Submitted:
03 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemical and Standards
2.2. ST-65 formulation
2.3. UHPLC-HRMS/MS analysis
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Exposure of SH-SY5Y to ST-65
2.7. Sample Extraction
2.8. NMR spectra acquisition
2.9. Statistical analysis
3. Results
3.1. UHPLC-HRMS/MS analysis
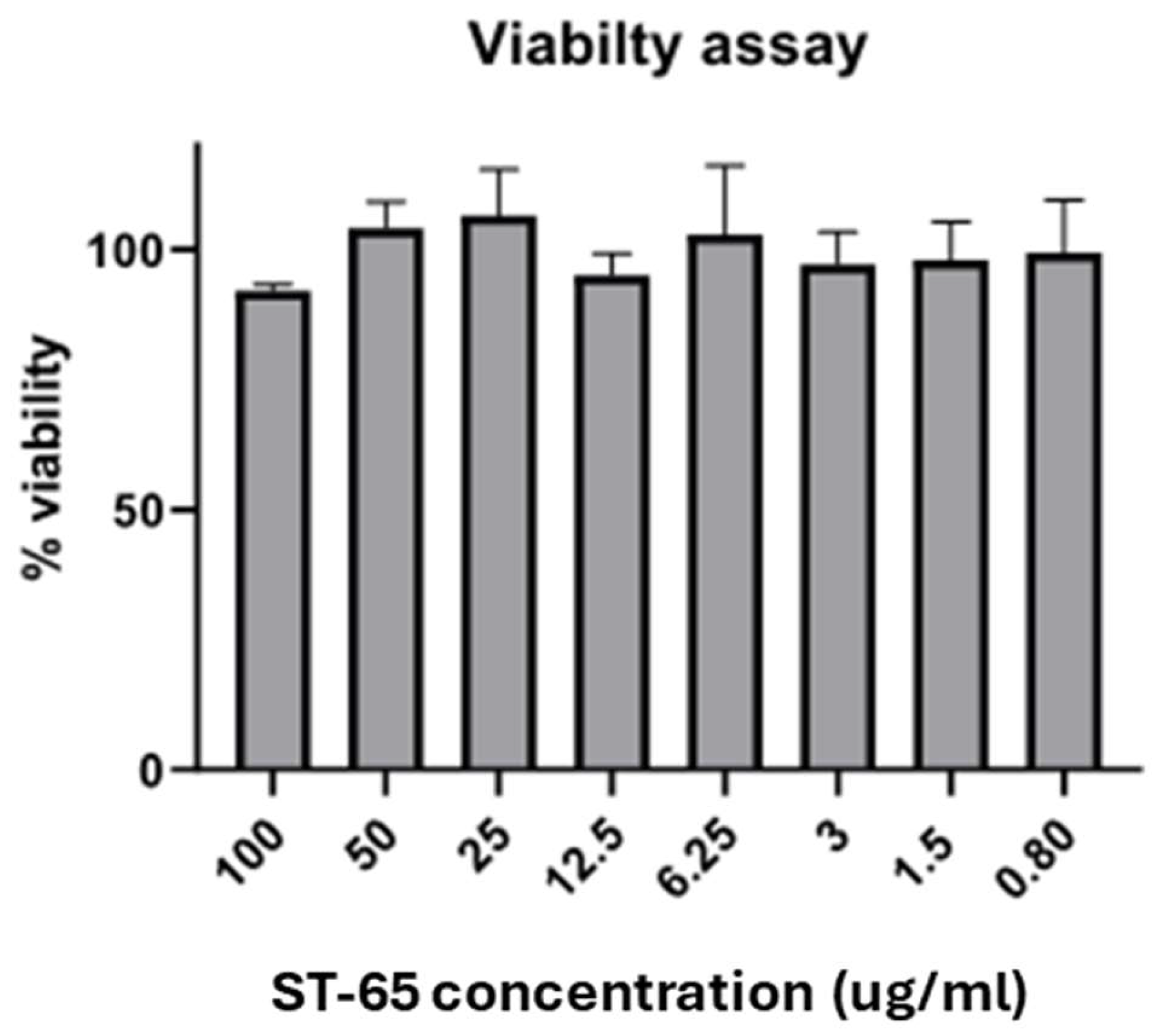
3.2. ST-65 viability
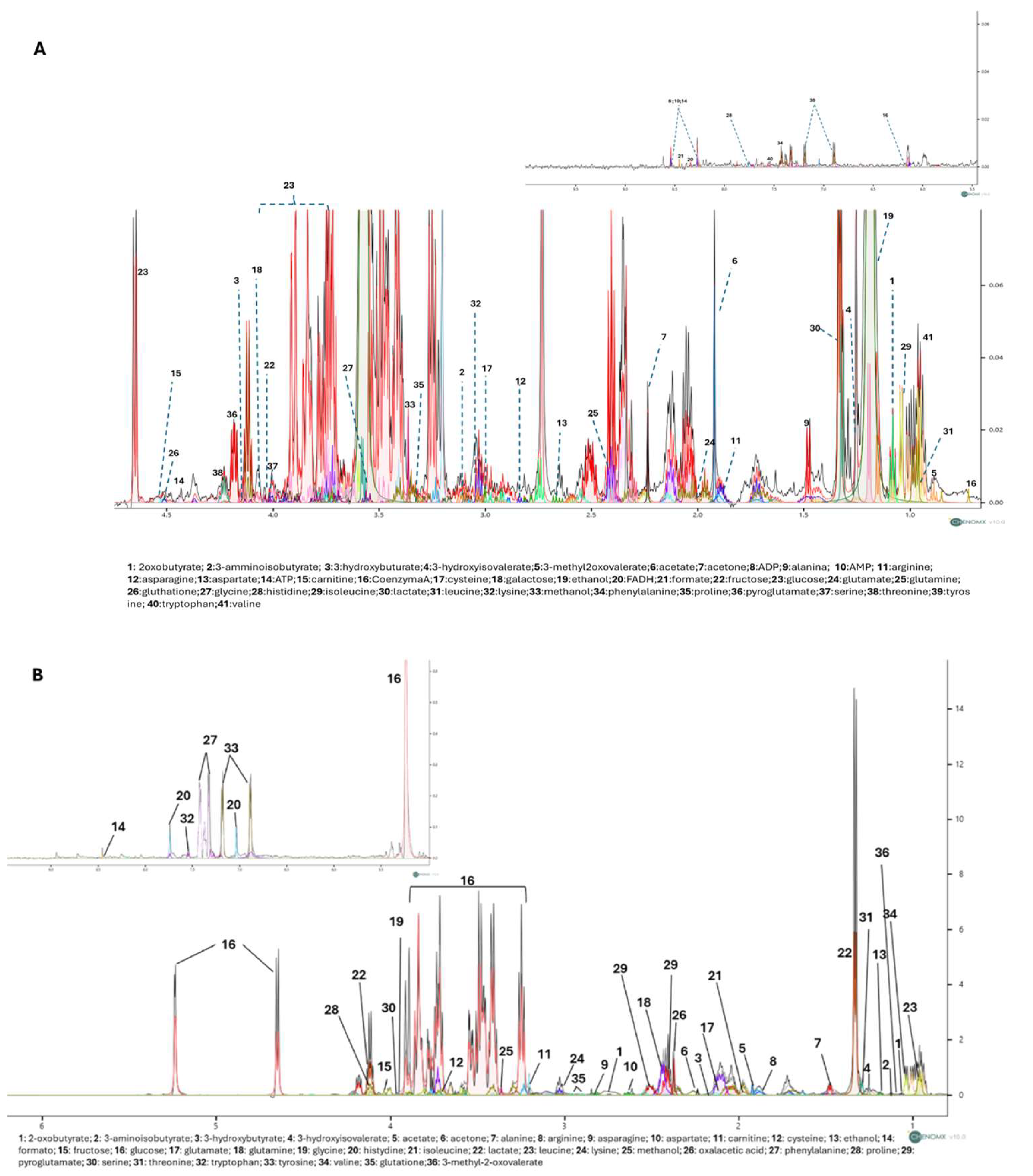
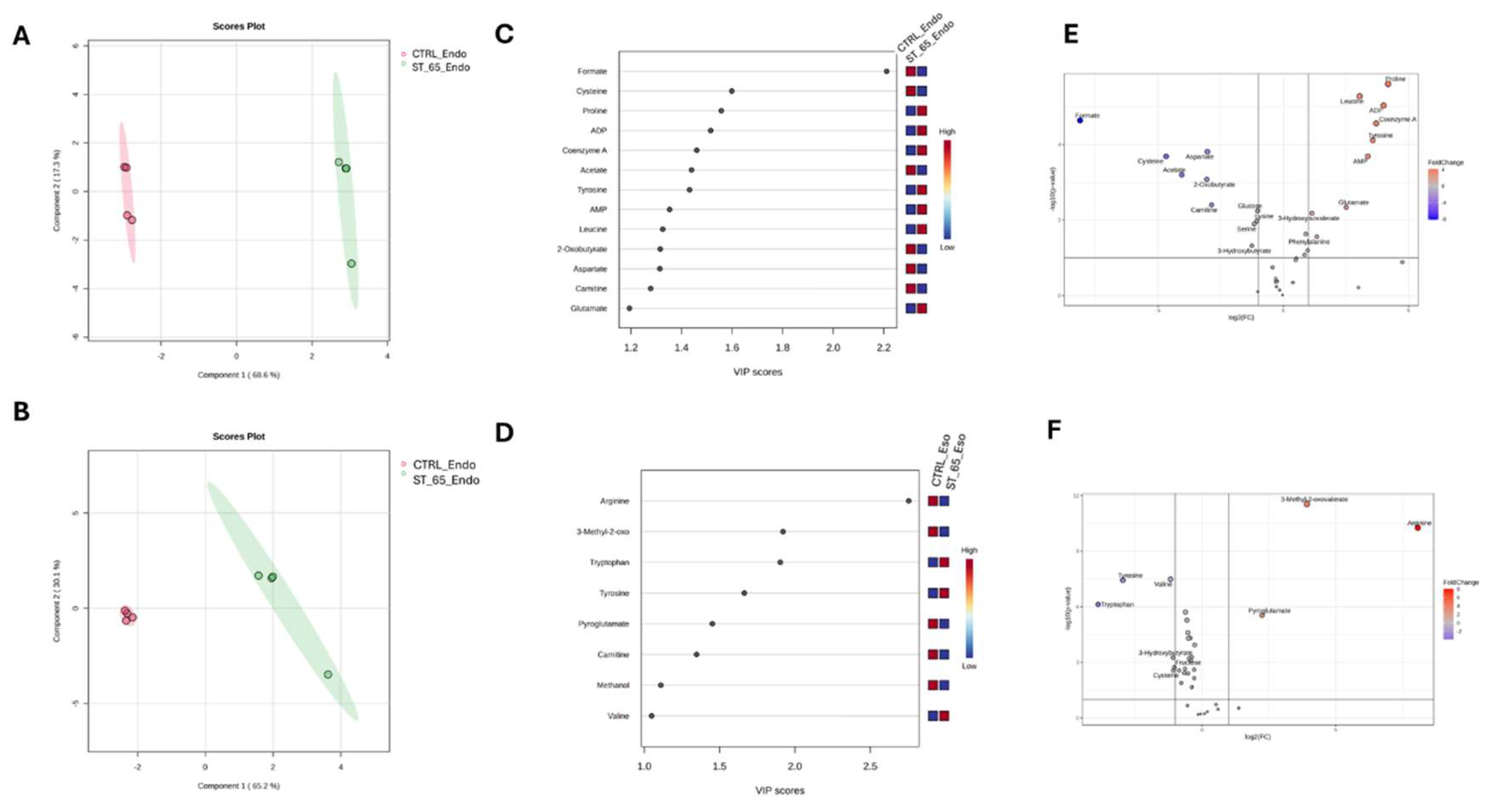
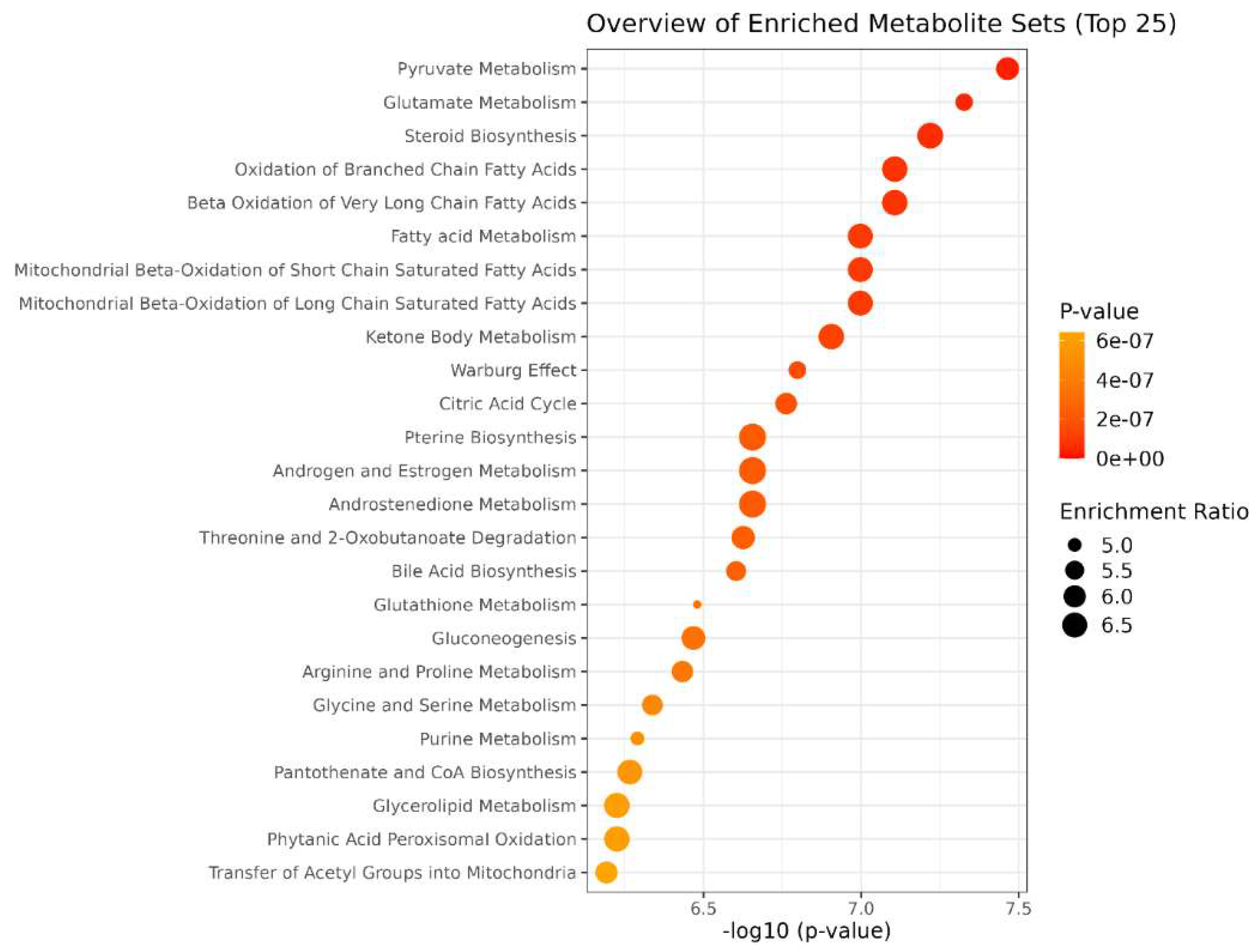
3.2. NMR Metabolomic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The role of cortisol in chronic stress, neurodegenerative diseases, and psychological disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.I.; Wu, A.M. Assessing the Role of Cortisol in Anxiety, Major Depression, and Neuroticism: A Mendelian Randomization Study Using SERPINA6/SERPINA1 Variants. Biological Psychiatry Global Open ScienceI 2024, 4, 100294. [Google Scholar] [CrossRef] [PubMed]
- Kuckuck, S.; van Der Valk, E.S.; Scheurink, A.J.; van Der Voorn, B.; Iyer, A.M.; Visser, J.A.; van Rossum, E.F.; et al. Glucocorticoids, stress and eating: The mediating role of appetite-regulating hormones. Obesity Reviews 2023, 24, e13539. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.S.; Youngstedt, S.D.; Rozales, A.A.R.C.; Ferreira, W.S.; De-Assis, D.E.; De-Assis, B.P.; Santana, M.G. Insomnia severity is associated with morning cortisol and psychological health. Sleep Science 2023, 16, 092–096. [Google Scholar]
- Luo, J.; Zhou, C.; Wang, S.; Tao, S.; Liao, Y.; Shi, Z.; Yang, P.; et al. Cortisol synergizing with endoplasmic reticulum stress induces regulatory T-cell dysfunction. Immunology 2023, 170, 334–343. [Google Scholar] [CrossRef]
- Kip, E.; Parr-Brownlie, L.C. Healthy lifestyles and wellbeing reduce neuroinflammation and prevent neurodegenerative and psychiatric disorders. Frontiers in Neuroscience 2023, 17, 1092537. [Google Scholar] [CrossRef]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Cielecka-Piontek, J.; et al. Ashwagandha (Withania somnifera)—Current research on the health-promoting activities: A narrative review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef]
- Cheah, K.L.; Yaacob, L.H.; Rahman, R.A. Effect of Ashwagandha (Withania somnifera) extract on sleep: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0257843. [Google Scholar] [CrossRef]
- Leonard, M.; Dickerson, B.; Estes, L.; Gonzalez, D.E.; Jenkins, V.; Johnson, S.; Kreider, R.B.; et al. Acute and Repeated Ashwagandha Supplementation Improves Markers of Cognitive Function and Mood. Nutrients 2024, 16, 1813. [Google Scholar] [CrossRef]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on stress and the stress-related neuropsychiatric disorders anxiety, depression, and insomnia. Current neuropharmacology 2021, 19, 1468. [Google Scholar] [CrossRef]
- Guo, S.; Rezaei, M.J. The benefits of ashwagandha (Withania somnifera) supplements on brain function and sports performance. Frontiers in Nutrition 2024, 11, 1439294. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.K.; Nagegowda, D.A. Biosynthesis of the triterpenoid withanolides in Withania somnifera. Current Opinion in Plant Biology 2024, 81, 102576. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Brimson, S.; Prasanth, M.I.; Thitilertdecha, P.; Malar, D.S.; Tencomnao, T. The effectiveness of Bacopa monnieri (Linn.) Wettst. as a nootropic, neuroprotective, or antidepressant supplement: Analysis of the available clinical data. Scientific reports 2021, 11, 596. [Google Scholar] [CrossRef]
- Sushma Sahu, M.R.; Murugan, N.A.; Mondal, A.C. Amelioration of Amyloid-β Induced Alzheimer's Disease by Bacopa monnieri through Modulation of Mitochondrial Dysfunction and GSK-3β/Wnt/β-Catenin Signaling. Molecular Nutrition & Food Research 2024, 68, 2300245. [Google Scholar]
- Lopresti, A.L.; Smith, S.J.; Ali, S.; Metse, A.P.; Kalns, J.; Drummond, P.D. Effects of a Bacopa monnieri extract (Bacognize®) on stress, fatigue, quality of life and sleep in adults with self-reported poor sleep: A randomised, double-blind, placebo-controlled study. Journal of Functional Foods 2024, 85, 104671. [Google Scholar] [CrossRef]
- Palollathil, A.; Najar, M.A.; Amrutha, S.; Pervaje, R.; Modi, P.K.; Prasad, T.S.K. Bacopa monnieri confers neuroprotection by influencing signaling pathways associated with interleukin 4, 13 and extracellular matrix organization in Alzheimer's disease: A proteomics-based perspective. Neurochemistry International 2024, 105864. [Google Scholar] [CrossRef]
- Gupta, V.; Prasad, S. Differential Alterations in the Expression of AMPA Receptor and Its Trafficking Proteins in the Hippocampus Are Associated with Recognition Memory Impairment in the Rotenone-Parkinson’s Disease Mouse Model: Neuroprotective Role of Bacopa monnieri Extract CDRI 08. Molecular neurobiology 2024, 1–19. [Google Scholar]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; dos Santos Buglio, D.; Barbalho, S.M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Bhandari, P.; Sendri, N.; Devidas, S.B. Dammarane triterpenoid glycosides in Bacopa monnieri: A review on chemical diversity and bioactivity. Phytochemistry 2020, 172, 112276. [Google Scholar] [CrossRef]
- Castaldo, G.; Schiavo, L.; Pagano, I.; Molettieri, P.; Conte, A.; Sarno, G.; Rastrelli, L.; et al. Clinical impact of enteral protein nutritional therapy on patients with obesity scheduled for bariatric surgery: A focus on safety, efficacy, and pathophysiological changes. Nutrients 2023, 15, 1492. [Google Scholar] [CrossRef]
- Pellegrini, P.; Lemasson, P.; Rastrelli, L.; D’Elia, M. Effectiveness of ketogenic therapy in patients with obesity and diabetes: A narrative review. Exploration of Foods and Foodomics 2024, 2, 313–325. [Google Scholar] [CrossRef]
- Castaldo, G.; Rastrelli, L.; Galdo, G.; Molettieri, P.; Aufiero, F.R.; Cereda, E. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: A proof-of-concept, single-arm, open-label clinical trial. Nutrition 2020, 74, 110757. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, G.; Pagano, I.; Grimaldi, M.; Marino, C.; Molettieri, P.; Santoro, A.; Rastrelli, L.; et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: A nuclear magnetic resonance-based metabolomic study. Journal of proteome research 2020, 20, 1509–1521. [Google Scholar] [CrossRef]
- Castaldo, G.; Marino, C.; Atteno, M.; D’Elia, M.; Pagano, I.; Grimaldi, M.; Rastrelli, L.; et al. Investigating the Effectiveness of a Carb-Free Oloproteic Diet in Fibromyalgia Treatment. Nutrients 2024, 16, 1620. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nature protocols 2007, 2, 2692. [Google Scholar] [CrossRef]
- Mckay, R.T. How the 1D-NOESY suppresses solvent signal in metabonomics NMR spectroscopy: An examination of the pulse sequence components and evolution. Concepts in Magnetic Resonance Part A 2011, 38, 197–220. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 1–5. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Current protocols in bioinformatics 2016, 55, 14.10. 1–14.10. 91. [Google Scholar] [CrossRef]
- Kumar, N.; Hoque, M.A.; Sugimoto, M. Robust volcano plot: Identification of differential metabolites in the presence of outliers. BMC bioinformatics 2018, 19, 1–11. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Xia, J.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Research 2024, gkae253. [Google Scholar] [CrossRef]
- Abdelwahed, M.T.; Hegazy, M.A.; Mohamed, E.H. Major biochemical constituents of Withania somnifera (ashwagandha) extract: A review of chemical analysis. Reviews in Analytical Chemistry 2023, 42, 20220055. [Google Scholar] [CrossRef]
- Nuengchamnong, N.; Sookying, S.; Ingkaninan, K. LC-ESI-QTOF-MS based screening and identification of isomeric jujubogenin and pseudojujubogenin aglycones in Bacopa monnieri extract. Journal of pharmaceutical and biomedical analysis 2016, 129, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Wang, S.Y.; Kuo, C.H.; Tseng, Y.J. Distribution-based classification method for baseline correction of metabolomic 1D proton nuclear magnetic resonance spectra. Analytical Chemistry 2013, 85, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wu, H.; Tjeerdema, R.S.; Viant, M.R.J.M. Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 2007, 3, 55–67. [Google Scholar] [CrossRef]
- Worley, B.; Halouska, S.; Powers, R. Utilities for quantifying separation in PCA/PLS-DA scores plots. Analytical biochemistry 2013, 433, 102–104. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Hoefsloot, H.C.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.; van Dorsten, F.A.J.M.; et al. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Akarachantachote, N.; Chadcham, S.; Saithanu, K. Cutoff threshold of variable importance in projection for variable selection. Int J Pure Appl Math 2014, 94, 307–322. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, S.; Baskin, E.; Patt, A.; Siddiqui, J.K.; Mathé, E.A. RaMP: A comprehensive relational database of metabolomics pathways for pathway enrichment analysis of genes and metabolites. Metabolites 2018, 8, 16. [Google Scholar] [CrossRef]
- Aquili, L. The Role of Tryptophan and Tyrosine in Executive Function and Reward Processing. Int J Tryptophan Res 2020, 13, 1178646920964825. [Google Scholar] [CrossRef]
- Fendt, S.M.; Verstreken, P. Neurons eat glutamate to stay alive. J Cell Biol 2017, 216, 863–865. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna) 2014, 121, 799. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.N.; Lemasters, J.J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 2014, 19 Pt A, 78–84. [Google Scholar] [CrossRef]
- Gómez Afonso, A.; Fernandez-Lazaro, D.; Adams, D.P.; Monserdà-Vilaró, A.; Fernandez-Lazaro, C.I. Effects of Withania somnifera (Ashwagandha) on Hematological and Biochemical Markers, Hormonal Behavior, and Oxidant Response in Healthy Adults: A Systematic Review. Current Nutrition Reports 2023, 12, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Choi, M.H.; Kim, J. Metabolic profiling of cholesterol and sex steroid hormones to monitor urological diseases. Endocr Relat Cancer 2016, 23, R455–R467. [Google Scholar] [CrossRef] [PubMed]
- Lamarre, S.G.; Morrow, G.; Macmillan, L.; Brosnan, M.E.; Brosnan, J.T. Formate: An essential metabolite, a biomarker, or more? 2013, 51, 571–578. [Google Scholar] [CrossRef]
- Leonardi, R.; Zhang, Y.M.; Rock, C.O.; Jackowski, S. Coenzyme A: Back in action. Progress in lipid research 2005, 44, 125–153. [Google Scholar] [CrossRef]
- Kalaiselvi, T.; Panneerselvam, C. Effect of L-carnitine on the status of lipid peroxidation and antioxidants in aging rats. The Journal of Nutritional Biochemistry 1998, 9, 575–581. [Google Scholar] [CrossRef]
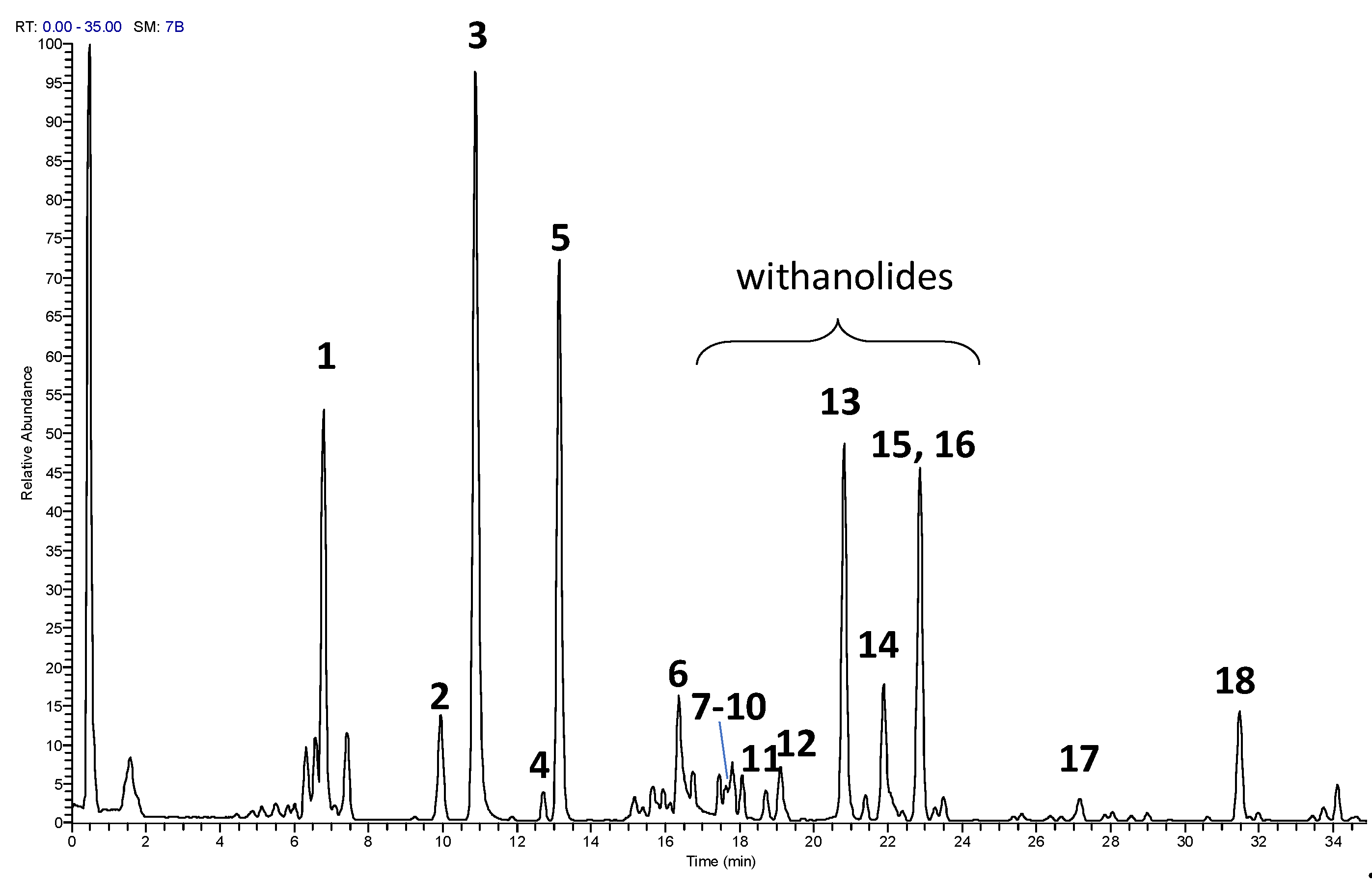
| N.a | RT [min] | m/z | Formula | ppm | MS/MS | Name |
|---|---|---|---|---|---|---|
| 1 | 6.8 | 224.1644 | C13 H22 O2 N | -0.47 | 124, 93 | unknown |
| 2 | 9.9 | 226.1801 | C13 H24 O2 N | -0.334 | 144, 126, 84 | unknown |
| 3 | 10.9 | 611.1597 | C27 H31 O16 | -1.524 | 465, 303 | Rutinb |
| 4 | 12.71 | 595.1658 | C27 H31 O15 | 0.241 | 449, 287 | Kaempferol hexose-deoxyhexose |
| 5 | 13.1 | 611.1959 | C28 H35 O15 | -1.745 | 447, 303 | Hesperidinb |
| 6 | 16.3 | 303.0497 | C15 H11 O7 | -0.723 | 153 | Quercetinb |
| 7 | 17.4 | 783.4157 | C40 H63 O15 | -0.482 | 459, 441, 423 | Withanoside IV/Withanoside X |
| 8 | 17.6 | 489.2847 | C28 H41 O7 | 0.225 | 317, 299, 281 | Vicosalactone B isomer |
| 9 | 17.8 | 489.2847 | C28 H41 O7 | 0.163 | 317, 299, 281 | Vicosalactone B isomer |
| 10 | 17.8 | 783.4156 | C40 H63 O15 | -0.635 | 459, 441, 423 | Withanoside IV/Withanoside X |
| 11 | 18.1 | 621.363 | C34 H53 O10 | -0.457 | 459, 441, 423 | Coagulin Q |
| 12 | 18.7 | 621.3632 | C34 H53 O10 | -0.264 | 459, 441, 423 | Coagulin Q isomer |
| 13 | 20.8 | 471.2737 | C28 H39 O6 | -0.733 | 435, 341, 299, 281 | Whitanolide A isomer |
| 14 | 21.8 | 471.2739 | C28 H39 O6 | -0.457 | 435, 341, 299, 281 | Whitanolide A isomer |
| 15 | 22.8 | 471.2737 | C28 H39 O6 | -0.797 | 435, 341, 299, 281 | Whitanolide A isomer |
| 16 | 22.8 | 767.4209 | C40 H63 O14 | -0.434 | 443, 425, 407, 389 | Withanoside V |
| 17 | 28.0 | 797.4688 | C42 H69 O14 | 0.861 | 599, 441, 423 | Bacopaside N |
| 18 | 31.4 | 931.5315 | C40 H83 O23 | -0.51 | 477 | unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





