Submitted:
03 December 2024
Posted:
03 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Impact of Standard Crude Protein Concentrations in Weaner Pig Diets: Effects on Growth, Gut Health and Immune Function
2.1. The Effects of Low Crude Protein in Weaner Pig Diets : Diarrhoea, Gut Health, Immune Function and Growth
3. The Role of Organic Acids in Weaner Pig Diets
3.1. The Potential of Organic Acid-Preserved Grain in Weaner Diets : Enhancing Grain Quality, Gut Health, Growth and Sustainability
4. The Protective Effects of Butyrate
4.1. The Effects of Supplementing Exogenous Butyrate on Post-Weaned Pig Growth and Gut Health
4.2. Promoting Endogenous Butyrate Production in Weaned Pigs via Prebiotic and Probiotic Supplementation: Effects on Growth, Gut Health and the Gut Microbiome
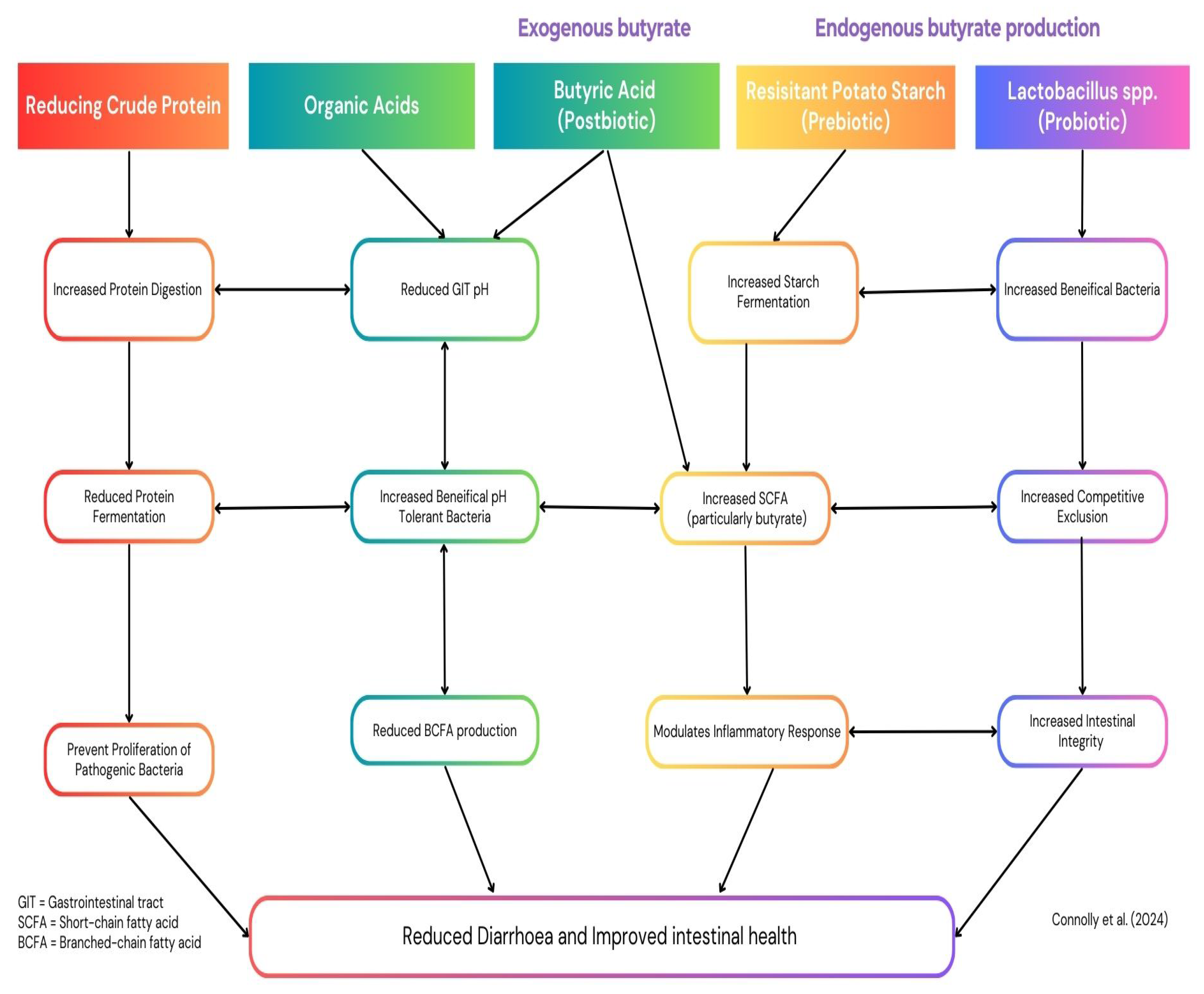
5. Exploring the Synergistic Effects of Combining these Dietary Strategies for Optimal Health and Growth in Weaner Pigs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbert, M.S.; Ijssennagger, N.; Kies, A.K.; Van Mil, S.W.C. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. American Journal of Physiology-Gastrointestinal and Liver Physiology 2018, 315, G159–G170. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds: Feeding strategies without using in-feed antibiotics. Journal of Animal Physiology and Animal Nutrition 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J Animal Sci Biotechnol 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.V.; Bouwhuis, M.A.; Sweeney, T. Novel marine polysaccharides and maternal nutrition to stimulate gut health and performance in post-weaned pigs. Anim. Prod. Sci. 2017, 57, 2376. [Google Scholar] [CrossRef]
- Pieper, R.; Tudela, C.V.; Taciak, M.; Bindelle, J.; Pérez, J.F.; Zentek, J. Health relevance of intestinal protein fermentation in young pigs. Anim. Health. Res. Rev. 2016, 17, 137–147. [Google Scholar] [CrossRef]
- Zhang, H.; Wielen, N.V.D.; Hee, B.V.D.; Wang, J.; Hendriks, W.; Gilbert, M. Impact of Fermentable Protein, by Feeding High Protein Diets, on Microbial Composition, Microbial Catabolic Activity, Gut Health and beyond in Pigs. Microorganisms 2020, 8, 1735. [Google Scholar] [CrossRef]
- Collins, C.L.; et al. Post-weaning and whole-of-life performance of pigs is determined by live weight at weaning and the complexity of the diet fed after weaning. Animal Nutrition 2017, 3, no. 4, pp. 372–379. [Google Scholar] [CrossRef]
- Veldkamp, T.; Vernooij, A.G. Use of insect products in pig diets. JIFF, 2021; 5. [Google Scholar] [CrossRef]
- Gloaguen, M.; Le Floc’h, N.; Corrent, E.; Primot, Y.; van Milgen, J. The use of free amino acids allows formulating very low crude protein diets for piglets1. Journal of Animal Science 2014, 92, 637–644. [Google Scholar] [CrossRef]
- NRC, Nutritional Requirements of Swine. Washington DC: National Academcic Press: 2012.
- Wensley, M.R.; et al. Maintaining continuity of nutrient intake after weaning. II. Review of post-weaning strategies. Translational Animal Science, 2021; 1. [Google Scholar] [CrossRef]
- Lallès, J.-P.; et al. Gut function and dysfunction in young pigs: physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livestock Production Science 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Fang, L.H.; et al. Effects of dietary energy and crude protein levels on growth performance, blood profiles, and nutrient digestibility in weaning pigs. Asian-Australas J Anim Sci. [CrossRef]
- Batson, K.L.; et al. Effects of feeding diets containing low crude protein and coarse wheat bran as alternatives to zinc oxide in nursery pig diets. Journal of Animal Science 2021, 99, skab090. [Google Scholar] [CrossRef]
- Williams, B.A.; Verstegen, M.W.A.; Tamminga, S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. NRR 2001, 14, 207. [Google Scholar] [CrossRef]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012; 1. [Google Scholar] [CrossRef]
- Gao, J.; et al. Protein Level and Infantile Diarrhea in a Postweaning Piglet Model. Mediators of Inflammation 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Wellock, I.J.; Fortomaris, P.D.; Houdijk, J.G.M.; Kyriazakis, I. The effect of dietary protein supply on the performance and risk of post-weaning enteric disorders in newly weaned pigs. Anim. Sci. 2006, 82, 327–335. [Google Scholar] [CrossRef]
- Bauer, E.; Metzler-Zebeli, B.U.; Verstegen, M.W.A.; Mosenthin, R. Intestinal gene expression in pigs: effects of reduced feed intake during weaning and potential impact of dietary components. Nutr. Res. Rev. 2011, 24, 155–175. [Google Scholar] [CrossRef]
- McCracken, B.A.; Spurlock, M.E.; Roos, M.A.; Zuckermann, F.A.; Gaskins, H.R. Weaning Anorexia May Contribute to Local Inflammation in the Piglet Small Intestine. The Journal of Nutrition 1999, 129, no. 3, pp. 613–619. [Google Scholar] [CrossRef]
- Xia, J.; et al. Research progress on diarrhoea and its mechanism in weaned piglets fed a high-protein diet. Animal Physiology Nutrition 2022, 106, 1277–1287. [Google Scholar] [CrossRef]
- Mayer, L. Mucosal immunity. Pediatrics 2003, 1595–1600. [Google Scholar] [CrossRef]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli1. Journal of Animal Science 2009, 87, 2833–2843. [Google Scholar] [CrossRef]
- Kim, J.C.; Heo, J.M.; Mullan, B.P.; Pluske, J.R. Efficacy of a reduced protein diet on clinical expression of post-weaning diarrhoea and life-time performance after experimental challenge with an enterotoxigenic strain of Escherichia coli. Animal Feed Science and Technology 2011, 170, 222–230. [Google Scholar] [CrossRef]
- Yue, L.Y.; Qiao, S.Y. Effects of low-protein diets supplemented with crystalline amino acids on performance and intestinal development in piglets over the first 2 weeks after weaning. Livestock Science 2008, 115, 144–152. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J Animal Sci Biotechnol 2018, 9, 60. [Google Scholar] [CrossRef]
- García, K.E.; de Souza, T.C.R.; Landín, G.M.; Barreyro, A.A.; Santos, M.G.B.; Soto, J.G.G. Microbial Fermentation Patterns, Diarrhea Incidence, and Performance in Weaned Piglets Fed a Low Protein Diet Supplemented with Probiotics. FNS 2014, 5, 1776–1786. [Google Scholar] [CrossRef]
- Tian, Z.; et al. Influence of low protein diets on gene expression of digestive enzymes and hormone secretion in the gastrointestinal tract of young weaned piglets. J. Zhejiang Univ. Sci. B 2016, 17, 742–751. [Google Scholar] [CrossRef]
- Opapeju, F.O.; Rademacher, M.; Blank, G.; Nyachoti, C.M. Effect of low-protein amino acid-supplemented diets on the growth performance, gut morphology, organ weights and digesta characteristics of weaned pigs. Animal 2008, 2, 1457–1464. [Google Scholar] [CrossRef]
- Deng, D.; et al. Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. The Journal of Nutritional Biochemistry 2009, 20, 544–552. [Google Scholar] [CrossRef]
- Yu, D.; Zhu, W.; Hang, S. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Archives of Animal Nutrition, 2019; 4. [Google Scholar] [CrossRef]
- Marchetti, R.; et al. Protein Content in the Diet Influences Growth and Diarrhea in Weaning Piglets. Animals, 7952; 5. [Google Scholar] [CrossRef]
- Rattigan, R.; Sweeney, T.; Vigors, S.; Rajauria, G.; O’Doherty, J.V. Effects of reducing dietary crude protein concentration and supplementation with laminarin or zinc oxide on the faecal scores and colonic microbiota in newly weaned pigs. J Anim Physiol Anim Nutr 2020, 104, no. 5, pp. 1471–1483. [Google Scholar] [CrossRef]
- Wang, Y.; et al. Dietary crude protein time-dependently modulates the bacterial community and metabolites and changes dietary nutrient efficiency in growing pigs. Animal Nutrition 2024, 17, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, M.; Yang, Y.; Mu, C.; Su, Y.; Zhu, W. Differential effect of early antibiotic intervention on bacterial fermentation patterns and mucosal gene expression in the colon of pigs under diets with different protein levels. Appl Microbiol Biotechnol 2017, 101, 2493–2505. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci Rep 2017, 7, 43412. [Google Scholar] [CrossRef]
- Rattigan, R.; Sweeney, T.; Maher, S.; Ryan, M.T.; Thornton, K.; O’Doherty, J.V. Effects of reducing dietary crude protein concentration and supplementation with either laminarin or zinc oxide on the growth performance and intestinal health of newly weaned pigs. Animal Feed Science and Technology 2020, 270, 114693. [Google Scholar] [CrossRef]
- Moreira, T.G.; et al. Dietary protein modulates intestinal dendritic cells to establish mucosal homeostasis. Mucosal Immunology 2024, S1933021924000606. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets1. Journal of Animal Science 2006, 84, 125–134. [Google Scholar] [CrossRef]
- Ferronato, G.; Prandini, A. Dietary Supplementation of Inorganic, Organic, and Fatty Acids in Pig: A Review. Animals 2020, 10, 1740. [Google Scholar] [CrossRef]
- Nowak, P.; Zaworska-Zakrzewska, A.; Frankiewicz, A.; Kasprowicz-Potocka, M. The effects and mechanisms of acids on the health of piglets and weaners – a review. Annals of Animal Science 2021, 21, 433–455. [Google Scholar] [CrossRef]
- Tugnoli; Giovagnoni; Piva; Grilli, “From Acidifiers to Intestinal Health Enhancers: How Organic Acids Can Improve Growth Efficiency of Pigs. Animals 2020, 10, 134. [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. Journal of Lipid Research 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Tsiloyiannis, V.K.; Kyriakis, S.C.; Vlemmas, J.; Sarris, K. The effect of organic acids on the control of porcine post-weaning diarrhoea. Research in Veterinary Science 2001, 70, 287–293. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chang, R.B.; Allgood, S.D.; Silver, W.L.; Liman, E.R. A TRPA1-dependent mechanism for the pungent sensation of weak acids. Journal of General Physiology 2011, 137, 493–505. [Google Scholar] [CrossRef]
- Hutchens, W.M.; et al. The effects of pharmacological levels of zinc, diet acidification, and dietary crude protein on growth performance in nursery pigs. Journal of Animal Science 2021, 99, skab259. [Google Scholar] [CrossRef]
- Su, Y.; Yao, W.; Perez-Gutierrez, O.N.; Smidt, H.; Zhu, W.-Y. Changes in abundance of Lactobacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning: Changes in the gastrointestinal microbiota of the weaning piglet. FEMS Microbiology Ecology 2008, 66, 546–555. [Google Scholar] [CrossRef]
- Schubert, M.L.; Peura, D.A. Control of Gastric Acid Secretion in Health and Disease. Gastroenterology 2008, 134, 1842–1860. [Google Scholar] [CrossRef]
- Wang, L.F.; Bergstrom, J.R.; Hahn, J.D.; Young, M.G.; Zijlstra, R.T. Acid-binding capacity of feed in swine nutrition. Animal Feed Science and Technology 2023, 295, 115519. [Google Scholar] [CrossRef]
- Hansen, C.F.; Riis, A.L.; Bresson, S.; Højbjerg, O.; Jensen, B.B. Feeding organic acids enhances the barrier function against pathogenic bacteria of the piglet stomach. Livestock Science 2007, 108, 206–209. [Google Scholar] [CrossRef]
- Roth, F.; Kirchgessner, M. Organic acids as feed additives for young pigs:Nutritional and gastrointestinal effects. J. Anim. Feed Sci. 1998, 7, 25–33. [Google Scholar] [CrossRef]
- Li, Z.; Yi, G.; Yin, J.; Sun, P.; Li, D.; Knight, C. Effects of Organic Acids on Growth Performance, Gastrointestinal pH, Intestinal Microbial Populations and Immune Responses of Weaned Pigs. Asian Australas. J. Anim. Sci 2008, 21, 252–261. [Google Scholar] [CrossRef]
- Kuang, Y.; et al. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs. Animal Feed Science and Technology 2015, 208, 145–157. [Google Scholar] [CrossRef]
- Mroz, Z.; Jongbloed, A.W.; Partanen, K.H.; Vreman, K.; Kemme, P.A.; Kogut, J. The effects of calcium benzoate in diets with or without organic acids on dietary buffering capacity, apparent digestibility, retention of nutrients, and manure characteristics in swine. Journal of Animal Science 2000, 78, 2622. [Google Scholar] [CrossRef]
- Ferrara, F.; Tedin, L.; Pieper, R.; Meyer, W.; Zentek, J. Influence of medium-chain fatty acids and short-chain organic acids on jejunal morphology and intra-epithelial immune cells in weaned piglets. J Anim Physiol Anim Nutr 2017, 101, 531–540. [Google Scholar] [CrossRef]
- Li, S.; et al. Supplementation with organic acids showing different effects on growth performance, gut morphology and microbiota of weaned pigs fed with highly or less digestible diets. Journal of Animal Science 2018. [CrossRef]
- Long, S.F.; et al. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Animal Feed Science and Technology 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Zeng, X.; et al. Dietary butyrate, lauric acid and stearic acid improve gut morphology and epithelial cell turnover in weaned piglets. Animal Nutrition 2022, 11, 276–282. [Google Scholar] [CrossRef]
- Namkung, H.; Gong, M.L.J.; Yu, H.; Cottrill, M.; de Lange, C.F.M. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can. J. Anim. Sci. 2004, 84, 697–704. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Sahibzada, S.; Pineda, L.; Han, Y.; Collins, A. Impacts of feeding organic acid-based feed additives on diarrhea, performance, and fecal microbiome characteristics of pigs after weaning challenged with an enterotoxigenic strain of Escherichia coli. Translational Animal Science 2021, 5, txab212. [Google Scholar] [CrossRef]
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr 2019, 14, 4. [Google Scholar] [CrossRef]
- Canibe, N.; Steien, S.H.; Overland, M.; Jensen, B.B. Effect of K-diformate in starter diets on acidity, microbiota, and the amount of organic acids in the digestive tract of piglets, and on gastric alterations. Journal of Animal Science 2001, 79, 2123. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. International Journal of Food Microbiology 2007, 119, 131–139. [Google Scholar] [CrossRef]
- Melin, P.; Sundh, I.; Håkansson, S.; Schnürer, J. Biological preservation of plant derived animal feed with antifungal microorganisms: safety and formulation aspects. Biotechnol Lett 2007, 29, 1147–1154. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Animal Nutrition, 6820. [Google Scholar] [CrossRef]
- Maher, S.; et al. Organic acid preservation of cereal grains improves grain quality, growth performance, and intestinal health of post-weaned pigs. Animal Feed Science and Technology 2024, 116078. [Google Scholar] [CrossRef]
- Jokiniemi, T.; Jaakkola, S.; Turunen, M.; Ahokas, J. Energy consumption in different grain preservation methods. Agronomy Research 2014, 12, 81–94. [Google Scholar]
- Jokiniemi, H.T.; Ahokas, J.M. Drying process optimisation in a mixed-flow batch grain dryer. Biosystems Engineering 2014, 121, 209–220. [Google Scholar] [CrossRef]
- Xu, X.; et al. A comparison of the nutritional value of organic-acid preserved corn and heat-dried corn for pigs. Animal Feed Science and Technology 2016, 214, 95–103. [Google Scholar] [CrossRef]
- Connolly, R.; Sweeney, T.; Maher, S. Organic acid and salt treatment of cereal at harvest improves growth performance in the post weaned pig. Animal-science proceedings 2022, 13, 204. [Google Scholar] [CrossRef]
- Dou, S.; et al. Characterisation of Early-Life Fecal Microbiota in Susceptible and Healthy Pigs to Post-Weaning Diarrhoea. PLoS ONE 2017, 12, e0169851. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Nowland, T.L.; Kirkwood, R.N.; Pluske, J.R. Review: Can early-life establishment of the piglet intestinal microbiota influence production outcomes? Animal 2021, 100368. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: the role of butyrate on colonic function: REVIEW: ROLE OF BUTYRATE ON COLONIC FUNCTION. Alimentary Pharmacology & Therapeutics 2007, 27, 104–119. [Google Scholar] [CrossRef]
- Leonel, A.J.; Alvarez-Leite, J.I. Butyrate: implications for intestinal function. Current Opinion in Clinical Nutrition and Metabolic Care 2012, 15, 474–479. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in Energy Metabolism: There Is Still More to Learn. Trends in Endocrinology & Metabolism 2021, 32, 159–169. [Google Scholar] [CrossRef]
- Geng, T.; et al. Probiotics Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 induce cytokine alterations by the production of TCDA, DHA, and succinic and palmitic acids, and enhance immunity of weaned piglets. Research in Veterinary Science 2021, 137, 56–67. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A. The Immunomodulatory Functions of Butyrate. JIR 2021, 14, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Piva, A.; Morlacchini, M.; Casadei, G.; Gatta, P.P.; Biagi, G.; Prandini, A. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Italian Journal of Animal Science 2002, 1, 35–41. [Google Scholar] [CrossRef]
- Feng, W.; et al. Sodium Butyrate Attenuates Diarrhea in Weaned Piglets and Promotes Tight Junction Protein Expression in Colon in a GPR109A-Dependent Manner. Cell Physiol Biochem 2018, 47, 1617–1629. [Google Scholar] [CrossRef]
- Biagi, G.; Piva, A.; Moschini, M.; Vezzali, E.; Roth, F.X. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate1. Journal of Animal Science 2007, 85, 1184–1191. [Google Scholar] [CrossRef]
- Jang, Y.D.; Lindemann, M.D.; Monegue, H.J.; Monegue, J.S. The effect of coated sodium butyrate supplementation in sow and nursery diets on lactation performance and nursery pig growth performance. Livestock Science 2017, 195, 13–20. [Google Scholar] [CrossRef]
- Nugent, A.P. Health properties of resistant starch. Nutrition Bulletin 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Desai, M.S.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353e21. [Google Scholar] [CrossRef]
- Heo, J.M.; Agyekum, A.K.; Yin, Y.L.; Rideout, T.C.; Nyachoti, C.M. Feeding a diet containing resistant potato starch influences gastrointestinal tract traits and growth performance of weaned pigs1. Journal of Animal Science 2014, 92, 3906–3913. [Google Scholar] [CrossRef]
- Yi, S.-W.; et al. Raw potato starch diet supplement in weaned pigs could reduce Salmonella Typhimurium infection by altering microbiome composition and improving immune status. Front. Vet. Sci. 2023, 10, 1183400. [Google Scholar] [CrossRef]
- Trachsel, J.; Briggs, C.; Gabler, N.K.; Allen, H.K.; Loving, C.L. Dietary Resistant Potato Starch Alters Intestinal Microbial Communities and Their Metabolites, and Markers of Immune Regulation and Barrier Function in Swine. Front. Immunol. 2019, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.K.; Nyachoti, C.M.; Krause, D.O. Raw potato starch in weaned pig diets and its influence on postweaning scours and the molecular microbial ecology of the digestive tract1. Journal of Animal Science 2009, 87, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.-W.; et al. Effect of feeding raw potato starch on the composition dynamics of the piglet intestinal microbiome. Anim Biosci 2022, 35, 1698–1710. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qian, K.; Wang, C.; Wu, Y. Roles of Probiotic Lactobacilli Inclusion in Helping Piglets Establish Healthy Intestinal Inter-environment for Pathogen Defense. Probiotics & Antimicro. Prot. 2018, 10, 243–250. [Google Scholar] [CrossRef]
- Pieper, R.; et al. Effect of Lactobacillus plantarum on intestinal microbial community composition and response to enterotoxigenic Escherichia coli challenge in weaning piglets. Livestock Science 2010, 133, 98–100. [Google Scholar] [CrossRef]
- Su, W.; Gong, T.; Jiang, Z.; Lu, Z.; Wang, Y. The Role of Probiotics in Alleviating Postweaning Diarrhea in Piglets From the Perspective of Intestinal Barriers. Front. Cell. Infect. Microbiol. 2022, 12, 883107. [Google Scholar] [CrossRef]
- Sultana, R.; McBain, A.J.; O’Neill, C.A. Strain-Dependent Augmentation of Tight-Junction Barrier Function in Human Primary Epidermal Keratinocytes by Lactobacillus and Bifidobacterium Lysates. Appl Environ Microbiol 2013, 79, 4887–4894. [Google Scholar] [CrossRef]
- Li, X.-Q.; et al. Risks Associated with High-Dose Lactobacillus rhamnosus in an Escherichia coli Model of Piglet Diarrhoea: Intestinal Microbiota and Immune Imbalances. PLoS ONE 2012, 7, e40666. [Google Scholar] [CrossRef]
- Dehghani, N. INTERACTIVE IMPACTS OF DIETARY ORGANIC ACIDS AND CRUDE PROTEIN LEVELS ON PERFORMANCE AND GUT MORPHOLOGY OF BROILER CHICKENS. Book of Abstracts 2012.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




