Submitted:
19 November 2024
Posted:
19 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods and Materials
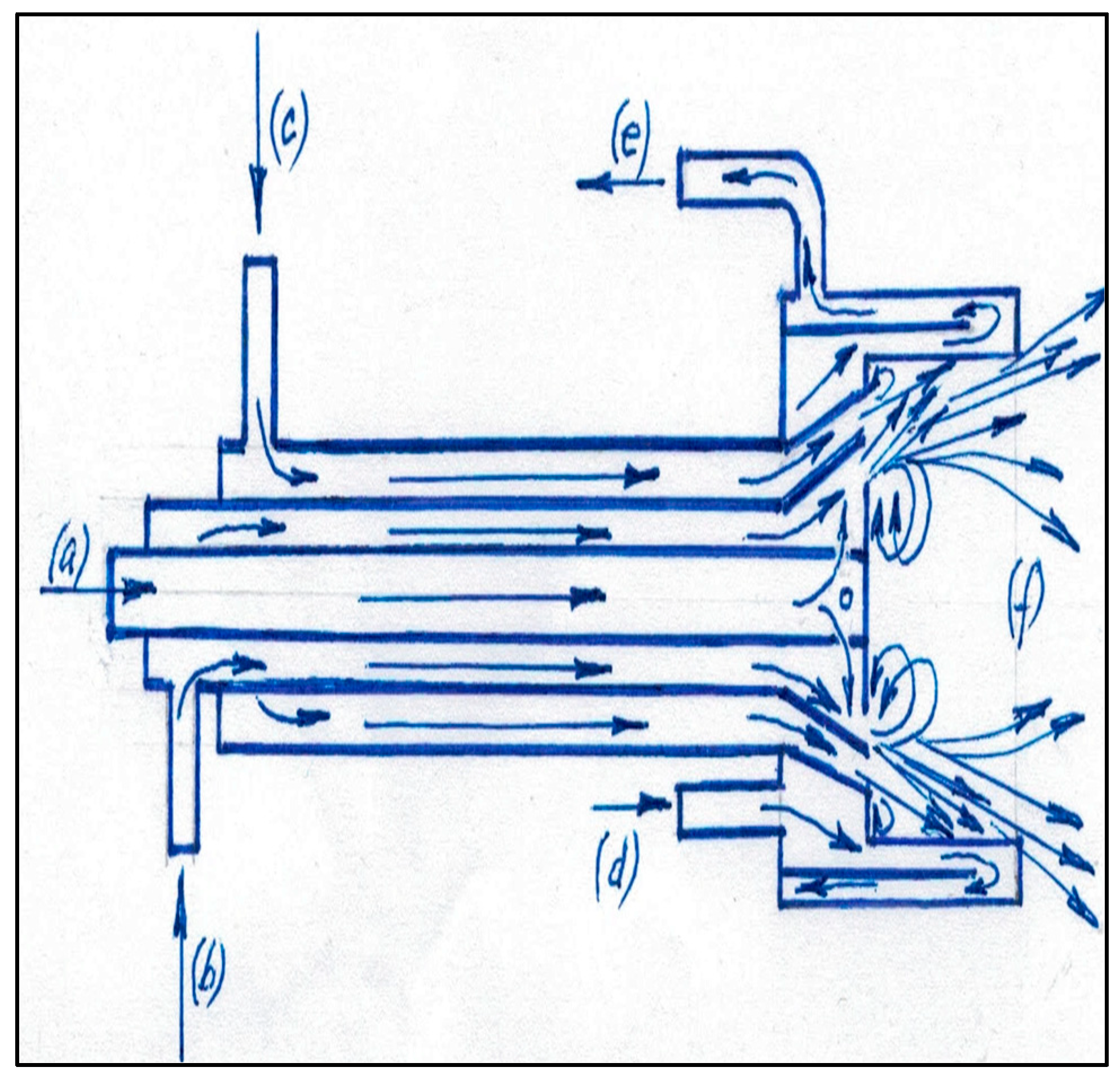
2.1. Methods
2.2. Materials
2.3. Methods for Determining the Technical Performances of the Burner
3. Results and Discussion
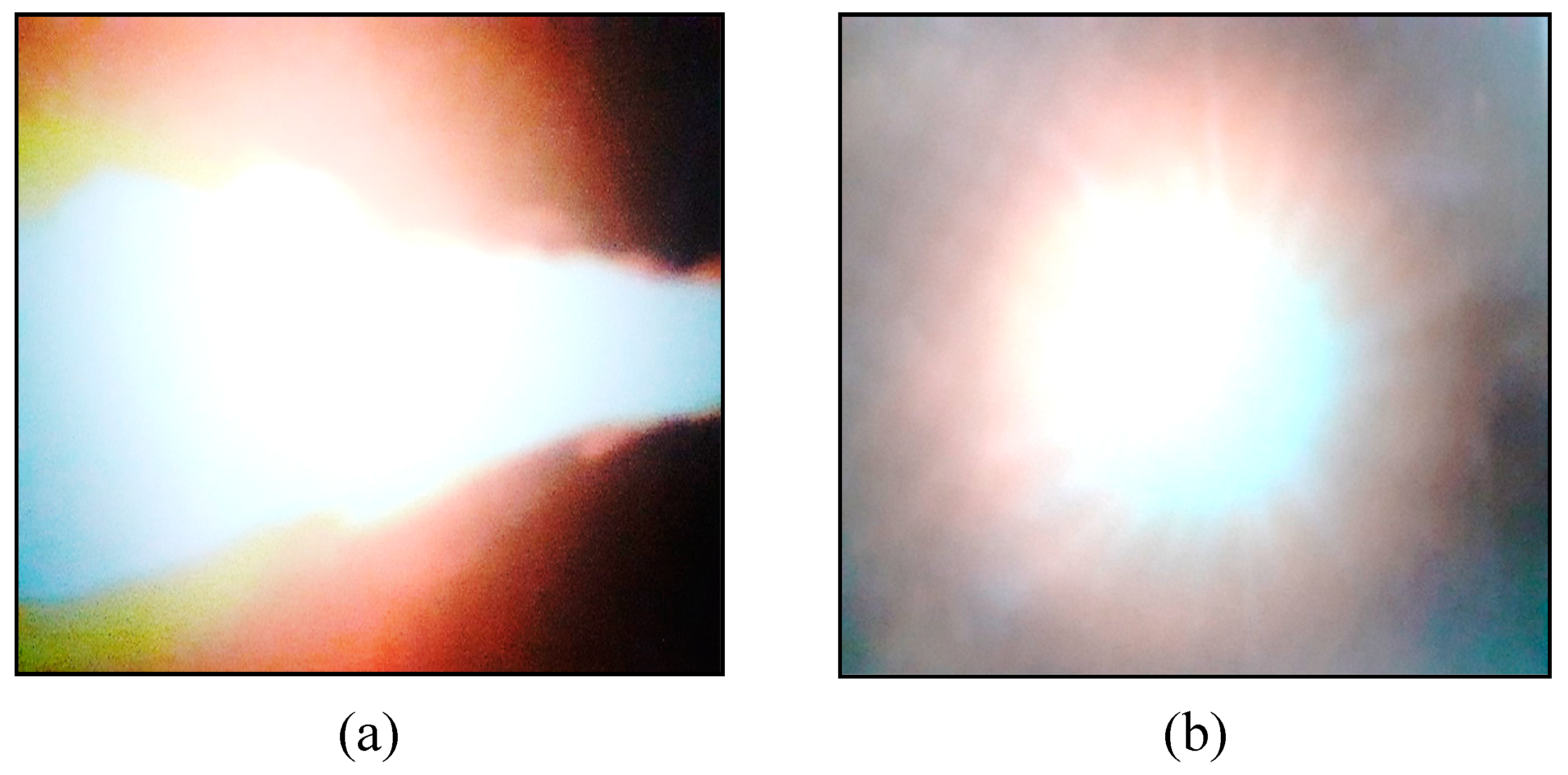
3.1. Results
3.2. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andres, R.J., Boden, T.A., Bréon, F.M., Ciais, P., Davis, S., Erickson, D., Gregg, J.S., Jacobson, A., Marland, G., Miller, J., Oda, T., Olivier, J.G.J., Rauparch, M.R., Rayner, P., Treanton, K., A synthesis of carbon dioxide emissions from fossil-fuel combustion, Journal of the European Geosciences Union, Copernicus Publications, Göttingen, Germany, 2012, vol. 9, no. 5, pp. 1845-1871. https://bg.copernicus.org/articles/9/1845/2012/.
- Chen, L., Msigwa, G., Yang, M., Osman, A.I., Fawzy, S., Rooney, D.W., Yap, P., Strategies to achieve a carbon neutral society: A review, Environmental Chemistry Letters, Springer Link, 2022, vol. 20, pp. 2277-2319. [CrossRef]
- Biofuel basics, Office of Energy Efficiency & Renewable Energy-US Department of Energy, Washington DC, the United States, 2023. https://www.energy.gov/eere/bioenergy/biofuel-basics.
- Sharmila, S., Jeyanthi, L.R., Ankush, S.D.R., Kowsalya, E., Extraction of bioethanol from plant leaves, Pharmacia Lettre, Scolars Research Library, 2016, vol. 8, no. 8, pp. 97-99, ISSN 0975-5071.
- Chandrakart, P., Bisaria, V.S., Simultaneous bioconversion of cellulose and hemicellulose to ethanol, Critical Reviews in Biotechnology, National Library of Medicine, 1998, vol. 18, no. 4, pp. 295-331. [CrossRef]
- Tsai, J., Ko, Y., Huang, C., Chiang, H., Effects of blending ethanol and gasoline on the performance of motorcycle catalysts and airborne pollutant emissions, Aerosol and Air Quality Research, 2019, vol. 19, no. 12. [CrossRef]
- Ethanol fuel basics, In: Alternative fuels data center, Energy Efficiences & Renewable Energy, US Department of Energy, Washington DC, the United States, 2023. https://afde.energy.gov/fuels/ethanol-fuel-basics.
- Nicol, R.W., Marchand, K., Lubitz, W.D., Bioconversion of crude glycerol by fungi, Applied Microbiology and Biotechnology, Springer Link, 2012, vol. 93, pp. 1865-1875. https://link.springer.com/article/10.1007/s00253-012-3921-7.
- US renewable diesel production growth drastically impacts global feedstock trade, International Agricultural Trade Report, US Department of Agriculture, Washington DC, the United States, June 2024.
- Christoph, R., Schmidt, B., Steinberner, U., Dilla, W., Karinen, R., Glycerol, In: Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012, vol. 17, pp. 67-82.
- Cornejo, A., Barrio, I., Campoy, M., Lazaro, J., Navarrete, B., Oxigenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: a critical review, Renewable and Sustainable Energy Reviews, Elsevier, 2017, vol. 79, pp. 1400-1413. [CrossRef]
- World Energy Outlook. Report Extract Data, The International Energy Agency (IEA), Paris (France), 2019.
- Agarwal, A.K., Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines, Progress in Energy and Combustion Science, Elsevier, 2007, vol. 33, pp. 233-271. [CrossRef]
- Caliskan, H., Yildiz, I., Mori, K., Chapter 10, Biofuels combustion in internal combustion engines, In: Advances in Biofuels Production, Optimization and Applications, Elsevier, 2024, pp. 185-205.
- Ramos, J.L., Valdivia, M., Garcia-Lorente, F., Segura, A., Benefits and perspectives on the use of biofuels, Microbial Biotechnology,2016, vol. 9, no. 4, pp. 435-440. [CrossRef]
- Global biofuel market overview, October 2024-2028, ReportLinker.com, 2024. https://www.reportlinker.com/market-report/Bioenergy/132956/Biofuel?term=biofuel%20outlook&matchtype=b&loc_interest=&8loc_physical=9194739&utm_gr.
- Paunescu, L., Surugiu, G., Volceanov, E., Experimentally use of glycerol as a biofuel in oxy-combustion thermal process, Nonconventional Technologies Review, 2022, vol. 26, no. 3, pp. 22-27. https://revtn/index.php/revtn/about.
- Yi, F., Axelbaum, R.L., Oxy-combustion of low-volatility liquid fuel with high water content, Energy & Fuels, 2015, vol. 29, no. 2, pp. 1137-1142. [CrossRef]
- Mihaescu, L., Cristea, E.D., Panoiu, N., Swirl burners: Theory, construction, use, 1st ed., Technical Publishing House, Bucharest, Romania, 1986 (in Romanian).
- Froud, D.Y., Syred, N., Characterisation of industrial swirl burners for efficient combustion of low calorific value gases, Proceedings of the Institute of Energy Conference Held, December 4-5, 1995, London, United Kingdom.
- Basu, P., Kefa, C., Jestin, L., Swirl burners, In: Boilers and Burners, Mechanical Engineering Series, Springer Link, 2000, pp. 212-242, ISBN 978-1-4612-1250-8_8. https://link.springer.com/chapter/10.1007/978-1-4612-1250-8_8.
- Ҫengel, Y.A., Boles, M.A., Kanoglu, M., Thermodynamics: An engineering approach, 10th ed., McGraw Hill, ISBN 9781266664489, 2024.
- Pei, S.K., Mohamed, K.A., Dand, A.W., Mohd, W., Conversion of crude and pure glycerol into derivates: A feasibility evaluation, Renewable and Sustainable Energy Reviews, 2016, vol. 63, pp. 533-555. [CrossRef]
- Kohse-Höinghaus, K., Osswald, P., Cool, T.A., Kasper, T., Hansen, N., Qi, F., Westbrook, C.K., Westmoreland, P.R., Biofuel combustion chemistry: From ethanol to biodiesel, Agewandte (International Edition), Journal of the German Chemical Society, Wiley Online Library, 2010, vol. 49, no. 21, pp. 3572-3579. [CrossRef]
- Ethanol-chemical compound, Britannica, available at: https://www.britannica/science/ethanol. Accessed: 15.08.2022.
- Moron, W., Rybak, W., NOx and SO2 emissions of coals, biomass and their blends under different oxy-fuel atmospheres, Atmospheric Environment, Elsevier, 2015, vol. 116, pp. 65-71. [CrossRef]
- Bohon, M.D., Metzger, B.A., Linak, W.P., King, C.J., Roberts, W.L., Glycerol combustion and emissions, Proceedings of the Combustion Institute, Elsevier, 2011, vol. 33, no. 2, pp. 2717-2724.
- Stainmetz, S.A., Herrington, J.S., Winterrowd, C.K., Roberts, W.L., Wendt, J.O.L., Crude glycerol combustion: Particulate, acrolein, and other volatile organic emissions, Proceedings of the Combustion Institute, Elsevier, 2012, vol. 34, no. 2, pp. 2749-2757.
| Parameter | Unit | Value |
|---|---|---|
| Burner heat power | kW | 51.5 |
| Glycerine hourly flow | kg·h-1 | 10.10 |
| Ethanol hourly flow | kg·h-1 | 1.15 |
| Glycerine and ethalon mix pressure | bar | 0.4 |
| Atomizing water flow | kg·h-1 | 79.5 |
| Atomizing water pressure | bar | 1.6 |
| Oxygen flow | m3N·h-1 | 11.73 |
| Oxygen pressure | mbar | 190 |
| Biofuel mix speed in radial orifice | m·s-1 | 177 |
| Biofuel spray speed in the annular section | m·s-1 | 210 |
| Oxygen speed in the annular section | m·s-1 | 215 |
| Waste gas speed at the exit from the burner | m·s-1 | 135 |
| Cooling water flow | m3·h-1 | 1.9 |
| Cooling water speed | m·s-1 | 1.4 |
| Parameter | Thermal Regime | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Biofuel mix hourly flow (m3N·h-1) |
11.33 | 11.25 | 10.97 | 10.00 | 8.97 | 7.95 | 7.01 | 6.08 |
| Oxygen flow (m3N·h-1) | 11.94 | 11.73 | 10.92 | 10.20 | 9.58 | 9.00 | 8.41 | 7.80 |
| Atomizing water flow (kg·h-1) |
82.71 |
79.50 |
73.11 |
68.01 |
62.70 |
57.68 |
52.51 |
47.40 |
| Waste gas pollution composition NO (mg·m3N-1) NO2 (mg·m3N-1) CO (vol. %) |
181 197 - |
178 193 - |
175 190 - |
171 186 - |
168 184 - |
165 180 - |
161 175 - |
154 169 - |
| Flame temperature (°C) | 1860 | 1850 | 1840 | 1835 | 1830 | 1820 | 1810 | 1790 |
| Flame length (mm) | 570 | 560 | 550 | 540 | 520 | 500 | 470 | 450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





