Submitted:
25 November 2024
Posted:
26 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
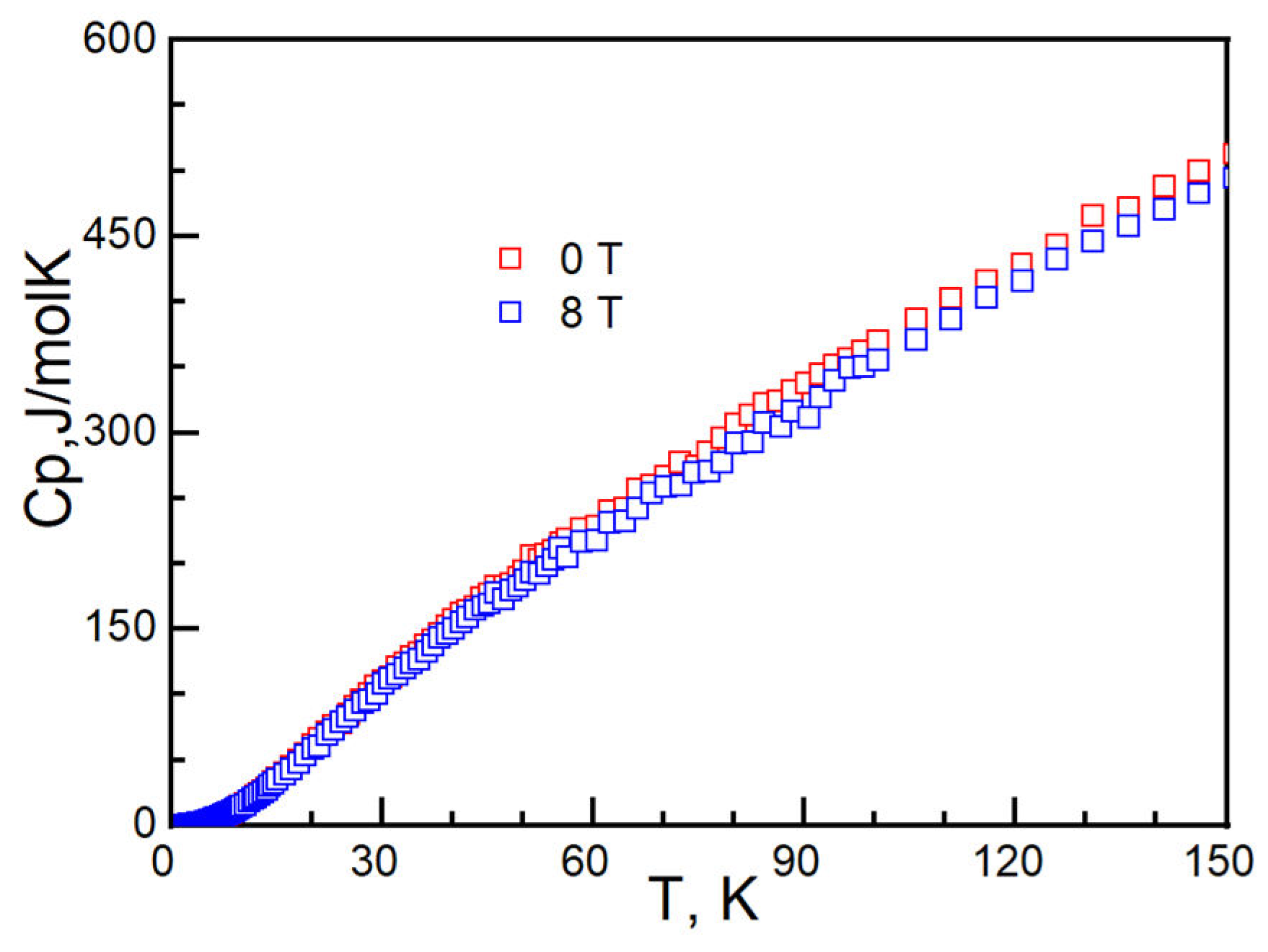
2. Experiment and Discussion
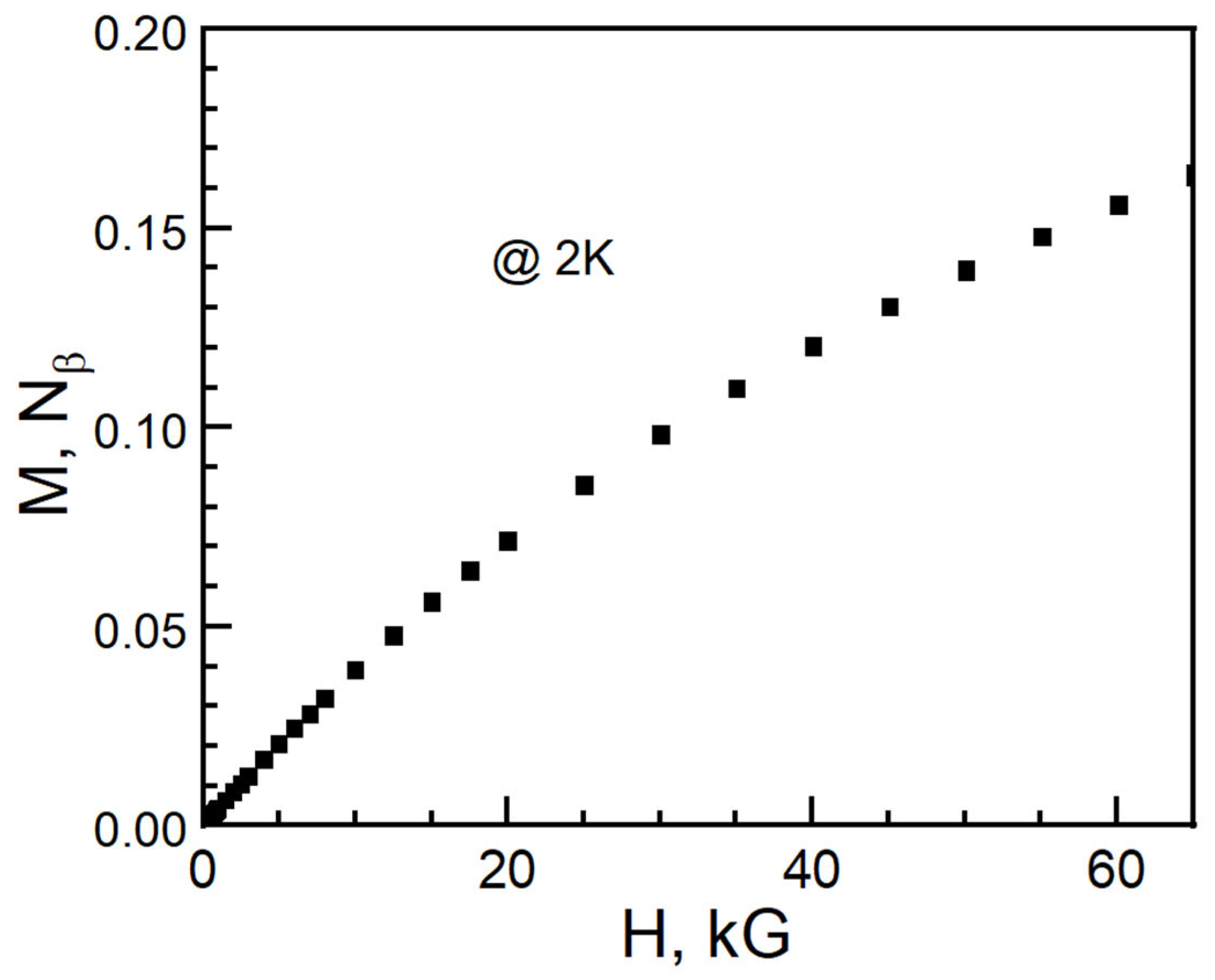
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, P.W. Resonating Valence Bonds: A New Kind of Insulator. Mater. Res. Bull. 1973, 153–160. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Muller, K.A. Possible High Tc Superconductivity in the Ba-La-Cu-O System. Z. Phys. B. Condensed Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Anderson, P.W. The Resonating Valence Bond State in La2CuO4 and Superconductivity. Science 1987, 235, 1197–1198. [Google Scholar] [CrossRef]
- Balents, L. Spin liquids in frustrated magnets. Nature 2010, 464, 199–208. [Google Scholar] [CrossRef]
- Broholm, C.; Cava, R.J.; Kivelson, S.A.; Nocera, D.G.; Norman, M.R.; Senthil, T. Quantum Spin Liquids. Science 2020, 367, eaay0668. [Google Scholar] [CrossRef]
- Williams, J.M.; Schultz, A.J.; Geiser, U.; Carlson, K.D.; Kini, A.M.; Wang, H.H.; Kwok, W.; Whangbo, M. Organic Superconductors – New Benchmarks. Science 1991, 252, 1501–1508. [Google Scholar] [CrossRef]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the charge distribution in BEDT-TTF salts. Synth. Met. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Pratt, F.L.; Baker, P.J.; Blundell, S.J.; Lancaster, T.; Ohira-Kawamura, S.; Baines, C.; Shimizu, Y.; Kanoda, K.; Watanabe, I.; Saito, G. Magnetic and non-magnetic phase of quantum spin liquid. Nature 2011, 471, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Hiramatsu, T.; Maesato, M.; Otsuka, A.; Yamochi, H.; Ono, A.; Itoh, M.; Yoshida, M.; Takigawa, M.; Yoshida, Y.; Saito, G. Pressure-Tuned Exchange Coupling of q Quan Spin Liquid in the Molecular Triangular Lattice k-(ET)2Ag2(CN)3. Phys. Rev. Lett. 2016, 117, 107203. [Google Scholar] [CrossRef]
- Yamashita, M.; Nakata, N.; Senshu, Y.; Nagata, M.; Yamamoto, H.M.; Kato, R.; Shibauchi, T.; Matsuda, Y. Highly Mobile Gapless Excitations in a Two-Dimensional Candidate Quantum Spin Liquid. Science 2010, 328, 1246. [Google Scholar] [CrossRef]
- Isono, T.; Kamo, H.; Ueda, A.; Takahashi, K.; Kimata, M.; Tajima, H.; Tsuchiya, S.; Terashima, T.; Uji, S.; Mori. Gapless Quantum Spin Liquid in an Organic Spin-1/2 Triangular-Lattice k-H3(cat-EDT-TTF)2, H. Phys. Rev. Lett. 2014, 112, 177201. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M.; Graham, A.W.; Day, D.; Simon, J.C.; Hursthouse, M.B.; Caufield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W.; Ducasse, L.; Guionneau, P. Superconducting and Semiconducting Magnetic Charge Transfer Salts: (BEDT-TTF)4AFe(C2O4)3·C6H5CN. J. Am. Chem. Soc. 1995, 117, 12209–12217. [Google Scholar] [CrossRef]
- Cassoux, P. A Molecular Paramagnetic Superconductor. Science 1996, 272, 1277–1278. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states-I—Orbital degeneracy. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1937, 161, 220–235. [Google Scholar] [CrossRef]
- Radaelli, P.G.; Cox, D.E.; Marezio, M.; Cheong, S.W.; Schiffer, P.E.; Ramirez, A.P. Simultaneous Structural, Magnetic, and Electronic Transitions in La1-xCaxMnO3 with x = 0.25 and 0.50. Phys. Rev. Lett. 1995, 75, 4488–4491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhu, D. (BEDT-TTF)3[Cu2(C2O4)3](CH3OH)2: an organic-inorganic hybrid antiferromagnetic semiconductor. Chem. Commun. 2012, 48, 197–199. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Gao, S.; Guo, Y.; Liu, F.; Zhu, D. BETS3[Cu2(C2O4)3](CH3OH)2: an organic–inorganic hybrid antiferromagnetic metal (BETS = bisethylene(tetraselenfulvalene)). CrystEngComm 2013, 15, 3529–3535. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Wang, D.; Baker, P.; Pratt, P.; Zhu, D. Candidate Quantum Spin Liquid due to Dimensional Reduction of a Two-Dimensional Honeycomb Lattice. Scientific Reports 2014, 4, 6451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Baker, P.; Zhang, Y.; Wang, D.; Wang, Z.; Su, S.; Zhu, D. Quantum Spin Liquid from a Three-Dimensional Copper-Oxalate Framework. J. Am. Chem. Soc. 2018, 140, 122–125. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-2012/7, University of Göttingen, Germany, 2014.
- Zhang, B.; Zhang, Y.; Zhu, D. [(C2H5)3NH]2Cu2(C2O4)3: a three-dimensional metal-oxalato framework showing structurally related dielectric and magnetic transitions at around 165 K. Dalton Trans. 2012, 14, 8509–8511. [Google Scholar] [CrossRef]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the charge distribution in BEDT-TTD salts. Synth. Met. 1997, 1973–1974. [Google Scholar] [CrossRef]
- Wang, H.H.; Ferraro, J.R.; Williams, J.M.; Geiser, U.; Schlueter, J.A. Rapid Raman Spectroscopic Determination of the Stoichiometry of Microscopic Quantities of BEDT-TTF-based Organic Conductors and Superconductors. J. Am. Chem. Soc. Chem. Commun. 1994, 1893–1894. [Google Scholar] [CrossRef]
- Coronado, E.; Day, P. Magnetic Molecular Conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar] [CrossRef] [PubMed]
- Price, C.C.; Perkins, N.B. Critical Properties of the Kitaev-Heisenberg Model. Phy. Rev. Lett. 2012, 109, 187201. [Google Scholar] [CrossRef]
- Cano, J.; Alemany, P.; Alvarez; Verdaguer, M.; Ruiz, E. Exchange Coupling in Oxalato-Bridged Copper(II) Binuclear Compounds: A Density Functional Study. Chem. Eur. J. 1998, 4, 476–484. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism, John Wiley & Sons Inc.; New York, 1993.
- Ramirez, A.P. Strongly geometrically frustrated magnets. Annu. Rev. Mater. Sci. 1994, 24, 453–480. [Google Scholar] [CrossRef]
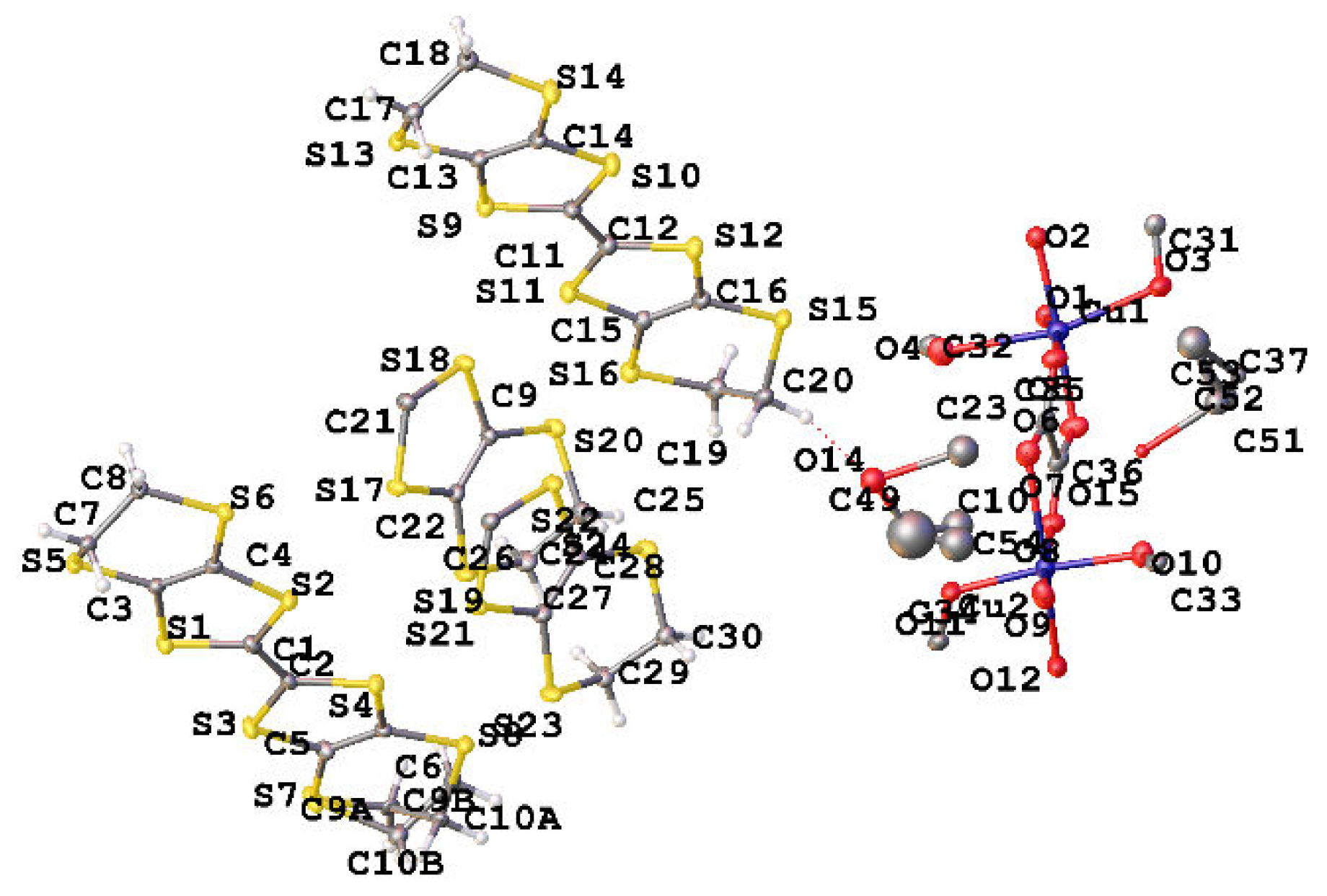
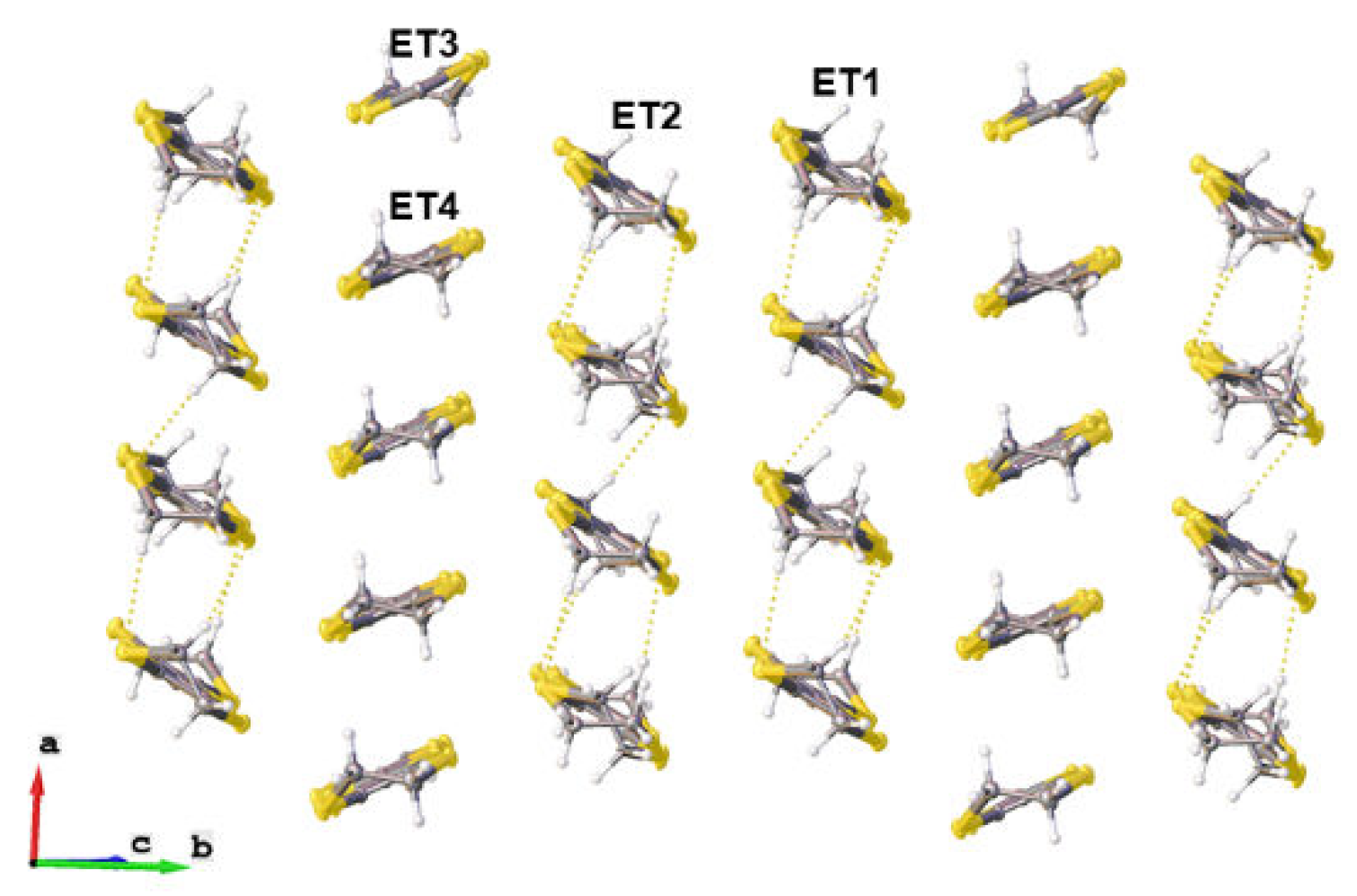
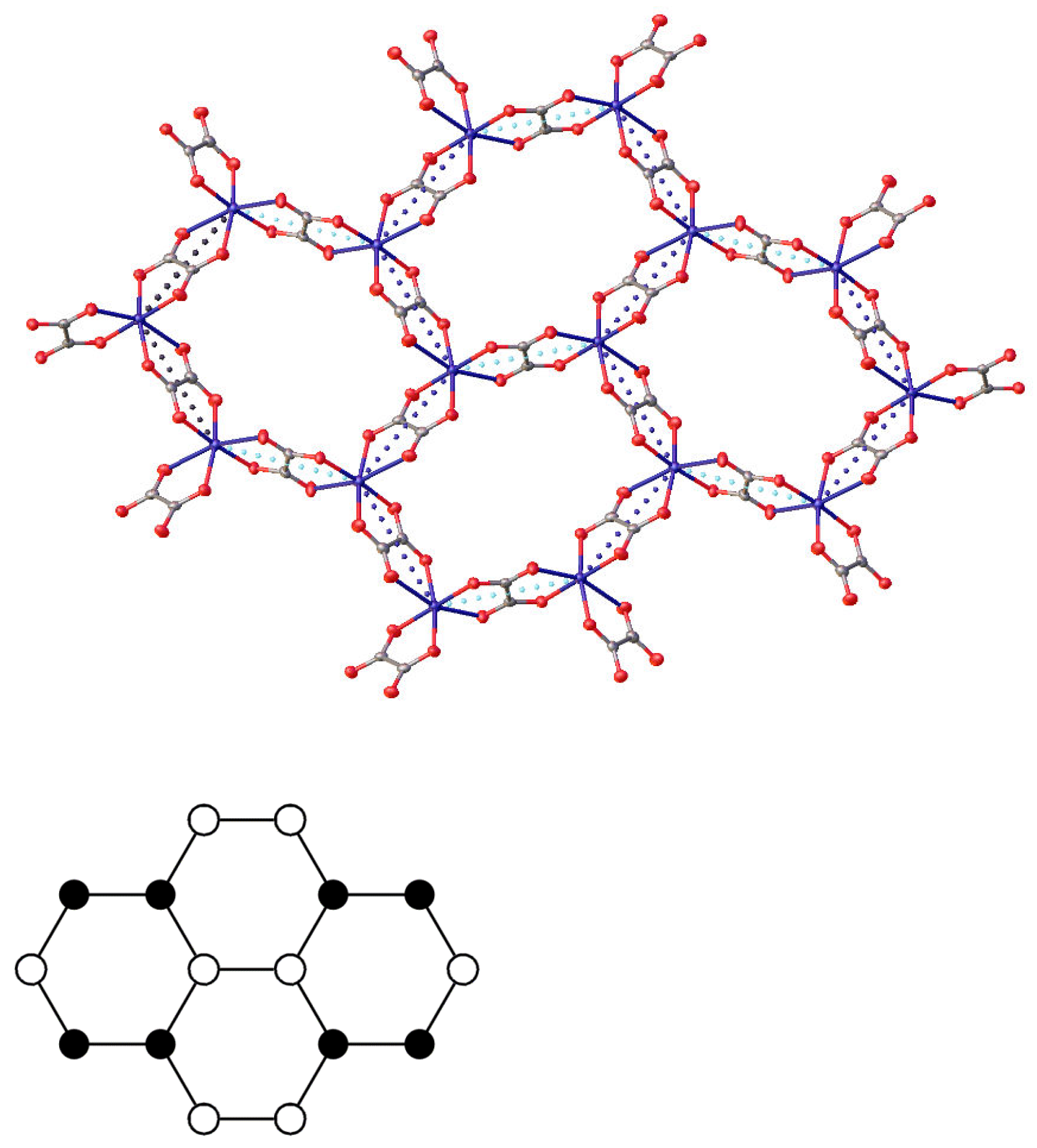
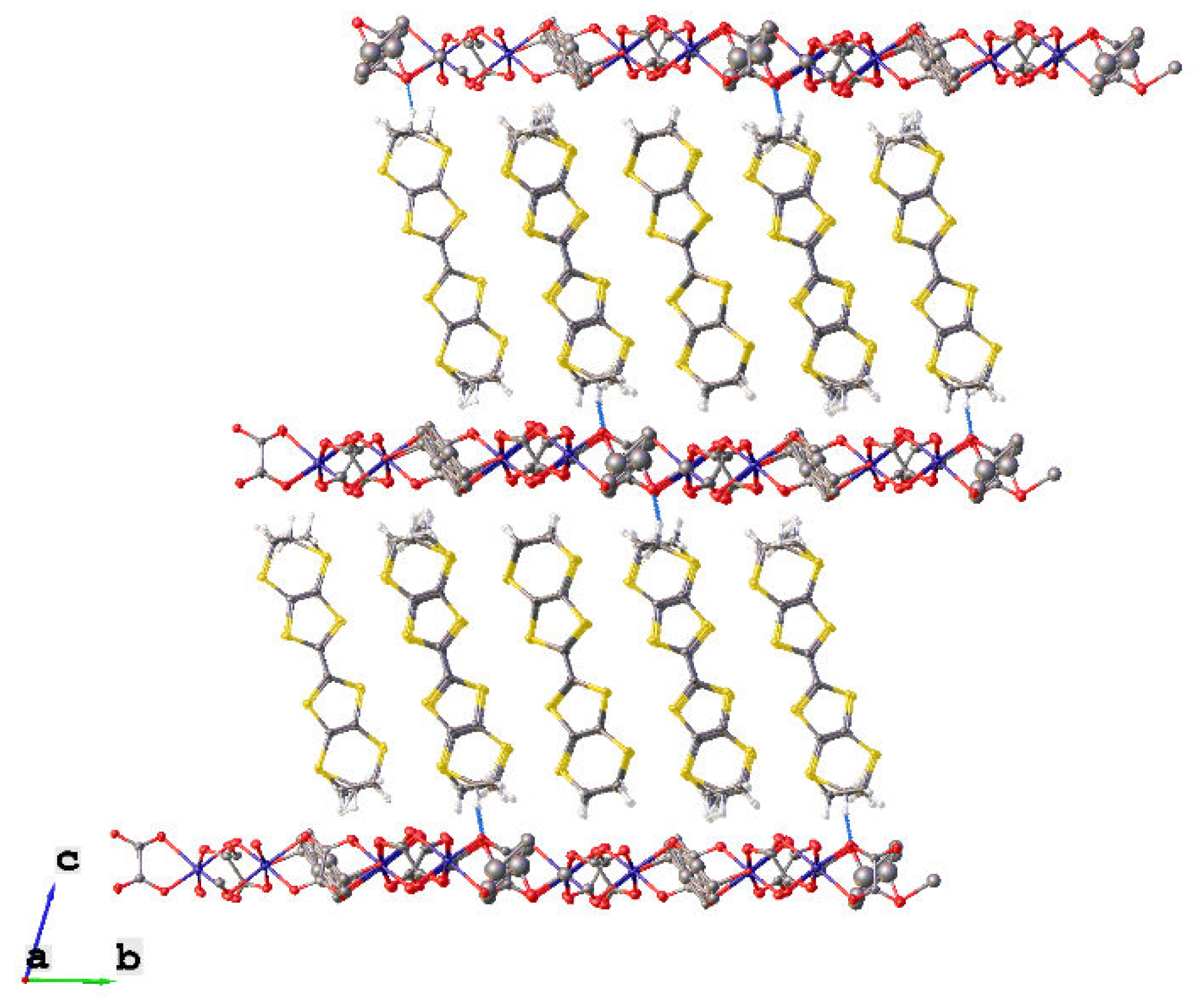
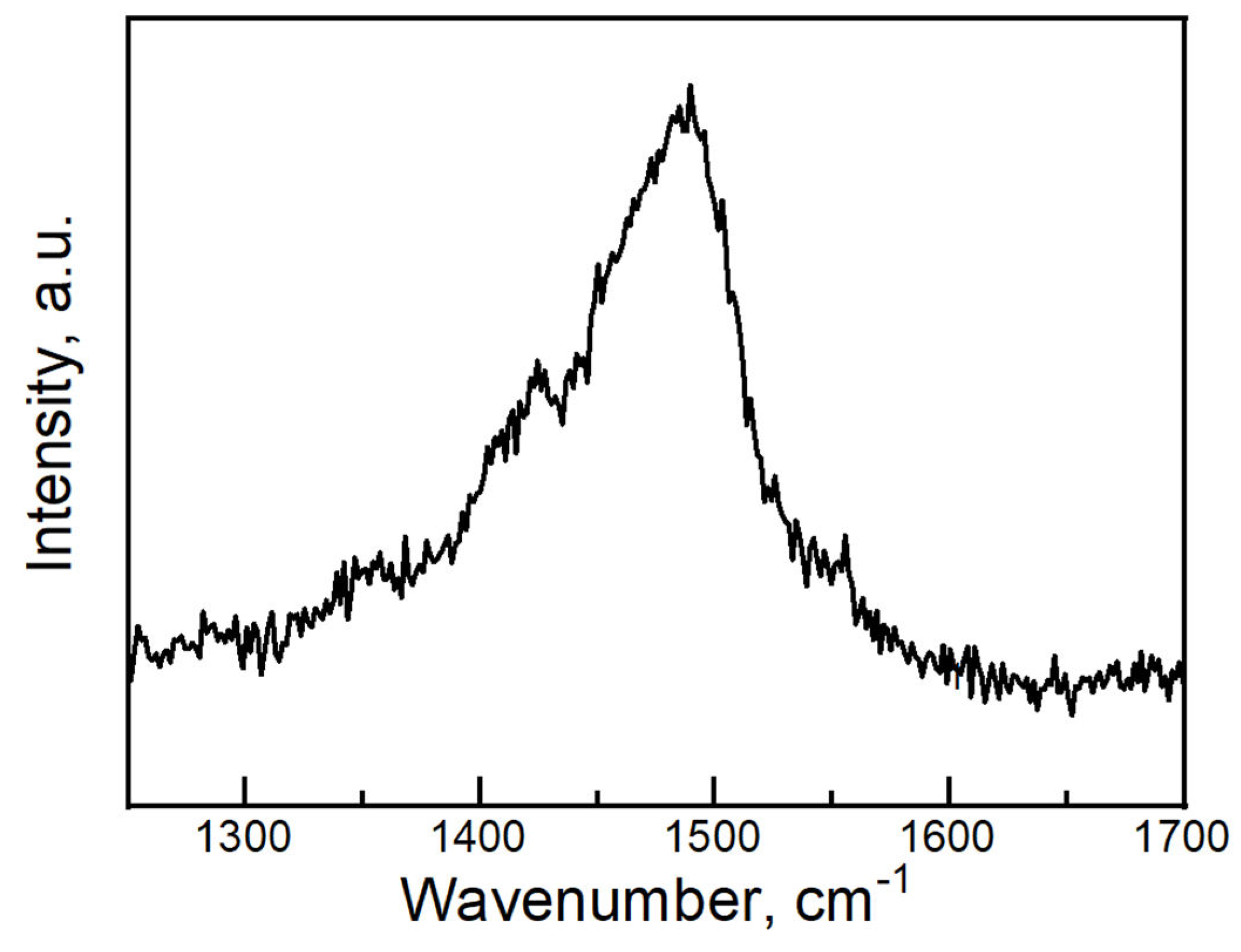
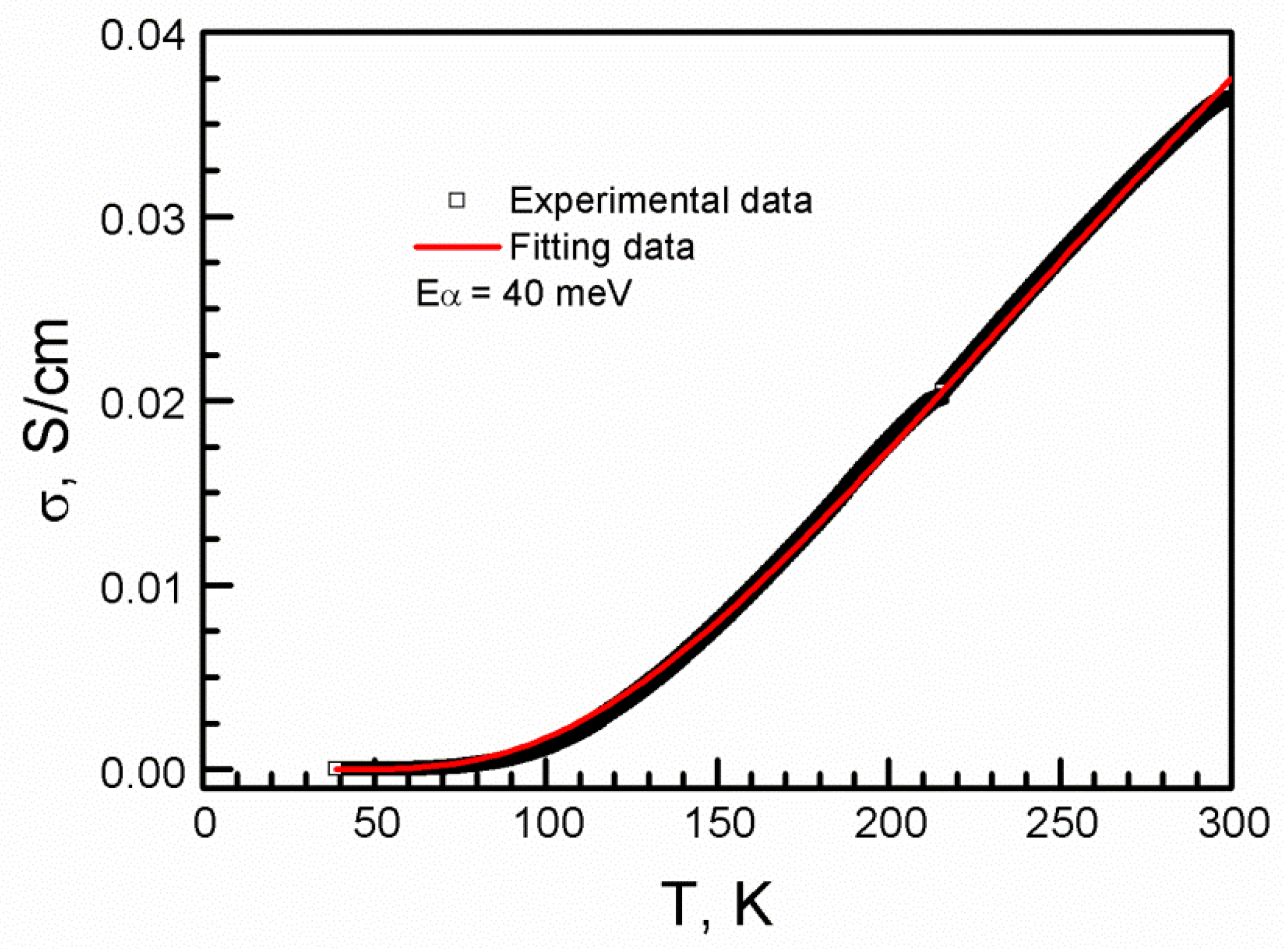
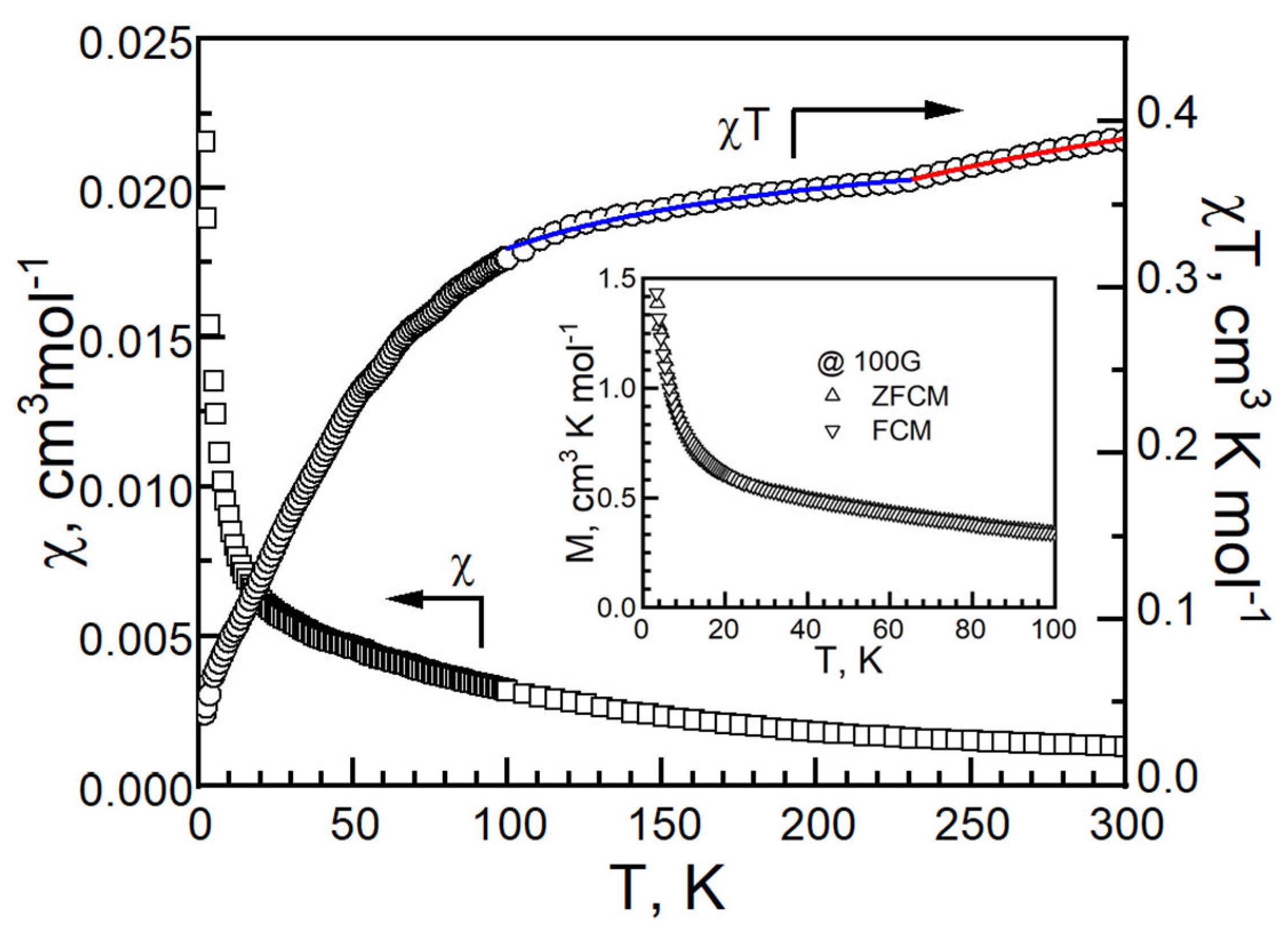
| T, K | 290 | 180 | 120 |
|---|---|---|---|
| Cell parameters | 8.8661(5) | 8.7230(8) | 8.6836(4) |
| 16.7819(8) | 16.7838(7) | 16.8123(7) | |
| 19.4803(9) | 19.3844(4) | 19.3959(7) | |
| 74.271(4) | 74.312(4) | 74.329(3) | |
| 88.962(4) | 89.053(3) | 88.991(4) | |
| 88.022(4) | 88.347(4) | 88.420(4) | |
| 2788.2(2) | 2731.0(3) | 2725.2(2) | |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Space group | P?1 | P?1 | P?1 |
| Z | 2 | 2 | 21.986 |
| Dc. g/cm3 | 1.945 | 1.986 | 1.990 |
| μ mm−1 | 1.726 | 1.762 | 1.766 |
| θ ° | 3.428, 27.877 | 3.446, 27.878 | 3.401, 27.878 |
| Completeness, % | 99.7 | 99.7 | 99.7 |
| no. total reflns | 45474 | 45148 | 44834 |
| no. unique reflns (Rint) | 13222(0.048) | 12961(0.0373) | 12934(0.0375) |
| no. obs. [I ≥ 2σ(I0)] | 8829 | 10020 | 10328 |
| no. params | 759 | 759 | 759 |
| R1, wR2 [I ≥ 2 σ(I0)] | 0.0610, 0.1488 | 0.0475, 0.1182 | 0.0469, 0.1163 |
| R1, wR2 (all data) | 0.0973, 0.1762 | 0.0669, 0.1325 | 0.0629, 0.1280 |
| GOF | 1.030 | 1.070 | 1.067 |
| Shift/error | 0.001/0.000 | 0.001/0.000 | 0.001/0.000 |
| Δρ, e/Å3 | 1.361(-0.849) | 1.017(-1.033) | 1.194(-1.156) |
| CCDC | 1431596 | 1431595 | 1431594 |
| T, K | 290 | 180 | 120 |
| Cu1-O, Å | 1.970, 1.976, 1.993 | 1.958, 1.967, 1.985 | 1.960, 1.971, 1.985 |
| 2.008, 2.259, 2.387 | 1.997, 2.239, 2.378 | 2.002, 2.239, 2.373 | |
| Cu2-O, Å | 1.962, 1.964, 1.979 | 1.953, 1.953, 1.974 | 1.952, 1.956, 1.975 |
| 2.010, 2.282, 2.378 | 1.991, 2.268, 2.383 | 1.988, 2.266, 2.382 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





