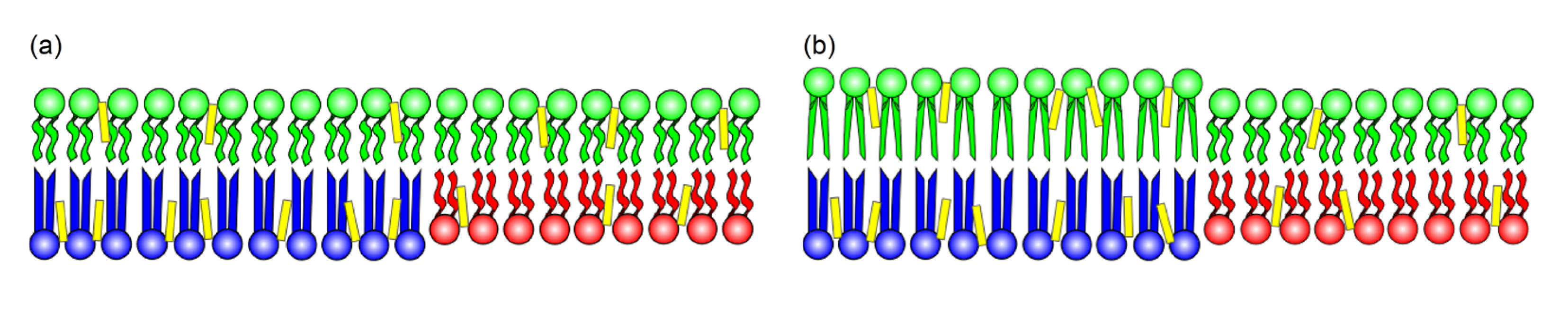
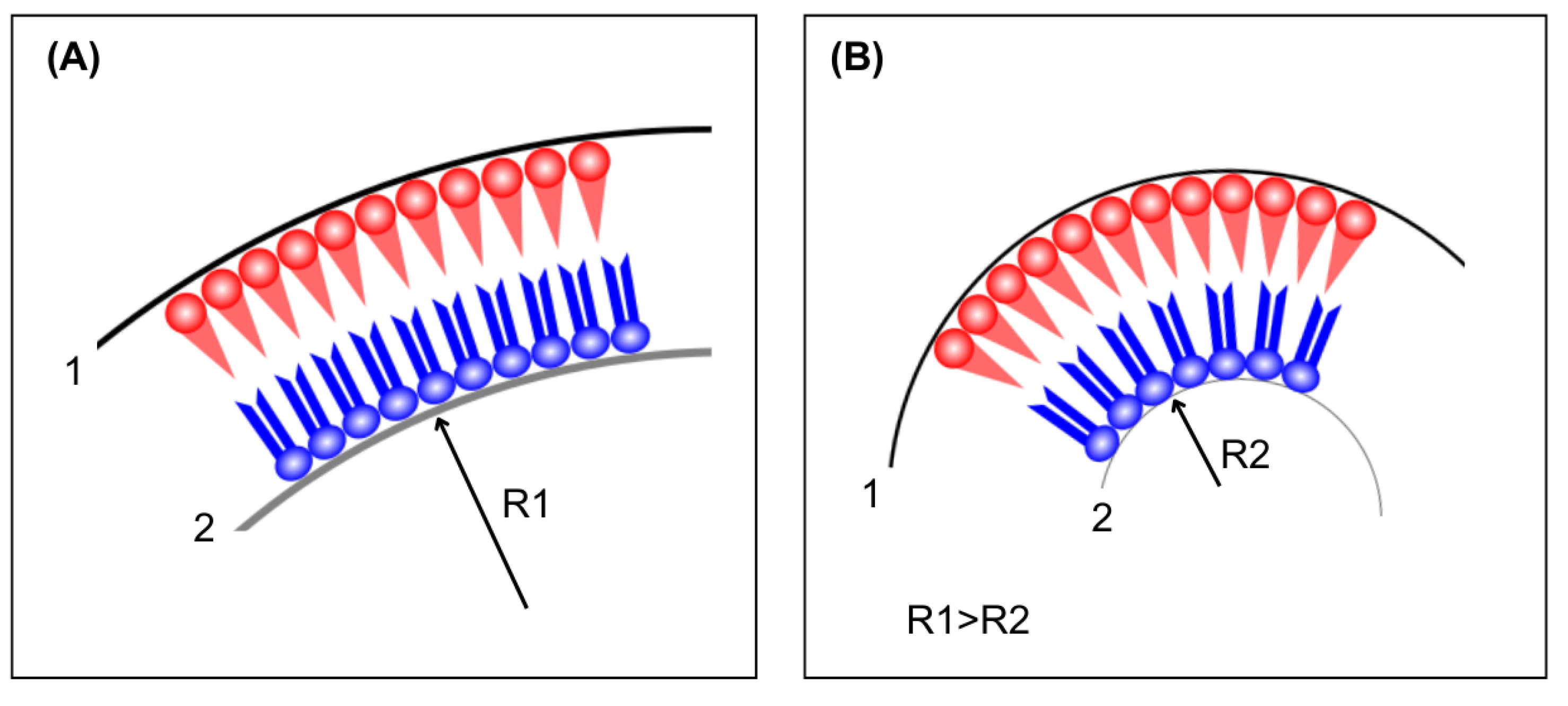
1. Introduction
Biological membranes rely on diverse lipid compositions to function as a lipid barrier protecting the cell and its organelles, if present, as well as on the regulation of cell function [
1]. Eukaryotic cells possess a wide variety of lipids, allocating significant resources to produce hundreds of lipid structures. As van Meer et al. (2008) [
2] pointed out, cells utilize 5% of their genetic material to produce a large number of different lipids. A question that poses itself is: Is there a purpose for such a significant expenditure of energy in terms of membrane function?
A relevant property of natural cell membranes is their bilayer asymmetry, which stems from the different lipid compositions of the individual leaflets forming the bilayer. Investigations from the last 50 years have shown that many organisms, including some types of bacteria [
3,
4,
5] and mammalian cells [
6,
7,
8,
9,
10], present asymmetric lipid bilayers enclosing their cells or organelles. These studies identified the main lipids present in the two leaflets in terms of their headgroups. More recently, Lorent et al. (2020) [
11] presented a detailed picture of the phospholipid distribution on each leaflet of the plasma membrane of red blood cells using an enzymatic digestion assay. The authors added information on the distribution of acyl chain length and unsaturation, which play an important role concerning order in the bilayer. A predominance of nearly saturated phosphatidylcholine (PC) and long chains sphingomyelin (SM) is found in the outer layer, while unsaturated phosphatidylcholine, phosphatidylethanolamine (PE) and ionizable phosphatidylserine (PS) compose most of the inner layer.
The lipid composition of the biological membrane has implications on its structure and lipid mobility, with the possibility of separating specific lipid domains. Order or fluidity of the lipid structure impacts membrane function in the cell [
12]. Phase properties are relevant for protein association and transport across the membrane and have been extensively explored in simple experimental (in vitro) or numerical (in silico) studies of symmetric bilayers. This has led to the development of a large set of accurate techniques both for the laboratory and for computers which allow recognition of the coexistence of phases, such as liquid ordered, liquid disordered or gel phases manifested in lipid bilayers. These techniques have been used in exploring the asymmetric bilayer in the last twenty years.
Knowledge of the main components of the cell membrane's inner and outer leaflet inspired specialists in molecular dynamics, and many numerical experiments have been conducted in the last twenty years, yielding significant advances in the field. The latter has been carefully reviewed and analyzed in the companion paper by Chaisson et al. (2023) [
13].
Experimental investigation of model asymmetric bilayers (in vitro) had to wait for the development of innovative techniques [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30].
A most relevant consequence of the multiplicity of lipids that constitute the cell-like model membrane is the presence of phase separation with domains of different lipid compositions allied to the ordering or disordering of the lipid molecules. Thus, in the case of asymmetric bilayers, an essential question is the effect of asymmetry on the phase behavior of the membrane. In order to probe this effect, the investigation could follow a sequence of studies:
Obtain data on the outer leaflet of the cell-like membrane from symmetric bilayers.
Obtain data on the inner leaflet of the cell-like membrane from symmetric bilayers.
Prepare the cell-like asymmetric membrane and investigate its overall phase behavior as compared to the behavior of the uncoupled individual leaflets.
This minireview is dedicated to the latest inquiries into lipid composition and phase behavior of the asymmetric cell-like membrane, as revealed by the study of giant unilamellar vesicles (GUVs). The use of simplified biomembranes allows for better control of variables and the use of simplified models to propose biophysical principles that could effectively explain observations. In
Section 2, we delve into the results for the structure of the exoplasmic leaflet, derived from data on symmetric bilayers with the lipid composition of the outer leaflet in the plasma membrane.
Section 3 presents the results corresponding to symmetric bilayers with the composition of the inner plasma membrane leaflet. Finally, in
Section 4, we explore the results of models for the asymmetric plasma membrane and the question of coupling between the two bilayer leaflets.
Section 5 offers some final considerations, highlighting the recent advancements in our understanding of lipid composition and phase behavior.
2. Modeling the Exoplasmic Leaflet of the Plasma Membrane
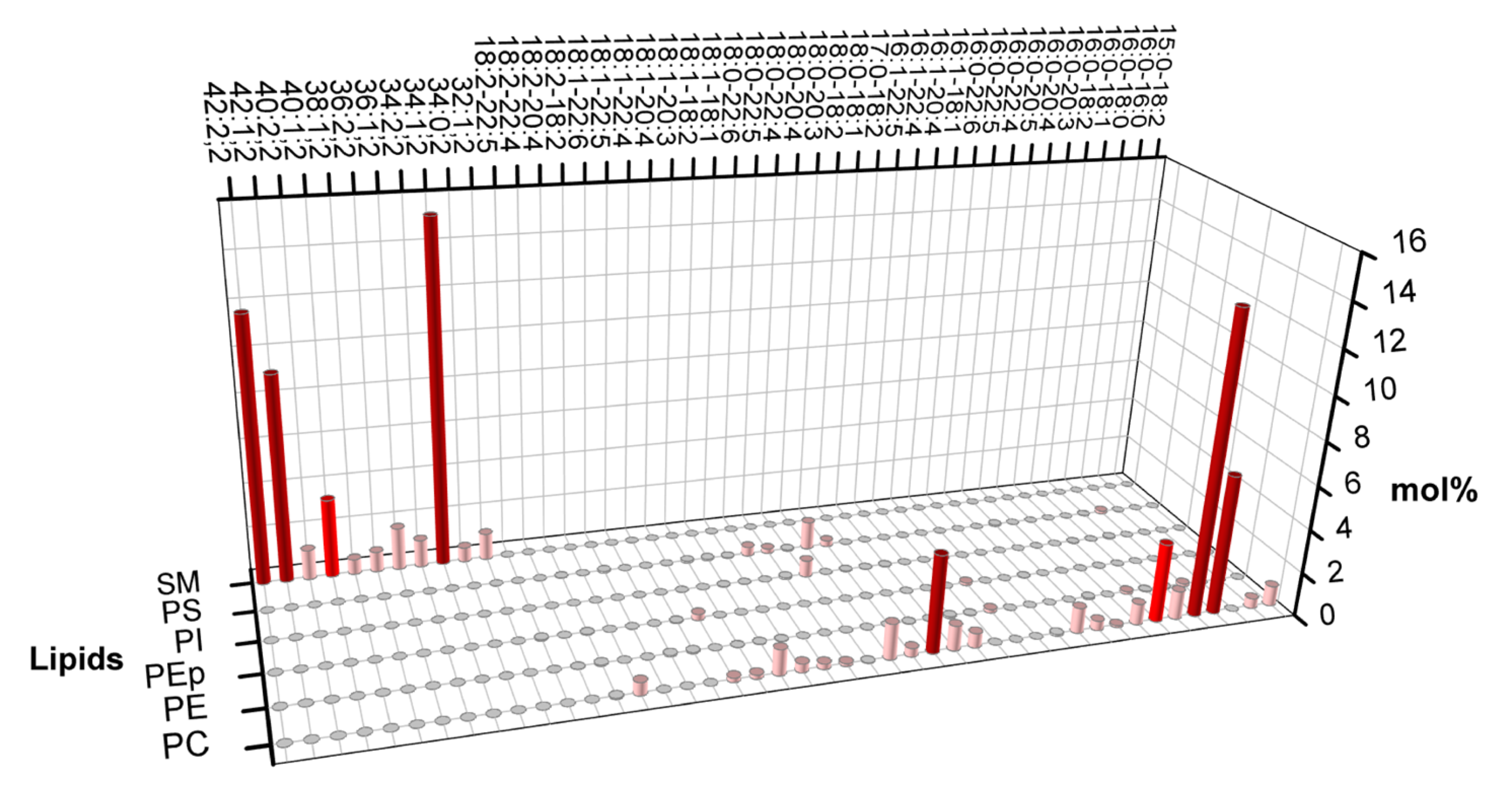
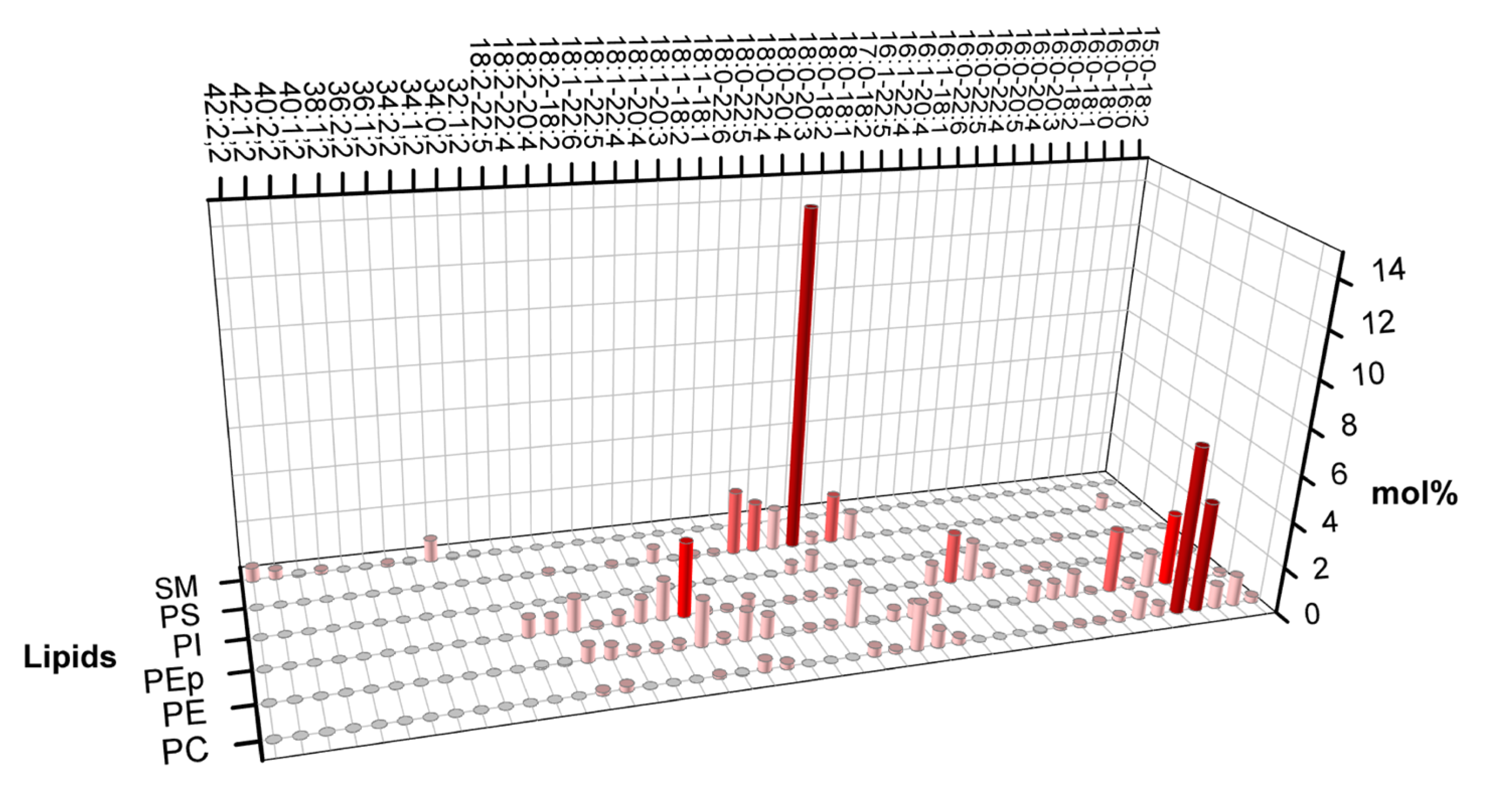
Lorent et al. (2020) obtained the phospholipid constitution of the plasma membrane's leaflets [
11].
Figure 1 shows that the exoplasmic leaflet has a significant fraction of high melting temperature lipids (High Tm lipid), such as long chain sphingomyelin (SM), for which, in general, the main gel-fluid transition temperature is above 40
oC.
Figure 1 also shows that the outer leaflet presents a considerable fraction of phosphatidylcholine lipids with much lower melting points due to chain unsaturation. Besides phospholipids, the plasma membrane also contains cholesterol (not shown in the plot), a lipid important in many cellular processes. The fraction of cholesterol between leaflets is still under debate.
In the following subsections, we shall present the main results of studies on the lipid structure of the symmetric membrane composed of some phospholipids used to model the outer leaflet of the plasma membrane plus cholesterol (see
Figure 1). The phase structure and lipid mobility depend on lipid-relative concentrations. Thus, an extensive range of phospholipid relative molar densities can be explored. A very efficient form of illustrating the results of such investigations is to present results in terms of phase diagrams. Here, we are interested in the phase coexistence of the liquid-ordered and -disordered phases, which are more relevant from the cell point of view and in the information on the size of domains that may reach nanoscales depending on the lipid composition. The presence of nanodomains may provide strong evidence in favor of the lipid raft hypothesis.
In the following, we shall revise a few phase diagrams relevant to rationalizing the outer leaflet behavior. Here, DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine) or bSM (Sphingomyelin (Brain, Porcine)) stand for the lipids with high-temperature melting points of the plasma membrane, both of which generally have long saturated acyl chains.
2.1. Triangular Phase Diagrams
Triangular phase diagrams play a crucial role in deciphering the complex phase behavior of ternary systems in general, and of lipid ternary systems, in particular [
31,
32,
33]. These systems involve the physical interaction of three chemical components combined in varied proportions. A typical example of such a system of interest in studying biomembranes is the mixture of phospholipids and cholesterol [
34,
35,
36] as illustrated in
Figure 2.
A triangle represents the full range of relative concentrations of the three-component system. Each vertex represents one of the pure components, while points on the sides represent binary mixtures. Each point inside the triangle indicates a specific three-component composition.
Different compositions of the ternary system may present different thermal phases at the same temperature. Thus, triangular phase diagrams are valuable for indicating the regions with lipid concentrations that present a single equilibrium phase or regions of coexistence between different phases at a specific temperature.
2.2. Mapping the Coexistence Region Ld+Lo Phases
Different experimental techniques may be used to investigate the coexistence of phases. The most straightforward method is fluorescence optical microscopy, which uses fluorescent labels that prefer one specific phase. In addition to microscopy techniques, one may investigate the presence of phase coexistence using different techniques, such as steady-state probe partition (SSP) fluorescence [
31,
34,
35,
36,
37,
38,
39,
40], electron spin resonance (ESR) [
38,
41], small-angle X-ray scattering (SAXS) [
42], small-angle neutron scattering (SANS) [
35,
43], cryo-EM [
44,
45], and atomic force microscopy (AFM) [
46,
47]. Here, we shall present mainly data based on fluorescence techniques.
Fluorescence techniques rely on information given by the fluorescence of dye molecules. In the presence of phase coexistence, dye molecules in the membrane may prefer one phase over the other, yielding a partition of such molecules. The experimental techniques based on fluorescence use the concept of partition to investigate the properties of different phases.
The partition coefficient (Kp) represents a measure of the preference of a specific molecule for one phase over another in terms of the ratio of the dye fractions at each of the two phases in equilibrium, equation (1). It is usual to define a partition coefficient greater than 1 to indicate a higher preference for the disordered phase. In contrast, a partition coefficient lower than 1 indicates a higher preference for the ordered phase (e.g., ([
36])).
where
and
are the fractions of fluorescent probes that partition into the Ld and the Lo phase, and
and
are the fractions of these phases, respectively.
We can estimate the partition coefficient for fluorescent dyes from confocal microscope images. Alternatively, equations (1) and (2) also display the mathematical equation, allowing us to extract Kp from steady-state probe partition SSPP-fluorescence experiments or SSPP-FRET [
48].
2.2.1. Fluorescence Microscopy
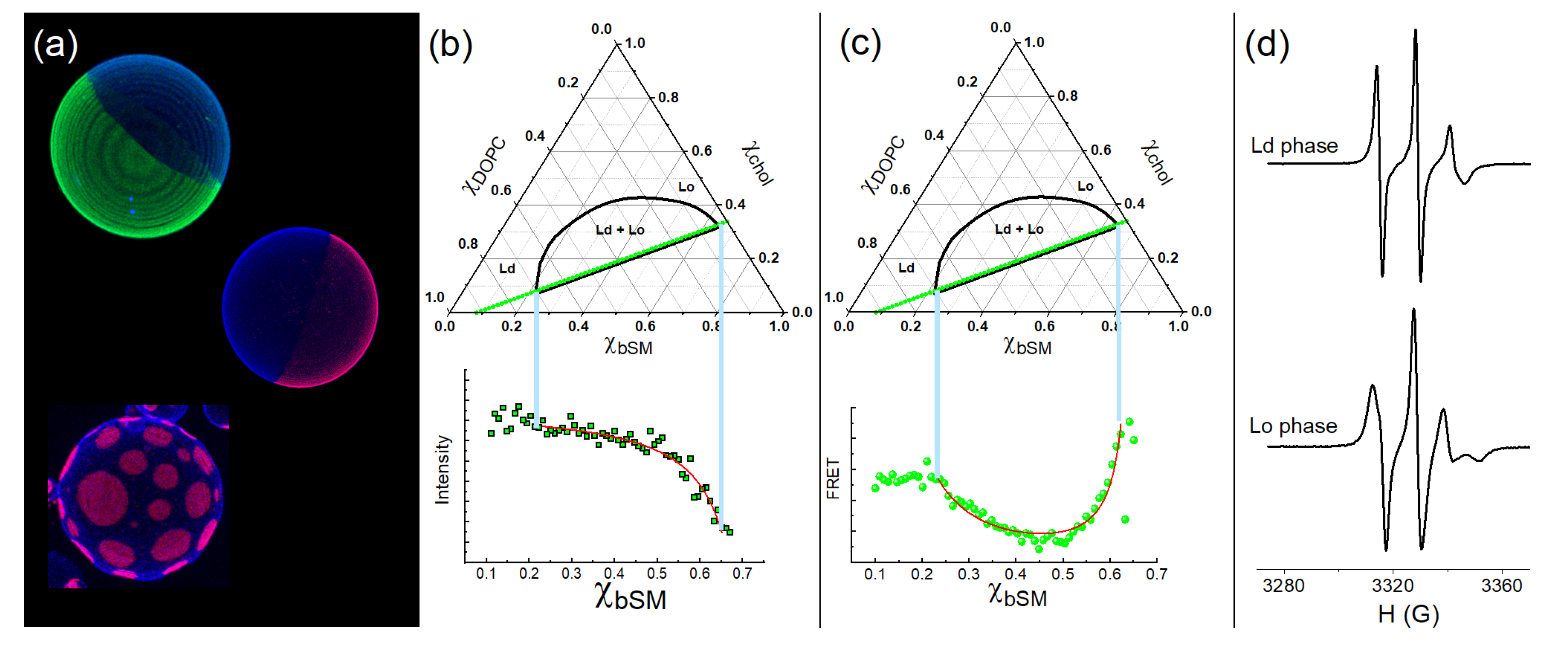
Figure 3 (a) displays examples of giant vesicles labeled with different fluorescent probes. The authors captured these images using confocal microscopy. The fluorophores indicate the coexistence of two phases, namely the Ld and Lo phases. In this example, the Ld phase is labeled green or pink.
The phase diagrams in
Figure 2 for the DSPC/DOPC/chol and bSM/DOPC/chol compositions correspond to samples that display visible domains under optical fluorescence microscopy [
35,
36,
37,
49,
50]. Note that DSPC and bSM are high Tm lipids, whereas DOPC is a low Tm lipid due to unsaturation on the acyl chains.
Interestingly, when POPC replaces DOPC, where POPC has one of the acyl chains unsaturated, samples of DSPC/POPC/chol and bSM/POPC/chol expected to show Ld+Lo domains, form giant vesicles with a uniform appearance [
51,
52]. Could there be phase domains whose sizes fall below the threshold of optical resolution? The study of various experimental methods was necessary to evaluate the presence of submicron domains, potentially on a nanoscale scale. Next, we briefly describe a few techniques that can be employed to investigate the coexistence of phases for nano-scale domains.
2.2.2. Steady-State Probe Partition Fluorescence
Fluorescent probes might exhibit different fluorescence intensities when embedded in distinct environments [
53]. This observation can relate to different fluorescence quantum yields in each phase. As fluorescent probes partition between different environments, we can calculate the partition coefficient based on the amount of dye in each phase, which is measured by the fluorescent signal in a trajectory of samples. In these experiments, samples have slightly different lipid compositions (e.g. the fraction of bSM is increasing by 0.01), as illustrated in the phase diagram, green dots.
Figure 3b shows the fluorescent signal of each composition. Note a brighter signal in the Ld phase compared to the Lo phase. Thus, the fluorescent signals from samples in the two-phase region can be described as a combination of signals from samples at the timeline endpoints, namely the compositions of a pure Ld phase on the tieline left hand side, and a pure Lo phase on the right hand side [
36]. The fluorescence intensity for the samples belonging to a tieline can be described by equation (2)
where
is the partition coefficient as described by equation (1), and
and
are the intensities measured on the tieline endpoints.
The steady-state probe partition fluorescence can measure the partition coefficient of fluorescent molecules that partition between the Ld and the Lo phases. However, based solely on this analysis, we cannot determine the domains' sizes for the different phases.
Förster Resonance Energy Transfer (FRET) employs the study of energy transfer between two dye molecules instead of monitoring the fluorescence signal of a single dye particle. This technique makes it possible to estimate distances on the nanometer scale. The energy transfer between fluorescent donors and fluorescent acceptors depends on the distance between both fluorophores. The FRET efficiency increases as dye molecules get closer and decreases when the donor and acceptor molecules are far apart. The distance between two fluorescent probes is affected by each fluorophore's preference for a specific phase [
36,
48].
2.2.3. Steady-State Probe Partition - Förster Resonance Energy Transfer
Figure 3 (c) shows an example where different probes, DHE and Bodipy-PC, prefer different phases. For this case, the region in the composition space of the phase diagram with a lower FRET efficiency indicates a region with the coexistence of phases. DHE prefers the Lo phases, whereas Bodipy-PC prefers the Ld phase. It can be seen that FRET efficiency (or the monitored FRET signal) varies abruptly at the phase coexistence endpoints at which the FRET pair (donor and acceptor probes) partition into their preferred phase. The FRET intensity for the samples belonging to a tieline can be described by equation (3)
where,
and
are the partition coefficients of the donor and the acceptor probes, and
and
are the FRET signal measured on the tieline endpoints.
A pair distribution function for donor-acceptors can also be used to calculate a theoretical FRET profile [
36,
54]. In these cases, comparing the experimental results to theoretical calculations allows us to estimate the sizes of nanodomains [
36,
54].
Other techniques are advantageous in investigating the coexistence of the Ld and the Lo phases. Electron spin resonance (ESR) can offer supplementary evidence regarding domains exhibiting distinct physical properties, such as lipid order.
Figure 3d shows ESR signals obtained for the Ld and the Lo phases of DSPC/DOPC/chol, as previously reported [
38]. When both phases coexist (Ld+Lo), the first derivative of the ESR absorption spectrum can be decomposed into a combination of Ld and Lo spectra [
36,
41].
2.3. Quaternary Phase Diagrams
Investigation of ternary lipid mixtures has shown that a lipid mixture such as DSPC/DOPC/chol forms macrodomains that we can observe under the microscope, whereas a different lipid mixture, such as DSPC/POPC/chol, forms phase domains of a nanometer scale, as observed with various spectroscopic techniques. As different lipid compositions yield domain sizes that might differ by several orders of magnitude, an interesting question arises: What happens when we mix these four lipids DSPC/DOPC/POPC/chol? What kinds of domains will we observe?
Feigenson's laboratory developed an answer to this question. The group studied the different four-component mixture, including DSPC/DOPC/POPC/chol, to investigate the morphology of the phase domains under relative fraction variations [
37,
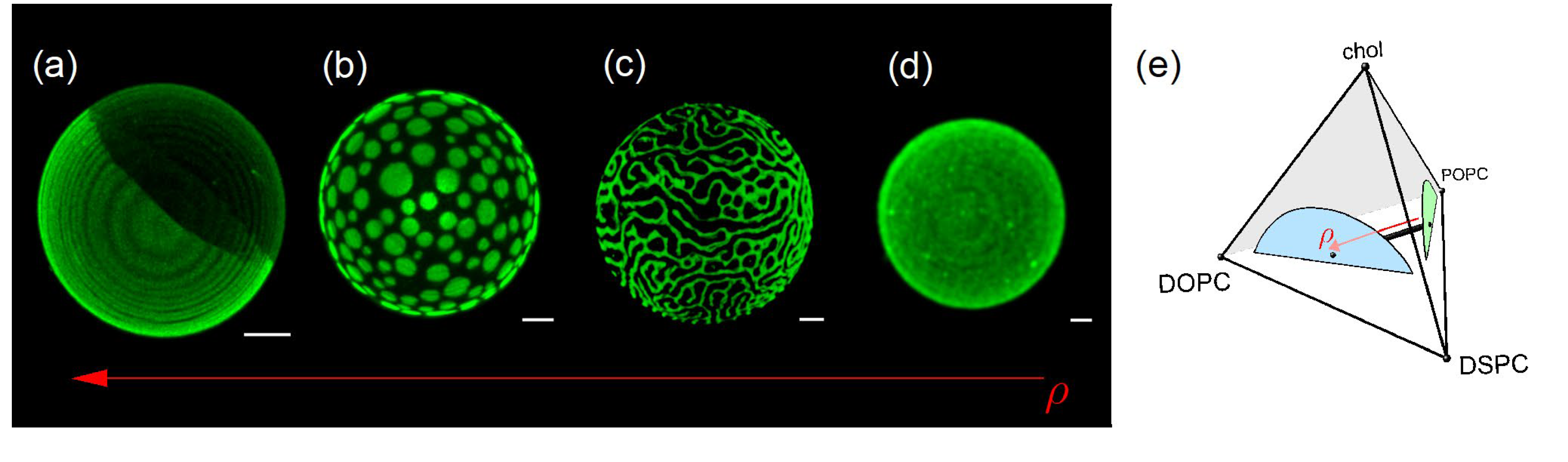
51]. The ratio between DOPC and POPC was varied, while the cholesterol and DSPC percentages were maintained fixed (χchol = 0.22 and χDSPC = 0.39). The authors defined a density variable, equation (4), with 0<ρ<1,
Experiments performed at room temperature yielded the formation of different morphologies of the Ld/Lo domains for different ranges of the relative fraction ρ.
Figure 4 illustrates microscopy results with different values of ρ. For ρ = 1 (Fig. 4a), we observe complete phase separation of the Lo (black) and Ld (green) phases. For ρ = 0.45 (Fig. 4b), we can see many circular Ld domains in a background "sea" of the Lo phase. For ρ = 0.32 (Fig. 4c), the domains form different patterns, with either a honeycomb appearance or strips, called modulated phases. Finally, for ρ = 0 (Fig. 4d), giant vesicles show a uniform appearance under the microscope. However, as mentioned above, domains have sizes of a few nanometers and cannot be observed under the microscope.
2.4. Energetic Factors Driving Phase Separation and Competing Interactions
As mentioned, various simplified lipid mixtures of 3 or 4 lipid components display liquid ordered-liquid disordered coexistence at a specific temperature [
35,
37,
38,
51,
56,
57,
58,
59]. For the examples forming macrodomains, such as those for ρ = 1, the phase separation minimizes the interface line between the two phases, which is a consequence of minimizing the system's free energy. At coexistence, the system's free energy includes contributions from the free energies of each bulk phase and from the interface. The interface free energy is proportional to the interface's length, with the interface tension as a linear coefficient. The minimum free energy corresponds to the minimal length of the interface line.
Modulated phases, however, have been seen for many lipid compositions (e.g., [
38]). The presence of modulated phases implies a longer interface line, given by the sum of the domain interface lines. Thus, there should be some compensation for the penalty for a larger interface.
The possibility of forming modulated phases exhibited by many physical systems, with length scales ranging from centimeters to nanometers, has been investigated by [
60]. The authors show that competing interactions, such as an additional repulsive interaction between particles, may yield modulated phases. The repulsive interaction raises the bulk free energy and thus favors the breaking-up of the two-phase domains, producing competition with the interface free energy. If the latter wins, the system will be unstable in terms of the formation of domains. Otherwise, if the repulsive interaction wins, the competition of interactions yields smaller domains (nanodomains) embedded in the other phase.
Inspired by the ideas presented by [
60], Feigenson and collaborators explored different possibilities for competitive interactions to reproduce domain morphologies observed in experiments with four-component lipid giant vesicles.
Numerical simulations were used to investigate the function of surface elastic deformation [
61] or the effect of a repulsive force between headgroup dipoles [
62].
Both effects, which compete with the line tension between the Ld and the Lo phases, can reproduce fragmentation of the phases, depending on model parameters. Numerical experiments used a discretized model of a spherical fluctuating surface (2013), whose energy was described by the Helfrich Hamiltonian [
63], which presents a positive contribution from local curvature and a negative contribution from local Gaussian curvatures. The authors adjusted the model parameters to produce modulated phases. Parameters thus obtained are about ten times smaller than the experimentally measured value for line tension, whereas the experimental data's elastic constants are within the same range.
In a second paper (2014), the authors probe the role of dipole-dipole interactions between the zwitterionic headgroups and the importance of surface curvature in the appearance of nanodomains or modulated phases [
62]. The authors use the radial distribution function to establish the different morphologies. On the other hand, by introducing dipole interactions, the repulsion between lipids favors Lo-Ld contacts. Thus, it competes with line tension, which favors Lo-Lo or Ld-Ld contacts. Consequently, dipole repulsion induces the formation of smaller domains. Additionally, the addition of curvature energy may lead to the emergence of modulated phases.
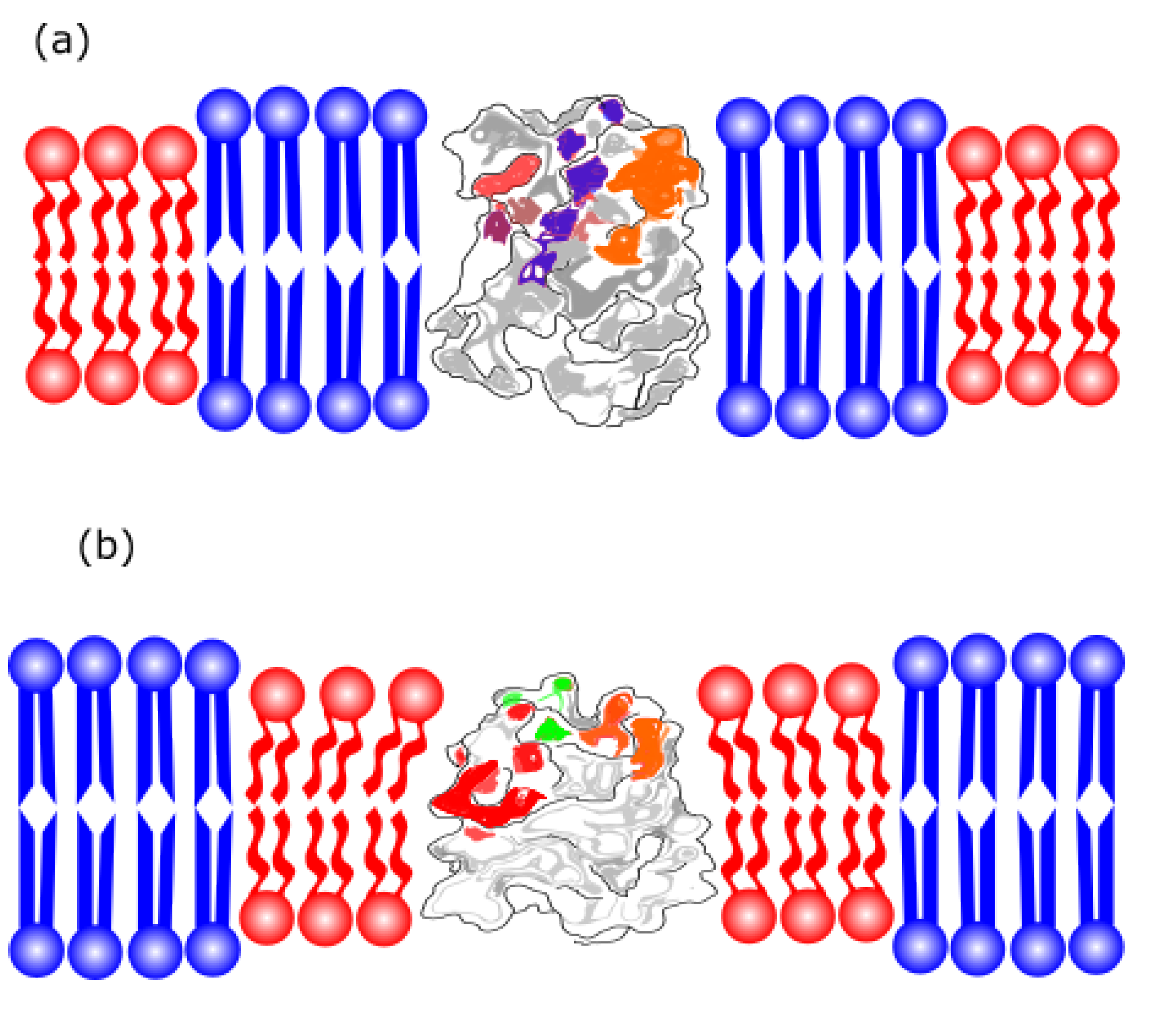
2.5. Membrane Phase Domains and Protein Partition
When two phases coexist, a direct implication is the molecular partition between these phases [
64,
65,
66]. As mentioned above, lipids display a partition described by a thermodynamic tieline. Experimentally, the lipid partition is obtained from the partition coefficients of molecular dyes since different fluorescent molecules move to the corresponding preferred environments, as we have seen in the previous sections. When we introduce peptides, proteins, or any external molecules into lipid membranes containing different phases, they may distribute into a specific phase due to physical interactions with lipids. Interestingly, in biomembranes, protein-lipid interactions could also drive transmembrane proteins to a particular phase domain.
The presence of specific environmental conditions can render proteins functional [
67]. The fluorescence assay methods mentioned above, which allow measurement of any labeled molecule's partition coefficient, are especially pertinent when investigating the partition of peptides and proteins. Thus, studies of model membranes incorporating synthetic peptides can improve the representation of cellular plasma membranes, especially regarding transmembrane proteins.
2.5.1. Transmembrane Peptide Mismatch
Synthetic peptides are used to represent the interactions between the proteins embedded in the membrane and the lipids that compose the lipid bilayer in a simplified manner. Thus, one may investigate the mismatch problem between protein length and membrane thickness using synthetic peptides. The disparity in thickness vs. length occurs when there is a greater chance that the hydrophobic section of the membrane bilayer or the peptide is more exposed to water because of a difference between the bilayer thickness and the peptide length [
68].
This discrepancy results in an energetically unfavorable interaction, prompting responses from both the peptide and the lipids in contact with the peptide to minimize the mismatch, as shown in
Figure 5. There are two types of mismatches: positive and negative. Positive mismatch occurs when the peptide is larger than the thickness of the bilayer, while negative mismatch occurs when the peptide is smaller than the thickness of the bilayer. Among the responses that peptides can exhibit are: (i) the formation of peptide oligomers, where peptides aggregate into "bundles" to reduce the contact surface of the peptide's hydrophobic part with the polar region; (ii) changes in their molecular structure, where peptides larger than the bilayer could, for example, reorganize into a π-helix structure (positive mismatch), and smaller peptides could reconfigure their molecular structure into a 3₁₀-helix; (iii) tilting in the direction normal to the bilayer, thus reducing their effective size within the bilayer and protecting the peptide's apolar region (occurring only for positive mismatch), as previously reported [
68] and (iv) reorientation of side chains, flanking residues, near the lipid/water interface, resulting in a reduction of the hydrophobic region's size.
Notably, the vesicle seeks to optimize energy balance. Among all adaptations, aggregation and reorientation of the side chains stand out as the most efficient responses to mismatch [
68]. Additionally, peptide flanking residues are crucial in adjusting the mismatch, with different interactions for positively charged and aromatic residues.
2.5.2. Protein Partition
Regarding the partition of proteins into Ld and Lo phases, Frewein et al. (2016) [
69] reported a mathematical model that considers the energetic contributions to protein partitioning based on the protein's size, shape, and overall oligomerization state. This model uses experimental values for the Lo/Ld domains, such as thickness, intrinsic curvature, bending rigidity, and Gaussian curvature modulus.
The findings show that proteins with a convex shape tend to cluster more in the Ld domains, while proteins with a concave shape have a greater tendency to locate in the Lo domains. This preference becomes more pronounced as the proteins increase in size [
69].
3. The Cytoplasmic Leaflet of the Plasma Membrane
The lipid composition of the cytoplasmic leaflet of the plasma membrane, which includes a significant fraction of PC, PE, PEp (PE plasmalogen), and PS head lipids, is a crucial factor. The absence of high Tm lipids and the abundant fraction of polyunsaturated lipids are the main differences between the cytoplasmic and the exoplasmic leaflets. The average number of unsaturation per lipid in the cytoplasmic leaflet is approximately twice as high as in the exoplasmic leaflet, underscoring the importance of this composition. According to Lorent et al. [
11],
Figure 7 shows the variety of lipids in the plasma membrane's cytoplasmic leaflet.
In addition, Wang and Silvius (2001) [
70] have investigated symmetric bilayers that mimic the cytoplasmic leaflet composition before the detailed knowledge of the acyl chain properties became available [
11]. Inspired by the information that the cytosolic leaflet of cell plasma membrane presented ordered rafts, the authors investigated the possibility of forming nano-scale domains, in lipid mixtures having PS, PE, and PC phospholipids plus cholesterol. None of these combinations composing the symmetric vesicles displayed ordered domains.
Studies of symmetric bilayers with the composition of either the outer or the inner leaflet of the plasma membrane reveal that the phase behavior of each leaflet is distinct. According to investigations on symmetric vesicles, the exoplasmic leaflet should present phase separation, while the exoplasmic leaflet would present a single fluid phase, highlighting the complexity and unique characteristics of each leaflet.
The study by Wang and Silvius [
70] raised a significant question that experimentalists have since pursued: How can these different leaflets interact with each other? This question serves as a crucial starting point for further exploration and understanding of the behavior of asymmetric bilayers.
One of the answers could be an interaction between the two different leaflets, a hypothesis that prompted inquiries into the possibility of mutual influence between the leaflets that compose an asymmetric bilayer.
4. The Asymmetric Membrane
Thus far, we have outlined several significant facets of symmetric vesicles with compositions reminiscent of the outer and inner leaflets of the plasma membrane. In this section, we will address the bilayer asymmetry and the differences in behavior that arise when the PM exoplasmic and cytoplasmic leaflets compose a model bilayer from experiments on in vitro asymmetrical membranes.
Comparing
Figure 1 and
Figure 7, the composition differences between the exoplasmic and cytoplasmic leaflets are notable. The exoplasmic leaflet has a sizeable fraction of lipids, which form ordered phases. As to the inner leaflet, lipids predominantly yield disordered phases at ambient temperatures. The study of symmetric lipid bilayers alone is limited in its ability to fully capture the complexity of natural cell membranes. Thus, better models of biological membranes must include their asymmetry. Experimental models of asymmetric bilayers could provide valuable insights into the cell membrane structure and function.
In part, the investigation of asymmetric membranes was delayed because preparing asymmetric bilayers in experimental models was difficult. However, the last 10-20 years saw advances in developing cutting-edge techniques to manage asymmetrical lipid bilayers. In the following sections, we will explore some of these methods.
4.1. Experimental Procedures to Make Asymmetric Lipid Bilayer
Different methods for preparing and studying asymmetric lipid bilayers have emerged in the last two decades. Krompers and Heerklotz (2023) [
71] and Scott et al. (2021) [
72] have summarized detailed studies of the literature on these novel methods.
One approach involves creating bilayer asymmetry by modifying the headgroup of some lipids on the outer leaflet with the help of specific enzymes [
73,
74]. An example of such an enzyme is phospholipase D (PLD), which can convert the PC headgroups into PS (or PE) headgroups via transphosphatidylation in case either serine (or ethanolamine) is present in the solution and acts on the PC lipids on the outer leaflet. Thus, one may reproduce a feature of natural plasma membranes where the PS and PE headgroups are more numerous in one of the leaflets. This method investigates the bilayer asymmetry regarding the role of lipid headgroups.
A different approach that has led to significant findings involves cyclodextrins (CDs), water-soluble cyclic oligosaccharides displaying amphiphilic properties. With a ring shape whose core is hydrophobic and whose outer side is hydrophilic, CD can involve and carry other molecules. Cyclodextrins come in various sizes, allowing them to interact with various lipids. One or two CDs can load and transport cholesterol if their ring is large, whereas smaller rings can more easily load fatty acids. To create asymmetric vesicles, we can place CDs in a solution with a small number of symmetric vesicles having the desired molecular composition for the inner leaflets and a large number of symmetric vesicles with the desired molecular composition for the outer leaflets (see Fig. 8 below). Cyclodextrins molecules randomly exchange the lipids on the outer leaflet between different vesicles, thus forming asymmetric bilayers [
23,
24,
25,
26,
75,
76,
77,
78,
79].
In this case, the authors reported calculating the lipid exchange percentage through an average value due to the challenge of monitoring individual vesicles. To prepare asymmetric GUVs, the authors recommend using MβCD.
A different strategy for mounting asymmetric bilayers relies on inserting lipids in a water-oil biphasic system. Lipids placed into the oil with water droplets form a reverse micelle, in which the hydrophobic chains turn to the oil phase; these are the lipids that resemble the composition of the inner layer of the asymmetric membrane. In addition, another mixture of lipids, chosen to represent the composition of the outer leaflet, is dissolved in a separate container at the interface of a two-phase water-oil system. The next step is to put the first solution with the reverse micelles onto this container. As water droplets fall from the oil to the water phase, the lipids of the inverted micelles interact with those of the oil-water interface and coat the micelles, forming an asymmetric bilayer vesicle in the water [
80]. However, an undesired effect is that oil residues remain between leaflets. Moreover, recent reports pointed out the asymmetry might be less than 100%, as initially expected [
81]. Nevertheless, a few studies report the percentage of asymmetry or possible contamination of lipids expected to be in the inner leaflet and stay in the outer leaflet and vice versa.
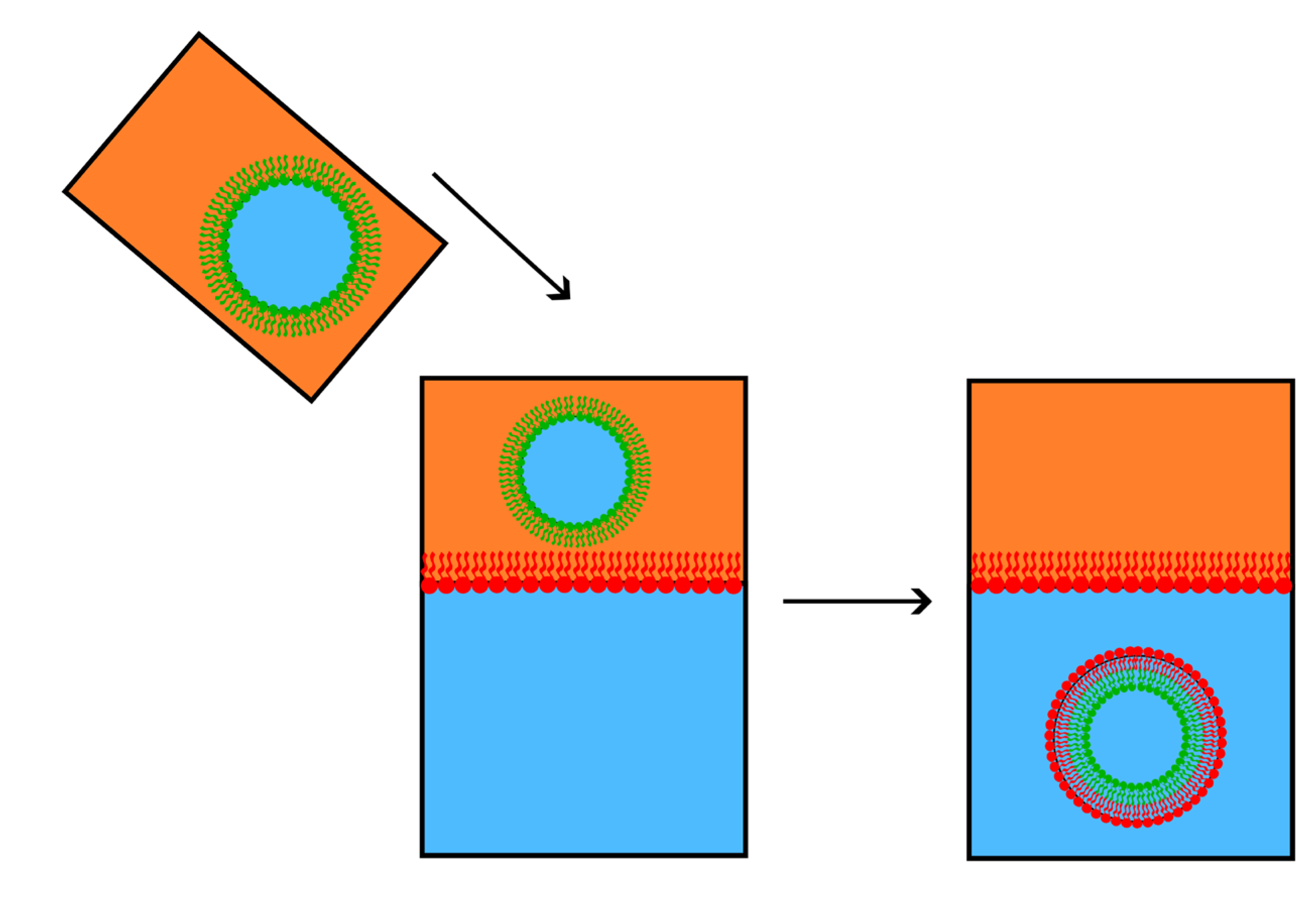
Figure 9.
The sequence represents the water-oil system technique for preparing asymmetric membranes. Oil is used as an apolar medium to prepare inverse micelles with one type of lipid (green). The oil-lipid solution is added to water without mixing. A second kind of lipid (in red) is added to the oil phase and arranges itself as a monolayer, with the polar heads turned to the water phase. The inverse (green) micelle in the oil phase precipitates through the monolayer into water. It gains an outer (red) monolayer, thus forming an asymmetric vesicle composed of red and green monolayers.
Figure 9.
The sequence represents the water-oil system technique for preparing asymmetric membranes. Oil is used as an apolar medium to prepare inverse micelles with one type of lipid (green). The oil-lipid solution is added to water without mixing. A second kind of lipid (in red) is added to the oil phase and arranges itself as a monolayer, with the polar heads turned to the water phase. The inverse (green) micelle in the oil phase precipitates through the monolayer into water. It gains an outer (red) monolayer, thus forming an asymmetric vesicle composed of red and green monolayers.
Hemifusion is a different technique for engineering asymmetric membranes [
18] , and other research groups have adopted this method to investigate asymmetric membranes [
30,
82]. This alternative method involves the connection of giant unilamellar vesicles (GUVs) and a Supported lipid bilayer (SLB) through their outer leaflets,
Figure 10. GUVs and the SLB are prepared with compositions meant for the inner and outer leaflets, respectively. Fusogenic agents, such as calcium ions, can induce hemifusion [
83,
84,
85]. In the process of hemifusion, lipid exchange occurs exclusively in the connected outer leaflets through lateral diffusion. This efficient exchange allows lipids from the supported lipid bilayer (SLB) to replace the lipids on the outer leaflet of giant unilamellar vesicles (GUVs). The SLB, acting as a substantial reservoir of lipids, plays a crucial role in this highly efficient process. Different fluorescent probes label the GUVs and the SLB, aiding in the identification of lipid exchange. Thus, after hemifusion and lipid exchange, the newly formed asymmetric GUV (aGUVs) comprises the two fluorophores originating in GUVs or the SLB. Quantifying both fluorescence signals allows us to determine the percentage of lipids exchanged for individual vesicles. This powerful tool allows for in-depth exploration of phase diagrams in asymmetric bilayers, offering a distinct advantage in this method.
Enoki and Feigenson (2019) conducted experiments to characterize hemifusion and prove that lipid transfer occurs only through the connected outer leaflets. The chelating of calcium ions after lipid exchange prevents complete bilayer fusion. The researchers have detailed the utilization of shear force to detach the aGUVs from the SLB [
18].
4.2. Electron Density Modeling for Asymmetric Bilayers
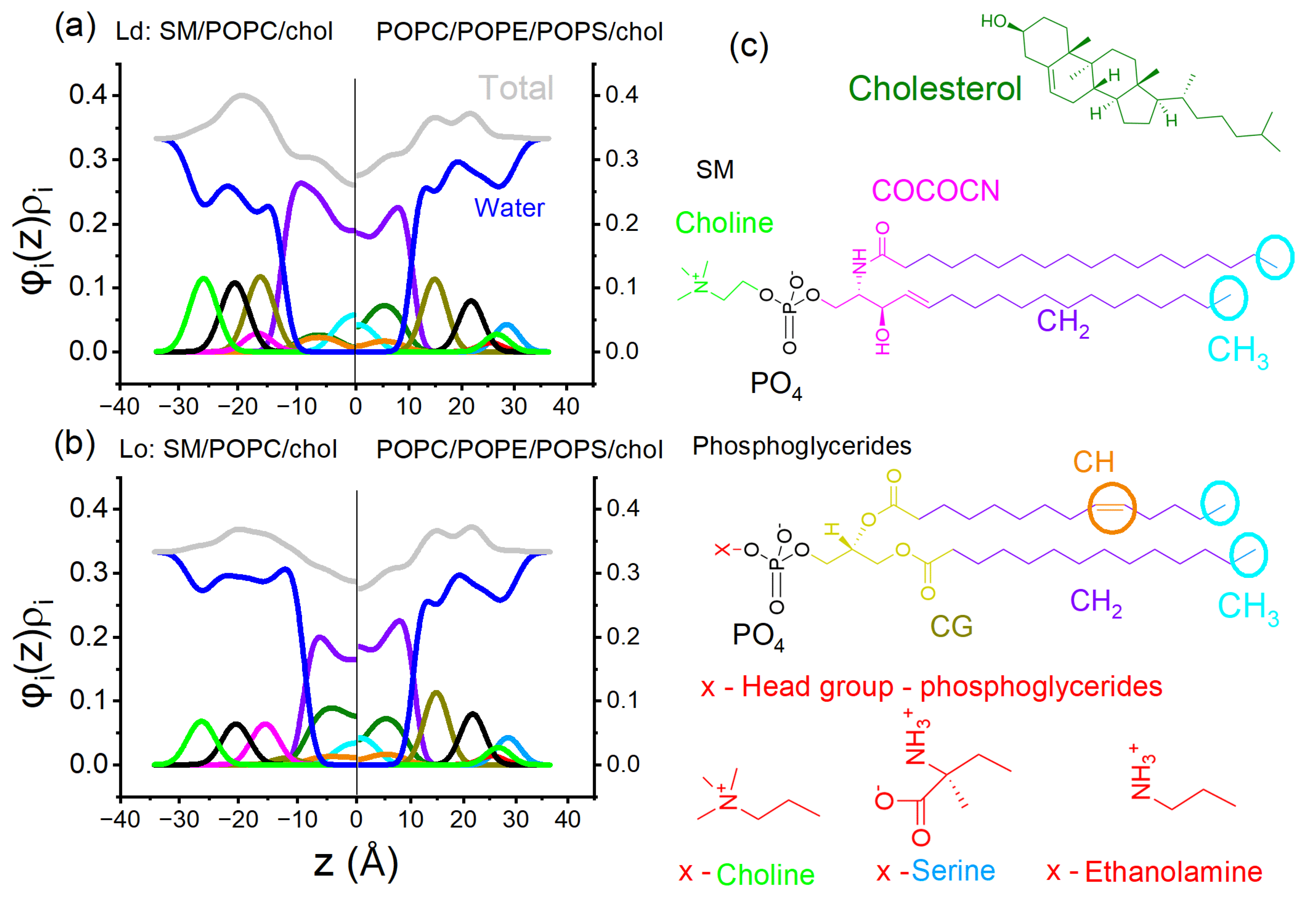
We can consider a straightforward model highlighting the differences between lipid composition modeling the plasma membrane leaflets. In this example, our asymmetric membrane has one leaflet with a composition that forms the coexistence of Ld and Lo phases, and another leaflet comprises a lipid composition that forms a single Ld phase.
Figure 13a shows a cartoon representing this asymmetric bilayer.
In order to enhance our understanding of the differences between leaflets, we evaluated the electron density profiles from leaflets containing different lipid compositions. Here, we simulate electron density profiles of symmetric bilayers to model 1) the Ld phase (leaflet Ld+Lo), 2) the Lo phase (leaflet Ld+Lo), and 3) the Ld phase (leaflet single phase) to build asymmetric bilayer. Note that in our model of the asymmetric bilayer, at the bilayer midplane, there will be the contact of compositions described by 1 and 3 and 2 and 3. (see cartoon in
Figure 13a).
In
Figure 11, the Lo and Ld compositions have the lipid molar fraction SM/POPC/chol = 0.22/0.71/0.07 (a, Ld phase), and SM/POPC/chol = 0.62/0.08/0.3 (b, Lo phase). These compositions are obtained from the phase diagram and represent the endpoint of the bottom tieline, as shown in
Figure 2c. In addition, in both panels (a and b), the model of the cytoplasmic leaflet comprises POPC/POPS/POPE/chol = 0.25/0.28/0.25/0.22. We note that we performed these calculations in symmetric bilayers, and here, we contrapose them together for comparison, though it may represent an asymmetric bilayer without any coupling.
Figure 11 shows the electron density profile ψi of each lipid chemical group weighted by its volume fraction ψi within the lipid bilayer. The total electron density profiles are represented by gray lines in the
Figure 11. The distribution of the chemical groups inside the bilayer was calculated through the Modified Scattering Density Profile (MSDP) model [
86] applied to small angle X-Ray scattering (SAXS) data, using GENFIT software [
87]. To further details, the reader must refer to references [
86,
88].
Interestingly, the asymmetric electron density profile may create distinct lateral pressure between leaflets, affecting the partition of transmembrane peptides and proteins. Thus,
Figure 11 emphasizes the potential impact of distinct lipid compositions on electron density profiles and resulting physical properties. However, it prompts the question of whether asymmetric leaflets retain their intrinsic characteristics despite significant differences in their properties, such as order and lipid packing.
4.3. Experimental Phase Diagram of Asymmetric Lipid Bilayers: Domains and Coupling
Different experiments performed by different research groups on asymmetric bilayers have disclosed the appearance of induced domains on a leaflet expected to be uniform. First, the observations were made on asymmetric bilayers assembled on support [
14,
15] and in (Montal-Mueller) membranes [
27], as well as asymmetric vesicles [
17,
18,
26,
55,
75,
89,
90]. Moreover, several experiments showed that asymmetric membranes prepared using cyclodextrin form induced domains but could also result in vanishing the phase separation [
16,
23,
30,
91], as discussed below. Previous theoretical reports investigating bilayer asymmetry observed what has been reported in the experiments by London's group [
92,
93].
Enoki and Feigenson (2019) and Enoki et al. (2021) report a particular example of induced domains [
18,
55]. In these experiments, the authors assembled asymmetric bilayers with one leaflet having a lipid composition DSPC/DOPC/chol = 0.39/0.39/0.22, which forms Ld and Lo domains. In contrast, the second leaflet is enriched with DOPC/chol = 0.8/0.2, which displays a fluid phase in the case of the symmetric membrane (
Figure 3a). A surprising result in these asymmetric bilayers revealed that the DOPC/chol-rich leaflet, which was expected to be uniform, showed ordered domains.
A different effect, previously observed by different research groups, occurs when a phase-separate and a fluid form an asymmetric membrane, and the fluid leaflet dictates the phase behavior of the entire bilayer. In particular, Enoki and Heberle (2023) reported a partial phase diagram from asymmetric DPPC/DOPC bilayers by contraposing theory and experiments [
30]. The saturated lipid DPPC is a gel-forming lipid at room temperature, while pure DOPC bilayers display a disordered phase at ambient temperature. The asymmetry of the system results from different relative concentrations on the two leaflets.
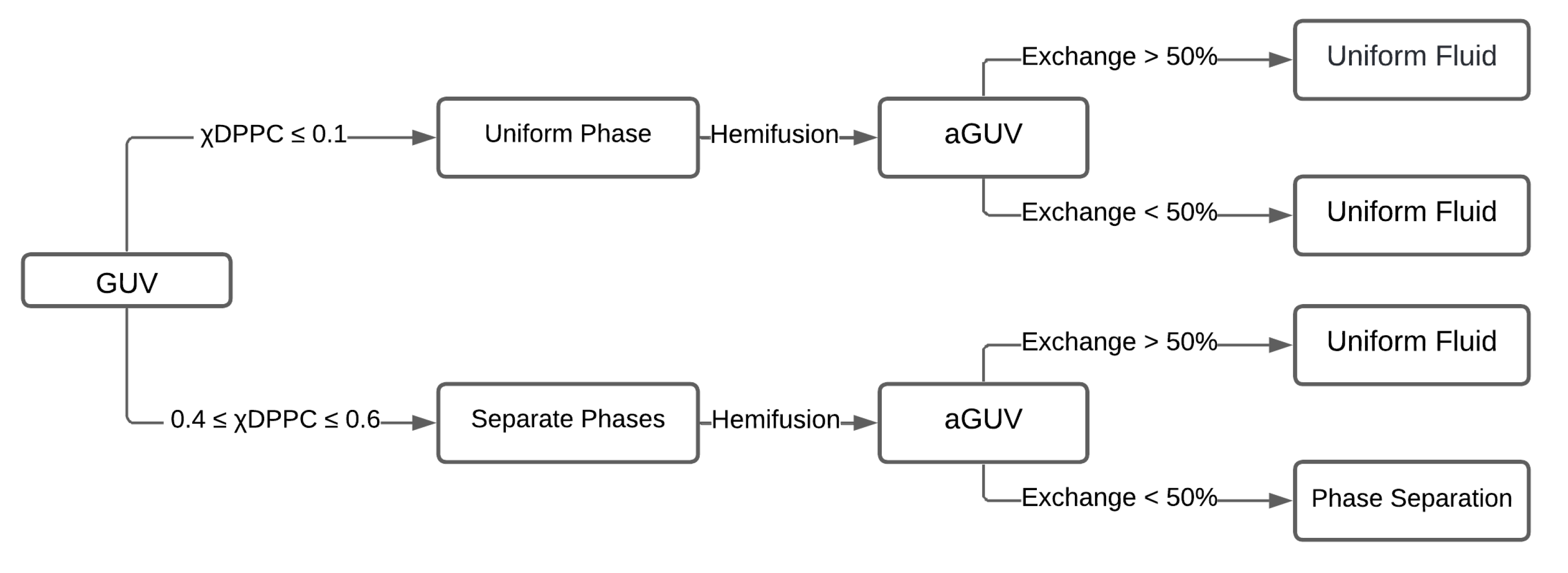
Figure 12 summarizes the phase behavior of the corresponding symmetric and asymmetric bilayers.
A necessary first step is investigating the phase behavior of symmetric membranes composed of DPPC/DOPC. A low fraction of the saturated lipid (DPPC ≤ 0.1) in symmetric bilayers yields a uniform, disordered phase. As the molar fraction of DPPC increases (0.2<DPPC<0.8), phase separation into gel + fluid phases sets in. Higher fractions of the saturated lipid (DPPC ≥ 0.9) result in faceted GUVs with a single uniform gel phase.
The second step is the preparation of aGUVs, engineered through the hemifusion technique. Briefly, the symmetric DPPC/DOPC GUVs were hemifused with a DOPC SLB, thus favoring the diffusion of DOPC into the GUV's outer leaflet. The inner leaflet maintains the initial lipid composition of the symmetric GUVs.
GUVs and the SLB were previously doped with different fluorescent probes to control the asymmetry: DiD fluorophore (red) labels the symmetric GUV, whereas TFPC (green) labels the DOPC SLB. After hemifusion, the green dyes inform about the behavior of the outer leaflet of aGUVs. The percentage of lipid exchange can be calculated by examining the signal of both dyes on individual aGUVs.
The fluorescence signals used to control asymmetry also report the presence or absence of phase separation. The authors thus establish the phase behavior of the asymmetric GUVs (aGUVs) and compare it with that of the symmetric GUVs.
The asymmetric vesicles followed the symmetric membrane behavior for a low fraction of the saturated lipid (molar fraction DPPC ≤ 0.1) in the symmetric GUV. They exhibited a uniform fluid phase, independently of the degree of outer leaflet exchange. Thus, for low DPPC fraction in the original symmetric vesicle, aGUVs present the exact morphology of the symmetric vesicles.
When the saturated lipid is present in intermediate fractions (0.4 ≤ DPPC ≤ 0.6) in the inner leaflet and less than 50% lipid exchange occurs for the outer leaflet, aGUVs exhibit phase separation in both leaflets; however, if the exchange in the outer leaflet is over 50%, aGUVs turn into uniform vesicles under observation in an ordinary microscope. Surprisingly, the phase domains before present in the inner leaflet vanish or have reduced domain sizes below 200 nm, the microscope resolution.
These experimental results suggest an interpretation in terms of interaction between leaflets: an energetic penalty in the midplane between ordered and disordered phases in opposing leaflets, which could compete with interactions driving phase separation.
The presence of induced domains, or the second case in which the asymmetric membranes show a single phase, has been described in terms of a "transbilayer coupling" [
92,
93]. The origin of this possible coupling is under discussion in different publications [
29,
92,
93,
94,
95,
96,
97].
4.4. Experimental Phase Diagram of Asymmetric Lipid Bilayers: Domains and Coupling
Theoretical models play a crucial role in elucidating lipid interactions, and our comprehension of this complex system has significantly advanced over the last 50 years [
98]. For example, intra-leaflet lipid-lipid interactions can explain the main gel-fluid transition for single lipid membranes or the ripple phase [
99,
100,
101,
102]. Furthermore, the fascinating dynamics of intra-leaflet interactions can be beautifully captured through pairwise interactions, offering us a powerful way to model line tension [
103].
The new experimental results on asymmetric membranes have triggered the interest of the membrane community with a new question: What is the rationale of the “transbilayer coupling”? Several hypotheses are under investigation: interdigitation, cholesterol distribution between leaflets, and inter-leaflet interactions, among others.
In this minireview, we focus on the hypothesis of a coupling interaction between leaflets of asymmetric bilayers since the domains that appear on the leaflet composed of low Tm lipids frustrate the previous idea of negligible coupling between the apposed monolayers. In this case, a plausible explanation could be an interaction between leaflets responsible for the formation of induced ordered domains within the leaflet of fluid (low Tm) lipid composition.
Figure 13 and 13b illustrate this hypothesis. In
Figure 13 a, each leaflet of the asymmetric bilayer displays an independent behavior, suggested by the phase diagrams of the two corresponding symmetric membranes. In contrast,
Figure 13 b illustrates the possible phase behavior of the two leaflets in accordance with the observed behavior in the experiments.
The likely cause of the formation of the induced ordered domain would be an energetic penalty between Lo/Ld contacts within the intermediate plane of the bilayer. The energy penalty would result in lipids rearranging, reducing unfavorable contacts between ordered and disordered chains, thereby decreasing interfacial tension. Enoki and Heberle (2023) have compared their experimental results for the phase diagram of the asymmetric lipid bilayer with predictions of a simple mean-field model [
30]. In order to probe the idea of an interaction that disfavors the presence of different thermal phases on the two leaflets, a regular solution model was considered, added with an interplane interaction parameter that penalizes the opposite phases on facing monolayers. In the model, the interplane Λ interaction, representing midplane interfacial tension, competes with an in-plane lipid-lipid interaction X. The theoretical model roughly reproduces the experimental phase diagram for intermediate values of the interplane Λ interaction (0.35<Λ<0.55) parameter at fixed X=2.25. Λ and X are dimensionless model parameters. However, Λ is proportional to the midplane surface tension parameter γ proposed by Wagner et. al. (2007) [
99], and our model parameters produce results for the midplane surface tension value compatible with values reported in Molecular Dynamics simulation studies [
104].
A different approach to interleaflet interaction relies on the competition between elastic energies. Lipids exhibit different intrinsic curvature depending on headgroup-tail sizes and on-chain rigidity [
105]. The presence of curvature involves elastic energy, which can be described as bending energy dependent on the bending modulus of the specific molecular structure. For symmetric vesicles, the bending rigidity is the same on the inner and outer leaflets, and the system relaxes to zero area tension on each leaflet. As to the asymmetric case, the situation is more complex. The difference in composition between the two leaflets is the source of additional area stress. In other words, different lipid geometries impair an ideal area relaxation for the two leaflets [
106,
107]. However, a second factor is present: each leaflet presents its own rigidity and, therefore, a preferential curvature, while, at the same time, each leaflet displays its own area tension. The two effects may oppose each other (as represented in the cartoon,
Figure 14), which can be described as competing terms in elastic energy.
Thus, bending stress remains if one considers a relaxed overall area tension. Otherwise, if bending stress is relaxed, area stress cannot be relaxed simultaneously. Hossein and Deserno (2020) have explored the two features and showed that even in the minimum energy situation, with zero overall stress, differential stress remains between the two leaflets [
108].
Cholesterol may be unable to balance this stress if cholesterol molecules prefer the leaflet modeling the exoplasmic leaflet due to its preference for saturated lipids. However, the distribution of cholesterol between leaflets is still controversial. Hossein and Deserno (2020) investigated the competition analytically and through coarse-grained models and have shown that the differential stress may contribute to an increased bending modulus and a transition to the gel phase of the compressed leaflet [
108]. The investigation of the possibility is an open road to exciting research for membrane experts, either through in vitro experiments, theory, or computational experiments.
5. Summary
In this short review, we have shown the importance of constructing lipid phase diagrams to guide the study of cell membranes of different compositions. Many techniques are available for identifying the coexistence of phases. An important question from the point of view of natural membranes is the establishment of the size of the ordered/disordered domains, which might be related to rafts seen in natural cell membranes.
Thus, as a first step in investigating lipid bilayers, experimentalists have studied different symmetric bilayers mimicking the cytoplasmic or exoplasmic leaflet of the plasma membrane. These studies allowed the construction of several ternary and quaternary phase diagrams and the discovery of nanoscopic domains.
As a second step, recent studies have approached binary lipid asymmetric systems. Hemifused asymmetric bilayers were shown to produce induced order (or induced disorder) domains, highlighting the necessity of considering interleaflet coupling in asymmetric bilayers. Initial modeling of this new phenomenon has been presented in terms of direct coupling due to interleaflet tension and elastic coupling.
In addition, the field is open to exciting new research in the laboratory, modeling design, and computer. Ternary asymmetric bilayers must be explored for phase and domain properties, the nature of coupling requires further testing, and the impact of asymmetry on transmembrane protein localization are just some of the open questions in new adventures for membrane scientists.
Author Contributions
TAE, VBH, ISO, GXP, MLBS, PHLOG, SUP and RI wrote the paper. SUP and RI worked on the electron density profiles and lipid volume fraction distribution modeling, and TAE, ISO, GXP, MLBS, PHLOG and SUP made the figures. All authors performed research and revised/edited the paper. Supervision, project administration, and funding acquisition, TAE. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The São Paulo Research Foundation, FAPESP (2022/04046-4, 2023/05540-5 and 2024/14296-3) and the Serrapilheira Institute (R-2401-46767) to TAE, FAPESP (2024/02663-1) to ISO, FAPESP (2023/11908-5) to GXP, FAPESP (2024/00346-9) to MLBS, FAPESP (2022/07024-1) to SUP. The APC was funded by Membranes, MDPI.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
RI and SUP thanks to Dr. Francesco Spinozzi for MSDP modeling into GENFIT software.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AFM |
Atomic Force Microscopy |
| aGUVs |
Asymmetric Giant Unilamellar Vesicle |
| bSM |
Brain Sphingomyelin |
| CDs |
Cyclodextrins |
| Chol |
Cholesterol |
| DLS |
Dynamic Light Scattering |
| DHE |
Dehydroergosterol |
| DOPC |
1,2-Dioleoyl-sn-glycero-3-phosphocholine |
| DOPE |
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine |
| DPPC |
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine |
| DSPC |
1,2-Distearoyl-sn-glycero-3-phosphocholine |
| ESR |
Electron Spin Resonance |
| FRET |
Förster Resonance Energy Transfer |
| GUV |
Giant Unilamellar Vesicle |
| Kp |
Partition Coefficient |
| LUV |
Large Unilamellar Vesicle |
| Ld |
Liquid-disordered phase |
| Lo |
Liquid-ordered phase |
| MSDP |
Modified Scattering Density Profile |
| PC |
Phosphatidylcholine |
| PE |
Phosphatidylethanolamine |
| Pep |
Phosphatidylethanolamine plasmalogen |
| PLD |
phospholipase D |
| PM |
Plasma Membrane |
| POPG |
1-Palmitoyl-2-Oleoyl-sn-glycero-3-[Phospho-rac-(1-glycerol)] |
| PS |
Phosphatidylserine |
| SANS |
Small-Angle Neutron Scattering |
| SAXS |
Small Angle X-ray Scattering |
| SLB |
Supported lipid bilayer |
| SM |
Sphingomyelin |
| SSPP |
Steady-State Probe Partition |
| TFPC |
1-palmitoyl-2-(dipyrrometheneboron difluoride)undecanoyl-sn-glycero-3-phosphocholine |
References
- Cockcroft, S. Mammalian Lipids: Structure, Synthesis and Function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Rees, D.A.; Young, G.; Walkinshaw, M.D.; Darke, A. Order-Disorder Transition for a Bacterial Polysaccharide in Solution. A Role for Polysaccharide Conformation in Recognition between Xanthomonas Pathogen and Its Plant Host. J. Mol. Biol. 1977, 110, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Paulowski, L.; Donoghue, A.; Nehls, C.; Groth, S.; Koistinen, M.; Hagge, S.O.; Böhling, A.; Winterhalter, M.; Gutsmann, T. The Beauty of Asymmetric Membranes: Reconstitution of the Outer Membrane of Gram-Negative Bacteria. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Bretscher, M.S. Asymmetrical Lipid Bilayer Structure for Biological Membranes. Nat. New Biol. 1972, 236, 11–12. [Google Scholar] [CrossRef]
- Verkleij, A. ; Zwaal, R.F..; Roelofsen, B.; Comfurius, P.; Kastelijn, D.; van Deenen, L.L.. The Asymmetric Distribution of Phospholipids in the Human Red Cell Membrane. A Combined Study Using Phospholipases and Freeze-Etch Electron Microscopy. Biochim. Biophys. Acta - Biomembr. 1973, 323, 178–193. [CrossRef]
- Sandra, A.; Pagano, R.E. Phospholipid Asymmetry in LM Cell Plasma Membrane Derivatives: Polar Head Group and Acyl Chain Distributions†. Biochemistry 1978, 17, 332–338. [Google Scholar] [CrossRef]
- Devaux, P.F. Static and Dynamic Lipid Asymmetry in Cell Membranes. Biochemistry 1991, 30, 1163–1173. [Google Scholar] [CrossRef]
- Wood, W.G.; Igbavboa, U.; Müller, W.E.; Eckert, G.P. Cholesterol Asymmetry in Synaptic Plasma Membranes. J. Neurochem. 2011, 116, 684–689. [Google Scholar] [CrossRef]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.; Lyman, E.; Levental, I. Plasma Membranes Are Asymmetric in Lipid Unsaturation, Packing and Protein Shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Gohrbandt, M.; Lipski, A.; Grimshaw, J.W.; Buttress, J.A.; Baig, Z.; Herkenhoff, B.; Walter, S.; Kurre, R.; Deckers-Hebestreit, G.; Strahl, H. Low Membrane Fluidity Triggers Lipid Phase Separation and Protein Segregation in Living Bacteria. EMBO J. 2022, 41. [Google Scholar] [CrossRef] [PubMed]
- Chaisson, E.H.; Heberle, F.A.; Doktorova, M. Building Asymmetric Lipid Bilayers for Molecular Dynamics Simulations: What Methods Exist and How to Choose One? Membranes (Basel). 2023, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, V.; Wan, C.; Tamm, L.K. Domain Coupling in Asymmetric Lipid Bilayers. Biochim. Biophys. Acta - Biomembr. 2009, 1788, 64–71. [Google Scholar] [CrossRef]
- Wan, C.; Kiessling, V.; Tamm, L.K. Coupling of Cholesterol-Rich Lipid Phases in Asymmetric Bilayers. Biochemistry 2008, 47, 2190–2198. [Google Scholar] [CrossRef]
- Feigenson, G.W.; Huang, J.; Enoki, T.A. An Unexpected Driving Force for Lipid Order Appears in Asymmetric Lipid Bilayers. J. Am. Chem. Soc. 2023, 145, 21717–21722. [Google Scholar] [CrossRef]
- Enoki, T.A.; Feigenson, G.W. Improving Our Picture of the Plasma Membrane: Rafts Induce Ordered Domains in a Simplified Model Cytoplasmic Leaflet. Biochim. Biophys. Acta - Biomembr. 2022, 1864, 183995. [Google Scholar] [CrossRef]
- Enoki, T.A.; Feigenson, G.W. Asymmetric Bilayers by Hemifusion: Method and Leaflet Behaviors. Biophys. J. 2019, 117, 1037–1050. [Google Scholar] [CrossRef]
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric Lipid Membranes: Towards More Realistic Model Systems. Membranes (Basel). 2015, 5, 180–196. [Google Scholar] [CrossRef]
- Eicher, B.; Marquardt, D.; Heberle, F.A.; Letofsky-Papst, I.; Rechberger, G.N.; Appavou, M.-S.; Katsaras, J.; Pabst, G. Intrinsic Curvature-Mediated Transbilayer Coupling in Asymmetric Lipid Vesicles. Biophys. J. 2018, 114, 146–157. [Google Scholar] [CrossRef]
- Heberle, F.A.; Marquardt, D.; Doktorova, M.; Geier, B.; Standaert, R.F.; Heftberger, P.; Kollmitzer, B.; Nickels, J.D.; Dick, R.A.; Feigenson, G.W.; et al. Subnanometer Structure of an Asymmetric Model Membrane: Interleaflet Coupling Influences Domain Properties. Langmuir 2016, 32, 5195–5200. [Google Scholar] [CrossRef] [PubMed]
- Frewein, M.P.K.; Piller, P.; Semeraro, E.F.; Batchu, K.C.; Heberle, F.A.; Scott, H.L.; Gerelli, Y.; Porcar, L.; Pabst, G. Interdigitation-Induced Order and Disorder in Asymmetric Membranes. J. Membr. Biol. 2022, 255, 407–421. [Google Scholar] [CrossRef] [PubMed]
- London, E. Membrane Structure-Function Insights from Asymmetric Lipid Vesicles. Acc. Chem. Res. 2019, 52, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; London, E. Preparation of Artificial Plasma Membrane Mimicking Vesicles with Lipid Asymmetry. PLoS One 2014, 9, e87903. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.T. ; Megha; London, E. Preparation and Properties of Asymmetric Vesicles That Mimic Cell Membranes. Effect upon Lipid Raft Formation and Transmembrane Helix Orientation. J. Biol. Chem. 2009, 284, 6079–6092. [Google Scholar] [CrossRef]
- Chiantia, S.; Schwille, P.; Klymchenko, A.S.; London, E. Asymmetric GUVs Prepared by MβCD-Mediated Lipid Exchange: An FCS Study. Biophys. J. 2011, 100, L1–L3. [Google Scholar] [CrossRef]
- Collins, M.D.; Keller, S.L. Tuning Lipid Mixtures to Induce or Suppress Domain Formation across Leaflets of Unsupported Asymmetric Bilayers. Proc. Natl. Acad. Sci. 2008, 105, 124–128. [Google Scholar] [CrossRef]
- Doktorova, M.; Heberle, F.A.; Eicher, B.; Standaert, R.F.; Katsaras, J.; London, E.; Pabst, G.; Marquardt, D. Preparation of Asymmetric Phospholipid Vesicles for Use as Cell Membrane Models. Nat. Protoc. 2018, 13, 2086–2101. [Google Scholar] [CrossRef]
- Enoki, T.A. The Use of Hemifusion to Create Asymmetric Giant Unilamellar Vesicles: Insights on Induced Order Domains. In Methods in Enzymology; Baumgart, T., Deserno, M., Eds.; Elsevier, 2024.
- Enoki, T.A.; Heberle, F.A. Experimentally Determined Leaflet–Leaflet Phase Diagram of an Asymmetric Lipid Bilayer. Proc. Natl. Acad. Sci. U. S. A. 2023, 120. [Google Scholar] [CrossRef]
- Feigenson, G.W.; Buboltz, J.T. Ternary Phase Diagram of Dipalmitoyl-PC/Dilauroyl-PC/Cholesterol: Nanoscopic Domain Formation Driven by Cholesterol. Biophys. J. 2001, 80, 2775–2788. [Google Scholar] [CrossRef]
- Feigenson, G.W. Phase Diagrams and Lipid Domains in Multicomponent Lipid Bilayer Mixtures. BBA - Biomembr. 2008, 1788, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, G.W. Phase Behavior of Lipid Mixtures. Nat. Chem. Biol. 2006, 2, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Heberle, F.A.; Feigenson, G.W.; Wu, J.; Goh, S.L.; Petruzielo, R.S.; Feigenson, G.W.; Zhao, J.; Wu, J.; Heberle, F.A.; Mills, T.T.; et al. Phase Studies of Model Biomembranes: Complex Behavior of DSPC/DOPC/Cholesterol. Biochim. Biophys. Acta 1768 2007, 2764–2776. [Google Scholar] [CrossRef]
- Petruzielo, R.S.; Heberle, F. a; Drazba, P.; Katsaras, J.; Feigenson, G.W. Phase Behavior and Domain Size in Sphingomyelin-Containing Lipid Bilayers Author ’ s Personal Copy. Biochim. Biophys. Acta 2013, 1828, 1302–1313. [Google Scholar] [CrossRef]
- Enoki, T.A.; Heberle, F.A.; Feigenson, G.W. FRET Detects the Size of Nanodomains for Coexisting Liquid-Disordered and Liquid-Ordered Phases. Biophys. J. 2018, 114, 1921–1935. [Google Scholar] [CrossRef]
- Konyakhina, T.M.; Wu, J.; Mastroianni, J.D.; Heberle, F. a.; Feigenson, G.W. Phase Diagram of a 4-Component Lipid Mixture: DSPC/DOPC/POPC/Chol. Biochim. Biophys. Acta - Biomembr. 2013, 1828, 2204–2214. [Google Scholar] [CrossRef]
- Usery, R.D.; Enoki, T.A.; Wickramasinghe, S.P.; Weiner, M.D.; Tsai, W.C.; Kim, M.B.; Wang, S.; Torng, T.L.; Ackerman, D.G.; Heberle, F.A.; et al. Line Tension Controls Liquid-Disordered + Liquid-Ordered Domain Size Transition in Lipid Bilayers. Biophys. J. 2017, 112, 1431–1443. [Google Scholar] [CrossRef]
- Heberle, F. a.; Buboltz, J.T.; Stringer, D.; Feigenson, G.W. Fluorescence Methods to Detect Phase Boundaries in Lipid Bilayer Mixtures. Biochim. Biophys. Acta - Mol. Cell Res. 2005, 1746, 186–192. [Google Scholar] [CrossRef]
- Konyakhina, T.M.; Feigenson, G.W. Phase Diagram of a Polyunsaturated Lipid Mixture: Brain Sphingomyelin/1-Stearoyl-2-Docosahexaenoyl-Sn-Glycero-3-Phosphocholine/Cholesterol. Biochim. Biophys. Acta - Biomembr. 2015, 1858, 153–161. [Google Scholar] [CrossRef]
- Heberle, F.A.; Wu, J.; Goh, S.L.; Petruzielo, R.S.; Feigenson, G.W. Comparison of Three Ternary Lipid Bilayer Mixtures: FRET and ESR Reveal Nanodomains. Biophys. J. 2010, 99, 3309–3318. [Google Scholar] [CrossRef]
- Heftberger, P.; Kollmitzer, B.; Heberle, F. a.; Pan, J.; Rappolt, M.; Amenitsch, H.; Kučerka, N.; Katsaras, J.; Pabst, G. Global Small-Angle X-Ray Scattering Data Analysis for Multilamellar Vesicles: The Evolution of the Scattering Density Profile Model. J. Appl. Crystallogr. 2014, 47, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Heberle, F.A.; Petruzielo, R.S.; Pan, J.; Drazba, P.; Kučerka, N.; Standaert, R.F.; Feigenson, G.W.; Katsaras, J. Bilayer Thickness Mismatch Controls Domain Size in Model Membranes. J. Am. Chem. Soc. 2013, 135, 6853–6859. [Google Scholar] [CrossRef] [PubMed]
- Heberle, F.A.; Doktorova, M.; Scott, H.L.; Skinkle, A.D.; Waxham, M.N.; Levental, I. Direct Label-Free Imaging of Nanodomains in Biomimetic and Biological Membranes by Cryogenic Electron Microscopy. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 19943–19952. [Google Scholar] [CrossRef]
- Cornell, C.E.; Mileant, A.; Thakkar, N.; Lee, K.K.; Keller, S.L. Direct Imaging of Liquid Domains in Membranes by Cryo-Electron Tomography. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 19713–19719. [Google Scholar] [CrossRef]
- Tokumasu, F.; Jin, A.J.; Feigenson, G.W.; Dvorak, J.A. Nanoscopic Lipid Domain Dynamics Revealed by Atomic Force Microscopy. Biophys. J. 2003, 84, 2609–2618. [Google Scholar] [CrossRef]
- Tokumasu, F.; Jin, A.J.; Feigenson, G.W.; Dvorak, J.A. Atomic Force Microscopy of Nanometric Liposome Adsorption and Nanoscopic Membrane Domain Formation. Ultramicroscopy 2003, 97, 217–227. [Google Scholar] [CrossRef]
- Buboltz, J.T. Steady-State Probe-Partitioning Fluorescence Resonance Energy Transfer: A Simple and Robust Tool for the Study of Membrane Phase Behavior. Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys. 2007, 76, 1–7. [Google Scholar] [CrossRef]
- Morales-Penningston, N.F.; Wu, J.; Farkas, E.R.; Goh, S.L.; Konyakhina, T.M.; Zheng, J.Y.; Webb, W.W.; Feigenson, G.W. GUV Preparation and Imaging: Minimizing Artifacts. Biochim. Biophys. Acta - Biomembr. 2010, 1798, 1324–1332. [Google Scholar] [CrossRef]
- Tsai, W.C.; Feigenson, G.W. Lowering Line Tension with High Cholesterol Content Induces a Transition from Macroscopic to Nanoscopic Phase Domains in Model Biomembranes. Biochim. Biophys. Acta - Biomembr. 2019, 1861, 478–485. [Google Scholar] [CrossRef]
- Konyakhina, T.M.; Goh, S.L.; Amazon, J.; Heberle, F. a.; Wu, J.; Feigenson, G.W. Control of a Nanoscopic-to-Macroscopic Transition: Modulated Phases in Four-Component DSPC/DOPC/POPC/Chol Giant Unilamellar Vesicles. Biophys. J. 2011, 101, L8–L10. [Google Scholar] [CrossRef]
- Goh, S.L.; Amazon, J.J.; Feigenson, G.W. Toward a Better Raft Model: Modulated Phases in the Four-Component Bilayer, DSPC/DOPC/POPC/CHOL. Biophys. J. 2013, 104, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy Principles of Fluorescence Spectroscopy; 2006; ISBN 978-0-387-31278-1 (Print) 978-0-387-46312-4 (Online).
- Frazier, M.L.; Wright, J.R.; Pokorny, A.; Almeida, P.F.F. Investigation of Domain Formation in Sphingomyelin/Cholesterol/POPC Mixtures by Fluorescence Resonance Energy Transfer and Monte Carlo Simulations. Biophys. J. 2007, 92, 2422–2433. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.A.; Wu, J.; Heberle, F.A.; Feigenson, G.W. Investigation of the Domain Line Tension in Asymmetric Vesicles Prepared via Hemifusion. Biochim. Biophys. Acta - Biomembr. 2021, 1863, 183586. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Heberle, F.A.; Miti, T.; Eicher, B.; London, E.; Katsaras, J.; Pabst, G. 1H NMR Shows Slow Phospholipid Flip-Flop in Gel and Fluid Bilayers. Langmuir 2017, 33, 3731–3741. [Google Scholar] [CrossRef] [PubMed]
- Heberle, F.A.; Doktorova, M.; Goh, S.L.; Standaert, R.F.; Katsaras, J.; Feigenson, G.W. Hybrid and Nonhybrid Lipids Exert Common Effects on Membrane Raft Size and Morphology. J. Am. Chem. Soc. 2013, 135, 14932–14935. [Google Scholar] [CrossRef]
- Veatch, S.L.; Keller, S.L. Separation of Liquid Phases in Giant Vesicles of Ternary Mixtures of Phospholipids and Cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef]
- Veatch, S.L.; Gawrisch, K.; Keller, S.L. Closed-Loop Miscibility Gap and Quantitative Tie-Lines in Ternary Membranes Containing Diphytanoyl PC. Biophys. J. 2006, 90, 4428–4436. [Google Scholar] [CrossRef]
- Seul, M.; Andelman, D. Domain Shapes and Patterns: The Phenomenology of Modulated Phases. Science (80-. ). 1995, 267, 476–483. [Google Scholar] [CrossRef]
- Amazon, J.J.; Goh, S.L.; Feigenson, G.W. Competition between Line Tension and Curvature Stabilizes Modulated Phase Patterns on the Surface of Giant Unilamellar Vesicles: A Simulation Study. Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys. 2013, 87, 1–10. [Google Scholar] [CrossRef]
- Amazon, J.J.; Feigenson, G.W. Lattice Simulations of Phase Morphology on Lipid Bilayers: Renormalization, Membrane Shape, and Electrostatic Dipole Interactions. Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys. 2014, 89, 1–11. [Google Scholar] [CrossRef]
- Helfrich, W. Elastic Properties of Lipid Bilayers: Theory and Possible Experiments. Zeitschrift fur Naturforsch. - Sect. C J. Biosci. 1973, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-Scale Fluid/Fluid Phase Separation of Proteins and Lipids in Giant Plasma Membrane Vesicles. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Levental, I. Structural Determinants of Protein Partitioning into Ordered Membrane Domains and Lipid Rafts. Chem. Phys. Lipids 2015, 192, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Diaz-Rohrer, B.; Lin, X.; Spring, K.; Gorfe, A.A.; Levental, K.R.; Levental, I. Structural Determinants and Functional Consequences of Protein Affinity for Membrane Rafts. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, H.J.; Stevens, T.J.; Munro, S. A Comprehensive Comparison of Transmembrane Domains Reveals Organelle-Specific Properties. Cell 2010, 142, 158–169. [Google Scholar] [CrossRef]
- Killian, J.A.A. Synthetic Peptides as Models for Intrinsic Membrane Proteins. FEBS Lett. 2003, 555, 134–138. [Google Scholar] [CrossRef]
- Frewein, M.; Kollmitzer, B.; Heftberger, P.; Pabst, G. Lateral Pressure-Mediated Protein Partitioning into Liquid-Ordered/Liquid-Disordered Domains. Soft Matter 2016, 12, 3189–3195. [Google Scholar] [CrossRef]
- Wang, T.-Y.Y.; Silvius, J.R. Cholesterol Does Not Induce Segregation of Liquid-Ordered Domains in Bilayers Modeling the Inner Leaflet of the Plasma Membrane. Biophys. J. 2001, 81, 2762–2773. [Google Scholar] [CrossRef]
- Krompers, M.; Heerklotz, H. A Guide to Your Desired Lipid-Asymmetric Vesicles. Membranes (Basel). 2023, 13, 267. [Google Scholar] [CrossRef]
- Scott, H.L.; Kennison, K.B.; Enoki, T.A.; Doktorova, M.; Kinnun, J.J.; Heberle, F.A.; Katsaras, J. Model Membrane Systems Used to Study Plasma Membrane Lipid Asymmetry. Symmetry (Basel). 2021, 13, 1356. [Google Scholar] [CrossRef]
- Drechsler, C.; Markones, M.; Choi, J.Y.; Frieling, N.; Fiedler, S.; Voelker, D.R.; Schubert, R.; Heerklotz, H. Preparation of Asymmetric Liposomes Using a Phosphatidylserine Decarboxylase. Biophys. J. 2018, 115, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, R.; Kurosaki, H.; Nakao, H.; Ikeda, K.; Nakano, M. Formation of Asymmetric Vesicles via Phospholipase D-Mediated Transphosphatidylation. Biochim. Biophys. Acta - Biomembr. 2018, 1860, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.T.; London, E. Preparation and Properties of Asymmetric Large Unilamellar Vesicles: Interleaflet Coupling in Asymmetric Vesicles Is Dependent on Temperature but Not Curvature. Biophys. J. 2011, 100, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Chiantia, S.; London, E. Acyl Chain Length and Saturation Modulate Interleaflet Coupling in Asymmetric Bilayers: Effects on Dynamics and Structural Order. Biophys. J. 2012, 103, 2311–2319. [Google Scholar] [CrossRef]
- Lin, Q.; London, E. Ordered Raft Domains Induced by Outer Leaflet Sphingomyelin in Cholesterol-Rich Asymmetric Vesicles. Biophys. J. 2015, 108, 2212–2222. [Google Scholar] [CrossRef]
- Huang, Z.; London, E. Effect of Cyclodextrin and Membrane Lipid Structure upon Cyclodextrin-Lipid Interaction. Langmuir 2013, 29, 14631–14638. [Google Scholar] [CrossRef]
- Kakuda, S.; Suresh, P.; Li, G.; London, E. Loss of Plasma Membrane Lipid Asymmetry Can Induce Ordered Domain (Raft) Formation. J. Lipid Res. 2022, 63. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Engineering Asymmetric Vesicles. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 10718–10721. [Google Scholar] [CrossRef]
- Leomil, F.S.C.; Stephan, M.; Pramanik, S.; Riske, K.A.; Dimova, R. Bilayer Charge Asymmetry and Oil Residues Destabilize Membranes upon Poration. Langmuir 2024, 40, 4719–4731. [Google Scholar] [CrossRef]
- McDonough, J.; Paratore, T.A.; Ketelhohn, H.M.; DeCilio, B.C.; Ross, A.H.; Gericke, A. Engineering Phosphatidylserine Containing Asymmetric Giant Unilamellar Vesicles. Membranes (Basel). 2024, 14, 181. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Nir, S.; Düzgünes, N. Molecular Mechanisms of Calcium-Induced Membrane Fusion. J. Bioenerg. Biomembr. 1990, 22, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Vail, W.J.; Newton, C.; Nir, S.; Jacobson, K.; Poste, G.; Lazo, R. Studies on Membrane Fusion. III. The Role of Calcium-Induced Phase Changes. Biochim. Biophys. Acta - Biomembr. 1977, 465, 579–598. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, J.; Stöckl, M.; Langosch, D.; Volkmer, R.; Herrmann, A. Direct Visualization of Large and Protein-Free Hemifusion Diaphragms. Biophys. J. 2010, 98, 1192–1199. [Google Scholar] [CrossRef]
- Rosa, R. De; Spinozzi, F.; Itri, R. Hydroperoxide and Carboxyl Groups Preferential Location in Oxidized Biomembranes Experimentally Determined by Small Angle X-Ray Scattering: Implications in Membrane Structure. Biochim. Biophys. Acta - Biomembr. 2018, 1860, 2299–2307. [Google Scholar] [CrossRef]
- Spinozzi, F.; Ferrero, C.; Ortore, M.G.; De Maria Antolinos, A.; Mariani, P. GENFIT: Software for the Analysis of Small-Angle X-Ray and Neutron Scattering Data of Macro-Molecules in Solution. J. Appl. Crystallogr. 2014, 47, 1132–1139. [Google Scholar] [CrossRef]
- Kučerka, N.; Nagle, J.F.; Sachs, J.N.; Feller, S.E.; Pencer, J.; Jackson, A.; Katsaras, J. Lipid Bilayer Structure Determined by the Simultaneous Analysis of Neutron and X-Ray Scattering Data. Biophys. J. 2008, 95, 2356–2367. [Google Scholar] [CrossRef]
- Enoki, T.A.; Wu, J.; Heberle, F.A.; Feigenson, G.W. Dataset of Asymmetric Giant Unilamellar Vesicles Prepared via Hemifusion: Observation of Anti-Alignment of Domains and Modulated Phases in Asymmetric Bilayers. Data Br. 2021, 35, 106927. [Google Scholar] [CrossRef]
- Feigenson, G.W.; Enoki, T.A. Nano-Scale Domains in the Plasma Membrane Are like Macroscopic Domains in Asymmetric Bilayers. Biophys. J. 2023, 122, 925–930. [Google Scholar] [CrossRef]
- Wang, Q.; London, E. Lipid Structure and Composition Control Consequences of Interleaflet Coupling in Asymmetric Vesicles. Biophys. J. 2018, 115, 664–678. [Google Scholar] [CrossRef]
- Wagner, A.J.; Loew, S.; May, S. Influence of Monolayer-Monolayer Coupling on the Phase Behavior of a Fluid Lipid Bilayer. Biophys. J. 2007, 93, 4268–4277. [Google Scholar] [CrossRef]
- May, S. Trans-Monolayer Coupling of Fluid Domains in Lipid Bilayers †. Soft Matter 2009, 5, 3148–3156. [Google Scholar] [CrossRef]
- Williamson, J.J.; Olmsted, P.D. Registered and Antiregistered Phase Separation of Mixed Amphiphilic Bilayers. Biophys. J. 2015, 108, 1963–1976. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.J.; Olmsted, P.D. Effects of Passive Phospholipid Flip-Flop and Asymmetric External Fields on Bilayer Phase Equilibria. Biophys. J. 2018, 115, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D. Interleaflet Coupling Mechanisms in Bilayers of Lipids and Cholesterol. Biophys. J. 2008, 94, L32–L34. [Google Scholar] [CrossRef]
- Putzel, G.G.; Schick, M. Phase Behavior of a Model Bilayer Membrane with Coupled Leaves. Biophys. J. 2008, 94, 869–877. [Google Scholar] [CrossRef]
- Heimburg, T. Thermal Biophysics of Membranes; Tutorials in Biophysics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; ISBN 9783527611591. [Google Scholar]
- Doniach, S. Thermodynamic Fluctuations in Phospholipid Bilayers. J. Chem. Phys. 1978, 68, 4912–4916. [Google Scholar] [CrossRef]
- Guidi, H.S.; Henriques, V.B. Lattice Solution Model for Order-Disorder Transitions in Membranes and Langmuir Monolayers. Phys. Rev. E 2014, 90, 052705. [Google Scholar] [CrossRef]
- Riske, K.A.; Barroso, R.P.; Vequi-Suplicy, C.C.; Germano, R.; Henriques, V.B.; Lamy, M.T. Lipid Bilayer Pre-Transition as the Beginning of the Melting Process. Biochim. Biophys. Acta 2009, 1788, 954–963. [Google Scholar] [CrossRef]
- Akabori, K.; Nagle, J.F. Structure of the DMPC Lipid Bilayer Ripple Phase. Soft Matter 2015, 11, 918–926. [Google Scholar] [CrossRef]
- Huang, J.; Hiraki, S.; Feigenson, G.W. Calculation of Liquid-Disordered/Liquid-Ordered Line Tension from Pairwise Lipid Interactions. J. Phys. Chem. B 2020, 124, 4949–4959. [Google Scholar] [CrossRef]
- Risselad, H.J.; Marrink, S.J. The Molecular Face of Lipid Rafts in Model Membranes. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 17367–17372. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Soft and Biological Structures. In Intermolecular and Surface Forces; Elsevier, 2011; pp. 535–576.
- Deserno, M. Fluid Lipid Membranes: From Differential Geometry to Curvature Stresses. Chem. Phys. Lipids 2015, 185, 11–45. [Google Scholar] [CrossRef] [PubMed]
- Deserno, M. Biomembranes Balance Many Types of Leaflet Asymmetries. Curr. Opin. Struct. Biol. 2024, 87, 102832. [Google Scholar] [CrossRef] [PubMed]
- Hossein, A.; Deserno, M. Spontaneous Curvature, Differential Stress, and Bending Modulus of Asymmetric Lipid Membranes. Biophys. J. 2020, 118, 624–642. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).