1. Introduction
With the rapid development of the industrial & commercial activities and the increase in population, the energy demand and the environmental issues are becoming more and more serious. Recycling all kinds of waste oil has attracted significant attention in recent years [
1]. These oil sources include engine oil (or mineral engine oil), lubricating oil, cutting oil, tank bottom oil, and so on. For example, the so-called waste engine oil refers to the engine oil that has been mixed with water, dust, metal-containing power produced from mechanical wear and tear like iron (Fe), lead (Pb), zinc (Zn), and magnesium (Mn) [
2]. Without waste oil collection and reuse by recycling into oil-related or oil-derived products, it can pose adverse environmental and health impacts due to its contents of petroleum-based components, heavy metals and other contaminants [
3]. Although the production of alternative fuels from waste oils can decline the consumption of petroleum-based fuels and thus reduce greenhouse gas (GHG) emissions [
4], the waste oil refining process still generates large amounts of oil-containing residue. Based on the current situation, the conversion of oil-containing residue from waste oil recycling plant into valuable products not only provided the total solutions in the waste oil recycling industry, but also made additional profits for these recycling enterprises.
In recent years, many researches have reviewed on different thermal, chemical and physical technologies to convert oil-containing residue or oily sludge into a variety of products [
5,
6,
7,
8,
9]. In brief, these studies focused on the recovery of oils (or residual oils) by adopting solvent extraction and ultrasonication, and the energy recovery by using thermal treatment and thermochemical processes like pyrolysis and gasification. Regarding the production of porous carbon-rich materials from oily sludge by activation process, only few studies performed the cases from the waste oil recycling plants [
10,
11], not those from the oil (petroleum) refinery plants like oil tanks (at the bottom of the tanks) [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. Furthermore, most of these researches adopted the chemical activation by using potassium hydroxide (KOH), less cases by using zinc chloride (ZnCl
2). Exceptionally, Li
et al. prepared the de-ashed char at 700°C and post-washing with hydrogen fluoride (HF) solution for 24 h to remove inorganic impurities [
18]. The authors used phosphoric acid (H
3PO
4) as an activating agent to promote the pore properties of the above-mentioned resulting char with different H
3PO
4/char ratios of 2:1, 2.5:1, and 3:1 at 400 °C for 1 h under a nitrogen flow [
18]. To obtain the highly porous carbon products, the crude products were washed with plenty of deionized (D.I.) water and then dried at 105°C for 24 h. In the previous studies [
10,
11], the pore properties of the resulting carbon products prepared from oil-containing residue by physical activation of carbon dioxide (CO
2) at 850°C for 0.5-1.5 h showed a decreasing trend [
11]. The maximal Brunauer–Emmett–Teller (BET) surface area only had 21.59 m
2/g. In addition, the data on the BET surface area can be enhanced to 40.53 m
2/g due to the acid-washing of the crude carbon products [
10].
Based on the results of the previous studies [
10,
11], it showed a feasible approach to prepare the carbon products from the residual sludge of waste oil recycling plant, even if the pore properties were not significant. In this work, we carried out the chemical activation experiments by using H
3PO
4 at a lower temperature (600°C). To enhance the pore properties by removing residual H
3PO
4 and inherent inorganic minerals, the post-washing of the crude carbon products were further performed by using deionized water and dilute acid. In this regard, the pore properties of the resulting carbon products were determined by the surface area and porosity analyzer based on the nitrogen isotherms (- 196°C). Their porous textures and elemental compositions on the surface were viewed by the scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS), respectively.
2. Materials and Methods
2.1. Materials
The starting precursor for producing carbon product was derived from a waste oil recycling plant, located in Neipu Industrial Park (Pingtung county, Taiwan). According to the official definition, the as-received oil-containing sludge in the form of dark and sticky slurry is a non-hazardous waste. The sample was analyzed to get the information about the thermochemical data on proximate analysis, calorific value, elemental content and thermogravimetric analysis (TGA). To perform the chemical activation experiments with phosphoric acid (or ortho-phosphoric acid), the concentration of ACS-grade activation agent from Merck KGaA (Darmstadt, Germany) was 85 wt%.
2.2. Thermochemical Properties of Oil-Containing Sludge
The baseline information about the thermochemical properties (including proximate analysis, calorific value and TGA) of the oil-containing sludge were first determined to get the thermal decomposition behavior and the potential for preparing carbon materials. Based on the official standard methods (i.e., “Standard Method for Determining Moisture Content of Industrial Waste” and “Standard Method for Determining Combustible and Ash Contents of Industrial Waste”, coded as NIEA R203.02C and NIEA R205.01C, respectively) in Taiwan, the proximate analysis of as-received sludge sample was performed to assess the quality of the precursor. Combustible is the component remaining after the determination of moisture and ash contents. Therefore, it is a measure of the solid combustible fraction. Calorific value is the energy content per unit mass of the sample (dry basis), which was indicative of the heat energy released while completely burning it by an adiabatic bomb calorimeter (Model: CALORIMETER ASSY 6200; Parr Co., Moline, IL, USA) in triplicate. It should be noted that the determined value represents gross calorific value or higher calorific value because it contains the latent heat of combustion product water vaporization. On the other hand, the TGA was performed by a precision instrument (Model: TGA-51; Shimadzu Co., Kyoto, Japan). The dried sludge sample was uniformly heated at four rates (5, 10, 15, and 20°C/min) under nitrogen gas (flowrate set by 50 cm3/min) up to 900°C. In addition, the main elemental contents (including carbon, oxygen, alkali metals, alkaline earth metals, silicon, and other main elements) on the surface of dried sludge sample were determined by using an energy-dispersive X-ray spectroscopy (Model: 7021-H; HORIBA Co., Kyoto, Japan).
2.3. Phosphoric Acid Activation Experiments
Chemical activation is a low energy-consumption process for producing porous carbon materials because it generally performed at lower temperatures (400–800°C) as compared to physical activation process at higher temperatures (700–1000°C) [
29]. In the former process, phosphoric acid is a commonly used activator acted as a dehydrating agent. According to the preliminary data on the pore properties of resulting carbon products prepared at 600-900°C for holding 30-60 min by a higher impregnation ratio of 2.0 (the mass ratio of phosphoric acid to oil-containing sludge), it only showed non-regular variations on specific surface area (BET model) ranging from 50.7 to 59.3 m
2/g. Therefore, the chemical activation experiments were designed at a lower temperature (600°C) by different impregnation ratios (i.e., 0.0, 0.5, 1.0, 1.5, and 2.0) in the present study. To carbonize the residual sludge, the inert atmosphere was carried out by purging nitrogen gas with a flowrate of 500 cm
3/g during all activation experiments. In order to evaluate the enhancement of the pore properties for the resulting carbon products, the post-washing treatment by deionized water and dilute acid solution (0.25 M HCl) were performed on a hot-plate at about 75°C for 30 min. The detailed procedures can refer to the previous studies [
11,
30,
31]. In this work, the resulting carbon products were stated as the specific code. For example, C-05-SL-600-30, C-05-SL-600-30-W and C-05-SL-600-30-A represent the carbon products (C) prepared from the sludge (SL) at 600°C for 30 min without washing, water-washing and acid-washing, respectively. To reduce the use of phosphoric acid, two acid-washing experiments with the impregnation ratios of 0.0 and 0.5 were carried out in the present study. That is, the resulting carbon products are C-0-SL-600-30-A and C-05-SL-600-30-A.
2.4. Characterization of Carbon Products
The nitrogen adsorption/desorption isotherm at –196°C were commonly used for determining the pore properties of a carbon material (or product), including specific surface area, pore volume, average pore size and pore size distribution [
32,
33]. An accelerated surface area & porosimeter (Model: ASAP 2020; Micromeritics Instrument Co., Norcross, GA, USA) was adopted for all pore property analyses. Prior to the determinations of pore properties, the degassed steps were performed under vacuum condition (1.33 Pa) at high temperature (200°C) for holding 10 h. Regarding the specific surface area, the Brunauer-Emmett-Teller (BET) surface area (S
BET) should be the most commonly used one, which is expressed in units of area per mass of sample (m
2/g). By comparison with the adsorption isotherm of a nonporous reference material, the data on micropore (pore size ˂ 2.0 nm) volume (V
micro) and micropore surface area (S
micro) can be calculated by using the “
t-plot” method [
33]. Herein, the
t-plot method was used to estimate the surface area and pore volume with micropores (pore diameter or width: ˂ 2.0 nm) based on the Harkins-Jura equation. The data on total pore volume (V
t) were estimated by assuming that all pores are filled with liquid adsorbate (i.e., nitrogen). Therefore, total nitrogen gas adsorbed at a saturated relative pressure (
ca. 0.995) was converted to liquid nitrogen volume by using its density at –196°C (i.e., 0.807 cm
3/g). By assuming uniform cylindrical pores, the following equation was generally used to calculate average pore diameter (D
av) of a porous sample for a given total pore volume and BET surface area.
On the other hand, the pore size distribution of a porous material was based on the Barrett–Joyner–Halenda (BJH) method, which can be represented both in differential and cumulative models [
33]. It should be noted that the BJH method was limited to display the pore size distributions in the range of mesopore (2.0 - 50.0 nm) to macropore (˃ 50.0 nm). In order to image the porous structures of the resulting carbon products, the scanning electron microscopy (SEM) (Model: S-3000N; Hitachi Co., Tokyo, Japan) was applied under an accelerating voltage of 15.0 kV. Prior to the SEM observations, the non-conductive carbon products were coated by a gold film in an ion sputter (Model: E1010; Hitachi Co., Tokyo, Japan).
3. Results and Discussion
3.1. Thermochemical Characterization of Oil-Containing Sludge
Table 1 listed the data on proximate analysis and calorific value of oil-containing sludge, which were used to evaluate the potential for the production of carbon products in the chemical activation process. Obviously, the as-received sample still contained large non-combustible fractions, including moisture content (65.44 wt%) and ash content (24.98 wt%). It showed high separation efficiency of residual oil in the waste oil recycling plant, resulting in only about 10 wt% of combustible component remained in the oil-containing sludge. Using the preliminary analysis by EDS,
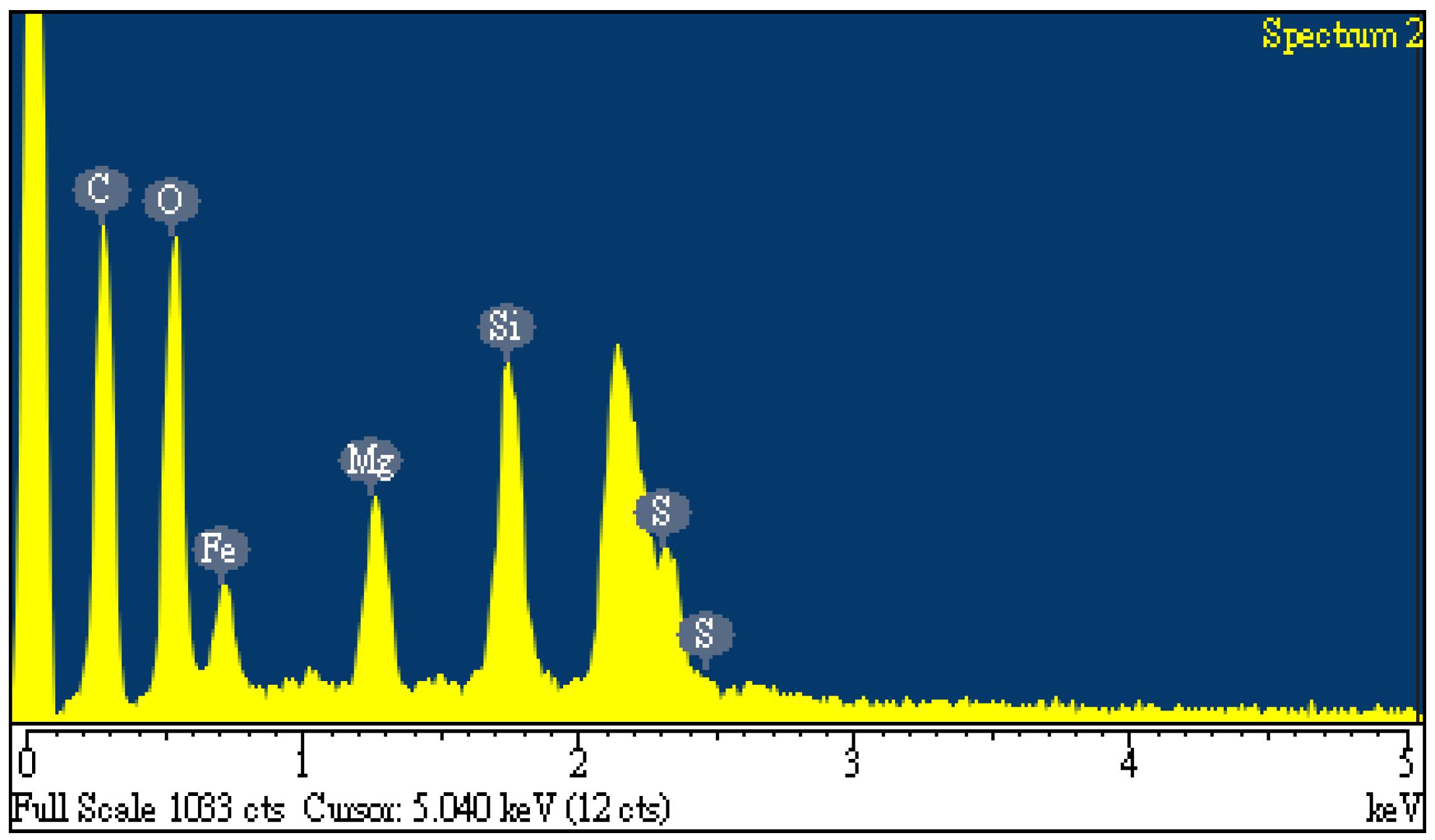
Figure 1 showed the elemental compositions on the surface of the dried oil-containing sludge, indicating high carbon and oxygen contents (38.81 wt% and 29.03 wt%, respectively) and other inorganic elements like iron (19.45 wt%), silicon (6.93 wt%), magnesium (4.50 wt%) and sulfur (1.28 wt%). In this regard, the origin of oil-containing sludge could be derived from the waste oil at the bottom of iron-made storage tank in the petroleum refinery plants. As seen in
Figure 1, the unidentified peaks at around 2.1 keV are assigned to the gold (Au) because this element was used to provide a conductive film on the surface by an ion sputter. Furthermore, the calorific value of dried oil-containing sludge only had 13.06 MJ/kg. This value was significantly lower than those of coal and petroleum-based fuels [
34,
35]. Although the oil-containing sludge can be treated by combustion method, the emissions of particulates and sulfur oxides (SOx) from the incineration plants or industrial boilers should be controlled by air pollution control systems like baghouse and flue gas desulfurization (FGD).
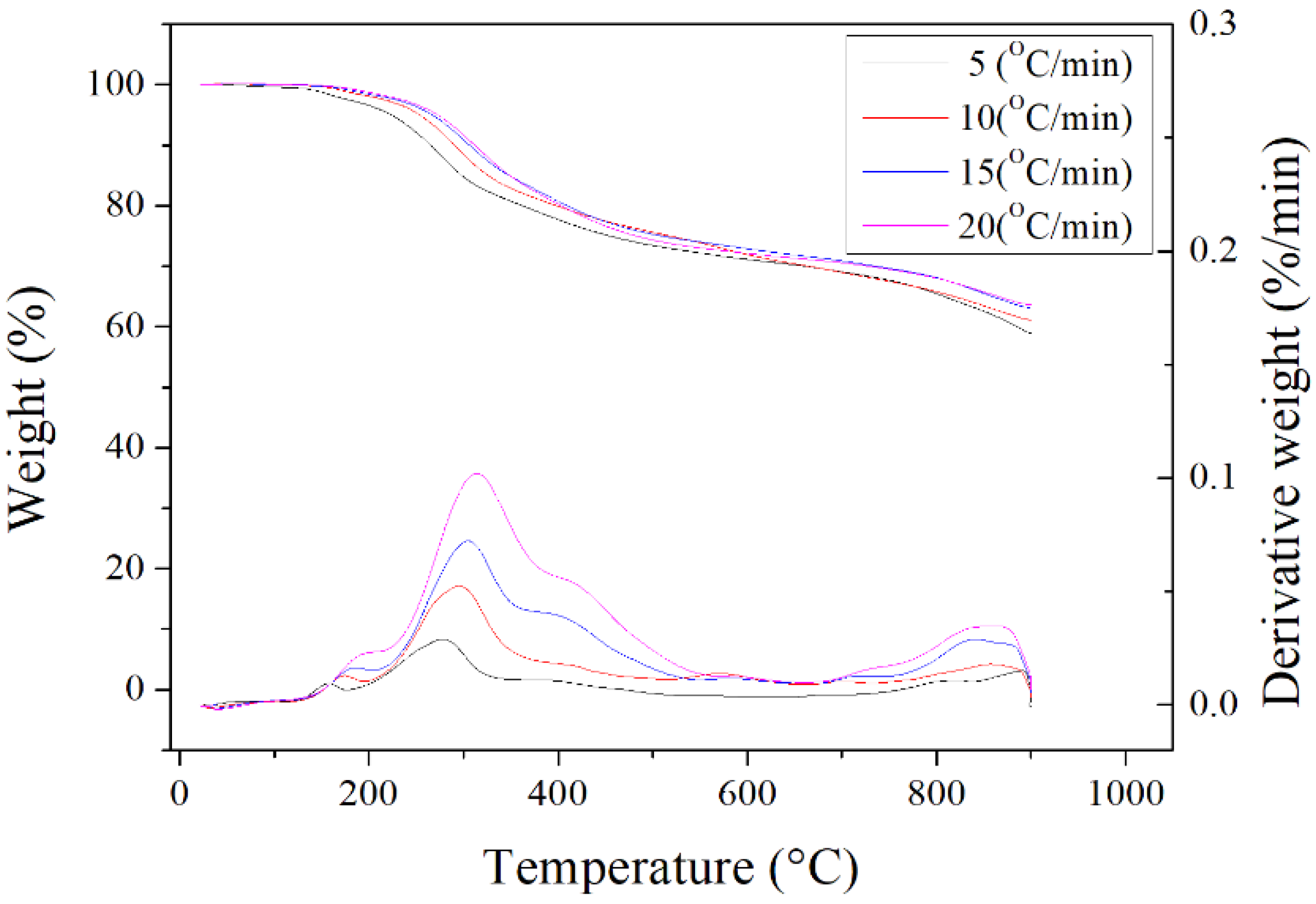
Figure 2 depicted the results of thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG) for the dried oil-containing sludge in the temperature range of 25°C to 900°C at four heating rates (5, 10, 15 and 20°C/min) under the inert nitrogen atmosphere. These curves have been normalized by the initial sample mass. Obviously, the significant weight loss of about 25-30% was observed in the temperature range of 200°C to 500°C. It can be attributed to the evaporation loss of residual heavy oil fractions still contained in the dried sludge. This value was close to the data on proximate analysis in
Table 1 (9.58 wt%/(1 - 0.6544) = 27.7 wt%). The weight loss at high temperatures (> 900°C) may be caused by the evaporation loss of inorganic fractions (or minerals) with low melting points.
3.2. Characterization of Resulting Carbon Products
The data on the pore properties of the resulting carbon products, including BET surface area (S
BET), micropore surface area (S
micro), external surface area (S
ext), total pore volume (V
t), micropore volume (V
micro) and average pore diameter (D
av), were summarized in
Table 2. Using BET surface area values as a porosity criterion, the main results can be concluded as follows:
The H3PO4 impregnation ratio had a positive impact on the pore properties of resulting carbon products. The BET surface area indicated a slight increasing trend with impregnation ratio increased. As compared to the carbon product without impregnation by H3PO4 (i.e., C-0-SL-600-30), this enhancement of pore development seemed to be not significant, thus showing that its BET surface area was slightly higher than that of carbon product (i.e., C-05-SL-600-30).
The pore properties of carbon products can be enhanced by post-washing by water and dilute acid solution; that is, 50.799 and 49.462 m
2/g (C-0-SL-600-30 and C-05-SL-600-30, respectively), 58.980 and 57.832 m
2/g (C-0-SL-600-30-W and C-05-SL-600-30-W, respectively), 63.132 and 69.132 m
2/g (C-0-SL-600-30-A and C-05-SL-600-30-A, respectively). On the other hand, the enhancement by acid-washing showed better pore properties than those by water-washing possibly due to more dissolution (leaching-off) of inorganic minerals and more pore developed and formed [
36,
37,
38].
As compared to the previous studies on the production of carbon products from oil-containing sludge [
10,
11], the results of H
3PO
4 chemical activation at 600°C in this work were superior to those by CO
2 physical activation at 850°C for holding same time (30 min). The maximal BET surface area value (69.13 m
2/g, seen in
Table 2) for the carbon product (C-05-SL-600-30-A) was larger than that (40.53 m
2/g) by CO
2 physical activation and post-acid washing [
10].
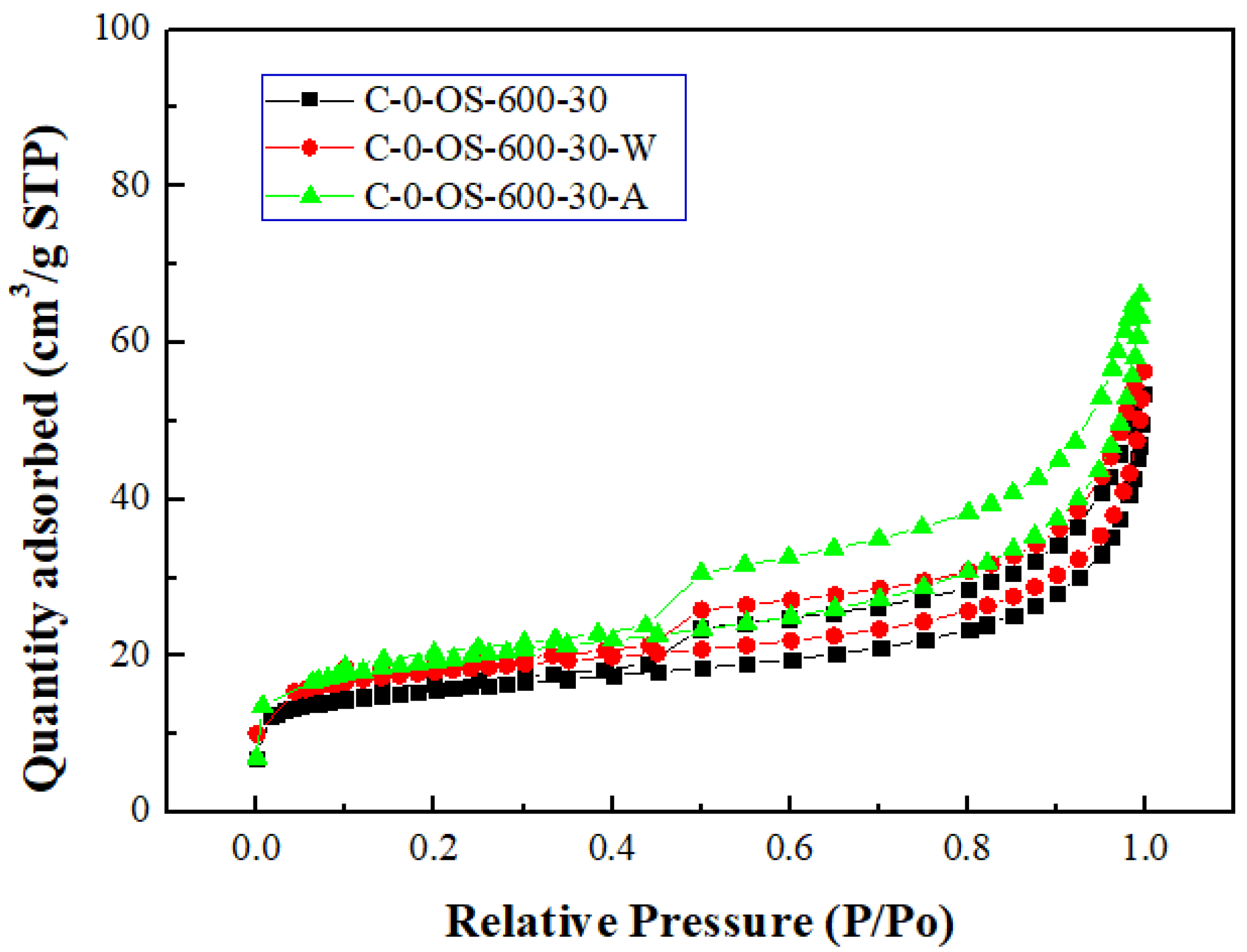
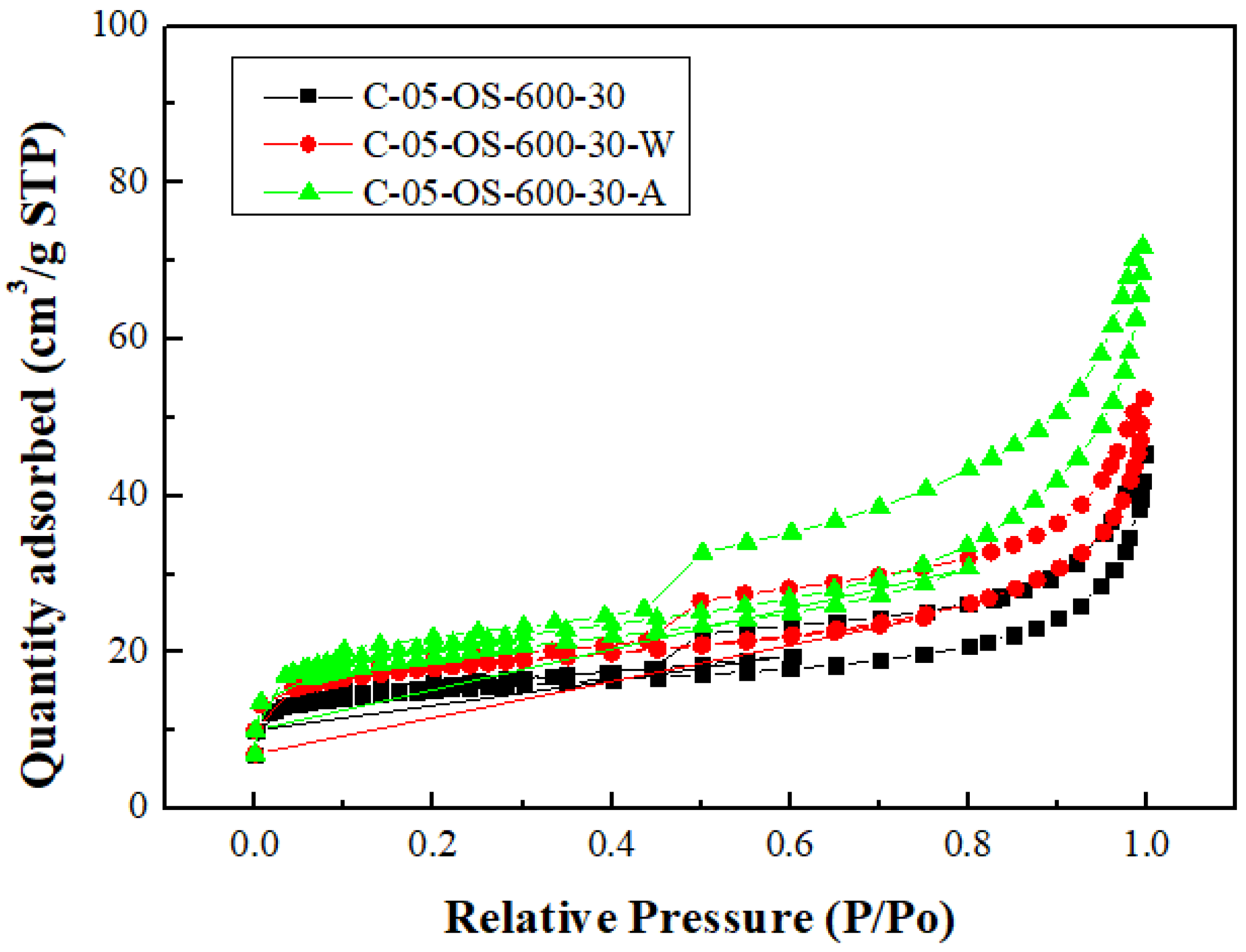
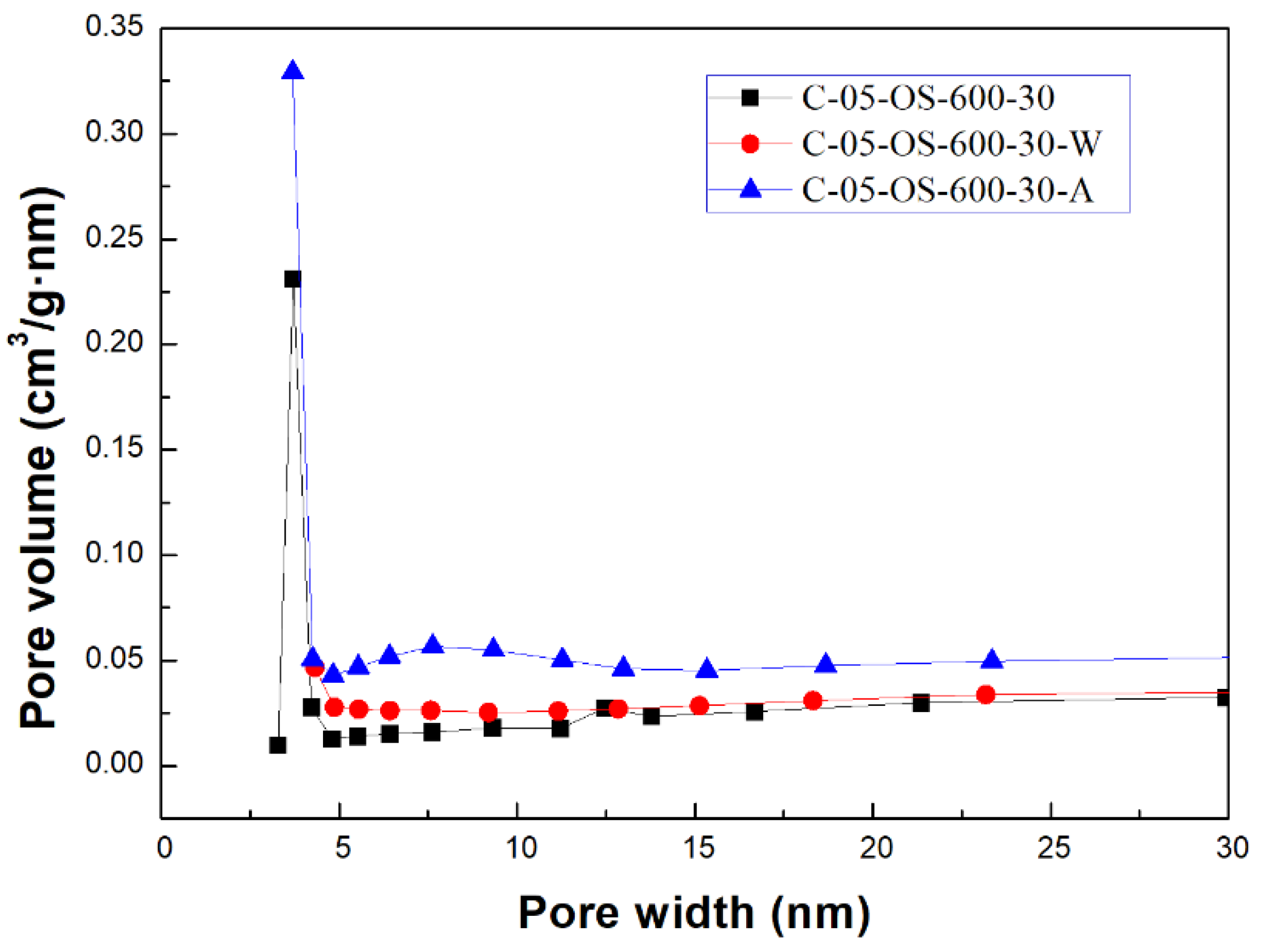
Figure 3 and
Figure 4 showed the N
2 adsorption & desorption isotherms and the pore size distributions for the carbon products without H
3PO
4 impregnation and with H
3PO
4 impregnation (using the lowest mass ratio of 0.5), respectively. Based on the classification of the International Union of Pure and Applied Chemistry (IUPAC) [
32,
33], the isotherms featured the hysteresis loop and the type IV, indicating mesoporous structures. As seen in
Figure 4, the mesopore size range concentrated on 3.0 to 4.5 nm, which was obtained by using the data on the branch of desorption isotherms and the Barrett-Joyner-Halenda (BJH) model [
33].
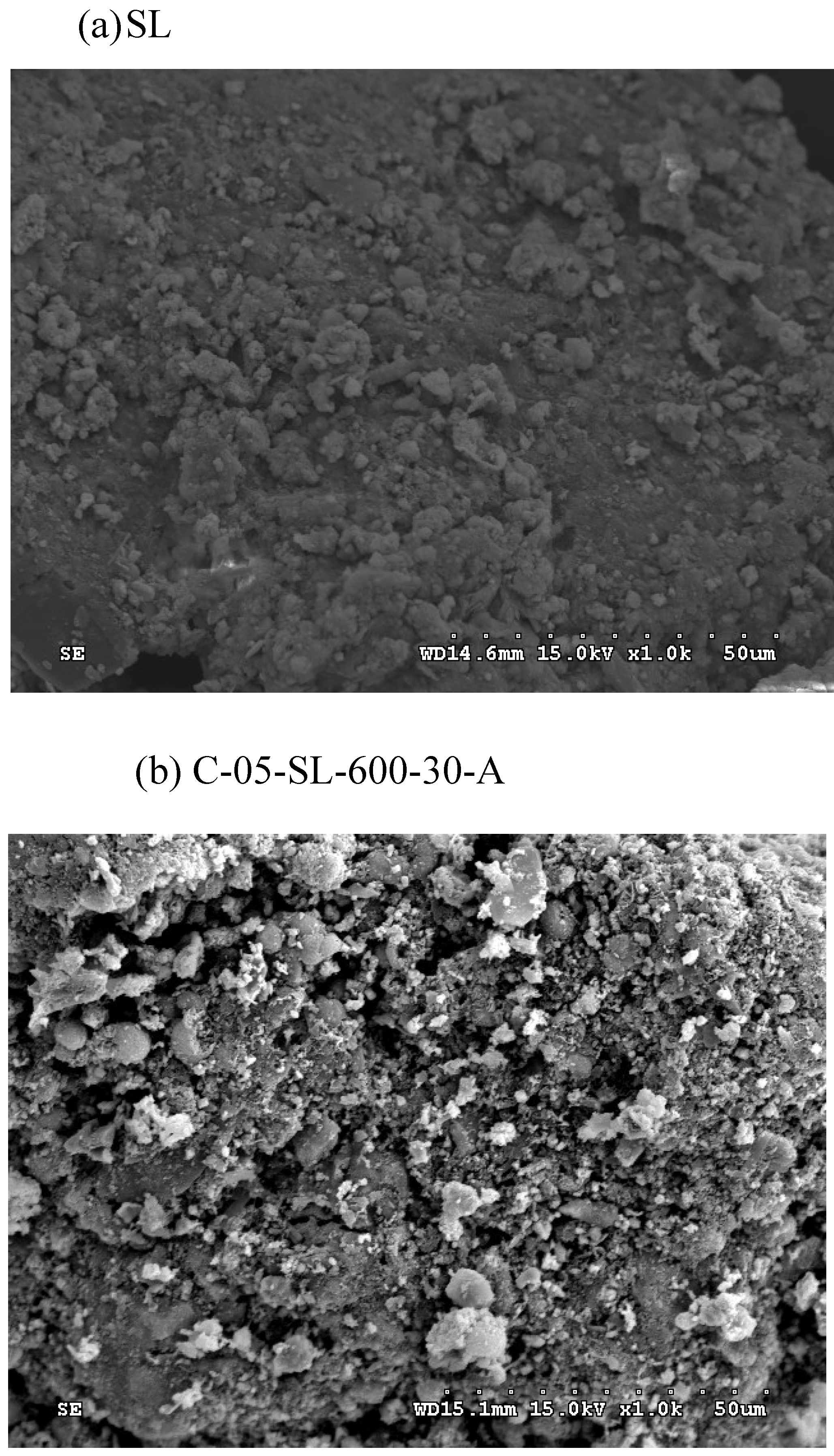
The SEM images of the dried oil-containing sludge (SL) and the optimal carbon product (i.e., C-05-SL-600-30-A) were shown in
Figure 5. The former seemed to be non-porous and heterogeneous (
Figure 5(a)) due to its low BET surface area (3.795 m
2/g). By contrast, the carbon product (
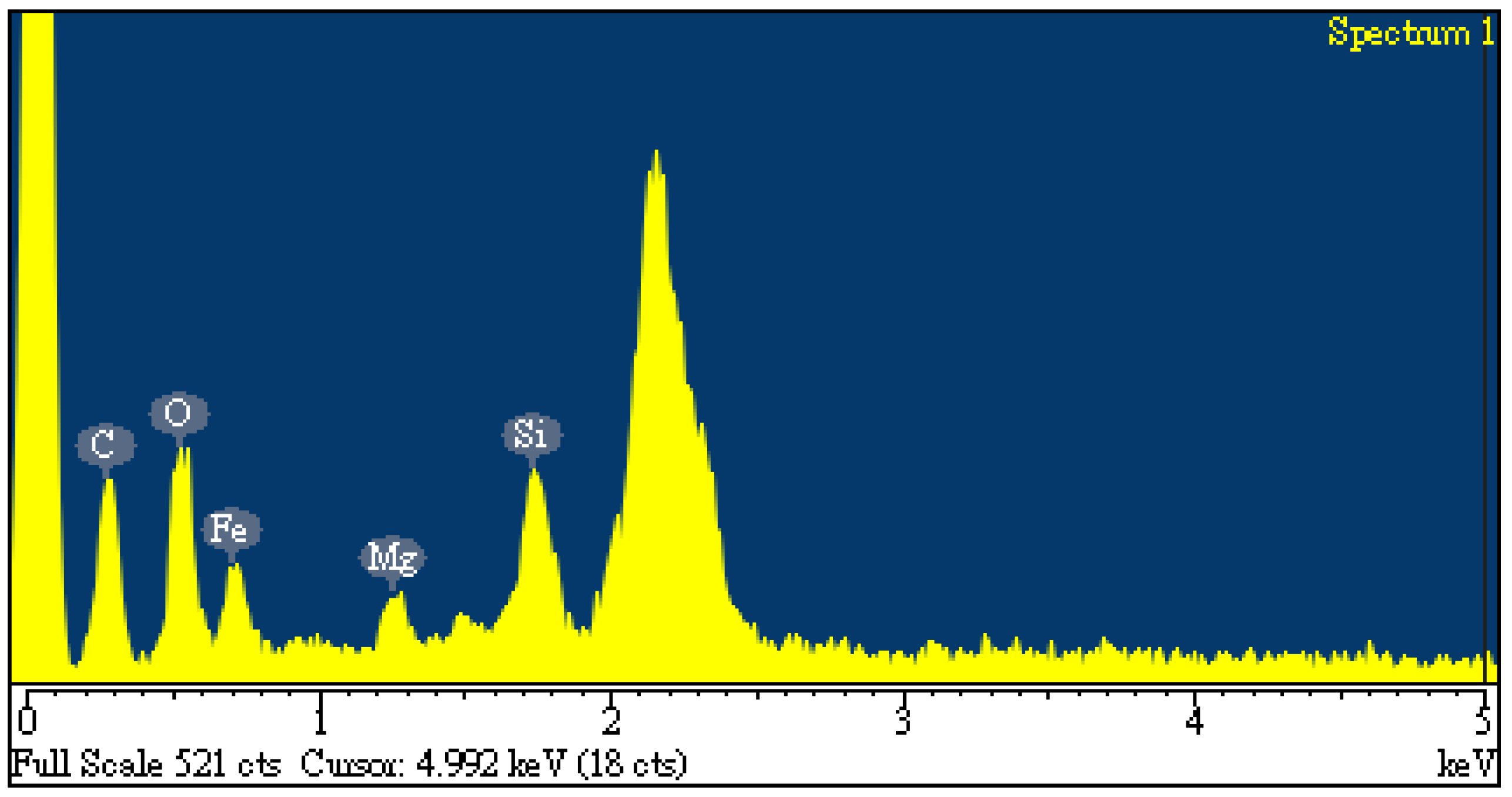
Figure 5(b)) indicated a porous texture on the surface, thus having higher BET surface area. In addition, the elemental compositions on the surface of the carbon products (i.e., C-05-SL-600-30-A) can be seen in
Figure 6. Herein, the significant peak on the far right is a background gold (Au) for providing a conductive film on the surface. The contents of carbon, oxygen, silicon, magnesium and iron were 24.22, 15.84, 5.04, 2.27 and 52.64 wt%, respectively. These results were consistent with the previous study [
10]. In comparison with the EDS data on the starting sludge (Sec. 3.1), the contents of carbon, silicon and magnesium were slightly reduced, the iron content was concentrated by increasing from 19.45 wt% to 52.64 wt%. As mentioned above, the carbon product with having mesoporous and magnetic properties may be used as an efficient adsorbents or catalyst supports. When it was disposed of or exhausted, it can be easily collected or separated by external magnets applied [
39,
40].
4. Conclusions
The waste oil recycling plants still generated large amounts of residual sludge with containing oils and inorganic minerals (e.g., iron oxides), which was used to produce porous carbon products by phosphoric acid (H3PO4) activation method in the present study. From the data on the pore properties, it was found that the H3PO4 impregnation ratio had a positive impact on the pore development or formation. The higher impregnation ratios, the larger pore properties. As predicted, the enhancement in the pore properties of resulting carbon products by acid-washing was superior to those by water-washing and without post-washing. It could be ascribed to the leaching removal of residual H3PO4 and inherent minerals. The carbon product with a maximal BET surface area (69.132 m2/g) can be produced at a lower temperature (600°C). More significantly, the resulting carbon product was a mesoporous and magnetic material, suggesting that it can be used as an efficient adsorbents or catalyst supports due to its simple recovery (or separation) if exhausted.
Author Contributions
Conceptualization, W.-T.T.; data collection, L.-A.K. and C.-H.T.; data analysis, L.-A.K. and Y.-C.Y.; writing-original draft preparation, W.-T.T.; writing—review and editing, W.-T.T.; supervision, W.-T.T.; sources, C.-C.P.; funding acquisition, C.-C.P.
Funding
This industrial/academic research received the funding from National Science and Technology Council and the De Jing Enterprise Co. (Pingtung, Taiwan) under the Project No. NSTC 113-2622-E-020-007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Sincere appreciation was also expressed to acknowledge the Instrumentation Center at National Pingtung University of Science and Technology for their assistances in scanning electron microscope - energy dispersive X-ray spectroscopy (SEM-EDS) observations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maceiras, R.; Alfonsín, V.; Morales, F.J. Recycling of waste engine oil for diesel production. Waste Manag. 2017, 60, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, B.; Wang, W.; Zhao, G.; Guo, P.; Ma, Q. Effect of waste engine oil and waste cooking oil on performance improvement of aged asphalt. Appl. Sci. 2019, 9, 1767. [Google Scholar] [CrossRef]
- Mishra, A.; Siddiqi, H.; Kumari, U.; Behera, I.D.; Mukherjee, S.; Meikap, B.C. Pyrolysis of waste lubricating oil/waste motor oil to generate high-grade fuel oil: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 150, 111446. [Google Scholar] [CrossRef]
- Hui, K.; Tang, J.; Lu, H.; Xi, B.; Qu, C.; Li, J. Status and prospect of oil recovery from oily sludge: A review. Arab. J. Chem. 2020, 13, 6523–6543. [Google Scholar] [CrossRef]
- Di, X.; Pan, H.; Li, D.; Hu, H.; Hu, Z.; Yan, Y. (2021) Thermochemical recycling of oily sludge by catalytic pyrolysis: A review. Scanning 2021, 2021, 1131858. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Zhang, D.; Yang, C. A review of the application of different treatment processes for oily sludge. Environ. Sci. Pollut. Res. 2021, 28, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Niu, A.; Sun, X.; Lin, C. Trend in research on characterization, environmental impacts and treatment of oily sludge: A systematic review. Molecules 2022, 27, 7795. [Google Scholar] [CrossRef]
- Chu, Z.; Li, Y.; Zhang, C.; Fang, Y.; Zhao, J. A review on resource utilization of oil sludge based on pyrolysis and gasification. J. Environ. Chem. Eng. 2023, 11(3), 109692. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Liang, H.; Chen, B.; Liu, Y.; Ma, Z.; Wang, Z. Characterization and treatment of oily sludge: A systematic review. Environ. Pollut. 2024, 344, 123245. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q. Preparation and characterization of porous carbon composites from oil-containing sludge by a pyrolysis-activation process. Processes 2022, 10, 834. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q.; Tsai, C.H.; Shen, Y.H. Production of mesoporous magnetic carbon materials from oily sludge by combining thermal activation and post-washing. Materials 2022, 15, 5794. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Mirghaffari, N. A preliminary study of the preparation of porous carbon from oil sludge for water treatment by simple pyrolysis or KOH activation. New Carbon Mater. 2015, 30, 310–318. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mirghaffari, N. Optimization and comparison of Cd removal from aqueous solutions using activated and non-activated carbonaceous adsorbents prepared by pyrolysis of oily sludge. Water Air Soil Pollut. 2015, 226, 2237. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.; Liu, Z.; Wang, Z.; Li, B.; Zhang, D. Hierarchical porous carbon from hazardous waste oily sludge for all-solid-state flexible supercapacitor. Electrochim. Acta 2017, 240, 43–52. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.L.; Huang, Q.X.; Ma, Z.Y.; Chi, Y.; Yan, J.H. Production and characterization of high quality activated carbon from oily sludge. Fuel Process. Technol. 2017, 162, 13–19. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Guo, L.; Han, D.; Li, B.; Gong, Z. Manganese oxide/hierarchical porous carbon nanocomposite from oily sludge for high-performance asymmetric supercapacitors. Electrochim. Acta 2018, 265, 71–77. [Google Scholar] [CrossRef]
- Gong, Z.; Meng, F.; Wang, Z.; Fang, P.; Li, X.; Liu, L.; Zhang, H. Study on preparation of an oil sludge-based carbon material and its adsorption of CO2- effect of the blending ratio of oil sludge pyrolysis char to KOH and urea. Energy Fuels 2019, 33, 10056–10065. [Google Scholar] [CrossRef]
- Li, X.; Han, D.; Zhang, M.; Li, B.; Wang, Z.; Gong, Z.; Liu, P.; Zhang, Y.; Yang, X. Removal of toxic dyes from aqueous solution using new activated carbon materials developed from oil sludge waste. Colloids Surf. A 2019, 578, 123505. [Google Scholar] [CrossRef]
- Meng, F.; Gong, Z.; Wang, Z.; Fang, P.; Li, X. Study on a nitrogen-doped porous carbon from oil sludge for CO2 adsorption. Fuel 2019, 251, 562–571. [Google Scholar] [CrossRef]
- Mojoudi, N.; Mirghaffari, N.; Soleimani, M.; Shariatmadari, H.; Belver, C.; Bedia, J. Phenol adsorption on high microporous activated carbons prepared from oily sludge - equilibrium, kinetic and thermodynamic studies. Sci. Rep. 2019, 9, 19352. [Google Scholar] [CrossRef] [PubMed]
- Mojoudi, N.; Soleimani, M.; Mirghaffari, N.; Belver, C.; Bedia, J. Removal of phenol and phosphate from aqueous solutions using activated carbons prepared from oily sludge through physical and chemical activation. Water Sci. Technol. 2019, 80, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, D.; Gong, Z.; Wang, Z. Nest-Like MnO2 nanowire/hierarchical porous carbon composite for high-performance supercapacitor from oily sludge. Nanomaterials 2021, 11, 2715. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, J.; McGill, W.B.; Whitcombe, T.W. Impact of pyrolysis temperature and activation on oily sludge-derived char for Pb(II) and Cd(II) removal from aqueous solution. Environ. Sci. Pollut. Res. 2021, 28, 5532–5547. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, B.; Han, D.; Gong, Z.; Li, X. Fe2O3/porous carbon composite derived from oily sludge waste as an advanced anode material for supercapacitor application. Nanomaterials 2022, 12, 3819. [Google Scholar] [CrossRef]
- Amari, A.; Noreen, A.; Osman, H.; Sammen, S.S.; Al-Ansari, N.; Salman, H.M. Investigation of the viable role of oil sludge-derived activated carbon for oily wastewater remediation. Front. Environ. Sci. 2023, 11, 1138308. [Google Scholar] [CrossRef]
- Cheng, H.; Peng, L.; Liu, J.; Ma, C.; Hao, F.; Zheng, B.; Yang, J. Adsorption separation of various polar dyes in water by oil sludge-based porous carbon. Appl. Sci. 2024, 14, 7283. [Google Scholar] [CrossRef]
- Jerez, S.; Pedersen, A.; Ventura, M.; Mazzoli, L.; Pariente, M.I.; Titirici, M.; Melero, J.A.; Barrio, J. Fe-N doped carbon materials from oily sludge as electrocatalysts for alkaline oxygen reduction reaction. Electrochim. Acta 2024, 483, 144045. [Google Scholar] [CrossRef]
- Long, J.; He, P.; Przystupa, K.; Wang, Y.; Kochan, O. Preparation of oily sludge-derived activated carbon and its adsorption performance for tetracycline hydrochloride. Molecules 2024, 29, 769. [Google Scholar] [CrossRef] [PubMed]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, 2006. [Google Scholar]
- Tsai, W.T.; Huang, P.C. Characterization of acid-leaching cocoa pod husk (CPH) and its resulting activated carbon. Biomass Convers. Biorefin. 2018, 8, 521–528. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q.; Tsai, C.H.; Chen, W.S.; Chang, Y.T. Enhancing the pore properties and adsorption performance of cocoa pod husk (CPH)-derived biochars via post-acid treatment. Processes 2020, 8, 144. [Google Scholar] [CrossRef]
- Condon, J.B. Surface Area and Porosity Determinations by Physisorption: Measurements and Theory; Elsevier: Amsterdam, 2006. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht (the Netherlands), 2006. [Google Scholar]
- Basu, P. ; Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Academic Press: San Diego (CA, USA), 2013. [Google Scholar]
- FACTS AND FIGURES - Heat Values of Various Fuels. Available online: https://world-nuclear.org/information-library/facts-and-figures/heat-values-of-various-fuels (accessed on 14 November 2024).
- Tsai, W.T.; Huang, P.C. Characterization of acid-leaching cocoa pod husk (CPH) and its resulting activated carbon. Biomass Convers. Biorefin. 2018, 8, 521–528. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q.; Tsai, C.H.; Chen, W.S.; Chang, Y.T. Enhancing the pore properties and adsorption performance of cocoa pod husk (CPH)-derived biochars via post-acid treatment. Processes 2020, 8, 144. [Google Scholar] [CrossRef]
- Tsai, C.H.; Tsai, W.T.; Kuo, L.A. Effect of post-washing on textural characteristics of carbon materials derived from pineapple peel biomass. Materials 2023, 16, 7529. [Google Scholar] [CrossRef] [PubMed]
- Yi, I.G.; Kang, J.K.; Lee, S.C.; Lee, C.G.; Kim, S.B. Synthesis of an oxidized mesoporous carbon-based magnetic composite and its application for heavy metal removal from aqueous solutions. Microporous Mesoporous Mater. 2019, 279, 45–52. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Fu, J.; Ao, W.; Siyal, A.A.; Zhou, C.; Liu, C.; Dai, J.; Yu, M.; Zhang, Y.; Jin, Y.; Yuan, Y.; Zhang, C. Iron-biochar production from oily sludge pyrolysis and its application for organic dyes removal. Chemosphere 2022, 301, 134803. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).