1. Introduction
Saliva plays a critical role in both oral and systemic health, acting as a fundamental component in maintaining oral hygiene, facilitating digestion, and serving as a diagnostic tool for various diseases. It is composed primarily of water, but also contains important organic and inorganic molecules that contribute to its protective functions, including buffering acids, remineralizing tooth enamel, and providing antimicrobial action [
1]. The production of saliva is influenced by daily rhythms, with peak secretion around meals and minimal flow during sleep, highlighting its dynamic nature in response to physiological needs. Additionally, saliva's diagnostic potential is underscored by its ability to reflect serum biomarkers, making it a valuable non-invasive medium for monitoring health conditions ranging from cardiovascular diseases to endocrine functions and infectious diseases [
2]. Its ease of collection and the correlation of many salivary parameters with serum levels offer a promising avenue for the non-invasive diagnosis and monitoring of systemic health [
3].
The study and analysis of saliva's biochemical components, including proteins, enzymes, hormones, and other critical biomolecules, providing a window into the health status of an individual [
4]. Among these components, mucins, immunoglobulins, cystatins, defensins, histatins, lactoferrin, agglutinin, lysozyme, and lactoperoxidase play pivotal roles in oral health by facilitating processes such as digestion, protection against caries, and combating oral infections [
5]. Each salivary gland—Parotid, Submandibular, and Sublingual—contributes uniquely to the saliva composition, producing a mixture of serous and mucinous secretions essential for maintaining oral tissues and initiating the digestion process. Furthermore, saliva's diagnostic potential extends beyond oral health, offering insights into systemic diseases through the transfer of biomolecules from blood to saliva via ultrafiltration and diffusion processes [
6]. This capability positions saliva as a non-invasive, accessible diagnostic tool for monitoring various health conditions, including cardiovascular diseases, endocrine functions, and infectious diseases, thereby underscoring the significance of understanding saliva's biochemistry and its relationship with physical health [
7].
The intricate relationship between various components within saliva, including enzymes like amylase and catalase, reflects the body's physiological and pathological states, influencing physical fitness and systemic health [
8]. The activities of these enzymes not only play a critical role in the oral cavity's defence mechanisms but also serve as potential indicators of broader health issues [
9]. For instance, variations in amylase activity have been linked to stress responses, whereas alterations in catalase activity could indicate oxidative stress levels, providing insights into the body's antioxidative capacity [
10]. This complex interplay underscores the necessity of a holistic approach to health monitoring, where understanding the biochemical properties of saliva and their connection to physical health can lead to innovative strategies for early diagnosis and intervention in various diseases. By analysing these relationships, researchers can better identify risk factors and develop targeted interventions aimed at improving physical fitness and mitigating disease risks, highlighting the potential of salivary analysis as a tool for comprehensive health assessment.
Given the foundational role of saliva in both oral and systemic health, as well as its diagnostic potential, this article proposes a focused study on the relationship between salivary biochemical parameters—specifically, salivary flow, protein levels, and the activities of enzymes such as amylase and catalase—and their collective impact on physical health. By delving into how these salivary components interact and their association with health outcomes, the objective is to harness saliva's diagnostic capabilities to develop simpler, more effective methods for health assessment and monitoring. This approach not only aims to enhance our understanding of saliva's comprehensive role in health and disease but also seeks to leverage its accessibility and non-invasive collection methods as a means to facilitate early detection, prevention, and management of various conditions. Through this study, we aim to elucidate the complex interconnections within salivary biochemistry, offering a new avenue for preventive health strategies and contributing to the broader field of personalized medicine.
2. Materials and Methods
2.1. Participants
A total of 31 participants were enrolled in the study. The mean age and body mass index (BMI) of people were 51.3 (10.4) years and 31.5 (7.9) kg/m2, respectively. The convenience sample was recruited from the Lusitania family health Unit in Évora (Portugal) and the University of Évora until April 2021. All procedures were conducted following the Helsinki Declaration (revised in Brazil, 2013) and approved by the University research ethic committee (GD/44902/2019).
2.2. Training Procedure
Participants performed a 1 h training intervention consisting of a mobility warm up (Rate of Perceived Exertion (RPE) = 3) followed by a strength training session (RPE = 7) composed of 3 bodyweight exercises (adapted push-ups, sit-to-stand, and glute bridge) and 1 banded exercise (standing one-hand row). Performing 3 sets of 10 reps for each exercise with 1 min of rest between each set and exercise [
11].
2.3. Salivary Biomarkers
Unstimulated whole saliva was collected at rest and after exercise for each participant, by direct draining into an ice-cold collection tube (pre-weighted), for 3 min. Subjects refrained from eating and drinking for at least 1 h before collection. After saliva collection, tubes with the samples were weighted (for saliva flux evaluation, mL/min), centrifuged at 1.500×
g, for 10 min, to remove food and cell debris, and supernatant were stored at -20 °C until analysis. Preceding analysis, saliva samples were thawed on ice and centrifuged for 30 min, 4 °C, 13,000×
g, for removal of mucinous material [
12]. Supernatant total protein concentration was assayed using the Bradford method [
13].
Dinitrosalicylic acid assay was used for measuring the starch-hydrolysing activity of salivary α-amylase [
14], minimized for 96 wells microplates. The reaction mixture consisted of 1% starch solution and saliva sample (diluted to 10 µg protein/ml protein in 20 mM phosphate buffer (pH 7.0)). After incubation at 37 °C for 20 min, the reaction was stopped by the addition of the DNS reagent. Samples were heated to 90 °C, for 30 min. Further, sodium and potassium tartrate (40%) were added to samples. Absorbance was measured at 530 nm. The absorbance values were then converted to glucose equivalent using a standard curve (3-18 mM). The results were expressed as µmol glucose/min/L saliva or as µmol glucose/min/mg salivary protein (specific activity).
Catalase activity was determined following the consumption of H
2O
2 at 240 nm [
15], measuring absorbance every 30 seconds for 6 minutes. Reactional mixture consisted in salivary samples diluted in 10 mM potassium phosphate buffer (pH 7.0) and 0.2% H
2O
2. Enzyme activity was expressed in mmol of H
2O
2 consumed per minute per L of saliva or in µmol of H
2O
2/min/mg of salivary protein (specific activity).
2.4. Statistical Analysis
All statistical analyses and graphics were conducted using Origin Pro 2017 SR2 (Origin Lab Corporation, USA). Linear Pearson correlation analysis was performed, and correlation coefficients were considered if slops prove to be different from zero by ANOVA (p < 0.05).
3. Results
The relationships between salivary flow parameters, protein concentration and salivary amylase and catalase enzymatic activities were studied.
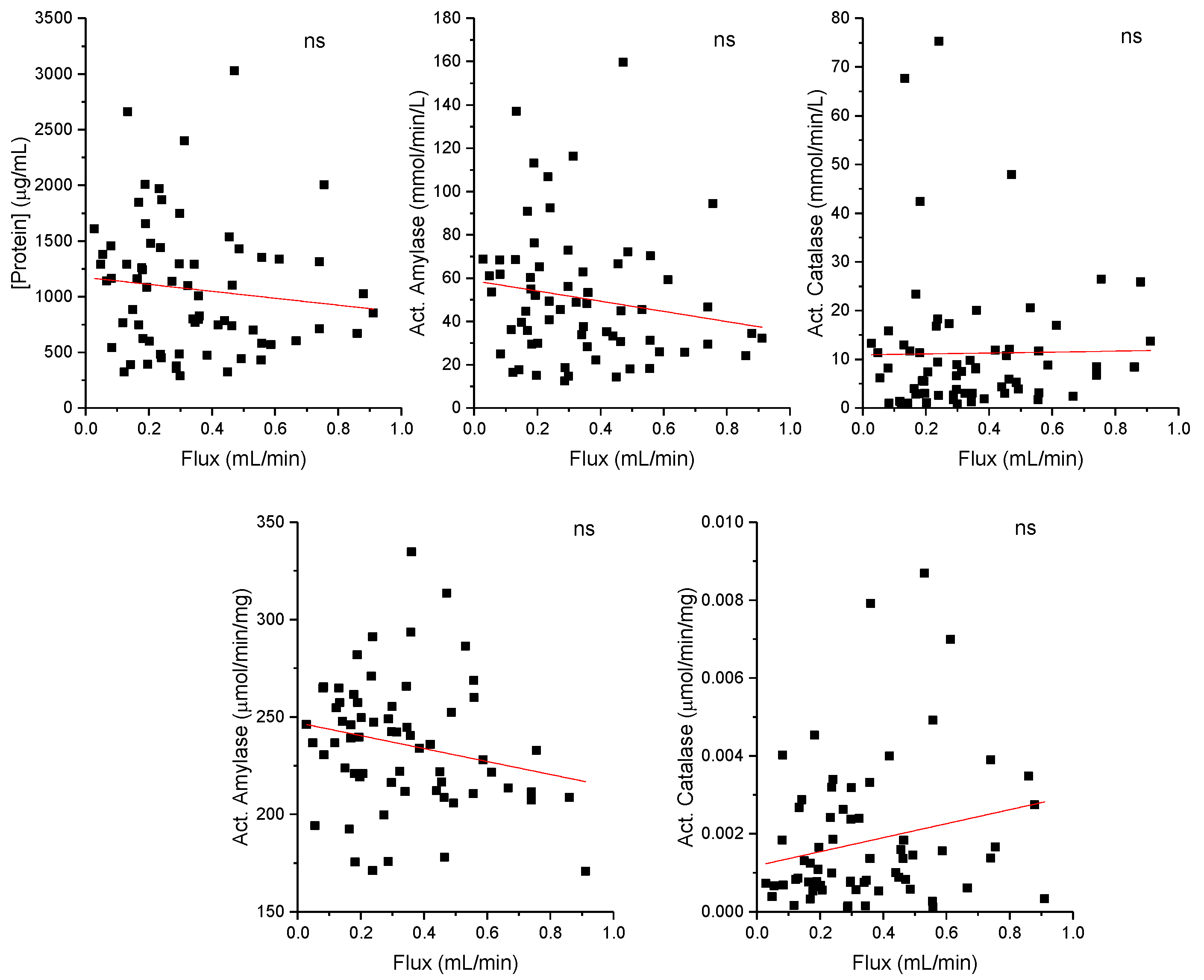
To evaluate the possibility of salivary flux to influence other salivary parameters, correlation plots are shown in
Figure 1. Enzymatic activities of catalase and amylase are expressed in function of saliva volume (upper panel) or in function of the total salivary protein concentration (lower panel).
The scatter plot shows individual data points representing different salivary flux and their corresponding concentrations of protein or enzymatic activity levels. The red line represents the trendline of the data, which mathematically describes the relationship between the two variables.
The results show that no correlations were observed, revealing that salivary flow does not influence protein concentration, amylase, or catalase activities. However, a tendency to flux influence negatively protein concentration, as well as amylase activity, can be observed, and inversely, a tendency to positively influence catalase activity.
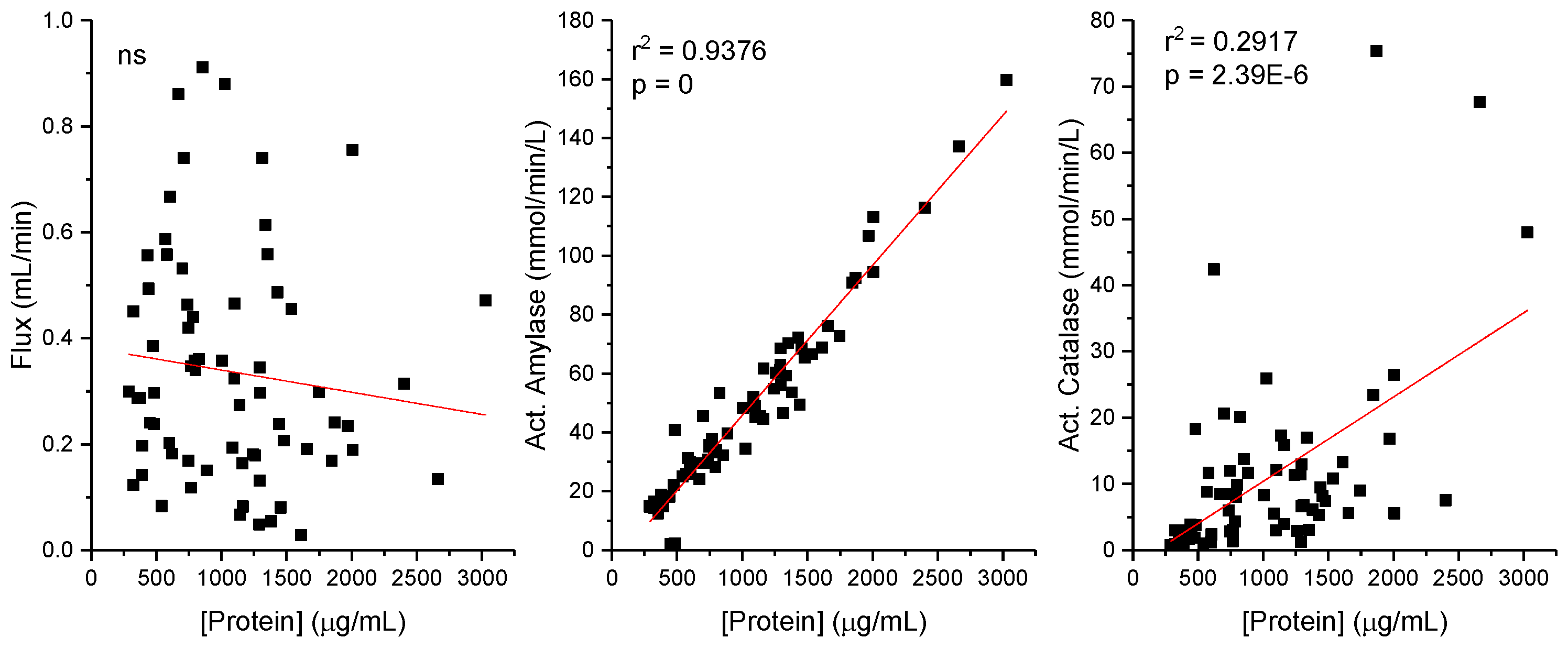
Figure 2 shows the study of the potential influence of total protein concentration (in µg/mL) over the enzymatic activities of amylase and catalase, that in this case must be expressed as mmol/L/min to guarantee the independency of the variables. Salivary flux is not correlated with protein concentration (as seen in
Figure 1), but in both enzymatic activities, positive correlations are observed (y = 0.0509x + 5.1176 and R2 = 0.93764, for amylase activity and (y = 0.01272x -2.34173 and R2 = 0.29169 for catalase activity). In the case of catalase, the correlation is week. However, for amylase activity, a strong linear relationship is observed, where the activity of amylase increases as the protein concentration increases. R
2 value suggests that approximately 93.76% of the variability in amylase activity can be explained by the protein concentration in this sample set.
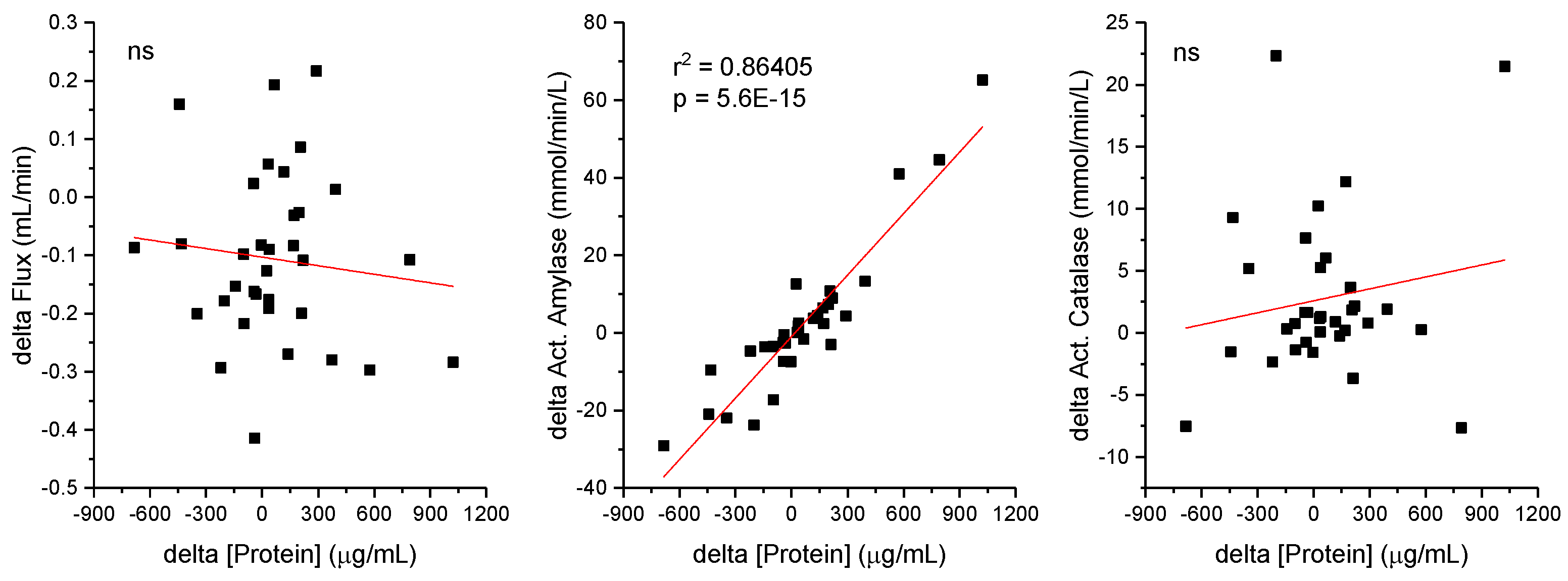
Exploring further the relation between salivary parameters and considering the positive correlations observed between protein concentrations and the amylase and catalase activities,
Figure 3 seek to correlate differences in parameters induced by the physical activity. The differences for individual parameters were calculates (delta = post – pre), and scatter plot shows individual data points representing the delta for concentrations of protein and their corresponding differences in the other parameters (flux, amylase, and catalase activity). A positive correlation between protein concentration deltas and amylase activity deltas (Act amylase in mmol/L/min) was observed. The equation of the line (y = 0.0529x + 0.9363) and the coefficient of determination (R
2 = 0.864) are displayed, indicating a strong linear relationship where the activity of amylase increases as the protein concentration increases. The R
2 value suggests that approximately 86.4% of the variability in amylase activity changes exercise-induced be explained by the protein concentration changes exercise-induced in this sample set. Although a positive correlation was observed between protein concentration and amylase activity (week, but statistically significative), the differenced in the variables induced by the physical activity are no correlated. In addition to the metabolites actively secreted into this fluid, contributing to its function in the digestive processes and maintaining oral hygiene.
4. Discussion
Saliva plays an indispensable role in systemic health, acting as a diagnostic tool for a wide array of diseases [
1]. In addition to the metabolites actively secreted into this fluid, contributing to its function in the digestive processes and maintaining oral hygiene, its diagnostic potential is further underscored by the transfer of biomolecules from blood to saliva through ultrafiltration and diffusion processes [
6], thereby reflecting the body's physiological and pathological states and influencing physical fitness and systemic health [
8]. Consequently, the aim of this study was to elucidate the intricate interconnections within salivary biochemistry, yielding profound insights into the biochemical dynamics of saliva.
Although there is a tendency for salivary samples with lower flow to have a higher concentration of protein, salivary flow and total protein concentration in saliva are not correlated. This may be a consequence of the fact that the mechanisms that control the flow (greater or lesser amount of water in the saliva) and protein secretion (such as amylase, for example) are regulated by different pathways. Typically, the parasympathetic nervous system regulates water volume in saliva and the sympathetic nervous system regulates secretion [
16].
Amylase and catalase enzymatic activities are also not correlated with salivary flow. However, the trends shown between the two enzymes are reversed. Amylase activity follows the same trend as protein concentration, while catalase follows the opposite trend. The explanation may lie in the fact that amylase is a protein actively secreted into saliva in response to stimuli (namely adrenergic), constituting the majority protein in saliva [
17], while catalase is an intracellular enzyme, which arises in saliva as a result of the passage of elements from the blood to saliva, so a greater flow can facilitate this transference.
Regarding the relationships between the variables and the salivary protein concentration, a pronounced positive correlation between protein concentration and amylase activity in saliva samples under basal conditions was found, as evidenced by a high coefficient of correlation (R
2 = 0.9376). This indicates that approximately 94% of the variability in amylase activity can be attributed to fluctuations in protein concentration. The equation of the trendline, y = 0.0509x + 51176, quantifies this relationship, suggesting a consistent escalation in amylase activity with increasing protein concentrations. Subsequently, examining the dynamic nature of salivary biochemistry in response to physical activity, it was demonstrated that changes in protein concentration and amylase activity exercise-induced are still strong correlated. The trendline equation, y = 0.0529x + 0.9363, with an R
2 value of 0.864, indicates a robust yet slightly less pronounced correlation compared to resting conditions. This variance intimates that physical activity modulates salivary biochemistry, potentially through mechanisms tied to hydration status, blood flow, and hormonal fluctuations, thereby affecting protein concentration and enzyme activity [
18]. The distinct correlation observed pre- and post-exercise elucidates the adaptability of salivary components to physiological alterations [
19], shedding light on the body's acute responses to stressors such as physical exertion.
The biological implications of these discoveries are multifaceted. Amylase, a pivotal enzyme in starch breakdown [
20], has been implicated in diverse physiological and stress-induced responses [
21,
22,
23]. The observed correlation may reflect a broader systemic reaction, wherein protein levels in saliva—modulated by nutritional, metabolic, and stress-related factors—directly influence enzymatic activity [
24]. This relationship holds the potential to serve as a biomarker for specific conditions or stress levels, offering a non-invasive portal into systemic health. Moreover, the correlations identified between protein concentration and amylase activity in saliva intimate that saliva could mirror not merely systemic health but also the body's responses to various stimuli, encompassing physical, emotional, or psychological stress. This premise suggests that amylase, as a stress-responsive enzyme [
25], might offer indirect insights into the sympathetic nervous system's activity, warranting further exploration into these relationships to unveil the complex interplay between physiological stressors and biochemical markers. Therefore, the robust correlations delineated in these findings underscore the potential of salivary analysis within clinical and research domains. By quantifying the relationship between protein concentration and amylase activity, this study augments the growing corpus of evidence advocating for the utilization of saliva as a diagnostic modality. The sensitivity of saliva to both resting and altered physiological states advocates for its utility in the non-invasive monitoring of health, stress responses, and potentially the efficacy of interventions aimed at ameliorating systemic health [
26]. These findings could refine the precision of extant diagnostic procedures by furnishing a more encompassing view of an individual's biochemical status, thereby facilitating the early detection of conditions that alter protein concentrations or enzymatic activities in saliva. Moreover, the discovery of these correlations prompts an array of future research directions. Longitudinal studies could explore how the correlations between salivary protein concentration and amylase activity evolve over time with chronic stress or disease progression. Comparative studies across diverse populations could ascertain whether these correlations are universally applicable or are modulated by genetic, environmental, or lifestyle variables. Addressing the challenges of specificity and sensitivity of these biomarkers for health conditions, alongside standardizing saliva collection and analysis methodologies, will be pivotal in actualizing the full potential of salivary analysis in health assessment and monitoring.
The pronounced correlations between protein concentration and amylase activity, evident both at rest and in response to physical activity, underscore the sensitivity of saliva to physiological changes. These insights herald a promising future for saliva's application in health monitoring and disease prevention, advocating for its incorporation into personalized medicine strategies and broader clinical practice. As research in this area progresses, the integration of salivary biomarkers into clinical practice could revolutionize non-invasive diagnostics and pave the way for innovative health monitoring solutions. By continuing to explore and understand the intricate biochemistry of saliva, we move closer to unlocking its full potential as a tool for enhancing human health and well-being.
5. Conclusions
Our research offers a compelling glimpse into the interconnexions between salivary parameters with diagnostic potential. It is worth noting that the high correlation found between protein concentration and amylase activity arise the possibility of using total protein concentration as an equivalent indicator of amylase activity when that is used as an indicator of adrenergic activation. It is a much simpler, inexpensive, and quick evaluation. However, the assessment of amylase activity remains relevant in the evaluation of the functional role of saliva.
Author Contributions
Conceptualization, A.R.-Z., V.J.C.-S., and J.A.P.; methodology, A.R.-Z., J.A.P., and A.C.; software, A.R.-Z., J.F.T.-A. and P.T.C.; validation, V.J.C.-S. and J.A.P.; formal analysis, A.R.-Z. and A.C.; investigation, A.R.-Z., J.F.T.-A., V.J.C.-S. and J.A.P.; data curation, J.F.T.-A., P.T.C. and A.C.; writing—original draft preparation, J.A.P. and A.C.; writing—review and editing, A.R.-Z., J.F.T.-A. and V.J.C.-S.; supervision, V.J.C.-S. and J.A.P.; project administration, J.F.T.-A., V.J.C.-S. and J.A.P.; funding acquisition, J.A.P. and P.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by national funds through the Foundation for Science and Technology, under the project UIDP/04923/2020.
Institutional Review Board Statement
All procedures were conducted following the Helsinki Declaration (revised in Brazil, 2013) and approved by the University of Évora research ethic committee (GD/44902/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Llena-Puy, C. The Rôle of Saliva in Maintaining Oral Health and as an Aid to Diagnosis. Medicina oral, patología oral y cirugía bucal 2006. [Google Scholar]
- Dodds, M.; Roland, S.; Edgar, M.; Thornhill, M. Saliva A Review of Its Role in Maintaining Oral Health and Preventing Dental Disease. BDJ Team 2015, 2(1–8). [Google Scholar] [CrossRef]
- Liao, C.; Chen, X.; Fu, Y. Salivary Analysis: An Emerging Paradigm for Non-invasive Healthcare Diagnosis and Monitoring. Interdisciplinary Medicine 2023, 1(3). [Google Scholar] [CrossRef]
- Mani Sundar, N.; Julius, A.; Valiathan, M.; Periyasamy, T.T.; Hemalatha, V.T. Human Saliva and Its Role in Oral and Systemic Health. Drug Invention Today 2019. [Google Scholar]
- Van’T Hof, W.; Veerman, E.C.I.; Amerongen, A.V.N.; Ligtenberg, A.J.M. Antimicrobial Defense Systems in Saliva. Monogr Oral Sci 2014, 24. [Google Scholar] [CrossRef]
- Pappa, E.; Vougas, K.; Zoidakis, J.; Vastardis, H. Proteomic Advances in Salivary Diagnostics. Biochimica et Biophysica Acta - Proteins and Proteomics. [CrossRef]
- Kumar, P.; Gupta, S.; Das, B.C. Saliva as a Potential Non-Invasive Liquid Biopsy for Early and Easy Diagnosis/Prognosis of Head and Neck Cancer. Transl Oncol 2024, 40, 101827. [Google Scholar] [CrossRef]
- Čižmárová, B.; Tomečková, V.; Hubková, B.; Hurajtová, A.; Ohlasová, J.; Birková, A. Salivary Redox Homeostasis in Human Health and Disease. International Journal of Molecular Sciences 2022. [Google Scholar] [CrossRef]
- Lynge Pedersen, A.M.; Belstrøm, D. The Role of Natural Salivary Defences in Maintaining a Healthy Oral Microbiota. J Dent 2019, 80. [Google Scholar] [CrossRef]
- Thoma, M.V.; Kirschbaum, C.; Wolf, J.M.; Rohleder, N. Acute Stress Responses in Salivary Alpha-Amylase Predict Increases of Plasma Norepinephrine. Biol Psychol 2012, 91. [Google Scholar] [CrossRef]
- Rubio-Zarapuz, A.; Apolo-Arenas, M.D.; Tomas-Carus, P.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J.; Parraca, J.A. Comparative Analysis of Psychophysiological Responses in Fibromyalgia Patients: Evaluating Neuromodulation Alone, Neuromodulation Combined with Virtual Reality, and Exercise Interventions. Medicina (B Aires) 2024, 60(3), 404. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Simões, C.; Rodrigues, L.; Costa, A.R.; Vitorino, R.; Amado, F.; Antunes, C.; do Carmo, I. Changes in the Salivary Protein Profile of Morbidly Obese Women Either Previously Subjected to Bariatric Surgery or Not. J Physiol Biochem 2015, 71(4), 691–702. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rodrigues, L.; Mouta, R.; Costa, A.R.; Pereira, A.; Capela E Silva, F.; Amado, F.; Antunes, C.M.; Lamy, E. Effects of High Fat Diet on Salivary α-Amylase, Serum Parameters and Food Consumption in Rats. Arch Oral Biol 2015, 60(6). [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in Vitro. Methods Enzymol 1984, 105(C). [Google Scholar] [CrossRef]
- Obayashi, K. Salivary Mental Stress Proteins. Clinica Chimica Acta 2013. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Breslin, P.A.S. Salivary Amylase: Digestion and Metabolic Syndrome. Current Diabetes Reports 2016. [Google Scholar] [CrossRef] [PubMed]
- Ntovas, P.; Loumprinis, N.; Maniatakos, P.; Margaritidi, L.; Rahiotis, C. The Effects of Physical Exercise on Saliva Composition: A Comprehensive Review. Dentistry Journal 2022. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Shaalan, A.M. Disease-Induced Changes in Salivary Gland Function and the Composition of Saliva. Journal of Dental Research 2021. [Google Scholar] [CrossRef]
- Butterworth, P.J.; Bajka, B.H.; Edwards, C.H.; Warren, F.J.; Ellis, P.R. Enzyme Kinetic Approach for Mechanistic Insight and Predictions of in Vivo Starch Digestibility and the Glycaemic Index of Foods. Trends in Food Science and Technology 2022. [Google Scholar] [CrossRef]
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int J Behav Med 2020, 27(3). [Google Scholar] [CrossRef] [PubMed]
- Petrakova, L.; Doering, B.K.; Vits, S.; Engler, H.; Rief, W.; Schedlowski, M.; Grigoleit, J.S. Psychosocial Stress Increases Salivary Alpha-Amylase Activity Independently from Plasma Noradrenaline Levels. PLoS One 2015, 10(8). [Google Scholar] [CrossRef] [PubMed]
- Strahler, J.; Fuchs, R.; Nater, U.M.; Klaperski, S. Impact of Physical Fitness on Salivary Stress Markers in Sedentary to Low-Active Young to Middle-Aged Men. Psychoneuroendocrinology 2016, 68. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Carpenter, G.; So, P.W. Salivary Metabolomics: From Diagnostic Biomarker Discovery to Investigating Biological Function. Metabolites 2020. [Google Scholar] [CrossRef] [PubMed]
- Kuras, Y.I.; McInnis, C.M.; Thoma, M.V.; Chen, X.; Hanlin, L.; Gianferante, D.; Rohleder, N. Increased Alpha-Amylase Response to an Acute Psychosocial Stress Challenge in Healthy Adults with Childhood Adversity. Dev Psychobiol 2017, 59(1). [Google Scholar] [CrossRef]
- Szabo, Y.Z.; Slavish, D.C. Measuring Salivary Markers of Inflammation in Health Research: A Review of Methodological Considerations and Best Practices. Psychoneuroendocrinology 2021. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).