
Figure 16.
Biopsy sample removed from the 3D scaffold of the S&S device 18 months post-surgery: highlighting a large area of mature muscle bundles (stained in red) near the S&S device structure (X), surrounded by viable connective tissue (colored in blue). AM 25X.
Figure 16.
Biopsy sample removed from the 3D scaffold of the S&S device 18 months post-surgery: highlighting a large area of mature muscle bundles (stained in red) near the S&S device structure (X), surrounded by viable connective tissue (colored in blue). AM 25X.
Figure 17.
Tissue sample excised from the 3D scaffold of the S&S device 18 months post-implantation: showing a large bundle of mature muscle elements (stained in red) within a loose and woven connective matrix (stained in blue), in close proximity to the S&S scaffold fabric (X). AM 25X.
Figure 17.
Tissue sample excised from the 3D scaffold of the S&S device 18 months post-implantation: showing a large bundle of mature muscle elements (stained in red) within a loose and woven connective matrix (stained in blue), in close proximity to the S&S scaffold fabric (X). AM 25X.
Figure 18.
Biopsy specimen excised 18 months post-implantation: showing a large zone of muscle bundles (coloured in red) in the stage of consolidated maturation, close to the 3D scaffold of the S&S device (X) and interspersed among well-perfused connective tissue (stained in blue). AM 50X.
Figure 18.
Biopsy specimen excised 18 months post-implantation: showing a large zone of muscle bundles (coloured in red) in the stage of consolidated maturation, close to the 3D scaffold of the S&S device (X) and interspersed among well-perfused connective tissue (stained in blue). AM 50X.
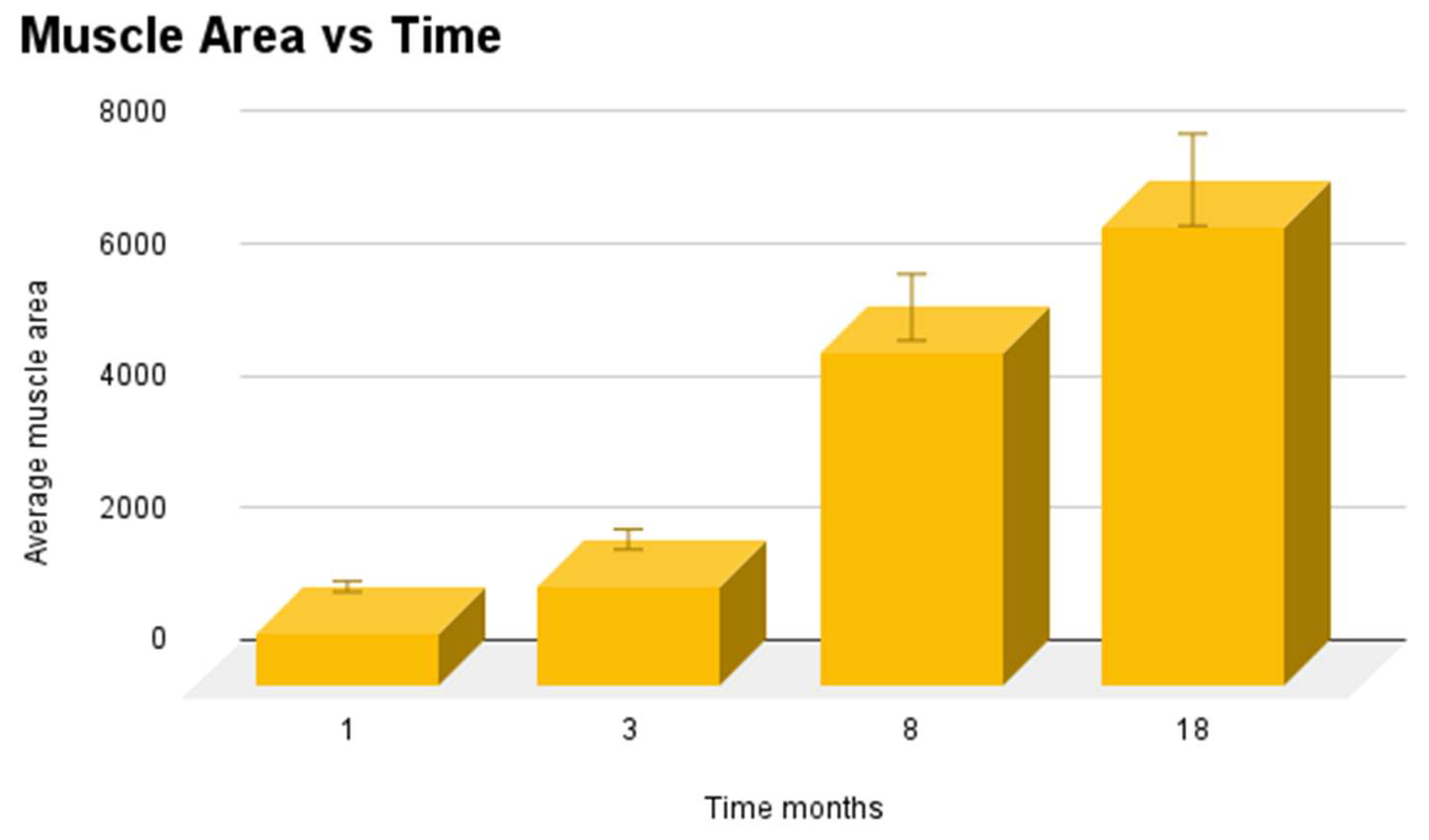
Figure 19.
Muscular ingrowth at different times post-surgery (1 month, 3 m, 8 m and 18 months).
Figure 19.
Muscular ingrowth at different times post-surgery (1 month, 3 m, 8 m and 18 months).
Table 1.
Mechanical properties of the Thermo-Polymer Elastomer (TPE) material utilized for the injection molding of the Stenting & Shielding Hernia System.
Table 1.
Mechanical properties of the Thermo-Polymer Elastomer (TPE) material utilized for the injection molding of the Stenting & Shielding Hernia System.
| TPE mechanical properties |
Value |
Unit |
Test Standard |
| ISO Data |
| Tensile Strength |
16 |
MPa |
ISO 37 |
| Strain at break |
650 |
% |
ISO 37 |
| Compression set at 70 °C, 24h |
54 |
% |
ISO 815 |
| Compression set at 100 °C, 24h |
69 |
% |
ISO 815 |
| Tear strength |
46 |
kN/m |
ISO 34-1 |
| Shore A hardness |
89 |
- |
ISO 7619-1 |
| Density |
890 |
kg/m³ |
ISO 1183 |
Table 2.
Muscle area in biopsy samples, µm2. Short-term: Tissue excision from the 3D scaffold of the S&S Hernia System 1 month after surgery. Mid-term: Tissue excision from the 3D scaffold of the S&S Hernia System 3 months after surgery. Long-term: Tissue excision from the 3D scaffold of the S&S Hernia System 8 months after surgery. Extra-long term: Tissue excision from the 3D scaffold of the S&S Hernia System 18 months after surgery.
Table 2.
Muscle area in biopsy samples, µm2. Short-term: Tissue excision from the 3D scaffold of the S&S Hernia System 1 month after surgery. Mid-term: Tissue excision from the 3D scaffold of the S&S Hernia System 3 months after surgery. Long-term: Tissue excision from the 3D scaffold of the S&S Hernia System 8 months after surgery. Extra-long term: Tissue excision from the 3D scaffold of the S&S Hernia System 18 months after surgery.
| |
Short term |
Mid-term |
Long-term |
Extra-long term |
| Time months |
≈1 |
3-4 |
6-8 |
18 |
| Media Muscle area µm2
|
807,25 |
1522 |
5038,1 |
6967,5 |
| DS Muscle area |
± 138,4013 |
± 303,0852 |
± 855,9019 |
± 870,4484 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).