Submitted:
12 November 2024
Posted:
13 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
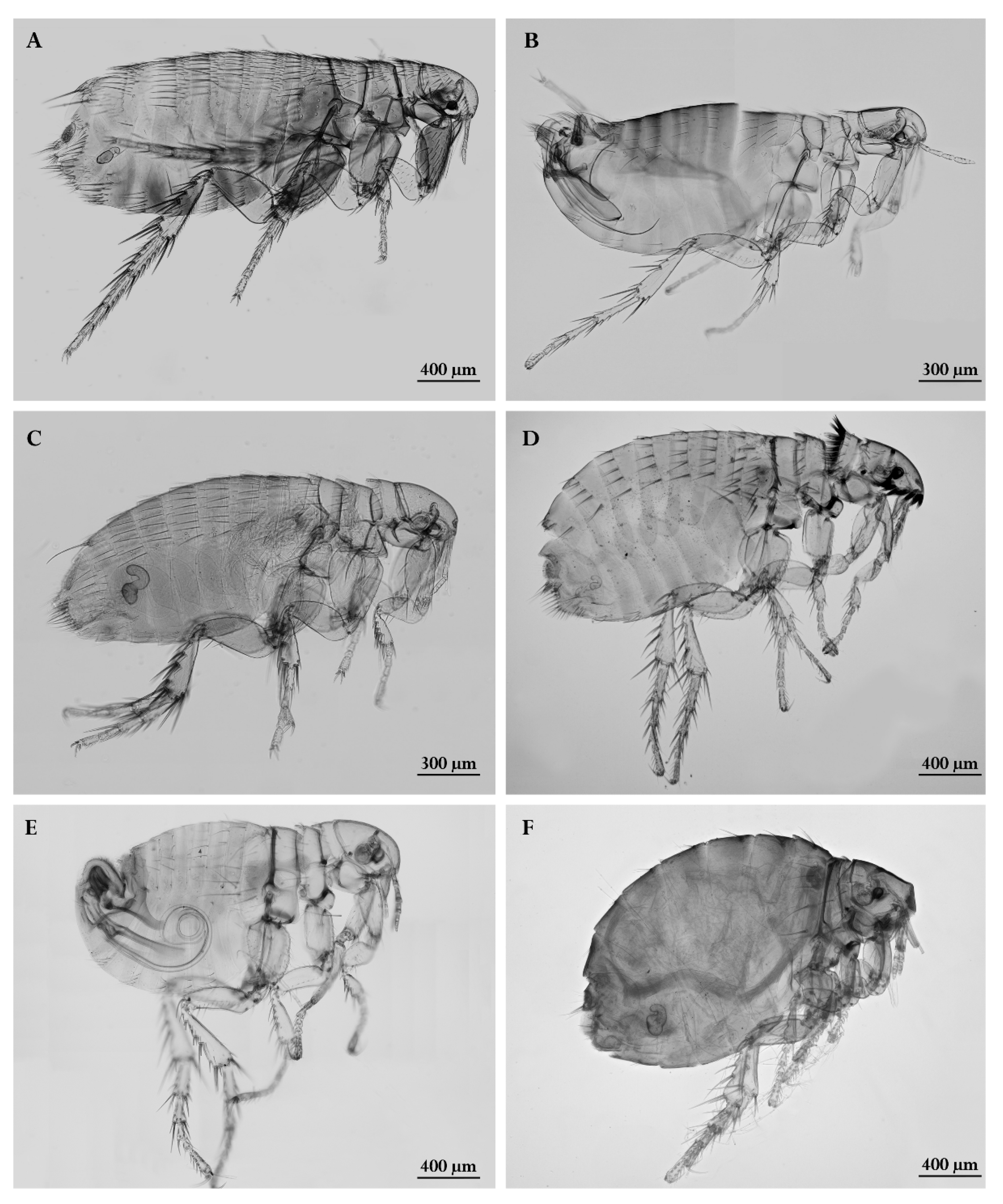
2.1. Parasite - Host Checklist
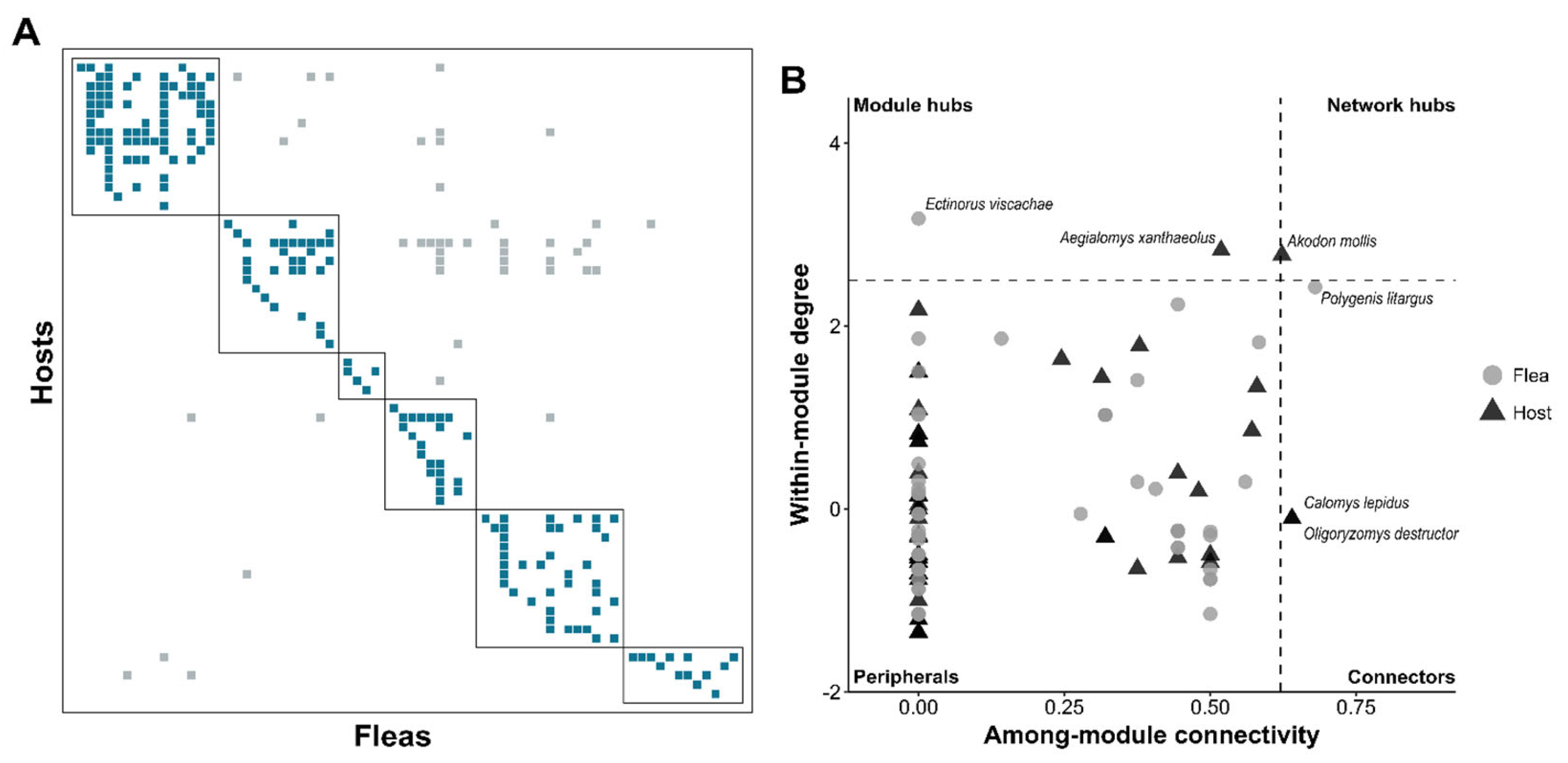
2.2. Flea-Host Interaction Network
3. Results
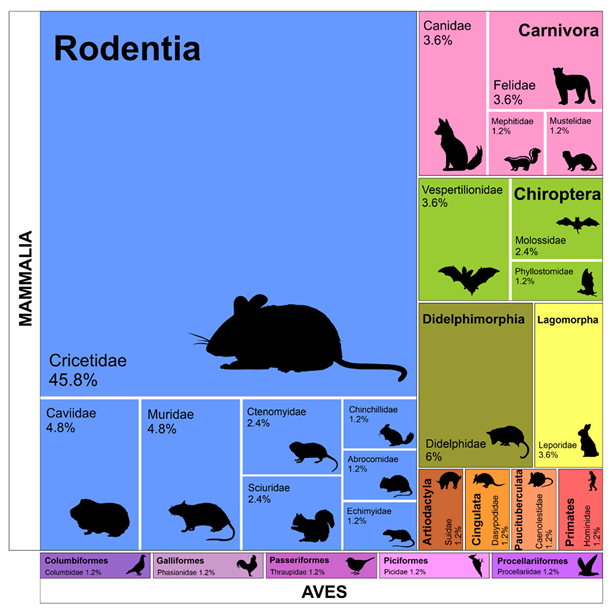
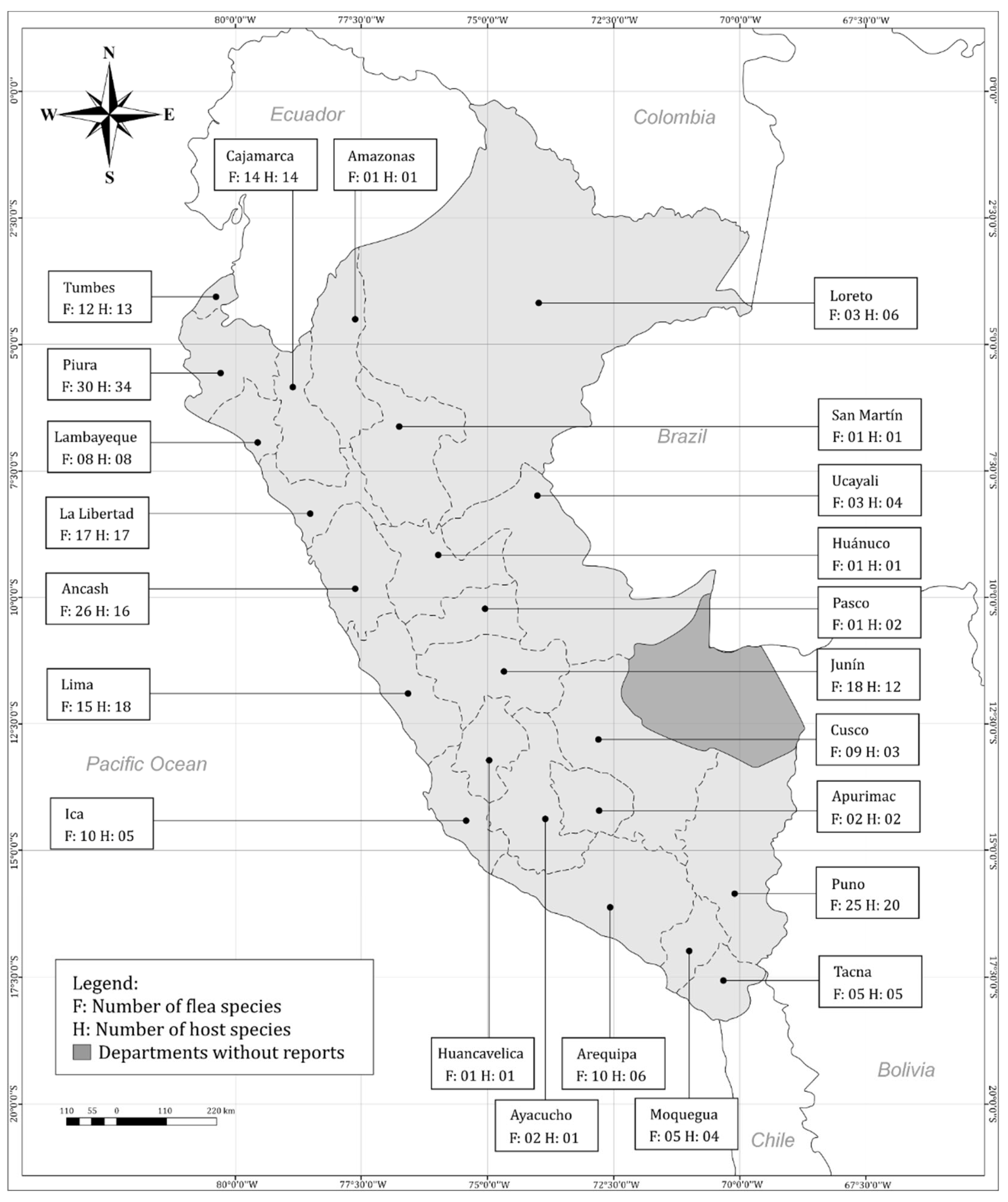
3.1. Parasite - Host Checklist
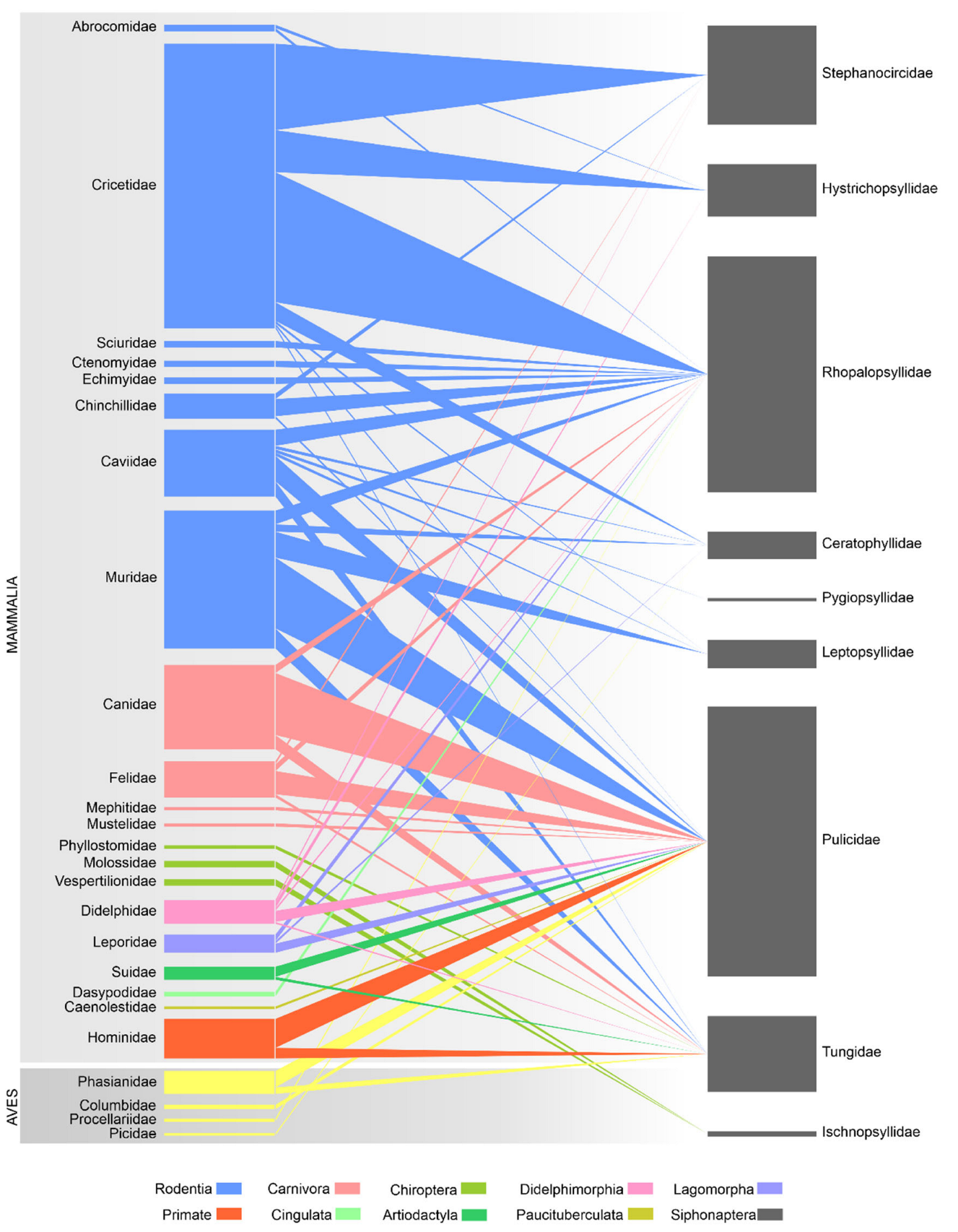
3.2. Flea-Host Interaction Network
4. Discussion
4.1. Parasite - Host Checklist
4.2. Flea-Host Interaction Network
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, R.E. Fleas (Siphonaptera). In: Lane RP, Crosskey RW (eds) Medical Insects and Arachnids.
- Linardi, P.M. Fleas and diseases. In: Marcondes C (ed) Arthropod Borne Diseases.
- Bossard, R.L.; Lareschi, M.; Urdapilleta, M.; Cutillas, C.; Zurita, A. Flea (Insecta: Siphonaptera) Family Diversity. Diversity (Basel) 2023, 15, 1096–10.3390. [Google Scholar] [CrossRef]
- Murray, M.D.; Orton, M.N.; Cameron, A.S. The Antarctic Flea Glaciopsyllus Antarcticus Smit and Dunnet. Antarct Res Ser 2013, 10, 393–395. [Google Scholar]
- Medvedev, S.G. Fauna and host-parasite associations of fleas (Siphonaptera) in different zoogeographical regions of the world. II. Entomologicheskoe Obozrenie 2000, 79, 640–655. [Google Scholar]
- Medvedev, S.G. Fauna and host-parasite associations of fleas (Siphonaptera) in different zoogeographical regions of the World. I. Entomologicheskoe Obozrenie 2000, 79, 409–435. [Google Scholar]
- Medvedev, S.G.; Krasnov, B.R. Fleas: Permanent satellites of small mammals. In: Morand S, Krasnov BR, Poulin R (eds) Micromammals and Macroparasites: From Evolutionary Ecology to Management.
- Beaucournu, J.C.; Moreno, L.; González-Acuña, D. Fleas (Insecta-Siphonaptera) of Chile: A review. Zootaxa 2014, 3900, 151–203. [Google Scholar] [CrossRef]
- Lareschi, M.; Sanchez, J.; Autino, A. A review of the fleas (Insecta: Siphonaptera) from Argentina. Zootaxa 2016, 4103, 239–258. [Google Scholar] [CrossRef]
- Sanchez, J.P.; Berrizbeitia, M.F.L.; Ezquiaga, M.C. Host specificity of flea parasites of mammals from the Andean Biogeographic Region. Med Vet Entomol 2023, 37, 511–522. [Google Scholar] [CrossRef]
- Macchiavello, A. Siphonaptera de la Costa Sur-Occidental de América: (Primera lista y distribución Zoo-Geográfica). Bol Of Sanit Panam (Engl) 1948, 27, 412–460. [Google Scholar]
- Johnson, P.T. A classification of the siphonaptera of South America: with descriptions of new species.
- Hopkins, G.H.E.; Rothschild, M. An illustrated catalogue of the Rothschild collection of fleas (Siphonaptera) in the British Museum (Natural History), Volume IV.
- Smit, F.G.A.M. A new species of flea from the Galapagos Islands. Entomolog Ber 1970, 30, 244–247. [Google Scholar]
- Smit, F.G.A.M. A New South American Ceratophyllid bird-flea. Entomolog Ber 1976, 36, 65–67. [Google Scholar]
- Smit, F.G.A.M. A new bird-flea from Peru. Zool J Linn Soc 1978, 62, 189–192. [Google Scholar] [CrossRef]
- Smit, F.G.A.M. An illustrated catalogue of the Rothschild collection of fleas in the British Museum (Natural History) Volume VII. T: Oxford University Press.
- Schramm, B.A.; Lewis, R.E. Four new species of Plocopsylla (Siphonaptera: Stephanocircidae) from South America. J Med Entomol 1987, 24, 399–407. [Google Scholar] [CrossRef]
- Schramm, B.A.; Lewis, R.E. A taxonomic revision of the Flea, genus Plocopsylla Jordan, 1931 (Siphonaptera: Stephanocircidae).
- Hastriter, M.W.; Zyzak, M.D.; Soto, R.; Fernandez, R.; Solorzano, N.; Whiting, M.F. Fleas (Siphonaptera) from Ancash Department, Peru with the description of a new species, Ectinorus Alejoi (Rhopalopsyllidae), and the description of the male of Plocopsylla pallas (Rothschild, 1914) (Stephanocircidae). Annals of Carnegie Museum 2002, 71, 87–106. [Google Scholar] [CrossRef]
- Krasnov, B.R. Functional and evolutionary ecology of fleas: A model for ecological parasitology. Functional and Evolutionary Ecology of Fleas: A Model for Ecological Parasitology. [CrossRef]
- Johnson, K.P.; Weckstein, J.D.; Bush, S.E.; Clayton, D.H. The evolution of host specificity in dove body lice. Parasitology 2011, 138, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.W.; Dittmar, K. Parasitic Bat Flies (Diptera: Streblidae and Nycteribiidae): Host Specificity and Potential as Vectors. In: Klimpel S., Melhorn H. (eds) Bats (Chiroptera) as vectors of diseases and parasites. Parasitology Research Monographs, Volumen 5. H: Springer.
- Mendez, E. Mammalian-siphonapteran associations, the environment, and biogeography of mammals of southwestern Colombia. Quaestiones Entomologicae 1977, 13, 91–182. [Google Scholar]
- Acosta, R. Relación huésped-parásito en pulgas (Insecta: Siphonaptera) y roedores (Mammalia: Rodentia) del estado de Querétaro, México. Folia Entomol Mex 2005, 44, 37–47. [Google Scholar]
- Runghen, R.; Poulin, R.; Monlleó-Borrull, C.; Llopis-Belenguer, C. Network Analysis: Ten Years Shining Light on Host–Parasite Interactions. Trends Parasitol 2021, 37, 445–455. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Peralta, G.; Cagnolo, L.; Santos, M. Ecological interaction networks. What we know, what we don’t, and why it matters. Ecologia Austral 2022, 32, 670–697. [Google Scholar] [CrossRef]
- Delmas, E.; Besson, M.; Brice, M.-H.; et al. Analyzing ecological networks of species interactions. Biological Reviews 2019, 94, 16–36. [Google Scholar] [CrossRef]
- Pilosof, S.; Fortuna, M.A.; Vinarski, M. V.; Korallo-Vinarskaya, N.P.; Krasnov, B.R. Temporal dynamics of direct reciprocal and indirect effects in a host-parasite network. Journal of Animal Ecology 2013, 82, 987–996. [Google Scholar] [CrossRef]
- Llaberia-Robledillo, M.; Balbuena, J.A.; Sarabeev, V.; Llopis-Belenguer, C. Changes in native and introduced host–parasite networks. Biol Invasions 2022, 24, 543–555. [Google Scholar] [CrossRef]
- dos Santos Cardoso, T.; de Andreazzi, C.S.; Junior, A.M.; Gentile, R. Functional traits shape small mammal-helminth network: patterns and processes in species interactions. Parasitology 2021, 148, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Pilosof, S.; Morand, S.; Krasnov, B.R.; Nunn, C.L. Potential parasite transmission in multi-host networks based on parasite sharing. PLoS One 2015, 10, e0117909–10.1371. [Google Scholar] [CrossRef] [PubMed]
- Bellay, S.; Oda, F.H.; Campião, K.M.; Yamada, F.H.; Takemoto, R.M.; de Oliveira, E.F. Host-Parasite Networks: An Integrative Overview with Tropical Examples. In: Dáttilo W, Rico-Gray V (eds) Ecological Networks in the Tropics:an integrative overview of species interactions from some of the most species-rich habitats on Earth.
- Poulin, R. Network analysis shining light on parasite ecology and diversity. Trends Parasitol 2010, 26, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.F.; Whiting, A.S.; Hastriter, M.W.; Dittmar, K. A molecular phylogeny of fleas (Insecta: Siphonaptera): Origins and host associations. Cladistics 2008, 24, 677–707. [Google Scholar] [CrossRef]
- Hastriter, M.W.; Bossard, R.L. Robert E. Lewis World Species Flea (Siphonaptera) List. 20. [CrossRef]
- Pacheco, V.; Diaz, S.; Graham-Angeles, L.; Flores-Quispe, M.; Calizaya-Mamani, G.; Ruelas, D.; Sánchez-Vendizú, P. Updated list of the diversity of mammals from Peru and a proposal for its updating. Rev Peru Biol 2021, 28, e21019–10.15381. [Google Scholar]
- Jameson, E.W. Pleioxenous host-restriction in fleas. J Nat Hist 1985, 19, 861–876. [Google Scholar] [CrossRef]
- Medvedev, S. Specific features of the distribution and host associations of fleas (Siphonaptera). Entomol Rev 2002, 82, 1165–1177. [Google Scholar]
- Llopis-Belenguer, C.; Balbuena, J.A.; Blasco-Costa, I.; Karvonen, A.; Sarabeev, V.; Jokela, J. Sensitivity of bipartite network analyses to incomplete sampling and taxonomic uncertainty. Ecology 2023, 104, e3974–10.1002. [Google Scholar] [CrossRef]
- Fründ, J.; Mccann, K.S.; Williams, N.M. Sampling bias is a challenge for quantifying specialization and network structure: Lessons from a quantitative niche model. Oikos 2016, 125, 502–513. [Google Scholar] [CrossRef]
- Beckett, S.J. Improved community detection in weighted bipartite networks. R Soc Open Sci 2016, 3, 140536–10.1098. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.M.; Pinheiro, R.B.P.; Poulin, R.; Krasnov, B.R.; Mello, M.A.R. The compound topology of host–parasite networks is explained by the integrative hypothesis of specialization. Oikos. [CrossRef]
- Vázquez, D.P.; Melián, C.J.; Williams, N.M.; Blüthgen, N.; Krasnov, B.R.; Poulin, R. Species abundance and asymmetric interaction strength in ecological networks. Oikos. [CrossRef]
- Felix, G.M.; Pinheiro, R.B.P.; Jorge, L.R.; Lewinsohn, T.M. A framework for hierarchical compound topologies in species interaction networks. Oikos 2022, 12, e09538–10.1111. [Google Scholar] [CrossRef]
- Messeder, J.V.S.; Guerra, T.J.; Dáttilo, W.; Silveira, F.A.O. Searching for keystone plant resources in fruit-frugivore interaction networks across the Neotropics. Biotropica 2020, 52, 857–870. [Google Scholar] [CrossRef]
- Guimerà, R.; Amaral, L.A.N. Functional cartography of complex metabolic networks. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc Natl Acad Sci U S A 2007, 104, 19891–19896. [Google Scholar] [CrossRef]
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite Package: Analysing Ecological Networks. R new 2008, 8, 8–11. [Google Scholar]
- Hastriter, M.W.; Schlatter, R.P. Revision of the fleas in the subgenus Dasypsyllus (Neornipsyllus) (Siphonaptera: Ceratophyllidae). Annals of Carnegie Museum 2006, 75, 247–257. [Google Scholar] [CrossRef]
- Zurita, A.; Callejón, R.; De Rojas, M.; Cutillas, C. Morphological and molecular study of the genus Nosopsyllus (Siphonaptera: Ceratophyllidae). Nosopsyllus barbarus (Jordan & Rothschild 1912) as a junior synonym of Nosopsyllus fasciatus (Bosc d’Antic, 1800). Insect Syst Evol 2018, 49, 81–101. [Google Scholar]
- Jordan, K. Notes on a collection of fleas from Peru. Bull World Hlth Org 1950, 2, 597–609. [Google Scholar]
- Smit, F.G.A.M. Key to the genera and subgenera of Ceratophyllidae. In: Traub R, Rothschild M, Haddow J (eds) The Rothschild collection of fleas. The Ceratophyllidae: key to the genera and host relationships.
- Johnson, P.T. Notes on Pleochaetis Jordan, 1933, from Colombia, with the description of a new species (Siphonaptera: Ceratophyllidae). Journal of the Washington Academy of Sciences 1954, 44, 289–296. [Google Scholar]
- Johnson, P.T. Myodopsylla setosa and Tiarapsylla bella, New Species of Fleas from Peru. Journal of the New York Entomological Society 1954, 62, 193–205. [Google Scholar]
- Autino, A.G.; Claps, G.L.; Barquez, R.M.; Díaz, M.M. Ectoparasitic insects (Diptera: Streblidae and siphonaptera: Ischnopsyllidae) of bats from iquitos and surrounding areas (Loreto, Peru). Mem Inst Oswaldo Cruz 2011, 106, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Darskaya, N.F.; Malygin, V.M. O blokhakh mlekopitaiushchikh iz basseĭna reki Ukaiali (Peruanskaia Amazoniia) [On the fleas of mammals from the Ucayali river basin, Peruvian Amazonia]. Parazitologiya 1996, 30, 187–190. [Google Scholar]
- Zurita, A.; Rivero, J.; García-Sánchez, Á.M.; Callejón, R.; Cutillas, C. Morphological, molecular and phylogenetic characterization of Leptopsylla segnis and Leptopsylla taschenbergi (Siphonaptera). Zool Scr 2022, 51, 741–754. [Google Scholar] [CrossRef]
- Traub, R. Records and descriptions of fleas from Peru. Proc Entomol Soc Wash 1952, 54, 1–22. [Google Scholar]
- López-Berrizbeitia, M.F.; Sanchez, J.; Barquez, R.M.; Díaz, M. Taxonomic revision of the flea genus Agastopsylla Jordan & Rothschild 1923 (Siphonaptera: Ctenophthalmidae). An Acad Bras Cienc 2020, 92, e20181136–10.1590. [Google Scholar]
- Jordan, K. Some Siphonaptera from South America. Novitates zoologicae : a journal of zoology in connection with the Tring Museum 1936, 39, 305–310.
- Sanchez, J.; Amor, V.; Bazán-León, E.A.; Vásquez, R.A.; Lareschi, M. Redescription of Neotyphloceras chilensis Jordan, new status (Siphonaptera: Ctenophthalmidae: Neotyphloceratini). Zootaxa 2012, 3259, 51–57. [Google Scholar] [CrossRef]
- Rothschild, N.C. New Siphonaptera from Peru. Novitates zoologicae : a journal of zoology in connection with the Tring Museum 1914, 21, 239–251. [Google Scholar] [CrossRef]
- Fuller, H.S. Notes on Neotropical Siphonaptera. Revista de Entomología 1942, 13, 39–44. [Google Scholar]
- Sanchez, J.; Lareschi, M. Two new species of Neotyphloceras (Siphonaptera: Ctenophthalmidae) from Argentinean Patagonia. Zootaxa 2014, 3784, 159–170. [Google Scholar] [CrossRef]
- López Berrizbeitia, M.F.; Sanchez, J.; Díaz, M.M.; Barquez, R.M.; Lareschi, M. Redescription of Neotyphloceras crassispina hemisus Jordan (Siphonaptera: Ctenophthalmidae: Neotyphloceratini). Journal of Parasitology 2015, 101, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Lareschi, M.; Salazar-Bravo, J.; Gardner, S.L. Fleas of the genus Neotyphloceras associated with rodents from Bolivia: new host and distributional records, description of a new species and remarks on the morphology of Neotyphloceras rosenbergi. Med Vet Entomol 2018, 32, 462–472. [Google Scholar] [CrossRef] [PubMed]
- López Berrizbeitia, M.F.; Hastriter, M.W.; Díaz, M.M. A new flea species of the genus cleopsylla (Siphonaptera: Stephanocircidae) from Northwestern Argentina. Journal of Parasitology 2016, 102, 514–519. [Google Scholar] [CrossRef]
- Pozo, E.J.; Troncos, G.; Palacios F, A.; Arévalo, F.; Carrión, G.; Laguna-Torres, A. Distribución y hospederos de pulgas (Siphonaptera) en la provincia de Ayabaca, Piura-1999. Rev Peru Med Exp Salud Publica 2005, 22, 316–320. [Google Scholar]
- Lareschi, M.; Autino, A.G.; Díaz, M.M.; Barquez, R.M. Taxonomy and distribution of Nonnapsylla wagner, 1938 (Siphonaptera: Stephanocircidae: Craneopsyllinae). Journal of Parasitology 2011, 97, 954–955. [Google Scholar] [CrossRef] [PubMed]
- Pucu, E.; Lareschi, M.; Gardner, S.L. Bolivian ectoparasites: A survey of the fleas of ctenomys (Rodentia: Ctenomyidae). Comp Parasitol 2014, 81, 114–118. [Google Scholar] [CrossRef]
- Jordan, K. Three new South American fleas. Novitates zoologicae : a journal of zoology in connection with the Tring Museum 1931, 36, 311–316.
- Estares P., L.; Chávez V., A.; Casas A., E. Ectoparásitos en caninos de los distritos de la zona climática norte de Lima Metropolitana. Revista de Investigaciones Veterinarias del Perú 2000, 11, 72–76. [Google Scholar] [CrossRef]
- Nuntón, J.; Quintana, H.; Vivar, E. Prevalencia de ectoparásitos y endoparásitos en Canis Familiaris sacrificados en Tumbes; julio - diciembre, 2013. Manglar 2015, 10, 93–97. [Google Scholar] [CrossRef]
- Flores-Mendoza, C.; Florin, D.; Felices, V.; Pozo, E.J.; Graf, P.C.F.; Burrus, R.G.; Richards, A.L. Detection of Rickettsia parkeri from within Piura, Peru, and the first reported presence of candidatus rickettsia andeanae in the tick Rhipicephalus sanguineus. Vector-Borne and Zoonotic Diseases 2013, 13, 505–508. [Google Scholar] [CrossRef]
- Rizzo, M.F.; Billeter, S.A.; Osikowicz, L.; Luna-Caipo, D. V.; Cáceres, A.G.; Kosoy, M. Fleas and Flea-associated Bartonella species in dogs and cats from Peru. J Med Entomol 2015, 52, 1374–1377. [Google Scholar] [CrossRef]
- Rizzo, M.F.; Osikowicz, L.; Cáceres, A.G.; Luna-Caipo, V.D.; Suarez-Puyen, S.M.; Bai, Y.; Kosoy, M. Identification of Bartonella rochalimae in Guinea Pigs (Cavia porcellus) and fleas collected from rural peruvian households. American Journal of Tropical Medicine and Hygiene 2019, 101, 1276–10.4269. [Google Scholar] [CrossRef] [PubMed]
- Dittmar De La Cruz, K.; Ribbeck, R.; Daugschies, A. Vorkommen und verbreitung von ektoparasiten bei meerschweinchen (Cavia spp.) in Peru, Südamerika. Berl Munch Tierarztl Wochenschr 2003, 116, 102–107. [Google Scholar]
- Naupay I., A.; Castro H., J.; Caro C., J.; Sevilla D., L.; Hermosilla J., J.; Larraín L., K.; Quispe S., C.; Panana R., O. Ectoparásitos en palomas Columba livia comercializadas en un mercado del distrito de San Martín de Porres, Lima, Perú. Revista de Investigaciones Veterinarias del Perú 2015, 26, 259–265. [Google Scholar] [CrossRef]
- Castro, J.; Naupay, A.; Orozco, K.; Rodríguez, S.; Díaz, Y.; Navarro, J.; Purca, N. Ectoparásitos de Columba livia linnaeus, 1758 (aves columbiformes) del distrito de Carmen de la Legua, callao, Perú. The Biologist 2018, 15, 425–435. [Google Scholar] [CrossRef]
- Ewing, H.E. (Henry E. New North American Siphonaptera. Proceedings of the Biological Society of Washington 1940, 53, 35–37. [Google Scholar]
- Lewis, R.E. Notes on the Geographical Distribution and Host Preferences in the Order Siphonaptera: Part 1. Pulicidae1, 2. J Med Entomol 1972, 9, 511–520. [Google Scholar] [CrossRef]
- Lareschi, M.; Venzal, J.M.; Nava, S.; Mangold, A.J.; Portillo, A.; Palomar-Urbina, A.M.; Oteo-Revuelta, J.A. The human flea Pulex irritans (Siphonaptera: Pulicidae) in northwestern Argentina, with an investigation of Bartonella and Rickettsia spp. Rev Mex Biodivers 2018, 89, 375–381. [Google Scholar] [CrossRef]
- Dittmar, K. Evaluation of ectoparasites on the guinea pig mummies of El Yaral and Moquegua Valley, in Southern Peru. Chungara 2000, 32, 123–125. [Google Scholar] [CrossRef]
- Dittmar, K.; Mamat, U.; Whiting, M.; Goldmann, T.; Reinhard, K.; Guillen, S. Techniques of DNA-studies on Prehispanic Ectoparasites (Pulex sp., Pulicidae, Siphonaptera) from Animal Mummies of the Chiribaya Culture, Southern Peru. Mem Inst Oswaldo Cruz 2003, 98, 53–58. [Google Scholar] [CrossRef]
- Ortiz-Cusma, J.F.; Martínez-Bravo, K.M.; Iglesias-Osores, S. Prevalencia de Xenopsylla Cheopis en Rattus Rattus en el distrito de Salas, Lambayeque. Revista de la Facultad de Medicina Humana 2022, 22, 258–265. [Google Scholar] [CrossRef]
- Boyer, S.; Gillespie, T.R.; Miarinjara, A. Xenopsylla cheopis (rat flea). Trends Parasitol 2022, 38, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Gárate, I.; Jiménez, P.; Flores, K.; Espinoza, B. Registro de Xenopsylla cheopis como hospedero intermediario natural de Hymenolepis diminuta en Lima, Perú. Rev Peru Biol 2011, 18, 249–252. [Google Scholar] [CrossRef]
- Smit, F.G.A.M. Siphonaptera (Fleas). In: Smit FGV (ed) Insect and other arthropods of medical importance.
- Jordan, K. On Parapsyllus and some closely related genera of Siphonaptera. Eos (Washington DC) 1942, 18, 7–29. [Google Scholar]
- Hastriter, M.W.; Sage, R.D. A description of two new species of Ectinorus (Siphonaptera: Rhopalopsyllidae) from Laguna Blanca National Park, Neuquén Province, Argentina. Proc Entomol Soc Wash 2009, 111, 581–597. [Google Scholar] [CrossRef]
- Wagner, J. Aphaniptera aus Süd-Peru sowie Bemerkungen über die Fam. Stephanocircidae Wagn. Zeitschrift für Parasitenkunde 1937, 9, 698–716. [Google Scholar] [CrossRef]
- López-Berrizbeitia, M.F.; Pérez, M.J.; Barquez, R.M. Description of two new species of Ectinorus (Ectinorus) (Siphonaptera, Rhopalopsyllidae) from Argentina, including a morphometric approach. Acta Trop 2020, 211, 105612–10.1016. [Google Scholar] [CrossRef]
- Hastriter, M. A description of four new species of fleas (Insecta, Siphonaptera) from Angola, Ethiopia, Papua New Guinea, and Peru. Zookeys 2009, 8, 39–61. [Google Scholar] [CrossRef]
- Beaucournu, J.-C.; Gallardo, M.H. Siphonaptères du Chili; description de quatre espèces nouvelles. Bull Soc Entomol France 1991, 96, 185–203. [Google Scholar] [CrossRef]
- Beaucournu, J.C.; Belaz, S.; Muñoz-Léal, S.; González-Acuña, D. A new flea, Ectinorus (Ectinorus) insignis n. sp. (Siphonaptera, Rhopalopsyllidae, Parapsyllinae), with notes on the subgenus Ectinorus in Chile and comments on unciform sclerotization in the superfamily Malacopsylloidea. Parasite 2013, 20, 1–12. [Google Scholar] [CrossRef]
- López Berrizbeitia, M.F.; Hastriter, M.W.; Barquez, R.M.; Díaz, M.M. Fleas of the genus Tetrapsyllus (Siphonaptera:Rhopalopsyllidae) associated with rodents from Northwestern Argentina. Int J Parasitol Parasites Wildl 2019, 9, 80–89. [Google Scholar] [CrossRef]
- Gimenez, D.F.; Ciccarelli, C.A.; de la Barrera, J.M. Siphonaptera de Mendoza. An Soc Cient Argent 1964, 178, 125–139. [Google Scholar]
- Beaucournu, J.-C.; Gallardo, M.H. Contribution à la faune du Chili. Puces nouvelles de la moitié nord [Siphonaptera]. Bull Soc Entomol France 1989, 94, 181–188. [Google Scholar] [CrossRef]
- Wells, E.A.; D’Alessandro, A.; Morales, G.A.; Angel, D. Mammalian wildlife diseases as hazards to man and livestock in an area of the Llanos Orientales of Colombia. J Wildl Dis 1981, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rafael, J.A. Notas & Comunicações Ocorrência de Polygenis Klagesi samuelis na Amazônia brasileira (Siphonaptera : Rhopaiopsyllidae). Acta Amazon 1982, 12, 231–232. [Google Scholar]
- Macchiavello, A. Estudios sobre peste selvetica en America del Sur: IV. Transmision experimental de la peste por polygenis litargus. Boletín de la Oficina Sanitaria Panamericana (OSP); 45 (2), ago 1958 1958, 45, 122–131. [Google Scholar]
- Zurita, A.; Lareschi, M.; Cutillas, C. New insights into the taxonomy of Malacopsylloidea superfamily (Siphonaptera) based on morphological, molecular and phylogenetic characterization of Phthiropsylla agenoris (Malacopsyllidae) and Polygenis (Polygenis) rimatus (Rhopalopsyllidae). Diversity (Basel) 2023, 15, 308–10.3390. [Google Scholar] [CrossRef]
- Lareschi, M.; Linardi, P.M.; Autino, A.G.; Barquez, R.M.; Díaz, M.M. First report of Polygenis (Polygenis) roberti beebei (Fox, 1947) (Siphonaptera: Rhopalopsyllidae) in Argentina, with a new host record and morphological data. Syst Parasitol 2003, 56, 183–187. [Google Scholar] [CrossRef]
- Traub, R.; Johnson, P.T. Fleas collected during a plague survey in Venezuela. Bol Oficina Sanit Panam 1952, 32, 111–135. [Google Scholar]
- Durden, L.A.; Campbell, D.C. Fleas, Lice, and Epifaunistic Pseudoscorpions of Some Native Mammals in Northwestern Costa Rica. Comp Parasitol 2016, 83, 240–244. [Google Scholar] [CrossRef]
- Ramos-Yana, V.S.; Mollericona-Quispe, J.L.; Arteaga, D.; Bernal-Hoverud, N. Distribución y caracterización morfológica y molecular de pulgas (Insecta, Siphonaptera), sus hospederos roedores e implicancia en salud humana, Parque Madidi. Bio Scientia 2021, 4, 4–5. [Google Scholar]
- Lareschi, M.; Linardi, P.M. New data on the morphology of Polygenis (Polygenis) rimatus (Jordan)(Siphonaptera: Rhopalopsyllidae). Neotrop Entomol 2005, 34, 121–125. [Google Scholar] [CrossRef]
- Durden, L.A.; Bermúdez, S.; Vargas, G.A.; Sanjur, B.E.; Gillen, L.; Brown, L.D.; Greiman, S.E.; Eremeeva, M.E. Fleas (Siphonaptera) Parasitizing Peridomestic and Indigenous Mammals in Panamá and Screening of Selected Fleas for Vector-Borne Bacterial Pathogens. J Med Entomol 2021, 58, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Rothschild, N.C. On the genera Rhopalopsyllus and Parapsyllus. Ectoparasites 1923, 1, 320–370. [Google Scholar]
- Jordan, K.; Rothschild, N.C. Revision of the non-combed eyed siphonaptera. Parasitology 1908, 1, 1–100. [Google Scholar] [CrossRef]
- Bermúdez, S.C.; Miranda, R.C. Distribution of ectoparasites of Canis lupus familiaris L. (Carnivora: Canidae) from Panama. Rev MVZ Cordoba 2011, 16, 2274–2282. [Google Scholar]
- Lewis, R.E. New species of Palaeopsylla Wagner, 1903, from Nepal, with a discussion of the Remota species group (Siphonaptera: Hystrichopsyllidae). J Parasitol 1973, 59, 187–197. [Google Scholar] [CrossRef]
- Del Ponte, E. Notas sobre Suctoria argentinos. V. Nuevos datos sobre Rhopalopsyllidae. Rhopalopsyllinae. Rev Soc Entomol Argent 1963, 26, 1–4. [Google Scholar]
- Tipton, V.J.; Machado-Allison, C.E. Fleas of Venezuela. Brigham Young University Science Bulletin - Biological Series 1972, 17, 1–115. [Google Scholar]
- Botelho, J.R.; Linardi, P.M.; Williams, P.; Nagem, R.L. Alguns hospedeiros reais de ectoparasitos do município de Caratinga, Minas Gerais, Brasil. Mem Inst Oswaldo Cruz 1981, 76, 57–59. [Google Scholar] [CrossRef]
- Linardi, P.M. Checklist de Siphonaptera (Insecta) do Estado de São Paulo. Biota Neotrop 2011, 11, 607–617. [Google Scholar] [CrossRef]
- da Costa, A.L.M.; Teixeira, R.H.F.; Paschoalotti, M.H.; Gomes, R.P.; Felippi, D.A.; Franco, P.N. Controle de pulgas em cervo-do-pantanal (Blastocerus dichotomus) de cativeiro com uso de coleira com imidacloprida e flumetrina. Veterinária e Zootecnia 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Dittmar, K. Arthropod and helminth parasites of the wild guinea pig, Cavia aperea, from the Andes and the Cordillera in Peru, South America. Journal of Parasitology 2002, 88, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.M.; Kutzscher, C.; Masello, J.F.; Pilgrim, R.L.C.; Quillfeldt, P. Stick-tight fleas in the nostrils and below the tongue: Evolution of an extraordinary infestation site in Hectopsylla (Siphonaptera: Pulicidae). Zool J Linn Soc 2007, 149, 117–137. [Google Scholar] [CrossRef]
- Macchiavello, A. Epidemiology of Plague in Ecuador. Am J Public Health Nations Health 1943, 33, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.J. Plague in the City of Ambato, Ecuador. Proceedings 6th Pacific Sci Congr 1942, 5, 139–143. [Google Scholar]
- Macchiavello, A. A Note on Plague in Chile. Bol Oficina Sanit Panam 1933, 11, 909–915. [Google Scholar]
- Autino, A.G.; Claps, G.L.; Barquez, R.M. Insectos ectoparásitos de murciélagos de las Yungas de la Argentina. Acta Zool Mex 1999, 78, 119–169. [Google Scholar] [CrossRef]
- Autino, A.G.; Claps, G.E. Catalogue of the ectoparasitic insects of the bats of Argentina. Insecta mundi 2000, 14, 193–209. [Google Scholar]
- Linardi, P.M.; Guimarães, L.R. Sifonápteros do Brasil. Universidade De São Paulo.
- Luz, J.L.; de Moraes Costa, L.; Gomes, L.A.C.; Esbérard, C.E.L. The chiggerflea Hectopsylla pulex (Siphonaptera: Tungidae) as an ectoparasite of free-tailed bats (Chiroptera: Molossidae). Mem Inst Oswaldo Cruz 2009, 104, 567–569. [Google Scholar] [CrossRef]
- Ramírez-Chaves, H.E.; Tamayo-Zuluaga, A.F.; Henao-Osorio, J.J.; Cardona-Giraldo, A.; Ossa-López, P.A.; Rivera-Páez, F.A. The chiggerflea hectopsylla pulex (Siphonaptera: Tungidae): Infestation on molossus molossus (chiroptera: Molossidae) in the central andes of Colombia. Zoologia 2020, 37, e53092–10.3897. [Google Scholar] [CrossRef]
- Fioravanti, M.L.; Gustinelli, A.; Onore, G.; Pampiglione, S.; Trentini, M. Presence of Tunga trimamillata (Insecta, Siphonaptera) in Peru. Parasite 2006, 13, 85–86. [Google Scholar] [PubMed]
- Maco, V.; Maco, V.P.; Gotuzzo, E. Case report: An ectopic case of Tunga spp. infection in Peru. American Journal of Tropical Medicine and Hygiene 2010, 82, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Linardi, P.M.; De Avelar, D.M.; Facury Filho, E.J. Establishment of Tunga trimamillata (Siphonaptera: Tungidae) in Brazil. Parasitol Res 2013, 112, 3239–3242. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, S.G.; Lobanov, A.L. Information-Analytic System of the World Fauna of Fleas (Siphonaptera): Results and Prospects. Entomologicheskoe Obozrenie 1999, 78, 732–748. [Google Scholar]
- Medvedev, S.G. An Attempted system analysis of the evolution of the order of fleas (Siphonaptera). Lectures in memoriam N.A.
- Medvedev, S.G. Host-parasite relations in fleas (Siphonaptera). I. Entomol Rev 1997, 77, 200–215. [Google Scholar]
- Medvedev S., G. Host-parasite relations of fleas (Siphonaptera). II. Entomologicheskoe Obozrenie 1997, 76, 511–521. [Google Scholar]
- Vatschenok, V.S. Fleas–vectors of pathogens causing diseases in humans and animals. N: Leningrad, USSR.
- Whitaker, J.O.; Wren, Jr.W.J.; Lewis, R.E. Parasites. In: Genoways HH, Brown JH (eds) Biology of the Heteromyidae. Special Publication. N. 10. P: American Society of Mammalogists.
- Linardi, P.M.; Botelho, J.R.; Ximenez, A.; Padovani, C.R. Notes on ectoparasites of some small mammals from Santa Catarina State, Brazil. J Med Entomol 1991, 28, 183–185. [Google Scholar] [CrossRef]
- De Moraes, L.B.; Paolinetti Bossi, D.E.; Linhares, A.X. Siphonaptera Parasites of Wild Rodents and Marsupials Trapped in Three Mountain Ranges of the Atlantic Forest in Southeastern Brazil. Mem Inst Oswaldo Cruz 2003, 98, 1071–1076. [Google Scholar] [CrossRef]
- Pinto, I.D.S.; Botelho, J.R.; Costa, L.P.; Leite, Y.L.R.; Linardi, P.M. Siphonaptera associated with wild mammals from the central atlantic forest biodiversity corridor in southeastern Brazil. J Med Entomol 2009, 46, 1146–1151. [Google Scholar] [CrossRef]
- Lareschi, M. The relationship of sex and ectoparasite infestation in the water rat Scapteromys aquaticus (Rodentia: Cricetidae) in La Plata, Argentina. Rev Biol Trop 2006, 54, 673–679. [Google Scholar] [CrossRef]
- Lareschi, M.; Krasnov, B.R. Determinants of ectoparasite assemblage structure on rodent hosts from South American marshlands: The effect of host species, locality and season. Med Vet Entomol 2010, 24, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Landaeta-Aqueveque, C.; Moreno Salas, L.; Henríquez, A.L.; Silva-de la Fuente, M.C.; González-Acuña, D. Parasites of Native and Invasive Rodents in Chile: Ecological and Human Health Needs. Front Vet Sci 2021, 8, 643742–10.3389. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.B.P.; Felix, G.M.F.; Lewinsohn, T.M. Hierarchical compound topology uncovers complex structure of species interaction networks. Journal of Animal Ecology 2022, 91, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.K.; Sukhdeo, M.V.K. Host centrality in food web networks determines parasite diversity. PLoS One 2011, 6, e26798–10.1371. [Google Scholar] [CrossRef] [PubMed]
- Dallas, T.; Cornelius, E. Co-extinction in a host-parasite network: Identifying key hosts for network stability. Sci Rep 2015, 5, 13185–10.1038. [Google Scholar] [CrossRef]
- Gómez, J.M.; Nunn, C.L.; Verdú, M. Centrality in primate-parasite networks reveals the potential for the transmission of emerging infectious diseases to humans. Proc Natl Acad Sci U S A 2013, 110, 7738–7741. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
| Network metric |
Value | z-score (free) |
p-value (free) |
z-score (rest) |
p-value (rest) |
|---|---|---|---|---|---|
| Q | 0.57 | 9.52 | <0.001 | NA | NA |
| NODFtotal | 10.57 | -0.56 | 0.69 | 1.54 | 0.05 |
| NODFsm | 33.26 | 14.36 | <0.001 | 0.71 | 0.30 |
| NODFdm | 5.00 | -6.66 | <0.001 | 2.17 | <0.001 |
| Normalized degree centrality | Betweenness centrality | ||
|---|---|---|---|
| Species | Value | Species | Value |
| Akodon mollis | 0.254 | Akodon mollis | 0.200 |
| Polygenis litargus | 0.250 | Neotyphloceras crassispina | 0.153 |
| Pulex irritans | 0.203 | Rattus norvegicus | 0.128 |
| Rattus rattus | 0.194 | Phyllotis andium | 0.124 |
| Neotyphloceras crassispina | 0.188 | Polygenis litargus | 0.123 |
| Cleopsylla townsendi | 0.188 | Pulex irritans | 0.123 |
| Echidnophaga gallinacea | 0.188 | Aegialomys xanthaeolus | 0.107 |
| Rattus norvegicus | 0.164 | Cleopsylla townsendi | 0.099 |
| Phyllotis andium | 0.149 | Phyllotis amicus | 0.089 |
| Ctenocephalides canis | 0.141 | Tiamastus cavicola | 0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





