1. Introduction
Amphoteric solids could play a decisive role in improving activity, selectivity and stability of catalytic materials. Over those bifunctional formulations new reaction pathways of well-known reactions could be provided and improved yields of desired products could be achieved. For instance, sol-gel ZrO
2 and corresponding Na-modified solids catalysts effectively cleaved the β-ether bond of phenethyl phenyl ether (from biomass-derived lignin valorization) to produce ethyl benzene, styrene and phenol as major products [
1]. Followed reaction pathways and thus product distribution were correlated to concentration of both surface acid (Lewis) and basic sites on the studied materials. Even more, some reactions require participation of both type of sites to take place. Otherwise, they could not be carried out at all on either acid or basic centers alone, for instance,
aldol condensation between 4-nitrobenzaldehyde and acetone, where also acid strength played a major role [
2]
. In this context, functionalization of rather inert SBA-15 mesoporous silica have been tried for some groups by using inorganic oxides [
3,
4]
. Even organic species have been used to that end [
2,
5]
. SBA-15 materials possess very good textural properties (large surface area, high pore volume, mesoporosity) that could be tuned depending on synthesis conditions used but lack both acidity and basicity [
6]
. Different strategies followed to improve surface properties have included modifications by adding Al and Mg salts during synthesis [
3]
, species that could provide (after calcination) Lewis or protonic acidity (depending on Al3+ location. framework or extra-framework [
7]
) and basic sites (as MgO). Zirconium and magnesium salts direct addition during direct mesoporous silica matrix formation have also been utilized to that end, by creation of acidic and basic domains, respectively, [
4]
. Considering zirconia surface amphoteric properties [
8]
, in this investigation a simpler approach was studied, i. e, direct addition during SBA-15 synthesis [
9]
of Zr salt alone. The main focus was determining if that strategy could provide enhanced acid and basic sites population on otherwise inert SiO2 surface. Binary solids at various Zr content prepared through methodology used in [
10]
(substituting Ti for Zr), were studied and characterized by textural, structural and surface instrumental techniques. Being a critical parameter that could strongly influence correct analytical data interpretation [
6]
, actual materials composition was also determined. As in previous reports, those sites were analyzed through ammonia and CO2 thermodesorption. It seems that ZrO2 domains alone could provide desired amphoteric characteristics to mesostructured silica matrices.
2. Results and Discussion
2.1. Materials Composition
According to data in
Table 1, degree of Zr incorporation in SBA-15 improved as the intended modifier concentration augmented. Probably, Zr species when at lower amount were not well-integrated to mesoporous framework. could be dissolved during washing of gel precursor [
6]. Originally, SBA-15 solid modified by 1 wt% Zr was also prepared but chemical analysis failed to identify zirconium domains in corresponding sample. This solid was then not further studied. Otherwise, when at higher concentration, Zr polymeric species of lower solubility could be formed [
11] remaining after gel washing. It is worth nothing that those extant Zr domains could not necessarily be properly integrated in SiO
2 tetrahedral network. In the case of SBA-15 modified by MgO and ZrO
2 Chen net al. [
4] identified Zr in the SBA-15 matrix by EDS but, unfortunately no quantitative data were provided making impossible to determine the amount of zirconia in the solid (20 wt% of equimolar ZrO
2/MgO).
2.2. Materials Texture
Although SBA-15 pristine matrix and corresponding Zr-modified materials all had type IV N
2 adsorption isotherms (Figure S1 (a)) with H1 hysteresis, typical profiles of mesostructured solids with cylindrical pores [
12], they showed distinctive features when compared one to another. Z3S displayed notably increased adsorbed N
2 volume meanwhile Z10S exhibited very marked hysteresis strongly suggesting presence of ink-bottle shaped pores. Thus, important textural alterations in studied samples could be envisaged. Z3S was the solid of the highest surface area (20% larger as to that of pristine SBA-15) where no definite trend related to modifier content was observed (Table S1). Pore volume remained unaltered by Zr addition but in the Z3S case which had strongly increased one (56%), respecting pristine mesoporous silica. As suggested by shifts to lower or higher partial pressures closing of their hysteresis (as to that of SBA-15), for Z10S and Z3S smaller and higher average pore size than that of S were registered (see corresponding maxima in Table S1 and Figure S1(b)). All in all, textural parameters suggested that certain pore obstruction were provoked in Z10S due to augmented Zr species content whereas the highest ZrO
2-SBA-15 interaction was expected in Z3S.
2.3. X-Ray Diffraction
Wide humps in the ~15-35° 2θ range in diffractograms of studied binary oxides (Figure S2) corresponded to amorphous mesoporous silica walls [
13]. Absence of diffraction signals related to zirconia phases (monoclinic one, according to annealing temperature samples were submitted to [
14]) suggested amorphous domains of well-dispersed ZrO
2 species (although not necessarily well-integrated to tetrahedral SiO
2 network) at any content studied.
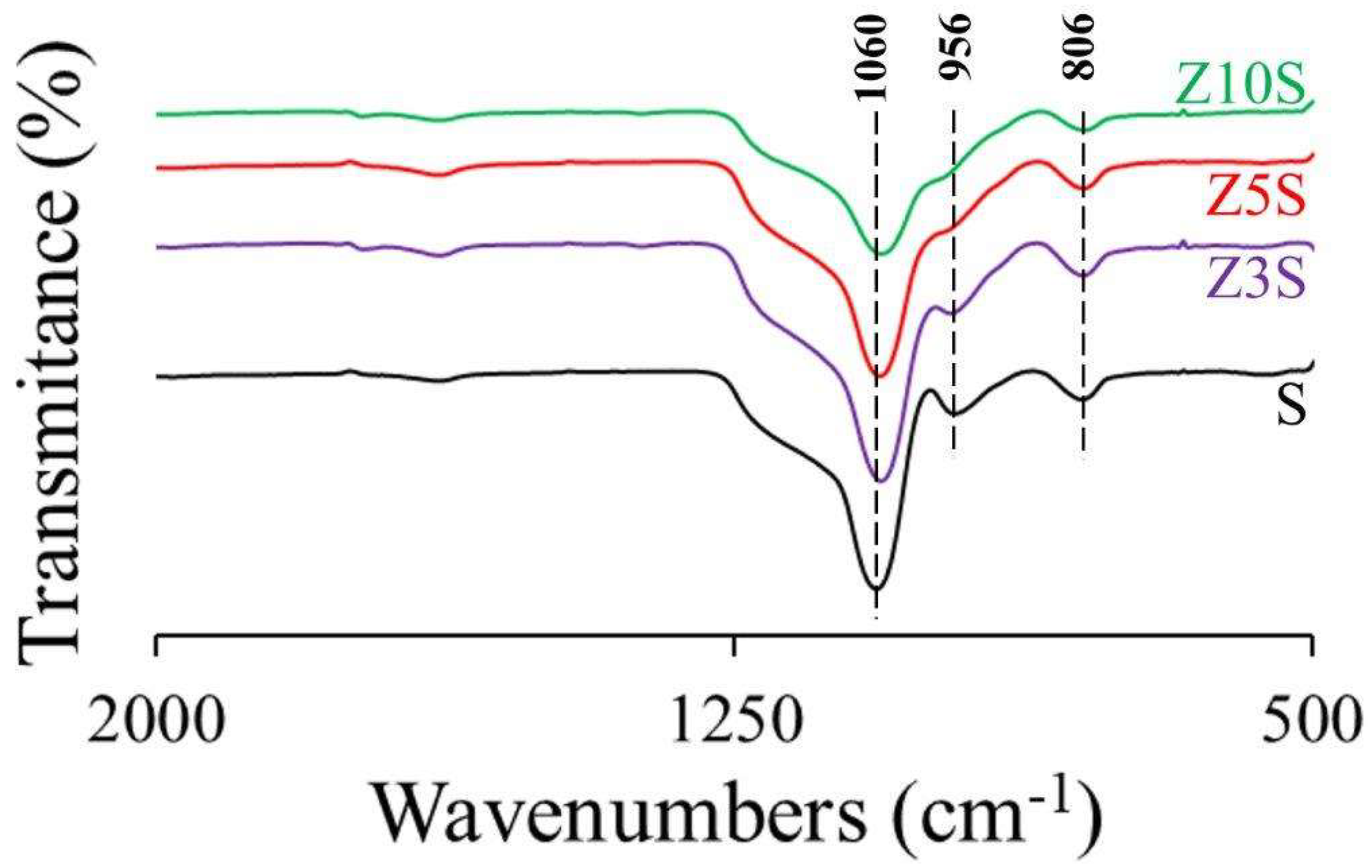
2.4. FTIR Spectroscopy
Peak at around 1060 cm
-1 corresponded to Si-O-Si asymmetric stretching vibration meanwhile band at ~806 originated by symmetric stretching of aforementioned bonds [
15],
Figure 1.
Meanwhile, non-bridged Si-OH stretching vibration [
15] was observed at 956 cm
-1 in pristine SBA-15 (S). This signal could present blue-shifts depending on interaction of those silanols to dopants [
6,
16]. Even more, Dong et al. [
15] reported disappearance of that signal after Zr deposition, being that attributed to Si-OH and Zr-OH groups association pointing out to Si-O-Zr linkages formation. In our case, progressive diminution in signal intensity was registered, as function of Zr concentration (
Figure 1), However, that did not necessarily imply effective integration of totality of zirconium into the mesoporous silica network. As mentioned, interaction could take place on silica surface. Adjusting pH to less acidic conditions have allowed enhanced degree of heteroatoms (Al and Ti) integration in SBA-15 when those species have been directly added during mesoporous matrix synthesis [
17]. However, in Zr-modified materials rather acceptable integration has been registered (Zr/Si=0.1, 13 wt%), even in materials prepared without augmented pH [
18], although, again, pH adjustment was reflected in augmented zirconium-silica interaction. In our samples acceptable integration of zirconium into the SBA-15 matrices (although not necessarily in the walls) was also observed (
Table 1 and
Figure 1), mainly in the case of solids of higher Zr content. However, that behavior is clearly dependent on properties of heteroatoms to be inserted. For instance, in the opposite to that found in this investigation La could not be integrated to mesoporous silica solids at any content [
6]. The rationale was that very acidic pH conditions during condensation stage of SBA-15 synthesis do not favor oxo form of heteroatomic species which remained as cationic ones precluding their proper integration to SBA-walls [
18,
19].
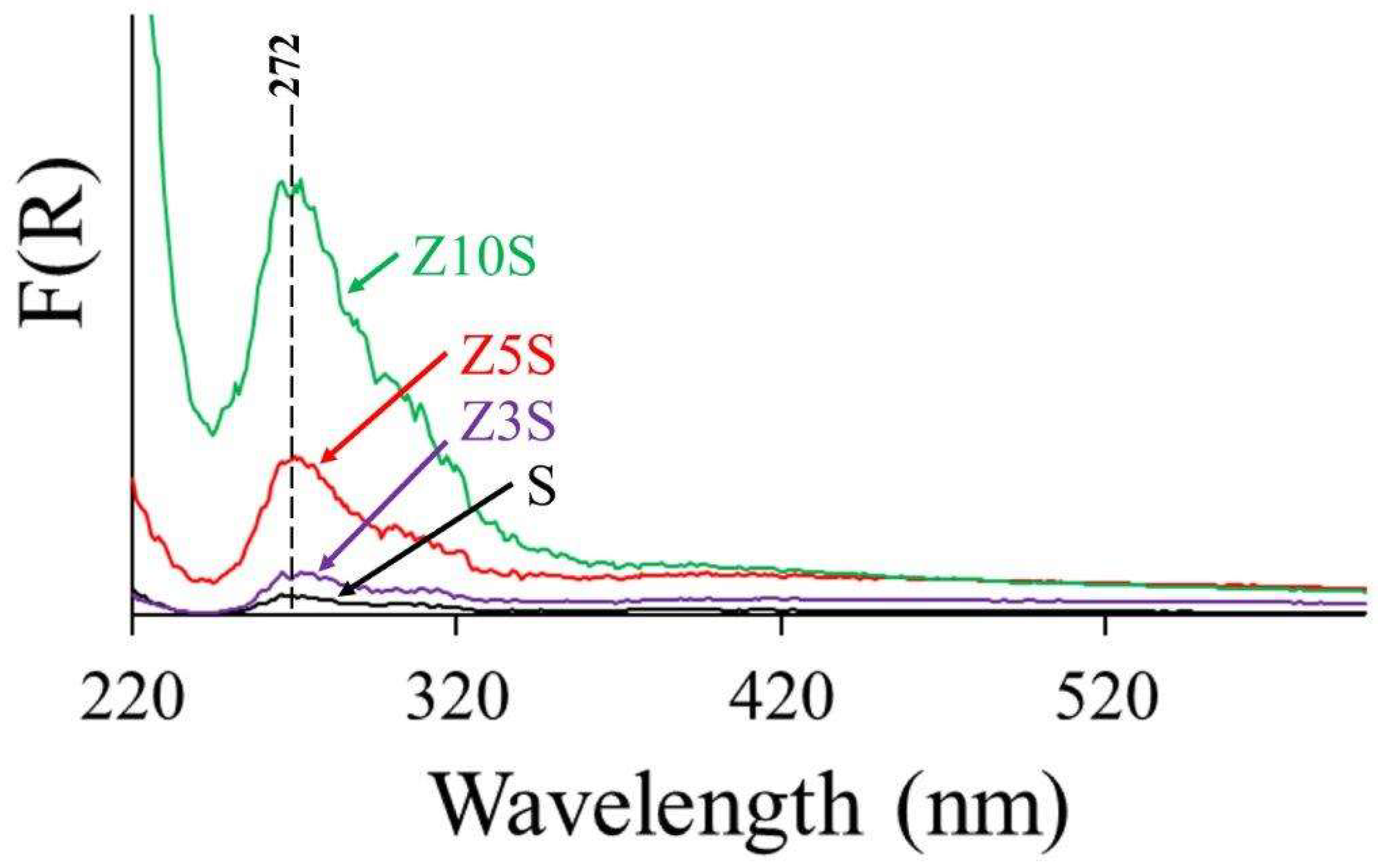
2.5. UV-Vis Spectroscopy
As expected, SBA-15 (S) did not absorb in the studied UV region [
19],
Figure 2. Absorption band at energies beyond that corresponding to electromagnetic radiation related to 220 nm could be due to Ligand-Metal Charge Transfer (LMCT) of tetrahedral oxidic Zr species, meanwhile absorption maximum centered at approximately 272 nm could be provoked by Zr
4+ in other coordination states. Those facts strongly suggested that the former peak originated in framework zirconia integrated to mesoporous silica tetrahedral network whereas the latter evidenced extra-framework species. Considering that, it seemed that Z10S contained the highest proportion of Zi well-integrated in SBA-15 walls, although important proportion could be also as ZrO
2 segregated domains. The later species that showed strong absorption at 272 nm could be provoked by LMCT O
2- to Zr
4+ in octahedral coordination [
19]. All in all, our UV spectra results pointed out to coexistence of framework and extra-framework Zr
4+ as well.
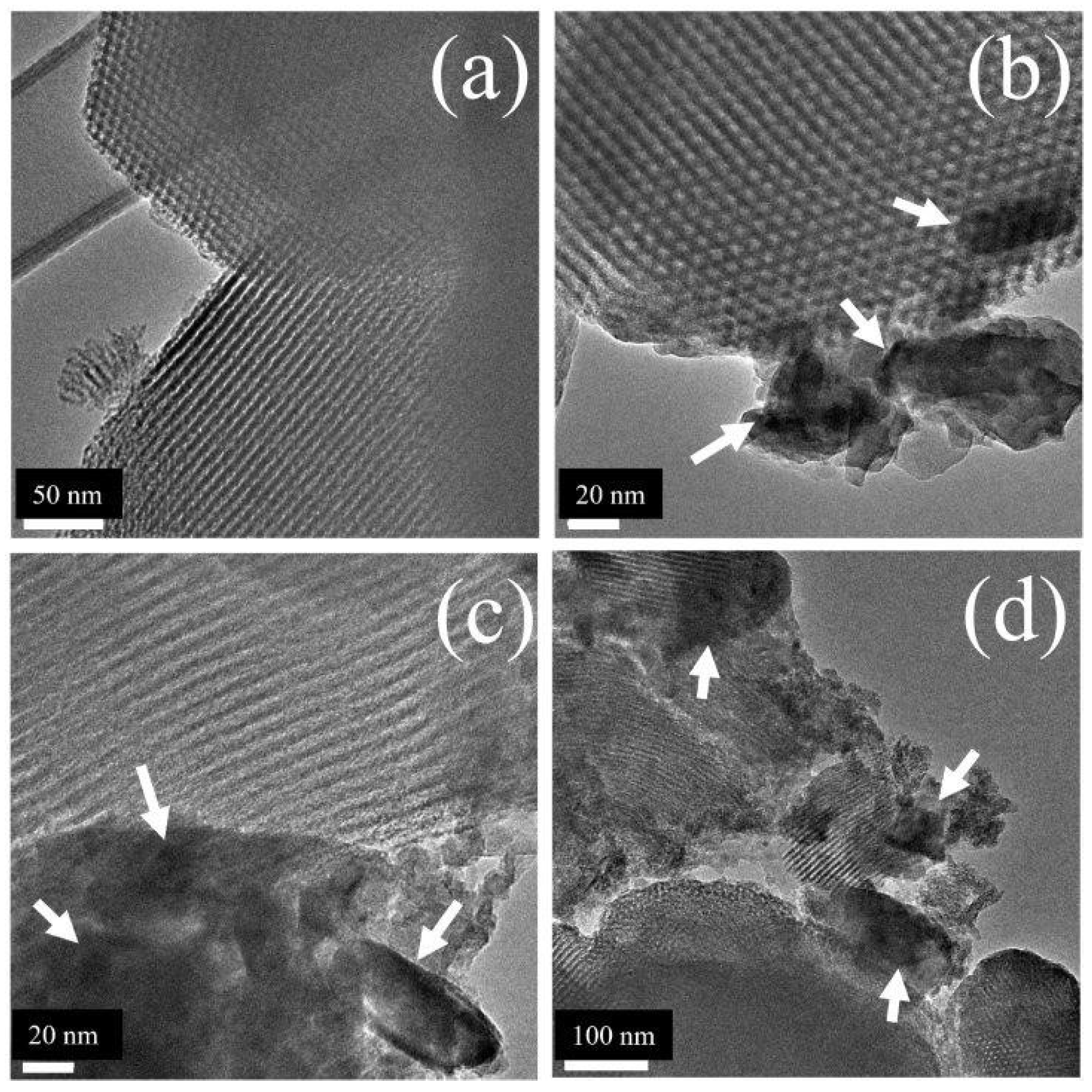
2.6. HR-TEM
Well-ordered structure with uniform channels along pores axes was clearly observed in pristine SBA-15 solids (
Figure 3 (a)), in full agreement with previous reports [
20]. As zirconium content in studied formulations progressively increased, amorphous (Figure S2) segregated ZrO
2 domains were evidenced (
Figure 3 (b)-(d)), in accordance with that registered by UV spectroscopy (
Figure 2).
Note that in all solids SBA-15 mesostructure remained well-ordered, differently to that reported by Colmenares-Zerpa et al. [
19] who found that Zr direct addition (Zr/Si=0.1, ~13 wt% Zr, not adjusted pH) resulted in significant SBA-15 structural losses in solids prepared from ZrO(NO
3)
2•xH
2O, suggesting important role of precursor salt used. Supporting that notion mesoporous network ordering was well-kept in the case of solids synthesized from Zr(SO
4)
2 (at Zr/Si=0.1) [
21]. In our case, extant segregated ZrO
2 domains formation could be favored by distinctive hydrolysis rate of Zr and Si precursors (tetraethyl orthosilicate and ZrOCl
2•8H
2O, respectively), under synthesis conditions used [
6].
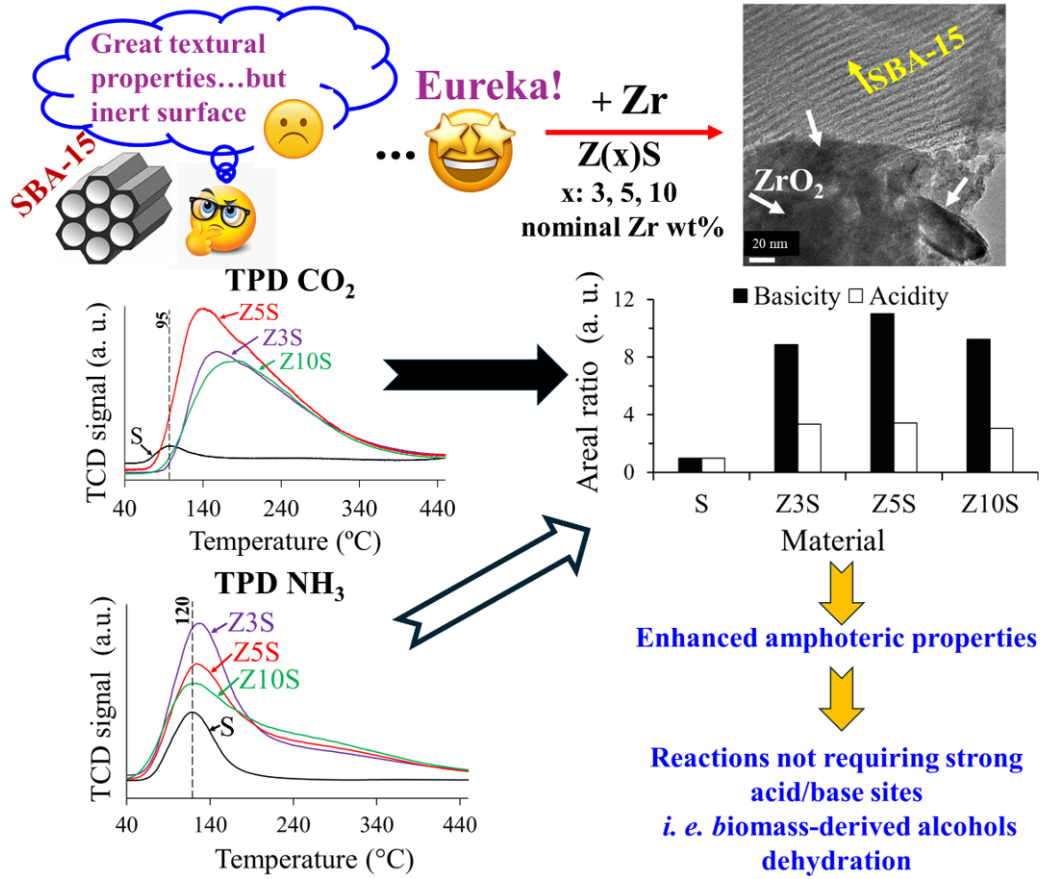
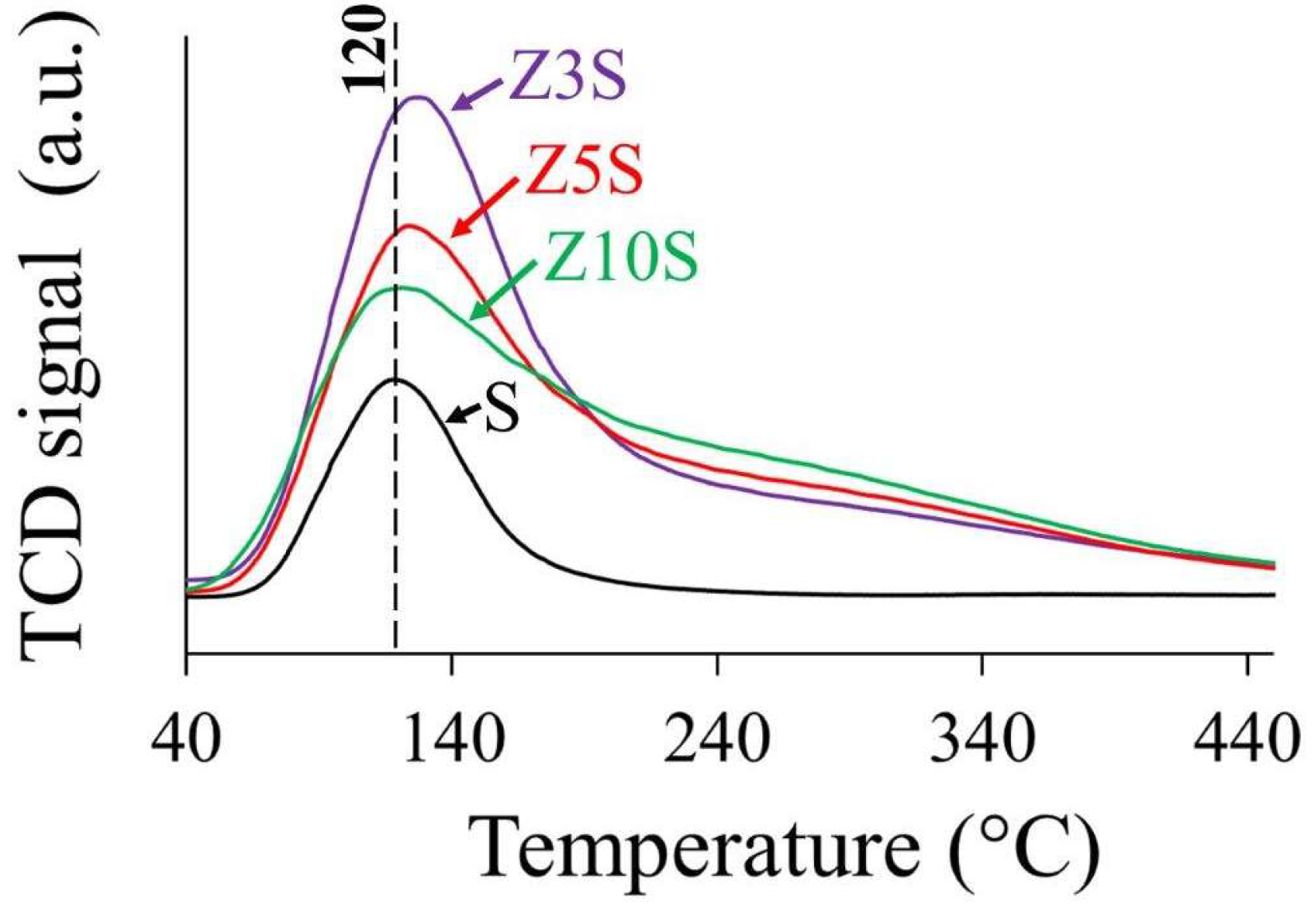
2.7. NH3 Thermodesorption
Weak signal at 120 °C in pristine SBA-15 (S) profile could be related to physisorbed NH
3 and very weak acid sites [
4,
6],
Figure 4.
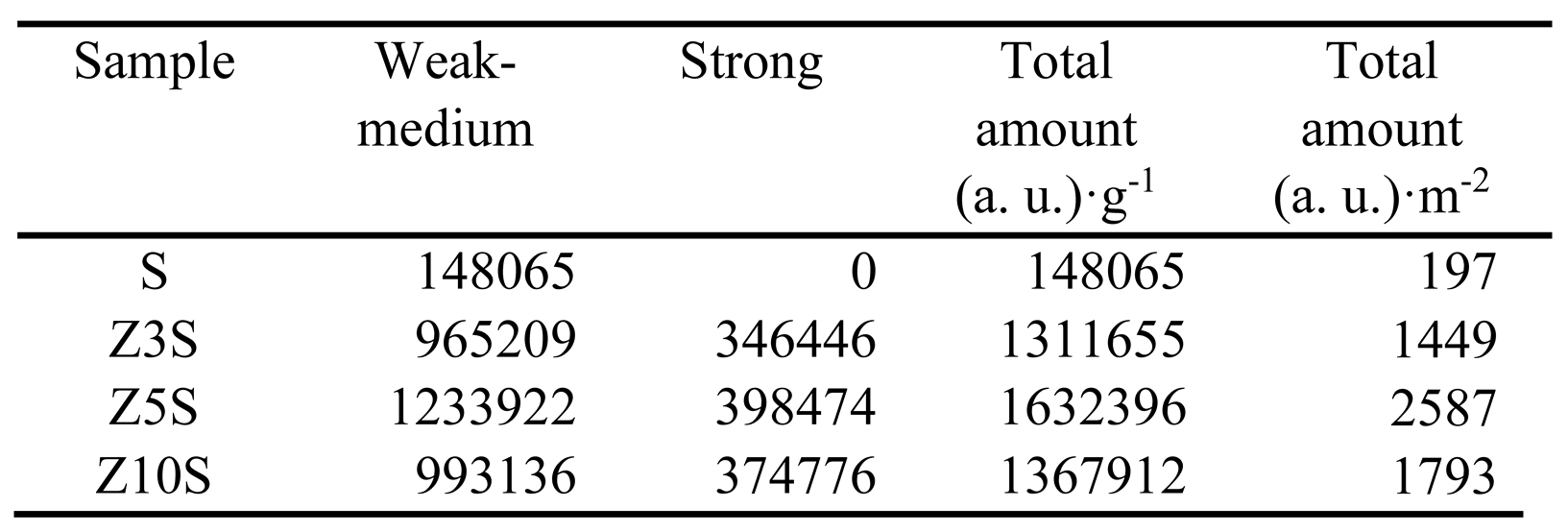
Amount and strength of acidic sites significantly enhanced in Zr-modified materials. Acid sites strength was arbitrarily divided according to desorption temperature (T
d), namely weak-medium (80 °C<T
d<250 °C) and strong (250 °C<T
d<380 °C), where most of the sites on Zr-modified solids were not strong (
Table 2).
Although the used technique did not allow differentiation between protonic and Lewis sites, Colmenares-Zerpa et al. [
19] found, by pyridine FTIR, that approximately 35-40% of Zr-modified solids (Si/Zr=2, 5 and 10, prepared through either direct heteroatom addition during silica synthesis or pH adjusted protocol) total acid sites corresponded to Brꬾnsted ones. Thus, a similar rationale could be applied extrapolating that to our case. As it is known that zirconia possesses just Lewis acidity related to coordinatively unsaturated sites [
1], protonic centers creation could be attributed to components interaction through Zr-O-Si linkages. On per mass basis, Z5S had the largest number of total sites,
Table 2. Total acidity was not directly related to Zr content in solids as combination of zirconium concentration (
Table 1) and dispersion (
Figure 3) could strongly influence observed trend. All in all, total acidity of Zr-containing samples was not very different from one to another. The lower population of sites on Z10S could probably be provoked by diminished dispersion as to that of the other solids (
Figure 3 (d)). Similar tendency (significantly augmented acidity originated by extant framework and extra-framework ZrO
2 domains) was observed by Chen at al. [
4] who studied series of SBA-15 solids modified by direct addition (during mesoporous silica matrix synthesis) of precursors of equimolar MgO-ZrO
2 mixture at high content (10-40 wt%). In surface area basis, the largest basicity was observed, again, for the Z5S formulation.
Table 2.
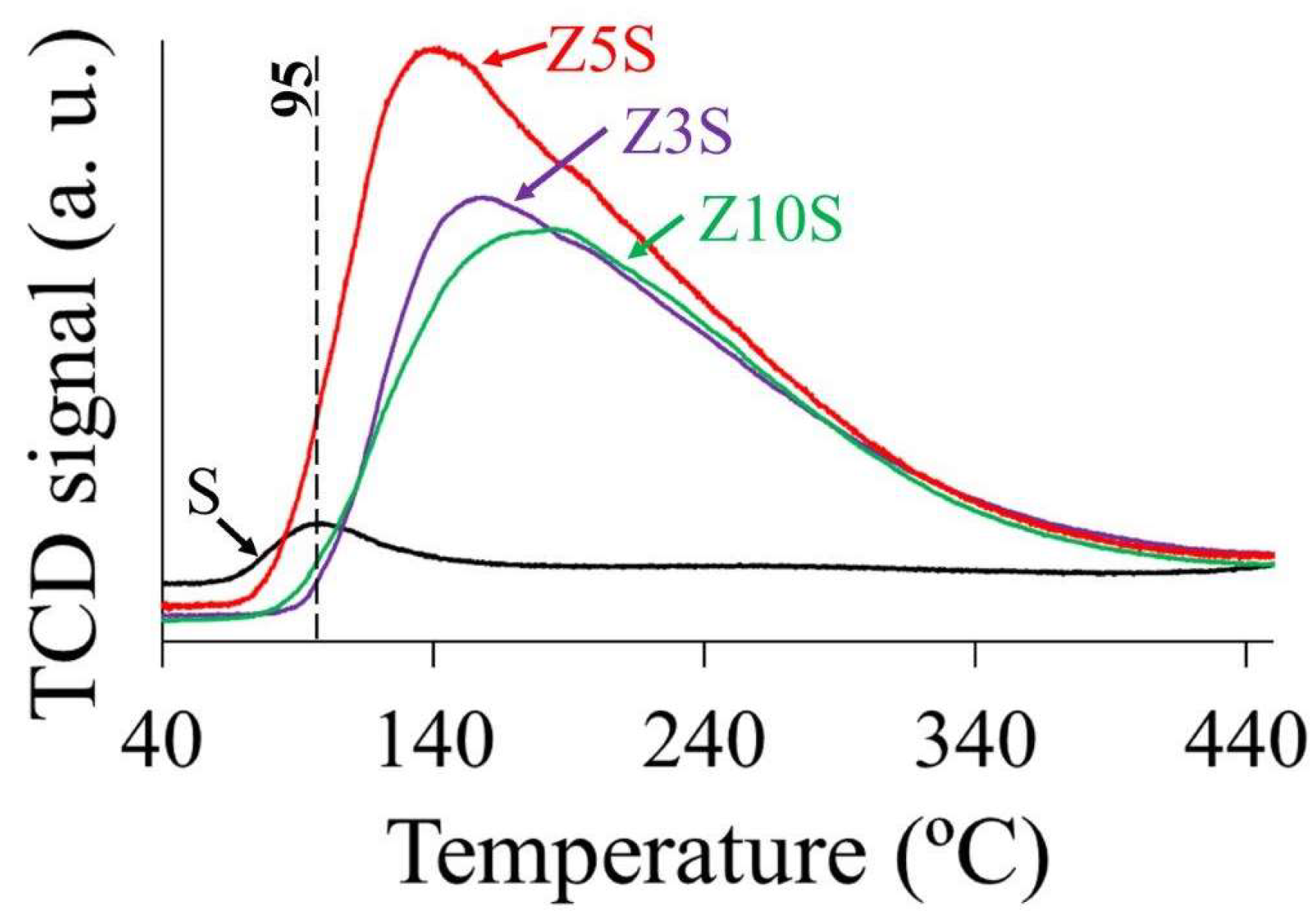
2.8. CO2 Thermodesorption
SBA-14 (S) just showed a small signal (95 °C) attributed to physisorbed CO
2 species [
6,
22]. The surface basic properties of the studied materials were estimated by integrating the area of corresponding TPD profiles,
Figure 5. Similarly to that found regarding acidity trends, basicity (number of sites and their strength) was significantly enhanced by Zr addition. However, most of those centers were either weak or of medium strength. The former could be related to bicarbonates formed by CO
2 adsorption on low-strength basic surface hydroxyls [
23], meanwhile the latter bidentate carbonates could be formed on Lewis acid-base pairs (Zr-O
2-) [
23], Scheme S1.
Zr addition resulted in basic sites amount increased by almost one order of magnitude being Z5S the sample with the highest sites population,
Table 3. In both bases (per mass or per surface area unit) tendencies were similar, where Z5S was the solid of the largest basicity,
Table 3. Again, not direct relationship with zirconia content in solids was found.
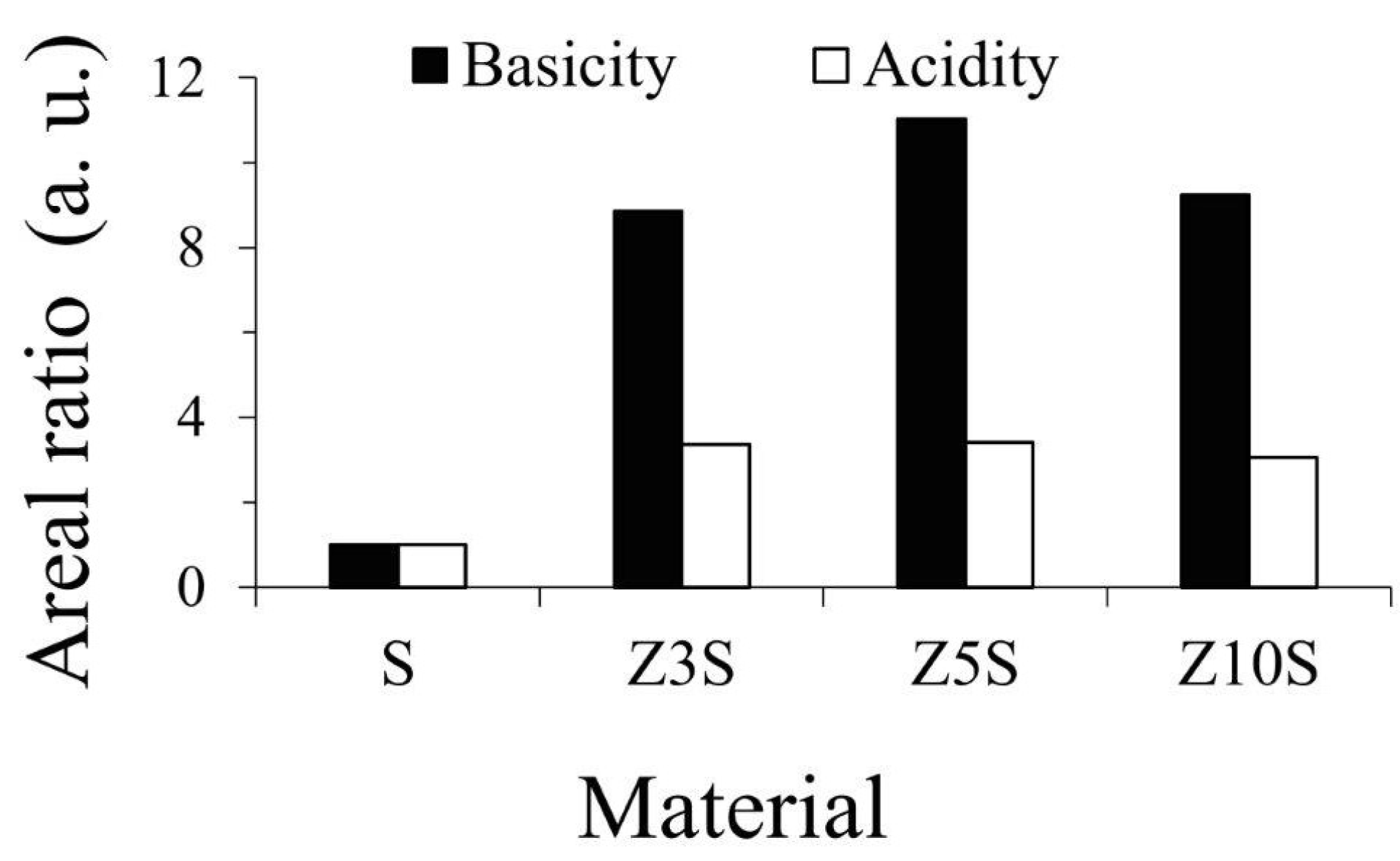
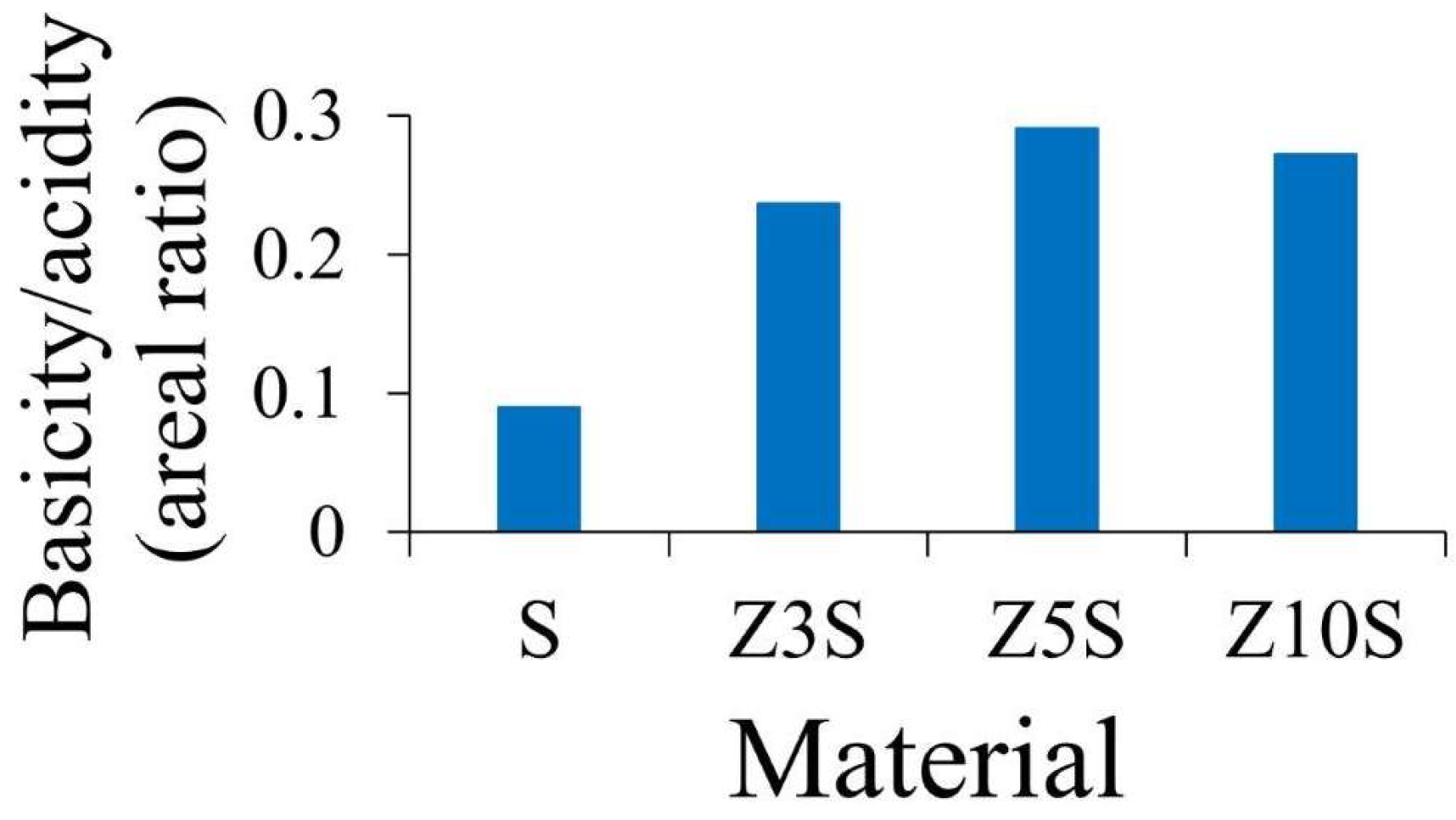
2.9. Acidic/Basic Properties
Amount (and strength) of SBA-15 surface acid and basic sites were significantly enhanced by extant Zr (
Figure 4 and
Figure 5, respectively), as observed in
Figure 6 where mesoporous silica was used as reference. Also, both protonic (related to Zr-O-Si bonds, framework sites) and Lewis (CUS, extra-framework zirconia) could be present on binary formulations.
The effect of zirconium doping was especially notable regarding medium-weak basic sites,
Figure 5 and
Figure 6. It seemed that the proportion of basic sites were lower than acidic ones,
Figure 7.
However, it is worth nothing that the last comparison was carried out in area units from TCD signals integration. Thus, that was affected by distinctive thermal conductivity of NH
3 and CO
2 (~2.6x10
-3 and ~16.1 x10
-3, both in W m
-1 K
-1, respectively). In any case, the existence of acid-basic pairs on Zr-modified SBA-15 was proven. Note that the synthesis method followed to prepare binary materials was quite simple and just included a single modifier (section 3.1). However, by utilizing pH-adjusted preparation technique, proportion of framework Zr (inserted in SBA-15 mesoporous network) could be increased [
18,
19], that being reflected in augmented protonic acidity (originated by higher proportion of Zr-O-Si bonds). Thus, the possibility of finely tuning corresponding amphoteric properties could be feasible. Santacesaria et al. have reported [
24] on the relevance of acid-basic pair on Y decationized zeolite applied in ethyl alcohol dehydration where proposed reaction mechanism required of vicinal acid-basic sites. Although the former were able to catalyze dehydration, reaction rate was increased by one order of magnitude by simultaneous presence of the latter ones. It was assumed that carbocation formation (on acid centers) was corresponding limiting step
Also, it was hypothesized that basic centers were required to stabilize carbocation through precursor [
24], Scheme S2. Also, amphoteric solids (containing Lewis acid-base pairs) have been recently applied as catalysts to produce biodiesel through fatty acid methyl esters (FAME) [
27,
28], where Jatropha curcas biodiesel yield was 100%. These results underline the relevance of those solids in the ongoing energy transition.
In our case, strong acidity was absent on Zr- containing solids. Avoidance of those centers could be very relevant as their existence has been linked to disintegration [
27] and coking reactions [
28].
Last but not least, corresponding catalytic tests of our Z-modified SBA-15 solids in reactions that demand amphoteric properties are ongoing and will be reported in due course.
3. Experimental
3.1. Materials Synthesis
Zr-Modified SBA-15
To prepare SBA-15 (S) method utilized in [
9] was used. Reactants and corresponding amounts of them were as previously detailed [
10]. Zr-modified materials (at 3, 5 and 10 wt%, ZxS, x; Zr nominal content) were prepared by directly adding pertinent amounts of ZrOCl
2•8H
2O, similarly to that carried out in [
6], regarding La-containing precursor.
3.2. Materials Characterization
Texture (surface area, pore size distribution (PSD) and pore volume) and structural order of various solids were studied by N
2 physisorption and X-ray diffraction, respectively. Corresponding details could be found elsewhere [
6]. Fourier transform infrared (FTIR) spectra of prepared materials were obtained by using Perkin Elmer Frontier equipment. UV-vis DRS spectra of several studied samples were acquired through Shimadzu UV-2600 equipment in the 200-600 nm wavelength range. Surface acidity and basicity of various materials were determined by temperature-programmed desorption (TPD) of NH
3 and CO
2, as described in [
6] Compositional analyses were carried out by EDS (Energy-Dispersive X-ray Spectroscopy) apparatus attached to scanning electron microscopy (SEM), JEOL JSM-6010LA (JEOL, Tokyo, Japan) operating at 20 kV accelerating voltage, The materials were characterized by high-resolution transmission electron microscopy (HR-TEM) in Titan 80-300 microscope with a Schottky-type field emission gun operating at 300 kV. The point resolution and the information limit were better than 0.085 nm. HR-TEM digital images were obtained through CCD camera. Prior to analysis powdered materials were ultrasonically dispersed (ethanol).
4. Conclusions
Simple Zr (nominal 3, 5 and 10 wt%, as ZrOCl2•8H2O) direct addition (n Ph ADJUSTEMENT= during SBA-15 synthesis resulted in binary materials of significantly enhanced amphoteric properties (as determined by NH3 and CO2 TPD titrating acid and basic sites, respective-y).), especially in the case of solid modified by nominal 5 wt% Zr content (actual 3.26 wt%, by EDS). Zirconium was integrated in BOTH framework (as found by FTIR and UV-vis spectroscopies) and extra-framework sites (UV-vis and HRTEM) of mesostructured silica network. Total Zr incorporated to mesoporous silica augmented with nominal content in binary formulations, although it was not necessarily integrated in SBA-15 walls forming Zr-O-Si linkages. Single ZrO2 domains (framework and extra-framework) provided suitable amphoteric properties due to augmented number and strength of both acid and basic sites, that effect being more notable in solid containing nominal 5 wt% Zr). The obtained binary oxides present great potential to be applied in reactions requiring vicinal non-strong acid-basic pairs (alcohols dehydration, for instance).
References
- Eom, H.-J.; Kim, M.-S.; Lee, D.-W.; Hong, Y.K.; Jeon, G.; Lee, K.Y. Zirconia catalysts (ZrO2 and Na-ZrO2) for the conversion of phenethyl phenyl ether (PPE) in supercritical water. Appl. Catal. A-Gen 2015, 493, 149–157. [Google Scholar] [CrossRef]
- Zeidan, R.K.; Davis. M. E. The effect of acid-base pairing on catalysis: An efficient acid-base functionalized catalyst for aldol condensation. J. Catal. 2007, 247, 379–382. [Google Scholar] [CrossRef]
- Shi, L. Y.; Wang, Y. M.; Ji, A.; Gao, L.; Wang, Y. In situ direct bifunctionalization of mesoporous silica SBA-15. J. Mater. Chem. 2005, 15, 1392–1396. [Google Scholar] [CrossRef]
- Chen, Y.; Han, J.; Zhan, H. Structure and acid-base properties of surface-modified mesoporous silica. Appl. Surf. Sci. 2007, 253, 9400–9406. [Google Scholar] [CrossRef]
- Derawi, D.; Razak, N.A.A.; Saadon, N.S.; Bar, N.A.; Taufiq-Yap, Y.H. Conversion of palm fatty acid distillate to bio-jet fuel-range hydrocarbons over bimetallic NiCo/SBA-15-NH2 catalyst. Fuel 2025, 380, 133192. [Google Scholar] [CrossRef]
- Morales-Hernández, G; Escobar, J. ; Pacheco-Sosa, J. G.; Guzmán-Cruz, M.A.; Torres-Torres, J.G.; del Ángel Vicente, P.; Barrera, M.C.; Santolalla-Vargas, C.E.; Pérez-Vidal, H. La-modified SBA-15 prepared by direct synthesis: importance of determining actual composition. Catalysts 2024, 14, 436. [Google Scholar] [CrossRef]
- Escobar, J.; Colín-Luna, J.A.; Barrera, M.C. Thioresistant PdPt/Al/SBA-15 for naphthalene hydrogenation. Ind. Eng. Chem. Res. 2024, 63, 1248–1260. [Google Scholar] [CrossRef]
- Rigney, M.P.; Funkenbush, E.F.; Carr, P.W. Physical and chemical characterization of microporous zirconia. J. Chrom. 1990, 499, 291–304. [Google Scholar] [CrossRef]
- Flodström, K.; Alfredsson, V. Influence of the block length of triblock copolymers on the formation of mesoporous silica. Microporous Mesoporous Mater. 2003, 59, 167–176. [Google Scholar] [CrossRef]
- Morales Hernández, G.; Pacheco Sosa, J. G.; Escobar Aguilar, J.; Torres Torres, J.G.; Pérez Vidal, H.; Lunagómez Rocha, M.A.; De la Cruz Romero, D.; del Ángel Vicente, P. Improving platinum dispersion on SBA-15 by titania addition. Rev. Mex. Ing. Quim. 2020, 19, 997–1010. [Google Scholar] [CrossRef]
- Kobayashi, T.; Sasaki, T.; Takagi, I.; Moriyama, H. J. Solubility of Zirconium (IV) Hydrous Oxides. Nucl. Sci. Technol. 2007, 44, 90–94. [Google Scholar] [CrossRef]
- Leofanti, S.G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–210. [Google Scholar] [CrossRef]
- Palcheva, R.; Kaluža, L.; Dimitrov, L.; Tyuliev, G.; Avdeev, G.; Jirátová, K.; Spojakina, A. NiMo catalysts supported on the Nb modified mesoporous SBA-15 and HMS: effect of thioglycolic acid addition on HDS. Appl. Catal. A-Gen 2016, 520, 24–34. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Chang, B.; Fu, J. ; Tian,Y.; Dong, X. Mesoporous solid acid catalysts of sulfated zirconia/SBA-15 derived from a vapor-induced hydrolysis route. Appl. Catal. A-Gen 2012. [Google Scholar] [CrossRef]
- Giraldo, L.; Bastidas-Barranco, M.; Moreno-Piraján, J.C. Vapour Phase Hydrogenation of Phenol over Rhodium on SBA-15 and SBA-16. Molecules 2014, 19, 20594–20612. [Google Scholar] [CrossRef]
- Wu, S.; Han, Y.; Zou, Y.C.; Song, J.W.; Zhao, L.; Di, Y.; Liu, S.Z.; Xiao, F.S. Synthesis of Heteroatom Substituted SBA-15 by the “pH-Adjusting” Method. Chem. Mater. 2004, 16, 486–492. [Google Scholar] [CrossRef]
- Colmenares-Zerpa, J.; Chimentão, R.J.; Gispert-Guirado, F.; Peixoto, A.F.; Llorca, J. Preparation of SBA-15 and Zr-SBA-15 materials by direct-synthesis and pH-adjustment methods. Mater. Lett. 2021, 301, 130326. [Google Scholar] [CrossRef]
- Colmenares-Zerpa, J.; Gajardo, J.; Peixoto, A.F.; Silva, D.S.A.; Silva, J.A.; Gispert-Guirado, F.; Llorca, J.; Urquieta-Gonzalez, E.A.; Santos, J.B.O.; Chimentão, R.J. ; High zirconium loads in Zr-SBA-15 mesoporous materials prepared by direct-synthesis and pH-adjusting approaches. J. Solid State Chem. 2022, 312, 123296. [Google Scholar] [CrossRef]
- Guo, X.; Feng, Y.; Ma, L.; Gao, D.; Jing, J.; Yu, J.; Sun, H.; Gong, H.; Zhang, Y. Phosphoryl functionalized mesoporous silica for uranium adsorption. Appl. Surf. Sci. 2017, 402, 53–60. [Google Scholar] [CrossRef]
- Qiang, T.; Zhao, J.; Li, J. Direct synthesis of homogeneous Zr-doped SBA-15 mesoporous silica via masking zirconium sulfate. Microporous Mesoporous Mater. 2018, 257, 162–174. [Google Scholar] [CrossRef]
- Terrab, I.; Ouargli, R.; Boukoussa, B.; Ghomari, K. , Hamacha, R.: Roy, R.; Azzouz, A.; Bengueddach, A. Res Chem. Intermed. 2017, 43, 3775–3786. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Diez, V.K.; Xu, M.; Iglesia, E.; Apesteguia, C.R. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Santacesaria, E.; Gelosa, D.; Giorgi, E.; Carrà, S. Role of basic and acid sites in the bimolecular dehydration of alcohols catalyzed by HY zeolite. J. Catal. 1984, 90, 1–1. [Google Scholar] [CrossRef]
- Zhang, Z.; Meng, P.; Luo, H.; Pei, Z.; Lu, X. Lewis Acid-Base Site-Assisted In Situ Transesterification Catalysis to Produce Biodiesel. Catalysts 2024, 14, 731. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Fang, Z.; Yang, X.-X.; Yang, Y.-T.; Luo, J.; Xu, K.; Bao, G.-R. One-step production of biodiesel from Jatropha oils with high acid value at low temperature by magnetic acid-base amphoteric nanoparticles. Chem. Eng. J. 2018, 348, 929–939. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, L.; Liu, R.; Wu, G.; Guan, N.; Li, L. Plate-Like ZSM-5 Zeolites as Robust Catalysts for the Cracking of Hydrocarbons. ACS Appl. Mater. Interfaces 2022, 14, 9–11415. [Google Scholar] [CrossRef]
- Venezia, A. M.; Murania, R.; La Parola, V.; Pawelec, B.; Fierro,J. L. G. Post-synthesis alumination of MCM-41: Effect of the acidity on the HDS activity of supported Pd Catalysts. Appl. Catal. A-Gen 2010, 383, 211–216. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).