Submitted:
12 November 2024
Posted:
13 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Transient Transfection with Synthetic miRNA-486-5p Mimics and Inhibitors
2.3. RNA Extraction from Cells
2.4. Retrotranscription and Real-Time PCR for miRNA Expression
2.5. Retrotranscription and Real-Time PCR assay for Stemness and EMT Genes Expression
2.6. Cell Viability Assay
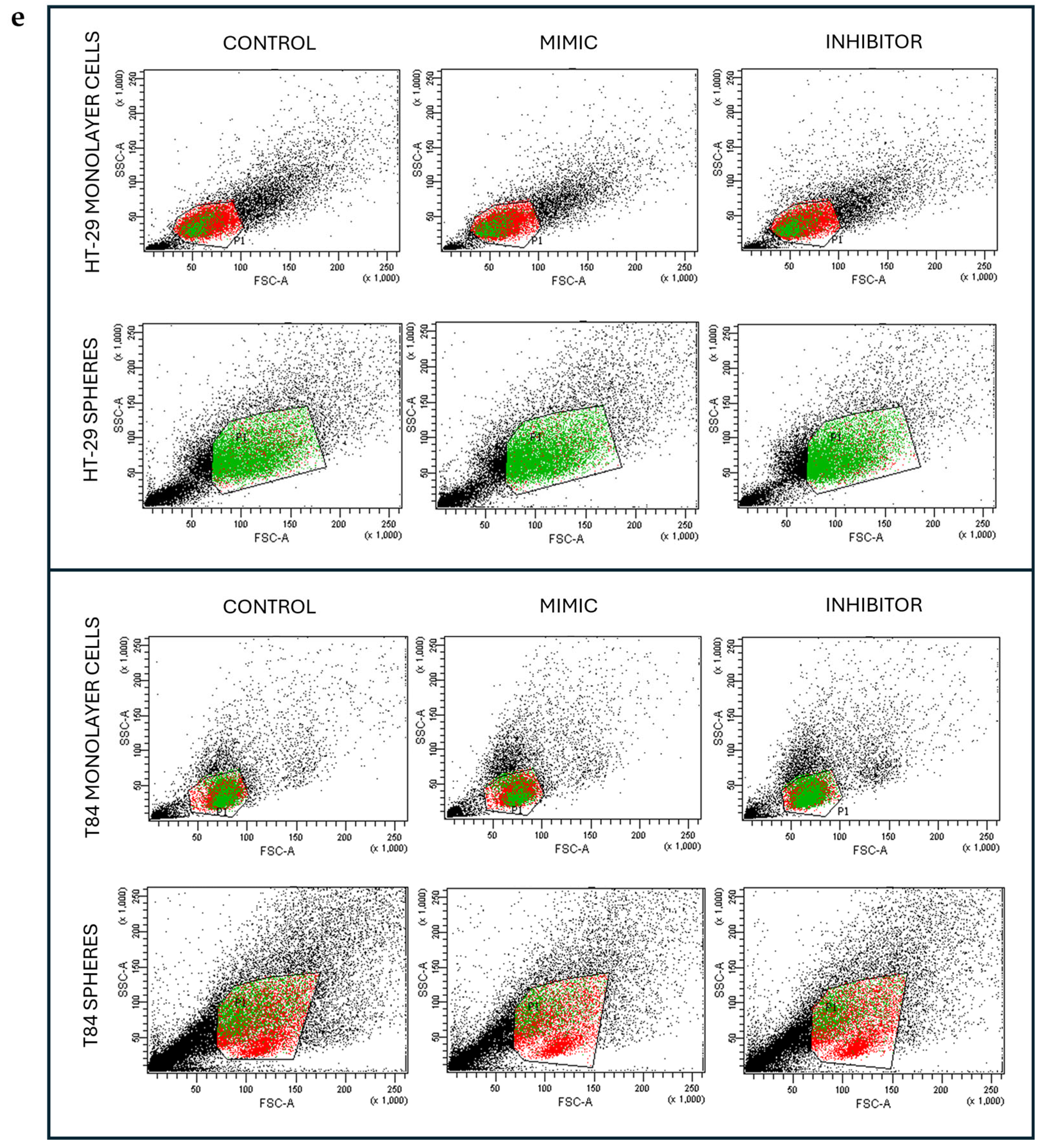
2.7. CSC Characterization
2.8. Soft Agar Colony Formation Assay
2.9. Statistical Analysis
3. Results
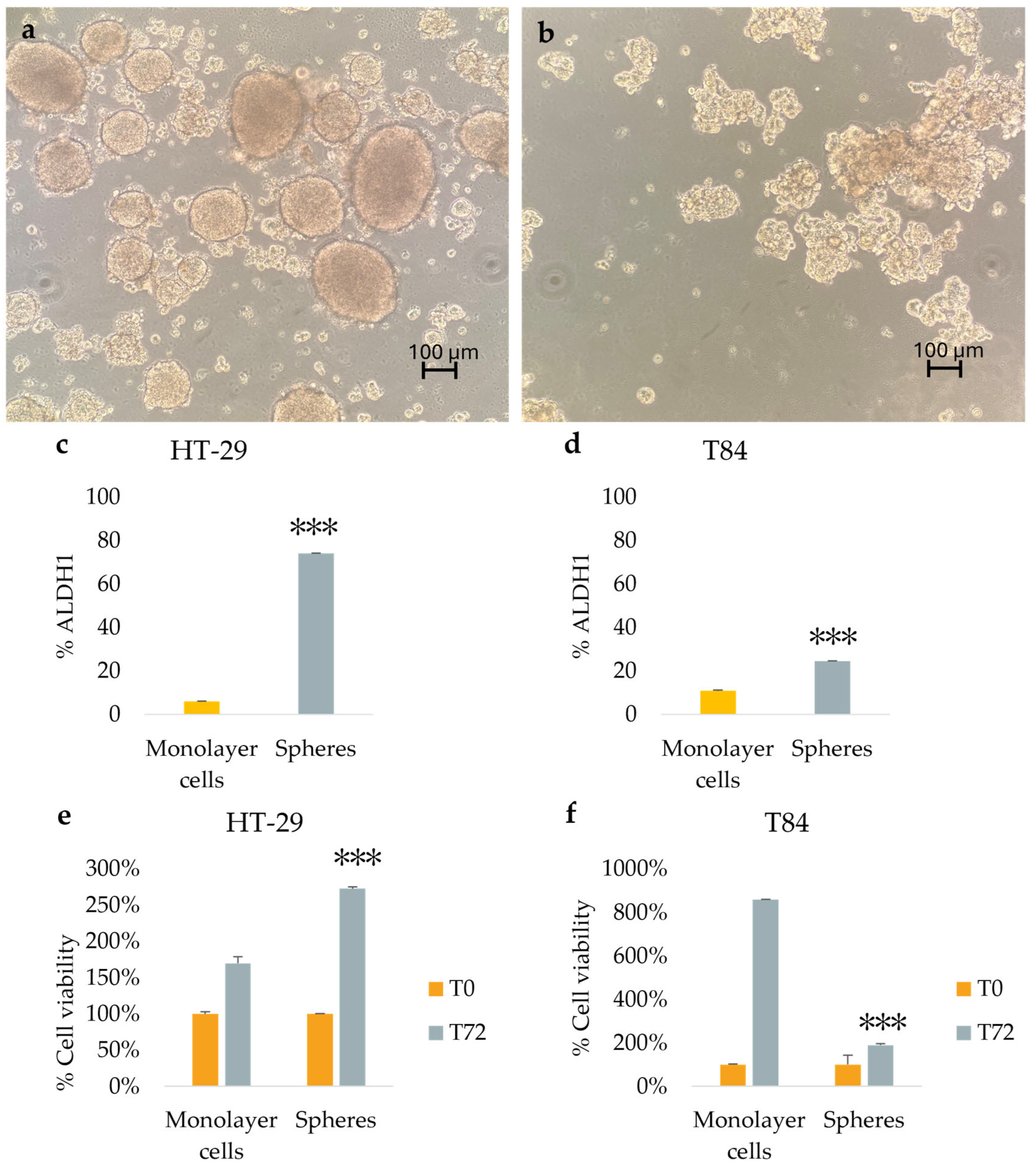
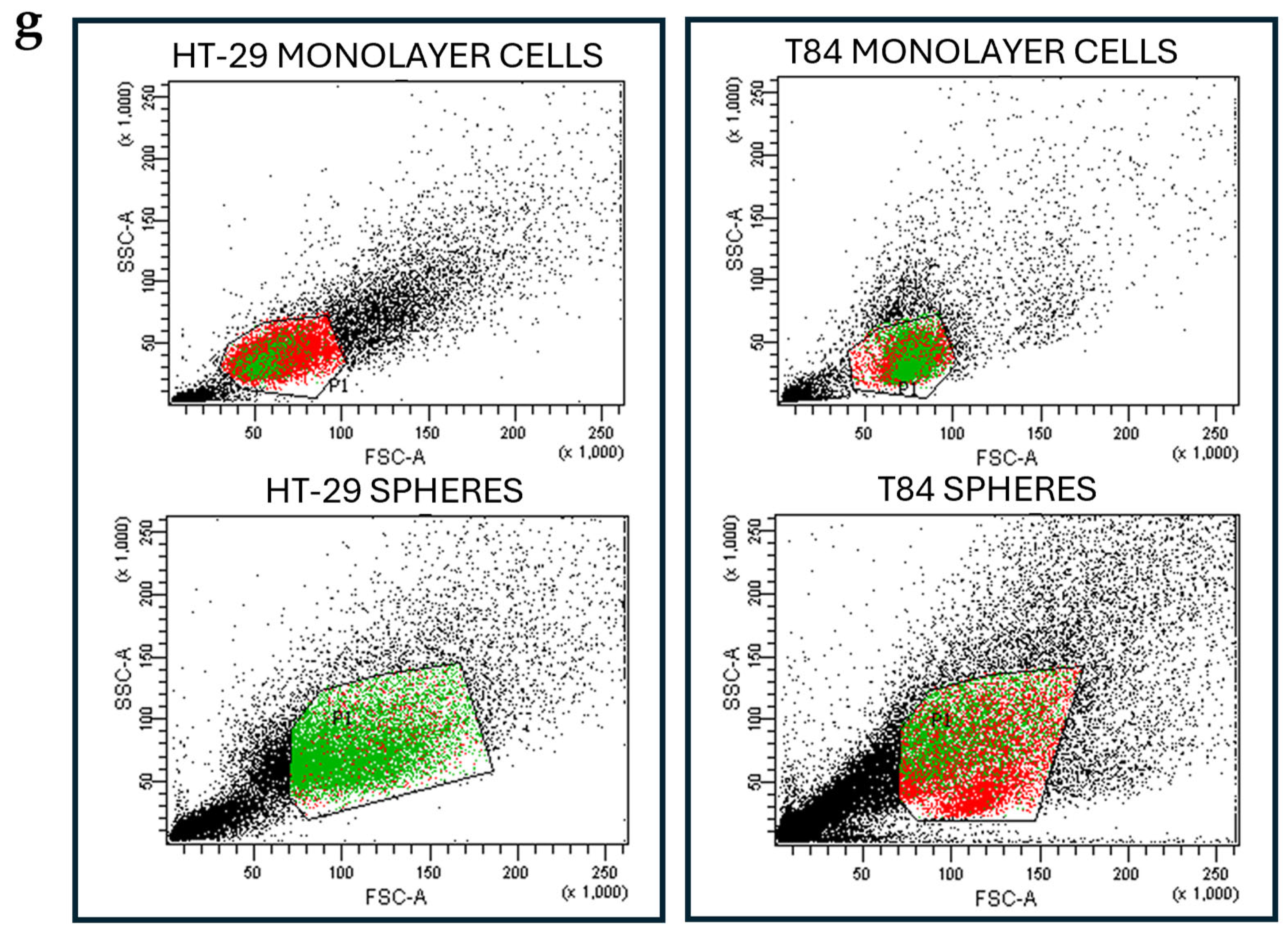
3.1. Spheroids and CSCs Enrichment in Colorectal Cell Lines HT-29 and T84
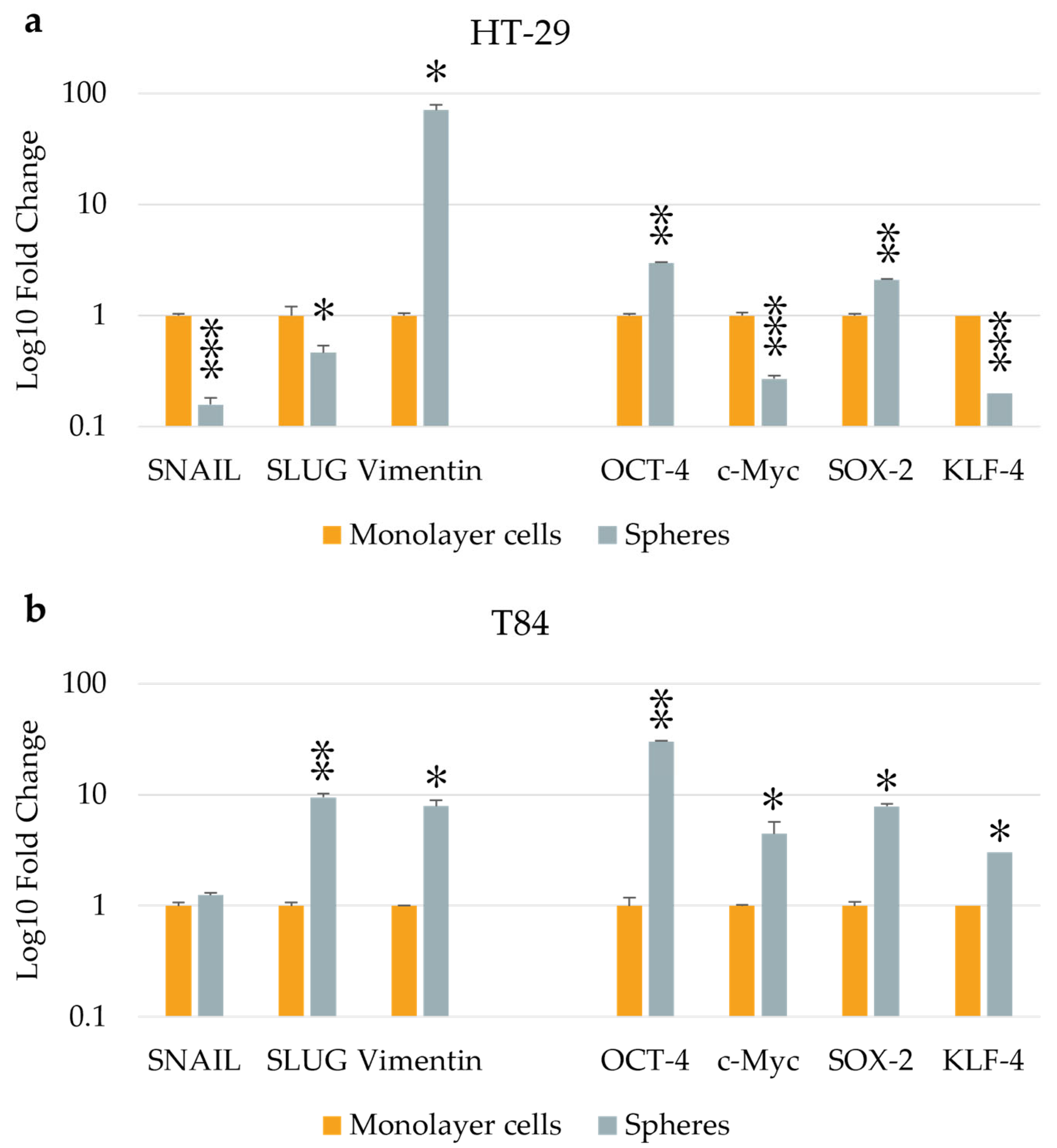
3.2. Expression Levels of EMT and Stemness Genes Change Between Adherent Cells and Colonospheres
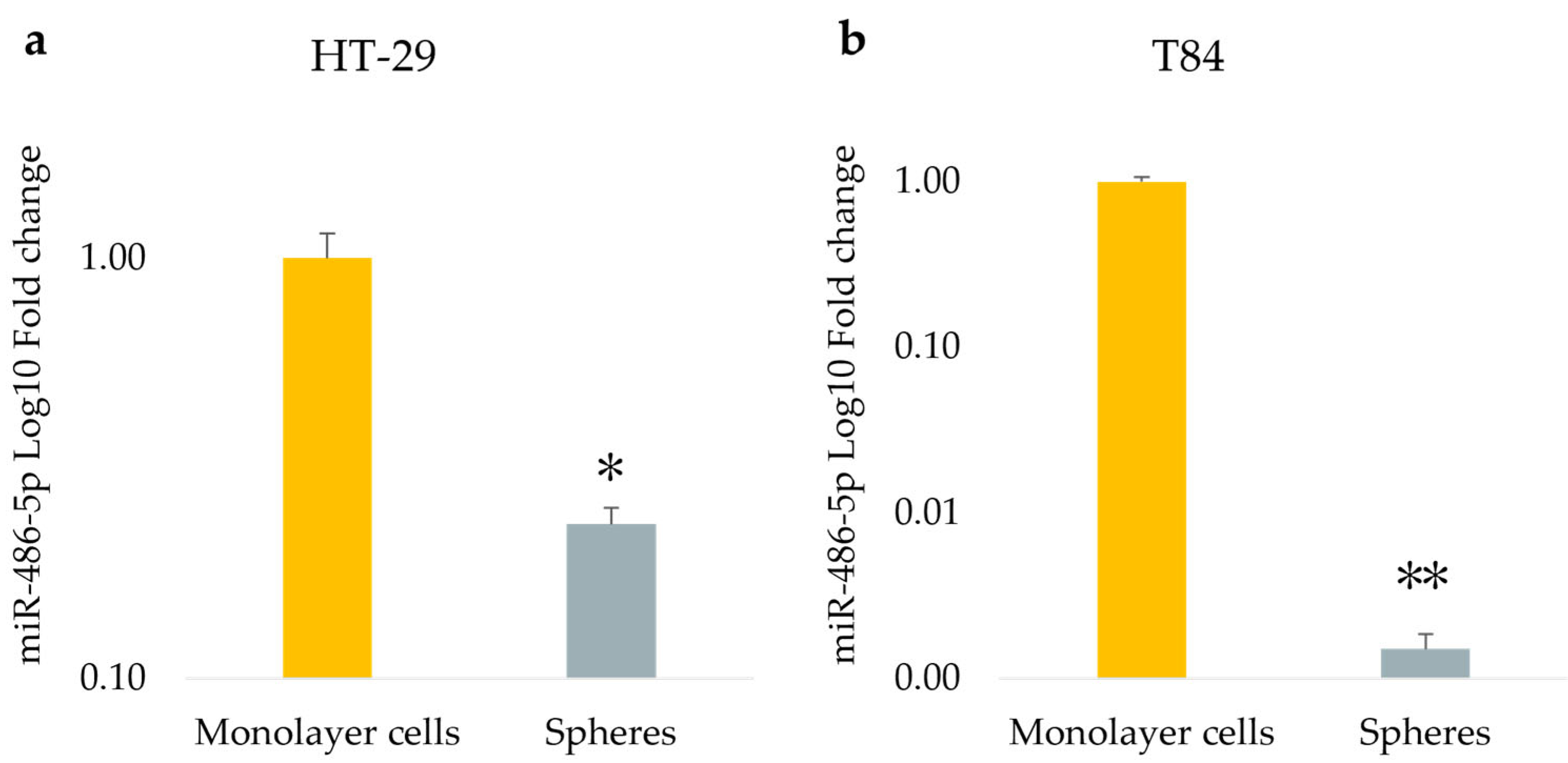
3.3. miR-486-5p Is Downregulated In Colonospheres Culture Model
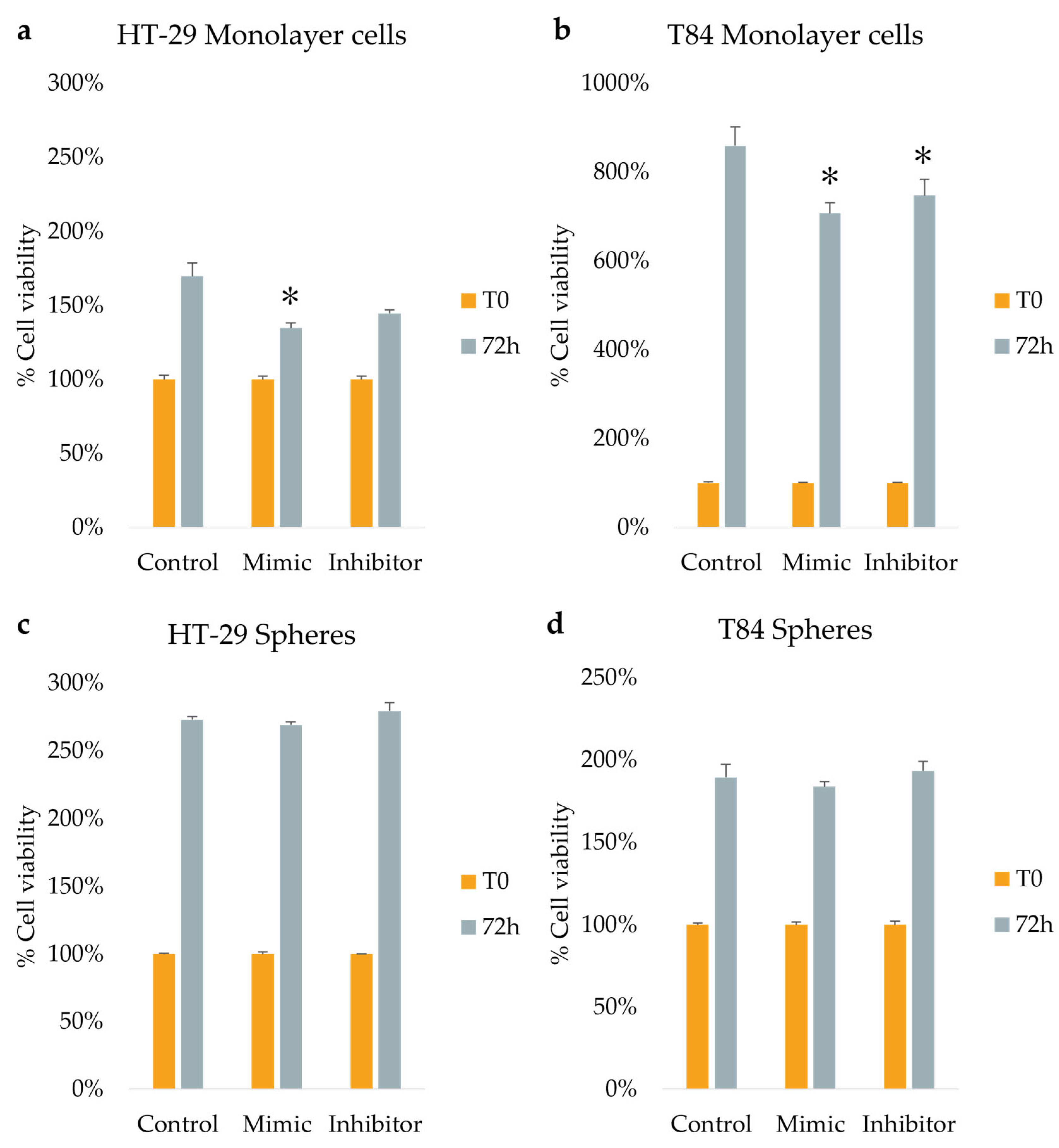
3.4. The Effect of Transfection on Cellular Viability
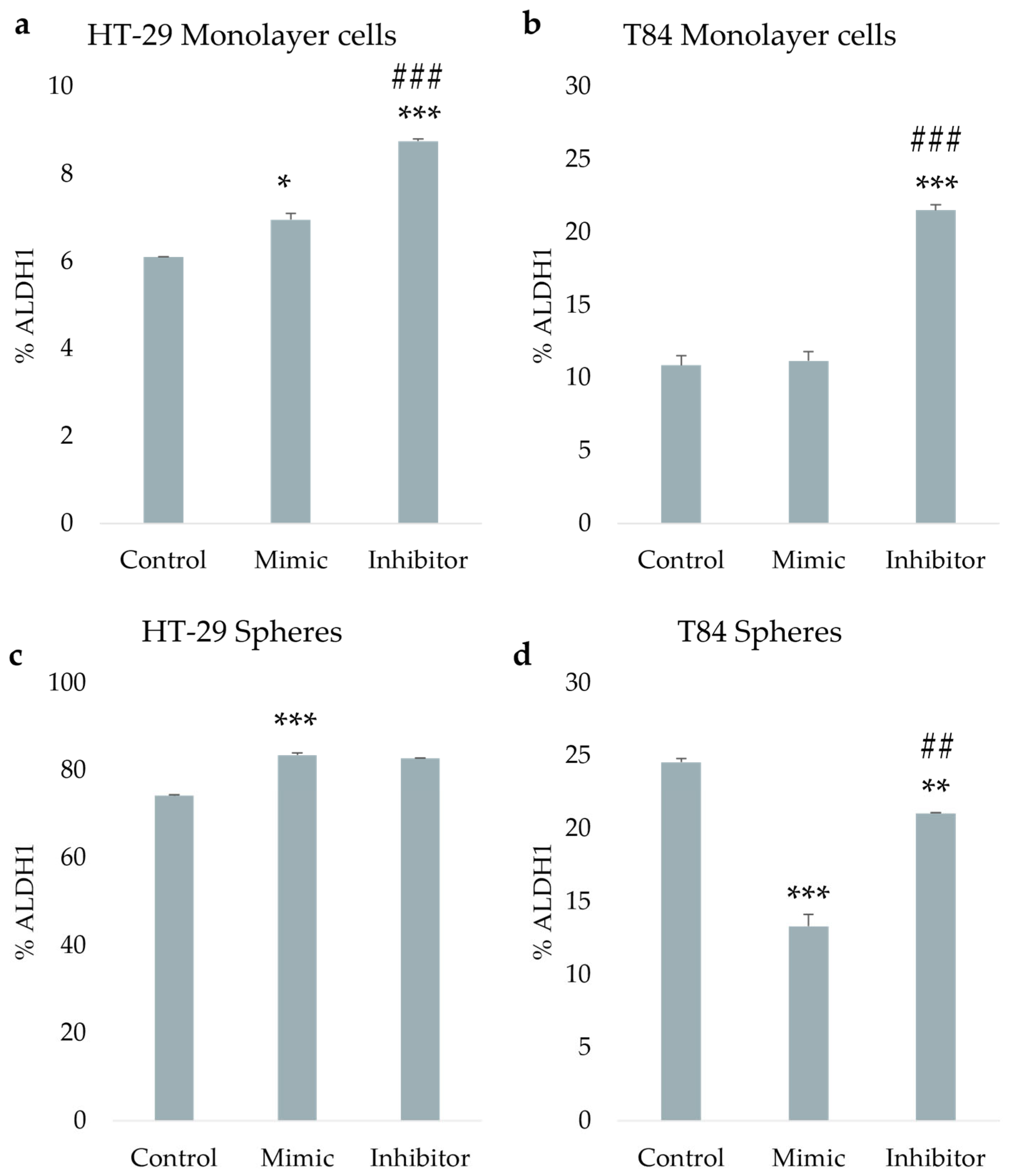
3.5. miR-486-5p Module the ALDH1 Activity in Both Adherent and CSCs
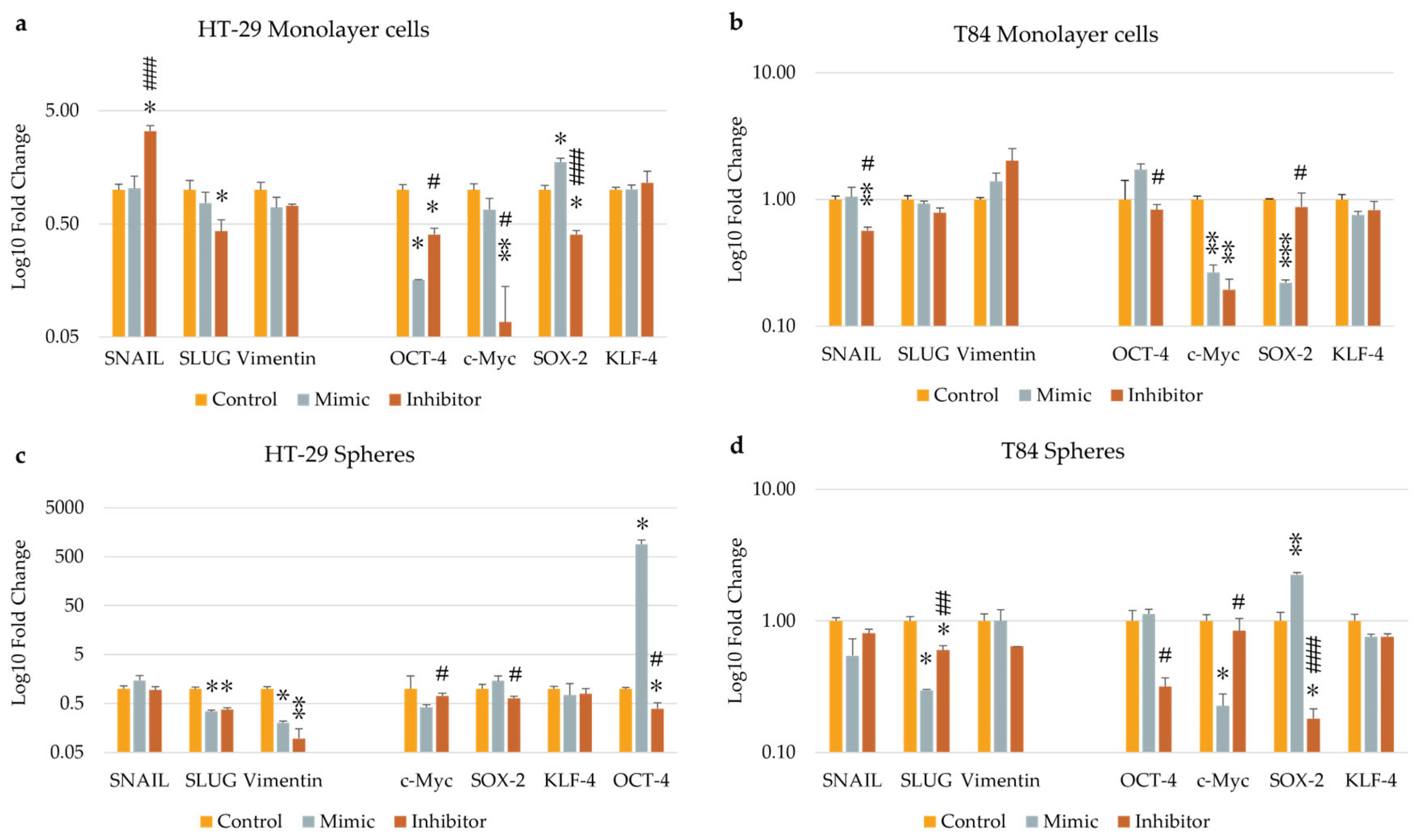
3.6. The Effect of Transfection on Epithelial-Mesenchimal Tansition (EMT) and Stemness Genes Expression
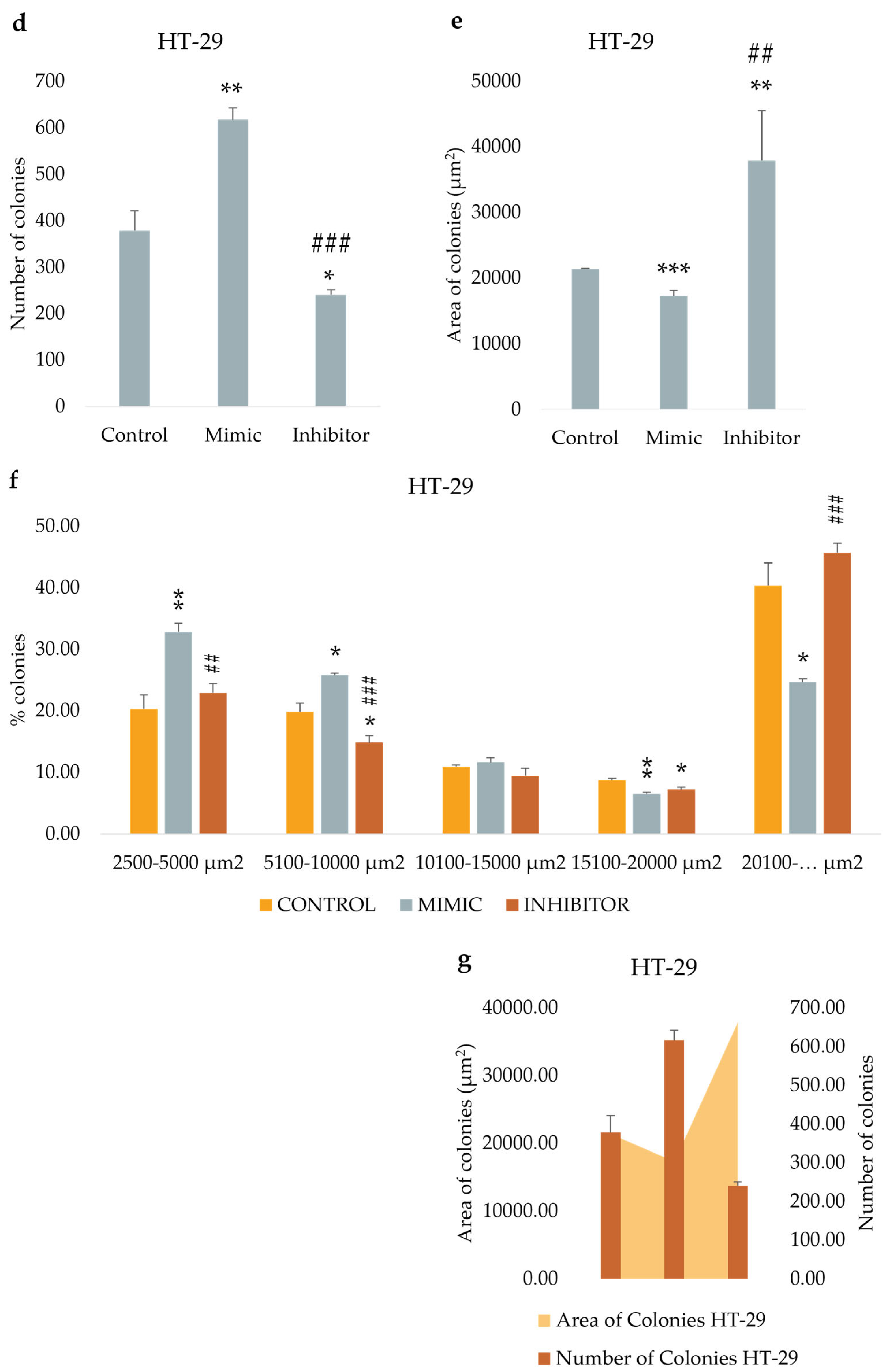
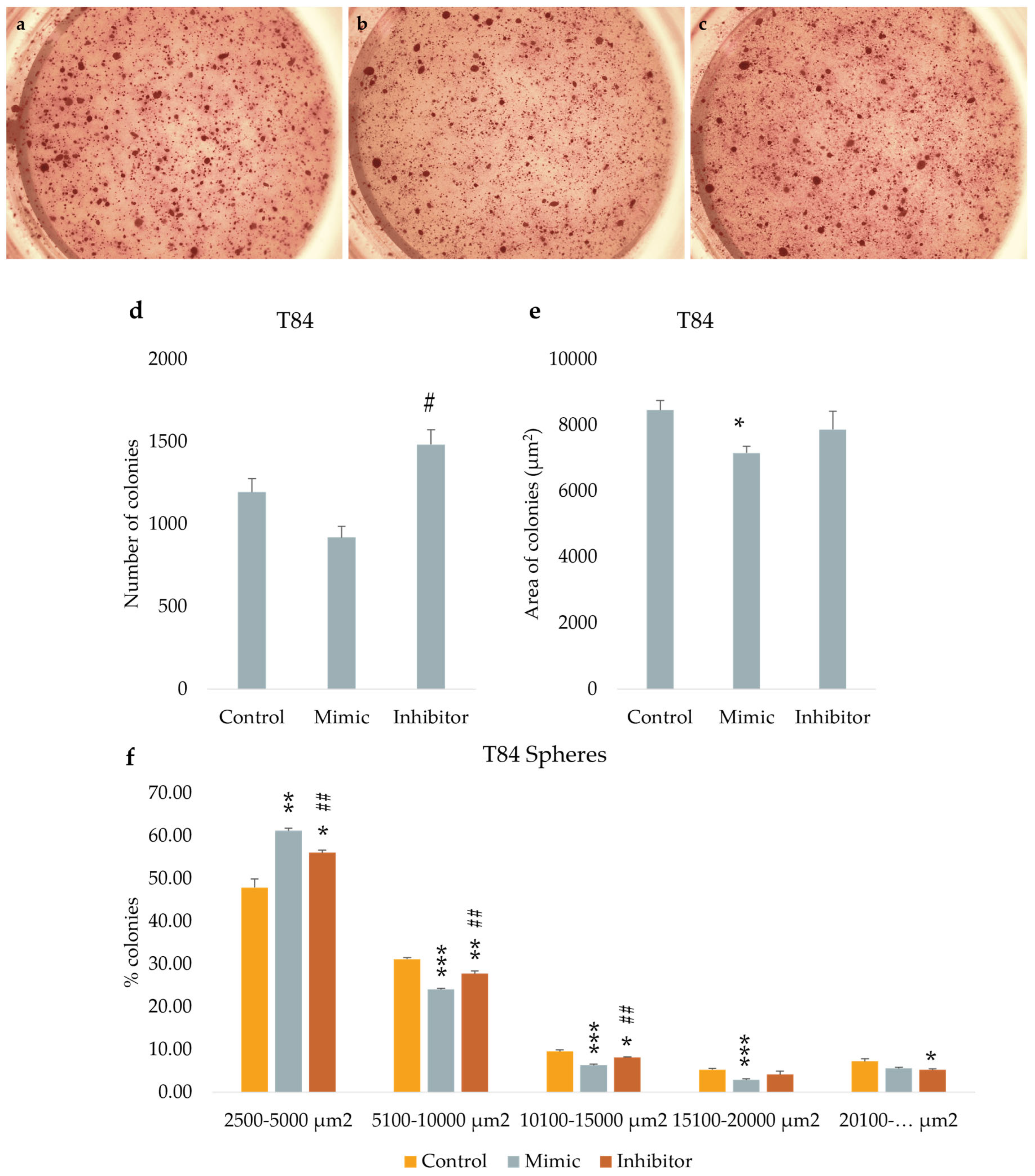
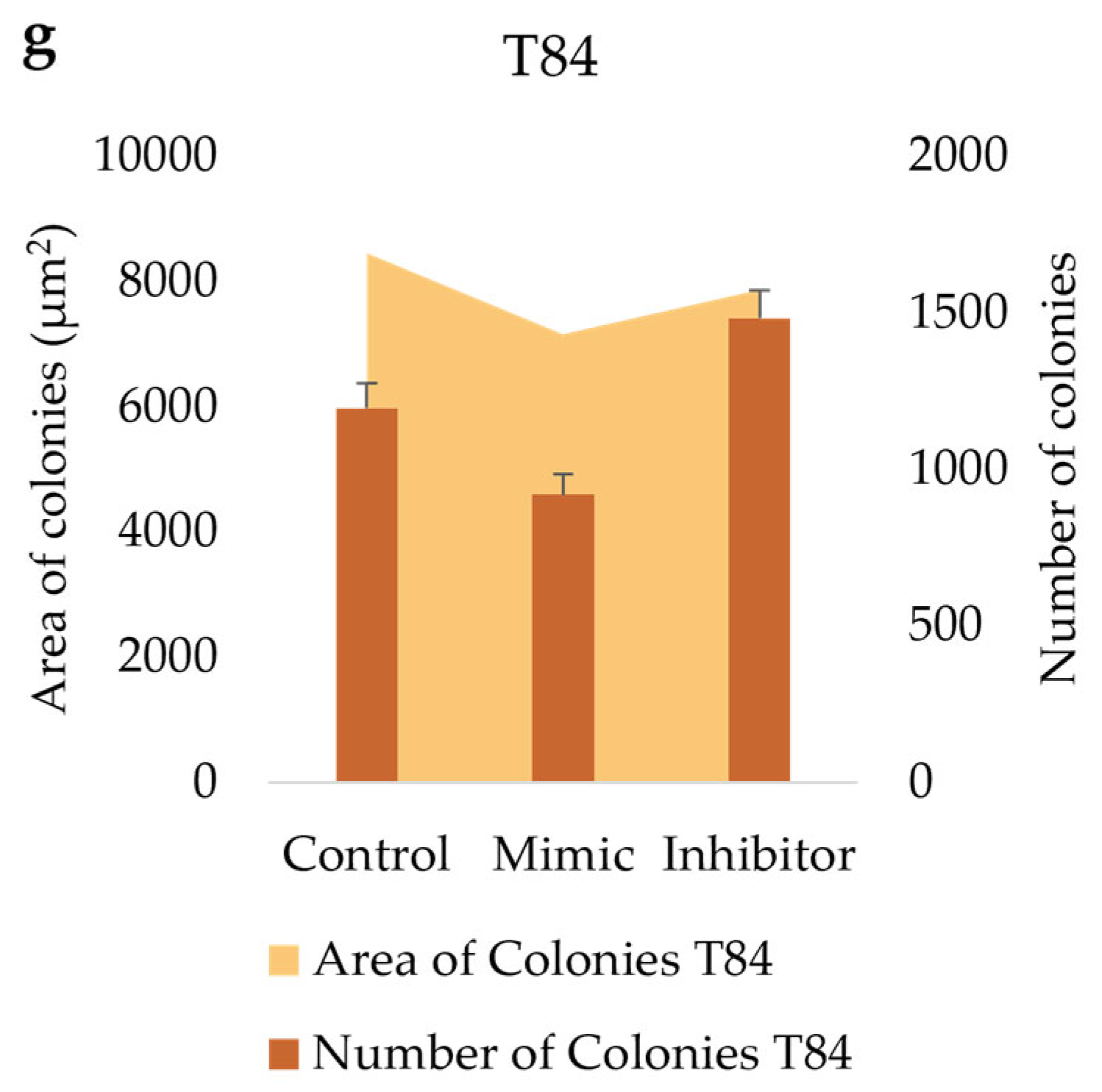
3.7. miR-486-5p Inhibition Enhances the Clonogenic Activity in Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024 May-Jun;74(3):229-263. [CrossRef] [PubMed]
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023 May-Jun;73(3):233-254. [CrossRef] [PubMed]
- Purandare NC, Dua SG, Arora A, Shah S, Rangarajan V. Colorectal cancer - patterns of locoregional recurrence and distant metastases as demonstrated by FDG PET / CT. Indian J Radiol Imaging. 2010 Nov;20(4):284-8. [CrossRef] [PubMed]
- Li J, Ma X, Chakravarti D, Shalapour S, DePinho RA. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021 Jun;35(11-12):787-820. [CrossRef] [PubMed]
- Tabuso M, Homer-Vanniasinkam S, Adya R, Arasaradnam RP. Role of tissue microenvironment resident adipocytes in colon cancer. World J Gastroenterol. 2017 Aug 28;23(32):5829-5835. [CrossRef] [PubMed]
- AlMusawi S, Ahmed M, Nateri AS. Understanding cell-cell communication and signaling in the colorectal cancer microenvironment. Clin Transl Med. 2021 Feb;11(2):e308. [CrossRef] [PubMed]
- Ebrahimi N, Afshinpour M, Fakhr SS, Kalkhoran PG, Shadman-Manesh V, Adelian S, Beiranvand S, Rezaei-Tazangi F, Khorram R, Hamblin MR, Aref AR. Cancer stem cells in colorectal cancer: Signaling pathways involved in stemness and therapy resistance. Crit Rev Oncol Hematol. 2023 Feb;182:103920. [CrossRef] [PubMed]
- Jiménez G, Hackenberg M, Catalina P, Boulaiz H, Griñán-Lisón C, García MÁ, Perán M, López-Ruiz E, Ramírez A, Morata-Tarifa C, Carrasco E, Aguilera M, Marchal JA. Mesenchymal stem cell’s secretome promotes selective enrichment of cancer stem-like cells with specific cytogenetic profile. Cancer Lett. 2018 Aug 10;429:78-88. [CrossRef] [PubMed]
- Gupta R, Bhatt LK, Johnston TP, Prabhavalkar KS. Colon cancer stem cells: Potential target for the treatment of colorectal cancer. Cancer Biol Ther. 2019;20(8):1068-1082. [CrossRef] [PubMed]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007 Jan 4;445(7123):111-5. [CrossRef] [PubMed]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007 Jan 4;445(7123):106-10. [CrossRef] [PubMed]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):10158-63. [CrossRef] [PubMed]
- Puglisi MA, Tesori V, Lattanzi W, Gasbarrini GB, Gasbarrini A. Colon cancer stem cells: controversies and perspectives. World J Gastroenterol. 2013 May 28;19(20):2997-3006. [CrossRef] [PubMed]
- Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011 Jun 15;474(7351):318-26. [CrossRef] [PubMed]
- Subramanian S, Steer CJ. Special Issue: MicroRNA Regulation in Health and Disease. Genes (Basel). 2019 Jun 15;10(6):457. [CrossRef] [PubMed]
- Tie Y, Liu B, Fu H, Zheng X. Circulating miRNA and cancer diagnosis. Sci China C Life Sci. 2009 Dec;52(12):1117-22. [CrossRef] [PubMed]
- Balacescu O, Sur D, Cainap C, Visan S, Cruceriu D, Manzat-Saplacan R, Muresan MS, Balacescu L, Lisencu C, Irimie A. The Impact of miRNA in Colorectal Cancer Progression and Its Liver Metastases. Int J Mol Sci. 2018 Nov 22;19(12):3711. [CrossRef] [PubMed]
- Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA Deregulation in Cancer Cells and the Tumor Microenvironment. Cancer Discov. 2016 Mar;6(3):235-46. [CrossRef] [PubMed]
- Farace C, Pisano A, Griñan-Lison C, Solinas G, Jiménez G, Serra M, Carrillo E, Scognamillo F, Attene F, Montella A, Marchal JA, Madeddu R. Deregulation of cancer-stem-cell-associated miRNAs in tissues and sera of colorectal cancer patients. Oncotarget. 2020 Jan 14;11(2):116-130. [CrossRef] [PubMed]
- Yan X, Liu X, Wang Z, Cheng Q, Ji G, Yang H, Wan L, Ge C, Zeng Q, Huang H, Xi J, He L, Nan X, Yue W, Pei X. MicroRNA-486-5p functions as a tumor suppressor of proliferation and cancer stem-like cell properties by targeting Sirt1 in liver cancer. Oncol Rep. 2019 Mar;41(3):1938-1948. [CrossRef] [PubMed]
- Zhang X, Zhang T, Yang K, Zhang M, Wang K. miR-486-5p suppresses prostate cancer metastasis by targeting Snail and regulating epithelial-mesenchymal transition. Onco Targets Ther. 2016 Nov 8;9:6909-6914. [CrossRef] [PubMed]
- Liu X, Chen X, Zeng K, Xu M, He B, Pan Y, Sun H, Pan B, Xu X, Xu T, Hu X, Wang S. DNA-methylation-mediated silencing of miR-486-5p promotes colorectal cancer proliferation and migration through activation of PLAGL2/IGF2/β-catenin signal pathways. Cell Death Dis. 2018 Oct 10;9(10):1037. [CrossRef] [PubMed]
- Pisano A, Griñan-Lison C, Farace C, Fiorito G, Fenu G, Jiménez G, Scognamillo F, Peña-Martin J, Naccarati A, Pröll J, Atzmüller S, Pardini B, Attene F, Ibba G, Solinas MG, Bernhard D, Marchal JA, Madeddu R. The Inhibitory Role of miR-486-5p on CSC Phenotype Has Diagnostic and Prognostic Potential in Colorectal Cancer. Cancers (Basel). 2020 Nov 19;12(11):3432. [CrossRef] [PubMed]
- Jiménez G, Hackenberg M, Catalina P, Boulaiz H, Griñán-Lisón C, García MÁ, Perán M, López-Ruiz E, Ramírez A, Morata-Tarifa C, Carrasco E, Aguilera M, Marchal JA. Mes-enchymal stem cell’s secretome promotes selective enrichment of cancer stem-like cells with specific cytogenetic profile. Cancer Lett. 2018 Aug 10;429:78-88. [CrossRef] [PubMed]
- De Lara-Peña L, Farace C, Pisano A, de Andrés JL, Fenu G, Etzi F, Griñán-Lisón C, Marchal JA, Madeddu R. Mimicking the Tumor Niche: Methods for Isolation, Culture, and Characterization of Cancer Stem Cells and Multicellular Spheroids. Methods Mol Biol. 2024;2777:145-161. [CrossRef] [PubMed]
- Sullivan BA, Noujaim M, Roper J. Cause, Epidemiology, and Histology of Polyps and Pathways to Colorectal Cancer. Gastrointest Endosc Clin N Am. 2022 Apr;32(2):177-194. [CrossRef] [PubMed]
- Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010 Jan;120(1):41-50. [CrossRef] [PubMed]
- Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005 Jul 15;122(1):6-7. [CrossRef] [PubMed]
- Yang S, Sui J, Liu T, Wu W, Xu S, Yin L, Pu Y, Zhang X, Zhang Y, Shen B, Liang G. Expression of miR-486-5p and its significance in lung squamous cell carcinoma. J Cell Biochem. 2019 Aug;120(8):13912-13923. [CrossRef] [PubMed]
- Oh HK, Tan AL, Das K, Ooi CH, Deng NT, Tan IB, Beillard E, Lee J, Ramnarayanan K, Rha SY, Palanisamy N, Voorhoeve PM, Tan P. Genomic loss of miR-486 regulates tumor progression and the OLFM4 antiapoptotic factor in gastric cancer. Clin Cancer Res. 2011;17(9):2657-67. [CrossRef] [PubMed]
- Yan X, Liu X, Wang Z, Cheng Q, Ji G, Yang H, Wan L, Ge C, Zeng Q, Huang H, Xi J, He L, Nan X, Yue W, Pei X. MicroRNA-486-5p functions as a tumor suppressor of proliferation and cancer stem-like cell properties by targeting Sirt1 in liver cancer. Oncol Rep. 2019 Mar;41(3):1938-1948. [CrossRef] [PubMed]
- He Y, Liu J, Wang Y, Zhu X, Fan Z, Li C, Yin H, Liu Y. Role of miR-486-5p in regulating renal cell carcinoma cell proliferation and apoptosis via TGF-β-activated kinase 1. J Cell Biochem. 2019 Mar;120(3):2954-2963. [CrossRef] [PubMed]
- Wen DY, Pan DH, Lin P, Mo QY, Wei YP, Luo YH, Chen G, He Y, Chen JQ, Yang H. Downregulation of miR-486-5p in papillary thyroid carcinoma tissue: A study based on microarray and miRNA sequencing. Mol Med Rep. 2018 Sep;18(3):2631-2642. [CrossRef] [PubMed]
- Ma H, Tian T, Liang S, Liu X, Shen H, Xia M, Liu X, Zhang W, Wang L, Chen S, Yu L. Estrogen receptor-mediated miR-486-5p regulation of OLFM4 expression in ovarian cancer. Oncotarget. 2016 Mar 1;7(9):10594-605. [CrossRef] [PubMed]
- Yang Y, Ji C, Guo S, Su X, Zhao X, Zhang S, Liu G, Qiu X, Zhang Q, Guo H, Chen H. The miR-486-5p plays a causative role in prostate cancer through negative regulation of multiple tumor suppressor pathways. Oncotarget. 2017 Aug 24;8(42):72835-72846. [CrossRef] [PubMed]
- Lopez-Bertoni H, Kotchetkov IS, Mihelson N, Lal B, Rui Y, Ames H, Lugo-Fagundo M, Guerrero-Cazares H, Quiñones-Hinojosa A, Green JJ, Laterra J. A Sox2:miR-486-5p Axis Regulates Survival of GBM Cells by Inhibiting Tumor Suppressor Networks. Cancer Res. 2020 Apr 15;80(8):1644-1655. [CrossRef] [PubMed]
- Svoronos AA, Engelman DM, Slack FJ. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016 Jul 1;76(13):3666-70. [CrossRef] [PubMed]
- Toledo-Guzmán ME, Hernández MI, Gómez-Gallegos ÁA, Ortiz-Sánchez E. ALDH as a Stem Cell Marker in Solid Tumors. Curr Stem Cell Res Ther. 2019;14(5):375-388. [CrossRef] [PubMed]
- Goossens-Beumer IJ, Zeestraten EC, Benard A, Christen T, Reimers MS, Keijzer R, Sier CF, Liefers GJ, Morreau H, Putter H, Vahrmeijer AL, van de Velde CJ, Kuppen PJ. Clinical prognostic value of combined analysis of Aldh1, Survivin, and EpCAM expression in colorectal cancer. Br J Cancer. 2014 Jun 10;110(12):2935-44. [CrossRef] [PubMed]
- Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009 Apr 15;69(8):3382-9. [CrossRef] [PubMed]
- Alowaidi F, Hashimi SM, Alqurashi N, Alhulais R, Ivanovski S, Bellette B, Meedenyia A, Lam A, Wood S. Assessing stemness and proliferation properties of the newly established colon cancer ‘stem’ cell line, CSC480 and novel approaches to identify dormant cancer cells. Oncol Rep. 2018 Jun;39(6):2881-2891. [CrossRef] [PubMed]
- Yue H, Hu Z, Hu R, Guo Z, Zheng Y, Wang Y, Zhou Y. ALDH1A1 in Cancers: Bidirectional Function, Drug Resistance, and Regulatory Mechanism. Front Oncol. 2022 Jun 22;12:918778. [CrossRef] [PubMed]
- Sládek NE, Kollander R, Sreerama L, Kiang DT. Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: a retrospective study. Rational individualization of oxazaphosphorine-based cancer chemotherapeutic regimens. Cancer Chemother Pharmacol. 2002 Apr;49(4):309-21. [CrossRef] [PubMed]
- Hadjimichael C, Chanoumidou K, Papadopoulou N, Arampatzi P, Papamatheakis J, Kretsovali A. Common stemness regulators of embryonic and cancer stem cells. World J Stem Cells. 2015 Oct 26;7(9):1150-84. [CrossRef] [PubMed]
- Biddle A, Mackenzie IC. Cancer stem cells and EMT in carcinoma. Cancer Metastasis Rev. 2012 Feb 3. Epub ahead of print. [CrossRef] [PubMed]
- van Schaijik B, Davis PF, Wickremesekera AC, Tan ST, Itinteang T. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: a review. J Clin Pathol. 2018 Jan;71(1):88-91. [CrossRef] [PubMed]
- Humphries HN, Wickremesekera SK, Marsh RW, Brasch HD, Mehrotra S, Tan ST, Itinteang T. Characterization of Cancer Stem Cells in Colon Adenocarcinoma Metastasis to the Liver. Front Surg. 2018 Jan 22;4:76. [CrossRef] [PubMed]
- Elbadawy M, Usui T, Yamawaki H, Sasaki K. Emerging Roles of C-Myc in Cancer Stem Cell-Related Signaling and Resistance to Cancer Chemotherapy: A Potential Therapeutic Target Against Colorectal Cancer. Int J Mol Sci. 2019;20(9):2340. [CrossRef] [PubMed]
- Anuja K, Kar M, Chowdhury AR, Shankar G, Padhi S, Roy S, Akhter Y, Rath AK, Banerjee B. Role of telomeric RAP1 in radiation sensitivity modulation and its interaction with CSC marker KLF4 in colorectal cancer. Int J Radiat Biol. 2020 Jun;96(6):790-802. [CrossRef] [PubMed]
- Wei D, Kanai M, Huang S, Xie K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis. 2006 Jan;27(1):23-31. [CrossRef] [PubMed]
- Darido C, Georgy SR, Cullinane C, Partridge DD, Walker R, Srivastava S, Roslan S, Carpinelli MR, Dworkin S, Pearson RB, Jane SM. Stage-dependent therapeutic efficacy in PI3K/mTOR-driven squamous cell carcinoma of the skin. Cell Death Differ. 2018 Jun;25(6):1146-1159. [CrossRef] [PubMed]
- Gross-Cohen M, Yanku Y, Kessler O, Barash U, Boyango I, Cid-Arregui A, Neufeld G, Ilan N, Vlodavsky I. Heparanase 2 (Hpa2) attenuates tumor growth by inducing Sox2 expression. Matrix Biol. 2021 May;99:58-71. [CrossRef] [PubMed]
- Wuebben EL, Rizzino A. The dark side of SOX2: cancer - a comprehensive overview. Oncotarget. 2017 Jul 4;8(27):44917-44943. [CrossRef] [PubMed]
- Su C, Li D, Li N, Du Y, Yang C, Bai Y, Lin C, Li X, Zhang Y. Studying the mechanism of PLAGL2 overexpression and its carcinogenic characteristics based on 3’-untranslated region in colorectal cancer. Int J Oncol. 2018 May;52(5):1479-1490. [CrossRef] [PubMed]
- Garte SJ. The c-myc oncogene in tumor progression. Crit Rev Oncog. 1993;4(4):435-49. [PubMed]
- Xu BS, Chen HY, Que Y, Xiao W, Zeng MS, Zhang X. ALKATI interacts with c-Myc and promotes cancer stem cell-like properties in sarcoma. Oncogene. 2020 Jan;39(1):151-163. [CrossRef] [PubMed]
- Vlachos IS, Kostoulas N, Vergoulis T, Georgakilas G, Reczko M, Maragkakis M, Paraskevopoulou MD, Prionidis K, Dalamagas T, Hatzigeorgiou AG. DIANA miRPath v.2.0: investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012 Jul;40(Web Server issue):W498-504. [CrossRef] [PubMed]
- Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011 Sep;68(18):3033-46. [CrossRef] [PubMed]
- Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda JI, Konishi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007 Mar 26;96(6):986-92. [CrossRef] [PubMed]
- Kroepil F, Fluegen G, Vallböhmer D, Baldus SE, Dizdar L, Raffel AM, Hafner D, Stoecklein NH, Knoefel WT. Snail1 expression in colorectal cancer and its correlation with clinical and pathological parameters. BMC Cancer. 2013 Mar 22;13:145. [CrossRef] [PubMed]
- Yanagawa J, Walser TC, Zhu LX, Hong L, Fishbein MC, Mah V, Chia D, Goodglick L, Elashoff DA, Luo J, Magyar CE, Dohadwala M, Lee JM, St John MA, Strieter RM, Sharma S, Dubinett SM. Snail promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009 Nov 15;15(22):6820-9. [CrossRef] [PubMed]
- Arumi-Planas M, Rodriguez-Baena FJ, Cabello-Torres F, Gracia F, Lopez-Blau C, Nieto MA, Sanchez-Laorden B. Microenvironmental Snail1-induced immunosuppression promotes melanoma growth. Oncogene. 2023 Sep;42(36):2659-2672. [CrossRef] [PubMed]
- Alves CC, Carneiro F, Hoefler H, Becker KF. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front Biosci (Landmark Ed). 2009 Jan 1;14(8):3035-50. [CrossRef] [PubMed]
- Wang Y, Ngo VN, Marani M, Yang Y, Wright G, Staudt LM, Downward J. Critical role for transcriptional repressor Snail2 in transformation by oncogenic RAS in colorectal carcinoma cells. Oncogene. 2010 Aug 19;29(33):4658-70. [CrossRef] [PubMed]
- Thiery, JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002 Jun;2(6):442-54. [CrossRef] [PubMed]
- Wang Y, Shi J, Chai K, Ying X, Zhou BP. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013 Nov;13(9):963-972. [CrossRef] [PubMed]
- Subbalakshmi AR, Sahoo S, Biswas K, Jolly MK. A Computational Systems Biology Approach Identifies SLUG as a Mediator of Partial Epithelial-Mesenchymal Transition (EMT). Cells Tissues Organs. 2022;211(6):689-702. [CrossRef] [PubMed]
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013 Nov;19(11):1438-49. [CrossRef] [PubMed]
- Jolly MK, Jia D, Boareto M, Mani SA, Pienta KJ, Ben-Jacob E, Levine H. Coupling the modules of EMT and stemness: A tunable ‘stemness window’ model. Oncotarget. 2015 Sep 22;6(28):25161-74. [CrossRef] [PubMed]
- Ren B, Liu H, Yang Y, Lian Y. Effect of BRAF-mediated PI3K/Akt/mTOR pathway on biological characteristics and chemosensitivity of NSCLC A549/DDP cells. Oncol Lett. 2021 Aug;22(2):584. [CrossRef] [PubMed]
- Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The Role of B-RAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-κB/Snail/RKIP/PTEN Circuit. Genes Cancer. 2010 May;1(5):409-420. [CrossRef] [PubMed]
- Moro M, Fortunato O, Bertolini G, Mensah M, Borzi C, Centonze G, Andriani F, Di Paolo D, Perri P, Ponzoni M, Pastorino U, Sozzi G, Boeri M. MiR-486-5p Targets CD133+ Lung Cancer Stem Cells through the p85/AKT Pathway. Pharmaceuticals (Basel). 2022 Feb 28;15(3):297. [CrossRef] [PubMed]
- Xiao, Y. MiR-486-5p inhibits the hyperproliferation and production of collagen in hypertrophic scar fibroblasts via IGF1/PI3K/AKT pathway. J Dermatolog Treat. 2021 Dec;32(8):973-982. [CrossRef] [PubMed]
- Zhang Y, Fu J, Zhang Z, Qin H. miR-486-5p regulates the migration and invasion of colorectal cancer cells through targeting PIK3R1. Oncol Lett. 2018 May;15(5):7243-7248. [CrossRef] [PubMed]
- Iourov IY, Vorsanova SG, Yurov YB. Pathway-based classification of genetic diseases. Mol Cytogenet. 2019 Feb 4;12:4. [CrossRef] [PubMed]
- Zorzan I, Pellegrini M, Arboit M, Incarnato D, Maldotti M, Forcato M, Tagliazucchi GM, Carbognin E, Montagner M, Oliviero S, Martello G. The transcriptional regulator ZNF398 mediates pluripotency and epithelial character downstream of TGF-beta in human PSCs. Nat Commun. 2020;11(1):2364. [CrossRef] [PubMed]
- Nakamura, D. The evaluation of tumorigenicity and characterization of colonies in a soft agar colony formation assay using polymerase chain reaction. Sci Rep. 2023 Apr 3;13(1):5405. [CrossRef] [PubMed]
- Horibata S, Vo TV, Subramanian V, Thompson PR, Coonrod SA. Utilization of the Soft Agar Colony Formation Assay to Identify Inhibitors of Tumorigenicity in Breast Cancer Cells. J Vis Exp. 2015;(99):e52727. [CrossRef] [PubMed]
- Gheytanchi E, Naseri M, Karimi-Busheri F, Atyabi F, Mirsharif ES, Bozorgmehr M, Ghods R, Madjd Z. Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int. 2021 Apr 13;21(1):204. [CrossRef] [PubMed]
- Chakraborty C, Chin KY, Das S. miRNA-regulated cancer stem cells: understanding the property and the role of miRNA in carcinogenesis. Tumour Biol. 2016 Oct;37(10):13039-13048. [CrossRef] [PubMed]
- Lobel GP, Jiang Y, Simon MC. Tumor microenvironmental nutrients, cellular responses, and cancer. Cell Chem Biol. 2023 Sep 21;30(9):1015-1032. [CrossRef] [PubMed]
- Rajendran V, Jain MV. In Vitro Tumorigenic Assay: Colony Forming Assay for Cancer Stem Cells. Methods Mol Biol. 2018;1692:89-95. [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




