Submitted:
03 December 2024
Posted:
03 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experiment
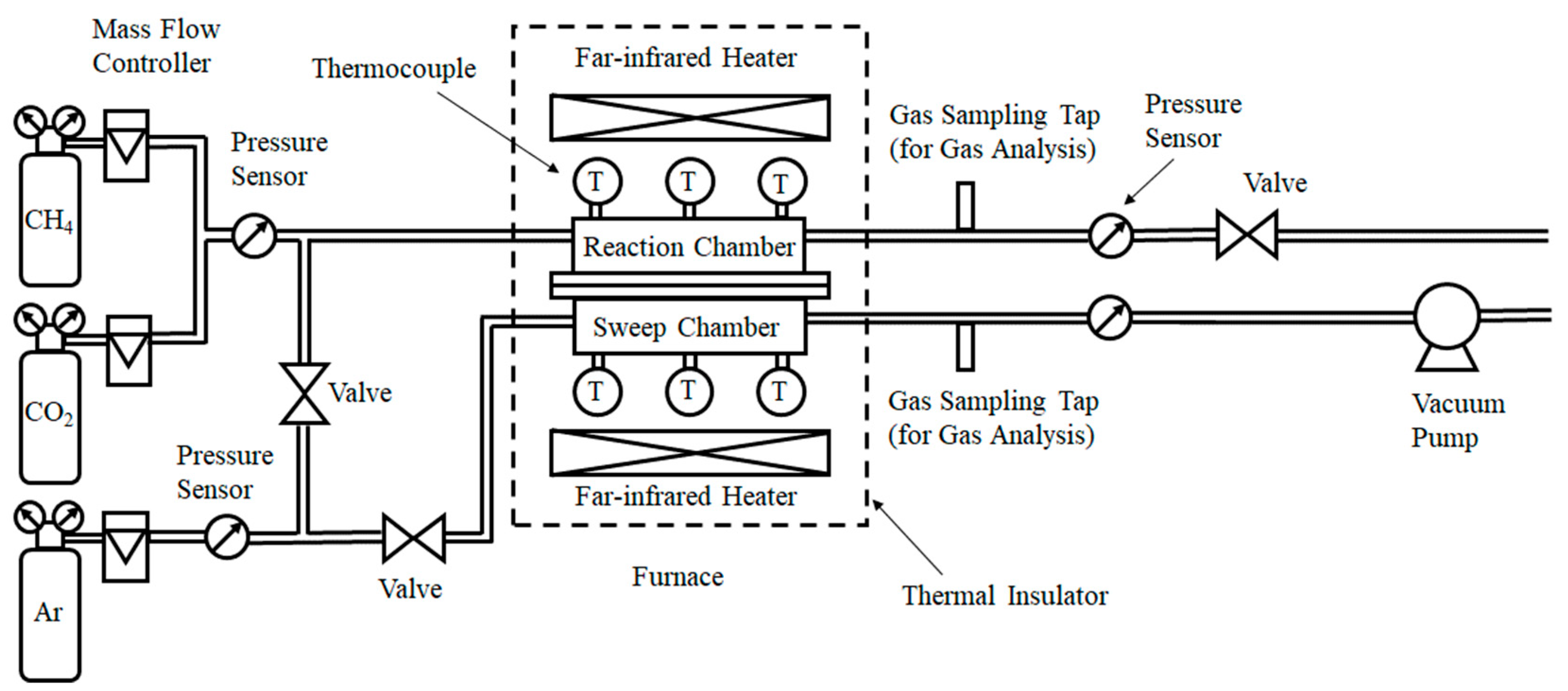
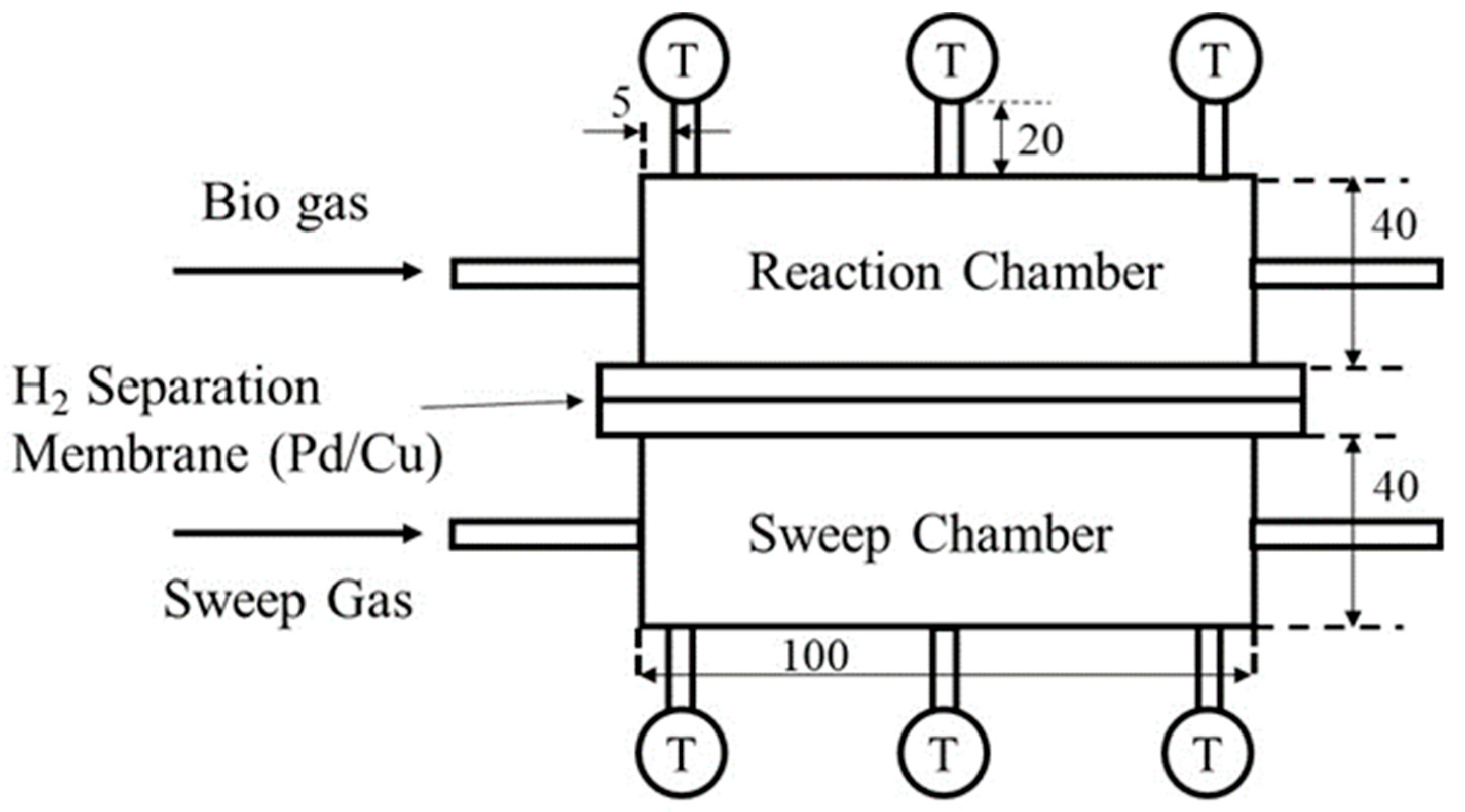
2.1. Experimental Set-Up
2.2. Assessment Factor to Evaluate the Performance of BDR Mmbrane Ractor in This Study
3. Results and Discussion
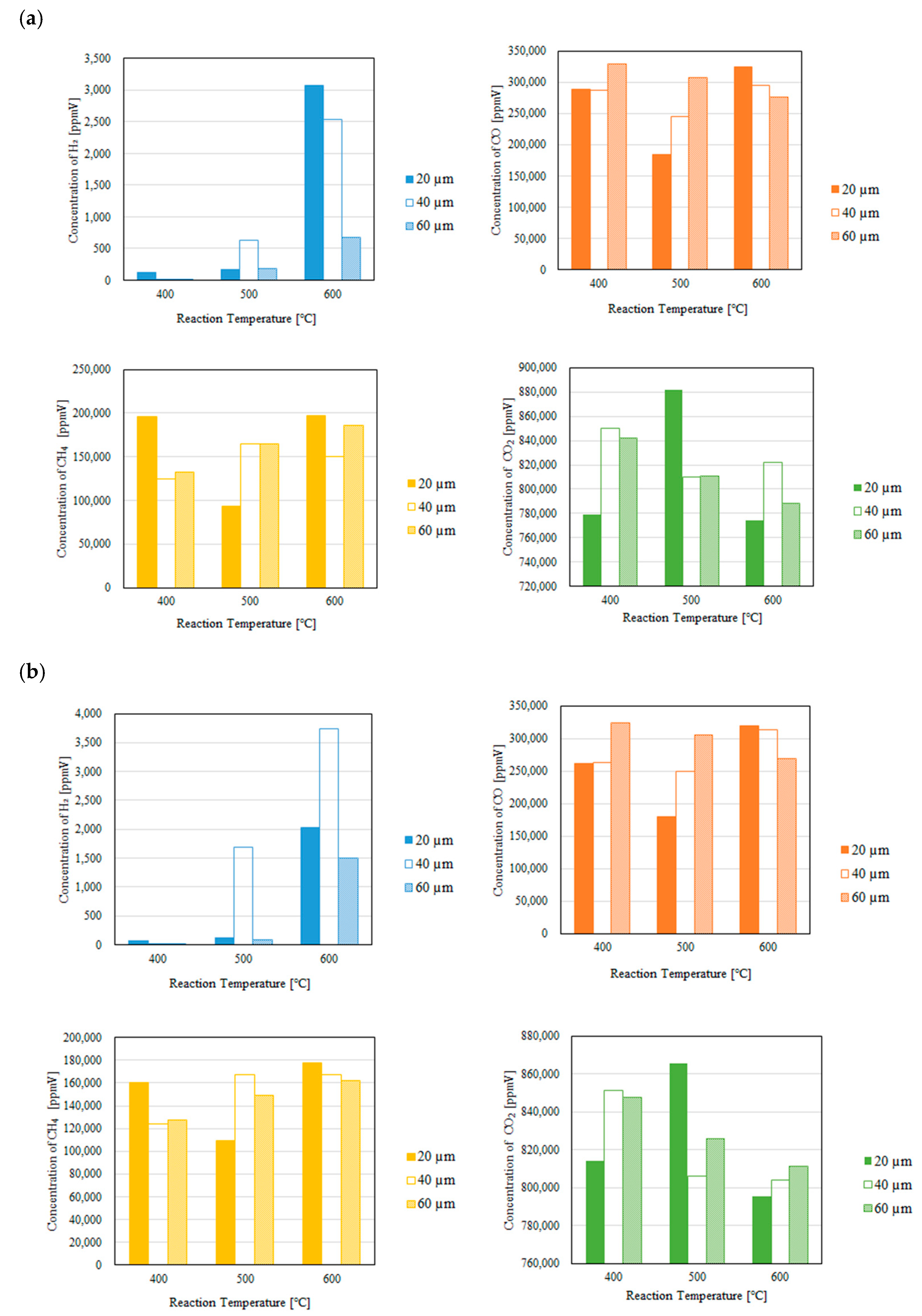
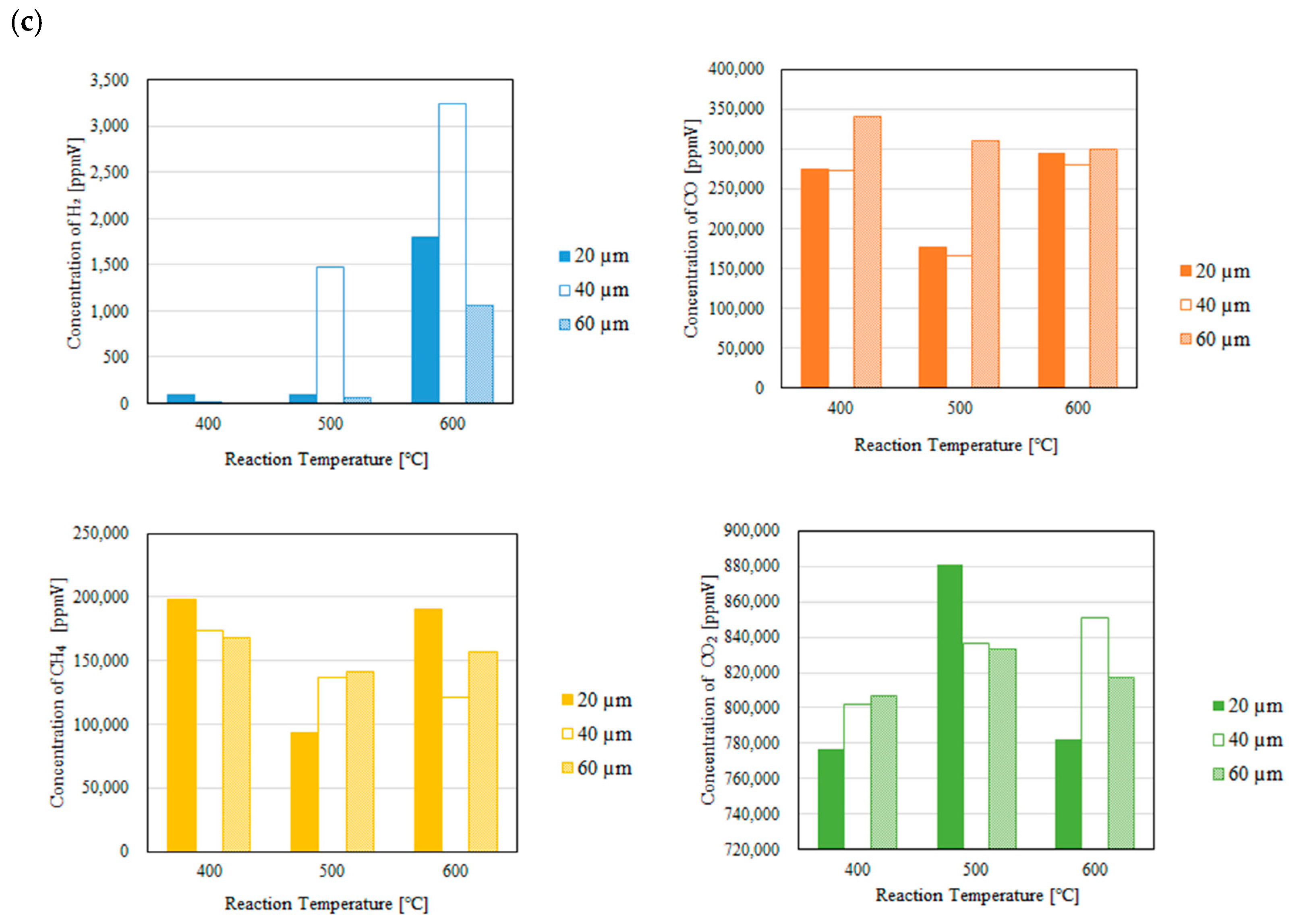
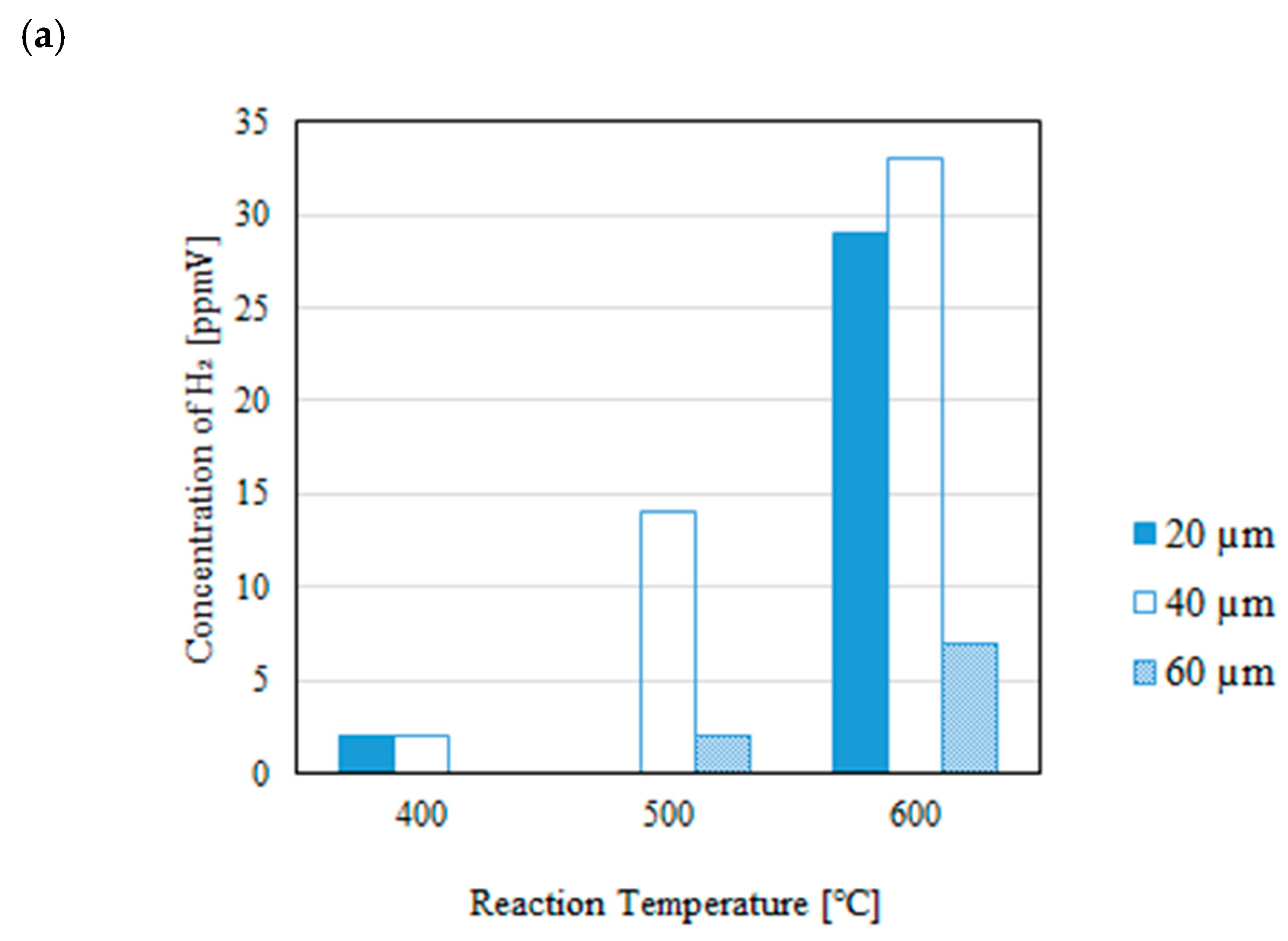
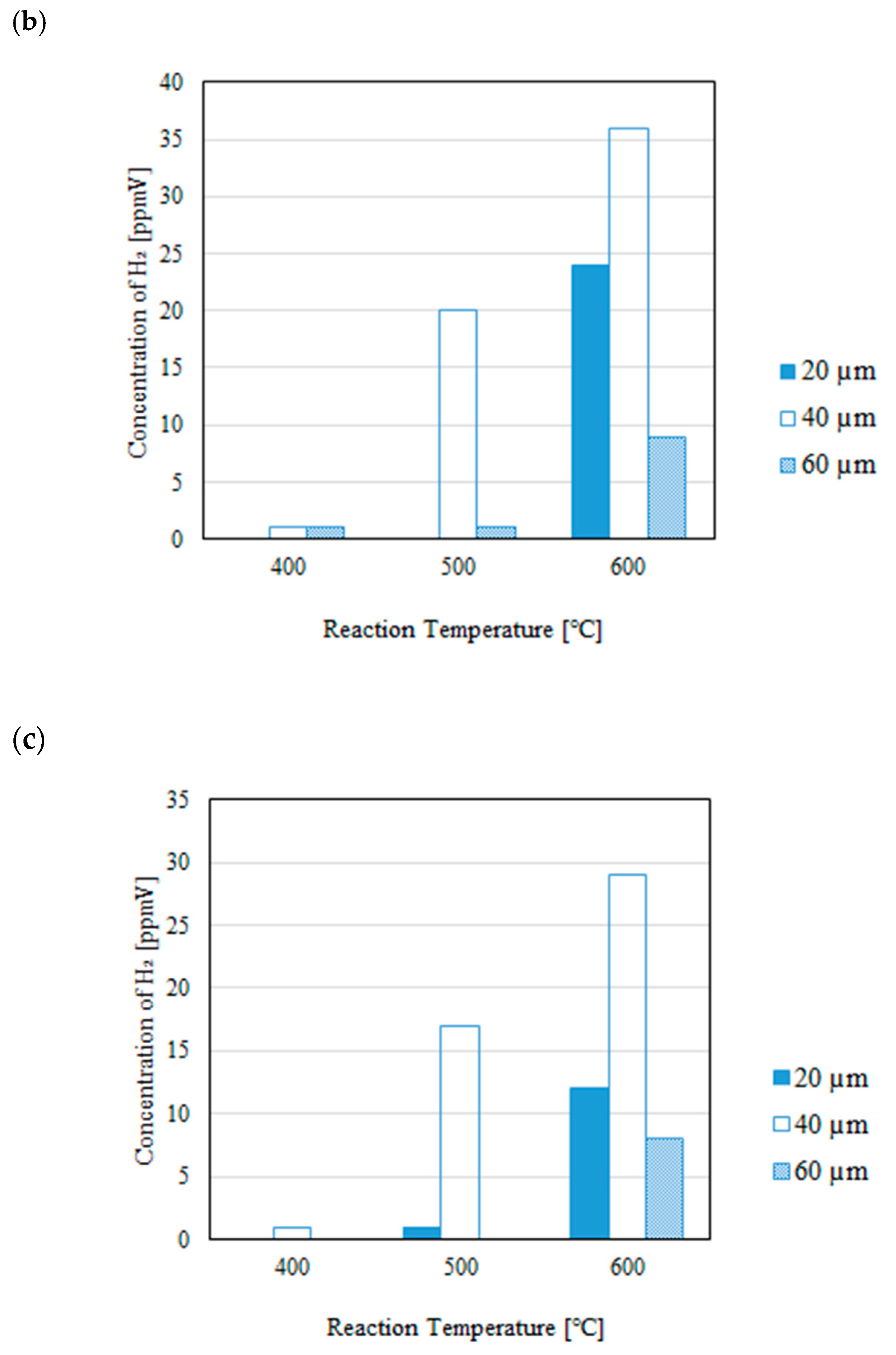
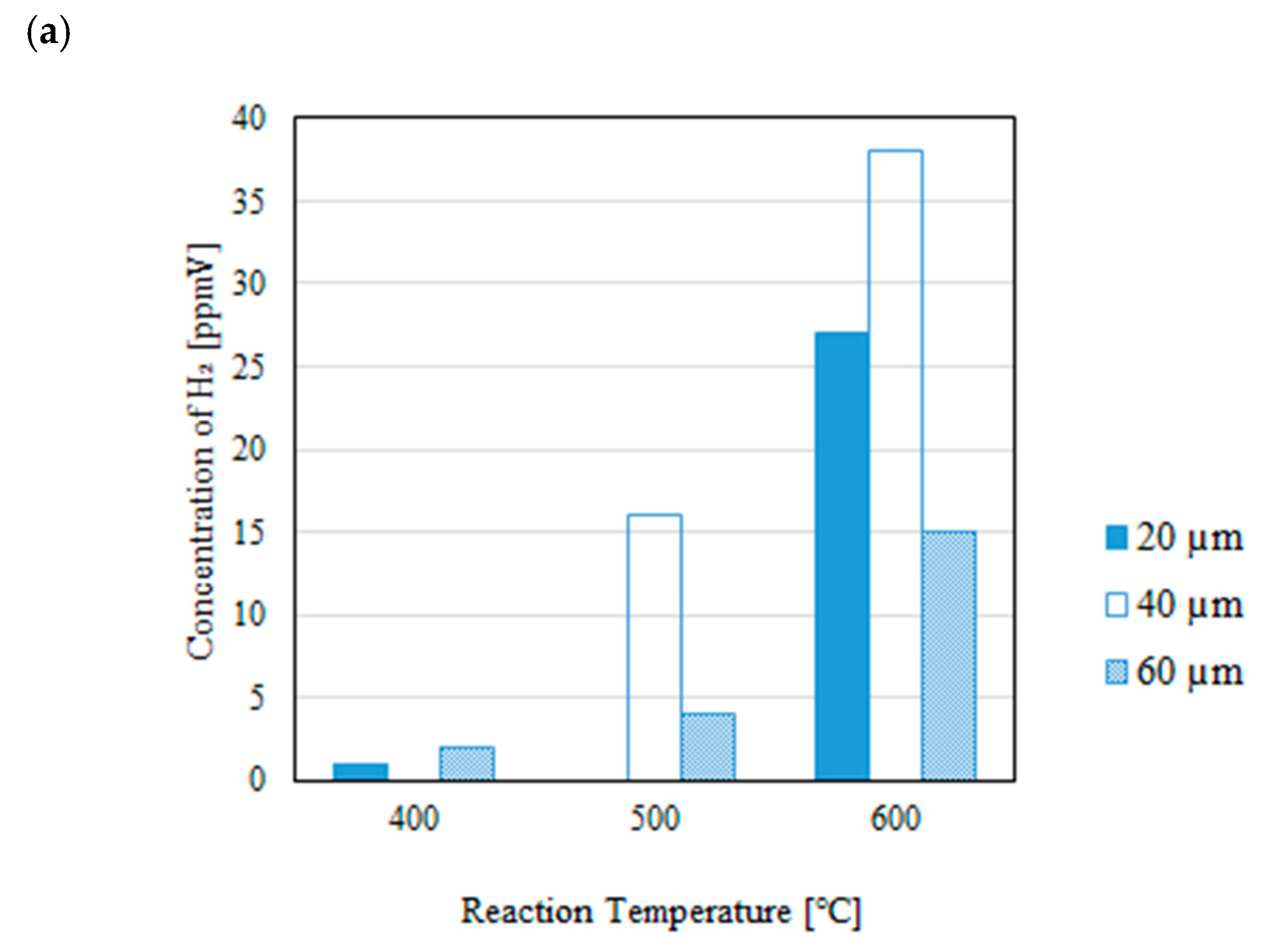
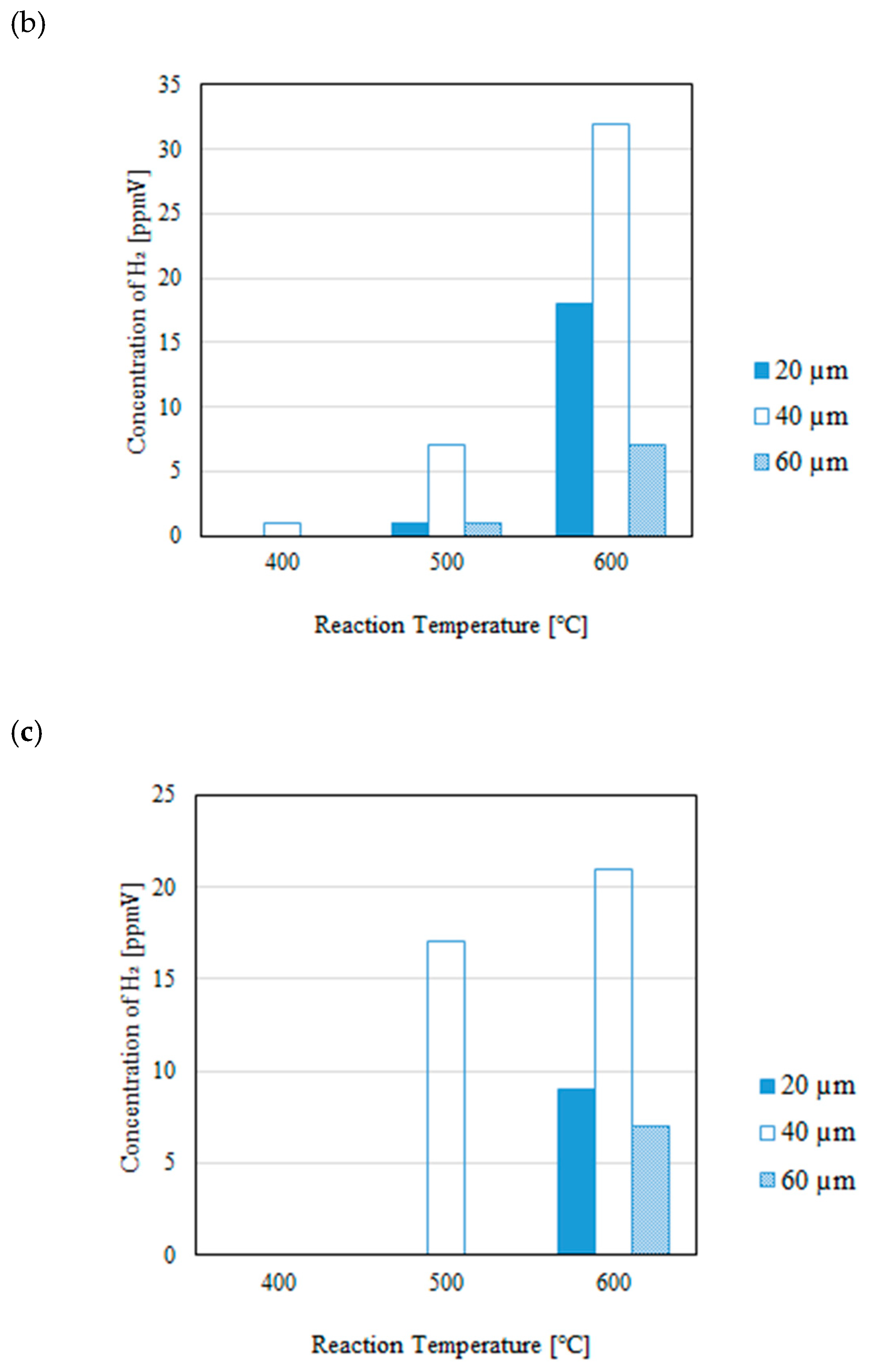
3.1. Impact of Thickness of Pd/Cu Membrane on Each Gas Concentration in the Reaction Chamber and the Concentration of H2 in the Sweep Chamber When Changing the Initial Reaction Temperature and the Differential Pressure Between the Reaction Chamber and the Sweep Chamber with and Without a Sweep Gas
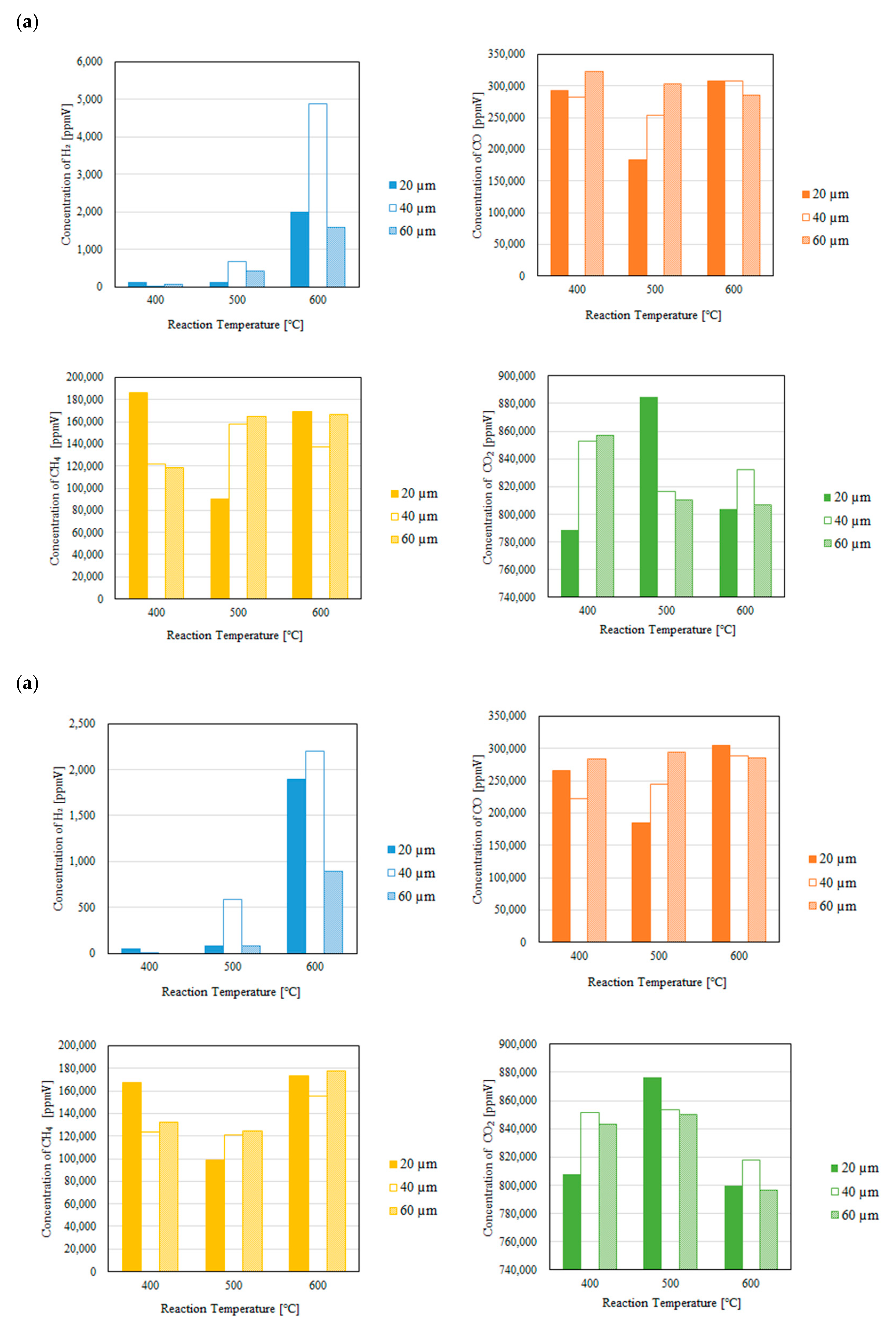
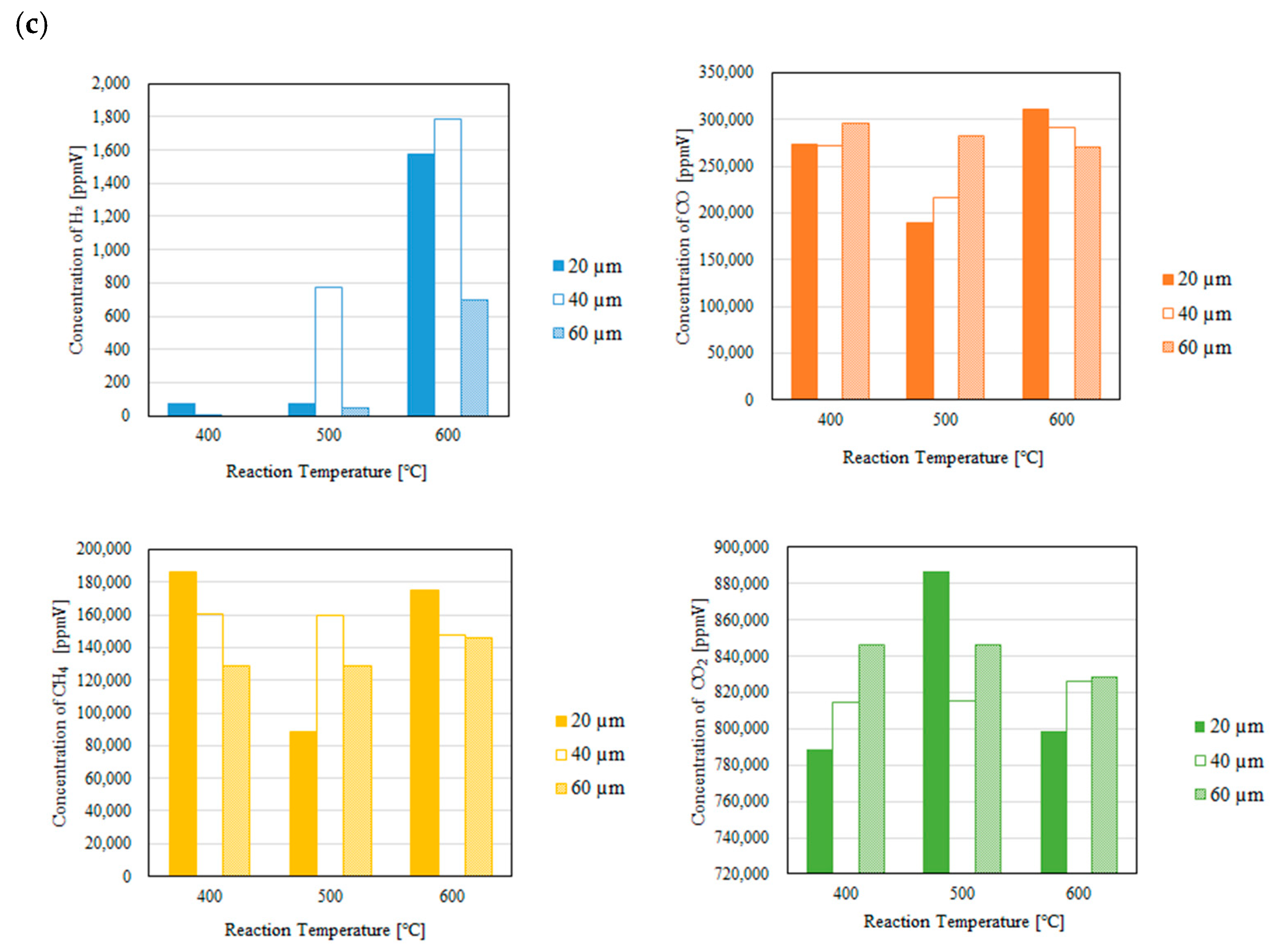
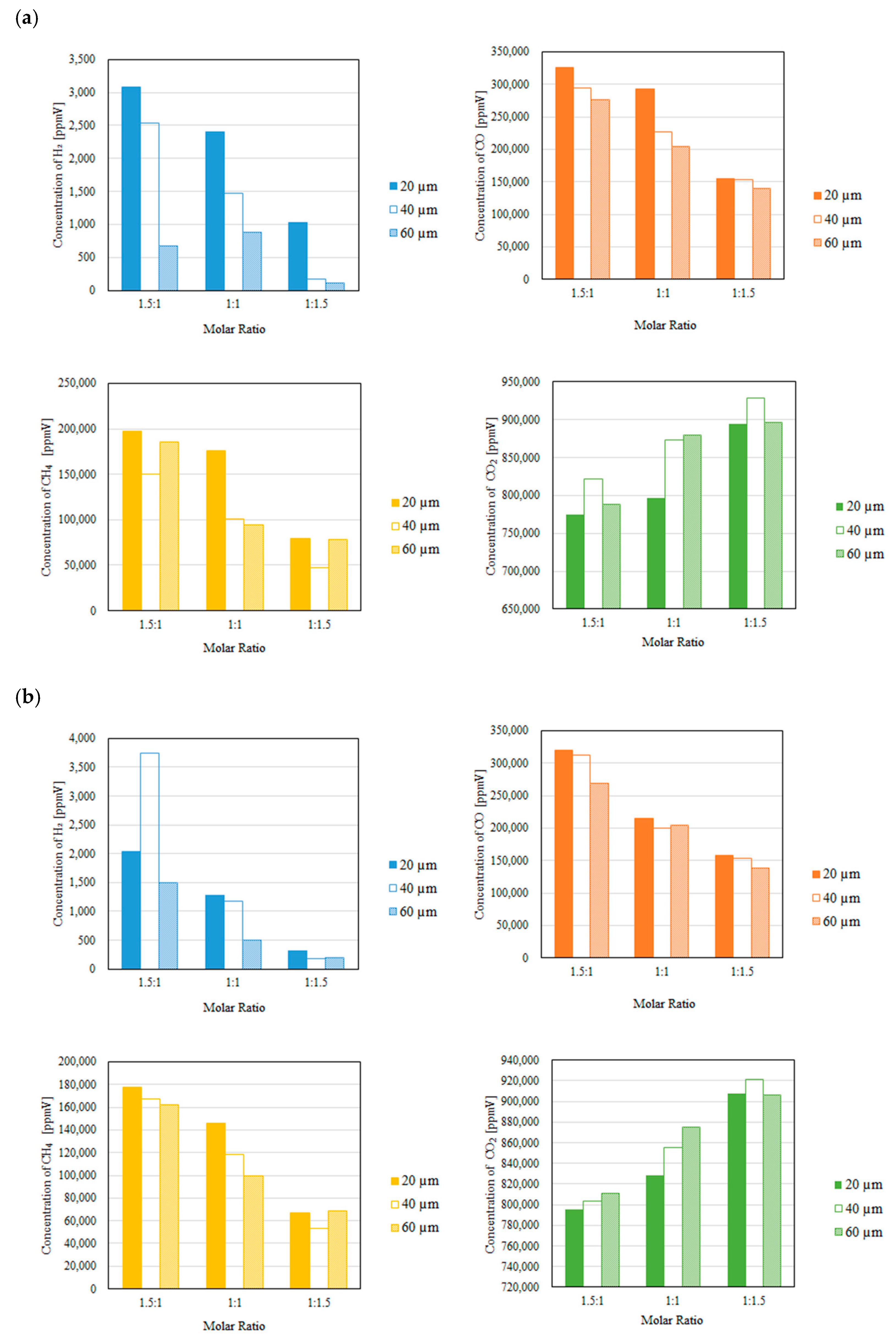
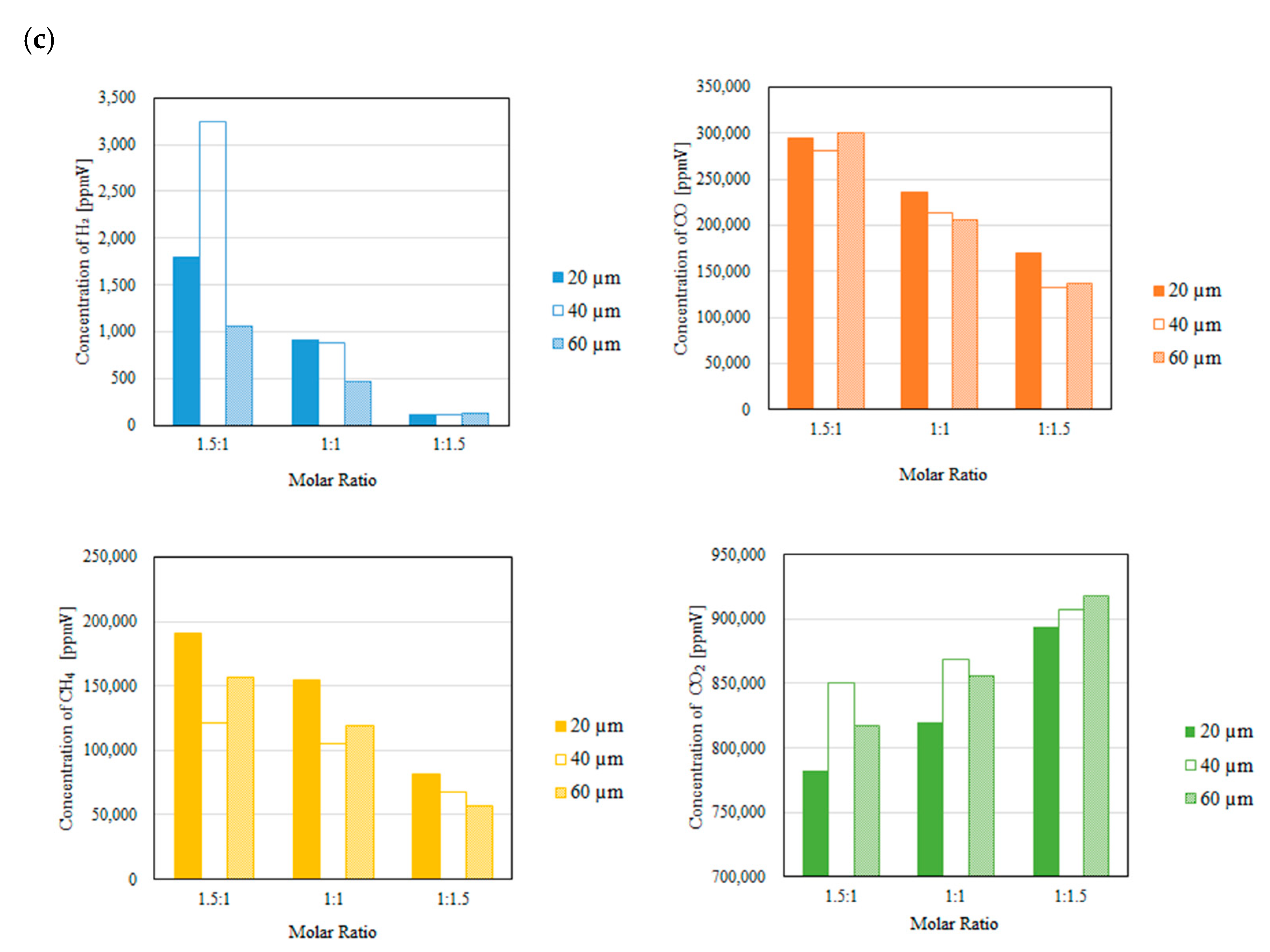
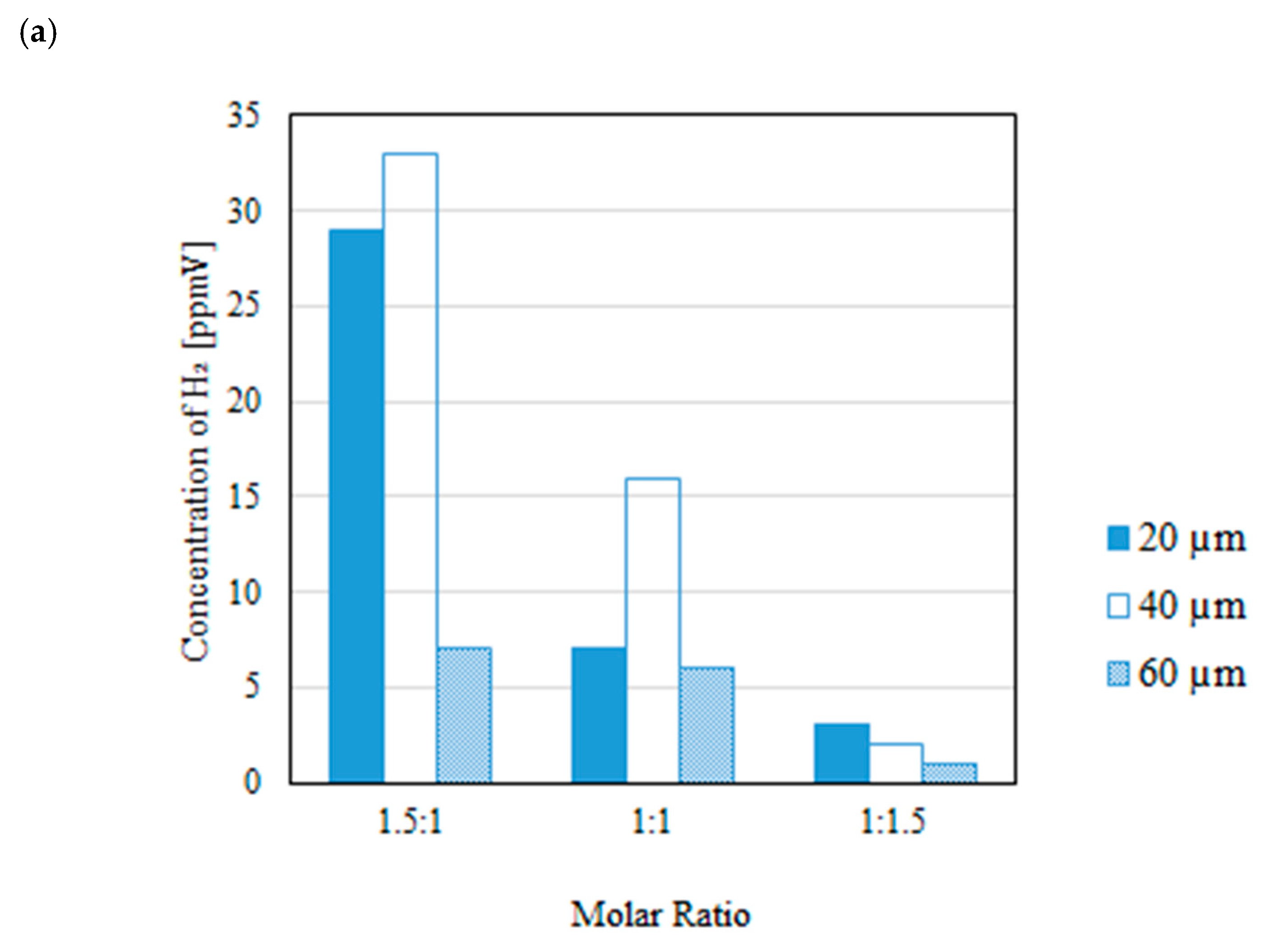
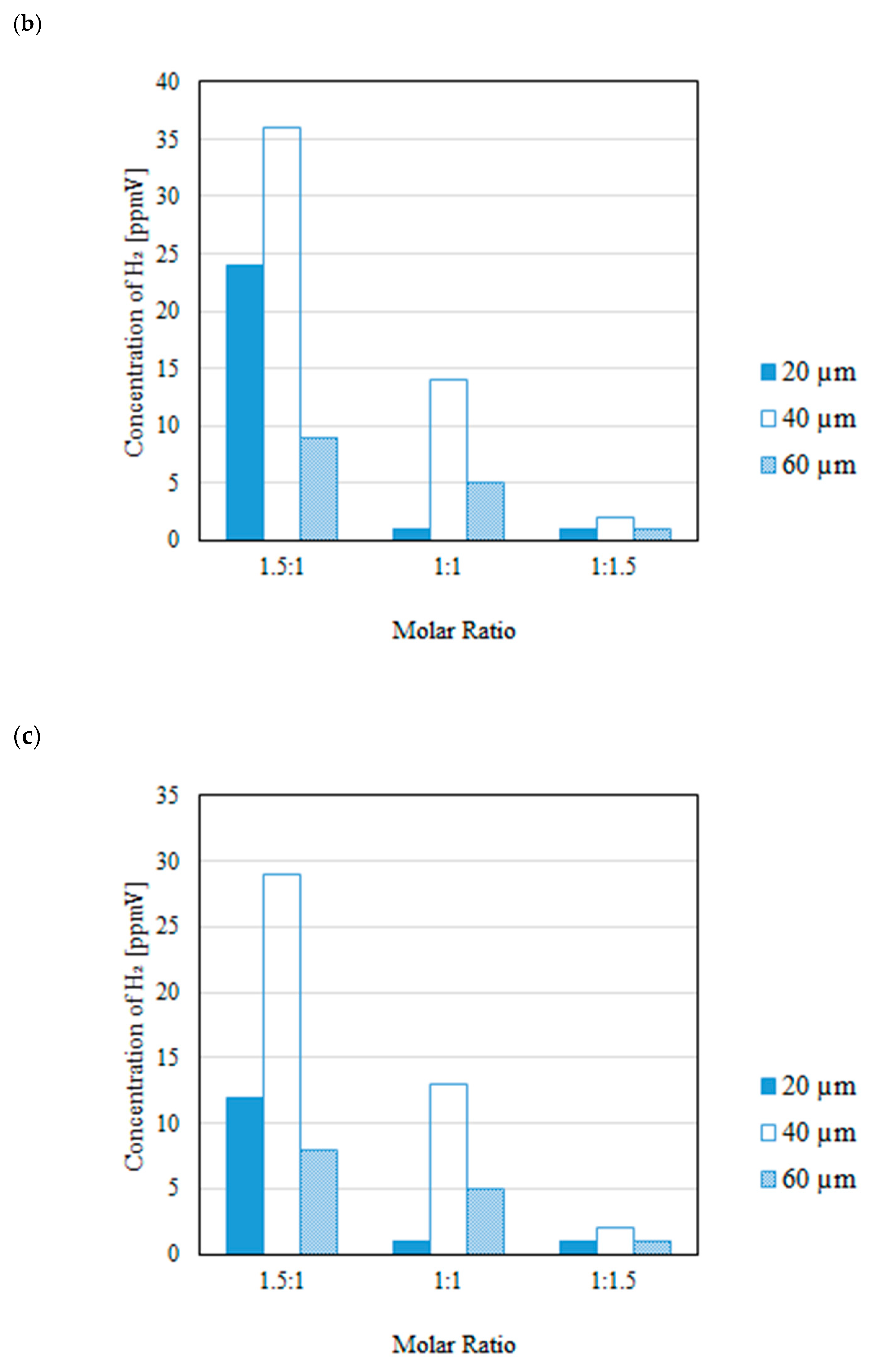
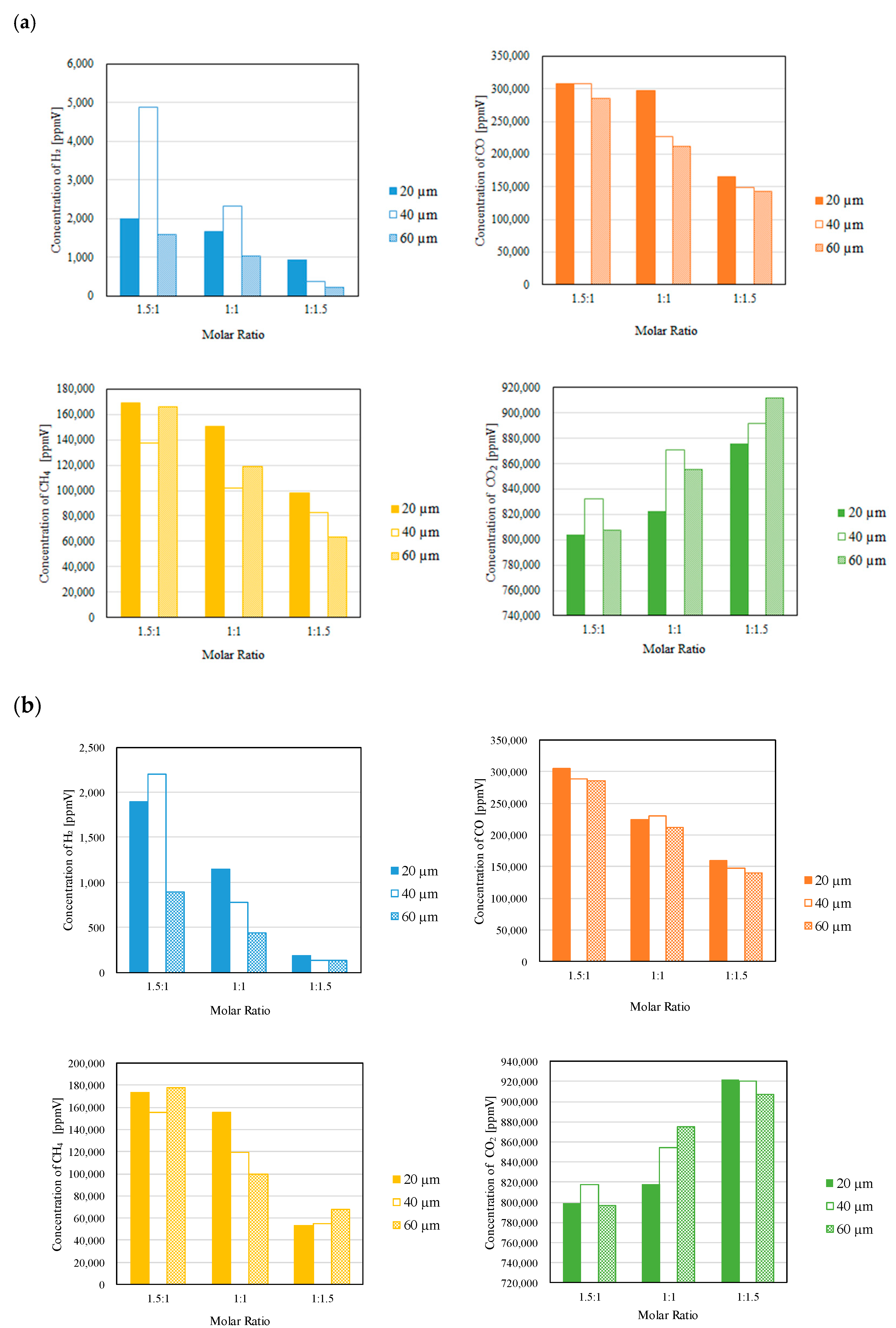
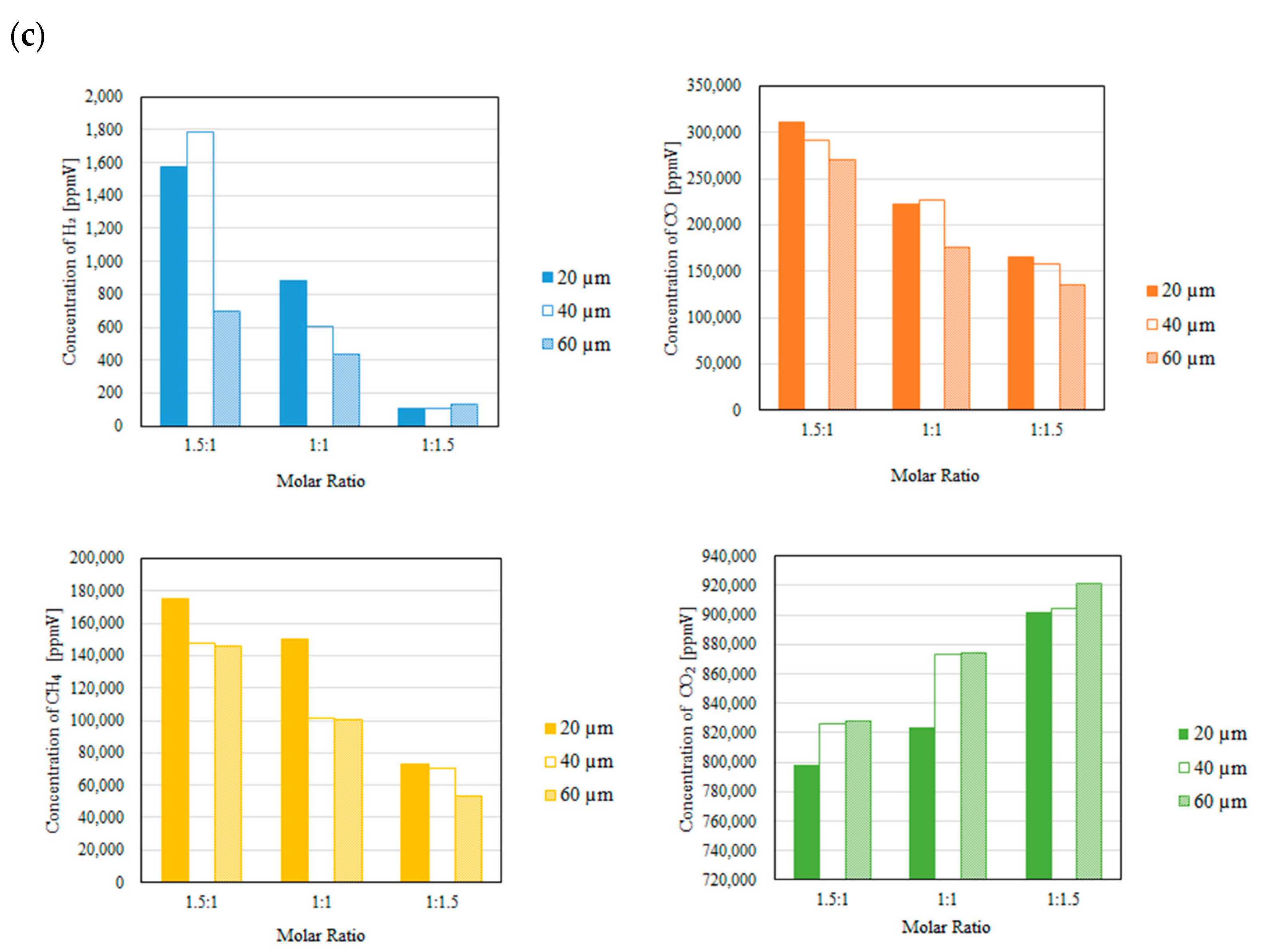
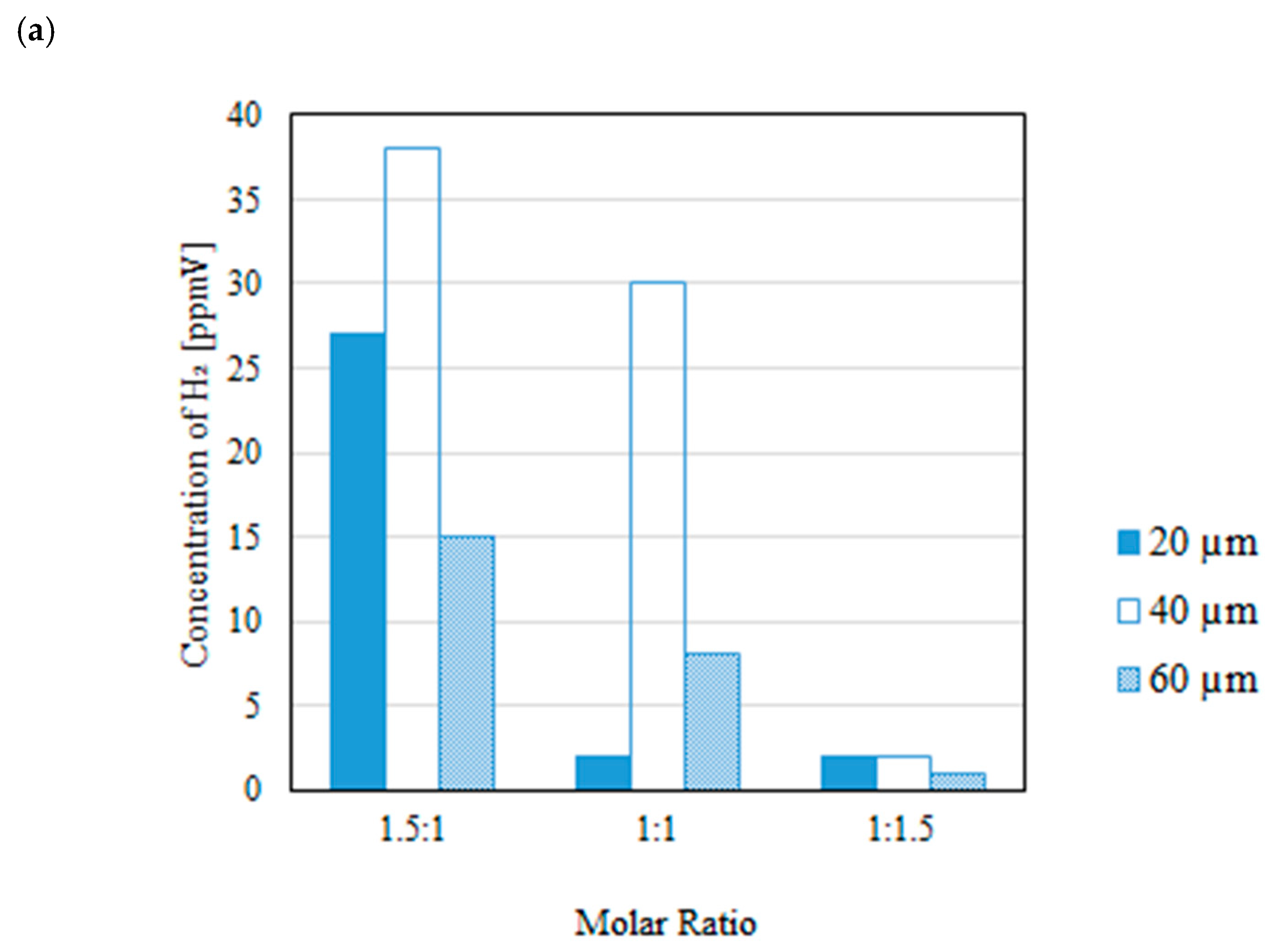
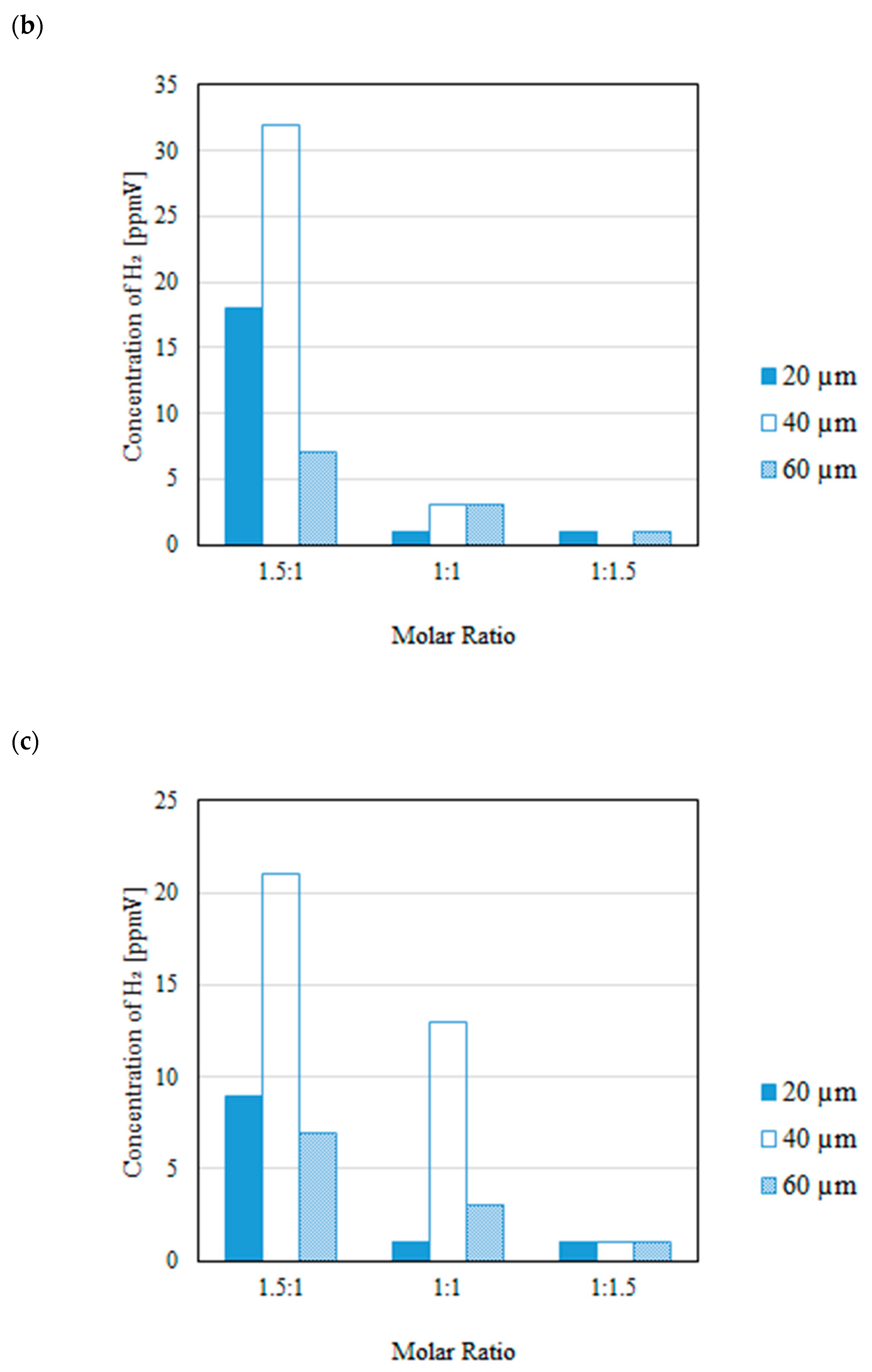
3.2. Impact of Thickness of Pd/Cu Membrane on Each Gas Concentration in the Reaction Chamber and the Concentration of H2 in the Sweep Chamber When Changing the Molar Ratio and the Differential Pressure Between the Reaction Chamber and the Sweep Chamber with and Without a Sweep Gas
- (i)
- H2 is produced by Equations (1) and (5).
- (ii)
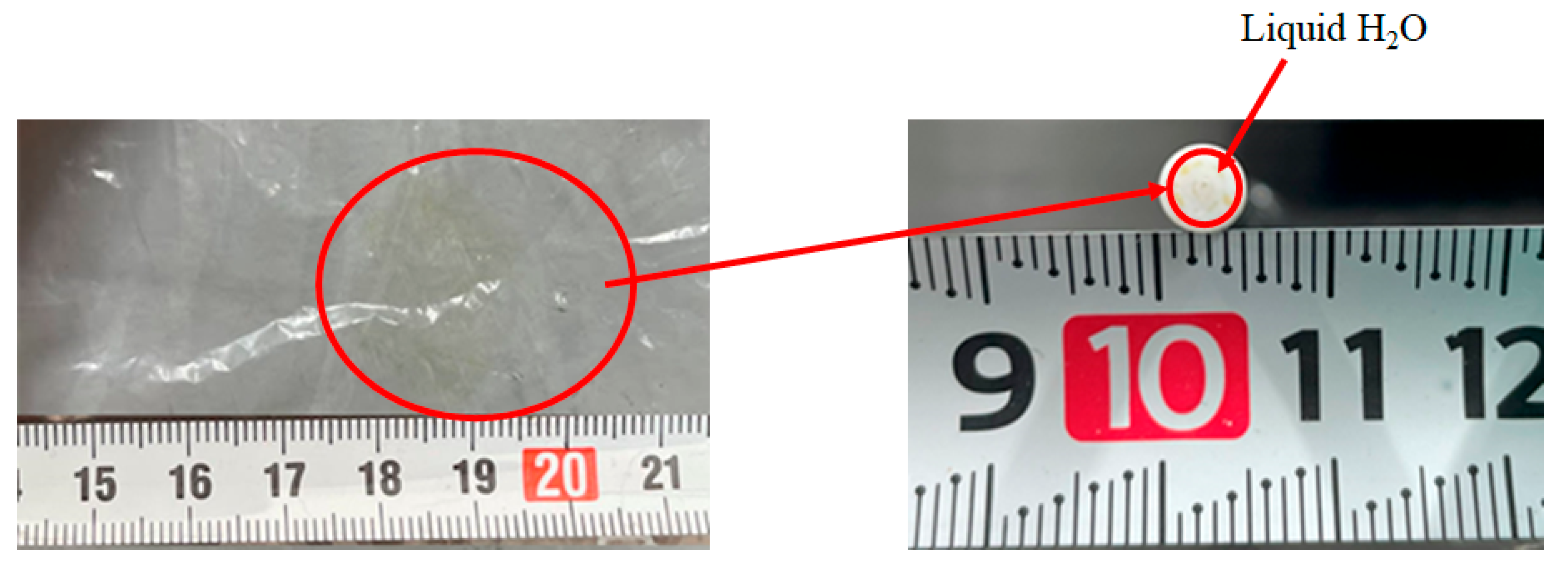
- The produced H2 is consumed by Equations (2) and (3), resulting that CO, CH4 and H2O are produced.
- (iii)
- The produced CO is consumed by Equations (6) and (8), resulting that C, CO2 and H2O are produced.
3.3. Comparison of Assessment Factors Among the Investigated Experimental Conditions
4. Conclusions
- (i)
- It is revealed that the concentration of H2 in the reaction chamber increases with the increase in the reaction temperature irrespective of the thickness of Pd/Cu membrane, the differential pressure between the reaction chamber and the sweep chamber and existing a sweep gas or not. The reaction progresses well with the increase in the reaction temperature since DR, RWGS and SR are endothermic reactions.
- (ii)
- It is revealed that the concentration in the sweep chamber increases with the increase in the reaction temperature irrespective of the thickness of Pd/Cu membrane, the differential pressure between the reaction chamber and the sweep chamber and existing a sweep gas or not. When the concentration of H2 in the reaction chamber is higher at higher reaction temperature, the driving force to penetrate through the Pd/Cu membrane is higher due to the high H2 partial differential pressure between the reaction chamber and the sweep chamber.
- (iii)
- It is revealed that the highest concentration of H2 in the reaction chamber as well as that in the sweep chamber are obtained for the molar ratio of CH4:CO2 = 1.5:1 among the investigated molar ratios irrespective of the thickness of Pd/Cu membrane, the differential pressure between the reaction chamber and the sweep chamber as well as existing a sweep gas or not.
- (iv)
- The highest concentration of H2 is obtained for the thickness of 40 μm mainly among the investigated conditions, i.e. the reaction temperature, the molar ratio of CH4:CO2, the differential pressure between the reaction chamber and the sweep chamber, and existing a sweep gas or not. This study claims that the optimum thickness is 40 μm.
- (v)
- It is clarified that the highest concentration of H2 is obtained for the thickness of 40 μm, the molar ratio of CH4:CO2 = 1.5:1 and the differential pressure between the reaction chamber and the sweep chamber of 0 MPa without a sweep gas, which is 4890 ppmV in the reaction chamber and 38 ppmV in the sweep chamber, respectively. Under this condition, CH4 conversion, H2 yield and thermal efficiency are 75.0 %, 0.214 % and 2.92 %, respectively.
- (vi)
- In the near future, the following subjects can be considered: to optimize the catalyst shape and composition (the pore size and the weight ratio of Ni, Cr and Ru); to optimize the thickness and the composition of Pd/Cu membrane; to match the H2 separation rate of Pd/Cu membrane and the H2 production rate of Ni/Cr/Ru catalyst, deciding the optimum operation condition.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kalai, D.Y.; Stangeland, K.; Jin, R.; Tucho, W.M.; Yu, Z. Biogas dry reforming for syngas production on La promoted hydrotalcitederived Ni Catalyst. Int. J. Hydrogen Energy 2018, 43, 19438–19450. [Google Scholar] [CrossRef]
- World Bioenergy Association. Available online: https://worldbioenergy.org/global-bioenergy-statistics (accessed on 7 November 2024).
- The Japan Gas Association. Available online: https://www.gas.or.jp/gas-life/biogas/ (accessed on 7 November 2024).
- Nishimura, A.; Takada, T.; Ohata, S.; Kolhe, M.L. Biogas dry reforming for hydrogen through membrane reactor utilizing negative pressure. Fuels 2021, 2, 194–209. [Google Scholar] [CrossRef]
- Nishimura, A.; Hayashi, Y.; Ito, S.; Kolhe, M.L. Performance analysis of hydrogen production for a solid oxide fuel cell system using a biogas dry reforming membrane reactor with Ni and Ni/Cr catalysts. Fuels 2023, 4, 295–313. [Google Scholar] [CrossRef]
- Nishimura, A.; Sato, R.; Hu, E. An energy production system powered by solar heat with biogas dry reforming reactor and solar heat with biogas dry reforming reactor and solid oxide fuel cell. Smart Grid and Renew. Energy 2023, 14, 85–106. [Google Scholar] [CrossRef]
- Yoo, E.; Choi, D.S.; Kim, J.; Kim, Y.H.; Kim, N.Y.; Joo, B. Effects of operating parameters and feed gas compositions on the dry reforming of methane over the Ni/Al2O3 catalyst. catalysts 2023, 13. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Deo, G. Process and catalyst improvements for the dry reforming of methane. Chemical Engineering and Science 2023, 276. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Bion, N.; Toniolo, F.S.; Noronha, B. Dry reforming of methane over embedded Ni nanoparticles in CeZrO2: effect of Ce/Zr ratio and H2O addition. International Journal of Hydrogen Energy 2024, 71, 1151–1163. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Ju, T.; Hao, S. Influence of H2S and NH3 on biogas dry reforming using Ni catalyst: a study on single and synergetic effect. Front. Environ. Sci. Eng. 2023, 17. [Google Scholar] [CrossRef]
- Kiani, P.; Meshksar, M.; Rahimpour, M.R. Biogas reforming over La-promoted Ni/SBA-16 catalyst for syngas production: catalytic structure and process activity investigation. International Journal of Hydrogen Energy. 2023, 48, 6262–6274. [Google Scholar] [CrossRef]
- Miao, C.; Chen, S.; Shang, K.; Liang, L.; Ouyang, J. Highly active Ni-Ru bimetallic catalyst integrated with MFI zeolite loaded cerium zirconium oxide for dry reforming of methane. ACS Applied Materials & Interfaces. 2022, 14, 47616–47632. [Google Scholar]
- Fontana, A.D.; Faroldi, B.; Cornaglia, L.M.; Tarditi, A.M. Development of catalytic membranes over PdAu selective films for hydrogen production through the dry reforming of methane. Molecular Catalysis. 2020, 481. [Google Scholar] [CrossRef]
- Andraos, S.; Abbas-Ghaleb, R.; Chlala, D.; Vita, A.; Italiano, C.; Lagana, M.; Pino, L.; Nakhl, M.; Specchia, S. Production of hydrogen by methane dry reforming over ruthenium-nickel based catalysts deposited on Al2O3, MgAl2O4, and YSZ. International Journal of Hydrogen Energy. 2019, 44, 25706–25716. [Google Scholar] [CrossRef]
- Nishimura, A.; Ichikawa, M.; Yamada, S.; Ichii, R. The characteristics of a Ni/Cr/Ru catalyst for a biogas dry reforming membrane reactor using a Pd/Cu membrane and a comparison of it with a Ni/Cr catalyst. hydrogen. 2024, 5, 414–435. [Google Scholar] [CrossRef]
- Nishimura, A.; Ohata, S.; Okukura, K.; Hu, E. The impact of operating conditions on the performance of CH4 dry reforming membrane reactor for H2 production. Journal of Energy and Power Technology. 2020, 2. [Google Scholar] [CrossRef]
- Cherbanski, R.; Kotkowski, T.; Molga, E. Thermogravimetiric analysis of coking during dry reforming methane. International Journal of Hydrogen Energy. 2023, 48, 7346–7360. [Google Scholar] [CrossRef]

| Parameters | Values |
| Initial reaction temperature [°C] | 400, 500, 600 |
| Pressure of supply gas [MPa] | 0.10 |
| Differential pressure between the reaction chamber and the sweep chamber [MPa] |
0, 0.010 and 0.020 |
| Molar ratio of provided CH4:CO2 (flow rate of provided CH4:CO2 [NL/min]) |
1.5:1, 1:1 and 1:1.5 (1.088:0.725, 0.725:0.725, 0.725:1.088) |
| Feed ratio of sweep gas to supply gas [-] | 0 (W/O), 1.0 (W) |
| Thickness of Pd/Cu membrane [μm] |
Sweep gas | CH4 conversion [%] |
CO2 conversion [%] |
H2 yield [%] |
H2 selectivity [%] |
CO selectivity [%] |
H2 recovery [%] |
Permeation flux [mol/(m2 ⋅ s)] | Thermal efficiency [%] |
| (a) | |||||||||
| 20 | W/O | 67.1 | -93.6 | 0.259 | 0.933 | 99.1 | 0.933 | 0 | 3.55 |
| W | 71.8 | -101 | 0.167 | 0.649 | 99.4 | 1.34 | 0 | 1.46 | |
| 40 | W/O | 75.0 | -106 | 0.214 | 0.766 | 99.2 | 1.29 | 0 | 2.92 |
| W | 77.1 | -108 | 0.411 | 1.57 | 98.4 | 0.771 | 0 | 3.59 | |
| 60 | W/O | 69.1 | -97.2 | 5.72×10-2 | 0.238 | 99.8 | 1.02 | 0 | 0.783 |
| W | 72.3 | -102 | 0.132 | 0.552 | 99.4 | 0.944 | 0 | 1.16 | |
| (b) | |||||||||
| 20 | W/O | 64.9 | -59.4 | 0.241 | 0.812 | 99.2 | 0.290 | 0 | 2.76 |
| W | 69.8 | -64.5 | 0.167 | 0.557 | 99.4 | 0.120 | 0 | 1.22 | |
| 40 | W/O | 79.9 | -74.6 | 0.148 | 0.585 | 99.4 | 1.08 | 0 | 1.69 |
| W | 79.7 | -74.2 | 0.236 | 1.030 | 99.0 | 1.27 | 0 | 1.71 | |
| 60 | W/O | 81.2 | -76.0 | 8.80×10-2 | 0.425 | 99.6 | 0.682 | 0 | 1.00 |
| W | 76.2 | -71.0 | 0.103 | 0.483 | 99.5 | 0.776 | 0 | 0.750 | |
| (c) | |||||||||
| 20 | W/O | 80.0 | -49.0 | 0.129 | 0.660 | 99.3 | 0.291 | 0 | 1.18 |
| W | 75.5 | -46.0 | 0.116 | 0.561 | 99.4 | 0.216 | 0 | 0.677 | |
| 40 | W/O | 88.2 | -54.6 | 2.21×10-2 | 0.102 | 99.9 | 1.13 | 0 | 0.200 |
| W | 79.3 | -48.6 | 4.49×10-2 | 0.239 | 99.8 | 0.557 | 0 | 0.261 | |
| 60 | W/O | 80.4 | -49.4 | 1.39×10-2 | 7.41×10-2 | 99.9 | 0.900 | 0 | 0.126 |
| W | 84.2 | -51.9 | 2.71×10-2 | 0.151 | 99.8 | 0.462 | 0 | 0.158 | |
| Thickness of Pd/Cu membrane [μm] |
Sweep gas | CH4 conversion [%] |
CO2 conversion [%] |
H2 yield [%] |
H2 selectivity [%] |
CO selectivity [%] |
H2 recovery [%] |
Permeation flux [mol/(m2 ⋅ s)] | Thermal efficiency [%] |
| (a) | |||||||||
| 20 | W/O | 70.4 | -98.9 | 0.171 | 0.638 | 99.4 | 1.17 | 5.00×10-4 | 2.34 |
| W | 71.0 | -99.8 | 0.160 | 0.624 | 99.4 | 0.938 | 5.00×10-4 | 1.40 | |
| 40 | W/O | 72.1 | -101 | 0.315 | 1.16 | 98.8 | 0.953 | 2.50×10-4 | 4.31 |
| W | 74.2 | -104 | 0.186 | 0.751 | 99.2 | 1.43 | 2.50×10-4 | 1.62 | |
| 60 | W/O | 72.9 | -103 | 0.126 | 0.549 | 99.5 | 0.595 | 1.67×10-4 | 1.73 |
| W | 70.5 | -99.2 | 7.47×10-2 | 0.311 | 99.7 | 0.781 | 1.67×10-4 | 0.653 | |
| (b) | |||||||||
| 20 | W/O | 70.8 | -65.6 | 0.128 | 0.595 | 99.4 | 7.80×10-2 | 5.00×10-4 | 1.47 |
| W | 68.9 | -63.7 | 0.115 | 0.509 | 99.5 | 8.70×10-2 | 5.00×10-4 | 0.841 | |
| 40 | W/O | 76.3 | -71.1 | 0.118 | 0.570 | 99.4 | 1.19 | 2.50×10-4 | 1.34 |
| W | 76.1 | -70.9 | 7.82×10-2 | 0.330 | 99.7 | 0.384 | 2.50×10-4 | 0.571 | |
| 60 | W/O | 80.1 | -75.0 | 4.97×10-2 | 0.240 | 99.8 | 1.01 | 1.67×10-4 | 0.565 |
| W | 80.1 | -75.0 | 4.40×10-2 | 0.200 | 99.8 | 0.682 | 1.67×10-4 | 0.320 | |
| (c) | |||||||||
| 20 | W/O | 83.2 | -51.2 | 4.01×10-2 | 0.202 | 99.8 | 0.312 | 5.00×10-4 | 0.366 |
| W | 86.7 | -53.6 | 2.34×10-2 | 0.117 | 99.9 | 0.535 | 5.00×10-4 | 0.136 | |
| 40 | W/O | 86.6 | -53.5 | 2.32×10-2 | 0.115 | 99.9 | 1.08 | 2.50×10-4 | 0.210 |
| W | 86.3 | -53.4 | 1.59×10-2 | 8.36×10-2 | 99.9 | 0 | 2.50×10-4 | 9.32×10-2 | |
| 60 | W/O | 82.9 | -51.1 | 2.47×10-2 | 0.140 | 99.9 | 0.506 | 1.67×10-4 | 0.225 |
| W | 83.0 | -51.2 | 1.68×10-2 | 9.51×10-2 | 99.9 | 0.743 | 1.67×10-4 | 9.77×10-2 | |
| Thickness of Pd/Cu membrane [μm] |
Sweep gas | CH4 conversion [%] |
CO2 conversion [%] |
H2 yield [%] |
H2 selectivity [%] |
CO selectivity [%] |
H2 recovery [%] |
Permeation flux [mol/(m2 ⋅ s)] | Thermal efficiency [%] |
| (a) | |||||||||
| 20 | W/O | 68.2 | -95.6 | 0.151 | 0.611 | 99.4 | 0.661 | 7.07×10-4 | 2.08 |
| W | 70.8 | -99.6 | 0.132 | 0.508 | 99.5 | 0.567 | 7.07×10-4 | 1.16 | |
| 40 | W/O | 79.8 | -113 | 0.273 | 1.12 | 98.9 | 0.885 | 3.54×10-4 | 3.74 |
| W | 75.4 | -106 | 0.151 | 0.601 | 99.4 | 1.16 | 3.54×10-4 | 1.31 | |
| 60 | W/O | 73.9 | -104 | 8.90×10-2 | 0.349 | 99.7 | 0.749 | 2.36×10-4 | 1.22 |
| W | 75.6 | -107 | 5.85×10-2 | 0.257 | 99.7 | 0.997 | 2.36×10-4 | 0.510 | |
| (b) | |||||||||
| 20 | W/O | 69.1 | -63.9 | 9.12×10-2 | 0.384 | 99.6 | 0.110 | 7.07×10-4 | 1.05 |
| W | 69.9 | -64.7 | 8.87×10-2 | 0.396 | 99.6 | 0.113 | 7.07×10-4 | 0.649 | |
| 40 | W/O | 78.9 | -73.8 | 8.91×10-2 | 0.405 | 99.6 | 1.46 | 3.54×10-4 | 1.01 |
| W | 79.8 | -74.6 | 6.17×10-2 | 0.265 | 99.7 | 2.11 | 3.54×10-4 | 0.442 | |
| 60 | W/O | 76.2 | -71.1 | 4.75×10-2 | 0.228 | 99.8 | 1.05 | 2.36×10-4 | 0.539 |
| W | 80.0 | -74.9 | 4.40×10-2 | 0.248 | 99.8 | 0.682 | 2.36×10-4 | 0.320 | |
| (c) | |||||||||
| 20 | W/O | 79.7 | -48.9 | 1.41×10-2 | 6.62×10-2 | 99.9 | 0.885 | 7.07×10-4 | 0.128 |
| W | 81.8 | -50.4 | 1.35×10-2 | 6.51×10-2 | 99.9 | 0.926 | 7.07×10-4 | 7.82×10-2 | |
| 40 | W/O | 83.0 | -51.2 | 1.50×10-2 | 8.68×10-2 | 99.9 | 1.67 | 3.54×10-4 | 0.135 |
| W | 82.5 | -50.8 | 1.35×10-2 | 6.64×10-2 | 99.9 | 0.923 | 3.54×10-4 | 7.85×10-2 | |
| 60 | W/O | 85.8 | -53.0 | 1.67×10-2 | 9.63×10-2 | 99.9 | 0.748 | 2.36×10-4 | 0.152 |
| W | 86.6 | -53.6 | 1.64×10-2 | 9.60×10-2 | 99.9 | 0.762 | 2.36×10-4 | 9.52×10-2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





