Submitted:
25 November 2024
Posted:
26 November 2024
You are already at the latest version
Abstract
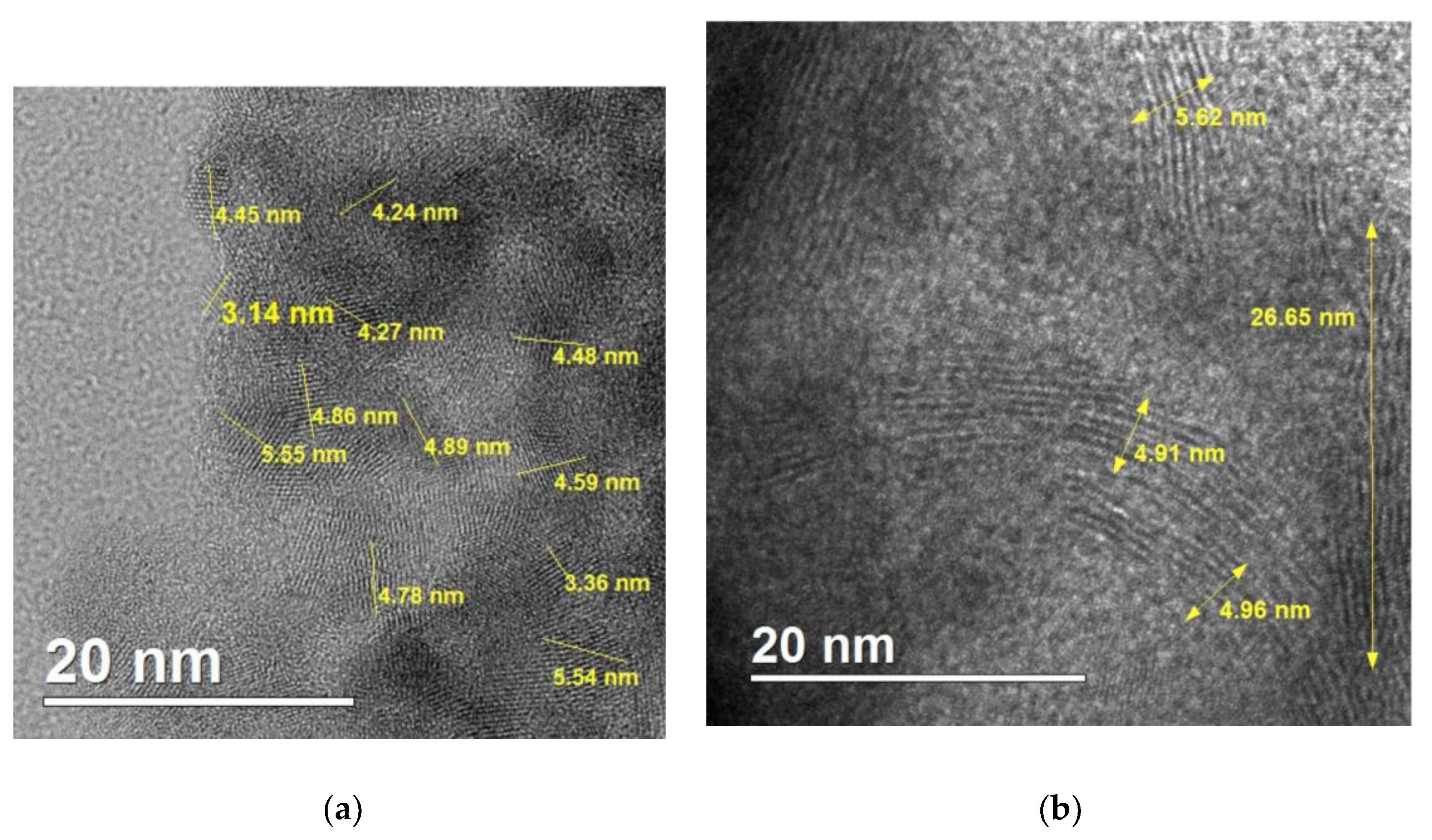
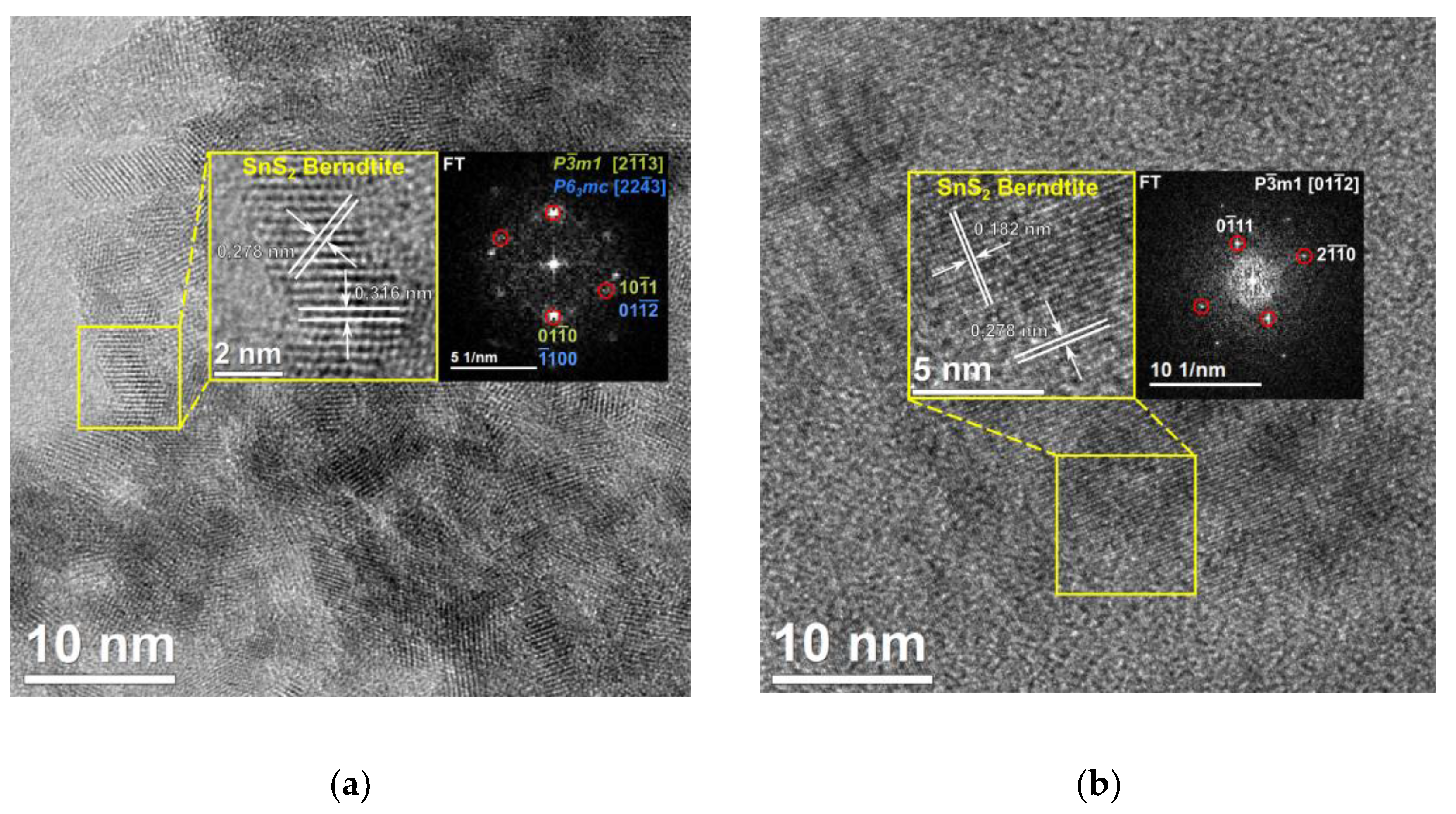
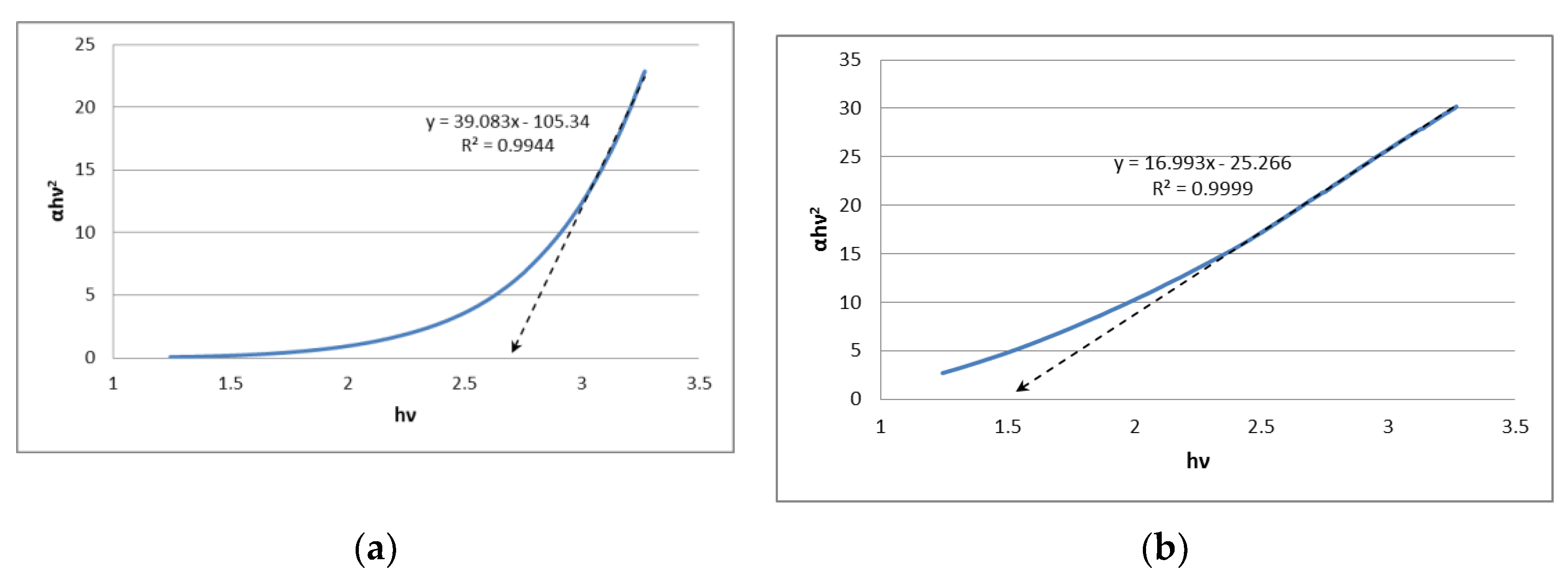
A sonochemical synthesis of SnS2 quantum dots using acetone as a solvent is investigated. Two different tin sources (SnCl2∙2H2O or SnCl4∙5H2O) as well as two different sulphur sources (thioacetamide or Na2S2O3) were applied. The sonication time was also varied between 60 and 120 minutes. Resulting products of syntheses were characterized with the following techniques: powder X-ray diffraction, electron microscopy (SEM and HR-TEM), Raman and FT-IR spectroscopies, the Tauc method, and X-ray photoelectron spectroscopy. Obtained SnS2 nanostructures were in the form of quantum dots in the case of synthesis lasting 60 minutes (size of crystallites in the range of 3.5 – 7 nm) and in the form of elongated nanorods of length c.a. 25-30 nm and width of 5-6 nm in the case of synthesis lasting 120 minutes. XPS analyses revealed that the surface of the obtained products contained significant amount of tin at the second oxidation state (i. e. SnS). The quantum dots produced in the synthesis lasting 60 minutes showed value of energy bandgap of 2.7 eV indicating potential applications in photocatalysis.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sonochemical Syntheses
2.3. UV-Vis Spectrophotometry
2.4. SEM Investigations
2.5. Raman Spectroscopy
2.6. FTIR Spectroscopy
2.7. HR-TEM Investigations
2.8. XPS Investigations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, L. A.; Colombara, D.; Abellon, R. D.; Grozema, F. C.; Peter, L. M.; Savenije, T. J.; Dennler, G.; Walsh, A. Synthesis, Characterization, and Electronic Structure of Single-Crystal SnS, Sn2S3, and SnS2. Chem. Mater. 2013, 25, 4908–4916. [Google Scholar] [CrossRef]
- Yuan, C.; Hou, L.; Yang, L.; Fan, C.; Li, D.; Li, J.; Shen, L.; Zhang, F.; Zhang, X. Interface-hydrothermal synthesis of Sn3S4/graphene sheet composites and their application in electrochemical capacitors. Mater. Lett. 2011, 65, 374–377. [Google Scholar] [CrossRef]
- Li, J.; Han, L.; Li, Y.; Li, J.; Zhu, G.; Zhang, X.; Lu, T.; Pan, L. MXene-decorated SnS2/Sn3S4 hybrid as anode material for high-rate lithium-ion batteries. Chem. Eng. J. 2020, 380, 122590. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhao, X.-G.; Lv, J.; Biswas, K.; Zhang, L. Computational Design of Mixed-Valence Tin Sulfides as Solar Absorbers. ACS Appl. Mater. Interfaces 2019, 11, 24867–24875. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N. N.; Earnshaw, A. The Chemistry of the Elements; Pergamon Press, 1984. [Google Scholar]
- Whittles, T. J.; Burton, L. A.; Skelton, J. M.; Walsh, A.; Veal, T. D.; Dhanak, V. R. Band Alignments, Valence Bands, and Core Levels in the Tin Sulfides SnS, SnS2, and Sn2S3: Experiment and Theory. Chem. Mater. 2016, 28, 3718–3726. [Google Scholar] [CrossRef]
- Lewis, D. J.; Kevin, P.; Bakr, O.; Muryn, C. A.; Malik, M. A.; O’Brien, P. Routes to tin chalcogenide materials as thin films or nanoparticles: a potentially important class of semiconductor for sustainable solar energy conversion. Inorg. Chem. Front. 2014, 1, 577–598. [Google Scholar] [CrossRef]
- Reddy, N. K.; Devika, M.; Gopal, E. S. R. Review on Tin (II) Sulfide (SnS) Material: Synthesis, Properties, and Applications. Crit Rev Solid State 2015, 40, 359–398. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Ji, Y.; Zhang, H.; Shen, X. Two-dimensional SnS nanosheets fabricated by a novel hydrothermal method. J. Mater. Sci. 2005, 40, 591–595. [Google Scholar] [CrossRef]
- An, C.; Tang, K.; Shen, G.; Wang, C.; Yang, Q.; Hai, B.; Qian, Y. Growth of belt-like SnS crystals from ethylenediamine solution. J. Cryst. Growth 2002, 244, 333–338. [Google Scholar] [CrossRef]
- Hickey, S. G.; Waurisch, C.; Rellinghaus, B.; Eychmüller, A. Size and Shape Control of Colloidally Synthesized IV-VI Nanoparticulate Tin(II) Sulfide. J. Am. Chem. Soc. 2008, 30, 14978–14980. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, D.; Wang, G. Synthesis and characterization of SnS nanowires in cetyltrimethylammoniumbromide (CTAB) aqueous solution. Chem. Phys. Lett. 2003, 379, 67–73. [Google Scholar] [CrossRef]
- Shen, G.; Chen, D.; Tang, K.; Huang, L.; Qian, Y.; Zhou, G. Novel polyol route to nanoscale tin sulfides flaky crystallines. Inorg. Chem. Commun. 2003, 6, 178–180. [Google Scholar] [CrossRef]
- Gajendiran, J.; Rajendran, V. Synthesis of SnS2 nanoparticles by a surfactant-mediated hydrothermal method and their characterization. Adv. Nat. Sci.: Nanosci. Nanotechnol. 2011, 2, 015001. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y. C. In air synthesis of SnS2 nanoplates from tin, sulfur and ammonium choride powders. Mater. Chem. Phys. 2008, 112, 742–744. [Google Scholar] [CrossRef]
- Giberti, A.; Gaiardo, A.; Fabbri, B.; Gherardi, S.; Guidi, V.; Malagu, C.; Bellutti, P.; Zonta, G.; Casotti, D.; Cruciani, G. Tin(IV) sulfide nanorods as a new gas sensing material. Sens Actuators B Chem 2016, 223, 827–833. [Google Scholar] [CrossRef]
- Okitsu, K.; Cavalieri, F. Sonochemical Production of Nanomaterials, SpringerBriefs in Molecular Science: Ultrasound and Sonochemistry; Springer Nature, 2018. [Google Scholar]
- Liu, Y.; Xu, J.; Ni, Z.; Fang, G.; Tao, W. One-step sonochemical synthesis route towards kesterite Cu2ZnSnS4 nanoparticles. J. Alloys Compd. 2015, 630, 23–28. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Gorai, S.; Chaudhuri, S. Sonochemical synthesis and characterization of cage-like β-indium sulphide powder. Mater. Chem. Phys. 2005, 89, 332–335. [Google Scholar] [CrossRef]
- Raju, N. P.; Tripathi, D.; Lahiri, S.; Thangavel, R. Heat reflux sonochemical synthesis of Cu3BiS3 quantum dots: Experimental and first-principles investigation of spin-orbit coupling on structural, electronic, and optical properties. Sol Energy 2023, 259, 107–118. [Google Scholar] [CrossRef]
- Pejova, B.; Sherif, E.; Minde, M. W. Sonochemically Synthesized Quantum Nanocrystals of Cubic CuInS2: Evidence for Multifractal Surface Morphology, Size-Dependent Structure, and Particle Size Distribution. J. Phys. Chem. C 2020, 124, 20240–20255. [Google Scholar] [CrossRef]
- Pollet, B. G. The use of ultrasound for the fabrication of fuel cell materials. Int J Hydrogen Energy 2010, 35, 11986–12004. [Google Scholar] [CrossRef]
- Islam, M. H.; Paul, M. T. Y.; Burheim, O. S.; Pollet, B. G. Recent developments in the sonoelectrochemical synthesis of nanomaterials. Ultrason. Sonochem. 2019, 59, 104711. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Mesbah, M.; Igwegbe, C. A.; Ezeliora, C. D.; Osagie, C.; Khan, N. A.; Dotto, G. L.; Salari, M.; Dehghani, M. H. Sono electro-chemical synthesis of LaFeO3 nanoparticles for the removal of fluoride: Optimization and modeling using RSM, ANN and GA tools. J. Environ. Chem. Eng. 2021, 9, 105320. [Google Scholar] [CrossRef]
- Matyszczak, G.; Jóźwik, P.; Polesiak, E.; Sobieska, M.; Krawczyk, K.; Jastrzębski, C.; Płociński, T. Sonochemical preparation of SnS and SnS2 nano- and micropowders and their characterization. Ultrason. Sonochem. 2021, 75, 105594. [Google Scholar] [CrossRef] [PubMed]
- Jamali-Sheini, F.; Yousefi, R.; Bakr, N. A.; Cheraghizade, M.; Sookhakian, M.; Huang, N. M. Highly efficient photo-degradation of methyl blue and band gap shift of SnS nanoparticles under different sonication frequencies. Materials Science in Semiconductor Processing 2015, 32, 172–178. [Google Scholar] [CrossRef]
- Cheraghizade, M.; Jamali-Sheini, F.; Yousefi, R.; Niknia, F.; Mahmoudian, M. R.; Sookhakian, M. The effect of tin sulfide quantum dots size on photocatalytic and photovoltaic performance. Materials Chemistry and Physics 2017, 195, 187–194. [Google Scholar] [CrossRef]
- Jamali-Sheini, F.; Cheraghizade, M.; Yousefi, R. Ultrasonic synthesis of In-doped SnS nanoparticles and their physical properties. Solid State Sciences 2018, 79, 30–37. [Google Scholar] [CrossRef]
- Sakthi, P.; Uma, J.; Siva, C.; Balraj, B. Sonochemical synthesis of interconnected SnS nanocrystals for supercapacitor and solar-physical conversion applications. Optical Materials 2022, 132, 112759. [Google Scholar] [CrossRef]
- García-Gómez, N. A.; de la Parra-Arcieniega, S. M.; Garza-Tovar, L. L.; Torres-González, L. C.; Sánchez, E. M. Ionic liquid-assisted sonochemical synthesis of SnS nanostructures. Journal of Alloys and Compounds 2014, 588, 638–643. [Google Scholar] [CrossRef]
- Park, J.; Hwang, Ch. H.; Lee, W. Y.; Kim, Y.; Kim, H.; Shim, I.-W. Preparation of size-tunable SnS nanoparticles by a sonochemical method under multibubble sonoluminescence conditions. Materials Letters 2014, 117, 188–191. [Google Scholar] [CrossRef]
- Khimani, A. J.; Chaki, S. H.; Chauhan, S. M.; Mangrola, A. V.; Meena, R. R.; Deshpande, M. P. Synthesis, characterization, antimicrobial and antioxidant study of the facile sonochemically synthesized SnS2 nanoparticles. Nano-Structures & Nano-Objects 2019, 18, 100286. [Google Scholar]
- Khimani, A. J.; Chaki, S. H.; Giri, R. Kr.; Meena, R. R.; Kannaujiya, R. M.; Deshpande, M. P. Thermal exploration of sonochemically achieved SnS2 nanoparticles: Elemental, structural, and morphological investigations of TG residual SnS2. Chemical Thermodynamics and Thermal Analysis 2023, 9, 100104. [Google Scholar] [CrossRef]
- Mukaibo, H.; Yoshizawa, A.; Momma, T.; Osaka, T. Particle size and performance of SnS2 anodes for rechargeable lithium batteries. Journal of Power Sources 2003, 119-121, 60–63. [Google Scholar] [CrossRef]
- Matyszczak, G.; Plocinski, T.; Dluzewski, P.; Fidler, A.; Jastrzebski, C.; Lawniczak-Jablonska, K.; Drzewiecka-Antonik, A.; Wolska, A.; Krawczyk, K. Sonochemical synthesis of SnS and SnS2 quantum dots from aqueous solutions, and their photo- and sonocatalytic activity. Ultrason. Sonochem. 2024, 105, 106834. [Google Scholar] [CrossRef]
- Mead, D. G.; Irwin, J. C. Raman spectra of SnS2 and SnSe2. Solid State Commun. 1976, 20, 885–887. [Google Scholar] [CrossRef]
- Bialoglowski, M.; Jastrzebski, C.; Podsiadlo, S.; Jastrzebski, D. J.; Gajda, R.; Gebicki, W.; Wrzosek, P. A.; Wozniak, K. Synthesis of tin disulfide single crystals for nano-layer exfoliation. Cryst. Res. Technol. 2015, 50, 695–699. [Google Scholar] [CrossRef]
- Huang, Y.; Sutter, E.; Sadowski, J. T.; Cotlet, M.; Monti, O. L. A.; Racke, D. A.; Neupane, M. R.; Wickramaratne, D.; Lake, R. K.; Parkinson, B. A.; Sutter, P. Tin Disulfide-An Emerging Layered Metal Dichalcogenide Semiconductor: Materials Properties and Device Characteristics. ACS Nano 2014, 8, 10743–10755. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, P. Origin of asymmetric broadening of Raman peak profiles in Si nanocrystals. Sci. Rep. 2017, 7, 43602. [Google Scholar] [CrossRef]
- Julien, C.; Mavi, H. S.; Jain, K. P.; Balkanski, M.; Perez-Vicente, C.; Morales, J. Resonant raman scattering studies of SnS2 crystals. Materials Science and Engineering: B 1994, 23, 98–104. [Google Scholar] [CrossRef]
- Burton, L. A.; Whittles, T. J.; Hesp, D.; Linhart, W. M.; Skelton, J. M.; Hou, B.; Webster, R. F.; O’Dowd, G.; Reece, C.; Cherns, D.; Fermin, D. J.; Veal, T. D.; Dhanak, V. R.; Walsh, A. Electronic and optical properties of single crystal SnS2: an earth-abundant disulfide photocatalyst. J. Mater. Chem. A 2016, 4, 1312–1318. [Google Scholar] [CrossRef]
- Schockley, W.; Queisser, H. J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
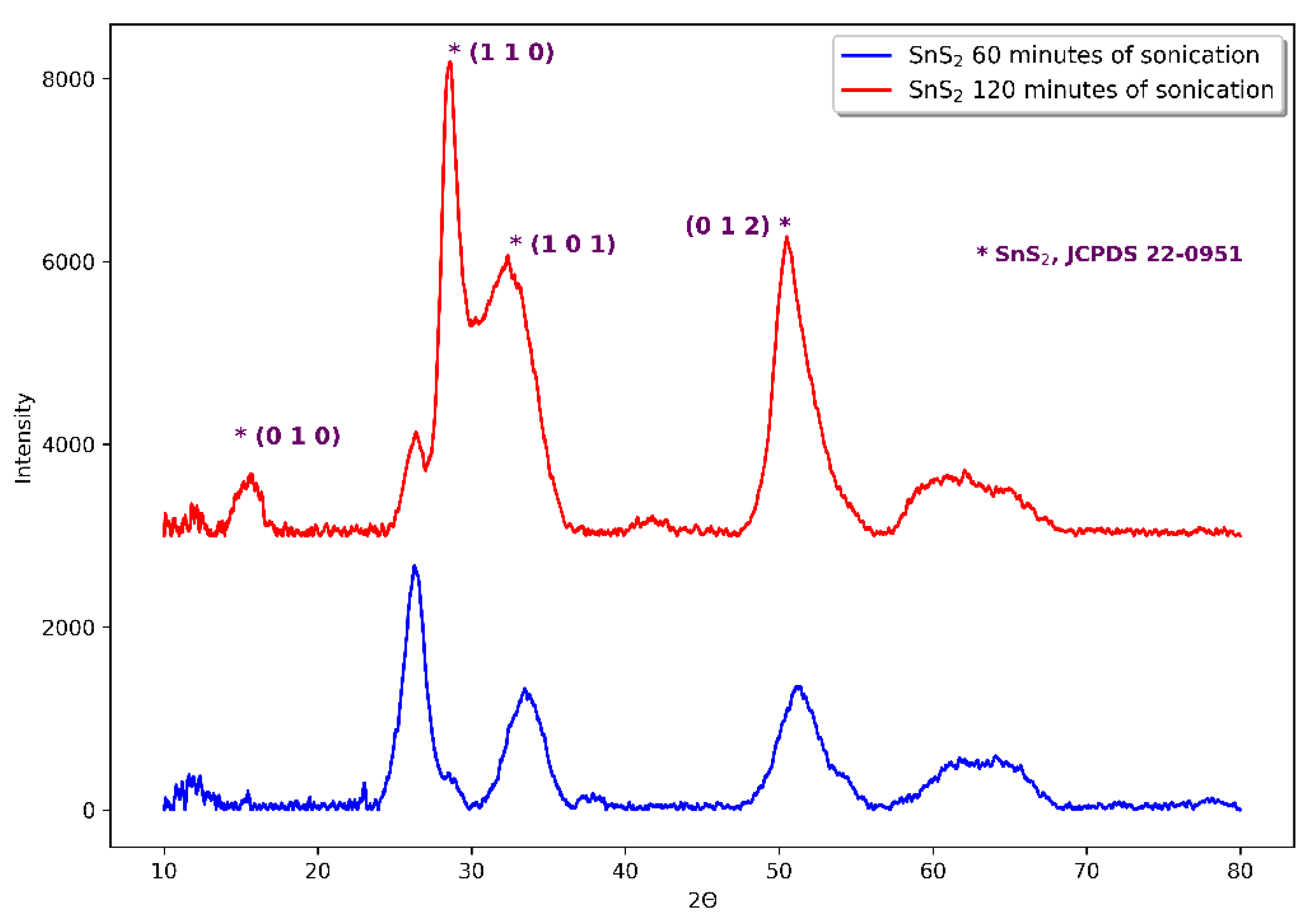
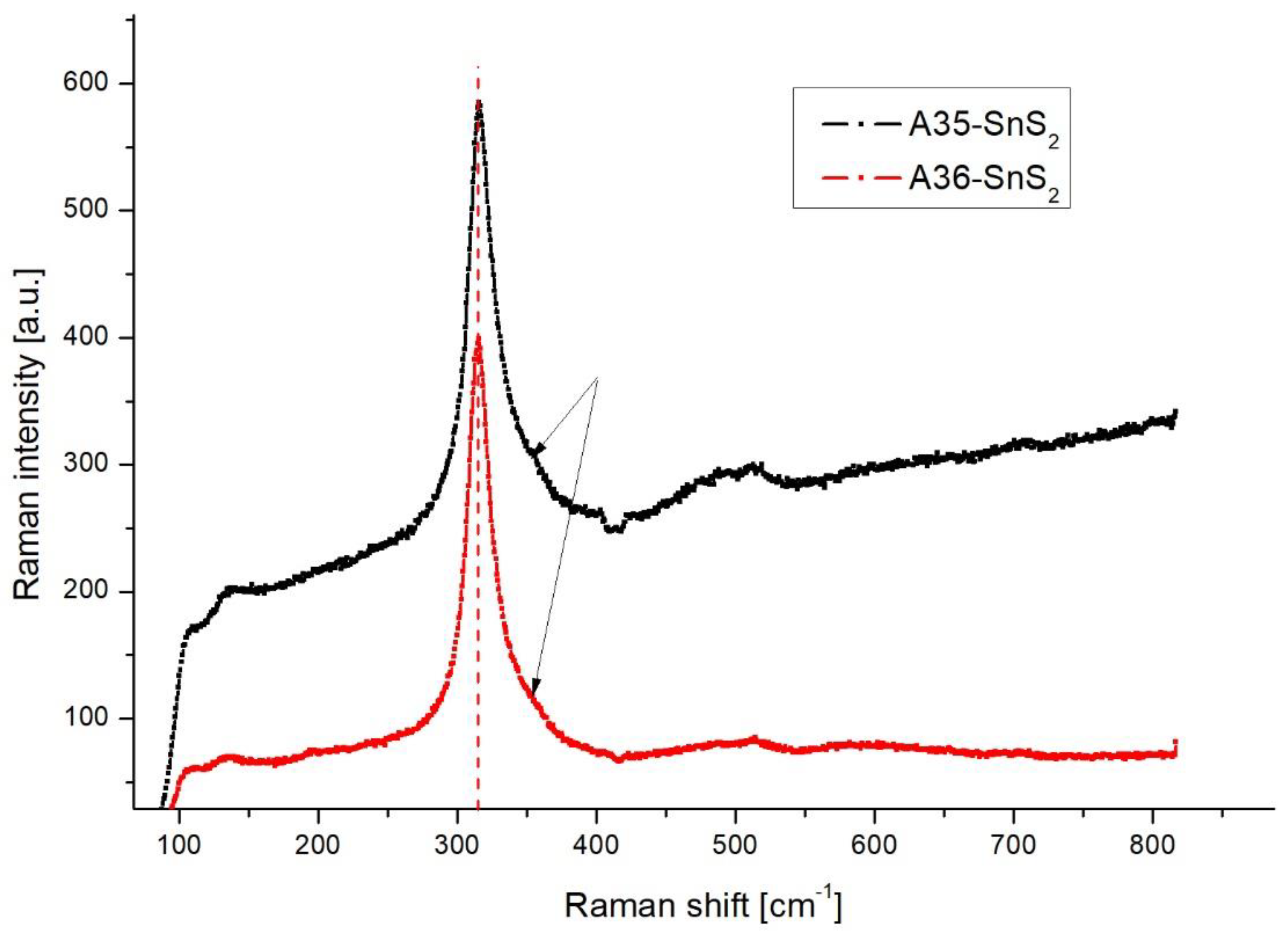
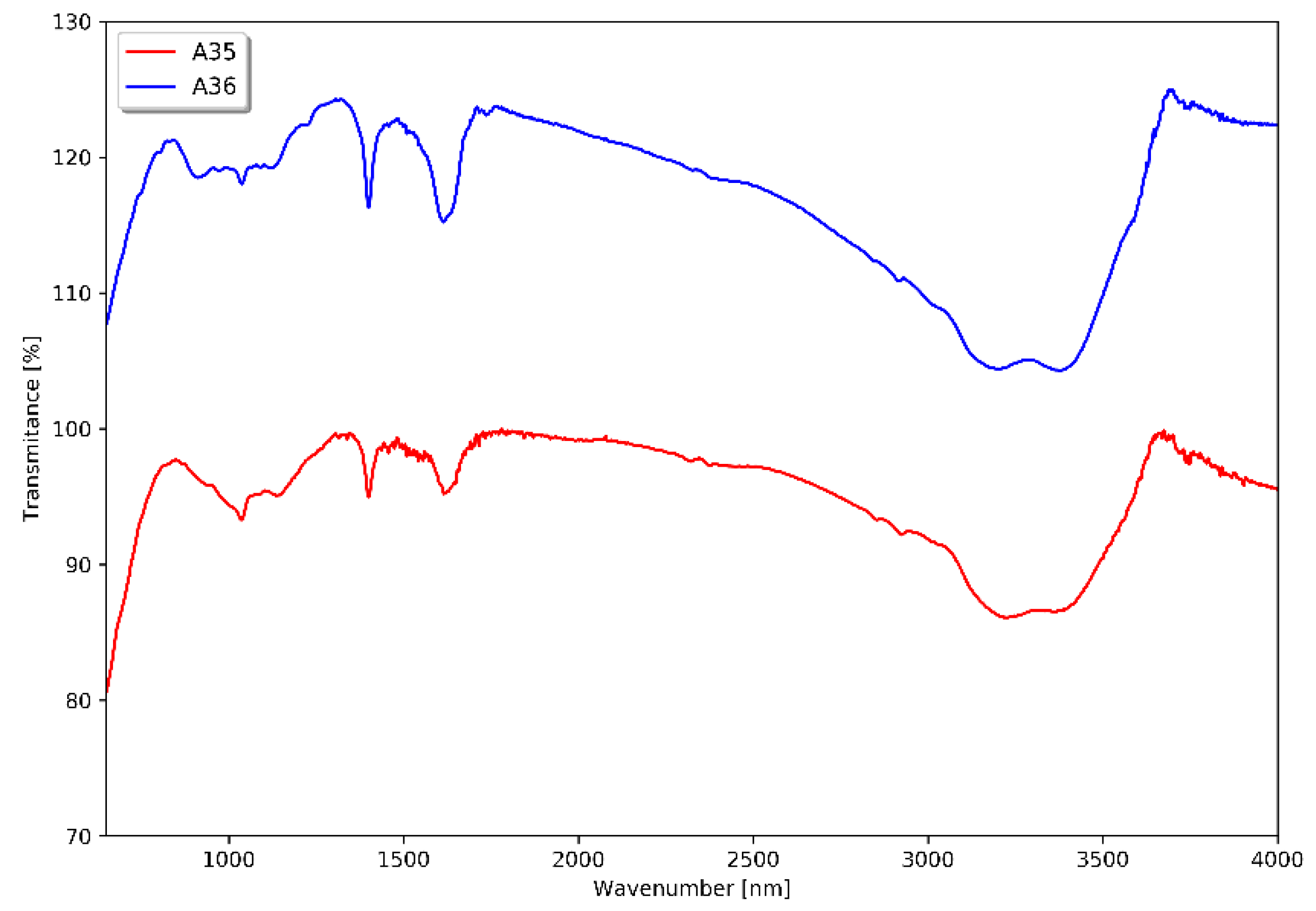
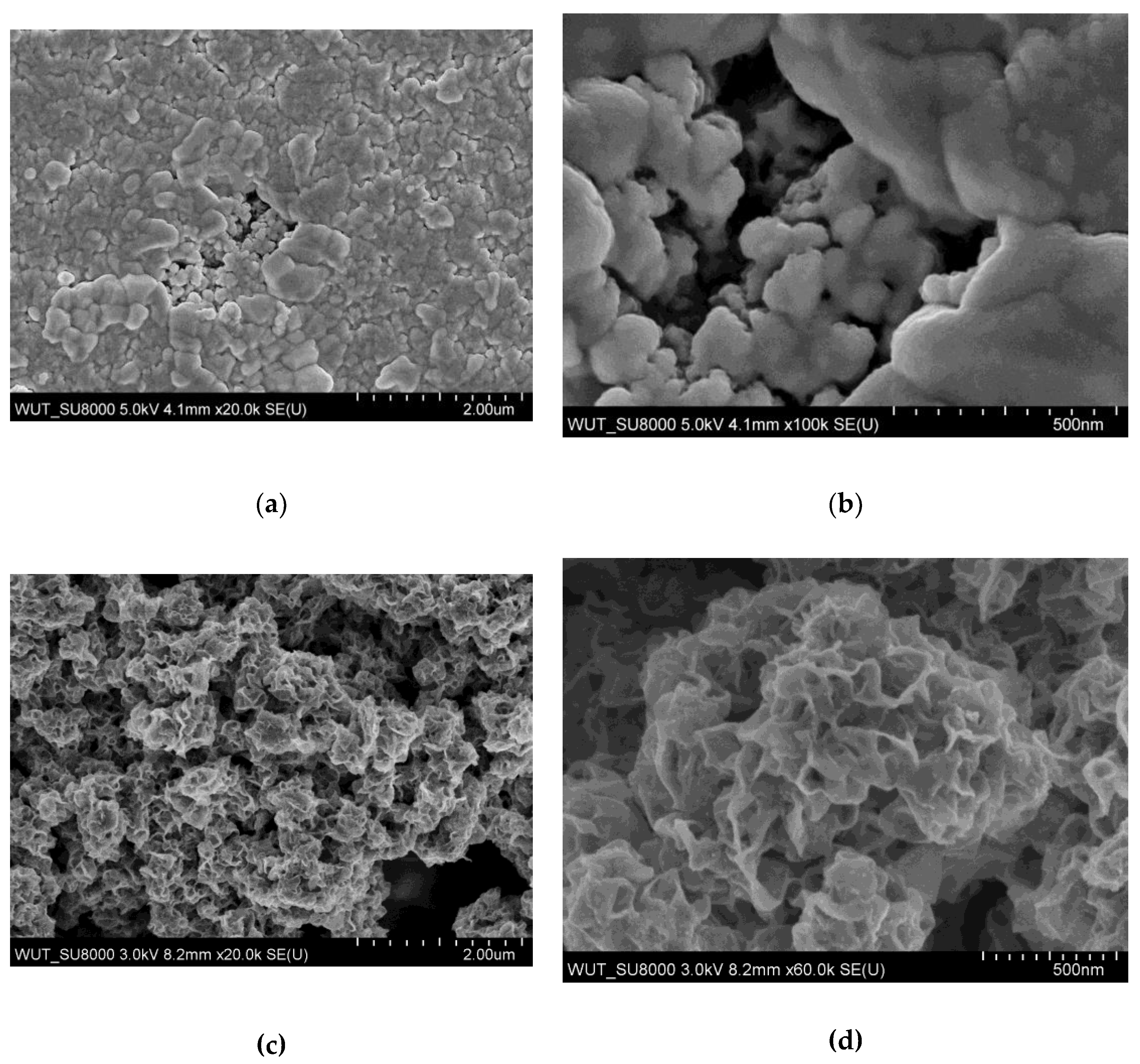

| No. (and sample name if available) | Tin source | Amount of tin source [mg] | Sulphur source | Amount of sulphur source [mg] | Sonication time [min] | Result of synthesis |
| 1 | SnCl4∙5H2O | 702 | Thioacetamide | 376 | 60 | Clear, yellow solution (no precipitate after centrifugation) |
| 2 | SnCl4∙5H2O | 702 | Thioacetamide | 376 | 120 | Yellow suspension (no precipitate after centrifugation) |
| 3 (A35) | SnCl2∙2H2O | 452 | Thioacetamide | 376 | 60 | Yellow precipitate |
| 4 (A36) | SnCl2∙2H2O | 452 | Thioacetamide | 376 | 120 | Yellow precipitate |
| 5 | SnCl4∙5H2O | 702 | Na2S2O3 | 1242 | 60 | Reaction mixture unchanged |
| 6 | SnCl4∙5H2O | 702 | Na2S2O3 | 1242 | 120 | Reaction mixture unchanged |
| 7 | SnCl2∙2H2O | 452 | Na2S2O3 | 1242 | 60 | Reaction mixture unchanged |
| 8 | SnCl2∙2H2O | 452 | Na2S2O3 | 1242 | 120 | Reaction mixture unchanged. |
| Sonication time [min] | Spectrum resolution | Sn | S | C | O | S:Sn |
| 60 | High | 14.7 | 12.5 | 17.5 | 55.3 | 0.85 |
| 60 | Low | 14.3 | 15.4 | 20.0 | 50.3 | 1.1 |
| 120 | High | 7.2 | 9.5 | 70.2 | 13.1 | 1.3 |
| 120 | Low | 0.74 | 1.6 | 77.5 | 20.2 | 2.2 |
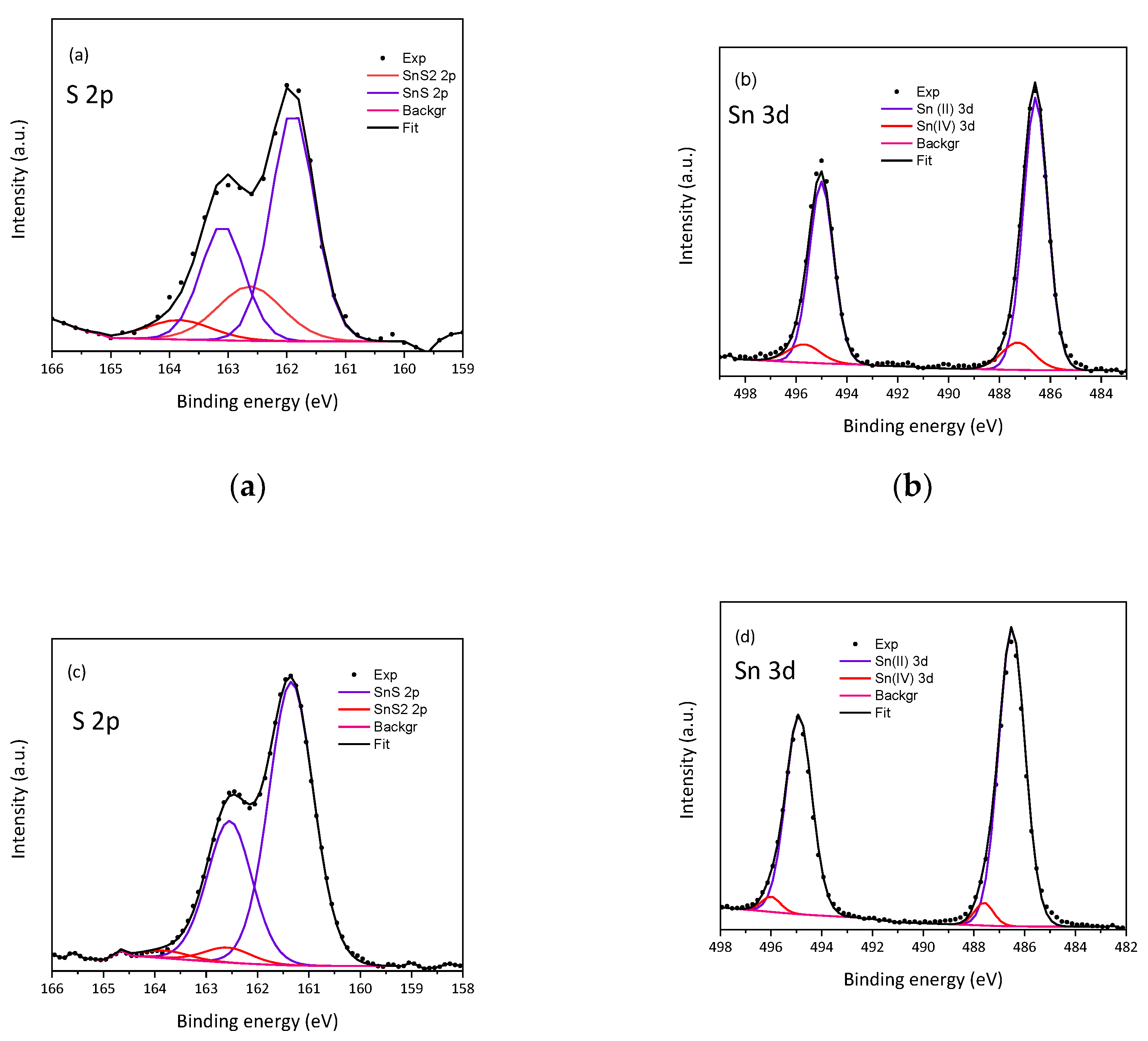
| Sonication time [min] | BE of S in SnS | FWHM | BE of S in SnS2 | FWHM | BE of Sn(II) in SnS | FWHM | BE of Sn(IV) in SnS2 | FWHM |
| 60 | 161.9; 74.1% | 0.9 | 162.6: 25.9% | 1.3 | 486.6; 88.2% | 1.1 | 487.3; 11.9% | 1.5 |
| 120 | 161.3; 94.8% | 1.0 | 162.6; 5.2% |
1.1 | 486.5; 94.8% |
1.2 | 487.6; 5.2% |
0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





