1. Introduction
Selenium compounds have garnered significant attention in the field of medicinal chemistry due to their unique biochemical properties and potential therapeutic applications.[
1,
2] Selenium, an essential trace element, is crucial for the proper functioning of several enzymes and proteins, particularly those involved in antioxidant defense and redox regulation.[
3,
4] The incorporation of selenium into organic molecules has led to the development of novel compounds with diverse pharmacological activities, including antifungal, anticancer, antiviral, antimicrobial, and anti-inflammatory effects. [
5,
6,
7,
8] These compounds often exhibit enhanced efficacy and selectivity compared to their sulfur or oxygen analogs. As our understanding of selenium’s role in human health deepens, the design and application of selenium-based therapeutics continue to expand, offering promising avenues for the treatment of various diseases.
The synthesis of organoselenium compounds has attracted a lot of interest in organic chemistry due to the unique chemical properties and biological activities of selenated compounds. Selenium, being chemically similar to sulfur, imparts distinct reactivity patterns that make its incorporation into organic molecules particularly valuable in synthetic strategies.[
9,
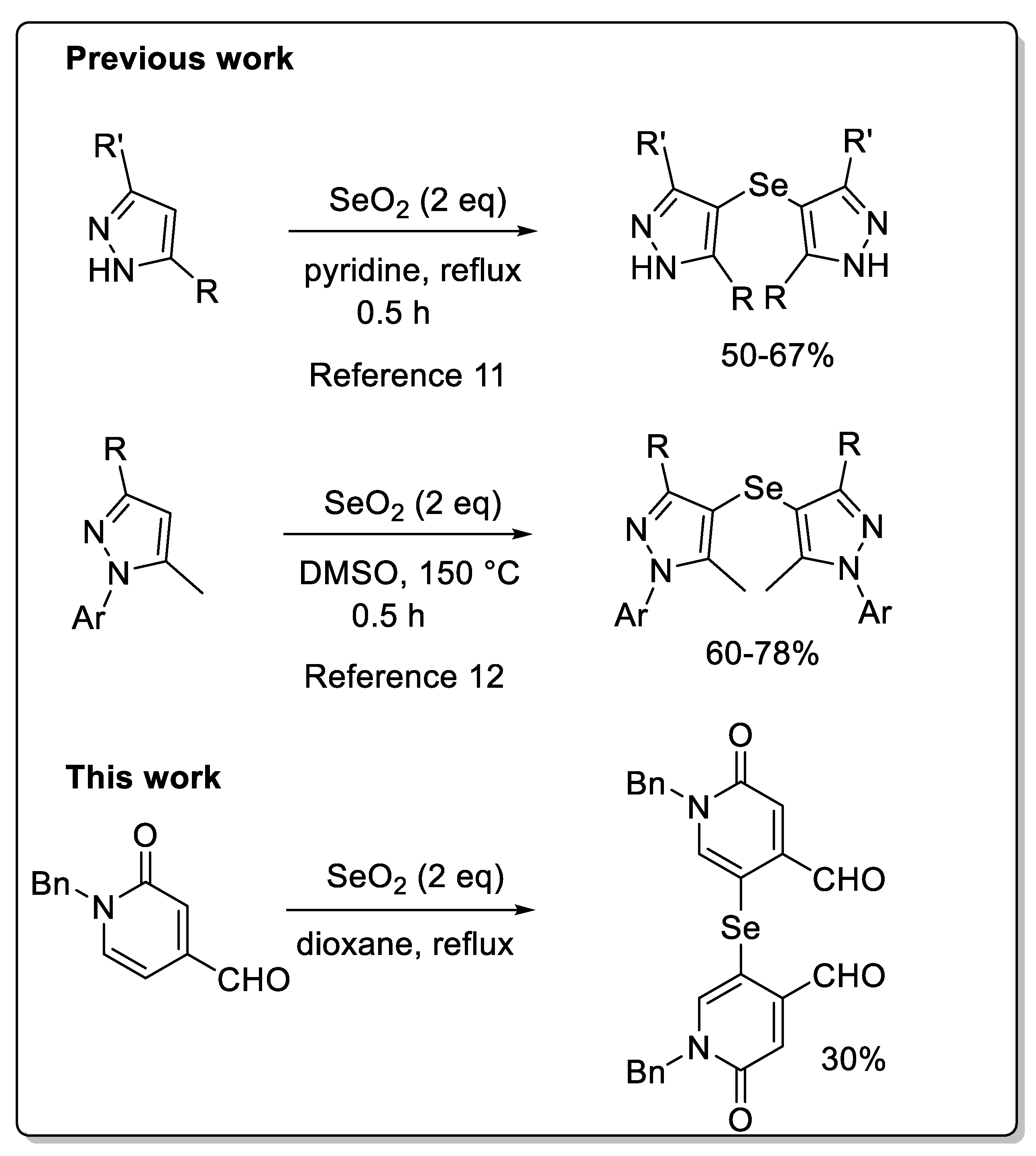
10] Gütlich et al. described a reaction between 3- and 3,5-substituted pyrazoles with selenium dioxide proceeds to afford bis(3R,5R0 -1H-pyrazol-4-yl)selenides in high yield (
Figure 1).[
11] Sawant et al. showed that mono- and di-selenylated bis-pyrazole derivatives could be obtained from arylated pyrazole in the presence of SeO2 and DMSO as solvent. [
12]
Continuing our research into the discovery of new antimycobacterials, [
13,
14,
15,
16] we have isolated an unusual selenium derivative as a by-product in an oxidation reaction. In this work, the synthesis and characterization of this compound is described.
2. Results and Discussion
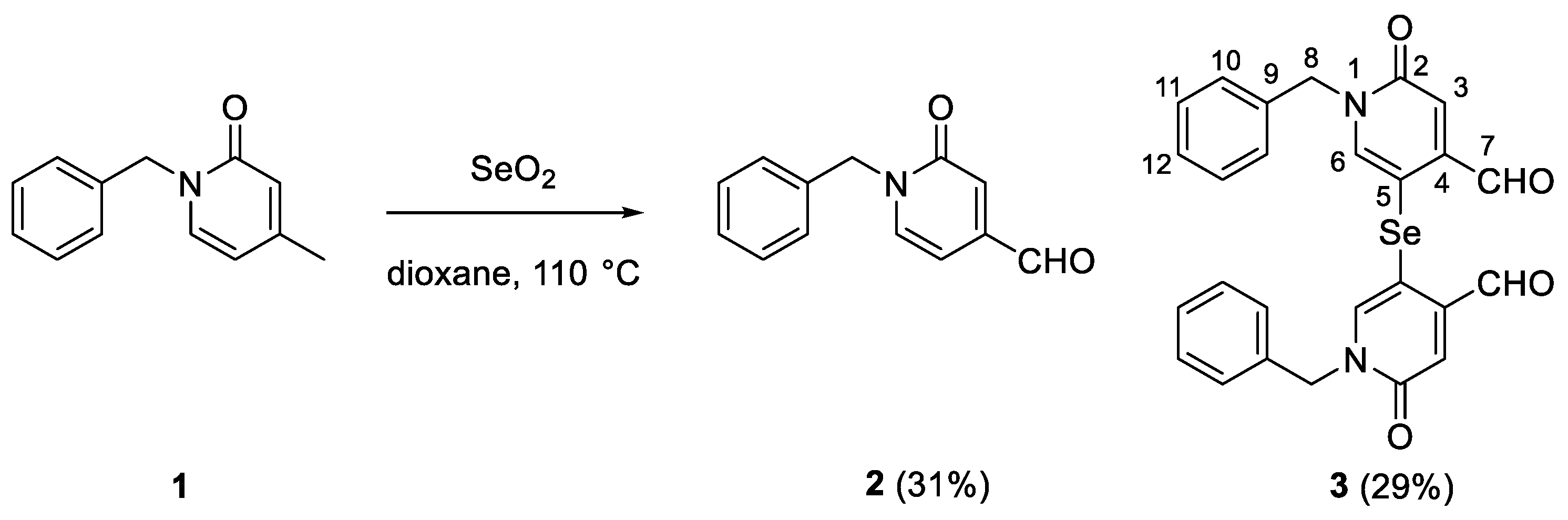
The synthesis of compound
2 involved the initial preparation of compound
1 (
Scheme 1).[
17] Then compound
1 was reacted with selenium oxide in dioxane at 110 °C to produce aldehyde
2 in 31% yield. During purification, another product was isolated in 29% yield which, on the basis of previous work and by comparison with the NMR of compound 2, we have attributed to the structure of the selenylated product
3 (
Scheme 1) [
11,
12].
Unfortunately, attempts to obtain suitable crystals of compound 3 for an X-ray structure determination using different solvents were not successful. Consequently, the structure was elucidated on the basis of 1H and 13C NMR analyses and mass spectrometry. The mass spectrum in negative mode of compound 3 showed a signal at m/z 503.0524 matching the theoretical mass of the deprotonated molecule (M-H+, 503.0510), with an additionnal signal at 549.0565 corresponding to the formic acid (used for the mass analy-sis) adduct (M-H+/HCOOH, theoretical mass 549.0565), both signals displaying the typi-cal pattern (
76Se, 9.37 %,
77Se, 7.63 %,
78Se, 23.77 %,
80Se, 49.61%,
82Se, 8.73 %) of molecules containing one selenium atom.
Heteronuclear Single Quantum Correlation spectroscopy (HSQC) was used to assign 13C signals of compound 3 as shown in Table 1.
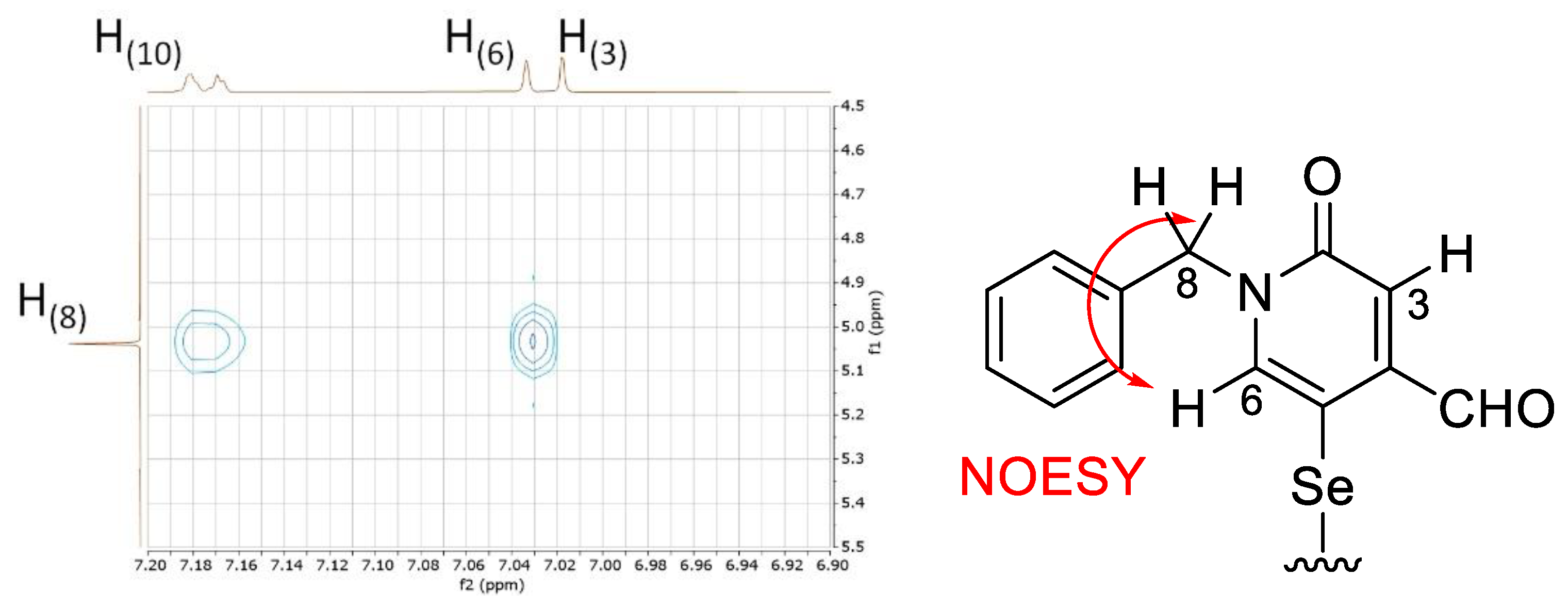
However, an ambiguity remained concerning the position of selenium on the heterocycle. On the
1H-NMR spectrum, the absence of coupling between the two protons of the heterocycle, which appear as singlets at 6.99 and 7.01 ppm, shows that selenium is not in position 3 on the ring. A correlation observed in the Nuclear Overhauser Effect spectroscopy (NOESY) spectrum between an heterocyclic proton (
i.e., H6 proton) and the benzylic protons (H8) leads to the conclusion that selenium should be located at position 5 (
Figure 2).
3. Materials and methods
All reagents and solvents were purchased from commercial sources (Sigma Aldrich or Alfa Aesar) and were used without further purification. 1H-NMR and 13C-NMR spectra were recorded on Bruker Advance 300 and 500 spectrometers, respectively, and the residual proton signals of deuterated CDCl3 were used as an internal reference (δ = 7.26 ppm and 77.00 ppm, respectively). Proton coupling patterns are abbreviated as “s” for singlet, “dd” for doublet of doublets and “m” for multiplet. The high-resolution mass spectrum (HRMS) was recorded on a Xevo G2 QTOF (Waters, Milford, USA).
Synthesis of compounds 2 and 3:
To a 25 mL round bottomed flask containing the 1-benzyl-4-methylpyridin-2(1H)-one (0.25 mmol, 50 mg, 1 equiv.), were added selenium oxide (0.3 mmol, 33.3 mg, 1.2 equiv.). under argon followed by anhydrous 1,4-dioxane (0.1 mL). The mixture was warmed up to reflux for 20 h, then cooled down at room temperature. Ethyl acetate was added and the resulting mixture was dried over magnesium sulfate and the solvent was concentrated under vacuum. The purification of the products was performed by using a 12 g prepacked column on a flash chromatography (25 mL/min, detector at 254 nm, 2 min at 40-60 petroleum ether – ethyl acetate, then gradient from 40-60 to 10-90 petroleum ether – ethyl acetate in 20 min).
1-Benzyl-2-oxo-1,2-dihydropyridine-4-carbaldehyde (2) – Lightly yellow powder (31%, 17 mg). 1H-NMR (300 MHz, DMSO-D6): δ ppm 9.87 (s, 1H); 7.40-7.28 (m, 6H); 7.04 (dd, J = 1.8 Hz, 0.5 Hz, 1H); 6.55 (dd, J = 7.0 Hz, 1.9 Hz, 1H); 5.16 (s, 2H); 13C-NMR (75 MHz, DMSO-D6): δ ppm 191.12; 162.64; 144.87; 138.55; 135.56; 129.03; 128.35; 128.21; 126.63; 101.05; 52.27; HRMS Calculated for C13H12NO2 (ESI, M+H+): 214.0868. Found: 214.0873.
3,3’-Selenobis(1-benzyl-2-oxo-1,2-dihydropyridine-4-carbaldehyde) (3) – Orange powder (18.5 mg, 29%). 1H-NMR (300 MHz, DMSO-D6): δ ppm 9.86 (s, 1H); 7.36 (m, 1H); 7.31 (m, 2H); 7.15 (m, 2H); 7.01 (s, 1H); 6.99 (s, 1H); 5.01 (s, 2H); 13C-NMR (150 MHz, DMSO-D6): δ ppm 191.78; 161.40; 143.05; 141.28; 134.48; 129.32; 128.95; 128.81; 126.06; 103.00; 52.31; HRMS Calculated for C26H19N2O4Se (ESI, M-H+ and M + HCOO-): 503.0510 and 549.0565. Found: 503.0524 and 549.0565.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, 1H, 13C spectra of compound 2; 1H, 13C, COSY, HSQC, NOESY spectra of compounds 3; HRMS spectrum of compound 3.
Author Contributions
EG: Investigation, Writing – original draft; JR: Investigation; SAA: Investigation; JAD: Investigation; PH: Investigation, Writing – review & editing; CL: Conceptualization, Investigation, Writing – review & editing.
Funding
This research was funded by the Centre Nationnal de la Recherche Scientifique (CNRS) and Toulouse 3 University.
Acknowledgements
The authors thank Marc Vedrenne and Nathalie Martins-Froment (Institut de Chimie de Toulouse platform, UAR2599, Université Toulouse 3 Paul Sabatier, CNRS, France-
https://ict.cnrs.fr) for their technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ramos-Inza, S.; Plano, D.; Sammartin, C. Metal-based compounds containing selinium : an appealing approach towards novel therapeutic drugs with cancer and antimicrobial effects. Eur. J. Med. Chem. 2022, 244, 114834. [Google Scholar] [CrossRef]
- Hou, W.; Xu, H. Incorporating selenium into heterocycles and natural products – From chemical properties to pharmacological activities. J. Med. Chem. 2022, 65, 4436–4456. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M. P. Selenium and human health. The Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, J.M.; Garje, R.; Sieren, J.C.; Buettner, G.R.; Zakharia, Y. Understanding the redox biology of selenium in the search of targeted cancer therapies. Antioxidants. 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D. L.; Tsuji, P. A.; Carlson, B. A.; Gladyshev, V. N. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Nogeira, C.W.; Barbosa, N.V.; Rocha, J.B. Toxicology and pharmacology of synthetic organoselenium compounds: an update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef] [PubMed]
- Chopade, S.M.; Phadnis, P.P.; Hodage, A.S.; Wadawale, A.; Jain, V.K. Synthesis, characterization, structures and cytotoxicity of platinum(II) complexes containing dimethylpyrazole based selenium ligands. Inorg. Chim. Acta. 2015, 427, 72–80. [Google Scholar] [CrossRef]
- Jeong, L.S.; Tosh, D.K.; Choi, W.J.; Lee, S.K.; Kang, Y.-J.; Choi, S.; Lee, J.H.; Lee, H.; Lee, H.W.; Kim, H.O. Discovery of a new template for anticancer agents: 2’-deoxy-2’-fluoro-4’-selenoarabinofuranosyl-cytosine (2’-F-4’-seleno-ara-C). J. Med. Chem. 2009, 52, 5303–5306. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Shaaban, S.; Ba-Ghazal, H.; Al-Faiyz, Y.S.; Al-Karmalawy, A.A.; Amri, N.; Youssef, I. Recent advanced in the synthesis of organoselenium heterocycle conjuguates. Tetrahedron 2024, 157, 133957. [Google Scholar] [CrossRef]
- Seredyuk, M.; Fritsky, I. O.; Krämer, R.; Kozlowski, H.; Haukka, M.; Gütlich, P. New reaction of 1H-pyrazoles with selenium dioxide: one-pot synthesis of bis(1H-pyrazol-4-yl)selenides. Tetrahedron 2010, 66, 8772–8777. [Google Scholar] [CrossRef]
- Kour, J.; Khajuria, P.; Verma, P. K.; Kapoor, N.; Kumar, A.; Sawant, S. D. Selective Synthesis of Bis-Heterocycles via Mono- and Di-Selenylation of Pyrazoles and Other Heteroarenes. ACS Omega 2022, 7, 13000–13009. [Google Scholar] [CrossRef] [PubMed]
- Tamhaev, R.; Grosjean, E.; Ahamed, H.; Chebaiki, M.; Rodriguez, F.; Recchia, D.; Degiacomi, G.; Pasca, M.R.; Maveyraud, L.; Mourey, L.; Lherbet, C. Exploring the plasticity of the InhA substrate-binding site using new diaryl ether inhibitors. Bioorg. Chem. 2024, 143, 107032. [Google Scholar] [CrossRef] [PubMed]
- Chebaiki, M.; Delfourne, E.; Tamhaev, R.; Danoun, S.; Rodriguez, F.; Hoffmann, P.; Grosjean, E.; Goncalves, F.; Azéma-Despeyroux, J.; Pál, A.; Korduláková, J.; Preuilh, N.; Britton, S.; Constant, P.; Marrakchi, H.; Maveyraud, L.; Mourey, L.; Lherbet, C. Discovery of new diaryl ether inhibitors against Mycobacterium tuberculosis targeting the minor portal of InhA. Eur. J. Med. Chem. 2023, 259, 115646. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Saffon, N.; Sammartino, J.C.; Degiacomi, G.; Pasca, M.R.; Lherbet, C. First triclosan-based macrocyclic inhibitors of InhA enzyme. Bioorg. Chem. 2020, 95, 103498. [Google Scholar] [CrossRef] [PubMed]
- Veau, D.; Krykun, S.; Mori, G.; Orena, B.S.; Pasca, M.R.; Frongia, C.; Lobjois, V.; Chassaing, S.; Lherbet, C.; Baltas, M. Triazolophthalazines: Easily accessible compounds with potent antitubercular activity. ChemMedChem. 2016, 11, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Li, Y.; Zhang, X.; Xie, H.; Cao, H.; Yu, L.; Xu, Q. Specific N-alkylation of hydroxypyridines achieved by a catalyst and base-free reaction with organohalides. J. Org. Chem. 2018, 83, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).