1. Introduction
The liver plays a central role in regulating systemic lipid metabolism and maintaining homeostasis. Disruptions in hepatic lipid metabolism lead to the development of NAFLD. NAFLD includes a spectrum of pathological changes in the liver, which can be histologically classified into two categories: (a) nonalcoholic fatty liver (NAFL), characterized by lipid accumulation (steatosis) in more than 5% of hepatocytes without signs of hepatocellular injury, and (b) nonalcoholic steatohepatitis (NASH), defined as hepatic steatosis and inflammation, along with hepatocyte injury with or without fibrosis [
1,
2]. Over the past two decades, the global incidence of NAFLD has increased significantly from 25% to 32% [
3], and this trend is expected to continue at an alarming rate. Nonetheless, until recently, there was no Federal Drug Administration (FDA)-approved pharmacological treatment for NAFLD or NASH [
4]. In March 2024, the FDA approved Resmetirom, a liver-targeted thyroid hormone receptor β-selective agonist for treating NASH. Regardless of its benefits, Resmetirom’s usage is associated with several side effects including nausea, diarrhea, gallbladder-related side effects, and drug-induced liver toxicity. Moreover, it is contraindicated in patients with decompensated cirrhosis [
5,
6]. Given the high prevalence and complications associated with NAFLD, the development of additional novel therapeutic drugs with minimal side effects is essential. In addition, dysregulated lipid metabolism in the liver elevates circulating levels of low-density lipoprotein (LDL) particles, which induces vascular endothelium toxicity, promotes LDL infiltration into the arterial wall, and contributes to plaque formation, thereby linking NAFLD to atherosclerosis development [
7]. Since NAFLD shares risk factors such as visceral obesity, insulin resistance, and dyslipidemia with ASCVD, patients with NAFLD are at higher risk for adverse ASCVD events. Notably, ASCVD is recognized as the major cause of death among NAFLD patients, independent of traditional risk factors [
8]. However, our knowledge about the endogenous molecular factors and downstream signaling mechanisms responsible for dysregulated hepatic lipid metabolism and their roles in atherosclerotic lesion formation remains limited.
NID2 is a glycoprotein present in the basement membrane, where it interacts with collagen IV, perlecan, and collagen I to stabilize the membrane structure [
9]. NID2 expression has been linked to the development of various cancers including ovarian, lung, gastric, pancreatic, and oral squamous cell carcinoma [
10,
11,
12,
13,
14]. Additionally, elevated
NID2 mRNA levels have been observed in human atherosclerotic echolucent calcified plaques [
15,
16], which was further confirmed by a study analyzing publicly available transcriptomic profiles of atherosclerotic arteries from the Gene Expression Omnibus database [
17]. Interestingly, in murine models of vascular calcification and neointima formation, NID2 demonstrated a protective role against vascular calcification and helped in the maintenance of the contractile phenotype of vascular smooth muscle cells (VSMCs), respectively [
18,
19]. This apparent contradiction suggests NID2's diverse role in different cell types and disease models, highlighting the need for further studies. Nevertheless, its role in regulating hepatosteatosis and associated atherosclerosis has never been explored.
In this study, we investigated the role of NID2 in the pathogenesis of NAFLD and atherosclerosis by overexpressing NID2 using an adeno-associated viral (AAV) vector in Apoe-/- mice and Western diet feeding. We observed that NID2 protein expression is increased in both steatotic livers and atherosclerotic vascular tissues. NID2 overexpression in male Apoe-/- mice promoted hepatic steatosis, fibrosis, and atherosclerosis development. Interestingly, female mice with or without NID2 overexpression exhibited no differences in atherosclerosis development and hepatic fibrosis. Mechanistically, we found attenuated AMPK activation in the livers of NID2-overexpressing mice compared with controls, with no effects on hepatic inflammation. These findings provide the first experimental evidence of NID2’s detrimental role in the development of NAFLD and atherosclerosis.
3. Discussion
The liver plays a key role in systemic lipid metabolism by regulating the synthesis, uptake, storage, and efflux of cholesterol [
8,
33]. The prevalent liver disease NAFLD and its inflammatory form NASH are associated with the development of coronary artery calcification, atherosclerotic plaques, and increased carotid intima-media thickness [
34,
35]. These associations have stimulated interest in identifying the shared molecular mechanisms, which drive these liver and vascular diseases. Nevertheless, our knowledge about the intrinsic molecular factors and downstream mechanisms responsible for dysregulated hepatic lipid metabolism and their roles in atherosclerotic lesion formation remains limited. Herein, we investigated the role of NID2 in regulating hepatosteatosis, fibrosis, and atherosclerotic lesion formation. Our results demonstrated (a) elevated NID2 levels in murine steatotic livers and human atherosclerotic vascular tissues, (b) increased hepatic steatosis and fibrosis in
NID2-overexpressing mice, (c) exacerbated atherosclerosis in mice with
NID2 overexpression, and (d) reduced AMPK activation in the livers of
NID2-overexpressing mice. Collectively, these findings suggest that NID2 contributes to the progression of both hepatosteatosis and atherosclerosis.
NID2 is a secretory glycoprotein, which is ubiquitously present in the basement membrane (BM) and helps to maintain BM stability [
9,
36]. Depending on the cell type and disease, NID2 plays both beneficial and detrimental roles. Previous studies have associated NID2 expression with various cancers, including gastric, ovarian, bladder, pancreatic, and esophageal squamous cell carcinoma [
11,
12,
14,
37]. Additionally, recent studies have linked
NID2 mRNA levels with human echolucent calcified plaques [
15,
16]. Further murine studies have identified the role of NID2 in non-atherosclerotic and atherosclerotic vascular calcification [
18,
38]. Interestingly, Chen et al. recently demonstrated a protective role of NID2 against aortic calcification induced by 5/6 nephrectomy, cholecalciferol-overload, and CaCl
2 administration [
18]. However, its role in regulating hepatosteatosis and atherosclerosis remains unknown. To address this gap, firstly, we investigated the expression of NID2 protein in human atherosclerotic arterial tissues and murine steatotic livers. Consistent with upregulated
NID2 expression in non-atherosclerotic and atherosclerotic calcified vascular tissues, increased NID2 protein expression was observed in atherosclerotic arteries and steatotic livers [
15,
16]. To explore NID2’s role in these diseases, we induced AAV-mediated overexpression of
NID2 gene in
Apoe-deficient mice. AAVs are widely used tools in research for their ability to efficiently transduce various cell types, allow long-term gene expression, and avoid integration into the host genome reducing the risk of mutagenesis. Further, this approach facilitates the rapid generation of mouse models without the need of breeding for several generations.
One of our notable observations was the significant increase in liver and epiWAT weight in male mice overexpressing
NID2 compared to controls. This increase in liver weight, a hallmark of hepatosteatosis [
39], indirectly suggests that NID2 may exacerbate hepatic fat accumulation in males following Western diet feeding. The lack of difference in plasma cholesterol, fasting glucose, and body fat composition between the groups indicates that NID2's effects on liver and epiWAT mass are independent of systemic metabolic changes, such as overall cholesterol or glucose regulation. Interestingly, female mice did not exhibit any differences in liver or adipose tissue weights despite
NID2 overexpression, which points to a protective sex hormone/chromosome-regulated mechanism in females or a differential response to
NID2 overexpression. Future studies are warranted to determine the mechanisms of observed sex-specific responses.
Our data demonstrate that
NID2 overexpression in male mice promotes hepatic steatosis, as evidenced by increased triglyceride, NEFA levels, and ORO staining of liver frozen sections, as well as increased plasma triglycerides. These findings are consistent with previous studies that implicate lipid dysregulation as a characteristic of NAFLD [
40,
41]. While plasma NEFA levels did not differ between control and
NID2-overexpressing mice, the elevated hepatic NEFA levels suggest that NID2 may alter the balance between hepatic lipid uptake and secretion. Free fatty acids, primarily derived from the lipolysis in adipose tissue, are known to be transported to the liver, where they contribute to the pathogenesis of NAFLD [
42]. In this context, the increased adipocyte size observed in
NID2-overexpressing male mice further supports the link between adipose tissue dysfunction and hepatic lipid accumulation, a well-established mechanism in NAFLD [
27]. Additionally, in male mice, overexpression of
NID2 increased liver fibrosis, which is a key determinant of the progression of simple steatosis to NASH [
43]. These findings align with a previous report suggesting a connection between hepatic lipid accumulation and fibrosis in NAFLD [
44]. Interestingly, though female mice exhibited increased hepatic lipid accumulation following
NID2 overexpression, no significant differences in fibrosis were noted. These sex-specific differences in fibrosis have also been observed in other models of liver disease and may be influenced by hormonal factors or differences in liver metabolism between males and females [
45]. It is known that the prevalence and severity of NAFLD is higher in men than in women during the reproductive age. However, after menopause, women develop NAFLD at a faster rate, suggesting a protective role of female sex hormone such as estrogen against NAFLD. Animal models also tend to follow a similar trend with higher severity and occurrence of hepatic steatosis, and pro-fibrotic/inflammatory cytokines in males than females, reinstating the differences we observed in our study [
45,
46].
The development of atherosclerosis is regulated by both systemic metabolic factors and local cellular responses [
47]. In line with our hepatic lipid metabolism studies,
NID2 overexpression aggravated aortic atherosclerosis progression in male mice, suggesting a pivotal role of NID2 in atherogenesis. Further,
NID2 overexpression led to increased lesion size, enhanced lipid accumulation, reduced collagen content, and enlarged necrotic cores in aortic root sections. These data are in compliance with the upregulated expression of
NID2 in atherosclerotic calcified vascular tissues, indicating the deleterious role of NID2 in vascular diseases [
15,
16]. In contrast, a recent study reported the protective role of NID2 in vascular calcification [
18]. The authors observed attenuated NID2 levels in murine calcified aortas and calcified primary rat VSMCs, and showed suppression of vascular calcification in global
Nid2-/- mice [
18]. Another study from the same research group highlighted NID2’s involvement in the maintenance of contractile phenotype of VSMCs [
19]. Both of these studies emphasized the beneficial role of NID2 in regulating VSMC phenotype. Arteries are composed of different layers with various cell types, including endothelial cells, VSMCs, adventitial fibroblasts, and lymphatic endothelial cells, etc. However, the specific role of NID2 in different vascular cell types in the context of vascular pathologies remain to be investigated utilizing cell-specific
NID2-deficient mice. It is possible that NID2 is playing differential role in different vascular cells. Further, NID2 is recognized as an endogenous ligand for leucine-rich repeat-containing G protein-coupled receptor 4 (LGR4) [
18]. Future studies are warranted to discover the other potential receptors of NID2 and investigate atherosclerosis in mice with cell-specific
Lgr4 deletion combined with NID2 overexpression to clarify the role of NID2-LGR4 axis in atherogenesis. Additionally, reduced hepatic cholesterol efflux capacity in individuals with NAFLD has been linked to the presence of subclinical atherosclerosis [
48], hinting that NID2 overexpression in hepatocytes may suppress cholesterol efflux, thereby leading to increased atherosclerosis. Comparable atherosclerosis in female control and
NID2-overexpressing mice suggests sex-specific responses and imply that factors such as hormonal differences or sex-specific gene regulation modulate the effects of NID2 in atherosclerosis. For instance, estrogen has been shown to exert protective effects against atherosclerosis by modulating lipid metabolism and reducing inflammation [
49,
50]. Therefore, it is possible that sex hormones may be counteracting pro-atherogenic effects of NID2 in females.
To investigate the molecular mechanisms by which NID2 regulates hepatosteatosis and atherosclerosis, we examined the expression of various proteins and genes involved lipid metabolism. AMPK is a master switch in hepatic metabolism and its activation via phosphorylation (Thr 172) reduces hepatosteatosis by promoting fatty acid oxidation and inhibiting lipid production in the liver [
51,
52,
53]. Phosphorylated AMPK inactivates ACC (Ser 79 phosphorylation), leading to increased fatty acid oxidation and reduced fatty acid synthesis [
30,
31]. Our data demonstrated reduced AMPK activation in the livers with
NID2 overexpression, with no changes in the activation status of ACC, hinting an ACC-independent function of AMPK in hepatosteatosis. These findings are consistent with a study by Zordoky et al., which also showed ACC-independent effects of AMPK in myocardial fatty acid oxidation [
54]. However, it is unknown that how NID2 regulates AMPK phosphorylation. It is possible that NID2 via inhibiting protein kinase C activation suppresses AMPK activation [
18]. Other AMPK-regulated factors such as liver X receptor, and sterol regulatory element-binding protein 1c (SREBP1c) may be mediating pro-steatotic/atherogenic effects of NID2 in mice [
55]. We observed no significant differences in hepatic mRNA levels of various lipid uptake, fatty acid synthesis, fatty acid transport, and fatty acid beta oxidation genes, except
Ldlr between control and
NID2-AAV-injected mice.
Ldlr mRNA expression was elevated in mice with
NID2 overexpression, which may represent a compensatory mechanism to clear excess lipids from circulation. Another possibility is that
NID2 overexpression increases proprotein convertase subtilisin/kexin type 9 levels, leading to degradation of LDLR protein and its reduced expression on the surface of hepatocytes, despite elevated mRNA levels [
56], potentially contributing to the development of hepatosteatosis in these mice.
A major limitation of the present study is the utilization of NID2-AAV under the control of the ubiquitous cytomegalovirus promoter, which drives gene expression in multiple organs in mice. To better understand the cell-specific effects of NID2 overexpression in NAFLD and atherosclerosis, future investigations with NID2-AAV with cell-specific promoter (hepatocytes/vascular cells) are required. Further, in vivo studies using cell-specific NID2 deficiency are needed to investigate the precise role of endogenous NID2 in the pathophysiology of these diseases. Additionally, further investigations into the sex-specific roles of NID2 are warranted, including the use of ovariectomized female mice or treatment of sex hormones, such as estradiol (E2), in male mice.
In conclusion, the present study for the first time demonstrates the detrimental role of NID2 in hepatosteatosis and atherosclerosis. The presented results suggest that blocking NID2-induced signaling may serve as potential therapeutic approach to suppress both NAFLD and atherosclerosis.
4. Materials and Methods
4.1. Animals and AAV Production
Eight- to ten-week-old male and female Apoe-/- mice (The Jackson Laboratory, Bar Harbor, stock # 002052) were used in the present study. One group of Apoe-/- mice designated as control, received an intraperitoneal injection of saline, while another group was administered a single dose of a recombinant AAV expressing human NID2 gene (AAV8-hNID2, referred to as NID2-AAV, 6.5 x 1013 viral genomes, IP) under the ubiquitous cytomegalovirus promoter, to induce NID2 overexpression. The plasmid DNA for NID2 vector construct (pAAV-hNID2-CMV-amp, # 31829101) was obtained from Applied Biological Materials Inc., Richmond, Canada. The plasmid was packaged into AAV serotype 8 capsid and purified using a density gradient iodixanol solution at the Cincinnati Children’s Hospital Medical Center Viral Vector Core [RRID:SCR_022641)-VVL]. Eight- to ten-week-old male wild-type C57BL/6J mice were fed a control diet (Research Diets, Inc., New Brunswick, D12450J) or a calorie-matched high-fat diet (Research Diets, Inc., D12492) for 12 weeks to determine NID2 protein levels using immunoblotting. All mice were housed in a climate-controlled vivarium with a 12-hour light/dark cycle. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center at Memphis, TN.
4.2. Atherosclerotic Lesion Analysis
Control and NID2-AAV-injected Apoe-/- mice were fed a Western diet (Inotiv, Indianapolis, IN, #TD.88137) for 12 weeks to induce hypercholesterolemia and atherosclerosis. In the twelfth week of feeding, whole-body fat and lean mass were measured using the EchoMRI Body Composition Analyzer. Fasting blood glucose (ReliOn Prime Blood Glucose Monitoring System) was determined following a 16-hour fast, just before euthanasia. Mice were anesthetized by isoflurane inhalation (3%), and blood (via cardiac puncture), heart, aorta, epididymal adipose tissue, and other tissues were collected for further analysis. Similar regions of the liver from each mouse were processed for histochemistry and molecular analysis. Plasma total cholesterol was determined utilizing the Amplex Red cholesterol assay (Molecular Probes, Eugene, A12216). In situ images of the abdominal area, aortic arch, and heart were captured using a Leica S6E stereomicroscope fitted with a camera.
To assess the atherosclerotic lesion burden in whole aortas, en face ORO staining was performed after fixing aortas in 4% paraformaldehyde (PFA). A 2% ORO solution (ThermoFisher Scientific, Ward Hill, #A12989.22) was used for staining. Aortas were opened longitudinally, and images were captured to quantify ORO-positive areas. To determine the lipid deposition in the aortic roots, the upper halves of fixed hearts were embedded in optimal cutting temperature (OCT) compound (Fisher Healthcare, Houston, TX, #23-730-571), and serial frozen cross-sections (7μm) were stained with 2% ORO solution. Images were captured using an Olympus BX43 inverted microscope. Four sections per mouse, spaced 90-100 μm apart were stained and analyzed, and the mean area of the four sections reported. ORO-positive areas were quantified using the Image Pro plus software (Media Cybernetics, Bethesda, MD).
4.3. Hepatic Lipid Accumulation, Triglyceride and Non-Esterified Fatty Acid Quantitation
To investigate the lipid accumulation in the liver, similar region of the PFA-fixed and sucrose-dehydrated liver from each mouse was embedded in an OCT compound and cryo-sectioning was performed. Frozen liver sections (7μm) were stained with 2% ORO for 10 min at room temperature and counterstained with hematoxylin (Fisher Healthcare, Houston, #22-220-100). For each mouse, at least two sections were stained and images of five to six random microscopic fields were captured. Image Pro Plus software was used for image analysis.
Liver homogenates were used to extract total lipids using the methanol and chloroform method. Plasma/hepatic triglyceride levels and NEFA levels were quantified following the standard protocols (Wako Pure Chemical Industries, Richmond, VA, 992-02892, 992-02892, 464-01601, 995-34791 and 999-34691), as described previously [
57].
4.4. Histochemistry
At least four aortic root sections per mouse, spaced 90-100 μm apart, were stained and analyzed, and the mean area of four sections was reported. Frozen serial cross-sections of aortic roots were washed twice with phosphate-buffered saline (PBS) and stained with H & E (Fisher Healthcare, Houston, #22-220-100 and #22-220-104) as described previously [
22,
58,
59] to evaluate the total lesion and necrotic area. Masson’s trichrome staining (Richard Allan Scientific LLC, Kalamazoo, #22-110-648) was performed following the standard protocol to analyze the collagen content.
Frozen or paraffin liver sections were stained with H & E and Sirius red (Fisher Scientific, #26357-02) as per the manufacturer’s instructions. H & E staining of liver sections was used to visualize the lipid globules, while Sirius red staining was employed to analyze the collagen content (fibrosis). A mean of five to six random microscopic fields per mouse was reported. All image analyses were performed utilizing the Image-Pro Plus software.
4.5. Western Blotting
Murine liver and human vascular tissue samples were homogenized in radio-immunoprecipitation assay lysis buffer (RIPA, ThermoScientific, #89900) supplemented with protease and phosphatase inhibitor cocktail (ThermoScientific, #A32959). Equal amounts of protein were separated on SDS-PAGE gels, and resolved bands were transferred onto nitrocellulose membranes (Li-Cor Biosciences, Lincoln). Membranes were blocked with Intercept blocking buffer (Li-Cor Biosciences, #927-60001) for 60 min at room temperature and incubated overnight at 4 °C with indicated primary antibodies. The next day, membranes were washed and probed with IRDye-conjugated secondary antibodies (Li-Cor Biosciences). After washing, membranes were scanned with an Odyssey DLx Infrared Imaging System (Li-Cor Biosciences), and band intensities were quantified using the NIH ImageJ software. The following primary antibodies were used : NID2 (Proteintech, #13530-1-AP), total ACC (Cell Signaling Technology, #3662S), pACCSer79 (Cell Signaling Technology, #11818S), total AMPKα (Cell Signaling Technology, #2793S), pAMPKαThr172 (Cell Signaling Technology, #2535S), IL-6 (Cell Signaling Technology, #12912S), TNFα (Cell Signaling Technology, #11948T), β-Tubulin (Cell Signaling Technology, #86298S) and GAPDH (Santa Cruz Biotechnology, Dallas, #sc-365062). All the antibodies except GAPDH and β-Tubulin were used at a 1:1000 dilution. GAPDH and β-Tubulin were utilized at a 1:2000 dilution.
4.6. Quantitative Reverse-Transcriptase PCR
Liver tissue samples were homogenized in TRIzol reagent (ThermoFisher Scientific, #15596018) and total RNA was extracted according to the manufacturer’s protocol. Complementary DNA was synthesized from total RNA (1 µg) using the RevertAid RT Reverse Transcription Kit (ThermoFisher Scientific, #K1691). The qRT-PCR was performed using PowerUp SYBR Green Master Mix (ThermoFisher Scientific, #A25742) in a QuantStudio 3 Real-Time PCR System (Applied Biosystems). Relative gene expression was calculated using the 2
-ΔΔCt method and
Gapdh as a housekeeping gene. Primer sequences used for qRT-PCR are listed in
Supplementary Table S1.
4.7. Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 10 (La Jolla, CA). Sample sizes (n) for each experiment are mentioned in the figure legends. The normality of the data was assessed by the Shapiro-Wilk test. Data are represented as mean ± SEM. Comparisons between the two groups were performed using a two-tailed student t-test for parametric data or a Mann-Whitney U test for non-parametric data. For parametric tests, the same standard deviation was assumed across groups. A p-value < 0.05 was considered statistically significant.
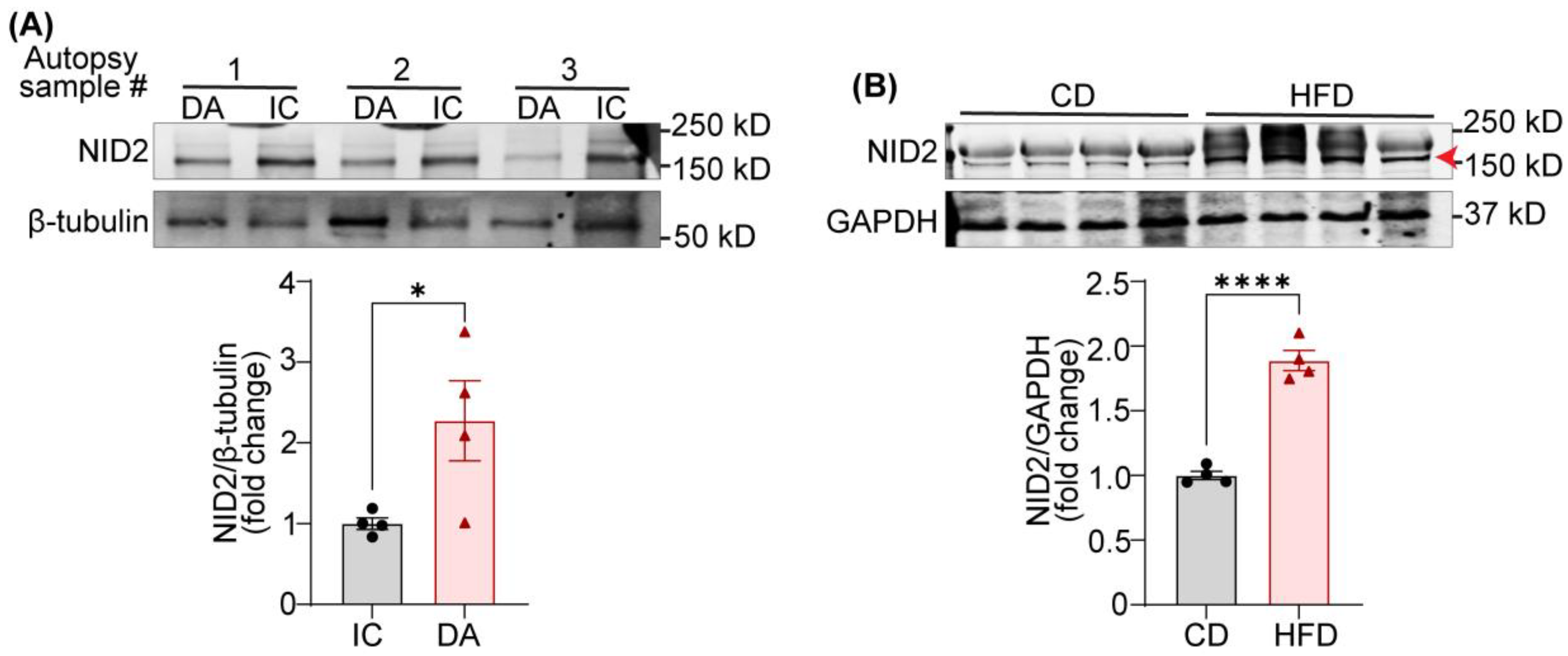
Figure 1.
Expression of NID2 protein is elevated in human atherosclerotic arteries and murine steatotic livers. (A) Representative western blot images for NID2 and β-tubulin protein expression in human atherosclerotic inner curvature (IC) and non-atherosclerotic descending aorta (DA) vascular tissue. The bar diagram shows mean protein levels along with individual data points calculated using densitometric analysis and expressed as a ratio of NID2 to β-tubulin. (B) Representative western blot images for NID2 and GAPDH in the livers of murine control diet (CD)- and calorie-matched high-fat diet (HFD, 12 weeks)-fed C57BL/6J mice. The bar diagram represents the mean NID2 protein levels normalized with GAPDH, (n = 4). Statistical analyses were performed using a two-tailed unpaired t-test (A and B). Data represents means ± SEM. *P < 0.05, and ****P < 0.0001.
Figure 1.
Expression of NID2 protein is elevated in human atherosclerotic arteries and murine steatotic livers. (A) Representative western blot images for NID2 and β-tubulin protein expression in human atherosclerotic inner curvature (IC) and non-atherosclerotic descending aorta (DA) vascular tissue. The bar diagram shows mean protein levels along with individual data points calculated using densitometric analysis and expressed as a ratio of NID2 to β-tubulin. (B) Representative western blot images for NID2 and GAPDH in the livers of murine control diet (CD)- and calorie-matched high-fat diet (HFD, 12 weeks)-fed C57BL/6J mice. The bar diagram represents the mean NID2 protein levels normalized with GAPDH, (n = 4). Statistical analyses were performed using a two-tailed unpaired t-test (A and B). Data represents means ± SEM. *P < 0.05, and ****P < 0.0001.
Figure 2.
NID2 overexpression enhances liver and epididymal white adipose tissue mass in male mice. (A) The schematic diagram illustrates the experimental plan. Eight- to ten-week-old male and female Apoe-/- mice were injected with control (Ctrl) and NID2-AAV intraperitoneally, fed a Western diet for 12 weeks, and analyzed. (B - E) Male control and NID2-AAV-injected Apoe-/- mice were utilized to measure NID2 mRNA levels in various organs by qRT-PCR at least in duplicate. Bar diagrams represent mRNA expression in liver (B, n = 10), kidney (C, n = 6), epididymal white adipose tissue (EpiWAT, D, n = 7 - 10), and heart (E, n = 10). Bar diagrams show body weight (F), plasma total cholesterol (G), fasting blood glucose (H), whole-body fat mass and lean mass (I), liver weight (J), adipose tissue weight (K), and spleen weight (L) (n = 5 - 6). Statistical analyses were performed using a two-tailed unpaired t-test (C and G - K), a two-tailed unpaired Mann-Whitney test (B, D, E and L), and a two-way ANOVA followed by Sidak post hoc test for multiple comparisons (F). Data represent mean ± SEM. *P < 0.05, ***P < 0.001 and ****P < 0.0001.
Figure 2.
NID2 overexpression enhances liver and epididymal white adipose tissue mass in male mice. (A) The schematic diagram illustrates the experimental plan. Eight- to ten-week-old male and female Apoe-/- mice were injected with control (Ctrl) and NID2-AAV intraperitoneally, fed a Western diet for 12 weeks, and analyzed. (B - E) Male control and NID2-AAV-injected Apoe-/- mice were utilized to measure NID2 mRNA levels in various organs by qRT-PCR at least in duplicate. Bar diagrams represent mRNA expression in liver (B, n = 10), kidney (C, n = 6), epididymal white adipose tissue (EpiWAT, D, n = 7 - 10), and heart (E, n = 10). Bar diagrams show body weight (F), plasma total cholesterol (G), fasting blood glucose (H), whole-body fat mass and lean mass (I), liver weight (J), adipose tissue weight (K), and spleen weight (L) (n = 5 - 6). Statistical analyses were performed using a two-tailed unpaired t-test (C and G - K), a two-tailed unpaired Mann-Whitney test (B, D, E and L), and a two-way ANOVA followed by Sidak post hoc test for multiple comparisons (F). Data represent mean ± SEM. *P < 0.05, ***P < 0.001 and ****P < 0.0001.
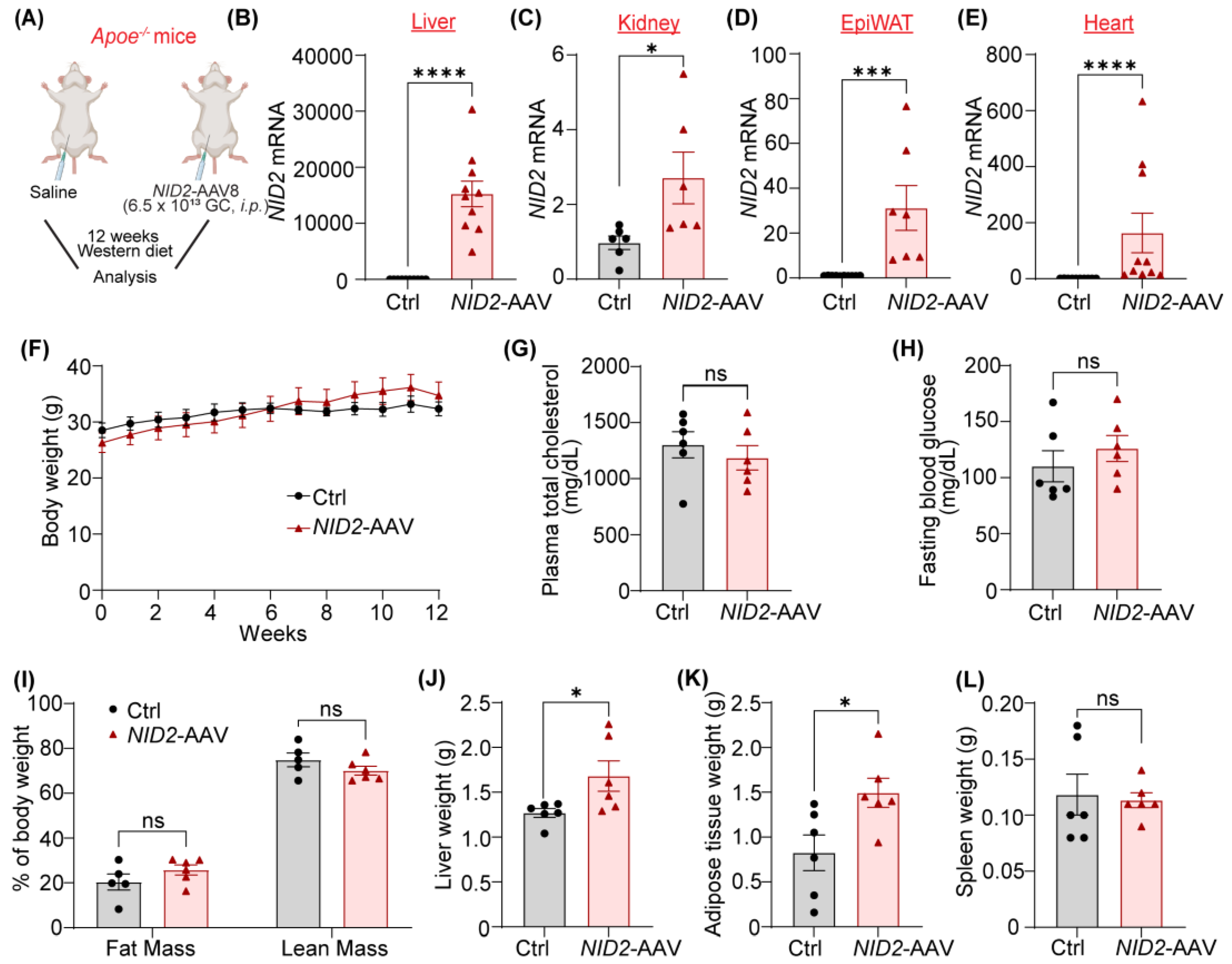
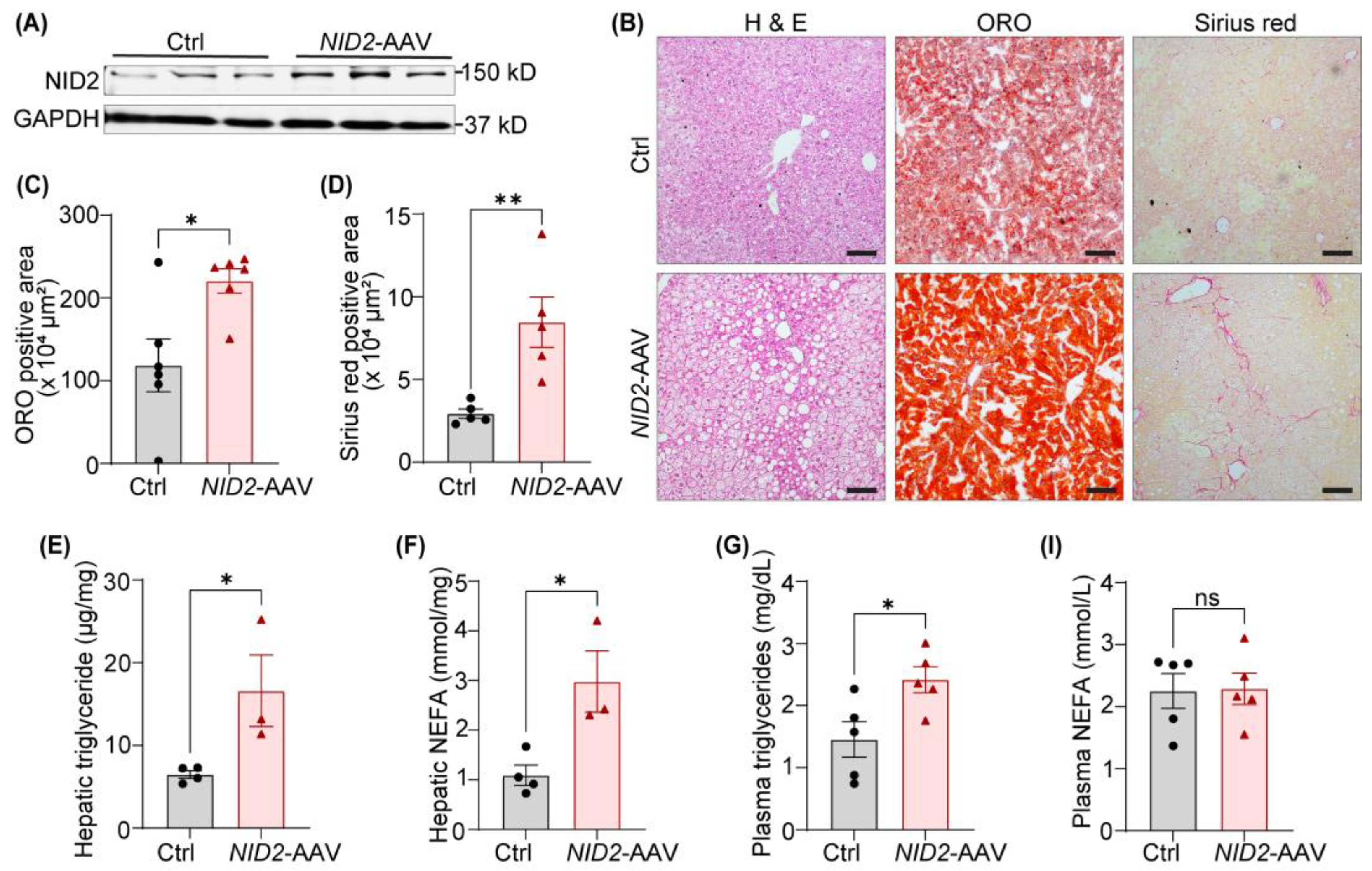
Figure 3.
NID2 overexpression in mice promotes hepatic lipid accumulation and fibrosis. Male Apoe-/- mice were injected with control and NID2-AAV intraperitoneally, fed a Western diet for 12 weeks, and analyzed. (A) Representative western blot images for NID2 and GAPDH protein expression in the livers of control and NID2-overexpressing mice (n = 3). (B) Representative images of liver sections stained with H & E (lipid droplets), ORO (neutral lipid accumulation), and Sirius red (fibrosis), scale bar 100 μm. (C - I) Bar diagrams represent lipid accumulation (C, n = 6), fibrosis area (D, n = 5), hepatic triglyceride (E, n = 3 - 4) and NEFA levels (F, n = 3 - 4), plasma triglyceride (G, n = 5) and NEFA levels (I, n = 5) in control and NID2-AAV-injected mice. Statistical analyses were performed using a two-tailed unpaired t-test (C - I). Data represent mean ± SEM. *P < 0.05, and **P < 0.01.
Figure 3.
NID2 overexpression in mice promotes hepatic lipid accumulation and fibrosis. Male Apoe-/- mice were injected with control and NID2-AAV intraperitoneally, fed a Western diet for 12 weeks, and analyzed. (A) Representative western blot images for NID2 and GAPDH protein expression in the livers of control and NID2-overexpressing mice (n = 3). (B) Representative images of liver sections stained with H & E (lipid droplets), ORO (neutral lipid accumulation), and Sirius red (fibrosis), scale bar 100 μm. (C - I) Bar diagrams represent lipid accumulation (C, n = 6), fibrosis area (D, n = 5), hepatic triglyceride (E, n = 3 - 4) and NEFA levels (F, n = 3 - 4), plasma triglyceride (G, n = 5) and NEFA levels (I, n = 5) in control and NID2-AAV-injected mice. Statistical analyses were performed using a two-tailed unpaired t-test (C - I). Data represent mean ± SEM. *P < 0.05, and **P < 0.01.
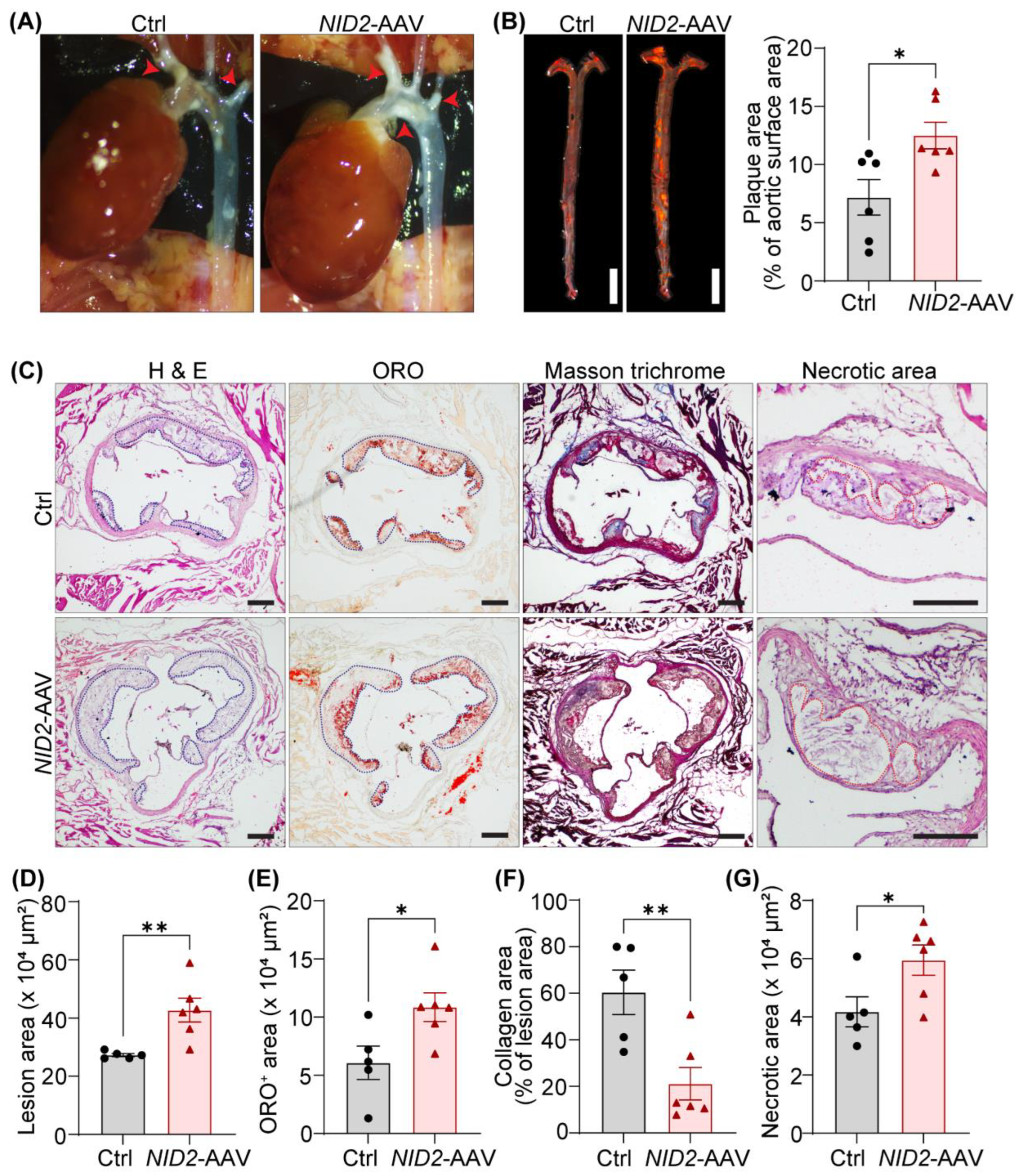
Figure 4.
NID2 overexpression augments atherosclerosis in male hypercholesterolemic mice. Male Apoe-/- mice were injected with control (Ctrl) and NID2-AAV intraperitoneally, fed a Western diet for 12 weeks and analyzed. (A) Representative in situ images of the aortic arch (red arrowheads point to atherosclerotic lesions). (B) Representative en face ORO staining of whole aortas, scale bar 5 mm. The bar diagram represents ORO-positive areas in whole aortas (n = 6). (C) Representative images of aortic root cross-sections stained with H & E (lesion area and necrotic core), ORO (lipid accumulation), and Masson’s trichrome (collagen content), scale bar 200 μm. (D - G) Bar diagrams show lesion area (D), lipid deposition (E), collagen content (F), and necrotic core area (G) (n = 5 - 6). Statistical analyses were performed using a two-tailed unpaired t-test (B and D - G). Data represent mean ± SEM. *P < 0.05, and **P < 0.01.
Figure 4.
NID2 overexpression augments atherosclerosis in male hypercholesterolemic mice. Male Apoe-/- mice were injected with control (Ctrl) and NID2-AAV intraperitoneally, fed a Western diet for 12 weeks and analyzed. (A) Representative in situ images of the aortic arch (red arrowheads point to atherosclerotic lesions). (B) Representative en face ORO staining of whole aortas, scale bar 5 mm. The bar diagram represents ORO-positive areas in whole aortas (n = 6). (C) Representative images of aortic root cross-sections stained with H & E (lesion area and necrotic core), ORO (lipid accumulation), and Masson’s trichrome (collagen content), scale bar 200 μm. (D - G) Bar diagrams show lesion area (D), lipid deposition (E), collagen content (F), and necrotic core area (G) (n = 5 - 6). Statistical analyses were performed using a two-tailed unpaired t-test (B and D - G). Data represent mean ± SEM. *P < 0.05, and **P < 0.01.
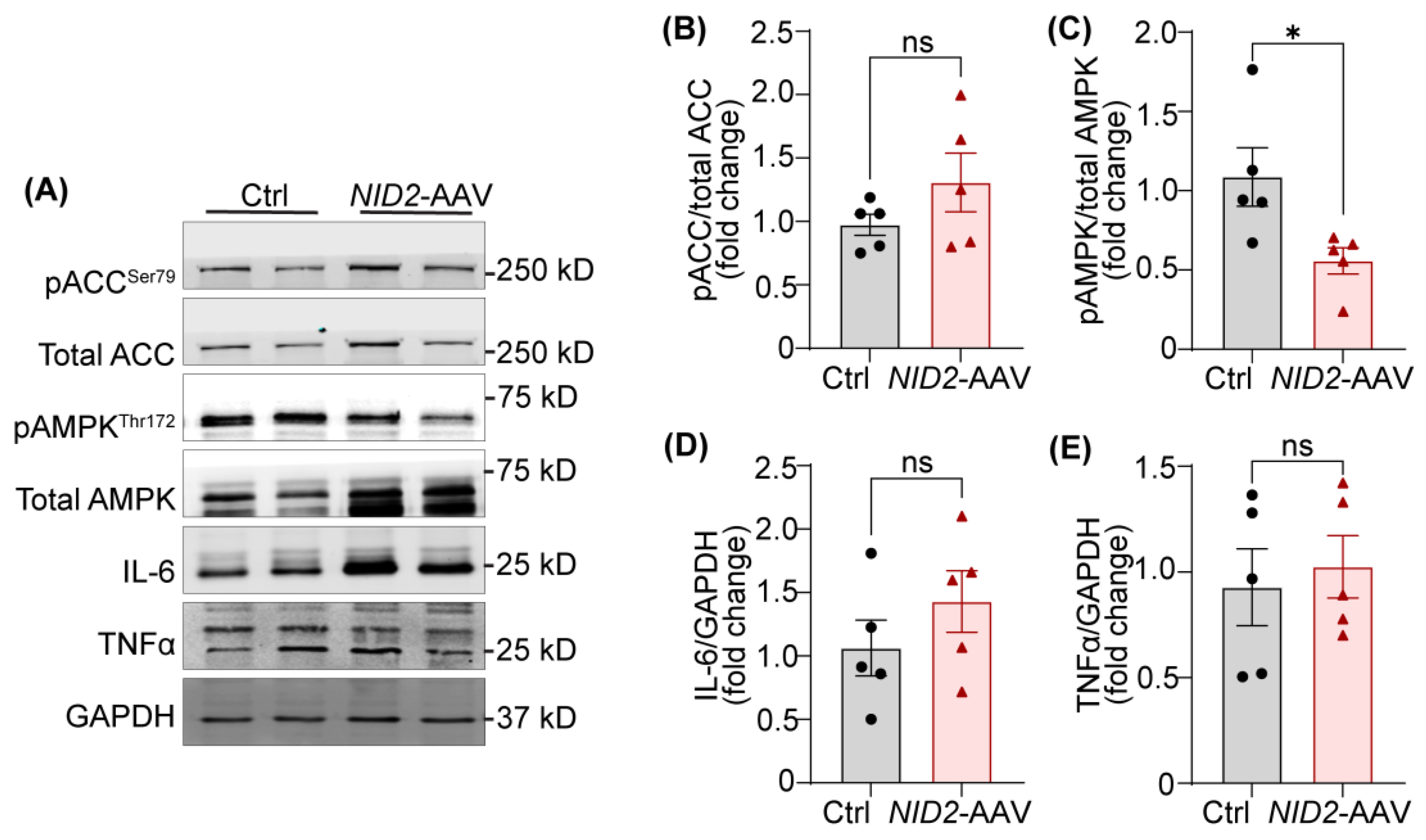
Figure 5.
NID2 overexpression inhibits the activation of the lipid metabolism-related protein AMPK. (A) Representative western blot images for lipid metabolism and pro-inflammatory proteins utilizing liver lysates from control and NID2-AAV-injected mice. Bar diagrams represent mean protein expression (B and C) as the ratios of phospho-total proteins, ACC (B) and AMPK (C), and protein levels of IL-6 (D) and TNFα (E) (n = 5). Statistical analyses were performed using a two-tailed unpaired t-test. Data represent mean ± SEM. *P < 0.05.
Figure 5.
NID2 overexpression inhibits the activation of the lipid metabolism-related protein AMPK. (A) Representative western blot images for lipid metabolism and pro-inflammatory proteins utilizing liver lysates from control and NID2-AAV-injected mice. Bar diagrams represent mean protein expression (B and C) as the ratios of phospho-total proteins, ACC (B) and AMPK (C), and protein levels of IL-6 (D) and TNFα (E) (n = 5). Statistical analyses were performed using a two-tailed unpaired t-test. Data represent mean ± SEM. *P < 0.05.