Submitted:
20 November 2024
Posted:
21 November 2024
You are already at the latest version
Abstract
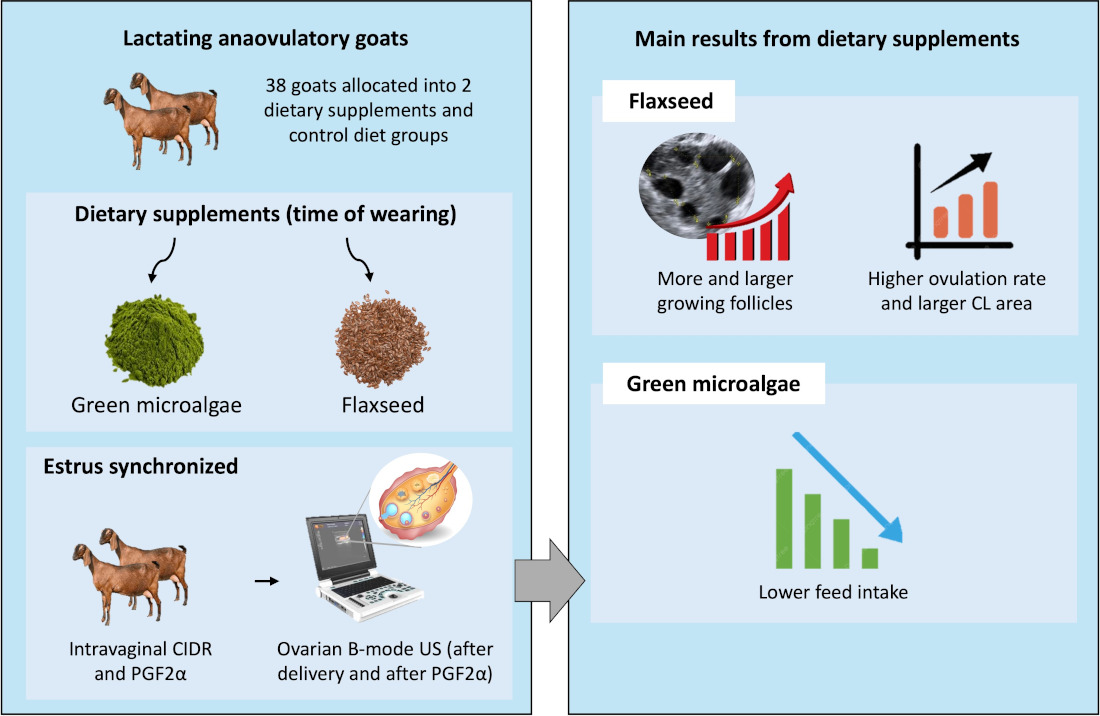
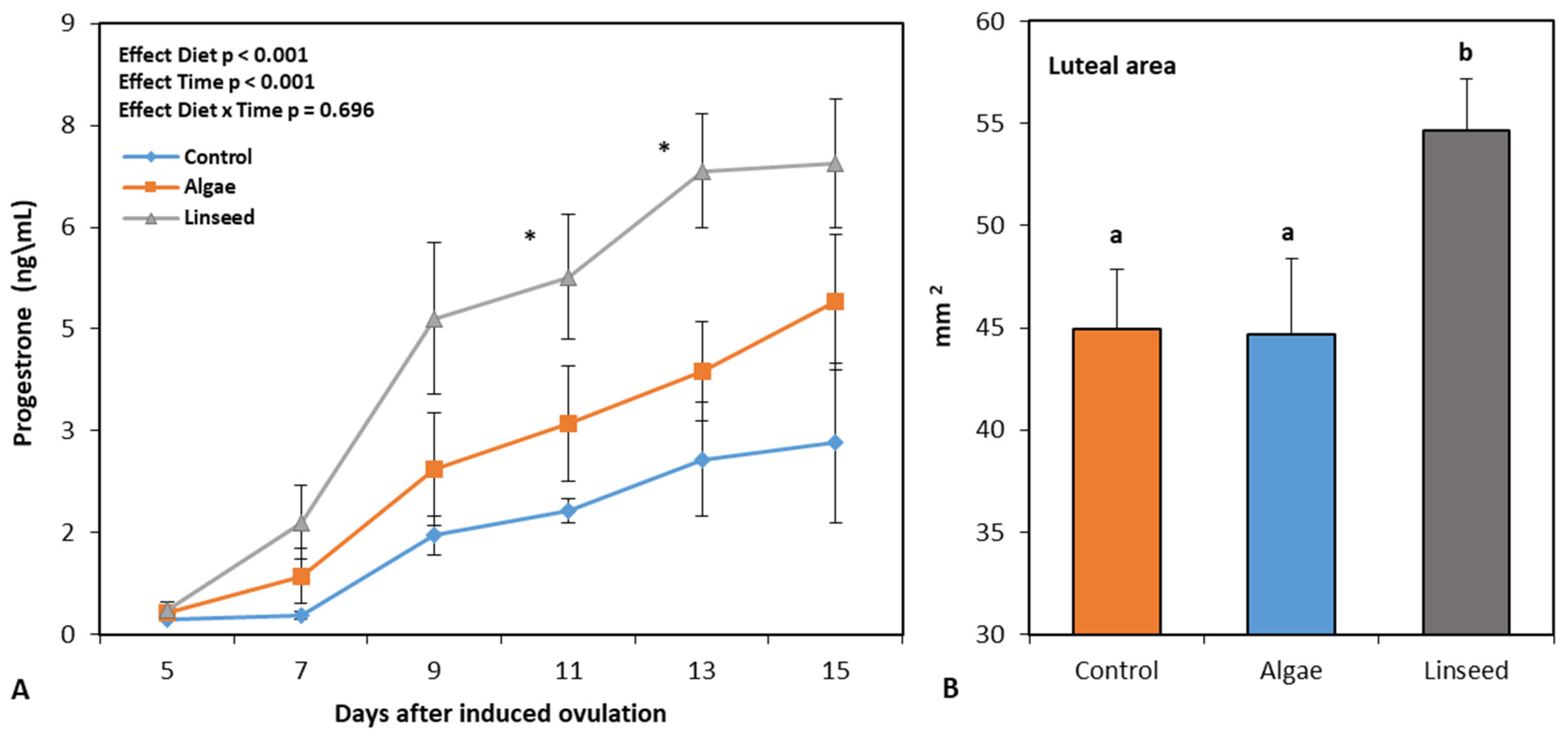
We investigated whether microalgae or linseed supply during the early postpartum period affects ovarian restimulation and supports the first postpartum ovulation in lactating anovulatory goats. Thirty-eight An-glo-Nubian-crossbred adult goats were allocated into three groups: con-trol diet (n=12), fed a total mixed ration (TMR) comprising chopped elephant grass and concentrate; algal diet (n=13), fed TMR+green microalgae (1% dry matter); and linseed diet (n=13), TMR+linseed (12% dry matter). Supplements were furnished from the 2nd to 5th week (time of weaning). Goats were estrus synchronized on day 40 by insertion of an intravaginal CIDR device for 5 days, after which 0.075mg PGF2α was applied to in-duce ovulation, and estrus was monitored for 72 hours. From the 5th-15th day of ovulation induction, the corpus luteum (CL) area and progesterone rate were monitored. The algal and linseed groups showed lower feed intake (P<0.001) and higher (P<0.001) triglyceride levels/follicle numbers, respectively. After estrus induction, no differences were ob-served in estrus response; however, the linseed group showed more and larger growing follicles (P=0.016 and P<0.01), a higher ovulation rate (P<0.05), larger CL area (P<0.05), and higher progesterone levels (P<0.001). Linseed after delivery stimulates follicular growth before and after ovulation induction, favoring better CL quality during the first ovulation.
Keywords:
1. Introduction
2. Materials and Methods
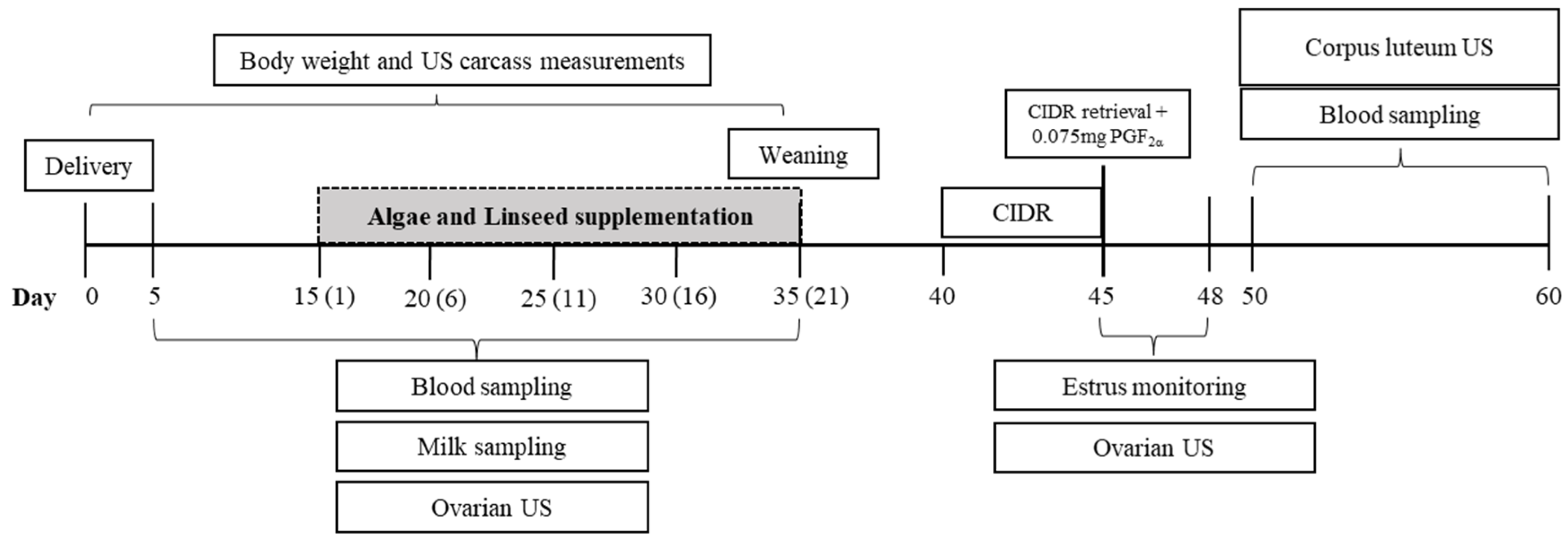
2.1. Animals and Diet Management
2.2. Nutritional Treatments and Experimental Design
2.3. Synchronization Program
2.4. In Vivo Performance and Adipose Carcass Markers Measurements
2.5. Ovarian Function
2.5.1. Follicular Dynamics, Ultrasonography Analysis, and Ovulatory Rate
2.6. Luteal Function
2.6.1. Progesterone Assay
2.6.2. Postpartum Luteal Activity
2.6.3. Corpus Luteum Ultrasonography
2.6.4. Metabolites, β-Hydroxybutyrate (BHB), Glutathione Peroxidase (GPx) Assays, and Fat-Protein Milk Ratio
2.7. Statistical Analysis
3. Results
3.1. Response During Supplementation
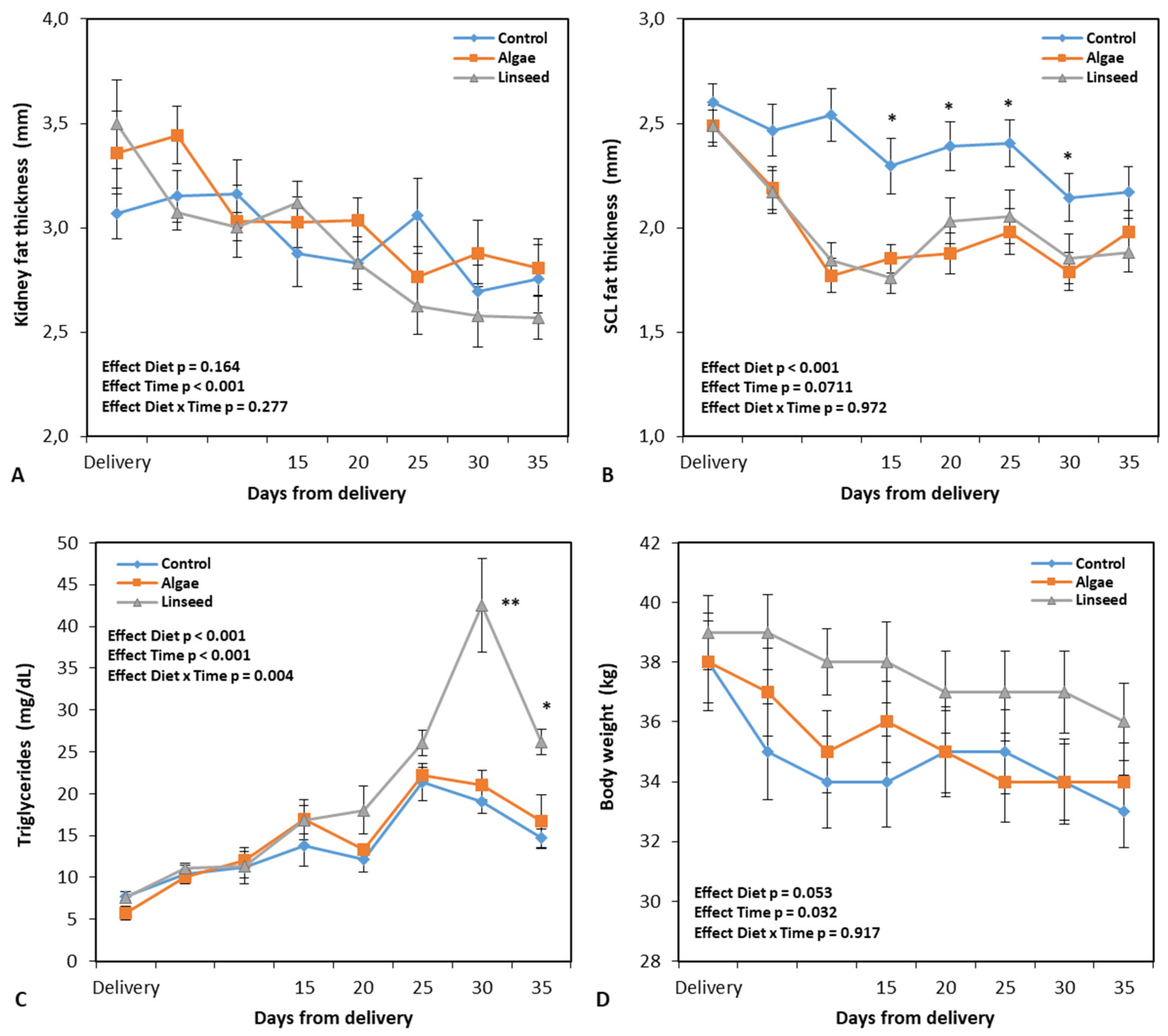
3.1.1. Changes in Feed Intake, Body-Weight, and Carcass Markers
3.1.2. Metabolic and Oxidative Damage Markers
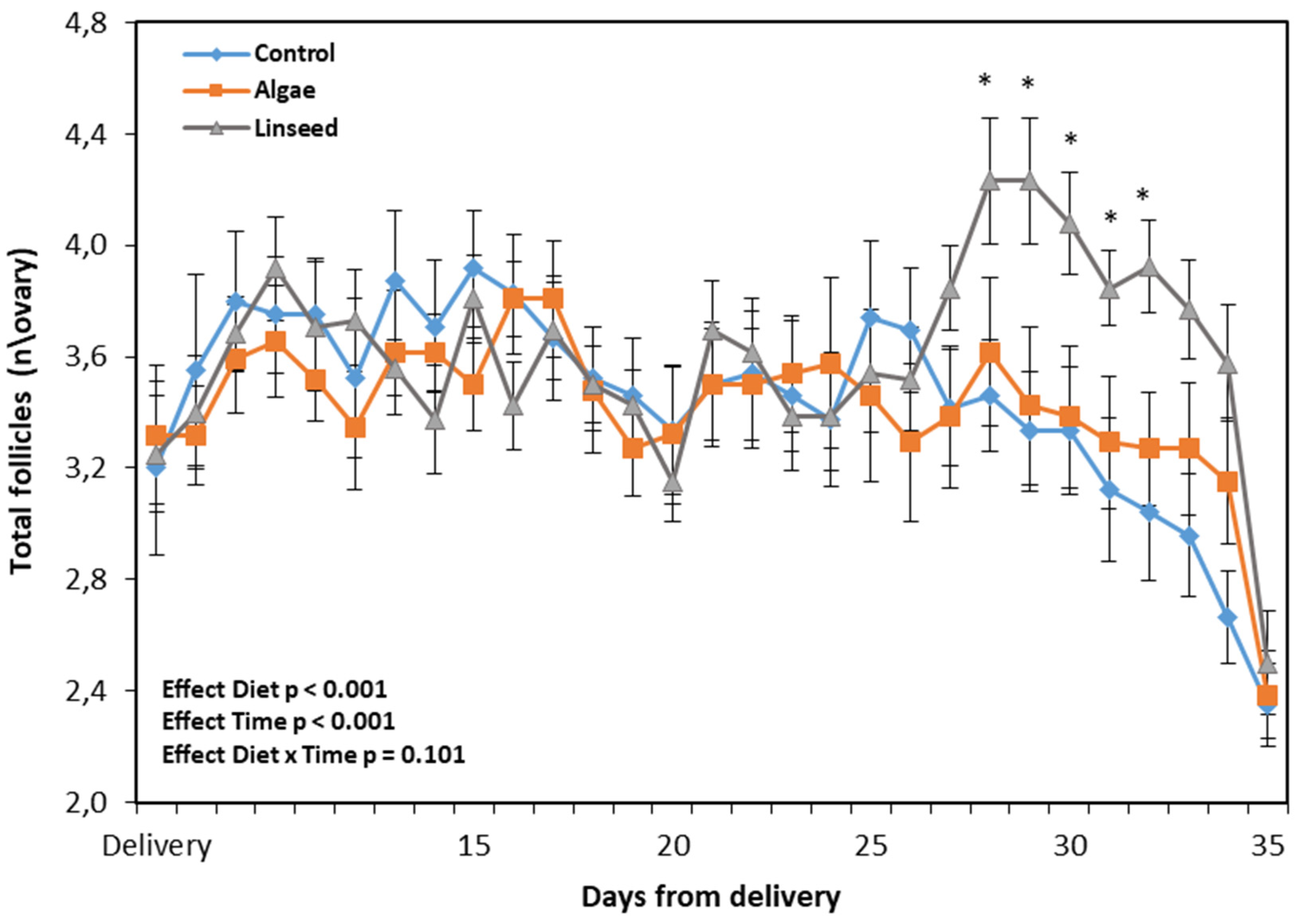
3.1.3. Ovarian and Luteal Function
3.2. Response After Ovulation Induction
3.2.1. Responsiveness to Estrus Synchronization
3.2.2. Follicles, Ovulatory Response, BHB, and GPx
3.2.3. Luteal Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banuelos, S.; Stevenson, J. S. Transition cow metabolites and physical traits influence days to first postpartum ovulation in dairy cows. Theriogenology. 2021, v. 173, p. 133–143. [CrossRef]
- Maranesi, M.; Castellini, C.; Dall’Aglio, C.; Petrucci, L.; Mattioli, S.; Boiti, C.; Zerani, M. Effects of PUFAs on animal reproduction: Male and female performances and endocrine mechanisms. Phytochemistry Reviews. 2018, v. 17, p. 801-814. [CrossRef]
- Sordillo, L. M. Nutritional strategies to optimize dairy cattle immunity. Journal of dairy science. 2016, v. 99, n. 6, p. 4967–4982. [CrossRef]
- Mezzetti, M.; Bionaz, M.; Trevisi, E. Interaction between inflammation and metabolism in periparturient dairy cows. Journal of Animal Science. 2020, v. 98, n. Supplement_1, p. S155-S174, 98(Supplement_1), S155–S174. [CrossRef]
- Kuhla, B. Pro-inflammatory cytokines and hypothalamic inflammation: Implications for insufficient feed intake of transition dairy cows. Animal. 2020, v. 14, n. S1, p. s65-s77. [CrossRef]
- Robertson, S. M.; Atkinson, T.; Friend, M. A.; Allworth, M. B.; Refshauge, G. Reproductive performance in goats and causes of perinatal mortality: A review. Animal Production Science. 2020, v. 60, n. 14, p. 1669-1680. [CrossRef]
- Menezes, L. M.; Sousa, W. H.; Cavalcanti-Filho, E. P.; Gama, L. T. Genetic parameters for reproduction and growth traits in Boer goats in Brazil. Small Ruminant Research. 2016, v. 136, p. 247-256. [CrossRef]
- Palmquist, D. L.; Jenkins, T. C. A 100-Year Review: Fat feeding of dairy cows. Journal of Dairy Science. 2017, v. 100, n. 12, p. 10061-10077. [CrossRef]
- Hristov, A. N.; Melgar, A.; Wasson, D.; Arndt, C. Symposium review: Effective nutritional strategies to mitigate enteric methane in dairy cattle. Journal of dairy Science.2022, v. 105, n. 10, p. 8543-8557. [CrossRef]
- Roque-Jiménez, J. A.; Rosa-Velázquez, M.; Pinos-Rodríguez, J. M.; Vicente-Martínez, J. G.; Mendoza-Cervantes, G.; Flores-Primo, A.; Relling, A. E. Role of long chain fatty acids in developmental programming in ruminants. Animals. 2021, v. 11, n. 3, p. 762. [CrossRef]
- Yadav, D.; Singh, A. K.; Kumar, B.; Mahla, A. S.; Singh, S. K.; Patra, M. K.; Krishnaswamy, N. Effect of n-3 PUFA-rich fish oil supplementation during late gestation on kidding, uterine involution and resumption of follicular activity in goat. Reproduction in domestic animals. 2019, 54(12), 1651-1659. [CrossRef]
- Mahla, A. S.; Bunkar, S. K.; Kumawat, B. L.; Saxena, V. K.; Selvaraju, S.; Bhatt, R. S.; Kumar, A. Dietary n-3 PUFA augments pre-ovulatory follicle turnover and prolificacy in well-fed ewes. Animal Reproduction Science. 2023, v. 252, p. 107231. [CrossRef]
- Elis, S.; Freret, S.; Desmarchais, A.; Maillard, V.; Cognié, J.; Briant, E.; Dupont, J. Effect of a long chain n-3 PUFA-enriched diet on production and reproduction variables in Holstein dairy cows. Animal reproduction Science. 2016, v. 164, p. 121-132. [CrossRef]
- Ribeiro, E. S.; Greco, L. F.; Bisinotto, R. S.; Lima, F. S.; Thatcher, W. W.; Santos, J. E. Biology of Preimplantation Conceptus at the Onset of Elongation in Dairy Cows1. Biology of Reproduction. 2016, v. 94, n. 4, p. 97, 1-18. [CrossRef]
- Cavalcanti, C. M.; Silva, M. R. L.; Conde, A. J. H.; Bezerra, A. F.; Alves, J. P. M.; Fernandes, C. C. L.; Rondina, D. Effect of peri-conception high-fat diets on maternal ovarian function, foetal and placentome growth, and vascular umbilical development in goats. Reproduction in Domestic Animals, 2022, v. 57, n. 12, p. 1481-1492. [CrossRef]
- Senosy, W.; Kassab, A.Y.; Mohammed, A.A. Effects of feeding green microalgae on ovarian activity, reproductive hormones and metabolic parameters of Boer goats in arid subtropics. Theriogenology. 2017, v. 96, p. 16-22. [CrossRef]
- Silva, M. R. L.; Alves, J. P. M.; Fernandes, C. C. L.; Cavalcanti, C. M.; Conde, A. J. H.; Bezerra, A. F.; Rondina, D. Use of green microalgae Chlorella as a nutritional supplement to support oocyte and embryo production in goats. Animal Reproduction Science. 2023a, v. 256, p. 107296. [CrossRef]
- Silva, M. R. L.; Alves, J. P. M.; Fernandes, C. C. L.; Cavalcanti, C. M.; Conde, A. J. H.; Bezerra, A. F.; Rondina, D. Effect of short-term nutritional supplementation of green microalgae on some reproductive indicators of Anglo-Nubian crossbred goats. Veterinary World. 2023b, v.16, n.3, p. 464. [CrossRef]
- Nudda, A.; Bee, G.; Correddu, F.; Lunesu, M. F.; Cesarani, A.; Rassu, S. P. G.; Battacone, G. Linseed supplementation during uterine and early post-natal life markedly affects fatty acid profiles of brain, liver and muscle of lambs. Italian Journal of Animal Science. 2022, v. 21, n.1, p. 361-377. [CrossRef]
- Akhtar, P.; Rajoriya, J. S.; Singh, A. K.; Ojha, B. K.; Jha, A. K.; Bisen, A.; Singh, M. Effects of dietary supplementation with omega-3 fatty acid-rich linseed on the reproductive performance of ewes in subtropical climates. Frontiers in Veterinary Science. 2024, v. 11, p. 1398961. [CrossRef]
- Till, B. E.; Huntington, J. A.; Posri, W.; Early, R.; Taylor-Pickard, J.;Sinclair, L. A. Influence of rate of inclusion of microalgae on the sensory characteristics and fatty acid composition of cheese and performance of dairy cows. Journal of dairy Science. 2019, v.102, n.12, p.10934-10946. [CrossRef]
- Ribeiro, E. S. Symposium review: Lipids as regulators of conceptus development: Implications for metabolic regulation of reproduction in dairy cattle 1. Journal of Dairy Science. 2018, v. 101, n.4, p. 3630–3641. [CrossRef]
- Izquierdo, D.; Roura, M.; Pérez-Trujillo, M.; Soto-Heras, S.; Paramio, M. T. Fatty acids and metabolomic composition of follicular fluid collected from environments associated with good and poor oocyte competence in goats. International journal of molecular sciences. 2022, v. 23, n.8, p. 4141. [CrossRef]
- Council, N. R. Nutrient requirements of small ruminants: Sheep, goats, cervids, and new world camelids. Nutrient requirements of small ruminants. 2007.
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem Physiol. 1959, v. 39, p. 911-917. [CrossRef]
- Sniffen, C. J.; O'connor, J. D.; Van Soest, P. J.; Fox, D. G.; Russell, J. B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of animal Science. 1992, v.70, n.11, p. 3562-3577. [CrossRef]
- Teixeira, A.; Joy, M., Delfa, R. In vivo estimation of goat carcass composition and body fat partition by real-time ultrasonography. J. Anim. Sci. 2008, v.86, n.9, p.369–2376. [CrossRef]
- Harter, C.J.; Silva, H.G.O.; Lima, L.D.; Castagnino, D.S.; Rivera, A.R.; Neto, O.B.; Gomes, R.A.; Canola, J.C.; Resende, K.T.; Teixeira, I.A.M.A. Ultrasonographic measurements of kidney fat thickness and Longissimus muscle area in predicting body composition of pregnant goats. Anim. Prod. Sci. 2014, v. 54, n. 9, p. 1481–1485. [CrossRef]
- Guido, S.I.; Andrade, J.C.O.; Guido, F.C.L.; Oliveira, M.A.L.; Lima, P.F.; Moura, R.T.D.; Santos, V.F.; Cavalcanti, C.C. Avaliação de corpos lúteos de receptoras caprinas. Revista Brasileira de Reprodução Animal. 2023, v. 27, n. 3, p. 491-492.
- Balaro, M.F.A,; Santos, A.S,; Moura, L.F.G.M,; Fonseca, J.F,; Brandão, F.Z. Luteal dynamic and functionality assessment in dairy goats by luteal blood flow, luteal biometry, and hormonal assay. Theriogenology. 2017, v. 95, p. 118-126. [CrossRef]
- Batista, A. P.; Gouveia, L.; Bandarra, N. M.; Franco, J. M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Research. 2013, v. 2, n. 2, p. 164-173. [CrossRef]
- Boeckaert, C.; Vlaeminck, B.; Dijkstra, J.; Issa-Zacharia, A.; Van Nespen, T.; Van Straalen, W.; Fievez, V. Effect of dietary starch or micro algae supplementation on rumen fermentation and milk fatty acid composition of dairy cows. Journal of Dairy Science. 2008, v. 91, n. 12, p. 4714-4727. [CrossRef]
- Pajor, F.; Egerszegi, I.; Szűcs, Á.; Póti, P., Bodnár, Á. Effect of marine algae supplementation on somatic cell count, prevalence of udder pathogens, and fatty acid profile of dairy goats’ milk. Animals. 2021, v.11, n.4, p. 1097. [CrossRef]
- Zeron, Y.; Sklan, D.; Arav, A. Effect of polyunsaturated fatty acid supplementation on biophysical parameters and chilling sensitivity of ewe oocytes. Molecular reproduction and development. 2002, v.61, n.2, p.271-278. [CrossRef]
- Zarrin, M.; De Matteis, L.; Vernay, M. C. M. B.; Wellnitz, O.; Van Dorland, H. A.; Bruckmaier, R. M. Long-term elevation of β-hydroxybutyrate in dairy cows through infusion: Effects on feed intake, milk production, and metabolism. Journal of dairy Science. 2013, v.96, n.5, p.2960-2972. [CrossRef]
- Song, Y.; Wang, Z.; Zhao, C.; Bai, Y.; Xia, C.; Xu, C. Effect of negative energy balance on plasma metabolites, minerals, hormones, cytokines and ovarian follicular growth rate in Holstein dairy cows. Journal of veterinary research. 2021, v. 65, n.3, p.361. [CrossRef]
- Pereira, G.; Simões, P.; Bexiga, R.; Silva, E.; Mateus, L.; Fernandes, T.; Lopes-da-Costa, L. Effects of feeding rumen-protected linseed fat to postpartum dairy cows on plasma n-3 polyunsaturated fatty acid concentrations and metabolic and reproductive parameters. Journal of Dairy Science. 2022, v. 105, n.1, p. 361-374. [CrossRef]
- Oliveira, F. B. B. D.; Fernandes, C. C. L.; Montenegro, A. R.; Oliveira, I. T. M.; Silva, C. P.; LIMA, F. W. R.; Rondina, D. Cured dry smoked shoulder meat quality from culled adult goats fed a high lipid diet. Food Science and Technology. 2021, v. 42, p. e19521. [CrossRef]
- Silva, J. R. V.; Lima, F. E. O.; Souza, A. L. P.; Silva, A. W. B. Interleukin-1β and TNF-α systems in ovarian follicles and their roles during follicular development, oocyte maturation and ovulation. Zygote. 2020, v. 28, n. 4, p. 270-277. [CrossRef]
- Yamamoto, Y.; Kuwahara, A.; Taniguchi, Y.; Yamasaki, M.; Tanaka, Y.; Mukai, Y.; Irahara, M. Tumor necrosis factor alpha inhibits ovulation and induces granulosa cell death in rat ovaries. Reproductive Medicine and Biology. 2014, v.14, n.3, p.107–115. [CrossRef]
- Crespo, D.; Mañanós, E. L.; Roher, N.; MacKenzie, S. A.; Planas, J. V. Tumor necrosis factor alpha may act as an intraovarian mediator of luteinizing hormone-induced oocyte maturation in trout. Biology of reproduction. 2012, v. 86, n.1, p. 5-1. [CrossRef]
- Ambrose, D. J.; Kastelic, J. P.; Corbett, R..; Pitney, P. A.; Petit, H. V.; Small, J. A.; Zalkovic, P. Lower Pregnancy losses in lactating dairy cows fed a diet enriched in α-linolenic acid. Journal of dairy Science. 2006, v. 89, n. 8, p. 3066-3074. [CrossRef]
- Martins, F. S.; Saraiva, M. V. A.; Celestino, J. J. H.; Bruno, J. B.; Almeida, A. P.; Cunha, R. M. S.; Figueiredo, J. R. Expression of protein and mRNA encoding Insulin Growth Factor-I (IGF-I) in goat ovarian follicles and the influence of IGF-I on in vitro development and survival of caprine preantral follicles. Animal Reproduction (AR). 2018, v.7, n.4, p. 349-361. [CrossRef]
- Magalhães-Padilha, D. M.; Duarte, A. B. G.; Araújo, V. R.; Saraiva, M. V. A.; Almeida, A. P.; Rodrigues, G. Q.; Figueiredo, J. R. Steady-state level of insulin-like growth factor-I (IGF-I) receptor mRNA and the effect of IGF-I on the in vitro culture of caprine preantral follicles. Theriogenology. 2012, v.77, n.1, p. 206-213. [CrossRef]
- Costa, S. L.; Costa, E. P.; Pereira, E.; Benjamin, L. A.; Rodrigues, M. T.; Mendes, V. R..; Silva, T. F. Influence of Insulin-like Growth Factor I (IGF-I) on the survival and the in vitro development of caprine preantral follicles. Pesquisa Veterinaria Brasileira. 2014, v. 34, p. 1037-1044. [CrossRef]
- Luz, V.B.; Chaves, R.N.; Alves, A.M.C.V.; Pinheiro, A.S.; Figueiredo, J.R. Role of insulin-like growth factor-I (IGF-I) and kit ligand (KL) in ovarian function. Acta Sci Vet. 2015, v. 43:1300. ISSN 1679-9216.
- Nudda, A.; Battacone, G.; Usai, M. G.; Fancellu, S.; Pulina, G. Supplementation with extruded linseed cake affects concentrations of conjugated linoleic acid and vaccenic acid in goat milk. Journal of Dairy Science. 2006, v. 89, n.1, p. 277-282. [CrossRef]
- Luna, P.; Bach, A.; Juárez, M.; De La Fuente, M. A. Effect of a diet enriched in whole linseed and sunflower oil on goat milk fatty acid composition and conjugated linoleic acid isomer profile. Journal of Dairy Science. 2008, v. 91, n.1, p. 20-28. [CrossRef]
- Bernard, L.; Bonnet, M.,; Leroux, C.; Shingfield, K. J.; Chilliard, YEffect of sunflower-seed oil and linseed oil on tissue lipid metabolism, gene expression, and milk fatty acid secretion in alpine goats fed maize silage–based diets. Journal of Dairy Science. 2009, v. 92, n. 12, p. 6083-6094. [CrossRef]
- Dutra, P. A.; Pinto, L. F. B.; Neto, B. C.; Gobikrushanth, M.; Barbosa, A. M.; Barbosa, L. P. Flaxseed improves embryo production in Boer goats. Theriogenology. 2019, v. 127, p. 26-31. [CrossRef]
- Zachut, M.; Dekel, I.; Lehrer, H.; Arieli, A.; Arav, A.; Livshitz, L.; Moallem, U. Effects of dietary fats differing in n-6: N-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte quality. Journal of Dairy Science. 2010, v.93, n.2, p. 529-545. [CrossRef]
- Ghaffarilaleh, V.; fouladi-nashta, A.; Paramio, M.-T. Effect of α-linolenic acid on oocyte maturation and embryo development of prepubertal sheep oocytes. Theriogenology. 2014, v. 82, n. 5, p. 686–696. [CrossRef]
- Fabjanowska, J.; Kowalczuk-Vasilev, E.; Klebaniuk, R.; Milewski, S.; Gümüş, H. N-3 Polyunsaturated Fatty Acids as a Nutritional Support of the Reproductive and Immune System of Cattle—A Review. Animals. 2023, v. 13, n. 22, p. 3589. [CrossRef]
- Surlis, C.; Cormican, P.; Waters, S. M.; Lonergan, P.; Keogh, K.; Doyle, D. N.; Kenny, D. A. Effects of dietary n-3-PUFA supplementation, post-insemination plane of nutrition and pregnancy status on the endometrial transcriptome of beef heifers. Scientific reports. 2020, v. 10, n. 1, p.20798, 2020. [CrossRef]
- AOAC (1990) AOAC-Official Methods of Analysis. AOAC, Maryland.
| Parameters | Diet | Algae | Linseed | |||
|---|---|---|---|---|---|---|
| Control | Algae | Linseed | ||||
| Ingredients, g/kg DM | ||||||
| Elephant grass | 600 | 600 | 600 | - | - | |
| Ground corn grain | 120 | 120 | 60 | - | - | |
| Soybean meal | 120 | 120 | 100 | - | - | |
| Wheat bran | 140 | 140 | 100 | - | - | |
| Ground Linseed | - | - | 120 | - | - | |
| Algae | - | 10 | - | - | - | |
| Mineral mixture | 20 | 20 | 20 | - | - | |
| Chemical fraction | ||||||
| Dry matter, g/kg as-fed basis | 568 | 523 | 573 | 892 | 929 | |
| Crude protein, g/kg of DM | 114 | 102 | 122 | 397 | 245 | |
| Ether extract, g/kg of DM | 28 | 28 | 57 | 210 | 283 | |
| Neutral detergent fiber, g/kg of DM | 546 | 507 | 555 | 178 | 331 | |
| Acid detergent fiber, g/kg of DM | 310 | 297 | 329 | 50 | 231 | |
| Ash, g/kg of DM | 89 | 83 | 88 | 44 | 34 | |
| Non-fibrous carbohydrates, g/kg of DM | 324 | 430 | 420 | 917 | 910 | |
| ME, Mcal/kg of DM | 2.23 | 2.27 | 2.35 | - | - | |
| Fatty acids, % | ||||||
| Saturated | - | - | - | 28.1 | 11.5 | |
| Unsaturated | - | - | - | 71.9 | 88.5 | |
| Monounsaturated | - | - | - | 45.0 | 23.1 | |
| Polyunsaturated | - | - | - | 26.9 | 65.4 | |
| UFA/SFA* | 2.6 | 7.7 | ||||
| *UFA: unsaturated fatty acids, SFA: saturated fatty acids. | ||||||
| Parameters | Diet | p Value | |||||
| Control | Algae | Linseed | SEM | Diet | Time | D vs T | |
| Feed intake | |||||||
| DMI*, kg\doe | 1.2a | 1.0b | 1.2a | 0.015 | < 0.001 | 0.231 | 0.997 |
| Metabolic markers and Oxidative stress | |||||||
| Glutathione peroxidase, U/L | 127.3 | 120.5 | 147.8 | 2.169 | 0.058 | 0.947 | 0.702 |
| BHB**, mmol/L | 0.3 | 0.3 | 0.4 | 0.019 | 0.878 | - | - |
| Cholesterol, mg\dL | 53.6 | 63.2 | 62.9 | 1.774 | 0.107 | < 0.001 | 0.180 |
| FPR***, ratio | 0.8a | 1.0b | 1.1b | 0.03 | 0.002 | 0.822 | 0.994 |
| Luteal function | |||||||
| Functional CL****, n/doe | 0B | 0B | 0B | - | - | - | - |
| Nonfunctional CL, n/doe | 0B | 0B | 1B | - | - | - | - |
| CL absent, n/doe | 12A | 13A | 12A | - | - | - | - |
| Progesterone, ng/mL | 0.2a | 0.2a | 0.3b | 0.011 | 0.025 | 0.945 | 0.988 |
| *DMI: dry matter intake; **BHB: β-hydroxybutyrate, measured at beginning of supplementation; ***Fat-protein milk ratio; ****CL: corpus luteum; Time, ANOVA effect for interval of assessment used. a,b P < 0.05 differences between diets. A,B P < 0.05 differences between CL classes in each diet. | |||||||
| Parameters | Diet | p Value | |||||
|---|---|---|---|---|---|---|---|
| Control | Algae | Linseed | SEM | Diet | Time | D vs T | |
| Follicle after ovulation induction* | |||||||
| Follicles < 3 mm, n\ovary | 2.0ab | 2.0a | 1.6b | 0.032 | 0.044 | 0.094 | 0.503 |
| Follicles ≥ 3 mm, n\ovary | 1.4ab | 1.2a | 1.6b | 0.025 | 0.016 | 0.009 | 0.404 |
| Total follicles, n\ovary | 3.3 | 3.2 | 3.3 | 0.025 | 0.461 | 0.681 | 0.503 |
| Max follicle size, mm | 4.2a | 4.6ab | 5.0b | 0.051 | 0.008 | 0.006 | 0.175 |
| Estrus and ovulatory response | |||||||
| No of does in estrus, % (n\n) | 25.0 (3/12) ǂ | 38.5 (5/13) | 46.2 (6/13) | - | 0.367 | - | - |
| Onset estrus**, h | 38.6 | 41.7 | 21.5 | 6.278 | 0.344 | - | - |
| Estrus length, h | 16.3 | 31.5 | 37.7 | 6.036 | 0.473 | - | - |
| No of does ovulate, % (n\n) | 25.0 (3/12) ǂ | 69.2 (9/13) | 61.5 (8/13) | - | 0.216 | - | - |
| Ovulatory rate, n\doe | 0.4a | 0.8ab | 1.0b | 0.132 | 0.046 | - | - |
| Metabolic marker and Oxidative stress*** | |||||||
| Glutathione peroxidase, U/L | 188.9 | 235.6 | 228.6 | 2.211 | 0.345 | - | - |
| BHB****, mmol/L | 0.2 | 0.2 | 0.3 | 0.038 | 0.715 | - | - |
| *Follicles traits, measured by ultrasonography in the 72 hours after ovulation induction; **interval between CIDR removal and estrus onset; *** performed at 5th day after prostaglandin injection; ****BHB: β-hydroxybutyrate; Time, ANOVA effect for interval of assessment used. a,b P < 0.05 differences between diets; ǂ P < 0.05, qui square test in each diet. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





