Submitted:
26 November 2024
Posted:
27 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Formulations Development
2.2. Preparation of Hair Locks Samples
2.3. Hair Diameter Measurement
2.4. Scanning Electronic Microscopy
2.4.1. Image Analysis of SEM Images
2.5. Hair Thermal Analysis
2.6. Assessment of Hair Loss by Breakage
2.7. Mechanical Resistance
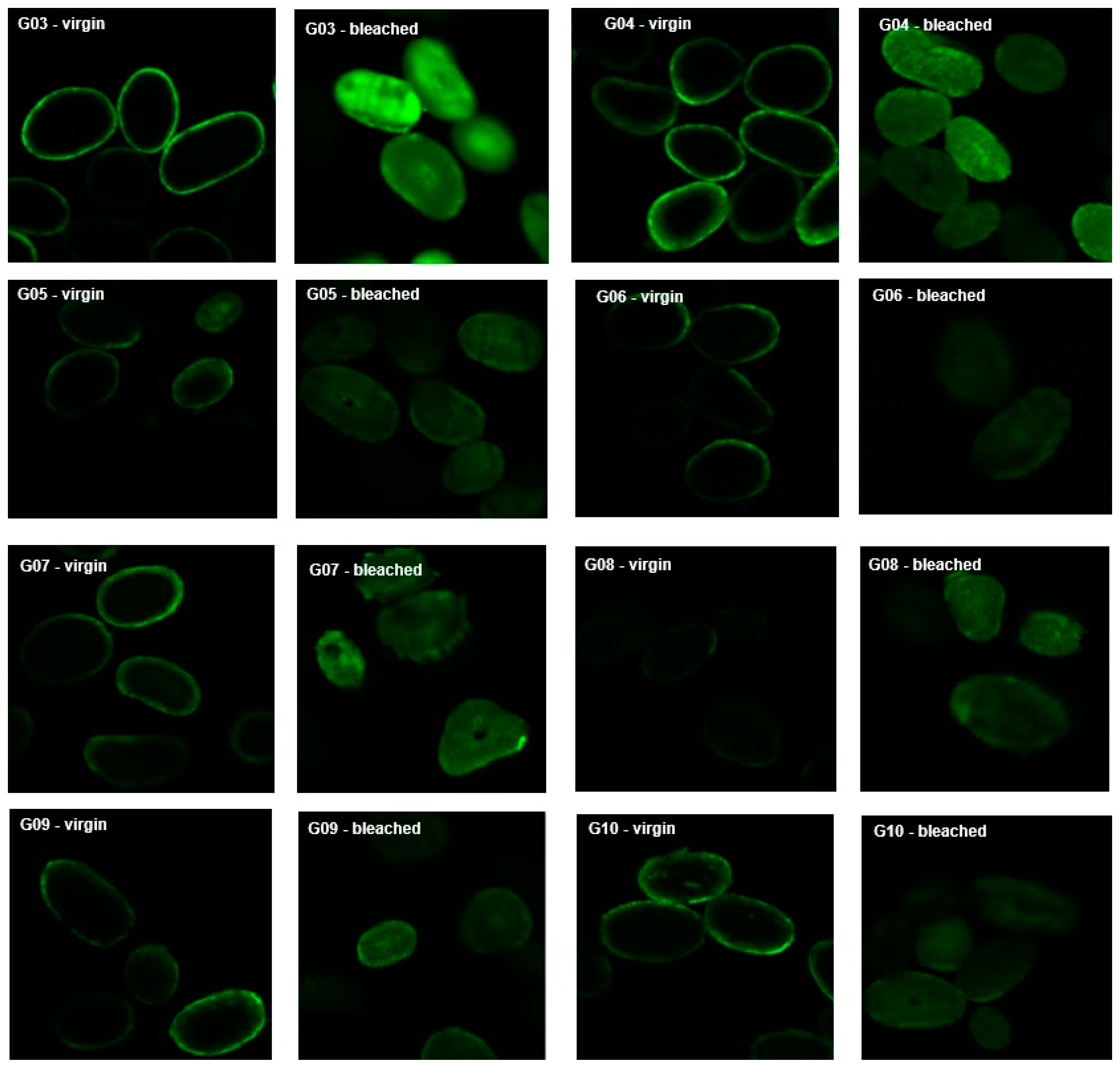
2.8. Fluorescence Confocal Microscopy
- a)
- “Virgin”: virgin locks were treated as described in item 2.2 with straightener products (G03 to G10) impregnated with rhodamine.
- b)
- “Bleached”: locks were previously bleached (as described for group G02 in item 2.2) and then treated as described in item 2.2 with straightener products (G03 to G10) impregnated with rhodamine.
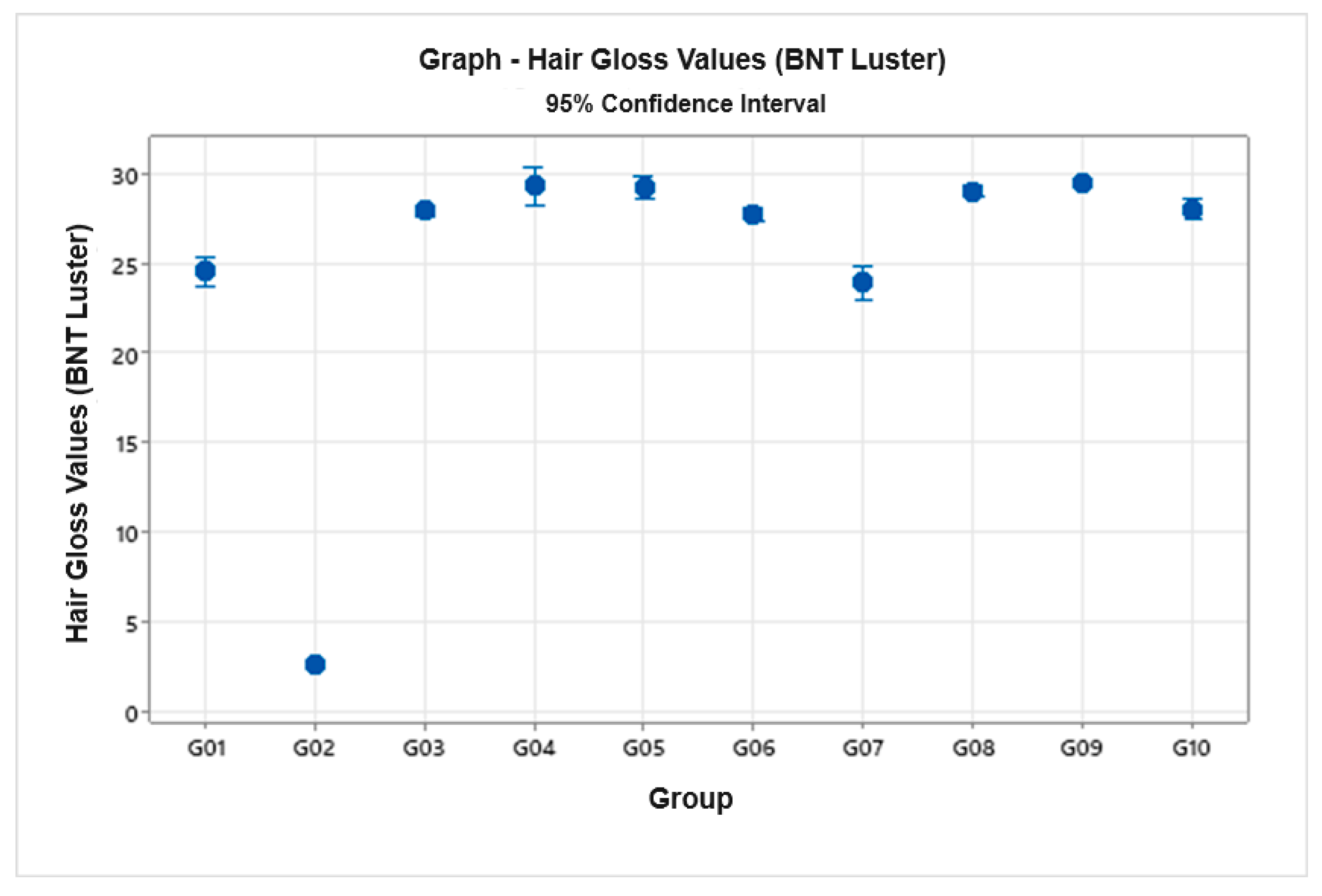
2.9. Hair Gloss Measurement
- LBNT = Bossa Nova Technologies Luster
- Sin = specular profile value in central light distribution
- Sout = specular profile value for extreme angle
- D = integral value of the diffuse profile
- Wvisual = average width of brightness band
3. Results
3.1. Formulations Development and Straightening Efficacy
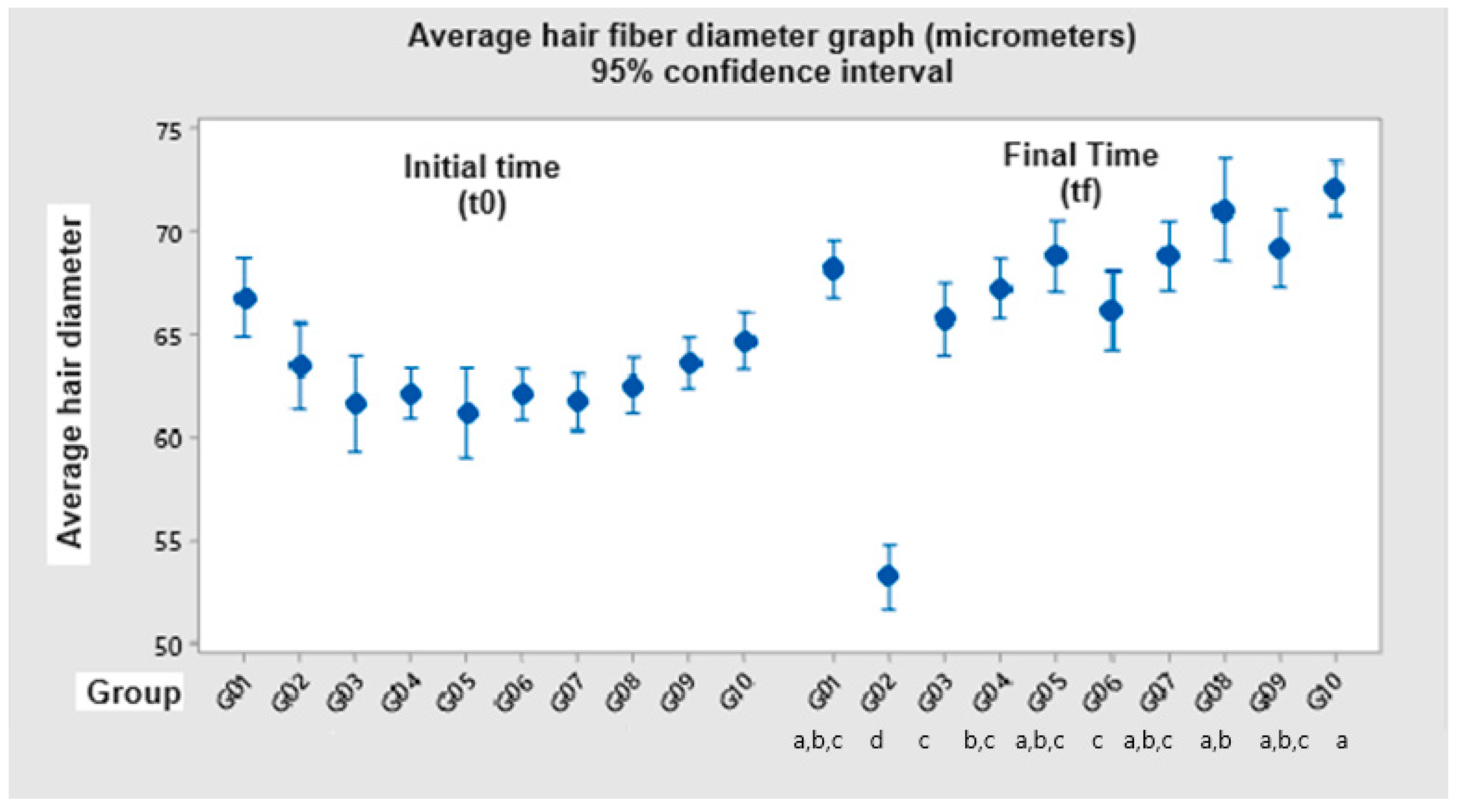
3.2. Hair Diameter Measurement
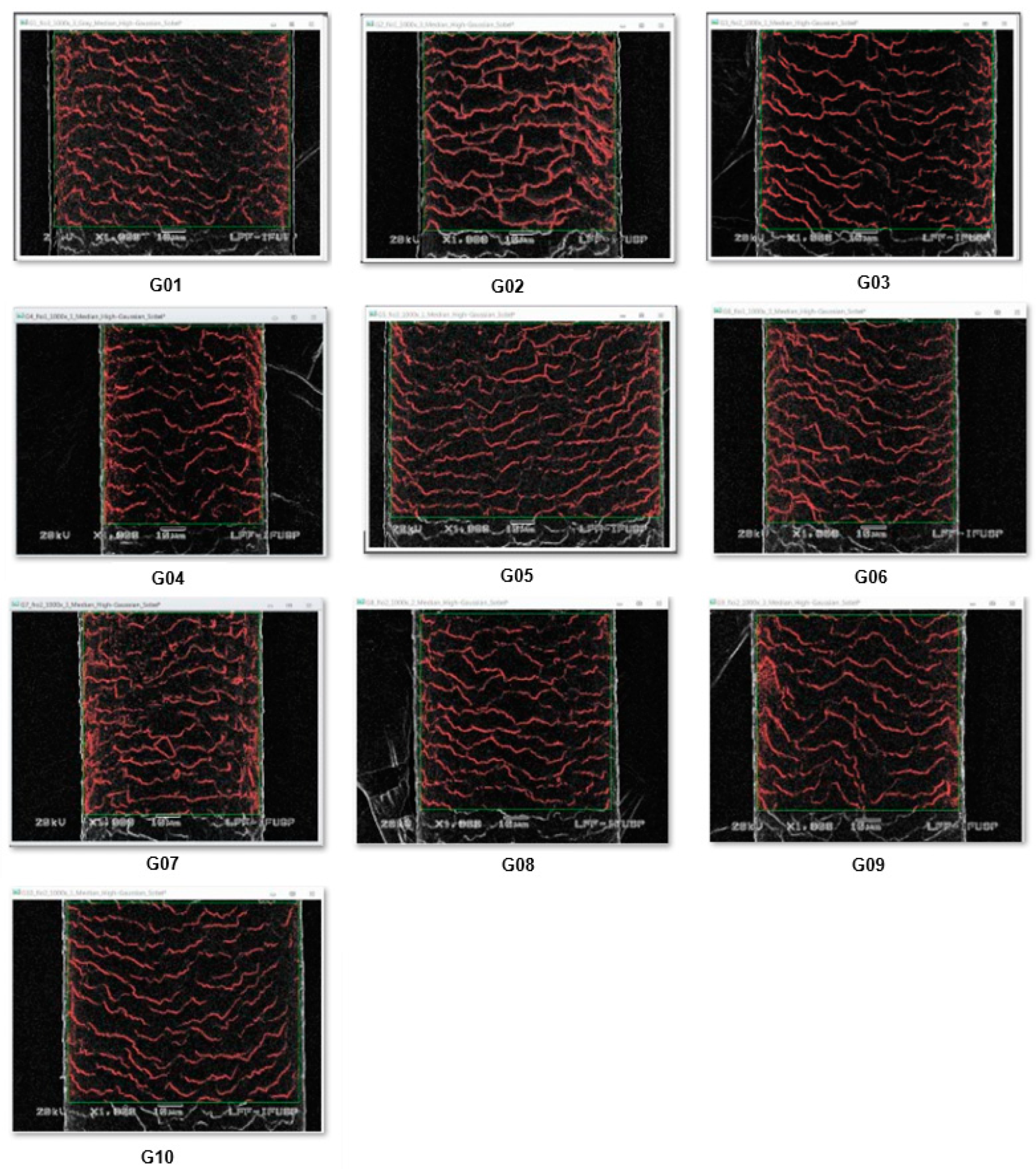
3.3. Scanning Electronic Microscopy (SEM) with Image Analysis
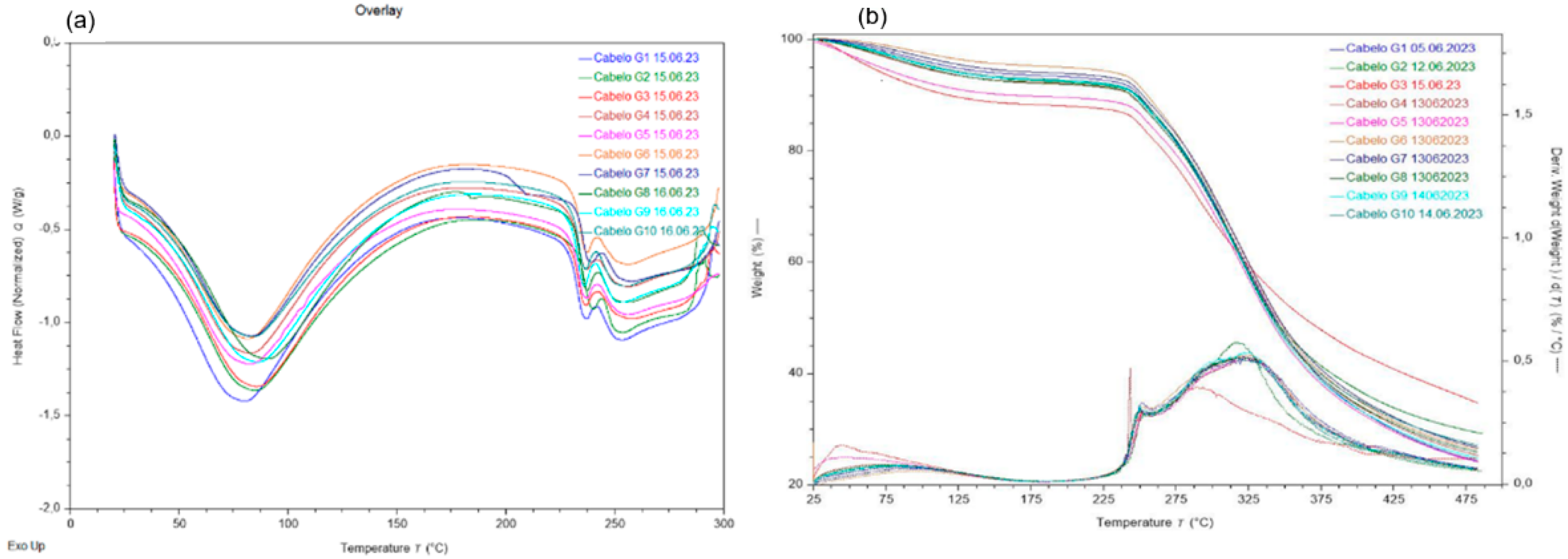
3.4. Hair Thermal Analysis
3.5. Assessment of Hair Breakage
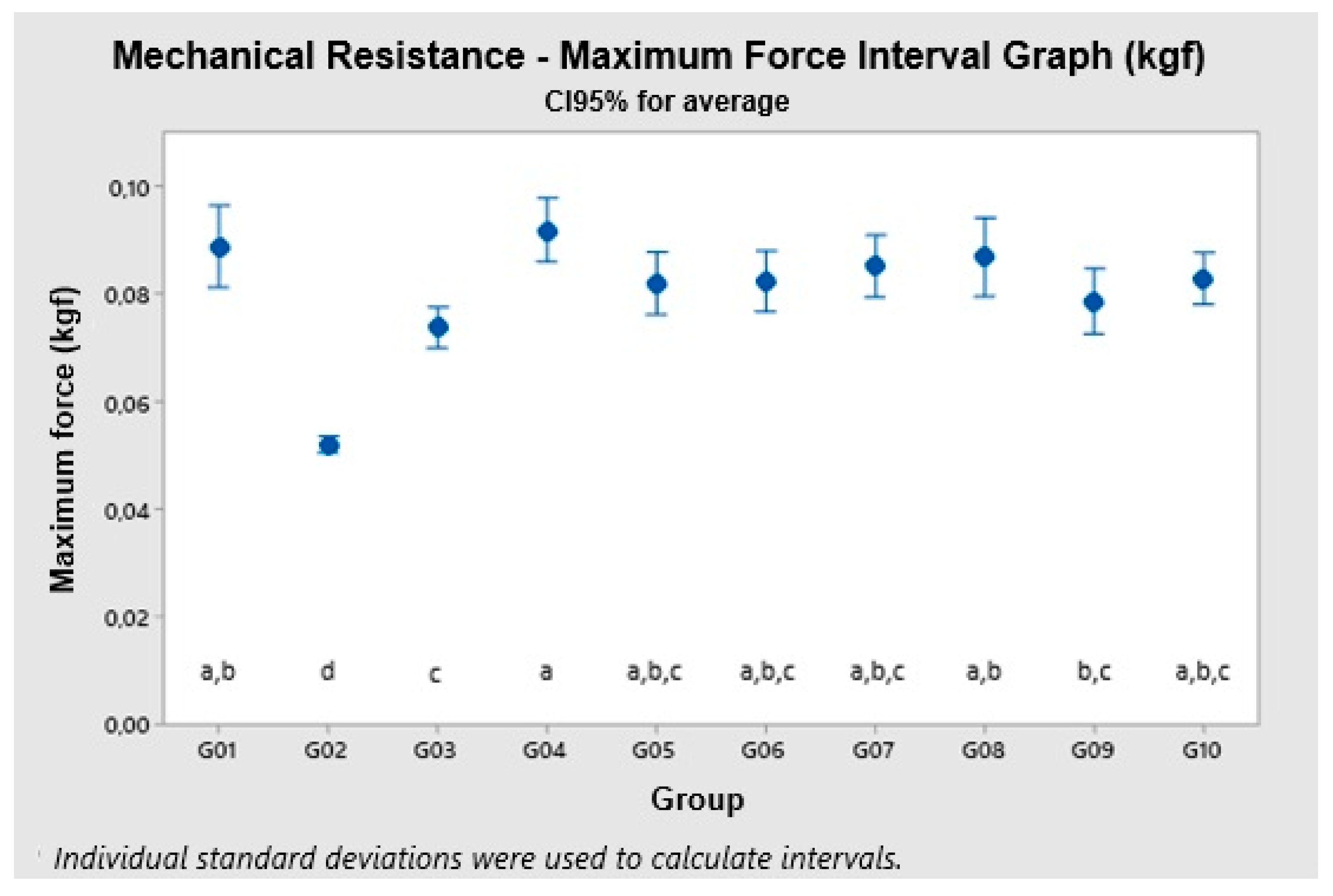
3.6. Mechanical Resistance
3.7. Fluorescence Confocal Microscopy
3.8. Hair Gloss Measurement
4. Discussion
- First event (peak 1): water loss at 50-150°C.
- Second event (peaks 2 and 3): onset of matrix pyrolysis and disorganization of the keratin structure at 250-350°C.
- Third event (peak 4): degradation of keratin’s carbon structure until 500°C.
- G03 permeated slightly more than G04, what was favored by the higher pH range and higher ammonium hydroxide’s (dilator) concentration.
- G05 and G06 penetrated the least among all treatments and had a very smooth action, what was expected, as they act at the lowest pH values (7.5-8.5) and their dilator (amino methyl propanol) is softer.
- When comparing G07 and G08, G07 permeated more, which could be attributed to the difference in active available in free form as well as a difference in active’s release kinetics due to the polymeric associations.
- G09 permeated slightly more than G10, what was probably favored by the increased pH range and higher amount of the dilator ammonium hydroxide. In these groups, sodium thioglycolate acted as a permeation accelerator and increased the liberation kinetics of the reductor active – thioglycolic acid.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fink, B.; Hufschmidt, C.; Hirn, T.; Will, S.; McKelvey, G.; Lankhof, J. Age, health and attractiveness perception of virtual (rendered) human hair. Front Psychol 2016, 7, 1–12. [CrossRef]
- Junior, C.M. Estudo da formação, aplicação e do desempenho do tioglicolato de amino metilpropanol como um novo composto químico para tratamento de controle e redução de volume dos cabelos. Masters Dissertation, Instituto de Pesquisas Tecnológicas do Estado de São Paulo, São Paulo, 2015.
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 5th ed.; Springer Berlin Heidelberg: Berlin, Germany, 2012.
- Colenci, A.V. Degradação do cabelo humano causada pelo uso de alisantes contemporâneos e outros processos químicos. Doctorate Thesis, Centro de Ciências Exatas e de Tecnologia, Universidade Federal de São Carlos, São Carlos, 2017.
- Barreto, T. et al. Straight to the point: What do we know so far on hair straightening? Skin Appendage Disorders 2021, 7, 265-271. [CrossRef]
- De Labbey, A. Composicion cosmetica reductora para la deformación permanente de los cabellos, a base de un éster de ácido tioglicólico y de N-acil (C2-C4) cisteamina y su procedimiento de realización. Depositor: L’Oreal. ES 2 057 791. Deposit: 07 aug. 1991. Concession: 16 oct. 1994.
- De Labbey, A. Y; Pataut, F. Composición reductora que comprende un ácido aminado básico y un polímero catiónico. Depositor: L’Oreal. 2 116 132. Deposit: 31 jul. 1996. Concession: 01 jul. 1998.
- Johnson, B. et al. Long-Lasting Care and Protection for Damaged Hair Help Restore Hair’s Hydrophobic State Featured DOWSILTM Brand Silicones. p. 7081, 2018.
- Neill, P. et al. Composición de ondulación permanente y procedimiento. Depositor: UNILEVER. 2 204 928. Deposit: 16 aug. 1995. Concession: 01 may 2004.
- Leonardi, G.R.; Elias, V.R.S. Cosmetologia e Empreendedorismo: Perspectivas para a criação de Novos Negócios; Editora Pharmabooks: São Paulo, Brazil; 2015.
- Quadflieg, J.M. Fundamental properties of Afro-American hair as related to their straightening/relaxing behaviour. Doctorate Thesis, Fakultät für Mathematik, Informatik und Naturwissenschaften der Rheinisch-Westfälischen Technischen Hochschule Aachen, Dortmund, 2003.
- National Health Surveillance Agency (ANVISA). Guia de Estabilidade de Produtos Cosméticos (Série Qualidade em Cosméticos; v. 1), 1st ed.; ANVISA: Brasília, Brazil, 2004, 54p. ISBN 85-88233-15-0.
- Velasco, M.V.R; De Sá Dias, T.C.; De Freitas, A.Z.; Júnior, N.D.V.; Pinto, C.A.S.O.; Kaneko, T.M., et al. Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Brazilian J Pharm Sci 2009, 45, 153–62. [CrossRef]
- Lima, C.R.R.C. Caracterização físico-química e analítica de fibras capilares e ingredientes cosméticos para proteção. Doctorate Thesis, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, 2016.
- Bossa Nova Vision. Samba Hair: Hair shine measurements for claims and research. Bossa Nova Vision: Los Angeles. Available at: https://www.bossanovavision.com/homepage/cosmetic-testing/samba-hair/. Access in: 7th jun. 2023.
- Bretzke, P.E. Caracterizacão e disponibilidade biológica in vitro e ex vivo de argilominerais utilizados como insumos farmacêuticos e cosméticos. Master Dissertation. Universidade do Vale do Itajaí (UNIVALI), Itajaí, 2015.
- Lima, C.R.R.C. et al. Alterations promoted by acid straightening and/or bleaching in hair microstructures. Journal of Applied Crystallography 2023,56, 1002–1014. [CrossRef]
- Sarruf F.D. Cosmetic attributes (oiliness reduction and firmness) from face masks composed of red, green and black clays. Doctorate Thesis, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, 2024.
- Damazio, M. G.; Makino, R. DE F. L. Terapia Capilar: Uma abordagem inter e multidisciplinar, 1st ed.; RED Publicações: São Paulo, Brazil, 2017.
- Lourenço, C.B. et al. Brief descriptions of the principles of prominent methods used to study the penetration of materials into human hair and a review of examples of their use. International Journal of Cosmetic Science 2021, 43, 113–122. [CrossRef]
- Frangie, C.M. et al. Milady Standard Cosmetology, 12th ed.; Cengage Learning: USA, 2012.
- Martins Junior C. Novo composto químico vantajoso para redução do volume e/ou ali- samento de cabelos, processo de obtenção do novo composto, solução vantajosa para redução do volume e alisamento de cabelos, processo de obtenção de solução, formulação. Depositant: Bruno Felipe Martins. BR 102022004790-1 A2. Concession: 2023.
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Nanoscale Molecular Characterization of Hair Cuticle Cells Using Integrated Atomic Force Microscopy–Infrared Laser Spectroscopy. Applied Spectroscopy 2020, 74, 1540–1550. [CrossRef]
- Wang, L. et al. Kinetics and Equilibrium of Solute Diffusion into Human Hair. Annals of Biomedical Engineering 2012, 40, 2719–2726. [CrossRef]
- Wortmann, F.J. et al. pH-equilibration of human hair: Kinetics and pH-dependence of the partition ratios for H+ − and OH− -ions based on a Freundlich isotherm. Biophysical Chemistry 2023, 297, 1-8.
- Wortmann, F.J.; Popescu, C.; Sendelbach, G. Effects of reduction on the denaturation kinetics of human hair. Biopolymers 2008, 89, 600-605. [CrossRef]
- Gao, T.; Pereira, A.; Zhu, S. Study of hair shine and hair surface smoothness. J Cosmet Sci 2009, 60, 187–197.
- Bloch, L.D. et al. Chemical and physical damage affect the perceptions of hair attributes: A quantitative sensory assessment by a trained panel. Journal of Sensory Studies 2021, 36, . [CrossRef]
- Goshima, A.M. Avaliação das Propriedades das Fibras Capilares Tratadas com Alisante Ácido com Diferentes Valores de pH. Master Dissertation. Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, 2019.
- Wortmann, F.J.; Deutz, H. Thermal-analysis of ortho-cortical and para-cortical cells isolated from wool fibers. Journal Applied Polymer Science 1998, 68, 1991-1995.
- Popescu, C.; Gummer, C. DSC of human hair: a tool for claim support or incorrect data analysis? International Journal of Cosmetic Science 2016, 38, 433-439. [CrossRef]
- Wortmann, F.J.; Wortmann, G.; Popescu, C. Linear and nonlinear relations between DSC parameters and elastic moduli for chemically and thermally treated human hair. Journal of Thermal Analysis and Calorimetry 2020, 140, 2171–2178. [CrossRef]
- Monteiro, V.F.; Maciel, A.P.; Longo, E. Thermal analysis of caucasian human hair. Journal of Thermal Analysis and Calorimetry 2005, 79, 289-293. [CrossRef]
- Junior, C.M. Desenvolvimento de uma nova geração de produtos para alisamento capilar contendo diferentes associações de ingredientes ativos. Doctorate Thesis, Universidade Estadual Paulista, Faculdade de Ciências Farmacêuticas, Araraquara, 2024.

| Formulations and Groups | Composition1 |
|---|---|
| Virgin hair (G01) - control | Not applicable |
| Market bleaching product (G02) - control | Association of ammonium, sodium and potassium persulfates + oxidizing cream 40V (12% H2O2) |
| Straightener with ammonium thioglycolate – high concentration (G03) | Ammonium thioglycolate 59%: 22.50% |
| Ammonium Hydroxide 29%: 2.50% | |
| Viscosity: 80000-120000 cPs | |
| pH: 9,0 – 9,5 | |
| Straightener with ammonium thioglycolate – low concentration (G04) | Ammonium thioglycolate 59%: 16.00% |
| Ammonium Hydroxide 29%: 1.50% | |
| Viscosity: 80000-120000 cPs | |
| pH: 9,0 – 9,5 | |
| Straightener with amino methyl propanol thioglycolate – high concentration (G05) | Amino methyl propanol: 12.50% |
| Thioglycolic acid: 11.00% | |
| Viscosity: 100000-200000 cPs | |
| pH: 7,5 – 8,5 | |
| Straightener with amino methyl propanol thioglycolate – low concentration (G06) | Amino methyl propanol: 7.90% |
| Thioglycolic acid: 7.50% | |
| Viscosity: 100000-200000 cPs | |
| pH: 7,5 – 8,5 | |
| Straightener with sodium cysteamine – high concentration (G07) | Cysteine: 5.00% |
| Sodium Hydroxide: 4.05% | |
| Sodium Metabisulfite: 0.50% | |
| Viscosity: 50000-100000 cPs | |
| pH: 11,0 – 13,0 | |
| Straightener with sodium cysteamine – low concentration (G08) | Cysteine: 4.00% |
| Sodium Hydroxide: 3.00% | |
| Sodium Metabisulfite: 0.50% | |
| Viscosity: 200000-300000 cPs | |
| pH: 11,0 – 13,0 | |
| Straightener with ammonium thioglycolate and sodium thioglycolate – high concentration (G09) | Ammonium thioglycolate 59%: 18.00% |
| Sodium Hydroxide: 2.75% | |
| Thioglycolic acid: 2.00% | |
| Viscosity: 100000-200000 cPs | |
| pH: 8,5 – 9,5 | |
| Straightener with ammonium thioglycolate and sodium thioglycolate – low concentration (G10) | Ammonium thioglycolate 59%: 20.00% |
| Sodium Hydroxide: 2.20% | |
| Thioglycolic acid: 1.00 | |
| Viscosity: 45000-80000 cPs | |
| pH: 8,5 – 9,5 |
| Group | pH value | Viscosity spindle and rotation (rpm) | Viscosity value (cPs) |
|---|---|---|---|
| G03 | 9,32 | S63 – 0.6 | 95180 |
| G04 | 9,23 | S63 – 1.0 | 112000 |
| G05 | 8,44 | S63 – 0.6 | 118000 |
| G06 | 7,84 | S63 – 0.6 | 174000 |
| G07 | 12,71 | S63 – 1.5 | 60707 |
| G08 | 11,27 | S63 – 0.3 | 250000 |
| G09 | 8,80 | S63 – 6.0 | 12157 |
| G10 | 9,00 | S63 – 1.5 | 51269 |
| Group | Selected Fiber | Selected Image | Percentage area of cuticle shadow |
|---|---|---|---|
| G01 | 3 | 3 | 9.06% |
| G02 | 1 | 3 | 8.09% |
| G03 | 2 | 1 | 5.38% |
| G04 | 1 | 1 | 7.35% |
| G05 | 3 | 1 | 6.10% |
| G06 | 1 | 3 | 7.08% |
| G07 | 2 | 3 | 7.82% |
| G08 | 2 | 2 | 5.95% |
| G09 | 2 | 3 | 7.04% |
| G10 | 2 | 1 | 5.51% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





