Submitted:
28 October 2024
Posted:
29 October 2024
You are already at the latest version
Abstract
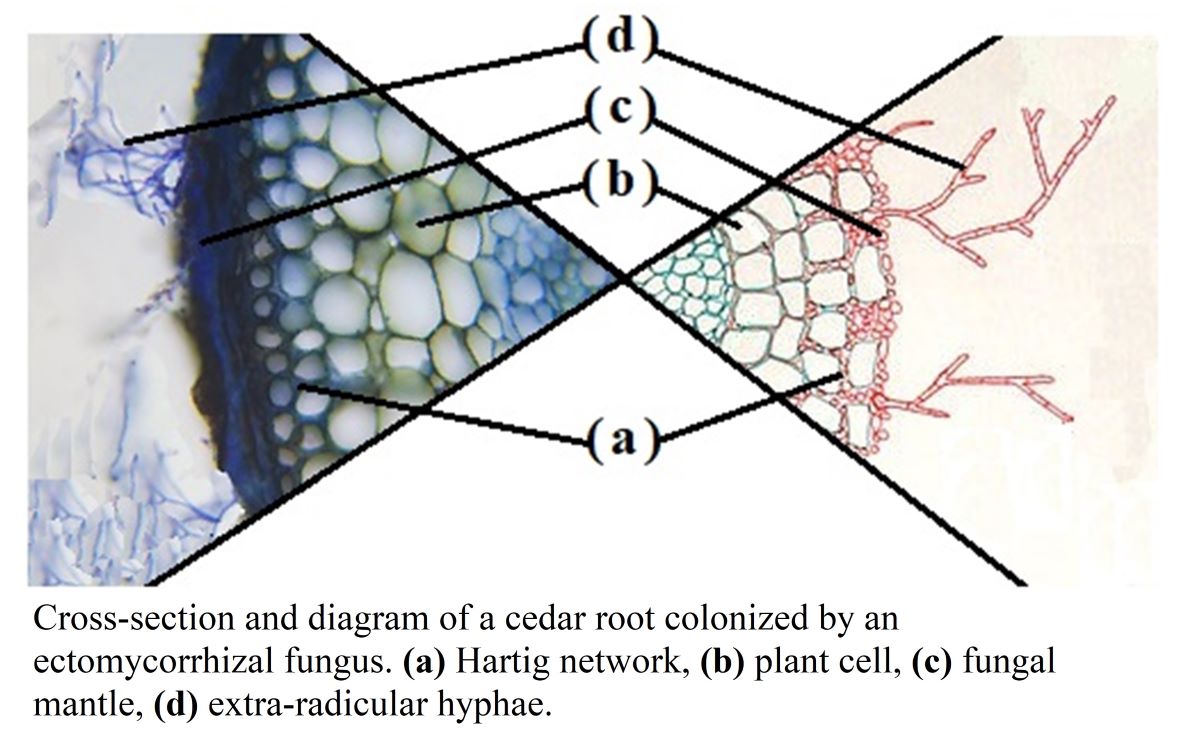
Keywords:
1. Background
2. Plant and Fungal Diversity
2.1. Host Plant Diversity and EM Fungal Diversity
2.2. Interactions Between Plant and Fungal Diversity
3. Host Specificity
3.1. Host Specificity and Ectomycorrhizal Associations
3.2. Host Specificity and Environmental Conditions
3.3. Biochemical mechanisms Host specificity
4. Microbial Interactions
4.1. Positive influences of bacteria on EM Formation
4.2. Negative Effects of Bacteria on Mycorrhizal Formation
4.3. Positive Interactions Between Fungi in EM Formation
4.4. Negative fungal interactions on EM Formation
5. Conclusion
Conflicts of Interest
References
- El Amrani, B. Effects of Soil Biotic and Abiotic Properties on the Growth and Mycorrhization of Cedars, Cedrus Atlantica Manetti. Bois & Forêts des Tropiques 2022, 351, 85–86. [CrossRef]
- Tunlid, A.; Floudas, D.; Op De Beeck, M.; Wang, T.; Persson, P. Decomposition of Soil Organic Matter by Ectomycorrhizal Fungi: Mechanisms and Consequences for Organic Nitrogen Uptake and Soil Carbon Stabilization. Front. For. Glob. Change 2022, 5, 934409 . [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytologist 2018, 220, 1108–1115. [CrossRef]
- Van Der Linde, S.; Suz, L.M.; Orme, C.D.L.; Cox, F.; Andreae, H.; Asi, E.; Atkinson, B.; Benham, S.; Carroll, C.; Cools, N.; et al. Environment and Host as Large-Scale Controls of Ectomycorrhizal Fungi. Nature 2018, 558, 243–248. [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural Functions of Lipopeptides from Bacillus and Pseudomonas : More than Surfactants and Antibiotics. FEMS Microbiol Rev 2010, 34, 1037–1062. [CrossRef]
- Tatsumi, C.; Taniguchi, T.; Du, S.; Yamanaka, N.; Tateno, R. Soil Nitrogen Cycling Is Determined by the Competition between Mycorrhiza and Ammonia-oxidizing Prokaryotes. Ecology 2020, 101, e02963. [CrossRef]
- Boddy, L.; Hiscox, J. Fungal Ecology: Principles and Mechanisms of Colonization and Competition by Saprotrophic Fungi. Microbiol Spectr 2016, 4, 4.6.17. [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal Fungi – Potential Organic Matter Decomposers, yet Not Saprotrophs. New Phytologist 2015, 205, 1443–1447. [CrossRef]
- Yang, T.; Adams, J.M.; Shi, Y.; He, J.; Jing, X.; Chen, L.; Tedersoo, L.; Chu, H. Soil Fungal Diversity in Natural Grasslands of the Tibetan Plateau: Associations with Plant Diversity and Productivity. New Phytologist 2017, 215, 756–765. [CrossRef]
- Santolamazza-Carbone, S. Ectomycorrhizal Fungal Community Structure in a Young Orchard of Grafted and Ungrafted Hybrid Chestnut Saplings. 2021, 31, 189–201. [CrossRef]
- Dai, D.-Q.; Suwannarach, N.; Bamunuarachchige, T.C.; Karunarathna, S.C. Editorial: Plant-Fungal Interactions. Frontiers in Microbiology 2023, 14. [CrossRef]
- Lei, X.; Shen, Y.; Zhao, J.; Huang, J.; Wang, H.; Yu, Y.; Xiao, C. Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants 2023, 12. [CrossRef]
- Rudawska, M.; Leski, T. Ectomycorrhizal Fungal Assemblages of Nursery-Grown Scots Pine Are Influenced by Age of the Seedlings. 2021, 12. [CrossRef]
- Fei, S.; Kivlin, S.N.; Domke, G.M.; Jo, I.; LaRue, E.A.; Philips, R.P. Coupling of Plant and Mycorrhizal Fungal Diversity: Its Occurrence, Relevance, and Possible Implications under Global Change. New Phytologist 2022, 234, 1960–1966. [CrossRef]
- O’Hanlon, R.; Harrington, T.J. Similar Taxonomic Richness but Different Communities of Ectomycorrhizas in Native Forests and Non-Native Plantation Forests. Mycorrhiza 2012, 22, 371–382. [CrossRef]
- Chai, D.-D.; Guo, S.-J.; Sun, X.-B.; Qin, T.-T. The Major Factors Affecting Ectomycorrhizal Fungi Diversity in the Forest Ecosystem. Advance Journal of Food Science and Technology 2013, 5, 879–890. [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root Biomass and Exudates Link Plant Diversity with Soil Bacterial and Fungal Biomass. Sci Rep 2017, 7, 44641. [CrossRef]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytologist 2020, 225, 87–104. [CrossRef]
- Bonito, G.; Reynolds, H.; Robeson, M.S.; Nelson, J.; Hodkinson, B.P.; Tuskan, G.; Schadt, C.W.; Vilgalys, R. Plant Host and Soil Origin Influence Fungal and Bacterial Assemblages in the Roots of Woody Plants. Molecular Ecology 2014, 23, 3356–3370. [CrossRef]
- Molina, R.; Horton, T.R. Mycorrhiza Specificity: Its Role in the Development and Function of Common Mycelial Networks. In Mycorrhizal Networks; Horton, T.R., Ed.; Ecological Studies; Springer Netherlands: Dordrecht, 2015; Vol. 224, pp. 1–39. [CrossRef]
- Heilmann-Clausen, J.; Maruyama, P.K.; Bruun, H.H.; Dimitrov, D.; Læssøe, T.; Frøslev, T.G.; Dalsgaard, B. Citizen Science Data Reveal Ecological, Historical and Evolutionary Factors Shaping Interactions between Woody Hosts and Wood-inhabiting Fungi. New Phytologist 2016, 212, 1072–1082. [CrossRef]
- Chen, Y.-L.; Xu, T.-L.; Veresoglou, S.D.; Hu, H.-W.; Hao, Z.-P.; Hu, Y.-J.; Liu, L.; Deng, Y.; Rillig, M.C.; Chen, B.-D. Plant Diversity Represents the Prevalent Determinant of Soil Fungal Community Structure across Temperate Grasslands in Northern China. Soil Biology and Biochemistry 2017, 110, 12–21. [CrossRef]
- Lewis, J.D. Mycorrhizal Fungi, Evolution and Diversification Of. In Encyclopedia of Evolutionary Biology; Elsevier, 2016; pp. 94–99. [CrossRef]
- Rúa, M.A.; Hoeksema, J.D. Interspecific Selection in a Diverse Mycorrhizal Symbiosis. Sci Rep 2024, 14, 12151. [CrossRef]
- Põlme, S.; Bahram, M.; Jacquemyn, H.; Kennedy, P.; Kohout, P.; Moora, M.; Oja, J.; Öpik, M.; Pecoraro, L.; Tedersoo, L. Host Preference and Network Properties in Biotrophic Plant-Fungal Associations. New Phytol 2018, 217, 1230–1239. [CrossRef]
- Tedersoo, L.; Sadam, A.; Zambrano, M.; Valencia, R.; Bahram, M. Low Diversity and High Host Preference of Ectomycorrhizal Fungi in Western Amazonia, a Neotropical Biodiversity Hotspot. The ISME Journal 2010, 4, 465–471. [CrossRef]
- Tedersoo, L.; Jairus, T.; Horton, B.M.; Abarenkov, K.; Suvi, T.; Saar, I.; Kõljalg, U. Strong Host Preference of Ectomycorrhizal Fungi in a Tasmanian Wet Sclerophyll Forest as Revealed by DNA Barcoding and Taxon-Specific Primers. New Phytologist 2008, 180, 479–490. [CrossRef]
- Ding, Q.; Liang, Y.; Legendre, P.; He, X.; Pei, K.; Du, X.; Ma, K. Diversity and Composition of Ectomycorrhizal Community on Seedling Roots: The Role of Host Preference and Soil Origin. Mycorrhiza 2011, 21, 669–680. [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using Network Analysis to Explore Co-Occurrence Patterns in Soil Microbial Communities. The ISME Journal 2012, 6, 343–351. [CrossRef]
- Courty, P.E.; Labbe, J.; Kohler, A.; Marc, B. Effect of Poplar Genotypes on Mycorrhizal Infection and Secreted Enzyme Activities in Mycorrhizal and Non-Mycorrhizal Roots. Journal of Experimental Botany 2011, 62, 12. [CrossRef]
- Gehring, C.; Bennett, A. Mycorrhizal Fungal–Plant–Insect Interactions: The Importance of a Community Approach. en 2009, 38, 93–102. [CrossRef]
- Compant, S.; Van Der Heijden, M.G.A.; Sessitsch, A. Climate Change Effects on Beneficial Plant-Microorganism Interactions: Climate Change and Beneficial Plant-Microorganism Interactions. FEMS Microbiology Ecology 2010, 73, 197–214. [CrossRef]
- Atkinson, P.; Blakeman, J.P. Seasonal Occurrence of an Antimicrobial Flavanone, Sakuranetin, Associated with Glands on Leaves of Ribes Nigrum. New Phytologist 1982, 92, 63–74. [CrossRef]
- Elhamouly, N.A.; Hewedy, O.A.; Zaitoon, A.; Miraples, A.; Elshorbagy, O.T.; Hussien, S.; El-Tahan, A.; Peng, D. The Hidden Power of Secondary Metabolites in Plant-Fungi Interactions and Sustainable Phytoremediation. Front. Plant Sci. 2022, 13, 1044896. [CrossRef]
- De Oliveira, T.L.C.; De Araújo Soares, R.; Ramos, E.M.; Das Graças Cardoso, M.; Alves, E.; Piccoli, R.H. Antimicrobial Activity of Satureja Montana L. Essential Oil against Clostridium Perfringens Type A Inoculated in Mortadella-Type Sausages Formulated with Different Levels of Sodium Nitrite. International Journal of Food Microbiology 2011, 144, 546–555. [CrossRef]
- Ito, S.; Ihara, T.; Tamura, H.; Tanaka, S.; Ikeda, T.; Kajihara, H.; Dissanayake, C.; Abdel-Motaal, F.F.; El-Sayed, M.A. α-Tomatine, the Major Saponin in Tomato, Induces Programmed Cell Death Mediated by Reactive Oxygen Species in the Fungal Pathogen Fusarium Oxysporum. FEBS Letters 2007, 581, 3217–3222. [CrossRef]
- Baetz, U.; Martinoia, E. Root Exudates: The Hidden Part of Plant Defense. Trends in Plant Science 2014, 19, 90–98. [CrossRef]
- Garcia, K.; Delaux, P.; Cope, K.R.; Ané, J. Molecular Signals Required for the Establishment and Maintenance of Ectomycorrhizal Symbioses. New Phytologist 2015, 208, 79–87. [CrossRef]
- Alam, B.; Lǐ, J.; Gě, Q.; Khan, M.A.; Gōng, J.; Mehmood, S.; Yuán, Y.; Gǒng, W. Endophytic Fungi: From Symbiosis to Secondary Metabolite Communications or Vice Versa? Front. Plant Sci. 2021, 12, 791033. [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple Elements of Soil Biodiversity Drive Ecosystem Functions across Biomes. Nat Ecol Evol 2020, 4, 210–220. [CrossRef]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A Global Synthesis Reveals Biodiversity Loss as a Major Driver of Ecosystem Change. Nature 2012, 486, 105–108. [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity Enhances Ecosystem Multifunctionality across Trophic Levels and Habitats. Nat Commun 2015, 6, 6936. [CrossRef]
- Koziol, L.; Bever, J.D. The Missing Link in Grassland Restoration: Arbuscular Mycorrhizal Fungi Inoculation Increases Plant Diversity and Accelerates Succession. Journal of Applied Ecology 2017, 54, 1301–1309. [CrossRef]
- Garbaye, J. Tansley Review No. 76 Helper Bacteria: A New Dimension to the Mycorrhizal Symbiosis. New Phytologist 1994, 128, 197–210. [CrossRef]
- Frey-Klett, P.; Garbaye, J. Mycorrhiza Helper Bacteria: A Promising Model for the Genomic Analysis of Fungal-Bacterial Interactions: Commentary. New Phytologist 2005, 168, 4–8. [CrossRef]
- Bonfante, P.; Anca, I.-A. Plants, Mycorrhizal Fungi, and Bacteria: A Network of Interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [CrossRef]
- Venturi, V.; Keel, C. Signaling in the Rhizosphere. Trends in Plant Science 2016, 21, 187–198. [CrossRef]
- Leveau, J.H.J.; Preston, G.M. Bacterial Mycophagy: Definition and Diagnosis of a Unique Bacterial–Fungal Interaction. New Phytologist 2008, 177, 859–876. [CrossRef]
- Martin, F. Molecular Mycorrhizal Symbiosis; Ed.; 1st ed.; Wiley, 2016. [CrossRef]
- Gamalero, E.; Lingua, G.; Berta, G.; Glick, B.R. Beneficial Role of Plant Growth Promoting Bacteria and Arbuscular Mycorrhizal Fungi on Plant Responses to Heavy Metal Stress. Can. J. Microbiol. 2009, 55, 501–514. [CrossRef]
- Boedicker, J.; Nealson, K. INVITED: Microbial Communication via Quorum Sensing. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2016, 1–1. [CrossRef]
- Pantigoso, H.A.; Newberger, D.; Vivanco, J.M. The Rhizosphere Microbiome: Plant–Microbial Interactions for Resource Acquisition. Journal of Applied Microbiology 2022, 133, 2864–2876. [CrossRef]
- Lee, J.-H.; Lee, J. Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol Rev 2010, 34, 426–444. [CrossRef]
- Pande, S.; Merker, H.; Bohl, K.; Reichelt, M.; Schuster, S.; De Figueiredo, L.F.; Kaleta, C.; Kost, C. Fitness and Stability of Obligate Cross-Feeding Interactions That Emerge upon Gene Loss in Bacteria. The ISME Journal 2014, 8, 953–962. [CrossRef]
- Hildebrandt, U.; Ouziad, F.; Marner, F.-J.; Bothe, H. The Bacterium Paenibacillus Validus Stimulates Growth of the Arbuscular Mycorrhizal Fungus Glomus Intraradices up to the Formation of Fertile Spores. FEMS Microbiology Letters 2006, 254, 258–267. [CrossRef]
- Selvakumar, G.; Krishnamoorthy, R.; Kim, K.; Sa, T.-M. Genetic Diversity and Association Characters of Bacteria Isolated from Arbuscular Mycorrhizal Fungal Spore Walls. PLoS ONE 2016, 11, e0160356. [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [CrossRef]
- Röttjers, L.; Faust, K. From Hairballs to Hypotheses–Biological Insights from Microbial Networks. FEMS Microbiology Reviews 2018, 42, 761–780. [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial Inoculants: Reviewing the Past, Discussing the Present and Previewing an Outstanding Future for the Use of Beneficial Bacteria in Agriculture. AMB Expr 2019, 9, 205. [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; 3rd ed.; Academic Press: Amsterdam Boston, 2008. [CrossRef]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in Fungal and Bacterial Physiology Alter Soil Carbon and Nitrogen Cycling: Insights from Meta-analysis and Theoretical Models. Ecology Letters 2013, 16, 887–894. [CrossRef]
- Wang, T.; Tian, Z.; Tunlid, A.; Persson, P. Nitrogen Acquisition from Mineral-associated Proteins by an Ectomycorrhizal Fungus. New Phytologist 2020, 228, 697–711. [CrossRef]
- Hartmann, M.; Niklaus, P.A.; Zimmermann, S.; Schmutz, S.; Kremer, J.; Abarenkov, K.; Lüscher, P.; Widmer, F.; Frey, B. Resistance and Resilience of the Forest Soil Microbiome to Logging-Associated Compaction. The ISME Journal 2014, 8, 226–244. [CrossRef]
- Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11, 2904. [CrossRef]
- Mavrodi, D.V.; Ksenzenko, V.N.; Bonsall, R.F.; Cook, R.J.; Boronin, A.M.; Thomashow, L.S. A Seven-Gene Locus for Synthesis of Phenazine-1-Carboxylic Acid by Pseudomonas Fluorescens 2-79. J Bacteriol 1998, 180, 2541–2548. [CrossRef]
- Prapagdee, B.; Kuekulvong, C.; Mongkolsuk, S. Antifungal Potential of Extracellular Metabolites Produced by Streptomyces Hygroscopicus against Phytopathogenic Fungi. Int. J. Biol. Sci. 2008, 330–337, doi:10.7150/ijbs.4.330.
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile Mediated Interactions Between Bacteria and Fungi in the Soil. J Chem Ecol 2012, 38, 665–703. [CrossRef]
- Behnsen, J.; Raffatellu, M. Siderophores: More than Stealing Iron. mBio 2016, 7, e01906-16. [CrossRef]
- Masri, M.; Sukmawaty, E.; Awalia Amir, A. Anti Fungal Activity of Chitinolytic Bacteria Lysinibacillus Fusiformis and Brevibacillus Reuszeri Against The Fungal Pathogens Rhizoctonia Solani and Fusarium Oxysporum. Microbiol indones 2022, 15, 3. [CrossRef]
- Zhang, Z.; Yuen, G.Y.; Sarath, G.; Penheiter, A.R. Chitinases from the Plant Disease Biocontrol Agent, Stenotrophomonas Maltophilia C3. Phytopathology® 2001, 91, 204–211. [CrossRef]
- Zhu, M.-L.; Wu, X.-Q.; Wang, Y.-H.; Dai, Y. Role of Biofilm Formation by Bacillus Pumilus HR10 in Biocontrol against Pine Seedling Damping-Off Disease Caused by Rhizoctonia Solani. Forests 2020, 11, 652. [CrossRef]
- Kaur, R.; Macleod, J.; Foley, W.; Nayudu, M. Gluconic Acid: An Antifungal Agent Produced by Pseudomonas Species in Biological Control of Take-All. Phytochemistry 2006, 67, 595–604. [CrossRef]
- Soti, P.G.; Jayachandran, K.; Koptur, S.; Volin, J.C. Effect of Soil pH on Growth, Nutrient Uptake, and Mycorrhizal Colonization in Exotic Invasive Lygodium Microphyllum. Plant Ecol 2015, 216, 989–998. [CrossRef]
- Zama, N.; Kirkman, K.; Mkhize, N.; Tedder, M.; Magadlela, A. Soil Acidification in Nutrient-Enriched Soils Reduces the Growth, Nutrient Concentrations, and Nitrogen-Use Efficiencies of Vachellia Sieberiana (DC.) Kyal. & Boatwr Saplings. Plants 2022, 11, 3564. [CrossRef]
- Neerincx, A.H.; Mandon, J.; Van Ingen, J.; Arslanov, D.D.; Mouton, J.W.; Harren, F.J.M.; Merkus, P.J.F.M.; Cristescu, S.M. Real-Time Monitoring of Hydrogen Cyanide (HCN) and Ammonia (NH3) Emitted by Pseudomonas Aeruginosa. J. Breath Res. 2015, 9, 027102. [CrossRef]
- Deepika, S.; Mittal, A.; Kothamasi, D. HCN-producing Pseudomonas Protegens CHA0 Affects Intraradical Viability of Rhizophagus Irregularis in Sorghum Vulgare Roots. J Basic Microbiol 2019, 59, 1229–1237. [CrossRef]
- Bahar, A.K.F.; Patandjengi, B.; Hardiansyah, M.Y.; Membalik, V. Characterization of Chitinolytic Bacteria Isolated from Ipomea Pes Caprae. IOP Conf. Ser.: Earth Environ. Sci. 2023, 1230, 012105. [CrossRef]
- Levy, A.; Salas Gonzalez, I.; Mittelviefhaus, M.; Clingenpeel, S.; Herrera Paredes, S.; Miao, J.; Wang, K.; Devescovi, G.; Stillman, K.; Monteiro, F.; et al. Genomic Features of Bacterial Adaptation Toplants. Nat Genet 2018, 50, 138–150. [CrossRef]
- Fernandez, C.W.; Kennedy, P.G. Revisiting the ‘Gadgil Effect’: Do Interguild Fungal Interactions Control Carbon Cycling in Forest Soils? New Phytologist 2016, 209, 1382–1394. [CrossRef]
- Sterkenburg, E.; Clemmensen, K.E.; Ekblad, A.; Finlay, R.D.; Lindahl, B.D. Contrasting Effects of Ectomycorrhizal Fungi on Early and Late Stage Decomposition in a Boreal Forest. The ISME Journal 2018, 12, 2187–2197. [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-Mediated Competition between Plants and Decomposers Drives Soil Carbon Storage. Nature 2014, 505, 543–545. [CrossRef]
- Bending, G.D.; Read, D.J. Lignin and Soluble Phenolic Degradation by Ectomycorrhizal and Ericoid Mycorrhizal Fungi. Mycological Research 1997, 101, 1348–1354. [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Karltun, E.; Lindahl, B.D. Below-ground Organic Matter Accumulation along a Boreal Forest Fertility Gradient Relates to Guild Interaction within Fungal Communities. Ecology Letters 2017, 20, 1546–1555. [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol Mol Biol Rev 2017, 81, e00063-16. [CrossRef]
- López-Mondéjar, R.; Brabcová, V.; Štursová, M.; Davidová, A.; Jansa, J.; Cajthaml, T.; Baldrian, P. Decomposer Food Web in a Deciduous Forest Shows High Share of Generalist Microorganisms and Importance of Microbial Biomass Recycling. The ISME Journal 2018, 12, 1768–1778. [CrossRef]
- Kubisch, P.; Hertel, D.; Leuschner, C. Fine Root Productivity and Turnover of Ectomycorrhizal and Arbuscular Mycorrhizal Tree Species in a Temperate Broad-Leaved Mixed Forest. Front. Plant Sci. 2016, 07. [CrossRef]
- Teste, F.P.; Jones, M.D.; Dickie, I.A. Dual-mycorrhizal Plants: Their Ecology and Relevance. New Phytologist 2020, 225, 1835–1851. [CrossRef]
- Dickie, I.A.; Moyersoen, B. Towards a Global View of Ectomycorrhizal Ecology. New Phytologist 2008, 180, 263–265. [CrossRef]
- Kadowaki, K.; Yamamoto, S.; Sato, H.; Tanabe, A.S.; Hidaka, A.; Toju, H. Mycorrhizal Fungi Mediate the Direction and Strength of Plant–Soil Feedbacks Differently between Arbuscular Mycorrhizal and Ectomycorrhizal Communities. Commun Biol 2018, 1, 196. [CrossRef]
- Averill, C.; Bhatnagar, J.M.; Dietze, M.C.; Pearse, W.D.; Kivlin, S.N. Global Imprint of Mycorrhizal Fungi on Whole-Plant Nutrient Economics. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 23163–23168. [CrossRef]
- Bergmann, J.; Weigelt, A.; Van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M.; et al. The Fungal Collaboration Gradient Dominates the Root Economics Space in Plants. Sci. Adv. 2020, 6, eaba3756. [CrossRef]
- Yang, G.; Wagg, C.; Veresoglou, S.D.; Hempel, S.; Rillig, M.C. How Soil Biota Drive Ecosystem Stability. Trends in Plant Science 2018, 23, 1057–1067. [CrossRef]
- Tedersoo, L.; Bahram, M. Mycorrhizal Types Differ in Ecophysiology and Alter Plant Nutrition and Soil Processes. Biological Reviews 2019, 94, 1857–1880. [CrossRef]
- Nickerson, M.N.; Moore, L.P.; U’Ren, J.M. The Impact of Polyphenolic Compounds on the in Vitro Growth of Oak-Associated Foliar Endophytic and Saprotrophic Fungi. Fungal Ecology 2023, 62, 101226. [CrossRef]
- Crowther, T.W.; Thomas, S.M.; Maynard, D.S.; Baldrian, P.; Covey, K.; Frey, S.D.; Van Diepen, L.T.A.; Bradford, M.A. Biotic Interactions Mediate Soil Microbial Feedbacks to Climate Change. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 7033–7038. [CrossRef]
- Yang, H.; Dai, Y.; Wang, X.; Zhang, Q.; Zhu, L.; Bian, X. Meta-Analysis of Interactions between Arbuscular Mycorrhizal Fungi and Biotic Stressors of Plants. The Scientific World Journal 2014, 2014, 1–7. [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [CrossRef]
- Akram, S.; Ahmed, A.; He, P.; He, P.; Liu, Y.; Wu, Y.; Munir, S.; He, Y. Uniting the Role of Endophytic Fungi against Plant Pathogens and Their Interaction. JoF 2023, 9, 72. [CrossRef]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of Plant Defense System in Response to Microbial Interactions. Front. Microbiol. 2020, 11, 1298. [CrossRef]
- Ali, S.; Tyagi, A.; Mir, Z.A. Plant Immunity: At the Crossroads of Pathogen Perception and Defense Response. Plants 2024, 13, 1434. [CrossRef]
- Raudaskoski, M.; Kothe, E. Novel Findings on the Role of Signal Exchange in Arbuscular and Ectomycorrhizal Symbioses. Mycorrhiza 2015, 25, 243–252. [CrossRef]
- Koide, R.T. Functional Complementarity in the Arbuscular Mycorrhizal Symbiosis. New Phytologist 2000, 147, 233–235. [CrossRef]
- Fernández, N.; Knoblochová, T.; Kohout, P.; Janoušková, M.; Cajthaml, T.; Frouz, J.; Rydlová, J. Asymmetric Interaction Between Two Mycorrhizal Fungal Guilds and Consequences for the Establishment of Their Host Plants. Front. Plant Sci. 2022, 13, 873204. [CrossRef]
- Shemesh, H.; Boaz, B.E.; Millar, C.I.; Bruns, T.D. Symbiotic Interactions above Treeline of Long-lived Pines: Mycorrhizal Advantage of Limber Pine ( Pinus Flexilis ) over Great Basin Bristlecone Pine ( Pinus Longaeva ) at the Seedling Stage. Journal of Ecology 2020, 108, 908–916. [CrossRef]
- Peay, K.G. The Mutualistic Niche: Mycorrhizal Symbiosis and Community Dynamics. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 143–164. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



