Submitted:
02 December 2024
Posted:
03 December 2024
You are already at the latest version
Abstract
Our study reports for the first time, over a 12-month period, the seasonal variations of chemical composition, antibacterial and antioxidant activity of Rosmarinus officinalis L. essential oil (RoEO) from southwestern Romania (Oltenia Region). To analyze the constituents of RoEO, a comprehensive gas chromatography/mass spectrometry (GC/MS) method was employed. The analysis aimed to identify and quantify the various components by comparing their mass spectra with reference spectra from the National Institute of Standards and Technology (NIST) Library 2020. Staphylococcus aureus minimum inhibitory concentration (MIC) values were determined using the microdilution method (96-well plates). The antioxidant activity was analyzed using 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and hydrogen peroxide (H2O2) radical scavenging assays. This analysis provided a detailed profile of the RoEO’s constituents, revealing significant monthly variations. Key compounds, such as camphor, eucalyptol, α-pinene, camphene and α-myrcene, were quantified, alongside lesser-studied constituents like β-pinene, α-terpinene, linalool, terpinolene, and carvacrol. Comparisons were made with a reference sample from Tunisia. Correlations between specific compounds and their bioactivity were explored to understand their contributions to the overall efficacy of the RoEO. This comprehensive analysis provides valuable insights into the potential applications and seasonal variability of RoEO from Romania.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material and Essential Oil Extraction
2.2. GC/MS Analysis
2.3. Assessment of the Antibacterial Activity
2.4. DPPH Free Radical Scavenging Assay
2.5. ABTS Radical Scavenging Assay
2.6. H2O2 Radical Scavenging Assay
2.7. Statistical Analysis
3. Results
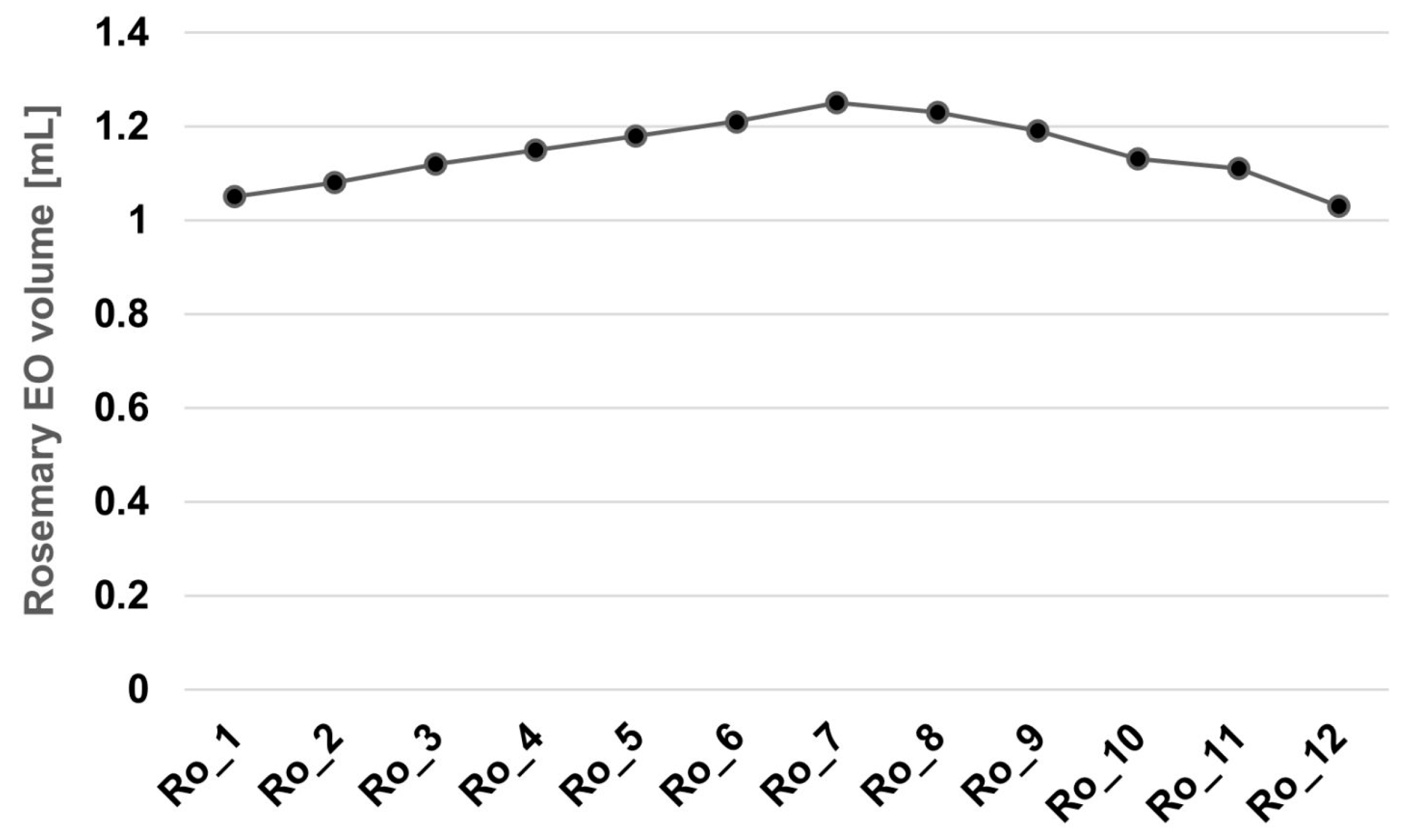
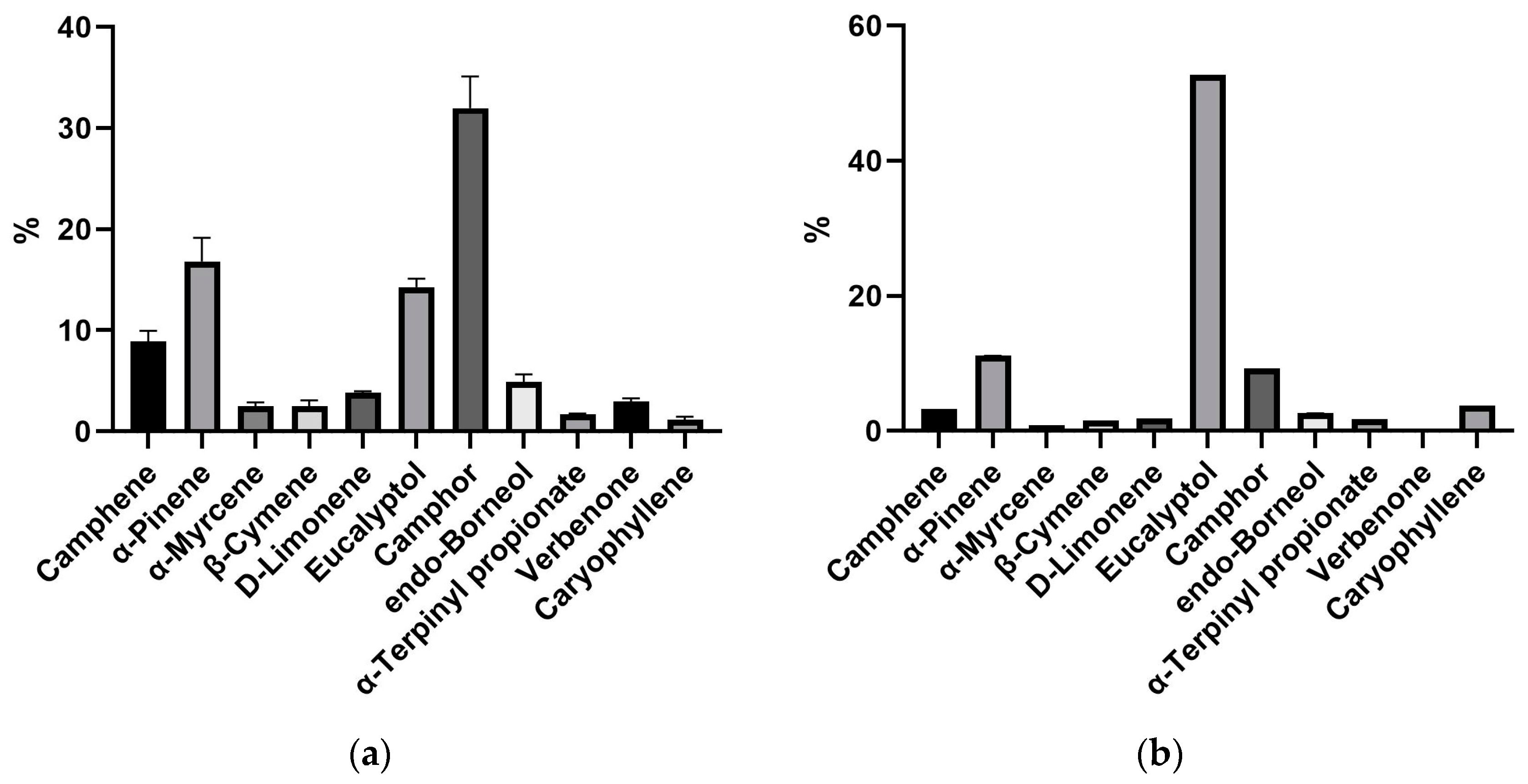
3.1. Extraction Yield and Chemical Profile of R. officinalis Essential Oil
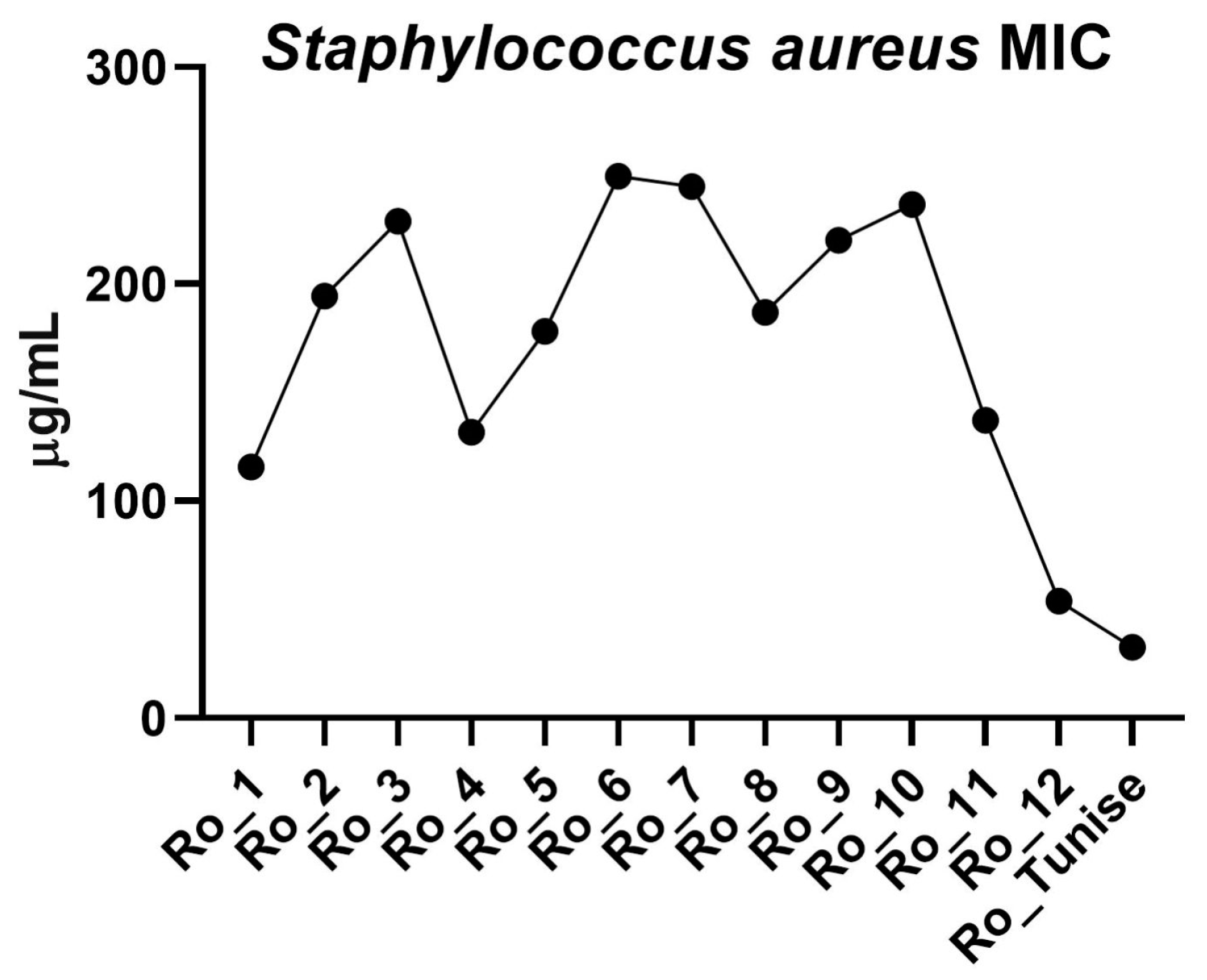
3.2. Antibacterial Activity
3.3. Antioxidant Activity
4. Discussion
4.1. Chemical Profile of R. officinalis Essential Oil
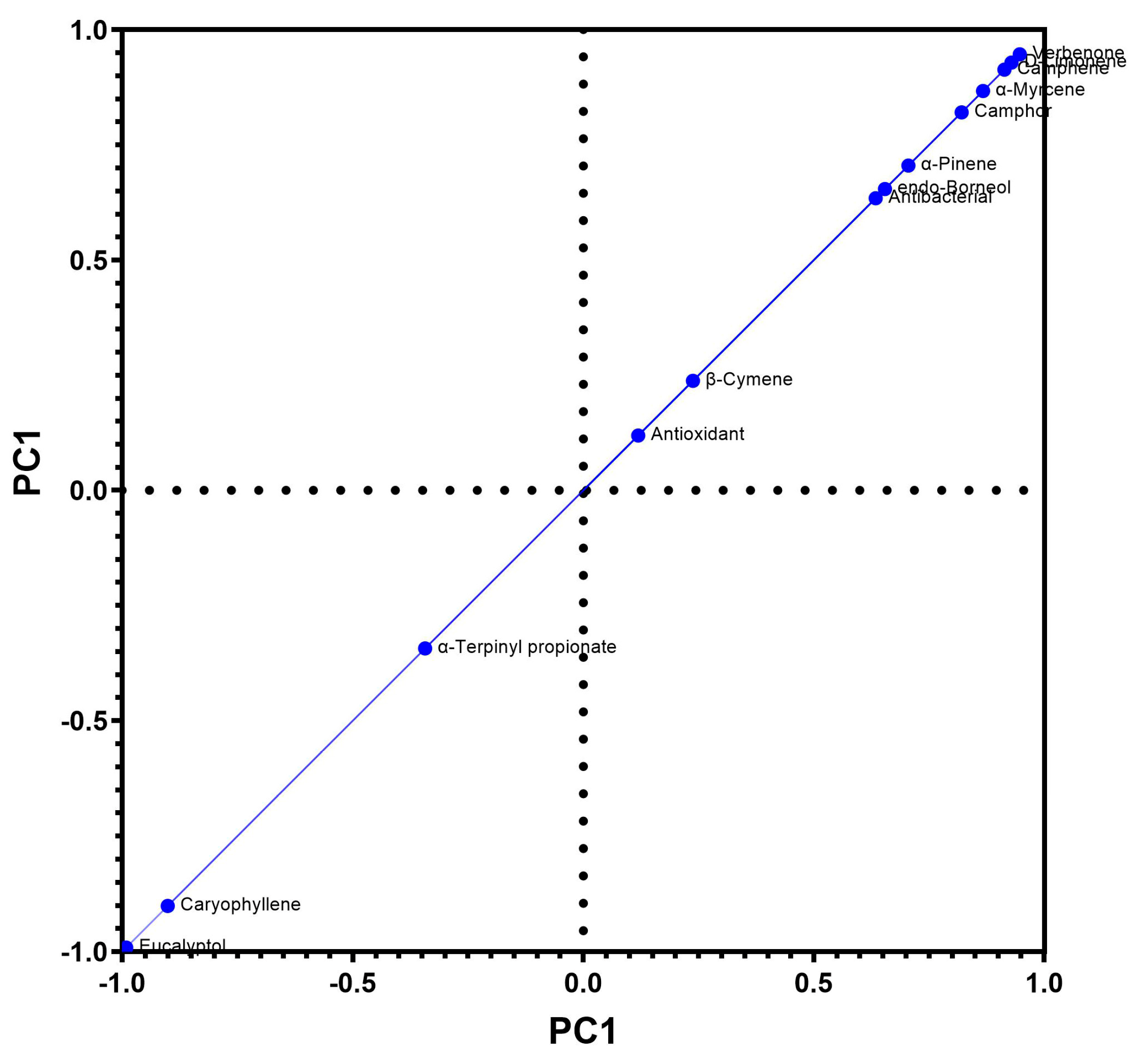
4.2. Antibacterial–Antioxidant Activity Correlation
4.3. Importance of Studying the Dynamics Over a Year
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, K.; Tamta, B.; Mukopadayay, S. A review on “pharmacological, phytochemical, and medicinal properties of Rosmarinus officinalis (Rosemary)”. Int. J. Health Sci. 2022, 6, 3491–3500. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds). Flora Europaea. Vol. 3: Diapensiaceae to Myoporaceae, 1st ed.; Cambridge University Press: Cambridge, UK, 1972; p. 187. [Google Scholar]
- Săvulescu, T. (Ed). Flora R.P.R., 1st ed.; Romanian Academy Publishing House: Bucharest, Romania, 1961; Volume VIII. (in Romanian) [Google Scholar]
- Popescu, G.; Iancu, T.; Popescu, C.A.; Stanciu, S.M.; Luca, R.; Imbrea, F.; Radulov, I.; Sala, F.; Moatăr, M.M.; Camen, D.D. The influence of soil fertilization on the quality and extraction efficiency of rosemary essential oil (Rosmarinus officinalis L.). Rom. Biotechnol. Lett. 2020, 25, 1961–1968. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.; Sandhya, S.; Syed Shaffath, A.; Vinod, K.R.; Swapna, R.; Banji, D. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. [Google Scholar]
- Borges, R.S.; Sánchez Ortiz, B.L.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Mersin, B.; Işcan, G.S. Rosmarinus officinalis L. In Novel Drug Targets with Traditional Herbal Medicines: Scientific and Clinical Evidence, 1st edition; Gürağaç Dereli, F.T., Ilhan, M., Belwal, T., Eds.; Springer: Cham, Switzerland, 2022; pp. 525–541. [Google Scholar] [CrossRef]
- Scheckel, K.A.; Degner, S.C.; Romagnolo, D.F. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J. Nutr. 2008, 138, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Karthik, D.; Viswanathan, P.; Anuradha, C.V. Administration of Rosmarinic Acid Reduces Cardiopathology and Blood Pressure Through Inhibition of p22phox NADPH Oxidase in Fructose-Fed Hypertensive Rats. J. Cardiovasc. Pharmacol. 2011, 58, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Kayashima, T.; Matsubara, K. Antiangiogenic Effect of Carnosic Acid and Carnosol, Neuroprotective Compounds in Rosemary Leaves. Biosci. Biotechnol. Biochem. 2012, 76, 115–119. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Flauzino, L.G.B.; Souza, M.G.M.; Turatti, I.C.C.; Andrade e Silva, M.L.; Martins, C.H.G.; da Silva Filho, A.A.; Cunha, W.R. Antibacterial Activity of the Essential Oil from Rosmarinus officinalis and its Major Components Against Oral Pathogens. Z. Naturforsch. C J. Biosci. 2010, 65, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, R.; Afzali, Z.; Afzali, D. Chemical Composition of Hydrodistillation Essential Oil of Rosemary in Different Origins in Iran and Comparison with Other Countries. Am.-Euras. J. Agric. Environ. Sci. 2009, 5, 78–81. [Google Scholar]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, I.A.; Osman, N.M. New Chemotype Rosmarinus officinalis L. (Rosemary) “R. officinalis ct. bornyl acetate”. Am. J. Res. Commun. 2014, 2, 232–240. [Google Scholar]
- Teja, Y.S.C.; Jyothika, L.S.; Vasavi, N.; Reddy, K.S.S.; Sudheer, A. Exploring the Therapeutic Potential of Rosemary: An In-Depth Review of its Pharmacological Properties. Asian J. Adv. Med. Sci. 2024, 6, 19–31. [Google Scholar]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef]
- Kosmopoulou, D.; Lafara, M.-P.; Adamantidi, T.; Ofrydopoulou, A.; Grabrucker, A.M.; Tsoupras, A. Neuroprotective Benefits of Rosmarinus officinalis and Its Bioactives against Alzheimer’s and Parkinson’s Diseases. Appl. Sci. 2024, 14, 6417. [Google Scholar] [CrossRef]
- Angourani, H.R.; Heydari, M.; Yousefi, A.R.; Pashaei, B.; Mastinu, A. Nanoparticles Based-Plant Protein Containing Rosmarinus officinalis Essential Oil; Fabrication, Characterization, and Evaluation. Appl. Sci. 2022, 12, 9968. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Pawłowska, K.; Janda, K.; Jakubczyk, K. Properties and use of rosemary (Rosmarinus officinalis L.). Pomeranian J. Life Sci. 2020, 66, 76–82. [Google Scholar] [CrossRef]
- Amar, Y.; Meddah, B.; Bonacorsi, I.; Costa, G.; Pezzino, G.; Saija, A.; Cristani, M.; Boussahel, S.; Ferlazzo, G.; Meddah, A.T. Phytochemicals, Antioxidant and Antiproliferative Properties of Rosmarinus officinalis L on U937 and CaCo-2 Cells. Iran J. Pharm. Res. 2017, 16, 315–327. [Google Scholar] [CrossRef]
- Bouammali, H.; Zraibi, L.; Ziani, I.; Merzouki, M.; Bourassi, L.; Fraj, E.; Challioui, A.; Azzaoui, K.; Sabbahi, R.; Hammouti, B.; et al. Rosemary as a Potential Source of Natural Antioxidants and Anticancer Agents: A Molecular Docking Study. Plants 2024, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Bejenaru, L.E.; Biţă, A.; Mogoşanu, G.D.; Segneanu, A.-E.; Radu, A.; Ciocîlteu, M.V.; Bejenaru, C. Polyphenols Investigation and Antioxidant and Anticholinesterase Activities of Rosmarinus officinalis L. Species from Southwest Romania Flora. Molecules 2024, 29, 4438. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Fernandes, D.; Silva, I.; Mateus, V. Potential Anti-Inflammatory Effect of Rosmarinus officinalis in Preclinical In Vivo Models of Inflammation. Molecules 2022, 27, 609. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines 2023, 11, 545. [Google Scholar] [CrossRef]
- Fernández, L.F.; Palomino, O.M.; Frutos, G. Effectiveness of Rosmarinus officinalis essential oil as antihypotensive agent in primary hypotensive patients and its influence on health-related quality of life. J. Ethnopharmacol. 2014, 151, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Jones, T.H.; Lopez, E.M.; McFeeters, R.L.; Ali, N.A.A.; Mansi, I.; Al-kaf, A.G.; Setzer, W.N. Chemotypic Characterization and Biological Activity of Rosmarinus officinalis. Foods 2017, 6, 20. [Google Scholar] [CrossRef]
- Madsen, S.; Bak, S.Y.; Yde, C.C.; Jensen, H.M.; Knudsen, T.A.; Bæch-Laursen, C.; Holst, J.J.; Laustsen, C.; Hedemann, M.S. Unravelling Effects of Rosemary (Rosmarinus officinalis L.) Extract on Hepatic Fat Accumulation and Plasma Lipid Profile in Rats Fed a High-Fat Western-Style Diet. Metabolites 2023, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Kabubii, Z.N.; Mbaria, J.M.; Mathiu, P.M.; Wanjohi, J.M.; Nyaboga, E.N. Diet Supplementation with Rosemary (Rosmarinus officinalis L.) Leaf Powder Exhibits an Antidiabetic Property in Streptozotocin-Induced Diabetic Male Wistar Rats. Diabetology 2024, 5, 12–25. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Vlavcheski, F.; Tsiani, E. Muscle Cell Insulin Resistance Is Attenuated by Rosmarinic Acid: Elucidating the Mechanisms Involved. Int. J. Mol. Sci. 2023, 24, 5094. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Peng, C.H.; Chyau, C.C.; Lin, Y.C.; Wang, H.E.; Peng, R.Y. Low-Density Lipoprotein, Collagen, and Thrombin Models Reveal that Rosmarinus officinalis L. Exhibits Potent Antiglycative Effects. J. Agric. Food Chem. 2007, 55, 2884–2891. [Google Scholar] [CrossRef]
- Shen, Y.; Han, J.; Zheng, X.; Ai, B.; Yang, Y.; Xiao, D.; Zheng, L.; Sheng, Z. Rosemary Leaf Extract Inhibits Glycation, Breast Cancer Proliferation, and Diabetes Risks. Appl. Sci. 2020, 10, 2249. [Google Scholar] [CrossRef]
- Saleh, A.; Al Kamaly, O.; Alanazi, A.S.; Noman, O. Phytochemical Analysis and Antimicrobial Activity of Rosmarinus officinalis L. Growing in Saudi Arabia: Experimental and Computational Approaches. Processes 2022, 10, 2422. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ciccarelli, D.; Giovanelli, S.; Pistelli, L.; Flamini, G.; Cervelli, C.; Mancianti, F.; Nardoni, S.; Bertelloni, F.; Ebani, V.V. Antimicrobial Activity and Composition of Five Rosmarinus (Now Salvia spp. and Varieties) Essential Oils. Antibiotics 2021, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Pyrovolou, K.; Konteles, S.J.; Trapali, M.; Krisilia, S.; Kokla, V.; Apostolaki, A.; Founda, V.; Houhoula, D.; Batrinou, A. Exploring the Antifungal Activity of Various Natural Extracts in a Sustainable Saccharomyces cerevisiae Model Using Cell Viability, Spot Assay, and Turbidometric Microbial Assays. Appl. Sci. 2024, 14, 1899. [Google Scholar] [CrossRef]
- Nolkemper, S.; Reichling, J.; Stintzing, F.C.; Carle, R.; Schnitzler, P. Antiviral Effect of Aqueous Extracts from Species of the Lamiaceae Family Against Herpes simplex Virus Type 1 and Type 2 in vitro. Planta Med. 2006, 72, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ferdousi, F.; Fukumitsu, S.; Kuwata, H.; Isoda, H. Antidepressant- and anxiolytic-like activities of Rosmarinus officinalis extract in rodent models: Involvement of oxytocinergic system. Biomed Pharmacother. 2021, 144, 112291. [Google Scholar] [CrossRef]
- Fonseca, E.C.M.; Ferreira, L.R.; Figueiredo, P.L.B.; Maia, C.d.S.F.; Setzer, W.N.; Da Silva, J.K.R. Antidepressant Effects of Essential Oils: A Review of the Past Decade (2012–2022) and Molecular Docking Study of Their Major Chemical Components. Int. J. Mol. Sci. 2023, 24, 9244. [Google Scholar] [CrossRef]
- Guimarães, N.S.S.; Ramos, V.S.; Prado-Souza, L.F.L.; Lopes, R.M.; Arini, G.S.; Feitosa, L.G.P.; Silva, R.R.; Nantes, I.L.; Damasceno, D.C.; Lopes, N.P.; et al. Rosemary (Rosmarinus officinalis L.) Glycolic Extract Protects Liver Mitochondria from Oxidative Damage and Prevents Acetaminophen-Induced Hepatotoxicity. Antioxidants 2023, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, N.H.M.; Fahmi, A.; Shams-Eldin, E.; Abdur-Rahman, M. Protective effect of rosemary (Rosmarinus officinalis) against diethylnitrosamine-induced renal injury in rats. Biomarkers 2020, 25, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Macarini, A.F.; Mariano, L.N.B.; Zanovello, M.; da Silva, R.d.C.V.; Corrêa, R.; de Souza, P. Protective Role of Rosmarinic Acid in Experimental Urolithiasis: Understanding Its Impact on Renal Parameters. Pharmaceuticals 2024, 17, 702. [Google Scholar] [CrossRef]
- Novi, S.; Vestuto, V.; Campiglia, P.; Tecce, N.; Bertamino, A.; Tecce, M.F. Anti-Angiogenic Effects of Natural Compounds in Diet-Associated Hepatic Inflammation. Nutrients 2023, 15, 2748. [Google Scholar] [CrossRef]
- Yousef, M.; Crozier, R.W.E.; Hicks, N.J.; Watson, C.J.F.; Boyd, T.; Tsiani, E.; MacNeil, A.J. Attenuation of allergen-mediated mast cell activation by rosemary extract (Rosmarinus officinalis L.). J. Leukoc. Biol. 2020, 107, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Rizvi, A.; Aatif, M.; Muteeb, G.; Khan, K.; Siddiqui, F.A. Dietary Polyphenols, Plant Metabolites, and Allergic Disorders: A Comprehensive Review. Pharmaceuticals 2024, 17, 670. [Google Scholar] [CrossRef]
- de Macedo, L.M.; Santos, É.M.d.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Del Baño, M.J.; Castillo, J.; Benavente-García, O.; Lorente, J.; Martín-Gil, R.; Acevedo, C.; Alcaraz, M. Radioprotective-Antimutagenic Effects of Rosemary Phenolics Against Chromosomal Damage Induced in Human Lymphocytes by Gamma-Rays. J. Agric. Food Chem. 2006, 54, 2064–2068. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Gościniak, A.; Formanowicz, D.; Cielecka-Piontek, J. Natural Guardians: Natural Compounds as Radioprotectors in Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 6937. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry, 4th edition; Allured Publishing Corp.: Carol Stream, IL, USA, 2017; pp. 1–788. [Google Scholar]
- Spadi, A.; Angeloni, G.; Guerrini, L.; Corti, F.; Maioli, F.; Calamai, L.; Parenti, A.; Masella, P. A Conventional VOC-PID Sensor for a Rapid Discrimination among Aromatic Plant Varieties: Classification Models Fitted to a Rosemary Case-Study. Appl. Sci. 2022, 12, 6399. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Enig, M.; Brook, I.; Elliott, T.B. Minimum inhibitory concentrations of herbal essential oils and monolaurin for Gram-positive and Gram-negative bacteria. Mol. Cell. Biochem. 2005, 272, 29–34. [Google Scholar] [CrossRef]
- Nefzi, K.; Ben Jemaa, M.; Baraket, M.; Dakhlaoui, S.; Msaada, K.; Nasr, Z. In Vitro Antioxidant, Antibacterial and Mechanisms of Action of Ethanolic Extracts of Five Tunisian Plants against Bacteria. Appl. Sci. 2022, 12, 5038. [Google Scholar] [CrossRef]
- Saha, S.; Bhattacharya, R.; Chaudhary, M.; Hazarika, T.K.; Mitra, A. Impact of geographical locations on essential oil composition and leaf histochemistry in Cinnamomum verum J. S. Presl. J. Essent. Oil Bear. Pl. 2023, 26, 1546–1562. [Google Scholar] [CrossRef]
- Rani, A.; Kanyal, B.; Aabha; Tewari, G.; Pande, C.; Prakash, O. Chemodiversity and antioxidant potential of the essential oil of fresh leaves of Cinnamomum zeylanicum Blume from Uttarakhand. J. Essent. Oil Bear. Pl. 2022, 25, 783–795. [Google Scholar] [CrossRef]
- Jawad, A.M.; Allawi, A.K.; Ewadh, H.M. Essential Oils of Rosemary as Antimicrobial Agent Against Three Types of Bacteria. Med. J. Babylon 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Brandt, C.C.M.; Lobo, V.S.; Fiametti, K.G.; Wancura, J.H.C.; Oro, C.E.D.; Oliveira, J.V. Rosemary essential oil microemulsions as antimicrobial and antioxidant agent in tomato paste. Food Chem. Adv. 2023, 2, 100295. [Google Scholar] [CrossRef]
- Konteles, S.J.; Stavropoulou, N.A.; Thanou, I.V.; Mouka, E.; Kousiaris, V.; Stoforos, G.N.; Gogou, E.; Giannakourou, M.C. Enriching Cured Meat Products with Bioactive Compounds Recovered from Rosa damascena and Rosmarinus officinalis L. Distillation By-Products: The Pursuit of Natural Antimicrobials to Reduce the Use of Nitrites. Appl. Sci. 2023, 13, 13085. [Google Scholar] [CrossRef]
- Oluwakayode, O.O.; Onocha, P.A. Antioxidant and Antimicrobial Activities of β-Caryophyllene Dominated Leaf Essential Oil of Trichilia monadelpha (Thonn.) JJ De Wilde. J. Nat. Sci. Res. 2020, 11, 35–42. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Garry, R.P.; Michet, A.; Benjilali, B.; Chabart, J.L. Essential Oils of Rosemary (Rosmarinus officinalis L.). The Chemical Composition of Oils of Various Origins (Morocco, Spain, France). J. Essent. Oil Res. 1993, 5, 613–618. [Google Scholar] [CrossRef]
- Salido, S.; Altarejos, J.; Nogueras, M.; Saánchez, A.; Luque, P. Chemical Composition and Seasonal Variations of Rosemary Oil from Southern Spain. J. Essent. Oil Res. 2003, 15, 10–14. [Google Scholar] [CrossRef]
- Yeddes, W.; Aidi Wannes, W.; Hammami, M.; Smida, M.; Chebbi, A.; Marzouk, B.; Saidani Tounsi, M. Effect of Environmental Conditions on the Chemical Composition and Antioxidant Activity of Essential Oils from Rosmarinus officinalis L. Growing Wild in Tunisia. J. Essent. Oil. Bear. Pl. 2018, 21, 972–986. [Google Scholar] [CrossRef]
- Lakušić, D.; Ristić, M.; Slavkovska, V.; Lakušić, B. Seasonal Variations in the Composition of the Essential Oils of Rosemary (Rosmarinus officinalis, Lamiaceae). Nat. Prod. Commun. 2013, 8, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Gouja, H.; Hannachi, H.; Isoda, H.; Neffati, M.; Najjaa, H. Seasonal changes in rosemary species: A chemotaxonomic assessment of two varieties based on essential oil compounds, antioxidant and antibacterial activities. PLoS One 2022, 17, e0273367. [Google Scholar] [CrossRef] [PubMed]
- Benomari, F.Z.; Sarazin, M.; Chaib, D.; Pichette, A.; Boumghar, H.; Boumghar, Y.; Djabou, N. Chemical Variability and Chemotype Concept of Essential Oils from Algerian Wild Plants. Molecules 2023, 28, 4439. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound | tR (min) | RI (NIST) | Ro_1 | Ro_2 | Ro_3 | Ro_4 | Ro_5 | Ro_6 | Ro_7 | Ro_8 | Ro_9 | Ro_10 | Ro_11 | Ro_12 | Ro_Tunise |
| 1. | Tricyclene | 6.56 | 933 | 0.23 | 0.30 | 0.33 | 0.31 | 0.31 | 0.34 | 0.29 | 0.35 | 0.41 | 0.36 | 0.38 | 0.36 | 0.11 |
| 2. | Camphene | 7.59 | 950 | 6.28 | 8.26 | 9.44 | 9.20 | 8.37 | 9.00 | 8.16 | 9.21 | 10.15 | 9.76 | 9.57 | 9.57 | 3.27 |
| 3. | 2,4-Thujadiene | 7.73 | 971 | 0.18 | 0.15 | 0.15 | 0.16 | 0.15 | 0.13 | 0.13 | 0.14 | 0.17 | 0.18 | 0.18 | 0.17 | 0.03 |
| 4. | β-Pinene | 8.73 | 972 | 0.17 | 0.22 | 0.28 | 0.28 | 0.31 | 0.30 | 0.35 | 0.39 | – | 0.34 | 0.32 | 0.38 | 5.33 |
| 5. | α-Pinene | 8.74 | 939 | 11.36 | 14.97 | 15.46 | 15.14 | 16.07 | 18.14 | 17.55 | 18.35 | 19.46 | 17.40 | 18.61 | 19.33 | 11.11 |
| 6. | 1-Octen-3-ol | 8.82 | 1078 | 0.16 | 0.15 | 0.13 | 0.12 | 0.14 | 0.17 | 0.18 | 0.15 | 0.15 | 0.14 | 0.17 | 0.17 | 0.02 |
| 7. | 3-Octanone | 9.07 | 1121 | 0.51 | 0.21 | 0.18 | 0.16 | 0.17 | 0.23 | 0.26 | 0.24 | 0.23 | 0.20 | 0.27 | 0.28 | 0.03 |
| 8. | α-Myrcene | 9.27 | 991 | 1.65 | 2.24 | 2.38 | 2.49 | 2.33 | 2.62 | 2.34 | 2.63 | 2.63 | 2.87 | 2.83 | 3.08 | 0.86 |
| 9. | β-Thujene | 9.36 | 964 | 0.63 | – | 0.15 | – | – | – | – | 0.19 | – | – | – | 0.17 | 0.23 |
| 10. | 3-Octanol | 9.61 | 1126 | 0.06 | – | – | – | – | 0.04 | 0.06 | – | – | – | – | – | – |
| 11. | α-Phellandrene | 10.04 | 1015 | – | 0.14 | – | – | 0.32 | 0.23 | 0.41 | – | 0.14 | 0.16 | 0.15 | – | 0.11 |
| 12. | 3-Carene | 10.14 | 1030 | 0.03 | – | – | – | 0.04 | 0.03 | 0.04 | 0.05 | 0.05 | 0.03 | 0.03 | 0.04 | 0.11 |
| 13. | α-Terpinene | 10.56 | 1016 | 0.95 | 0.40 | 0.46 | 0.68 | 0.66 | 0.59 | – | 0.60 | – | 0.63 | – | – | 0.36 |
| 14. | β-Cymene | 10.95 | 1018 | 4.27 | 2.49 | 2.60 | 2.69 | 2.59 | 2.36 | 2.40 | 2.15 | 2.07 | 2.02 | 1.95 | 2.12 | 1.57 |
| 15. | D-Limonene | 11.19 | 1031 | 4.13 | 3.75 | 3.87 | 3.95 | 3.79 | 3.99 | 3.90 | 3.89 | 3.75 | 3.59 | 3.63 | 3.85 | 1.86 |
| 16. | Eucalyptol | 11.35 | 1034 | 16.16 | 15.42 | 14.16 | 14.31 | 13.92 | 13.42 | 13.70 | 14.23 | 13.07 | 14.29 | 14.22 | 14.47 | 52.77 |
| 17. | β-cis-Ocimene | 12.12 | 1049 | – | – | – | – | – | – | – | – | – | – | – | – | 0.03 |
| 18. | γ-Terpinene | 12.72 | 1062 | 0.08 | 0.12 | 0.22 | 0.33 | 0.31 | 0.31 | 0.32 | 0.48 | 0.52 | 0.62 | 0.55 | 0.39 | 0.60 |
| 19. | Linalool | 15.15 | 1095 | 0.54 | 0.82 | 0.77 | 0.80 | 0.85 | 0.89 | 0.88 | 0.87 | 0.74 | 0.77 | 0.80 | 0.84 | 0.57 |
| 20. | Crysanthenone | 16.31 | 1102 | – | 0.03 | 0.05 | 0.07 | 0.06 | 0.05 | 0.06 | 0.06 | 0.08 | 0.08 | 0.07 | 0.10 | – |
| 21. | Terpinolene | 17.47 | 1088 | 0.10 | 0.14 | 0.17 | 0.25 | 0.25 | 0.30 | 1.09 | 0.37 | 0.93 | 0.38 | 0.95 | 0.82 | 0.26 |
| 22. | Camphor | 17.90 | 1146 | 40.03 | 34.23 | 30.85 | 32.21 | 33.13 | 31.22 | 34.24 | 29.62 | 29.29 | 29.69 | 29.86 | 29.41 | 9.27 |
| 23. | Camphenilanol | 18.31 | 1151 | 0.08 | 0.11 | 0.09 | 0.08 | 0.03 | 0.07 | 0.08 | 0.06 | 0.04 | – | 0.03 | – | – |
| 24. | Sabinone | 18.76 | 1162 | 0.07 | – | – | – | – | 0.02 | – | – | – | 0.05 | – | 0.06 | – |
| 25. | Pinocarvone | 18.76 | 1191 | – | 0.04 | – | – | 0.04 | – | – | – | – | – | 0.05 | – | – |
| 26. | D-Pinocamphone | 18.85 | 1206 | 0.33 | 0.04 | 0.14 | – | 0.12 | 0.13 | – | – | 0.07 | 0.09 | 0.08 | 0.07 | 0.02 |
| 27. | endo-Borneol | 19.40 | 1163 | 3.87 | 6.40 | 6.09 | 4.79 | 4.63 | 4.22 | 3.83 | 5.01 | 4.78 | 4.70 | 4.98 | 5.32 | 2.60 |
| 28. | Terpinen-4-ol | 19.95 | 1176 | 0.64 | 0.75 | 0.71 | 0.65 | 0.65 | 0.69 | 0.61 | 0.66 | 0.61 | 0.59 | 0.60 | 0.69 | 0.58 |
| 29. | α-Terpinyl propionate | 20.91 | 1333 | 1.83 | 1.91 | 1.64 | 1.53 | 1.66 | 1.74 | 1.66 | 1.65 | 1.51 | 1.58 | 1.58 | 1.74 | 1.78 |
| 30. | Verbenone | 21.56 | 1204 | 2.86 | 2.77 | 2.68 | 2.18 | 2.64 | 3.20 | 3.37 | 2.97 | 2.94 | 3.19 | 3.08 | 3.33 | 0.04 |
| 31. | trans-Shisool | 23.76 | 1326 | – | – | – | – | – | – | – | – | – | – | 0.03 | 0.03 | – |
| 32. | (+)-Borneol acetate | 25.45 | 1330 | 0.34 | 0.72 | 2.72 | 3.05 | 2.88 | 2.97 | 2.23 | 3.22 | 3.55 | 3.48 | 2.16 | 0.86 | 0.81 |
| 33. | (+)-cis-Verbenol acetate | 25.68 | 1351 | – | – | – | – | – | – | – | – | – | – | – | – | 0.01 |
| 34. | Thymol | 25.91 | 1290 | 0.09 | – | – | – | – | – | 0.04 | – | – | 0.07 | 0.04 | – | – |
| 35. | Piperitenone | 26.92 | 1303 | 0.03 | 0.05 | 0.06 | 0.06 | 0.05 | 0.07 | 0.03 | 0.04 | 0.05 | 0.05 | 0.02 | 0.03 | – |
| 36. | α-Cubebene | 27.61 | 1374 | – | – | – | – | – | – | – | 0.03 | – | – | – | 0.04 | 0.02 |
| 37. | Ylangene | 27.68 | 1395 | 0.05 | 0.07 | 0.17 | 0.16 | 0.15 | 0.06 | 0.04 | 0.05 | 0.06 | 0.06 | 0.07 | 0.05 | 0.05 |
| 38. | Copaene | 27.82 | 1415 | 0.04 | 0.03 | 0.07 | 0.07 | 0.07 | 0.04 | 0.03 | – | 0.03 | 0.04 | 0.05 | – | 0.22 |
| 39. | Carvacrol | 28.04 | 1298 | 0.07 | 0.15 | 0.08 | – | 0.07 | 0.06 | 0.04 | 0.06 | 0.05 | 0.05 | 0.04 | 0.04 | – |
| 40. | Methyleugenol | 28.34 | 1396 | – | – | – | – | – | – | – | – | – | – | – | – | 0.01 |
| 41. | Caryophyllene | 28.71 | 1420 | 0.68 | 1.14 | 1.33 | 1.43 | 1.29 | 1.01 | 0.60 | 1.12 | 1.20 | 1.47 | 1.56 | 1.10 | 3.77 |
| 42. | Humulene | 29.36 | 1454 | – | – | – | 0.24 | 0.26 | – | 0.16 | 0.13 | 0.14 | – | 0.18 | 0.13 | 0.38 |
| 43. | p-Thymol | 29.58 | 1465 | – | – | 0.08 | 0.15 | 0.07 | 0.06 | – | 0.07 | 0.04 | – | – | 0.05 | – |
| 44. | 7-epi-α-Cadinene | 29.76 | 1491 | 0.05 | 0.05 | 0.11 | 0.11 | 0.10 | 0.04 | 0.03 | 0.04 | 0.03 | 0.04 | 0.03 | 0.03 | 0.02 |
| 45. | α-Bisabolene | 30.20 | 1506 | – | – | – | 0.08 | 0.05 | – | – | – | – | – | – | – | 0.05 |
| 46. | (-)-δ-Cadinene | 30.36 | 1520 | 0.09 | – | 0.19 | 0.18 | 0.18 | 0.11 | – | – | 0.09 | 0.11 | 0.12 | 0.08 | 0.23 |
| 47. | trans-Calamenene | 30.41 | 1542 | 0.04 | – | – | – | – | – | – | 0.03 | – | 0.02 | – | 0.02 | – |
| 48. | α-Calacorene | 30.72 | 1561 | 0.04 | 0.06 | 0.14 | 0.12 | 0.11 | 0.05 | 0.04 | 0.04 | – | 0.04 | 0.04 | 0.03 | 0.01 |
| 49. | (+)-Sativen | 30.94 | 1583 | 0.02 | – | – | 0.05 | – | 0.03 | – | – | 0.01 | – | – | – | – |
| 50. | Cubenol | 32.01 | 1600 | 0.05 | 0.07 | 0.11 | – | 0.10 | – | 0.03 | – | – | – | 0.02 | – | – |
| 51. | α-Bisabolol | 32.62 | 1621 | 0.03 | 0.04 | – | 0.01 | 0.08 | 0.03 | – | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 |
| 52. | Levomenthol | 32.62 | 1632 | – | – | 0.06 | 0.05 | – | – | 0.02 | – | – | 0.02 | – | – | – |
| 53. | Caryophyllene oxide | 38.74 | 1652 | 0.03 | 0.06 | 0.05 | 0.07 | 0.05 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.10 |
| Total No. of compounds identified | 41 | 36 | 37 | 36 | 41 | 40 | 37 | 37 | 36 | 38 | 40 | 39 | 38 | |||
| Total (%) | 98.85 | 98.50 | 98.26 | 98.21 | 99.05 | 98.99 | 99.22 | 99.19 | 99.06 | 99.19 | 99.34 | 99.28 | 99.21 | |||
| Monoterpene hydrocarbons (%) | 30.06 | 33.18 | 35.51 | 35.48 | 35.50 | 38.34 | 36.98 | 38.80 | 40.28 | 38.34 | 39.15 | 40.28 | 25.84 | |||
| Oxygenated monoterpenes (%) | 66.94 | 63.44 | 60.18 | 59.93 | 60.80 | 58.81 | 60.79 | 58.52 | 56.82 | 58.70 | 57.64 | 57.04 | 68.46 | |||
| Sesquiterpene hydrocarbons (%) | 1.01 | 1.35 | 2.10 | 2.44 | 2.21 | 1.34 | 0.90 | 1.44 | 1.56 | 1.78 | 2.05 | 1.48 | 4.75 | |||
| Oxygenated sesquiterpenes (%) | 0.11 | 0.17 | 0.16 | 0.08 | 0.23 | 0.06 | 0.05 | 0.04 | 0.02 | 0.03 | 0.06 | 0.03 | 0.11 | |||
| Other compounds (%) | 0.73 | 0.36 | 0.31 | 0.28 | 0.31 | 0.44 | 0.50 | 0.39 | 0.38 | 0.34 | 0.44 | 0.45 | 0.05 | |||
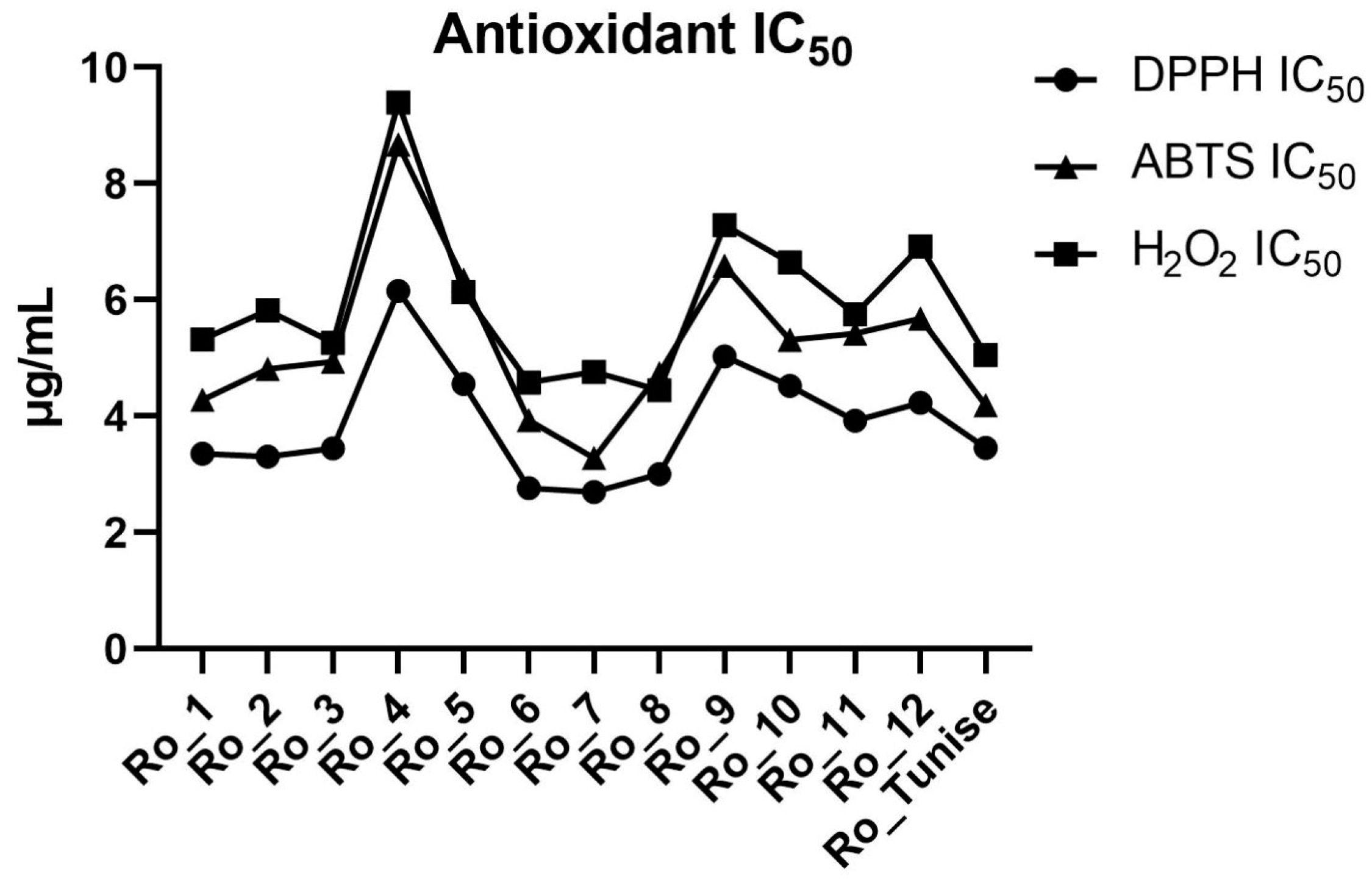
| Activity | Ro_1 | Ro_2 | Ro_3 | Ro_4 | Ro_5 | Ro_6 | Ro_7 | Ro_8 | Ro_9 | Ro_10 | Ro_11 | Ro_12 | Ro_Tunise |
| S. aureus MIC (μg/mL) | 115.6 | 194.4 | 228.8 | 131.5 | 178.2 | 249.7 | 244.9 | 186.8 | 220.2 | 236.7 | 137.1 | 53.87 | 32.63 |
| DPPH IC50 (μg/mL) | 3.351 | 3.307 | 3.447 | 6.158 | 4.556 | 2.763 | 2.692 | 3.008 | 5.033 | 4.517 | 3.917 | 4.233 | 3.456 |
| ABTS IC50 (μg/mL) | 4.283 | 4.812 | 4.941 | 8.672 | 6.348 | 3.926 | 3.281 | 4.738 | 6.591 | 5.316 | 5.423 | 5.679 | 4.190 |
| H2O2 IC50 (μg/mL) | 5.317 | 5.824 | 5.265 | 9.398 | 6.137 | 4.579 | 4.764 | 4.448 | 7.289 | 6.642 | 5.756 | 6.919 | 5.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





