Submitted:
22 November 2024
Posted:
27 November 2024
You are already at the latest version
Abstract
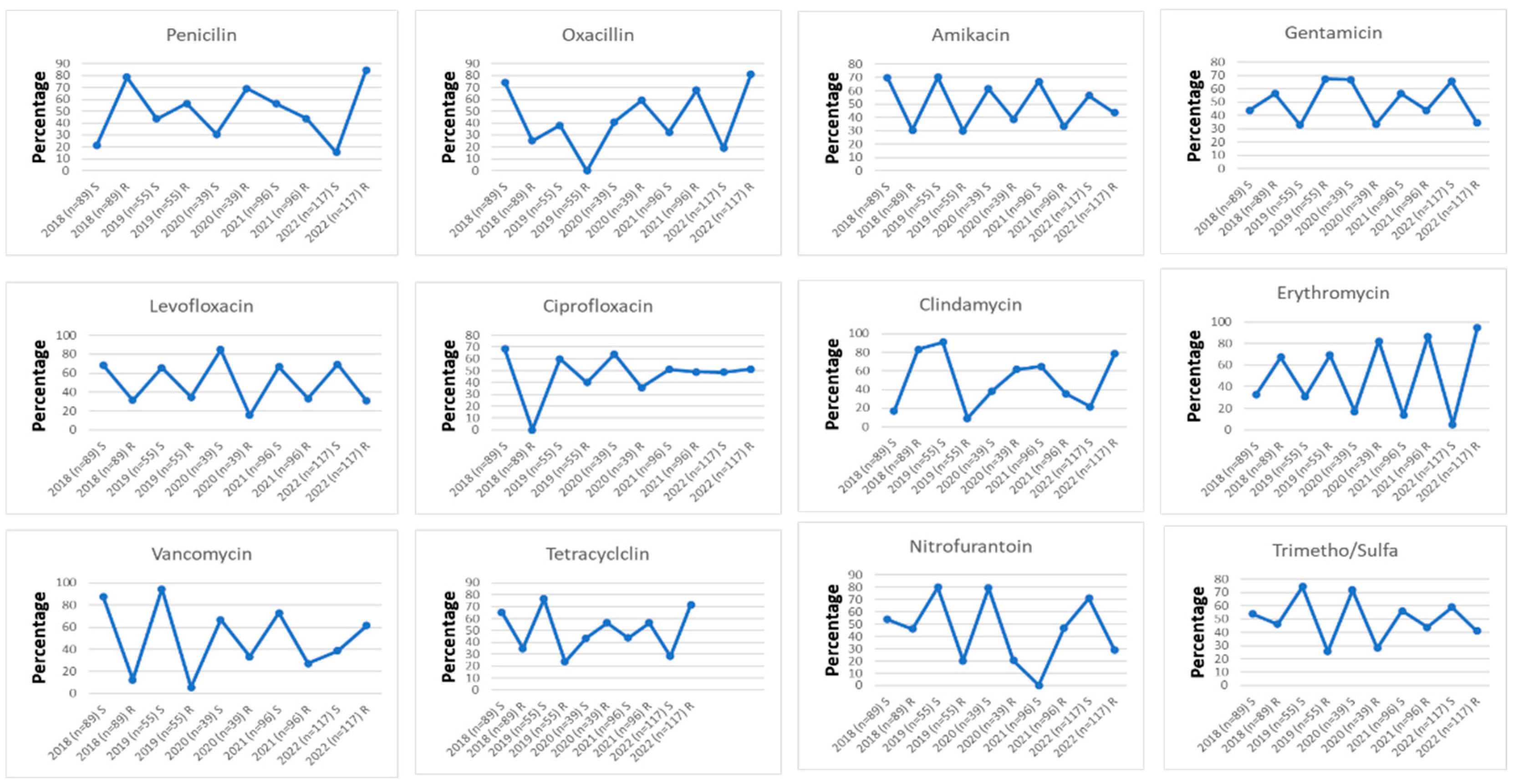
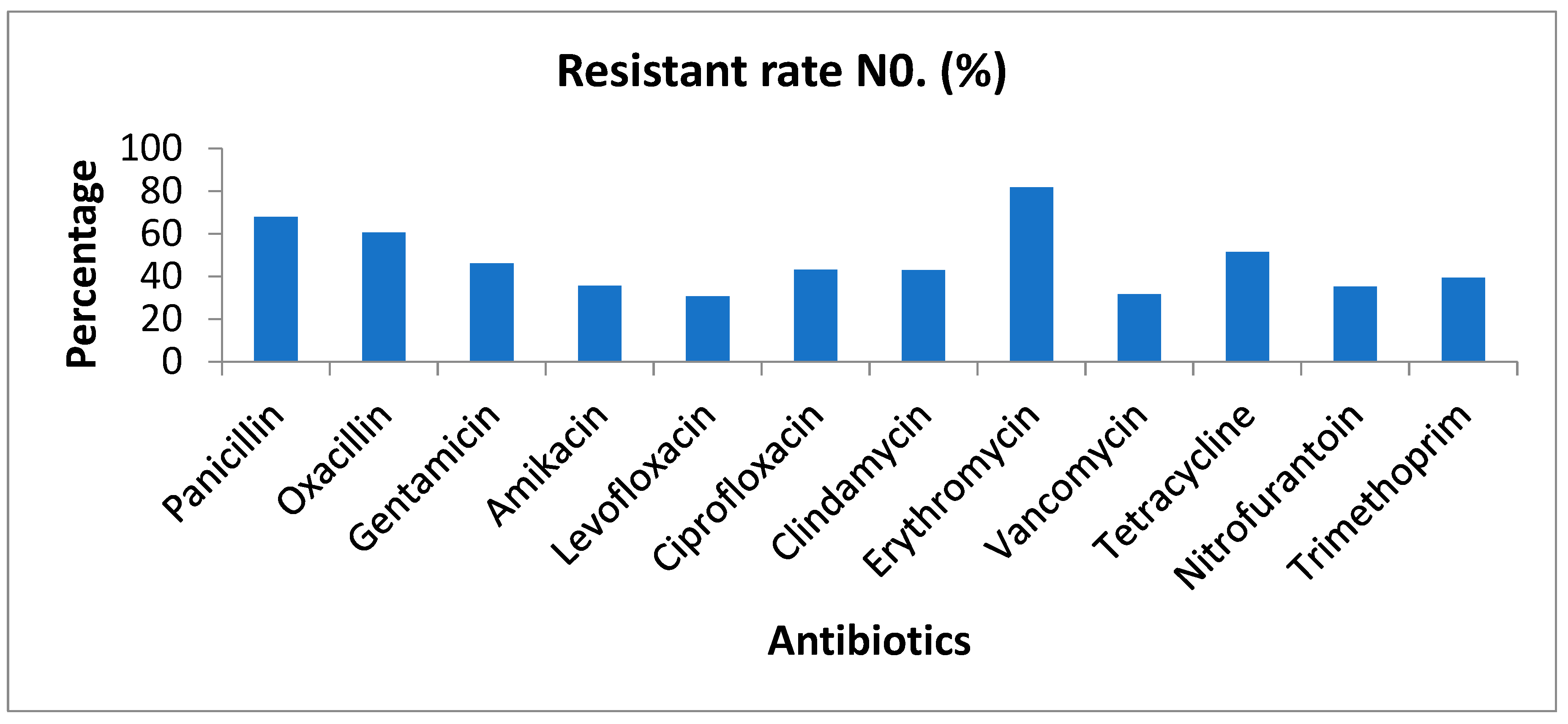
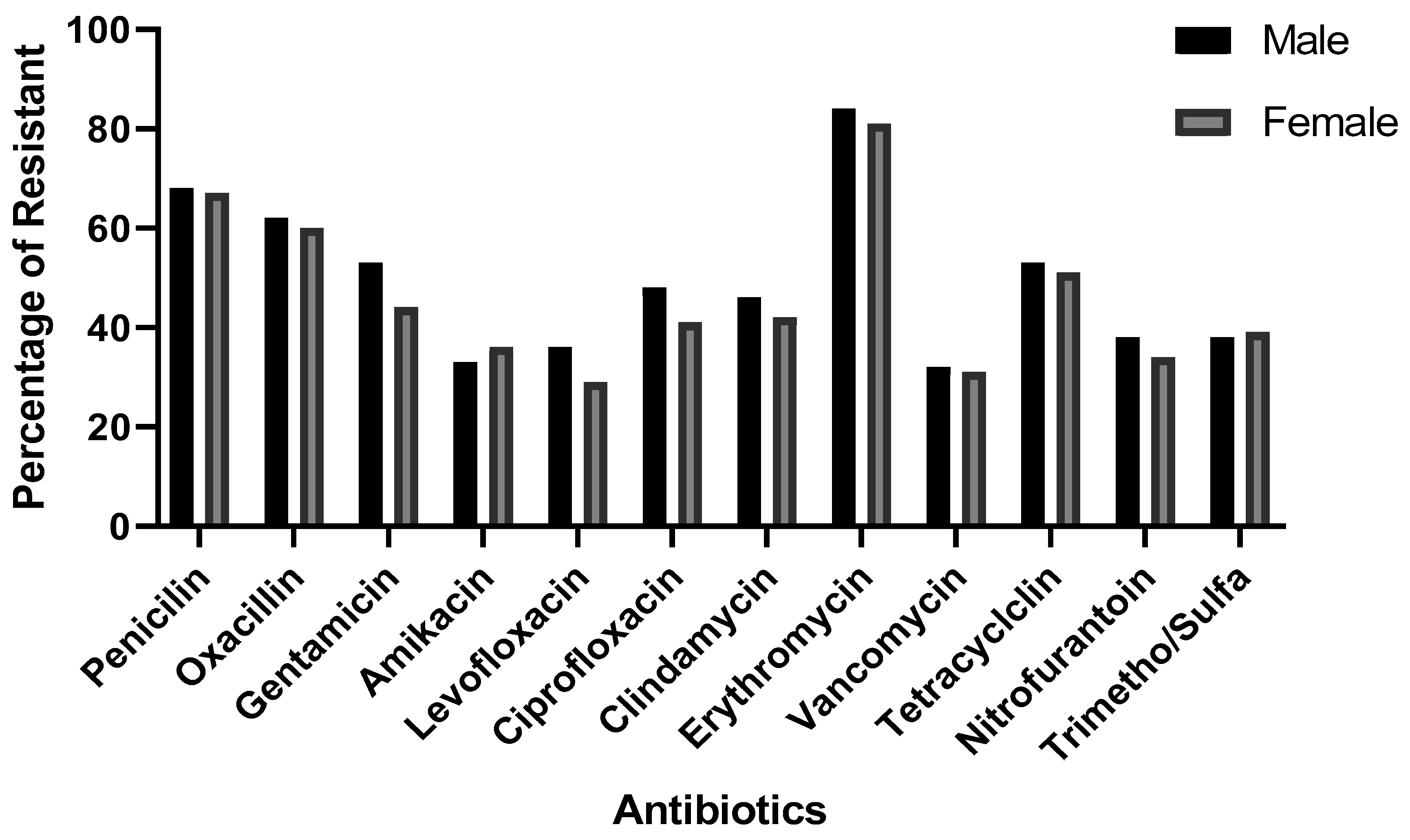
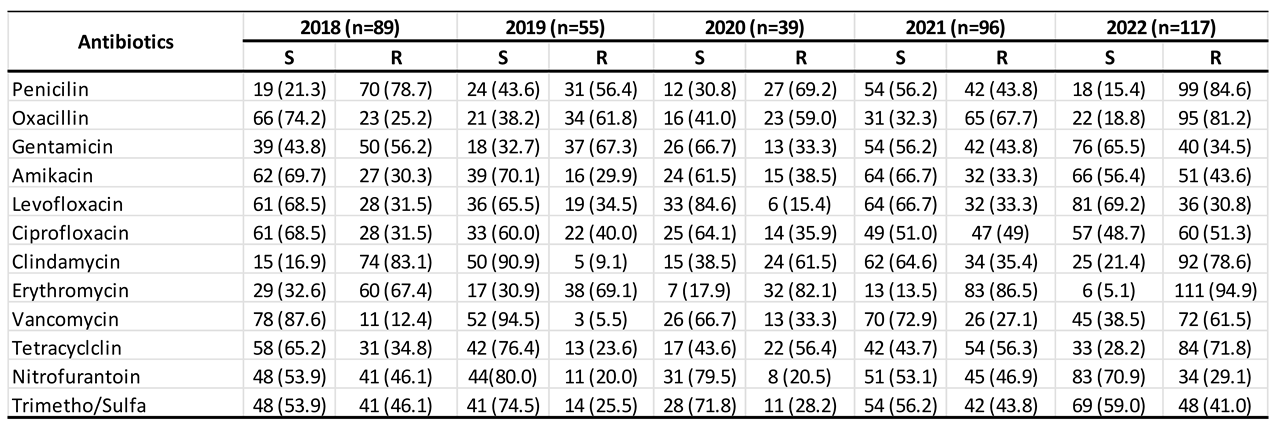
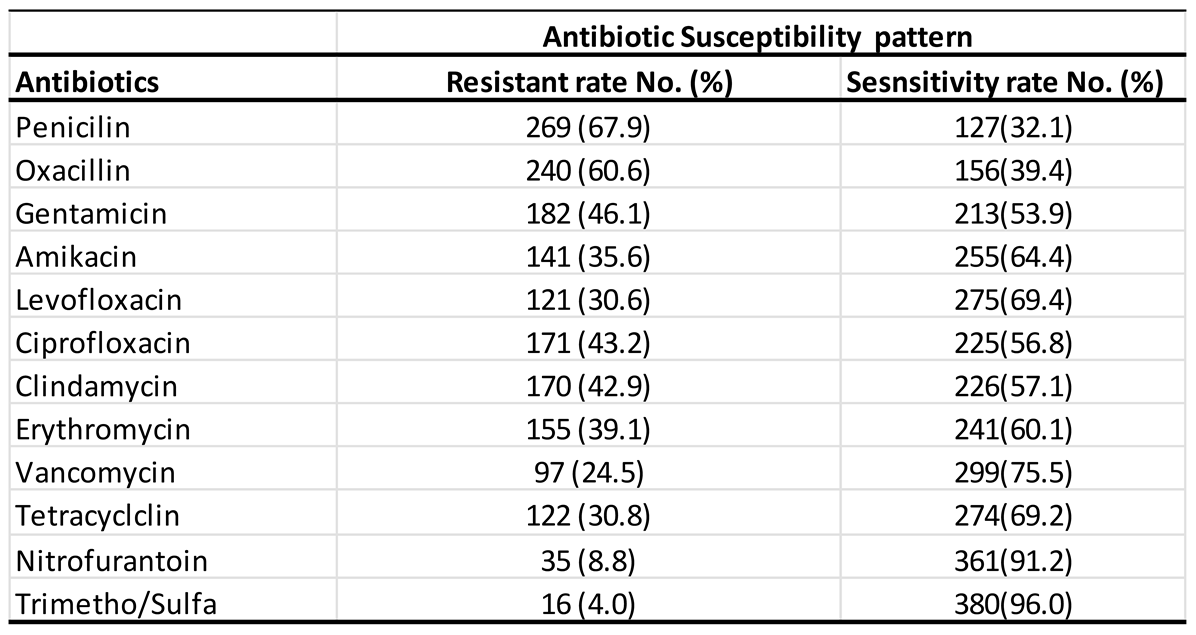
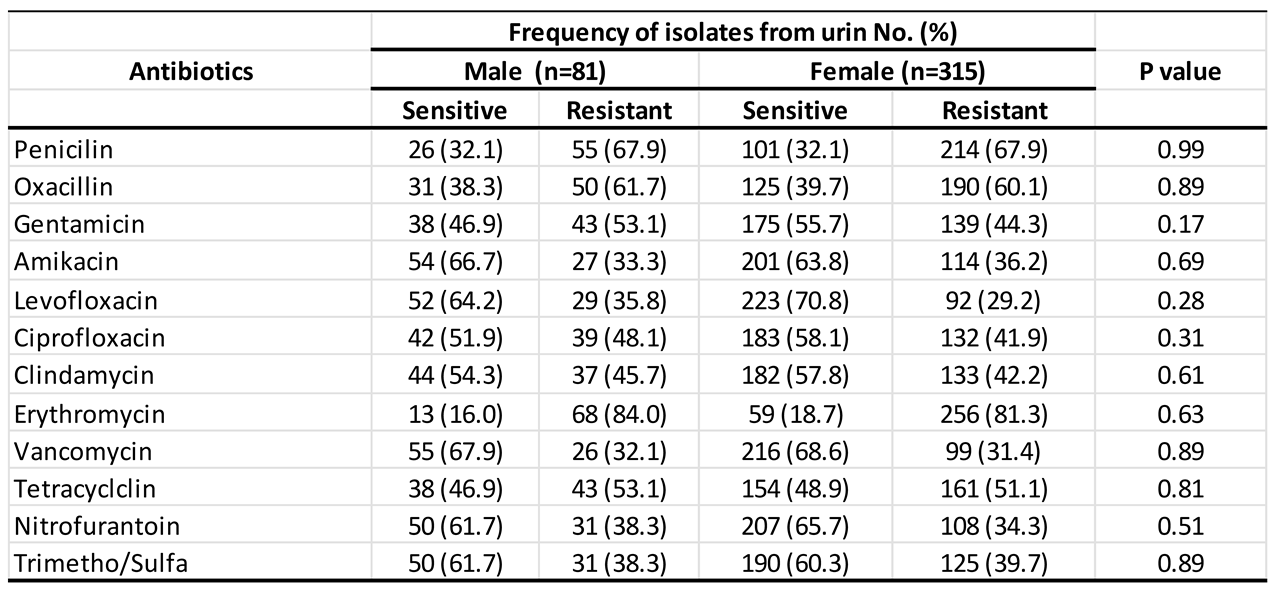
Background and aims: Staphylococcus aureus is a gram-positive bacterium responsible for a wide range of infections, colonizing multiple sites in the human body. The rise in antimicrobial resistance poses a significant global public health threat, necessitating urgent intervention from governments and communities. This study aimed to evaluate the antibiotic susceptibility of Staphylococcus aureus isolated from urine samples collected at Azadi Teaching Hospital in Duhok, Iraq, focusing on commonly used antibiotics.Methods: Conducted between January 2018 and February 2022, this study analyzed 396 urine samples obtained from patients at Azadi Teaching Hospital, Duhok province, Iraq. The Staphylococcus aureus strains were identified and tested for antibiotic susceptibility using the VITEK system.Results: Over the 5-year period, Staphylococcus aureus isolates exhibited the highest resistance rates in 2022 and the highest sensitivity rates in 2019. Erythromycin demonstrated the highest resistance (81.82%), while levofloxacin showed the greatest sensitivity (69.4%). Although gender-based differences in susceptibility were minimal, males generally exhibited slightly higher resistance rates than females.Conclusion: The Staphylococcus aureus isolates from urine samples demonstrated varied antibiotic susceptibility patterns, with high resistance to erythromycin, penicillin, and oxacillin, and high sensitivity to levofloxacin, nitrofurantoin, and vancomycin. Vancomycin exhibited the highest sensitivity, while erythromycin had the lowest. These findings may aid physicians and healthcare providers in selecting appropriate antimicrobial treatments in the region.
Keywords:
1. Introduction
Aim of Study
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection and Processing
2.3. Staphylococcus aureus Identification and Antibiotic Sensitivity Testing
2.5. Statistical Analysis
3. Results
3.1. Trend of Antimicrobial Resistance Among Participants
3.2. Overall Resistance Rate of Antibiotics
3.3. Antimicrobial Sensitivity Profile of Staph aureus According to Gender
4. Discussion
5. Conclusions
References
- Rasheed, N.A.; Hussein, N.R. Staphylococcus aureus: An Overview of Discovery, Characteristics, Epidemiology, Virulence Factors and Antimicrobial Sensitivity Short Title: Methicillin Resistant Staphylococcus aureus: An overview.
- Bitrus, A.; Olabode, M.; Abbas, M.; Dauda Goni, M. Staphylococcus aureus: A Review of Antimicrobial Resistance Mechanism. Veterinary Sciences: Research and Reviews 2018, 4. [Google Scholar] [CrossRef]
- Abduljabar, S.S.; Naqid, I.A. Staphylococcus aureus Among Athletes In Zakho City, Kurdistan Region, Iraq: Nasal Carriage Rate, Risk Factors, and Antibiotic Sensitivity Profile. Science Journal of University of Zakho 2022, 10, 112–118. [Google Scholar] [CrossRef]
- Abdulkareem WH, N.; Mohammed, A.; Arif, S.; Naqid, I. Risk Factors Association for MRSA Nasal Colonization in Preoperative Patients in Azadi Teaching Hospital-Duhok, Kurdistan Region, Iraq. Science Journal of University of Zakho 2020, 8, 88–91. [Google Scholar] [CrossRef]
- Abduljabar, S.S.; Naqid, I.A. Factors Associated With The Nasal Carriage Rate of Methicillin-Resistant Staphylococcus aureus (MRSA) and the Molecular Detection of the mecA Gene Among Athletes. Egyptian Academic Journal of Biological Sciences. C, Physiology and Molecular Biology 2023, 15, 749–760. [Google Scholar] [CrossRef]
- Rasheed, N.; Hussein, N. The Nasal Carriage of Staphylococcus aureus and Its Antimicrobial Susceptibility Pattern in Secondary School Students in Kurdistan Region, Iraq. Journal of Kermanshah University of Medical Sciences, In Press. [CrossRef]
- Naqid, I.A.; Balatay, A.A.; Hussein, N.R.; Ahmed, H.A.; Saeed, K.A.; Abdi, S.A. Bacterial Strains and Antimicrobial Susceptibility Patterns in Male Urinary Tract Infections in Duhok Province, Iraq. Middle East J Rehabil Health Stud 2020, 7, e103529. [Google Scholar] [CrossRef]
- Naqid, I.A.; Balatay, A.A.; Hussein, N.R.; Saeed, K.A.; Ahmed, H.A.; Yousif, S.H. Antibiotic Susceptibility Pattern of Escherichia coli Isolated from Various Clinical Samples in Duhok City, Kurdistan Region of Iraq. Int J Infect 2020, 7, e103740. [Google Scholar] [CrossRef]
- Naqid, I.A.; Hussein, N.R.; Balatay, A.; Saeed, K.A.; Ahmed, H.A. Antibiotic Susceptibility Patterns of Uropathogens Isolated from Female Patients with Urinary Tract Infection in Duhok Province, Iraq. Jundishapur J Health Sci 2020, 12, e105146. [Google Scholar] [CrossRef]
- Naqid, I.A.; Hussein, N.R.; Balatay, A.A.; Saeed, K.A.; Ahmed, H.A. The Antimicrobial Resistance Pattern of Klebsiella pneumonia Isolated from the Clinical Specimens in Duhok City in Kurdistan Region of Iraq. J Kermanshah Univ Med Sci 2020, 24, e106135. [Google Scholar] [CrossRef]
- Hussein, N.; Salih, R.S.; Rasheed, N.A. Prevalence of Methicillin-Resistant Staphylococcus aureus in Hospitals and Community in Duhok, Kurdistan Region of Iraq. Int J Infect 2019, 6, e89636. [Google Scholar] [CrossRef]
- Hussein, N.R.; Assafi, M.S.; Ijaz, T. Methicillin-resistant Staphylococcus aureus nasal colonisation amongst healthcare workers in Kurdistan Region, Iraq. J Glob Antimicrob Resist 2017, 9, 78–81. [Google Scholar] [CrossRef]
- Haydar, S.O.; Naqid, I. A Study of Bacterial Vaginosis and Associated Risk Factors among Married Women in Zakho City, Kurdistan Region, Iraq. Journal of Life and Bio Sciences Research 2022, 3, 33–39. [Google Scholar] [CrossRef]
- Haydar, S.O.; Naqid, I.A. EVALUATION OF CANDIDA ALBICANS AND ITS ASSOCIATED RISK FACTORS AMONG MARRIED WOMEN OF REPRODUCTIVE AGE IN ZAKHO CITY, KURDI-STAN REGION, IRAQ. Science Journal of University of Zakho 2023, 11, 202–208. [Google Scholar] [CrossRef]
- Naqid, I.A.; Al-Brefkani, A.M.T.; Hussein, N.R. A study of prevalence and risk factors for Helicobacter pylori infection among Adult in Duhok Province, Kurdistan Region, Iraq. Archives of Razi Institute, 2023. [Google Scholar]
- Naqid, I.A.; Yousif, S.H.; Balatay, A.A.; Khasho, D.A.; HUSSEIN, N.R. Study on Anticardiolipin Antibodies in Women With Recurrent Abortion in Duhok Province, Kurdistan Region, Iraq. ACTA MEDICA IRANICA 2020, 58. [Google Scholar] [CrossRef]
- Naqid, I.A.; Yousif, S.H.; Hussein, N.R. Serological Study of IgG and IgM Antibodies to Cytomegalovirus and Toxoplasma Infections in Pregnant Women in Zakho City, Kurdistan Region, Iraq. Women’s Health Bulletin 2019, 6, 8–12. [Google Scholar] [CrossRef]
- Naqid, A.I.; Yousif, S.H.; Hussein, N.R. Seroprevalence of Rubella and Herpes Simplex Virus in Women with Miscarriage and Stillbirth in Zakho City, Kurdistan Region, Iraq: A Cross- Sectional Study. Women’s Health Bulletin 2020, 7, 18–22. [Google Scholar] [CrossRef]
- Naqid, I.; Abdi, B.; Ahmed, R.; Ibrahim, N.; Musa, D.; M.Saleem, Z.; Saleem, M.; Chafrash, A.; Hussein, N.; Saeed, K. Public knowledge, attitudes, and practices regarding the coronavirus disease pandemic: a cross-sectional study in the Kurdistan region, Iraq Running title: Public KAP toward the COVID-19 pandemic. 2021.
- Naqid, I.A. Epidemiological study of Intestinal protozoan Infections: A Cross-sectional study in Zakho City, Kurdistan Region, Iraq during 2018-2022. Archives of Razi Institute 2024, 79, 587–592. [Google Scholar] [CrossRef]
- Mosa, A.A.; Ibrahim, S.V.; Naqid, I.A.; Hawezy, D.J.; Al-Jaf, S.M.A.; Hussein, N.R. The Impact of SARS-CoV-2 Pandemic on Medical Students: Knowledge, Attitudes, and Practices towards E-Learning: An Online Cross-Sectional Study in the Kurdistan Region, Iraq. Galician Medical Journal 2023, 30, E202314. [Google Scholar] [CrossRef]
- Khalid, F.K.; Rasheed, N.A.; Hussein, N.R.; Naqid, I.A. A study of HBV infection and its risk factors in pregnant women in Zakho city, Iraq. PLOS ONE 2022, 17, e0273362. [Google Scholar] [CrossRef]
- Hussein, N.R. Prevalent Genotypes of Staphylococcus aureus Strains Isolated From Healthcare Workers in Duhok City, Kurdistan Region, Iraq. Int J Infect 2016, 3, e35375. [Google Scholar] [CrossRef]
- Hussein, N.R.; Daniel, S.; Salim, K.; Saleh Assafi, M. Urinary Tract Infections and Antibiotic Sensitivity Patterns Among Women Referred to Azadi Teaching Hospital, Duhok, Iraq. Avicenna J Clin Microbiol Infect 2018, 5, 27–30. [Google Scholar] [CrossRef]
- Al-Naqshbandi, A.A.; Chawsheen, M.A.; Abdulqader, H.H. Prevalence and antimicrobial susceptibility of bacterial pathogens isolated from urine specimens received in rizgary hospital - Erbil. Journal of infection and public health 2019, 12, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Qodrati, M.; SeyedAlinaghi, S.; Dehghan Manshadi, S.A. Antimicrobial susceptibility testing of Staphylococcus aureus isolates from patients at a tertiary hospital in Tehran, Iran, 2018-2019. 2022, 27, 152. [CrossRef]
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




