1. Introduction
Tumor cells grow in a dynamic and complex microenvironment termed as tumor microenvironment (TME). TME comprises of cytokines, hormones, enzymes, growth factors, diverse cell types like fibroblasts, immune inflammatory cells, pericytes and endothelial cells (Hanahan & Weinberg, 2011; Hasan et al., 2018). Hypoxia is referred to be a condition of insufficient oxygen when the tumor vascular supply is interrupted or the tumor outgrows its vascular supply (Challapalli et al., 2017). Tumor cells farther than 180micrometer from the vessel die by necrosis (Thomlinson & Gray, 1955)whereas the cells in the immediate vicinity tend to survive in a chronic hypoxic condition eliciting strong cellular hypoxic response(Ivan et al., 2022). Hypoxia leads to metabolic reprogramming like a shift from aerobic to anerobic respiration, pH regulation, growth factors production and cell proliferation (Maldonado & Melendez-Zajgla, 2022). It is a classical feature of tumor microenvironment that profoundly impact gene expression leading to higher phenotypic heterogeneity tumor progression and therapeutic response (Shih & Kung, 2017).Different transcriptional and epigenetic mechanisms regulate hypoxia induced gene expression and tumor metastasis. Cells respond to hypoxia via activation of Hypoxia Signalling Pathway (HSP). This cellular response to hypoxia is governed by a family of transcriptional factors called as Hypoxia inducible factors (HIFs) (Iyer et al., 1998).
2. HIF Subunits and Composition
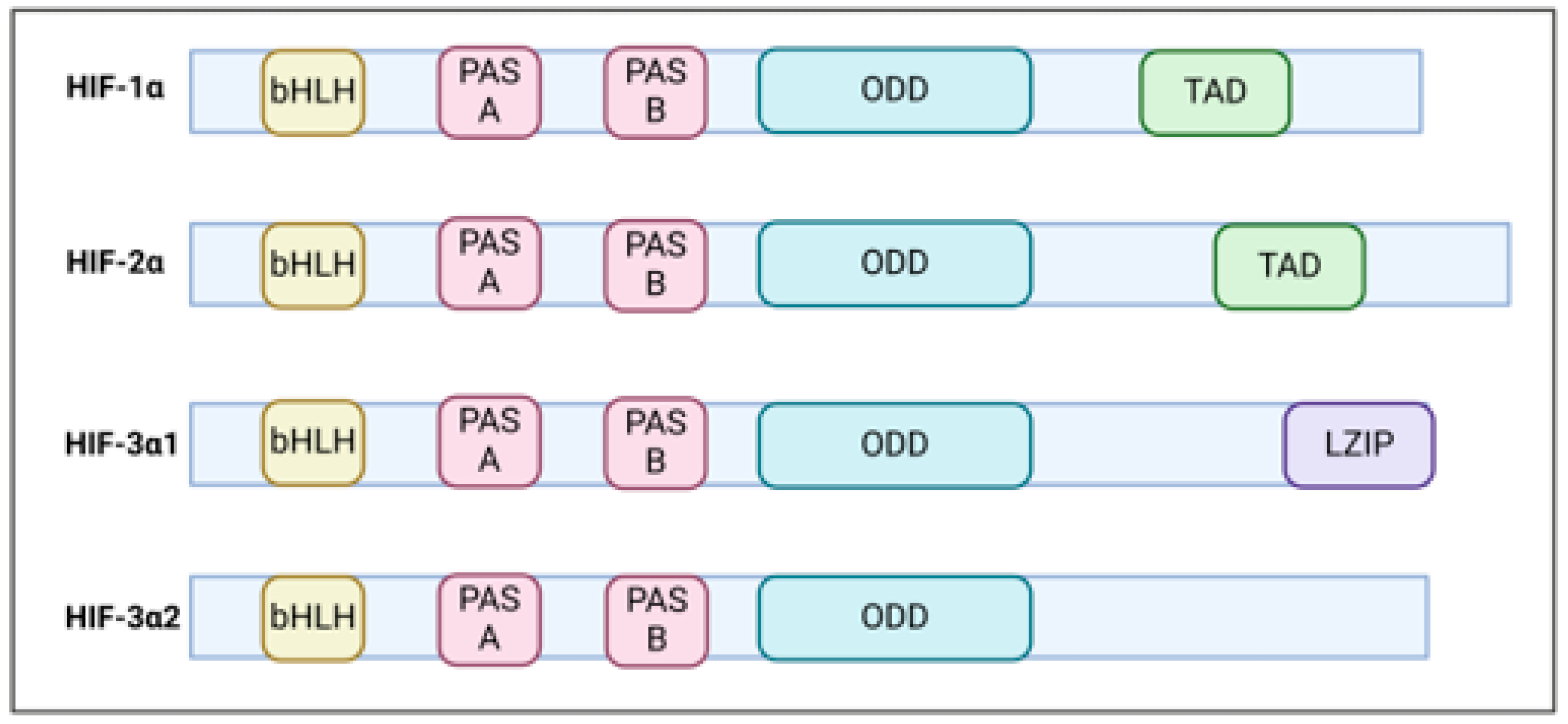
HIF is a heterodimer comprising of bHLH-PAS (basic helix loop helix and PERN-ARNT-SIM family) proteins comprising of an inducible oxygen –regulated α subunit and a stable constitutive β subunit (Wang et al., 1995). In humans there are three α (HIF1-α,HIF2-α, HIF3-α) and two beta (ARNT, ARNT2) paralogs (Graham Presnell, 2017). HIF1-α was initially identified by the expression of erythropoietin gene (Semenza et al., 1991). Structurally all HIF alpha subunits share common functional domains (
Figure 1). The N-terminal domain comprising of bHLH serves as DNA binding domain (Wang et al., 1995)whereas the PAS domain harbouring two vital regions PAS-A and PAS-B plays a crucial role in its dimerization (
Figure 1).
The alpha subunit bears a oxygen-dependent degradation domain (ODD) rendering the HIF1-α sensitivity towards oxygen (HuangLE & SchauM, 1998) whereas the C-terminal transcriptional activation domain (TAD) is crucial for full transcriptional activation (Lando et al., 2002). While HIF1-α and HIF2-α are master regulators of hypoxic responses that bear a significant degree of sequence similarity and share common target genes. However, they exhibit distinct DNA binding patterns and also modulate distinct gene targets (Ratcliffe, 2007). HIF1-α exhibit ubiquitous expression whereas HIF2-α demonstrate tissue specific pattern and varied activation kinetics (Jaśkiewicz et al., 2022). Both isoforms are overexpressed in different cancers with unfavourable prognosis and associated with chemo/radiotherapy resistance (Keith et al.). HIF3-α is the least explored and tissue specific factor demonstrating complex regulation due to generation of multiple variants due to the presence of different promoters, transcription initiation sites and alternative splicing (Duan, 2016). Some of these variants lack oxygen sensitivity and DNA binding ability (S.-L. Yang et al., 2015). Recent studies have shown that, contrary to prior assumptions that HIF-3 mainly suppresses the functions of HIF1-/2, HIF-3α actually acts as an oxygen-sensitive transcriptional activator, managing a unique array of target genes in response to low oxygen levels (Zhang et al., 2014). Recently, HIF-3α has been shown to promote pancreatic cancer metastasis via transcriptional activation of RhoC-ROCK1 pathway (Zhou et al., 2018). HIFs are vital in numerous physiological and pathological situations, such as congenital defects, ongoing inflammation, and cancer, which positions them as promising targets for therapeutic interventions.
3. Oxygen Dependent Regulation of HIF
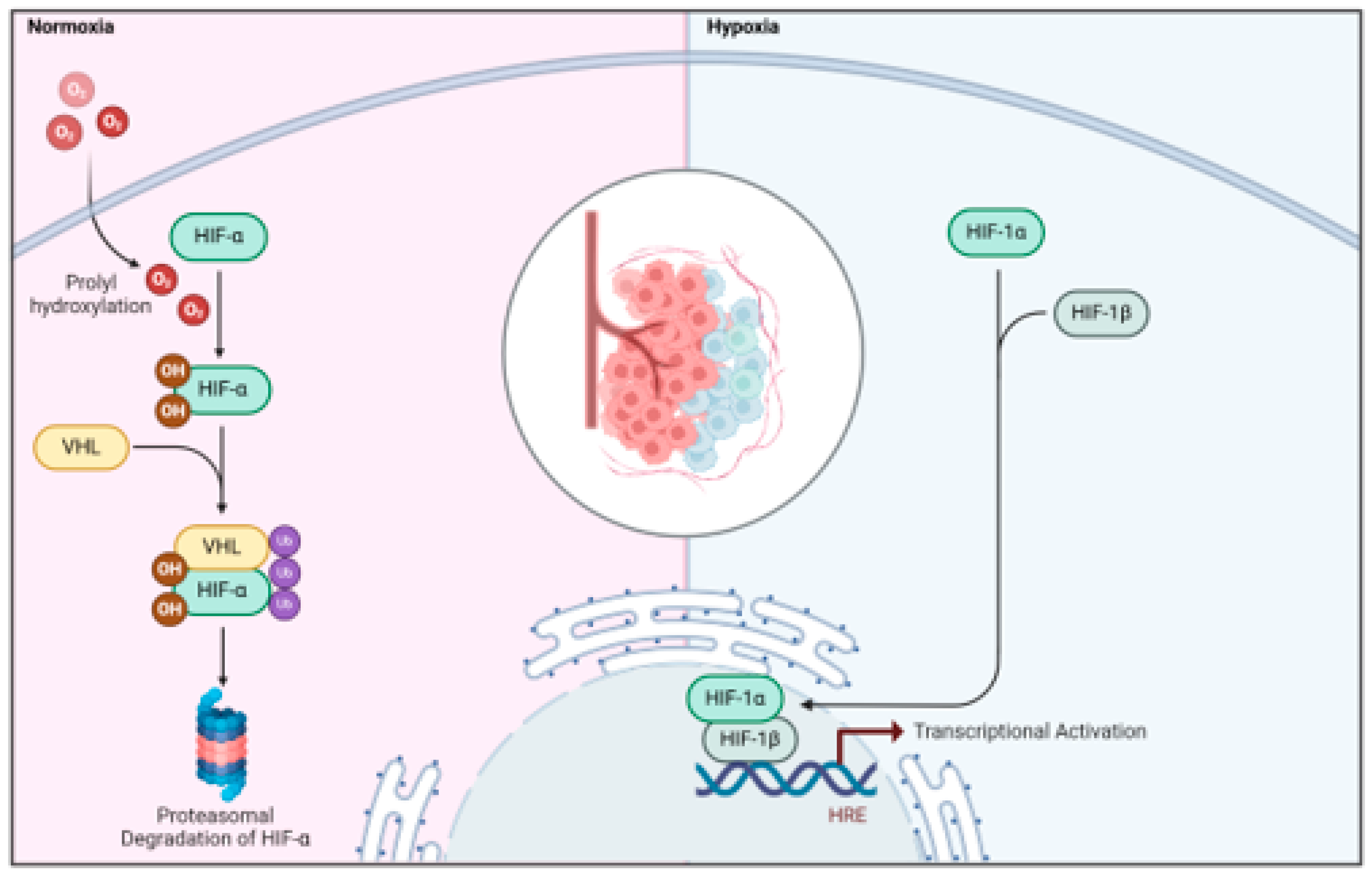
Under normoxic conditions, the HIF1-α subunits are quickly hydroxylated by a group of dioxygenases known as prolyl hydroxylase domain-containing enzymes (PHD1, 2, and 3, or EGLN1–3) belonging to the Egl-9 family, which are then recognized by the VHL (von Hippel-Lindau tumor suppressor protein) E3 ubiquitin ligase complex, resulting in the swift degradation of HIF1-α via the proteasome pathway (
Figure 2) (Shih & Kung, 2017). When encountering hypoxia, the activity of dioxygenases PHD is inhibited, leading to the stabilization of the HIF1-α subunit, which accumulates in the nucleus and forms a stable complex with the β subunit. This complex engages with specific DNA sequences that contain the consensus nucleotide sequence 5′-(A/G)CGTG-3′ located within the hypoxia response elements (HREs) in the promoter regions of HIF1 target genes, thereby facilitating the stimulation of downstream transcription (
Figure 2) . Notably, in mammalian systems, it has been demonstrated that HIF1- can function as either an activator or repressor depending on the cellular context, and the set of genes regulated by HIF1- varies among different cell types (Kelly et al., 2003). Overall, hypoxia induces a finely tuned HIF-dependent transcriptional activation that triggers a wide range of cellular adaptations, including metabolic reprogramming, increased proliferation, reduced apoptosis, angiogenic growth, and the promotion of cell survival under these conditions (Semenza, 2010, 2014).
Besides posttranscriptional regulation, the expression of HIF is also delicately adjusted at the translational stage through a complex interaction with the mammalian target of rapamycin (mTOR) complex. mTOR is a serine/threonine kinase that acts as a sensor and integrator of signals from the surrounding extracellular environment (Liu & Sabatini, 2020). It consists of two functionally distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). While mTORC1 mainly controls cell growth and metabolism by affecting protein translation, mTORC2 is involved in regulating cell survival and proliferation (Kim & Guan, 2019). In various cancer types, mTOR is often over- activated due to the loss of tumour suppressors like phosphatase and tensin homologue (PTEN), activation of oncogenes such as phosphoinositide 3-kinase (PI3K), and metabolic reprogramming (Xu et al., 2018). Numerous studies have highlighted the crucial role of the mTORC1 complex in modulating HIF1-α protein expression at both translational and transcriptional levels (Dodd et al., 2015). Conversely, hypoxia and the presence of HIF1-α negatively regulate mTOR activity through the transcriptional control of the mTOR inhibitor REDD1, which leads to a halt in translation (Brugarolas et al., 2004). Interestingly, the activation of HIF2- has the opposite effect, as the stabilization of HIF2- enhances mTORC activity by regulating the amino acid transporter SLC7A5 through transcription, resulting in increased amino acid uptake, activation of mTORC1, and enhanced tumor growth in xenograft models (Elorza et al., 2012).
The activation of HIF1- pathways is correlated with a more aggressive tumour phenotype and unfavourable clinical outcomes across various cancer types. Thus, investigating the regulatory mechanisms that govern HIF1--mediated transcriptional control could enhance our understanding of the role of hypoxia in tumour progression. The genes produced as a result not only influence various biological processes, such as cellular metabolism, growth, apoptosis, and migration, but also include several oncogenes and tumour suppressor genes. Given that these processes and genes play pivotal roles in carcinogenesis, it is not unexpected that hypoxia serves as a crucial factor in the tumour microenvironment associated with cancer development.
4. Hypoxia and Chemotherapy Resistance
The hypoxia-inducible factors (HIF) function as sensors for low oxygen levels, coordinating a collective response that enhances the survival and invasiveness of cancer cells, while also influencing a wide range of metabolic adaptations. This results in promote tumor growth and resistance to chemotherapy. The rise in the uptake of glucose and amino acids, the flow of glycolysis, and the production of lactate; the changes in glutamine metabolism, the tricarboxylic acid cycle, and oxidative phosphorylation; the elevated levels of reactive oxygen species in mitochondria; and the adjustments in both fatty acid synthesis and breakdown are characteristic features of the metabolic reconfiguration caused by hypoxia.
In addition to factors independent of metabolism, one fundamental reason for the ineffectiveness of chemotherapy is the structure of tumors. Specifically, in solid tumors, cells located in hypoxic regions, often distant from blood vessels, experience restricted delivery of chemotherapeutic agents due to irregular vascularization (Karakashev & Reginato, 2015). Furthermore, HIF1-α enhances the expression of multiple genes that contribute to resistance against chemotherapy. One such gene is MDR1, which encodes for Pgp (Comerford et al., 2002). Pgp is a member of the adenosine triphosphate (ATP)-binding cassette (ABC) transporter family and acts to efflux various chemotherapeutic drugs, regardless of their structure and mechanism of action, thus resulting in a multidrug resistance (MDR) phenotype (Gottesman et al., 2002). The upregulation of Pgp driven by HIF1-α has been observed in several cancers, including liver (Zhu et al., 2012), laryngeal , lung(Xie et al., 2013), and breast (Salaroglio et al., 2018)cancers, along with malignant pleural mesothelioma (Kopecka et al., 2016) and chronic lymphocytic leukemia in B-cells (Rigoni et al., 2015). The wide variety of tumors exhibiting this phenomenon indicates that it is a fairly conserved mechanism. Additionally, other ABC transporters involved in drug efflux, such as MDR related protein 1 (MRP1) (Chen et al., 2009), lung resistance protein (LRP) (Zhu et al., 2005), and breast cancer resistance protein (BCRP) (He et al., 2019), are also regulated at the transcriptional level by HIF1-α and HIF2-α, further increasing the range of drugs that are effluxed and thus lose effectiveness in hypoxic areas .
The changes in metabolism within glycolysis and the mitochondria triggered by low oxygen levels contribute to chemoresistance through different mechanisms like increased pro-survival mechanisms and diminished apoptosis, activation of epithelial-to-mesenchymal transition (EMT), heightened DNA repair capabilities, modifications in drug metabolism, and alterations in drug targets (Hasan et al., 2018; Pan et al., 2016). The heightened acidification of the tumor microenvironment resulting from increased glycolytic enzyme activity and MCT4 expression is a primary factor contributing to chemoresistance. On one hand, the decreased extracellular pH (pHe) promotes the protonation of weak bases like anthracyclines, leading to their inactivation and entrapment in lysosomes after they have infiltrated the cancer cell; this process is referred to as “ion trapping.”(Pillai et al., 2019). Additionally, a common characteristic of tumor areas that are hypoxic and acidic is the heightened expression of enzymes that promote alkalinization. The Na+/H+ exchanger (NHE) serves as a prime example of a transporter that is upregulated in response to the surrounding acidity: its activity leads to an increase in intracellular pH (pHi), establishing optimal conditions for the efficient efflux of Pgp, which operates best at a pH of 7.6-7.8 (Äänismaa et al., 2008). Chemoresistance in regions of tumors with low oxygen has also been linked to changes in mitochondrial metabolism, as well as in fusion, fission, and mitophagy processes (Alexa-Stratulat et al., 2019). On one hand, because ATP is essential for the functioning of ABC transporters, it could be anticipated that cells in hypoxic conditions—characterized by diminished oxidative phosphorylation (OXPHOS) (Kung-Chun Chiu et al., 2019) and increased mitophagy (Jung et al., 2019)—would generate less ATP for ABC transporters, potentially making them more sensitive to chemotherapy. The generation of ROS, commonly linked to changes in OXPHOS within hypoxic areas of tumors, can lead to damage in mtDNA, which in turn diminishes the efficiency of OXPHOS(Yu, 2011). In general, the mitochondrial-related factors (reduced OXPHOS and ATP production, elevated ROS, heightened mitophagy, and increased mtDNA mutations) that define hypoxic tumor cells contribute to chemoresistance. Cells that express HIF1-α display significant metabolic flexibility, enabling them to survive more effectively when faced with limited glucose and oxygen supplies, or during chemotherapy treatments. Thus, the diverse impacts of hypoxia on metabolic reprogramming collectively play a role in chemoresistance through various but interrelated mechanisms.
5. Hypoxia Associated lncRNA in Breast Cancer
The wide spectrum of hypoxic responsive genome is not limited to only protein coding genes but also non-coding RNAs like microRNAs, lncRNAs and circular RNAs. Hypoxia responsive ncRNAs play a pivotal role in modulating hypoxic gene expression at transcriptional, post-transcriptional level either by regulating the HIF transcriptional cascade or acting as HIF guided effector molecules (Camps et al., 2014). Recent studies have implicated that long non-coding RNA (lncRNA) play pivotal role in gene expression regulation at chromatin, transcriptional and posttranscriptional levels in diverse cellular processes. LncRNAs have recently gained attention as hypoxia regulated lncRNA are involved in different cellular processes like migration, invasion, anti-apoptosis, angiogenesis and tumor progression (Kapinova et al., 2018).
6. Regulation of Gene Expression by Hypoxia Inducible lncRNA
LncRNA regulate gene expression or protein levels via different mechanisms. They may interact with protein –protein complex leading its degradation or stability, dissociate protein binding to promoter, formation of cellular structures like paraspeckles trigger protein glycosylation, regulate RNA splicing or serve as a coactivator of gene expression (Kuo et al., 2020). LncRNA NEAT1 is a hypoxia inducible lncRNA critical for paraspeckles formation. These paraspeckles can sequester RNA transcripts and transcriptionally active proteins leading to modulation of gene expression of proteins involved in tumor metastasis (Choudhry et al., 2015). LncRNA MALAT1 reduce the degradation of HIF1-α/ HIF2-α by inhibiting the binding of VHL with HIF1-α/ HIF2-α (Luo et al., 2016). LncRNA H19 is responsible for nuclear localization of HIF1-α (Corrado et al., 2019).Another hypoxia inducible lncRNA LUCAT1 interacts with poly-pyrimidine tract binding protein (PTPB1) to alter alternative splicing of DNA damage genes (Huan et al., 2020).
7. Role of Hypoxia Associated LncRNA in Cancer Metastasis
7.1. Breast Cancer
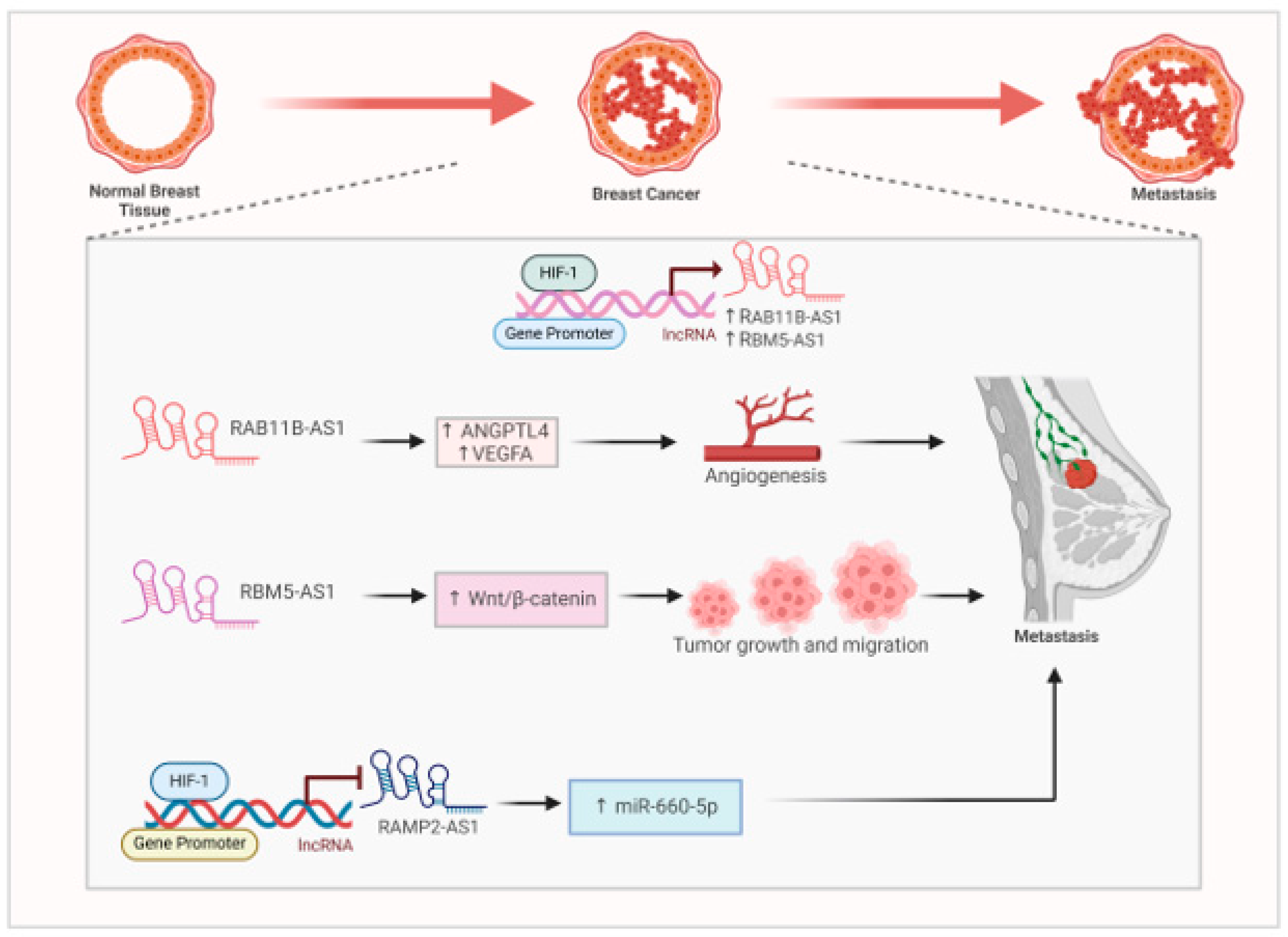
Metastatic breast cancer spreads from breast to different parts of the body like lungs, liver, bone and brain. Hypoxia is a hallmark of breast tumour microenvironment playing pivotal role cancer progression. Long non-coding RNAs (lncRNAs) are essential in the progression of breast cancer in low-oxygen environments. LncRNAs that respond to hypoxia are linked to unfavorable prognoses and influence tumor biology, suggesting they could be used as prognostic indicators and treatment targets. Hypoxia associated lncRNA contribute towards cancer metastasis by diverse mechanisms. Hypoxia induces the expression of RAB11B-AS1 in MDA-MB231 cells by binding of the HIF1-α to its promoter(Niu et al., 2020). Overexpression of RAB11B-AS1 promoted invasion and migration. This promotion of angiogenesis and breast cancer metastasis is mediated by proangiogenic factors like ANGPTL4 and VEGFA(French & Edwards, 2020) .Silencing of RAB11-B-AS1 promotes cancer cell apoptosis and reduces breast cancer metastasis (French & Edwards, 2020) (
Figure 3).
Hypoxia induces the expression of a lncRNA RBM5-AS1 via RUNX2 transcription factor (X. Li et al., 2022). RBM5-AS1 initiate Wnt/ β-catenin signaling by upregulating and interacting with the β-catenin. RBM5-AS1 downregulates the expression of AXIN1 leading to the accumulation of β-cateninin cytoplasm followed by its nuclear translocation(X. Li et al., 2022). RBM5-AS1 bears binding site for β-catenin/ TCF4 (X. Li et al., 2022). It acts a scaffold and facilitate the organization β-catenin-TCF4 transcriptional complex. Thus, RBM5-AS1, plays a crucial role in the growth, maintenance of stemness, migration, and invasion of breast cancer by enhancing the accumulation of β-catenin and strengthening the interaction between TCF4 and β-catenin, which activates the Wnt/β-catenin pathway(X. Li et al., 2022) (
Figure 3). Contrary, to hypoxia induced lncRNAs , a recent study documented a hypoxia suppressed lncRNA RAMP2-AS1. RAMP2-AS1 has been previously documented as a tumor suppressor in breast cancer by downregulating CXCL11 by DNMT1 and DNMT3b(L. Li et al., 2022) . Knockdown of RAMP2-AS1 consequently upregulates miR-660-5p leading to decreased expression of ATM in MCF-7 and MDA-MB231 cells. This lncRNA is downregulated in breast cancer as compared to normal controls. It regulated the miR-660/ATM axis in breast cancer (Lou et al., 2024) (
Figure 3). These research findings underscore the intricate relationship between long non-coding RNAs (lncRNAs) and hypoxia in breast cancer, stressing their possible influence on prognosis, treatment strategies, and patient outcomes. Additional studies could result in enhanced diagnostic, prognostic, and therapeutic resources for individuals with breast cancer.
7.2. Hepatocellular Carcinoma (HCC)
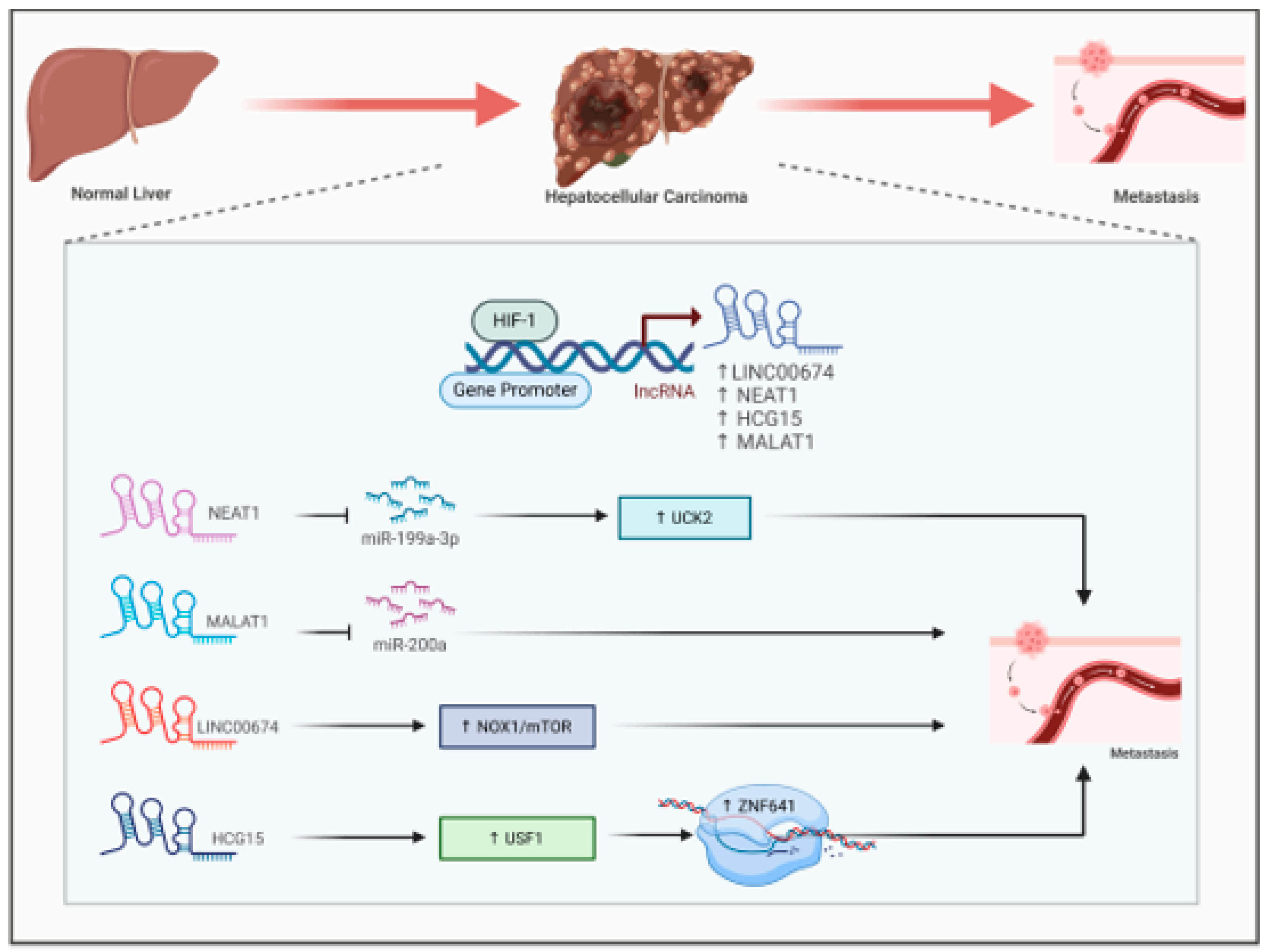
Recent research has underscored the important function of hypoxia-associated long non-coding RNAs (lncRNAs) in hepatocellular carcinoma (HCC). These lncRNAs participate in multiple processes such as glucose metabolism, maintenance of cancer stem cells, cell apoptosis, proliferation, and immune evasion, all of which contribute to unfavorable outcomes for HCC patients (Wang & Wang, 2024). Scientists have discovered and confirmed lncRNA signatures associated with hypoxia as prognostic models for HCC, showing their ability to serve as independent predictors that surpass conventional clinical factors (Cheng et al., 2022; Zhou et al., 2021). Hypoxia induces the expression of LINC00674 by increasing the occupancy of HIF1-α to the HRE region of its promoter. Overexpression of this lncRNA stimulates HCC cells proliferation and metastasis by activating the NOX1/mTOR signaling (Zhu et al., 2022) (
Figure 4). LncRNA NEAT1 is another hypoxia induced RNA that interact with the tumor suppressive miR-199a-3p to sustain HCC growth by regulating miR-199a-3p/UCK2 pathway (
Figure 4)., suggesting its potential to be a plausible therapeutic target (Zhang et al., 2020). LncRNA MALAT1 regulate Hep3B cell proliferation, migration, invasion and metastasis by sponging miR-200a (
Figure 4). (Zhao et al., 2019).Another hypoxia responsive lncRNA HLA complex group 15 (HCG) facilitate HCC cells migration and invasion by enhancing ZNF641transcription. Knockdown of HCG15 abolished USF1 mediated ZNF641transcription (
Figure 4). leading to reduced Hep3B cell proliferation, migration and invasion (Yan et al., 2022). These findings imply that lncRNAs associated with hypoxia may act as promising biomarkers and therapeutic targets for the diagnosis, prognosis, and treatment of liver cancer.
7.3. Gastric Cancer (GC)
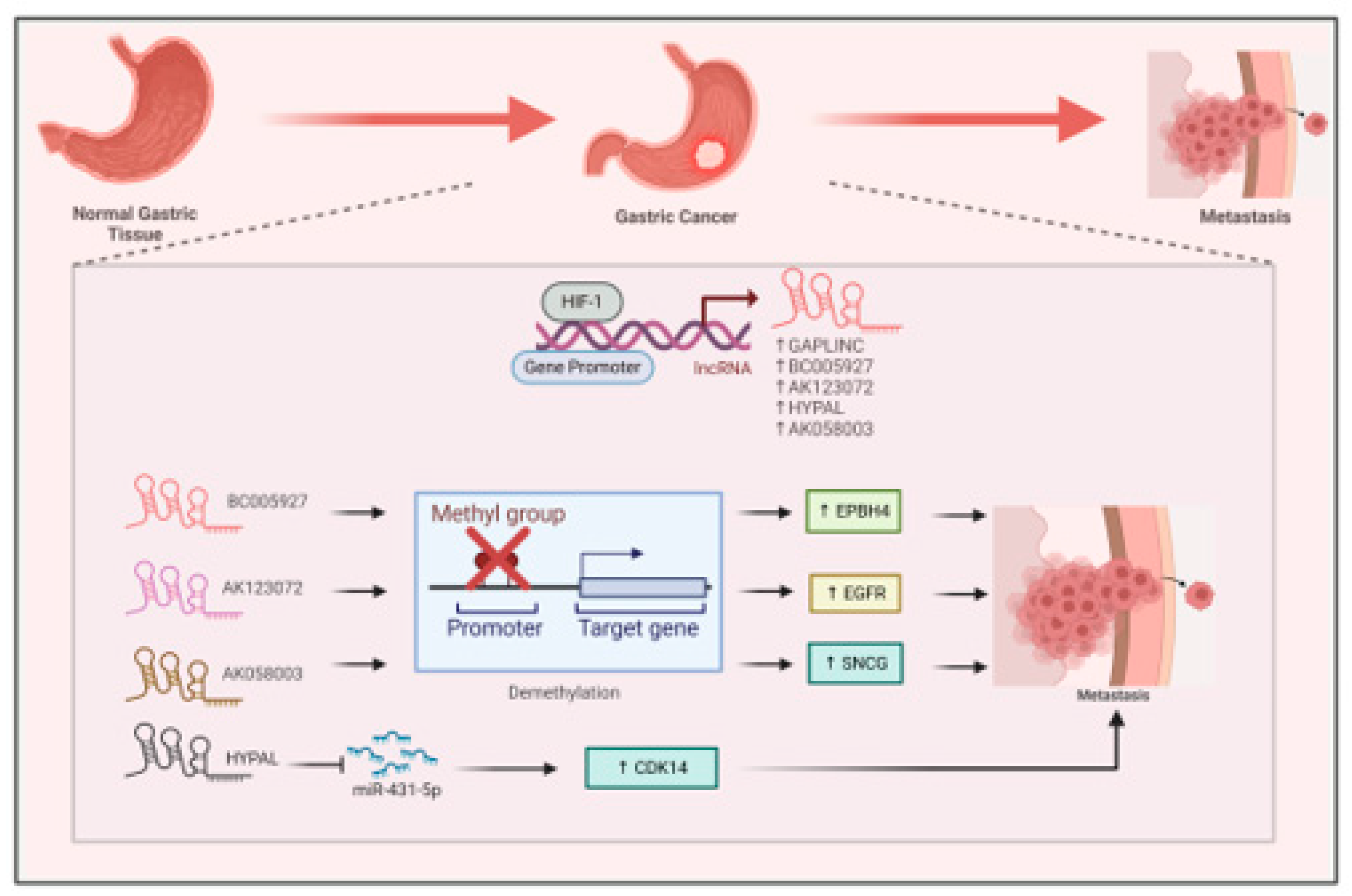
Hypoxia plays a crucial role in gastric cancer progression by inducing the expression of different lncRNAs via HIF1-α mediated transcriptional activation. LncRNas like GAPLINC are overexpressed in gastric cancer on hypoxia promotes its malignancy (
Figure 5) (Liu et al., 2016). LncRNA BC005927 is a direct transcriptional target of HIF1-α. BC005927 upregulates the expression of metastasis associated gene EPBH4 by modulating its DNA methylation promoting gastric cancer metastasis (
Figure 5) (Liu et al., 2018) . In another study, hypoxia induces the expression of lncRNA AK123072. High expression of this lncRNA promoted gastric cancer cells migration and invasion by modulating the expression of metastasis related gene EGFR (
Figure 5). Depletion of AK123072, leads to increased methylation of the CpG island in the promoter region (Z. Yang et al., 2015) . Another lncRNA, HYPAL promotes GC metastasis under hypoxic conditions by sponging miR-431-5p leading to upregulation of CDK14. This HIF1-α/HYPAL/ miR-431-5p /CDK14axis activates the Wnt- β-catenin pathway (
Figure 5) promoting gastric cancer cells proliferation and inhibiting apoptosis (Piao et al., 2022). In gastric cancer, hypoxia induce the expression of lncRNA AK058003. This lncRNA resides upstream of SNCG (synuclein gamma, a synuclein family member). It promotes the expression of SNCG via demethylation of its promoter (
Figure 5) leading to increase in cell proliferation, migration and invasion (Wang et al., 2014). Thus, the lncRNAs that are regulated by hypoxia play various roles in metabolism, autophagy, invasion, and metastasis within the hypoxic microenvironment of gastrointestinal cancers, positioning them as potential targets for therapy(Huang et al., 2019).
7.4. Pancreatic Cancer (PC)
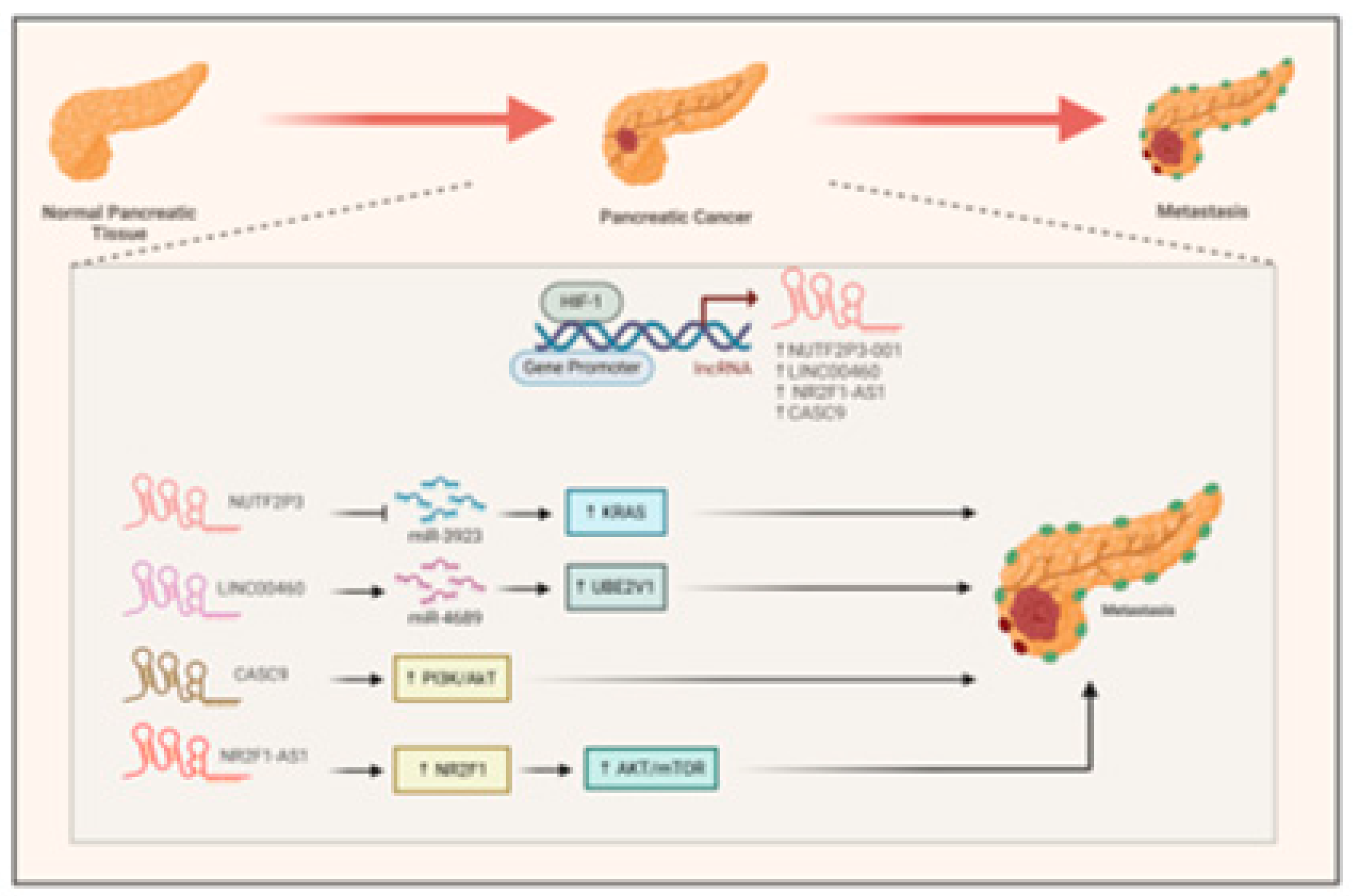
Recent research has underscored the important function of long non-coding RNAs (lncRNAs) induced by hypoxia in the advancement of pancreatic cancer. Scientists have pinpointed various lncRNAs, such as NUTF2P3-001 (Li et al., 2016), LINC00460(Zhang et al., 2023), and CASC9, which are elevated in hypoxic environments and play a role in tumor development (
Figure 6). The hypoxia-inducible factor 1-α (HIF1-α) is crucial for the regulation of these lncRNAs as it attaches to their promoter regions These lncRNAs facilitate the proliferation, invasion, and metastasis of cancer cells via several mechanisms, including the modulation of the miR-3923/KRAS pathway (
Figure 6) (Li et al., 2016), the regulation of the miR-4689/UBE2V1 axis(Zhang et al., 2023), and the enhancement of glycolysis and epithelial-mesenchymal transition (EMT) via activation of PI3k/AKT pathway (
Figure 6). (Zhang et al., 2021).
In hypoxia, HIF1-α also induces the expression of NR2F1-AS1that positively regulated its neighboring gene NR2F1, to promote cancer cell proliferation, migration and invasion by activating the AKT/mTOR signaling (
Figure 6). (Liu et al., 2022). This evidence indicates that lncRNAs associated with hypoxia may act as potential therapeutic targets and prognostic markers for pancreatic cancer.
7.5. Lung Cancer
Long non-coding RNAs (lncRNAs) are essential in driving cancer progression under hypoxic conditions, especially in lung cancer. Hypoxia-responsive lncRNAs (HRLs) influence gene expression through various mechanisms and affect HIF1- signaling (Shih & Kung, 2017)In non-small cell lung cancer (NSCLC), LINC01436 functions as an oncogene by sequestering miR-30a-3p and is regulated by E2F6 in response to hypoxia (Yuan et al., 2019). LINC01436 sponges miR-30a-3p to regulate the expression of its target gene EPAS1(
Figure 7) (Yuan et al., 2019) promoting lung cancer cells migration, invasion and tumour metastasis (Yuan et al., 2019).
A set of seven hypoxia-related lncRNAs has been identified as a prognostic indicator for patients with lung adenocarcinoma, showing a connection to immunosuppressive environments(Shao et al., 2021).The relationship between lncRNAs and hypoxia affects numerous facets of tumor development, including growth, metabolism, angiogenesis, and metastasis(Dong et al., 2016)These results underscore the potential of hypoxia-related lncRNAs as biomarkers and therapeutic targets in lung cancer, paving the way for innovative diagnostic and treatment strategies.
8. Role of Hypoxia Induced LncRNA Chemoresistance
The presence of low oxygen levels in the tumor microenvironment plays a role in the development of resistance to chemotherapy across different types of cancer. Numerous studies have pointed out that long non-coding RNAs (lncRNAs) are significant factors in this phenomenon. Profiling methods and bioinformatics analysis have enabled us to discover an increasing number of hypoxia-regulated non-coding RNAs by identifying the presence of hypoxia response elements (HREs) in their promoter regions (Slemc & Kunej, 2016). Additionally, various studies have shown that certain non-coding RNAs can be induced by hypoxia even in the absence of HREs, suggesting an indirect regulatory mechanism that often involves epigenetic factors; HIF may influence the expression of non-coding RNAs by activating histone deacetylases or impacting the machinery responsible for miRNA maturation (Lai et al., 2018). Research indicates that lncRNAs induced by hypoxia play a role in chemoresistance through multiple mechanisms, such as modulation of drug efflux proteins, promotion of epithelial-mesenchymal transition, and sponging of miRNAs, highlighting possible therapeutic targets to address chemoresistance in tumors affected by hypoxia. In this review , some of the hypoxia induced lncRNAs associated with chemoresistance.
8.1. LncRNA H19
In non-small lung cancer (NSCLC) cell lines (A549 and H1650) , hypoxia induce the expression of lncRNA H19, HIF1-α,HO-1, MRP-1, Pgp resulting in increased migration and invasion of cancer cells alongwith increase in cisplatin resistance (H. Li et al., 2022). In another study, increased expression of H19 has been reported in colorectal cancer (CRC) under hypoxic conditions or oxiplatin treated CRC cells. H19 played a pivotal role in oxaliplatin resistance in CRC both in vitro and in vivo. H19 acts a competitive endogenous lncRNA (celncRNA) of miR-675-3p to regulate EMT in CRC(H. Li et al., 2022) . Thus, targeting H19 may overcome the chemotherapy resistance in NSCLC and CRC.
8.2. LncRNA EMS
LncRNA EMS is an hypoxia induced lncRNA in esophageal cancer . Hypoxia also induce the expression of the protein WTAP and reduces the expression of miR-758-3p. The high expression of EMS and WTAP leads to cisplatin resistance in Esophageal cells. Moreover, high level of EMS / WTAP or low level of miR-758-3p is associated with poor patient survival.
8.3. LncRNA ANRIL
Hypoxia also induced the expression of the antisense-long non-coding RNA in the INK4 locus (ANRIL) through the binding of HIF1-α to its promoter (Yin et al., 2020). Dual luciferase assay showed direct interaction of miR-328 with its direct target, the drug resistance gene ABCG2 and ANRIL with miR-328 proving that ANRIL sponges miR-328 resulting in increased expression of ABCG2. Thus the hypoxia induced lncRNA ANRIL increased the resistance of retinoblastoma cells by promoting cell proliferation, inhibiting apoptosis and boosting the expression of ABCG2 and MDR1 (Yin et al., 2020).
A study conducted on Midkine (MK), a hairpin-binding growth factor, indicates that MK promotes chemoresistance and carcinogenesis. lncRNA ANRIL knockdown in tumor cells depicted reduced apoptosis, proliferation, and increased levels of cisplatin sensitivity by impairing drug transporters MPRP1 and ABCC2. Midkine derived from cancer-associated fibroblasts (CAFs) promotes cisplatin resistance by upregulating the lncRNA ANRIL(Zhang et al., 2017) .
8.4. LncRNA HOTAIR
Dysregulated expression of HOX anti-sense intergenic RNA (HOTAIR) is associated with different cancers like breast cancer (Qian et al., 2020), liver cancer (Topel et al., 2020)and lung cancer (Li et al., 2021). In breast cancer it is associated with radiotherapy resistance due to recruitment of EZH2 to MYC promoter (Qian et al., 2020). HOTAIR over expression led to increased expression of DNA repair factors like KU70, KU80, DNA-PK and ATM regulating breast cancer cell proliferation by regulating apoptosis and cell cycle (Qian et al., 2020). In NSCLC, HOTAIR induces geftinib resistance through epigenetic regulation of EZH2/H3K27me3. In PC9 lung cancer cells over expression of HOTAIR enhances the H3K27me3 recruitment to p16and p21 promoters. Silencing of HOTAIR induced the expression of p16 and p21 whereas CDK4, cyclinD, E2F1 and LSD1 expression declined (Li et al., 2021). Moreover, in glioblastoma (GBM) HOTAIR competitively binds to miR-526b-3p limiting its ability to bind EVA1. Upregulation of EVA1 led to temozolomide (TMZ) resistance in GBM(Li et al., 2021). In human cervical cell line (HeLA) HOTAIR evades the inhibitory effect of Wnt/ β-catenin pathway blocker ICRT14 due to its interaction with β-catenin (Trujano-Camacho et al., 2021).In nasopharangeal carcinoma, HOTAIR contributes towards cisplatin resistance. It sponges miR-106a-5p and upregulates the expression of SRY box transcription factor 4 (SOX4) promoting NOC cell proliferation (Cao et al., 2022). High expression of HOTAIR in colorectal cancer (CRC) is associated with poor clinical prognosis. CRC cells with high HOTAIR expression display oxaliplatin resistance whereas silencing of HOTAIR significantly increased its sensitivity. HOTAIR acts as a ceRNA for miR-1277-5p resulting in upregulation of ZEB1. Thus, HOTAIR/ miR-1277-5p/ZEB1 axis modulate oxaliplatin resistance in CRC (Weng et al., 2022).
8.5. LncRNA CBSLR
CBSmRNA-stabilizing lncRNA (CBSLR) is a hypoxia induced lncRNA in gastric cancer cells. CBS can act as oncogenic or tumor suppressor depending on the tumor type. It promotes colon cancer via autocrine and paracrine signaling whereas its reduced expression promotes glioma tumorigenesis (Phillips et al., 2017; Takano et al., 2014). CBS has a distinct role in protecting different cancer cells like breast cancer, lung cancer, hepatocellular carcinoma and fibrosarcoma from ferroptosis (Hayano et al., 2016; Wang et al., 2018). Ferroptosis is an ROS dependent form of cell death in which iron accumulation and lipid peroxidation are the main changes. Aberrant ferroptosis is associated with diverse pathological conditions and resistance to cancer therapies(Yan et al., 2021). Overexpression of CBSLR is associated with poor prognosis and worse chemotherapy response in gastric cancer (GC). HIF1-α induces lncRNA CBSLR to recruit YTHFD2 and CBS to make CBSLR/YTHFD2/CBS complex leading to decreased CBS mRNA stability via m6A modification (Yang et al., 2022). Low expression of CBS favours the ubiquitin mediated degradation of ACSL4 due to its reduced methylation. Silencing of CBSLR upregulated ACSL4 at post transcriptional level increase the phosphotidyl ethanolamine level leading to ferroptosis (Yang et al., 2022). Thus, CBSLR/ /CBS/ ACSL4 prmotes ferroptosis resistance in the GC cells under hypoxia (Yang et al., 2022).
8.6. LncRNA NORAD
Hypoxia induces vasculogenic mimicry resulting in increased plasticity and heterogeneity in tumours. Upregulation of NORAD is associated with tumour metastasis and poor patient prognosis in colorectal cancer (CRC) cells. NORAD is positively associated with HIF1-α. 5-Fluorouracil (5-FU) is the standard chemotherapy treatment of CRC. Upon hypoxia, the upregulation of NORAD leads to 5-FU resistance. NORAD acts as a ceRNA by sponging miR-495-3p to regulate HIF1-α expression (Zhang et al., 2022).Knockdown of NORAD inhibited the hypoxia induced VM and the 5FU resistance of CRC cells. Moreover, elevated NORAD expression has also been reported in cisplatin resistant NSCLC cells .NORAD sponges miR-129-1-3p and boosts SOX4 expression whereas its inhibition decreases cisplatin resistance in NSCLC cells (Huang et al., 2020). Thus, targeting NORAD may overcome chemotherapy resistance in CRC and NSCLC.
8.7. NLUCAT1
A combined profiling study was conducted on early-stage lung adenocarcinoma (LUAD) and A549 cell lines cultured in hypoxic and normoxic conditions. The study suggested that the upregulation of NLUCAT1, a particularly new nuclear-hypoxia-regulated lncRNA transcript, was strongly upregulated by hypoxia in vitro and correlated with poor prognosis and other hypoxia markers in LUAD. A CRISPR-Cas9-mediated inactivation of NLUCAT1 showed decreased invasive and proliferative properties, an increased state of oxidative stress, and high sensitivity to cisplatin-induced apoptosis. Transcriptome analysis of NLUCAT1-knockdown cells showed repressed genes present within antioxidant/ cisplatin-response networks. Partial mimic of NLUCAT1 inactivation in LUAD cells and increased ROS-dependent caspase activation were observed in cells with consequent knockdown of particularly four gene products PDK4, GLRX, GPX2, and ALDH3A1. In short, NLUCAT1 promotes aggressive phenotype in early-stage hypoxic tumors(Moreno Leon et al., 2019).
8.8. LUCAT1
Another study demonstrated the underlying drug resistance mechanism of LUCAT1 which facilitates the growth of CRC cells both in vivo and in vitro. In CRC cells, mechanistically, LUCAT1 interacts with the polypyrimidine tract binding protein 1 (PTBP1) which results in the association of genes responsible for DNA damage with PTBP1. This phenomenon results in alternative splicing of DNA damage-related genes. A combination of LUCAT1 knockdown through antisense oligonucleotide (ASO) combined with chemotherapeutic drug delivery showed better outcomes in vivo when compared to the group treated with only chemotherapeutic drugs. Thus, CRC patients with a higher LUCAT1 expression have correlated with poor prognosis and resistance to chemotherapy drugs clinically(Moreno Leon et al., 2019).
In Glioblastoma stem-like cells (GSC), LUCAT1 was found to be induced by nuclear factor erythroid 2-related factor 2 (NRF2) and hypoxia-inducible factor 1 (HIF1α). LUCAT1 was found to be highly expressed in hypoxic regions of glioblastoma (GBM). HIF1α and its co-activator CBP form a complex with LUCAT1 and regulate the genetic expression of HIF1α target and GSC’s adaptation to hypoxia. In GBM xenograft models, silencing of LUCAT1 decreases tumor growth and promotes a longer survival rate in mouse models (Huang et al., 2024).
9. Conclusion
Hypoxia contributes to resistance to therapy through multiple mechanisms, such as inhibiting apoptosis, regulating autophagy, responding to DNA damage, and facilitating drug efflux. The expression of lncRNAs specific to different tissues and their role in distinct resistance mechanisms to various drug types highlight their potential as therapeutic targets This review highlights the intricate relationships among lncRNAs, hypoxia and their target genes in the mechanisms behind chemoresistance. Gaining insight into these interactions is essential for creating new therapeutic approaches to combat drug resistance in cancer therapy. targeting lncRNAs, it may be possible to overcome drug resistance in cancer patients, indicating their promise as effective therapeutic targets and enhancers of chemotherapy.
10. Future Directions
Further, research on deciphering the role of lncRNA-related drug resistance could lead to new strategies for enhancing the outcomes of cancer treatment.
Author Contributions
Conceptualization, A.T. (Aamira Tariq) and M.A.E. (Mohammad Affan Elahi); writing—original draft preparation, A.M. (Ambrin Malik), M.Z. (Mahmoud Zahra), A.T. (Aamira Tariq); writing—review and editing, A.T. (Aamira Tariq), M.A.E. (Mohammad Affan Elahi); All authors have read and agreed to the published version of the manuscript.
Funding
This work is not supported by any research grant.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
All the images have been drawn using BioRender software.
References
- Alexa-Stratulat, T., Pešić, M., Gašparović, A. Č., Trougakos, I. P., & Riganti, C. (2019). What sustains the multidrug resistance phenotype beyond ABC efflux transporters? Looking beyond the tip of the iceberg. Drug resistance updates, 46, 100643. [CrossRef]
- Brugarolas, J., Lei, K., Hurley, R. L., Manning, B. D., Reiling, J. H., Hafen, E.,…Kaelin, W. G. (2004). Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes & development, 18(23), 2893-2904.
- Camps, C., Saini, H. K., Mole, D. R., Choudhry, H., Reczko, M., Guerra-Assunção, J. A.,…Hatzigeorgiou, A. G. (2014). Integrated analysis of microRNA and mRNA expression and association with HIF binding reveals the complexity of microRNA expression regulation under hypoxia. Molecular cancer, 13, 1-21. [CrossRef]
- Cao, W., Sun, Y., Liu, L., Yu, J., Ji, J., Wang, Y., & Yang, J. (2022). HOTAIR mediates cisplatin resistance in nasopharyngeal carcinoma by regulating miR-106a-5p/SOX4 axis. Bioengineered, 13(3), 6567-6578. [CrossRef]
- Challapalli, A., Carroll, L., & Aboagye, E. O. (2017). Molecular mechanisms of hypoxia in cancer. Clinical and translational imaging, 5, 225-253. [CrossRef]
- Chen, L., Feng, P., Li, S., Long, D., Cheng, J., Lu, Y., & Zhou, D. (2009). Effect of hypoxia-inducible factor-1α silencing on the sensitivity of human brain glioma cells to doxorubicin and etoposide. Neurochemical research, 34, 984-990. [CrossRef]
- Cheng, M., Zhang, J., Cao, P.-B., & Zhou, G.-Q. (2022). Prognostic and predictive value of the hypoxia-associated long non-coding RNA signature in hepatocellular carcinoma. Yi Chuan= Hereditas, 44(2), 153-167. [CrossRef]
- Choudhry, H., Albukhari, A., Morotti, M., Haider, S., Moralli, D., Smythies, J.,…Buffa, F. (2015). Tumor hypoxia induces nuclear paraspeckle formation through HIF2-α dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene, 34(34), 4482-4490. [CrossRef]
- Comerford, K. M., Wallace, T. J., Karhausen, J. r., Louis, N. A., Montalto, M. C., & Colgan, S. P. (2002). Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer research, 62(12), 3387-3394.
- Corrado, C., Costa, V., Giavaresi, G., Calabrese, A., Conigliaro, A., & Alessandro, R. (2019). Long non coding RNA H19: a new player in hypoxia-induced multiple myeloma cell dissemination. International Journal of Molecular Sciences, 20(4), 801. [CrossRef]
- Dodd, K. M., Yang, J., Shen, M. H., Sampson, J. R., & Tee, A. R. (2015). mTORC1 drives HIF1-α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene, 34(17), 2239-2250.
- Dong, J., Xu, J., Wang, X., & Jin, B. (2016). Influence of the interaction between long noncoding RNAs and hypoxia on tumorigenesis. Tumor Biology, 37(2), 1379-1385. [CrossRef]
- Duan, C. (2016). Hypoxia-inducible factor 3 biology: complexities and emerging themes. American Journal of Physiology-Cell Physiology, 310(4), C260-C269. [CrossRef]
- Elorza, A., Soro-Arnáiz, I., Meléndez-Rodríguez, F., Rodríguez-Vaello, V., Marsboom, G., de Cárcer, G.,…Serrano-Oviedo, L. (2012). HIF2α acts as an mTORC1 activator through the amino acid carrier SLC7A5. Molecular cell, 48(5), 681-691. [CrossRef]
- French, J. D., & Edwards, S. L. (2020). A new hypoxia-responsive lncRNA in metastatic breast cancer. Non-coding RNA Investigation, 4. [CrossRef]
- Gottesman, M. M., Fojo, T., & Bates, S. E. (2002). Multidrug resistance in cancer: role of ATP–dependent transporters. Nature reviews cancer, 2(1), 48-58. [CrossRef]
- Graham, A. M., & Presnell, J. S. (2017). Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PloS one, 12(6), e0179545. [CrossRef]
- Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. cell, 144(5), 646-674. [CrossRef]
- Hasan, S., Taha, R., & El Omri, H. (2018). Current opinions on chemoresistance: An overview. Bioinformation, 14(2), 80. [CrossRef]
- Hayano, M., Yang, W., Corn, C., Pagano, N., & Stockwell, B. (2016). Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death & Differentiation, 23(2), 270-278.
- He, M., Wu, H., Jiang, Q., Liu, Y., Han, L., Yan, Y.,…Chen, H. (2019). Hypoxia-inducible factor-2α directly promotes BCRP expression and mediates the resistance of ovarian cancer stem cells to adriamycin. Molecular oncology, 13(2), 403-421.
- Huan, L., Guo, T., Wu, Y., Xu, L., Huang, S., Xu, Y.,…He, X. (2020). Hypoxia induced LUCAT1/PTBP1 axis modulates cancer cell viability and chemotherapy response. Molecular cancer, 19, 1-17.
- Huang, H., Shah, H., Hao, J., Lin, J., Prayson, R. A., Xie, L.,…Yu, J. S. (2024). Long non-coding RNA lung cancer-associated transcript-1 promotes glioblastoma progression by enhancing Hypoxia-inducible factor 1 alpha activity. Neuro-Oncology, 26(8), 1388-1401. [CrossRef]
- Huang, L., Wang, W., Hu, Z., Guan, C., Li, W., & Jiang, X. (2019). Hypoxia and lncRNAs in gastrointestinal cancers. Pathology-Research and Practice, 215(12), 152687. [CrossRef]
- Huang, Q., Xing, S., Peng, A., & Yu, Z. (2020). NORAD accelerates chemo-resistance of non-small-cell lung cancer via targeting at miR-129-1-3p/SOX4 axis. Bioscience reports, 40(1), BSR20193489. [CrossRef]
- HuangLE, G., & SchauM, B. (1998). Regulationofhypoxiainducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin proteasome pathway. Proc Natl Acad Sci USA, 95, 7987-7992.
- Ivan, M., Fishel, M. L., Tudoran, O. M., Pollok, K. E., Wu, X., & Smith, P. J. (2022). Hypoxia signaling: challenges and opportunities for cancer therapy. Seminars in cancer biology,. [CrossRef]
- Iyer, N. V., Kotch, L. E., Agani, F., Leung, S. W., Laughner, E., Wenger, R. H.,…Aimee, Y. Y. (1998). Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes & development, 12(2), 149-162.
- Jaśkiewicz, M., Moszyńska, A., Króliczewski, J., Cabaj, A., Bartoszewska, S., Charzyńska, A.,…Bartoszewski, R. (2022). The transition from HIF1- to HIF2- during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cellular & molecular biology letters, 27(1), 109. [CrossRef]
- Jung, J., Zhang, Y., Celiku, O., Zhang, W., Song, H., Williams, B. J.,…Gilbert, M. R. (2019). Mitochondrial NIX promotes tumor survival in the hypoxic niche of glioblastoma. Cancer research, 79(20), 5218-5232. [CrossRef]
- Kapinova, A., Kubatka, P., Zubor, P., Golubnitschaja, O., Dankova, Z., Uramova, S.,…Richnavsky, J. (2018). The hypoxia-responsive long non-coding RNAs may impact on the tumor biology and subsequent management of breast cancer. Biomedicine & Pharmacotherapy, 99, 51-58. [CrossRef]
- Karakashev, S. V., & Reginato, M. J. (2015). Progress toward overcoming hypoxia-induced resistance to solid tumor therapy. Cancer management and research, 253-264. [CrossRef]
- Keith, B., Johnson, R., & HIF1α, M. S. HIF2α: Sibling rivalry in hypoxic tumour growth and progression., 2011, 12, 9-22. [CrossRef]
- Kelly, B. D., Hackett, S. F., Hirota, K., Oshima, Y., Cai, Z., Berg-Dixon, S.,…Semenza, G. L. (2003). Cell type–specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circulation research, 93(11), 1074-1081. [CrossRef]
- Kim, J., & Guan, K.-L. (2019). mTOR as a central hub of nutrient signalling and cell growth. Nature cell biology, 21(1), 63-71. [CrossRef]
- Kopecka, J., Rankin, G. M., Salaroglio, I. C., Poulsen, S.-A., & Riganti, C. (2016). P-glycoprotein-mediated chemoresistance is reversed by carbonic anhydrase XII inhibitors. Oncotarget, 7(52), 85861. [CrossRef]
- Kung-Chun Chiu, D., Pui-Wah Tse, A., Law, C.-T., Ming-Jing Xu, I., Lee, D., Chen, M.,…Wai-Hung Ho, D. (2019). Hypoxia regulates the mitochondrial activity of hepatocellular carcinoma cells through HIF/HEY1/PINK1 pathway. Cell death & disease, 10(12), 934.
- Kuo, T.-C., Kung, H.-J., & Shih, J.-W. (2020). Signaling in and out: long-noncoding RNAs in tumor hypoxia. Journal of Biomedical Science, 27(1), 59. [CrossRef]
- Lai, H.-H., Li, J.-N., Wang, M.-Y., Huang, H.-Y., Croce, C. M., Sun, H.-L.,…Hung, M.-C. (2018). HIF1-α promotes autophagic proteolysis of Dicer and enhances tumor metastasis. The Journal of Clinical Investigation, 128(2), 625-643. [CrossRef]
- Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J., & Whitelaw, M. L. (2002). Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science, 295(5556), 858-861. [CrossRef]
- Li, H., Wang, J., Jin, Y., Lin, J., Gong, L., & Xu, Y. (2022). Hypoxia upregulates the expression of lncRNA H19 in non-small cell lung cancer cells and induces drug resistance. Translational Cancer Research, 11(8), 2876. [CrossRef]
- Li, L., Gan, Y.-P., & Peng, H. (2022). RAMP2-AS1 inhibits CXCL11 expression to suppress malignant phenotype of breast cancer by recruiting DNMT1 and DNMT3B. Experimental Cell Research, 416(2), 113139. [CrossRef]
- Li, W., Li, Y., Zhang, H., Liu, M., Gong, H., Yuan, Y.,…Chen, C. (2021). HOTAIR promotes gefitinib resistance through modification of EZH2 and silencing p16 and p21 in non-small cell lung cancer. Journal of cancer, 12(18), 5562. [CrossRef]
- Li, X., Deng, S.-j., Zhu, S., Jin, Y., Cui, S.-p., Chen, J.-y.,…Zhao, S.-f. (2016). Hypoxia-induced lncRNA-NUTF2P3-001 contributes to tumorigenesis of pancreatic cancer by derepressing the miR-3923/KRAS pathway. Oncotarget, 7(5), 6000.
- Li, X., Yang, J., Ni, R., Chen, J., Zhou, Y., Song, H.,…Pan, Y. (2022). Hypoxia-induced lncRNA RBM5-AS1 promotes tumorigenesis via activating Wnt/β-catenin signaling in breast cancer. Cell death & disease, 13(2), 95. [CrossRef]
- Liu, G. Y., & Sabatini, D. M. (2020). mTOR at the nexus of nutrition, growth, ageing and disease. Nature reviews Molecular cell biology, 21(4), 183-203.
- Liu, L., Zhao, X., Zou, H., Bai, R., Yang, K., & Tian, Z. (2016). Hypoxia promotes gastric cancer malignancy partly through the HIF1-α dependent transcriptional activation of the long non-coding RNA GAPLINC. Frontiers in Physiology, 7, 420. [CrossRef]
- Liu, X., Wang, Y., Sun, L., Min, J., Liu, J., Chen, D.,…Zhou, Y. (2018). Long noncoding RNA BC 005927 upregulates EPHB 4 and promotes gastric cancer metastasis under hypoxia. Cancer Science, 109(4), 988-1000. [CrossRef]
- Liu, Y., Chen, S., Cai, K., Zheng, D., Zhu, C., Li, L.,…Sun, C. (2022). Hypoxia-induced long noncoding RNA NR2F1-AS1 maintains pancreatic cancer proliferation, migration, and invasion by activating the NR2F1/AKT/mTOR axis. Cell death & disease, 13(3), 232. [CrossRef]
- Lou, W., Xiao, S., & Lin, K. (2024). Identification of a hypoxia-suppressed lncRNA RAMP2-AS1 in breast cancer. Non-coding RNA Research, 9(3), 782-795. [CrossRef]
- Luo, F., Sun, B., Li, H., Xu, Y., Liu, Y., Liu, X.,…Wei, S. (2016). A MALAT1/HIF2-α feedback loop contributes to arsenite carcinogenesis. Oncotarget, 7(5), 5769.
- Maldonado, V., & Melendez-Zajgla, J. (2022). The role of hypoxia-associated long non-coding RNAs in breast cancer. Cells, 11(10), 1679. [CrossRef]
- Moreno Leon, L., Gautier, M., Allan, R., Ilié, M., Nottet, N., Pons, N.,…Rezzonico, R. (2019). The nuclear hypoxia-regulated NLUCAT1 long non-coding RNA contributes to an aggressive phenotype in lung adenocarcinoma through regulation of oxidative stress. Oncogene, 38(46), 7146-7165. [CrossRef]
- Niu, Y., Bao, L., Chen, Y., Wang, C., Luo, M., Zhang, B.,…Kumar, A. (2020). HIF2-induced long noncoding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and breast cancer metastasis. Cancer research, 80(5), 964-975.
- Pan, S. T., Li, Z. L., He, Z. X., Qiu, J. X., & Zhou, S. F. (2016). Molecular mechanisms for tumour resistance to chemotherapy. Clinical and Experimental Pharmacology and Physiology, 43(8), 723-737. [CrossRef]
- Phillips, C. N. M., Zatarain, J. R., Nicholls, M. E., Porter, C., Widen, S. G., Thanki, K.,…Randall, J. W. (2017). Upregulation of cystathionine-β-synthase in colonic epithelia reprograms metabolism and promotes carcinogenesis. Cancer research, 77(21), 5741-5754. [CrossRef]
- Piao, H.-Y., Liu, Y., Kang, Y., Wang, Y., Meng, X.-Y., Yang, D., & Zhang, J. (2022). Hypoxia associated lncRNA HYPAL promotes proliferation of gastric cancer as ceRNA by sponging miR-431-5p to upregulate CDK14. Gastric Cancer, 25(1), 44-63. [CrossRef]
- Pillai, S. R., Damaghi, M., Marunaka, Y., Spugnini, E. P., Fais, S., & Gillies, R. J. (2019). Causes, consequences, and therapy of tumors acidosis. Cancer and Metastasis Reviews, 38, 205-222. [CrossRef]
- Qian, L., Fei, Q., Zhang, H., Qiu, M., Zhang, B., Wang, Q.,…Mei, M. (2020). lncRNA HOTAIR promotes DNA repair and radioresistance of breast cancer via EZH2. DNA and Cell Biology, 39(12), 2166-2173. [CrossRef]
- Ratcliffe, P. J. (2007). HIF1- and HIF2-: working alone or together in hypoxia? The Journal of clinical investigation, 117(4), 862-865.
- Rigoni, M., Riganti, C., Vitale, C., Griggio, V., Campia, I., Robino, M.,…Buondonno, I. (2015). Simvastatin and downstream inhibitors circumvent constitutive and stromal cell-induced resistance to doxorubicin in IGHV unmutated CLL cells. Oncotarget, 6(30), 29833. [CrossRef]
- Salaroglio, I. C., Gazzano, E., Abdullrahman, A., Mungo, E., Castella, B., Abd-Elrahman, G. E. F. A.-E.,…Riganti, C. (2018). Increasing intratumor C/EBP-β LIP and nitric oxide levels overcome resistance to doxorubicin in triple negative breast cancer. Journal of Experimental & Clinical Cancer Research, 37, 1-20. [CrossRef]
- Semenza, G. L. (2010). Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene, 29(5), 625-634. [CrossRef]
- Semenza, G. L. (2014). Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annual Review of Pathology: Mechanisms of Disease, 9(1), 47-71.
- Semenza, G. L., Nejfelt, M. K., Chi, S. M., & Antonarakis, S. E. (1991). Hypoxia-inducible nuclear factors bind to an enhancer element located 3'to the human erythropoietin gene. Proceedings of the National Academy of Sciences, 88(13), 5680-5684. [CrossRef]
- Shao, J., Zhang, B., Kuai, L., & Li, Q. (2021). Integrated analysis of hypoxia-associated lncRNA signature to predict prognosis and immune microenvironment of lung adenocarcinoma patients. Bioengineered, 12(1), 6186-6200. [CrossRef]
- Shih, J.-W., & Kung, H.-J. (2017). Long non-coding RNA and tumor hypoxia: new players ushered toward an old arena. Journal of biomedical science, 24, 1-19. [CrossRef]
- Slemc, L., & Kunej, T. (2016). Transcription factor HIF1A: downstream targets, associated pathways, polymorphic hypoxia response element (HRE) sites, and initiative for standardization of reporting in scientific literature. Tumor Biology, 37, 14851-14861. [CrossRef]
- Takano, N., Sarfraz, Y., Gilkes, D. M., Chaturvedi, P., Xiang, L., Suematsu, M.,…Semenza, G. L. (2014). Decreased expression of cystathionine β-synthase promotes glioma tumorigenesis. Molecular Cancer Research, 12(10), 1398-1406. [CrossRef]
- Thomlinson, R. H., & Gray, L. H. (1955). The histological structure of some human lung cancers and the possible implications for radiotherapy. British journal of cancer, 9(4), 539. [CrossRef]
- Topel, H., Bagirsakci, E., Comez, D., Bagci, G., Cakan-Akdogan, G., & Atabey, N. (2020). lncRNA HOTAIR overexpression induced downregulation of c-Met signaling promotes hybrid epithelial/mesenchymal phenotype in hepatocellular carcinoma cells. Cell Communication and Signaling, 18(1), 110. [CrossRef]
- Trujano-Camacho, S., Cantú-de León, D., Delgado-Waldo, I., Coronel-Hernández, J., Millan-Catalan, O., Hernández-Sotelo, D.,…Campos-Parra, A. D. (2021). Inhibition of Wnt-β-catenin signaling by ICRT14 drug depends of post-transcriptional regulation by HOTAIR in human cervical cancer HeLa cells. Frontiers in oncology, 11, 729228. [CrossRef]
- Wang, G. L., Jiang, B.-h., Rue, E. A., & Semenza, G. L. (1995). Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the national academy of sciences, 92(12), 5510-5514. [CrossRef]
- Wang, L., Cai, H., Hu, Y., Liu, F., Huang, S., Zhou, Y.,…Wu, F. (2018). A pharmacological probe identifies cystathionine beta-synthase as a new negative regulator for ferroptosis. Cell Death Dis 9: 1005. In.
- Wang, X., & Wang, X. (2024). The regulation of hypoxia-related lncRNAs in hepatocellular carcinoma. Discover Oncology, 15(1), 144.
- Wang, Y., Liu, X., Zhang, H., Sun, L., Zhou, Y., Jin, H.,…Guo, H. (2014). Hypoxia-inducible lncRNA-AK058003 promotes gastric cancer metastasis by targeting γ-synuclein. Neoplasia, 16(12), 1094-1106.
- Weng, X., Liu, H., Ruan, J., Du, M., Wang, L., Mao, J.,…Huang, Y. (2022). HOTAIR/miR-1277-5p/ZEB1 axis mediates hypoxia-induced oxaliplatin resistance via regulating epithelial-mesenchymal transition in colorectal cancer. Cell Death Discovery, 8(1), 310. [CrossRef]
- Xie, J., Li, D.-W., Chen, X.-W., Wang, F., & Dong, P. (2013). Expression and significance of hypoxia-inducible factor-1α and MDR1/P-glycoprotein in laryngeal carcinoma tissue and hypoxic Hep-2 cells. Oncology letters, 6(1), 232-238.
- Xu, Z., Hu, J., Cao, H., Pilo, M. G., Cigliano, A., Shao, Z.,…Calvisi, D. F. (2018). Loss of Pten synergizes with c-Met to promote hepatocellular carcinoma development via mTORC2 pathway. Experimental & Molecular Medicine, 50(1), e417-e417. [CrossRef]
- Yan, H., He, N., & He, S. (2022). HCG15 is a hypoxia-responsive lncRNA and facilitates hepatocellular carcinoma cell proliferation and invasion by enhancing ZNF641 transcription. Biochemical and Biophysical Research Communications, 608, 170-176. [CrossRef]
- Yan, H.-f., Zou, T., Tuo, Q.-z., Xu, S., Li, H., Belaidi, A. A., & Lei, P. (2021). Ferroptosis: mechanisms and links with diseases. Signal transduction and targeted therapy, 6(1), 49. [CrossRef]
- Yang, H., Hu, Y., Weng, M., Liu, X., Wan, P., Hu, Y.,…Lv, K. (2022). Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. Journal of Advanced Research, 37, 91-106.
- Yang, S.-L., Wu, C., Xiong, Z.-F., & Fang, X. (2015). Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function. Molecular medicine reports, 12(2), 2411-2416. [CrossRef]
- Yang, Z., Wang, R., Zhang, T., & Dong, X. (2015). Hypoxia/lncRNA-AK123072/EGFR pathway induced metastasis and invasion in gastric cancer. International Journal of Clinical and Experimental Medicine, 8(11), 19954.
- Yin, X., Liao, Y., Xiong, W., Zhang, Y., Zhou, Y., & Yang, Y. (2020). Hypoxia-induced lncRNA ANRIL promotes cisplatin resistance in retinoblastoma cells through regulating ABCG2 expression. Clinical and Experimental Pharmacology and Physiology, 47(6), 1049-1057. [CrossRef]
- Yu, M. (2011). Generation, function and diagnostic value of mitochondrial DNA copy number alterations in human cancers. Life sciences, 89(3-4), 65-71. [CrossRef]
- Yuan, S., Xiang, Y., Wang, G., Zhou, M., Meng, G., Liu, Q.,…Wu, N. (2019). Hypoxia-sensitive LINC 01436 is regulated by E2F6 and acts as an oncogene by targeting miR-30a-3p in non-small cell lung cancer. Molecular oncology, 13(4), 840-856. [CrossRef]
- Zhang, D., Ding, L., Li, Y., Ren, J., Shi, G., Wang, Y.,…Hou, Y. (2017). Midkine derived from cancer-associated fibroblasts promotes cisplatin-resistance via up-regulation of the expression of lncRNA ANRIL in tumour cells. Sci Rep, 7(1), 16231. [CrossRef]
- Zhang, L., Wu, H., Zhang, Y., Xiao, X., Chu, F., & Zhang, L. (2022). Induction of lncRNA NORAD accounts for hypoxia-induced chemoresistance and vasculogenic mimicry in colorectal cancer by sponging the miR-495-3p/hypoxia-inducible factor-1α (HIF1-α). Bioengineered, 13(1), 950-962. [CrossRef]
- Zhang, P., Yao, Q., Lu, L., Li, Y., Chen, P.-J., & Duan, C. (2014). Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell reports, 6(6), 1110-1121. [CrossRef]
- Zhang, Q., Cheng, Q., Xia, M., Huang, X., He, X., & Liao, J. (2020). Hypoxia-induced lncRNA-NEAT1 sustains the growth of hepatocellular carcinoma via regulation of miR-199a-3p/UCK2. Frontiers in oncology, 10, 998.
- Zhang, R., Wang, X., Ying, X., Huang, Y., Zhai, S., Shi, M.,…Li, F. (2023). Hypoxia-induced long non-coding RNA LINC00460 promotes p53 mediated proliferation and metastasis of pancreatic cancer by regulating the miR-4689/UBE2V1 axis and sequestering USP10. International Journal of Medical Sciences, 20(10), 1339.
- Zhang, Z., Fang, E., Rong, Y., Han, H., Gong, Q., Xiao, Y.,…Zhu, Z. (2021). Hypoxia-induced lncRNA CASC9 enhances glycolysis and the epithelial-mesenchymal transition of pancreatic cancer by a positive feedback loop with AKT/HIF1-α signaling. American Journal of Cancer Research, 11(1), 123.
- Zhao, Z.-B., Chen, F., & Bai, X.-F. (2019). Long noncoding RNA MALAT1 regulates hepatocellular carcinoma growth under hypoxia via sponging MicroRNA-200a. Yonsei Medical Journal, 60(8), 727-734. [CrossRef]
- Zhou, C., Zhang, H., & Lu, L. (2021). Identification and validation of hypoxia-related lncRNA signature as a prognostic model for hepatocellular carcinoma. Frontiers in Genetics, 12, 744113. [CrossRef]
- Zhou, X., Guo, X., Chen, M., Xie, C., & Jiang, J. (2018). HIF-3α promotes metastatic phenotypes in pancreatic cancer by transcriptional regulation of the RhoC–ROCK1 signaling pathway. Molecular Cancer Research, 16(1), 124-134. [CrossRef]
- Zhu, H., Chen, X., Luo, S., Guan, J., Zhang, W., Zhang, B., & Wang, H. (2005). Hypoxia-inducible factor-1 alpha dependent expression and significance of the related multidrug resistance genes induced by hypoxia in human hepatocarcinoma cell. Zhonghua wai ke za zhi [Chinese Journal of Surgery], 43(5), 277-281.
- Zhu, H., Luo, S.-f., Wang, J., Li, X., Wang, H., Pu, W.-y.,…Zhuang, Z.-x. (2012). Effect of environmental factors on chemoresistance of HepG2 cells by regulating hypoxia-inducible factor-1α. Chinese medical journal, 125(06), 1095-1103.
- Zhu, N., Chen, X., Zhao, J., Fang, L., Yao, Y., Zhou, F.,…Xu, Q. (2022). Hypoxia-induced LINC00674 facilitates hepatocellular carcinoma progression by activating the NOX1/mTOR signaling pathway. Journal of Cancer, 13(11), 3177.
- Äänismaa, P. i., Gatlik-Landwojtowicz, E., & Seelig, A. (2008). P-glycoprotein senses its substrates and the lateral membrane packing density: consequences for the catalytic cycle. Biochemistry, 47(38), 10197-10207. [CrossRef]
Figure 1.
The composition of HIF subunits varies. The N terminal structure is conserved among different HIF proteins whereas the C terminal is different in different Hif isoforms. The structure of various HIF isoforms is illustrated as follows. The basic Helix-Loop-Helix (bHLH) and Per-ARNT-Sim (PAS) domains are crucial for both DNA binding and dimerization. Within the HIF-α subunits, the oxygen-dependent degradation domain (ODD) provides sensitivity to oxygen levels. Importantly, the ODD region consists of conserved proline residues that are hydroxylated in an oxygen-dependent manner by prolyl hydroxylase (PHD) enzymes. Furthermore, the transactivation domain (TAD) plays a vital role in achieving maximum transcriptional activity. HIF-3α exhibits greater complexity due to its multiple splice variants that have varying biological effects. Significantly, HIF-3 is distinct among the subunits as it contains a unique LZIP motif that also allows for DNA binding.
Figure 1.
The composition of HIF subunits varies. The N terminal structure is conserved among different HIF proteins whereas the C terminal is different in different Hif isoforms. The structure of various HIF isoforms is illustrated as follows. The basic Helix-Loop-Helix (bHLH) and Per-ARNT-Sim (PAS) domains are crucial for both DNA binding and dimerization. Within the HIF-α subunits, the oxygen-dependent degradation domain (ODD) provides sensitivity to oxygen levels. Importantly, the ODD region consists of conserved proline residues that are hydroxylated in an oxygen-dependent manner by prolyl hydroxylase (PHD) enzymes. Furthermore, the transactivation domain (TAD) plays a vital role in achieving maximum transcriptional activity. HIF-3α exhibits greater complexity due to its multiple splice variants that have varying biological effects. Significantly, HIF-3 is distinct among the subunits as it contains a unique LZIP motif that also allows for DNA binding.
Figure 2.
Regulation of HIF by oxygen levels. In normal oxygen situations, prolyl hydroxylase (PHD) enzymes hydroxylate HIF-α subunits in a process that depends on oxygen. This hydroxylation tags them for degradation via the proteasome by increasing their affinity for the E3 Ligase VHL. Conversely, in low oxygen conditions, PHD activity is suppressed, resulting in the stabilization and movement of HIF-α subunits into the nucleus. Once there, they form a heterodimer with HIF-1β and initiate the transcription of genes that facilitate adaptation to hypoxic environments. HIF refers to hypoxia-inducible factor, and VHL stands for von Hippel-Lindau.
Figure 2.
Regulation of HIF by oxygen levels. In normal oxygen situations, prolyl hydroxylase (PHD) enzymes hydroxylate HIF-α subunits in a process that depends on oxygen. This hydroxylation tags them for degradation via the proteasome by increasing their affinity for the E3 Ligase VHL. Conversely, in low oxygen conditions, PHD activity is suppressed, resulting in the stabilization and movement of HIF-α subunits into the nucleus. Once there, they form a heterodimer with HIF-1β and initiate the transcription of genes that facilitate adaptation to hypoxic environments. HIF refers to hypoxia-inducible factor, and VHL stands for von Hippel-Lindau.
Figure 3.
Interaction between HIF1-α and hypoxia associated lncRNA in breast cancer (BC). HIF1-α directly binds to the promoter of RAB11B-AS1 and RBM5-AS1 to induce their expression. Both these lncRNAs lead to breast cancer metastasis. HIF1-α suppresses the expression of tumor suppressor RAMP2-AS1 to upregulate oncogenic miR-660-5p to further promote BC metastasis.
Figure 3.
Interaction between HIF1-α and hypoxia associated lncRNA in breast cancer (BC). HIF1-α directly binds to the promoter of RAB11B-AS1 and RBM5-AS1 to induce their expression. Both these lncRNAs lead to breast cancer metastasis. HIF1-α suppresses the expression of tumor suppressor RAMP2-AS1 to upregulate oncogenic miR-660-5p to further promote BC metastasis.
Figure 4.
Interaction between HIF1-α and hypoxia associated lncRNA in Heptocellular carcinoma (HCC). HIF1-α directly binds to the promoter of LINC00674, NEAT1, HCG15 and MALAT1 to induce their expression. NEAT1 and MALAT1lncRNAs promote HCC metastasis by sponging miR-1991-3p and miR200a. lead to breast cancer metastasis. LINC00674 activate the NOX/mTOR pathway and HCG15 enhance ZNF641 transcription to promote HCC metastasis.
Figure 4.
Interaction between HIF1-α and hypoxia associated lncRNA in Heptocellular carcinoma (HCC). HIF1-α directly binds to the promoter of LINC00674, NEAT1, HCG15 and MALAT1 to induce their expression. NEAT1 and MALAT1lncRNAs promote HCC metastasis by sponging miR-1991-3p and miR200a. lead to breast cancer metastasis. LINC00674 activate the NOX/mTOR pathway and HCG15 enhance ZNF641 transcription to promote HCC metastasis.
Figure 5.
Interaction between HIF1-α and hypoxia associated lncRNA in Gastric cancer. HIF1-α directly binds to the promoter of GAPLINC, BC005927 , AK123072, HYPAL and AK058003 to induce their expression. BC005927, AK123072, and AK0580031lncRNAs boost the expression of oncogenic genes like EPBH4, EGFR and SNCG by demethylating their promoters. LncRNA HYPAL act as ceRNA for miR-431-5p resulting in overexpression of CDK14 leading to metastasis.
Figure 5.
Interaction between HIF1-α and hypoxia associated lncRNA in Gastric cancer. HIF1-α directly binds to the promoter of GAPLINC, BC005927 , AK123072, HYPAL and AK058003 to induce their expression. BC005927, AK123072, and AK0580031lncRNAs boost the expression of oncogenic genes like EPBH4, EGFR and SNCG by demethylating their promoters. LncRNA HYPAL act as ceRNA for miR-431-5p resulting in overexpression of CDK14 leading to metastasis.
Figure 6.
Interaction between HIF1-α and hypoxia associated lncRNA in Pancreatic cancer. HIF1-α directly binds to the promoter of NUTF2P3-001, LINC00460, CASC9, and NR2F1-AS1 to induce their expression. NUTF2P3-001 and LINC00460 sponge miR-3923 and miR-4689 to enhance the expression of KRAS and UBE2V1 respectively leading to pancreatic cancer metastasis. CASC9, and NR2F1-AS1activate the PI3K/AKT and AKT/mTOR pathway to promote pancreatic cancer metastasis.
Figure 6.
Interaction between HIF1-α and hypoxia associated lncRNA in Pancreatic cancer. HIF1-α directly binds to the promoter of NUTF2P3-001, LINC00460, CASC9, and NR2F1-AS1 to induce their expression. NUTF2P3-001 and LINC00460 sponge miR-3923 and miR-4689 to enhance the expression of KRAS and UBE2V1 respectively leading to pancreatic cancer metastasis. CASC9, and NR2F1-AS1activate the PI3K/AKT and AKT/mTOR pathway to promote pancreatic cancer metastasis.
Figure 7.
Interaction between HIF1-α and hypoxia associated lncRNA in Lung cancer. Hypoxia induce the expression of LINC01436 by the binding of HIF1-α to its promoter. LINC01436 sponge miR-30a-3p to activate E2F6 leading to lung cancer metastasis.
Figure 7.
Interaction between HIF1-α and hypoxia associated lncRNA in Lung cancer. Hypoxia induce the expression of LINC01436 by the binding of HIF1-α to its promoter. LINC01436 sponge miR-30a-3p to activate E2F6 leading to lung cancer metastasis.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).