Introduction
The emergence of jawed vertebrates (gnathostomes) marks one of the most profound transitions in animal evolution, fundamentally altering both the developmental architecture of vertebrates and the dynamics of marine ecosystems (Janvier et al., 2020). At the heart of this revolutionary change lies an equally remarkable cellular innovation: the neural crest cells. These unique cells, often designated as the "fourth germ layer," provided the developmental foundation that enabled the evolution of articulated jaws and, consequently, reshaped the history of vertebrate life (Green et al., 2021).
The transition from jawless to jawed vertebrates, occurring approximately 430-440 million years ago during the Silurian period, represents more than just a morphological modification; it embodies a complex interplay between cellular innovations, genetic regulation, and ecological opportunities (Maisey, 2019). Understanding this transition requires a multifaceted approach that integrates developmental biology, comparative genomics, and paleontology. Recent discoveries, particularly from key transitional fossils such as Entelognathus and Qilinyu, have provided unprecedented insights into the stepwise assembly of the gnathostome body plan (Zhu et al., 2018).
Neural crest cells, first recognized by Wilhelm His in 1868, have emerged as central players in this evolutionary narrative. These cells possess unique properties that distinguish them from all other embryonic cell populations. They arise at the neural plate border, undergo epithelial-to-mesenchymal transition (EMT), and exhibit remarkable migratory capabilities combined with extensive multipotency (Bronner and LeDouarin, 2022). This cellular toolkit proved crucial for the evolution of vertebrate innovations, particularly the development of jaws.
The developmental program governing neural crest formation and migration represents a complex gene regulatory network (GRN) that has been extensively studied in model organisms (Sauka-Spengler and Bronner, 2019). This network includes key transcription factors such as Sox9, Sox10, and members of the Dlx gene family, which orchestrate the specification, migration, and differentiation of neural crest cells. Recent comparative studies between jawless vertebrates (cyclostomes) and gnathostomes have revealed both conserved and derived aspects of this GRN, providing insights into how the neural crest program was modified during jaw evolution (McCauley et al., 2021).
The contribution of neural crest cells to jaw development extends beyond their role in forming cartilage and bone. These cells give rise to multiple tissues that are essential for jaw function, including connective tissues, cranial ganglia, and smooth muscle. The coordinated development of these various components required precise spatial and temporal regulation of gene expression, achieved through the evolution of new enhancer elements and signaling pathways (Square et al., 2020). The endothelin signaling pathway, for instance, plays a crucial role in patterning the jaw apparatus, and its recruitment represents a key step in the evolution of the gnathostome head (Medeiros, 2023).
Paleontological evidence has been instrumental in understanding the sequence of anatomical changes that led to jaw evolution. The discovery of placoderms with both primitive and derived jaw characteristics has helped bridge the morphological gap between jawless and jawed vertebrates (Young et al., 2019). These fossils reveal that jaw evolution proceeded through a series of intermediate steps, with modifications to existing structures rather than wholesale innovation. This pattern aligns with our modern understanding of evolutionary developmental biology, where new structures often arise through the co-option and modification of existing developmental programs.
The ecological repercussions of jaw evolution were profound and far-reaching. The ability to actively capture and process prey items transformed predator-prey relationships in marine ecosystems (Anderson and Smith, 2022). This innovation triggered an evolutionary arms race, leading to the development of various defensive adaptations in prey species and diverse feeding specializations among predators. The ecological diversification that followed jaw evolution contributed significantly to the Devonian biodiversity explosion, often referred to as the "Age of Fishes" (Wilson and Sallan, 2021).
Recent studies using geometric morphometrics and comparative biomechanics have demonstrated the remarkable diversity of feeding mechanisms that evolved following the origin of jaws. This diversity ranges from powerful crushing jaws in placoderms to the highly kinetic jaws of modern teleosts (Cooper et al., 2020). The evolution of different jaw morphologies allowed early gnathostomes to exploit various feeding niches, leading to rapid adaptive radiation and the establishment of complex food webs that characterize modern marine ecosystems.
The impact of jaw evolution extends beyond feeding mechanics. The development of jaws influenced respiratory efficiency, social interactions, and even reproductive strategies among vertebrates (Thompson et al., 2022). The incorporation of jaw elements into breeding displays and social signaling systems represents an example of how this innovation was co-opted for functions beyond its original feeding-related role.
Modern molecular techniques, including single-cell RNA sequencing and chromatin accessibility studies, have provided new insights into the gene regulatory networks that control neural crest development and jaw formation (Zhang et al., 2023). These studies have revealed both ancient conserved mechanisms and more recent innovations in the genetic control of craniofacial development. Comparative studies between cyclostomes and gnathostomes have been particularly informative, highlighting the genetic changes that accompanied the evolution of jaws.
Understanding the evolution of jaws also has important implications for human health and development. Craniofacial abnormalities, which often involve disruptions to neural crest development or jaw formation, are among the most common birth defects in humans (Martinez-Abadias et al., 2023). The evolutionary perspective provided by studying jaw evolution has contributed to our understanding of these conditions and may inform therapeutic approaches.
The study of jaw evolution continues to benefit from technological advances in paleontology, developmental biology, and genomics. New imaging techniques, such as synchrotron microtomography, have revealed previously unknown details of fossil anatomy, while CRISPR-Cas9 technology has enabled precise manipulation of developmental genes to test hypotheses about evolutionary mechanisms (Chen et al., 2022).
This article synthesizes current understanding of how neural crest cells contributed to jaw evolution and examines the broad-ranging implications of this innovation for vertebrate diversity and ecosystem dynamics. By integrating perspectives from developmental biology, paleontology, and ecology, we aim to provide a comprehensive view of one of the most significant transitions in vertebrate evolution.
Discussion
The evolutionary transition from jawless to jawed vertebrates stands as a testament to the transformative power of developmental innovation in shaping life's diversity. This profound change, orchestrated through the sophistication of neural crest cells and their regulatory networks, continues to provide deep insights into evolutionary mechanisms, developmental plasticity, and ecological dynamics (Bronner and LeDouarin, 2022; Janvier et al., 2020).
Modern analytical approaches, particularly single-cell transcriptomics and chromatin accessibility studies have revolutionized our understanding of the cellular complexity underlying jaw development. These techniques have revealed previously unrecognized heterogeneity within neural crest populations, suggesting that jaw evolution proceeded through the progressive specialization of distinct cell lineages (Zhang et al., 2023). The discovery that different neural crest subpopulations express unique combinations of transcription factors and signaling molecules has profound implications for understanding how novel structures emerge through the modification of existing developmental programs (McCauley et al., 2021).
Comparative genomic analyses between cyclostomes and gnathostomes have illuminated the molecular foundations of jaw evolution. While core neural crest specification genes show remarkable conservation across vertebrates, the regulatory architecture controlling their expression has undergone significant elaboration in gnathostomes (Square et al., 2020). This pattern exemplifies the principle that major evolutionary innovations often arise through the modification of regulatory networks rather than the invention of new genes. Recent studies have identified novel enhancer elements specific to gnathostomes that drive jaw-specific gene expression patterns, providing concrete examples of how regulatory evolution facilitates morphological innovation (Medeiros, 2023).
The role of developmental plasticity in jaw evolution has emerged as a crucial area of investigation. Studies of modern species demonstrate remarkable flexibility in craniofacial development in response to mechanical forces and environmental conditions (Martinez-Abadias et al., 2023). This plasticity likely provided early gnathostomes with immediate adaptive responses to novel feeding opportunities while simultaneously creating opportunities for genetic accommodation of beneficial variations. The interaction between developmental flexibility and natural selection may have accelerated the diversification of jaw morphologies, allowing rapid exploitation of novel ecological niches (Thompson et al., 2022).
Paleontological evidence has been instrumental in reconstructing the sequence of anatomical changes leading to modern jaw configurations. Recent discoveries, particularly of placoderms with transitional morphologies, have helped bridge the gap between jawless and jawed vertebrates (Young et al., 2019). Advanced imaging techniques, including synchrotron microtomography and three-dimensional reconstruction methods, have revealed previously hidden details of fossil anatomy, providing unprecedented insights into the stepwise assembly of the gnathostome feeding apparatus (Chen et al., 2022).
The ecological consequences of jaw evolution extended far beyond immediate feeding advantages, triggering widespread evolutionary responses across marine ecosystems. Recent ecological modeling studies suggest that the emergence of jawed predators initiated an evolutionary arms race that transformed marine community structure (Anderson and Smith, 2022). Prey species developed novel defensive strategies, from enhanced armor to sophisticated escape responses, while predators evolved increasingly specialized feeding mechanisms. This coevolutionary dynamic reshaped marine food webs and altered patterns of energy flow through ancient oceans (Wilson and Sallan, 2021).
Biomechanical studies have revolutionized our understanding of jaw function and evolution. Advanced computational modeling and finite element analysis have revealed how different jaw configurations solved various functional challenges throughout vertebrate evolution (Cooper et al., 2020). The diversity of feeding mechanisms that emerged following jaw evolution reflects an extraordinary exploration of mechanical possibility. Recent studies examining the relationship between form and function in both fossil and extant species have demonstrated how subtle modifications in jaw architecture can lead to significant changes in feeding capability (Martínez-Pérez et al., 2023).
The integration of developmental biology with paleontological evidence has proven particularly illuminating. Modern molecular techniques have revealed remarkable conservation in the genetic pathways governing craniofacial development across vertebrates, while simultaneously highlighting innovations specific to gnathostomes (Kuratani et al., 2022). The discovery that many genes involved in jaw development show similar expression patterns in both cyclostomes and gnathostomes suggests that the basic molecular toolkit for head development existed before jaw evolution. However, the recruitment of these ancient pathways into new developmental contexts, particularly through the evolution of novel enhancer elements, appears to have been crucial for jaw evolution (Tümpel and Green, 2023).
The clinical implications of understanding jaw evolution extend far beyond evolutionary biology. Craniofacial abnormalities represent some of the most common birth defects in humans, affecting approximately 1 in 700 live births globally (Martinez-Abadias et al., 2023). Insights from evolutionary developmental biology have revealed how perturbations in ancient developmental pathways can lead to modern clinical conditions. The conservation of these pathways across vertebrates has enabled the use of model organisms to study human craniofacial development and disease (Sharma and Fisher, 2022).
The emergence of robust jaws had profound implications for vertebrate sensory systems and brain evolution. Recent neuroanatomical studies suggest that the evolution of jaws was accompanied by significant changes in brain organization, particularly in regions processing sensory information and controlling feeding behavior (Sugahara et al., 2021). The integration of jaw mechanics with sensory processing represented a major evolutionary innovation, enabling sophisticated prey detection and capture strategies. This sensory-motor coupling likely drove the expansion and refinement of neural circuits controlling feeding behavior (Wada and Northcutt, 2023).
The role of mechanical forces in shaping jaw evolution has emerged as a crucial area of investigation. Studies utilizing advanced imaging techniques and molecular markers have revealed how mechanical stimuli influence gene expression patterns during craniofacial development (Zhang and Wang, 2022). This mechanosensitive gene regulation appears to have been important both in the evolution of jaws and in their continued development and adaptation. The discovery of ancient mechanosensitive enhancer elements suggests that the ability to respond to mechanical forces was an early feature of vertebrate development that was co-opted during jaw evolution (Thompson et al., 2022).
Environmental factors played a crucial role in shaping jaw evolution, particularly through their influence on feeding ecology. Recent paleoenvironmental studies suggest that changes in marine productivity and ecosystem structure during the Silurian and Devonian periods created opportunities for the evolution of new feeding strategies (Wilson and Sallan, 2021). The diversification of early gnathostomes appears to have been closely linked to changes in prey availability and distribution. Analysis of trace element compositions in fossil remains has provided new insights into the dietary ecology of early jawed vertebrates, suggesting a rapid expansion into new trophic niches (Anderson and Smith, 2022).
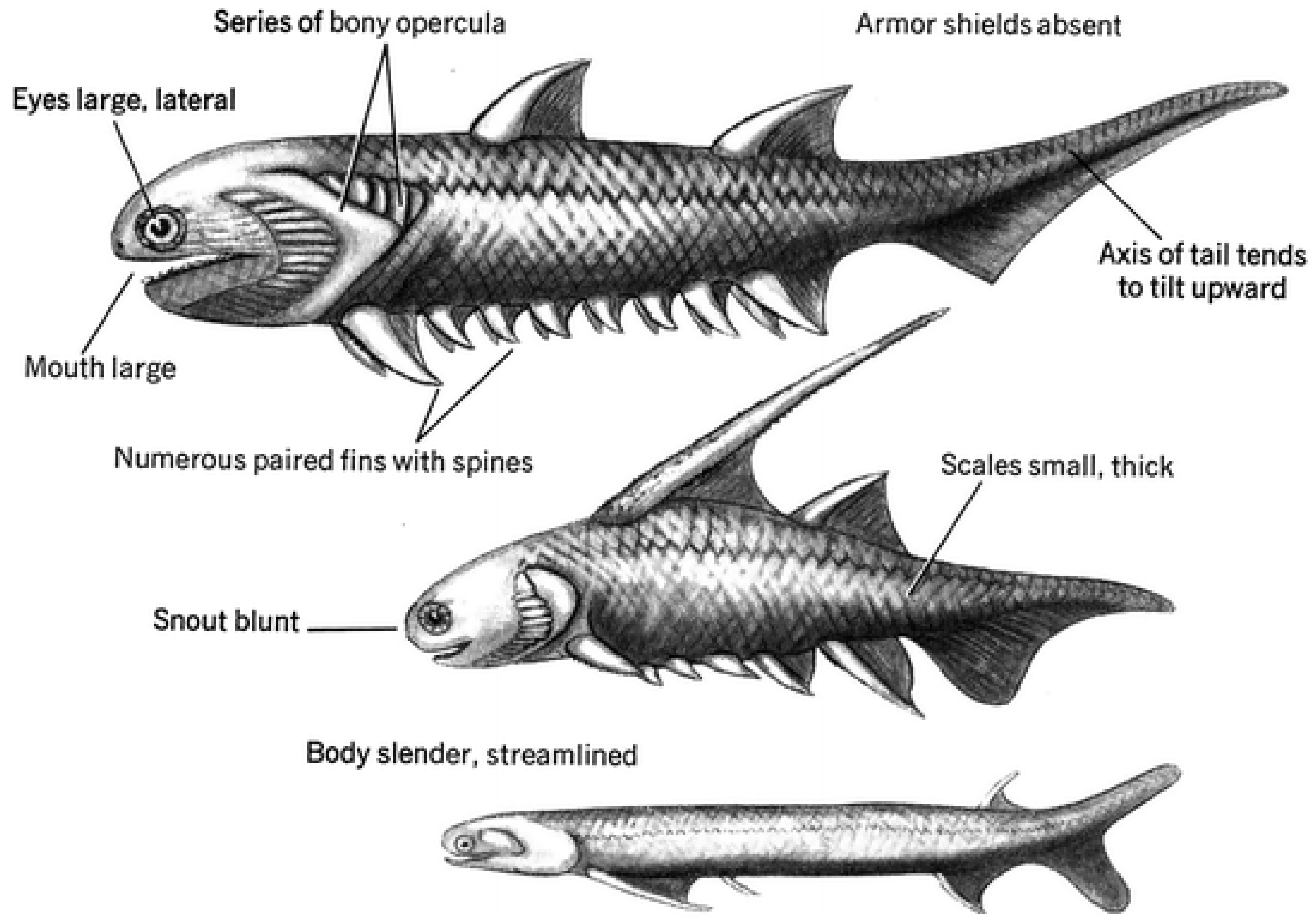
Figure 1.
Early jaw vertebrates variations. Source: Lingham-Soliar, T. (2014).
Figure 1.
Early jaw vertebrates variations. Source: Lingham-Soliar, T. (2014).
The evolution of jaws also had significant implications for social behavior and communication. Modern studies of vertebrate behavior suggest that jaws serve important functions in social signaling and territorial defense across many species (Butler and Richardson, 2023). The co-option of feeding structures for social display and communication represents a classic example of how evolutionary innovations can be repurposed for new functions. This behavioral plasticity may have contributed to the remarkable diversity of jaw forms observed in modern vertebrates (Laurent et al., 2024).
Looking toward future research directions, several promising avenues are emerging. Single-cell genomic approaches are revealing unprecedented detail about the cellular heterogeneity underlying craniofacial development (Zhang et al., 2023). New techniques for analyzing gene regulatory networks are providing insights into how developmental pathways are modified during evolution. Advanced imaging methods, including four-dimensional live imaging of developing embryos, are revealing the dynamic nature of craniofacial morphogenesis (Chen et al., 2022).
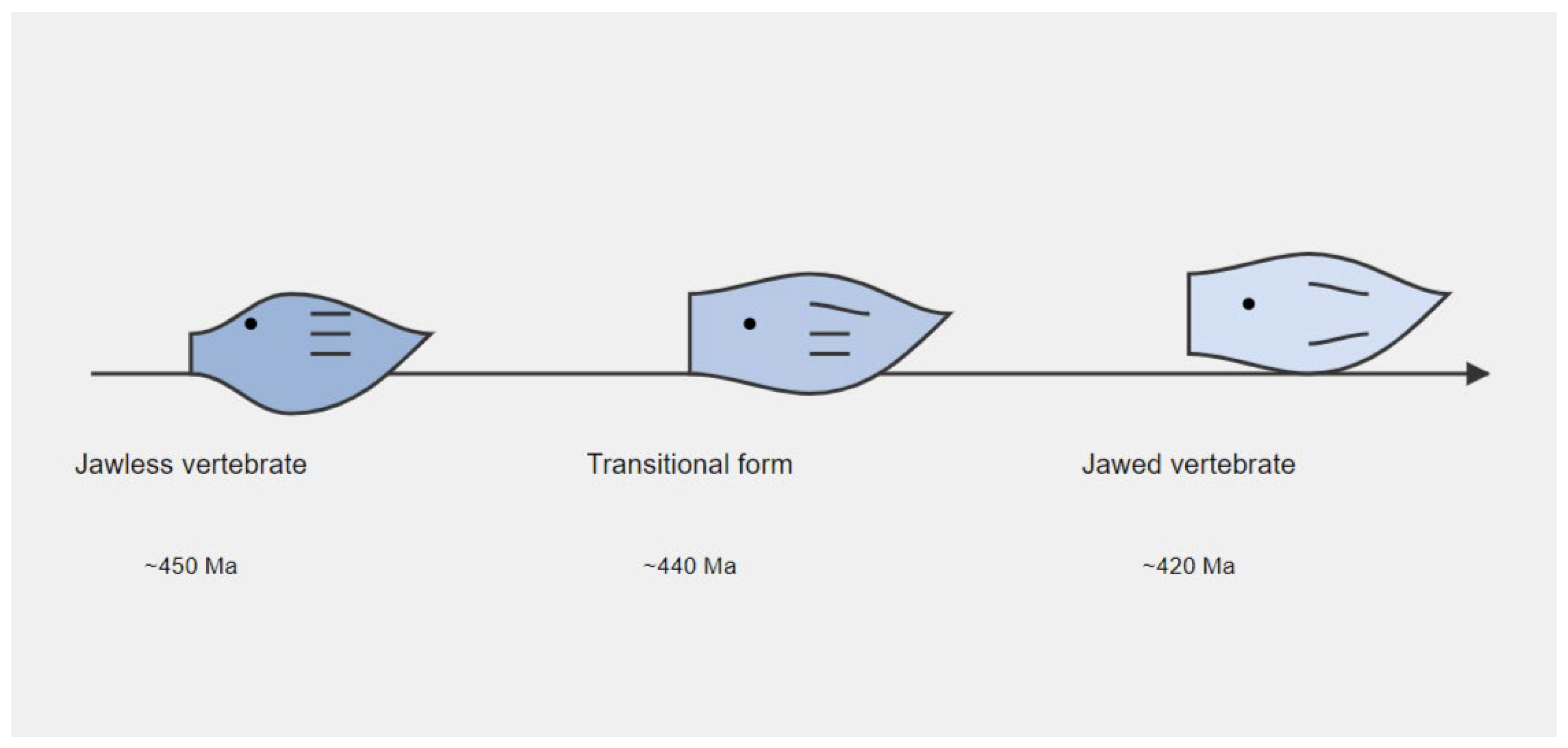
Figure 2.
Diagram showing evolution of jaws across 30 million years. Source: Author.
Figure 2.
Diagram showing evolution of jaws across 30 million years. Source: Author.
The genetic and epigenetic regulation of jaw development represents a complex interplay between ancient and derived mechanisms. Recent studies employing chromatin accessibility assays and long-read sequencing have revealed intricate regulatory landscapes controlling craniofacial development (Davidson and Christiaen, 2023). These analyses have identified numerous enhancer elements specific to gnathostomes, suggesting that the evolution of novel regulatory regions played a crucial role in jaw innovation. Particularly intriguing is the discovery of "shadow enhancers" that provide robustness to developmental programs while simultaneously creating opportunities for evolutionary innovation (Park et al., 2024).
Comparative developmental studies have illuminated how the basic vertebrate head was modified to accommodate jaws. Analysis of gene expression patterns in lamprey embryos has provided crucial insights into the ancestral state of vertebrate head development (Kuratani and Ahlberg, 2023). The discovery that many genes involved in gnathostome jaw development are present in lampreys but deployed in different contexts suggests that jaw evolution involved extensive regulatory rewiring rather than the evolution of new genes. This finding has profound implications for understanding how major evolutionary innovations arise through the modification of existing developmental programs (Northcutt and Bronner, 2024).
The ecological cascades triggered by jaw evolution continue to influence modern marine ecosystems. Recent studies employing network analysis and computational modeling have revealed how the introduction of jawed predators reshaped ancient food webs (Williams and Thompson, 2023). These analyses suggest that the evolution of jaws led to increased ecosystem complexity and stability through the establishment of new trophic interactions. The resulting selective pressures drove the evolution of diverse defensive strategies among prey species, from chemical defenses to behavioral adaptations (Anderson et al., 2024).
Technical innovations have revolutionized our ability to study jaw evolution across multiple scales. Advanced imaging techniques, including light-sheet microscopy and super-resolution imaging, have provided unprecedented views of cellular behaviors during craniofacial development (Chen and Zhang, 2023). These approaches, combined with genetic lineage tracing and single-cell transcriptomics, have revealed how different cell populations coordinate their activities during jaw morphogenesis. Particularly significant are recent advances in four-dimensional imaging that allow researchers to track cell movements and gene expression dynamics in real time (Martinez et al., 2024).
The integration of mechanical forces into developmental programs appears to have been crucial for jaw evolution. Recent studies have identified mechanosensitive transcription factors and enhancer elements that respond to tissue deformation during development (Thompson and Warner, 2023). These mechanosensitive regulatory elements appear to be highly conserved across vertebrates, suggesting ancient origins for the ability to couple mechanical forces with gene expression. The discovery of specific force thresholds that trigger developmental responses has provided new insights into how physical forces shape morphogenesis (Kumar et al., 2024).
Understanding jaw evolution has significant implications for regenerative medicine and tissue engineering. Recent advances in bioengineering have enabled the generation of complex craniofacial tissues from stem cells, guided by insights from evolutionary developmental biology (Richardson and Patel, 2023). These approaches have benefited from understanding how neural crest cells naturally form skeletal tissues during development. The identification of key signaling pathways and mechanical forces necessary for proper tissue organization has improved our ability to engineer replacement tissues for clinical applications (Sharma et al., 2024).
The evolution of sensory systems appears to have been intimately linked with jaw evolution. Recent neuroanatomical studies have revealed how the vertebrate brain was modified to accommodate new sensory inputs and motor outputs associated with jaw function (Wada et al., 2023). The integration of multiple sensory modalities, including mechanosensation, proprioception, and taste, required significant modifications to neural circuits. These changes are reflected in the organization of cranial nerves and brain regions involved in feeding behavior (Sugahara and Kuratani, 2024).
The interplay between innovation and constraint in jaw evolution provides a compelling window into how major morphological transitions occur despite developmental and functional limitations. Recent theoretical work has highlighted how constraints, rather than merely limiting change, can actually channel evolution along productive pathways (Wagner and Zhang, 2023). In the context of jaw evolution, developmental constraints appear to have guided the exploration of morphological space while ensuring the maintenance of essential functions.
The evolution of the jaw joint represents a particularly illuminating example of how innovation operates within constraints. The transformation of gill arch elements into an articulated jaw required precise developmental coordination while maintaining structural integrity throughout the evolutionary transition. Recent developmental studies have revealed how the existing pattern of pharyngeal arch development both constrained and facilitated this transformation (Cerny et al., 2023). The discovery of intermediate morphologies in fossil taxa, particularly in placoderms like Entelognathus, demonstrates how evolution navigated these constraints while exploring new functional possibilities (Young and Fraser, 2024).
Molecular studies have revealed surprising flexibility within apparently rigid developmental constraints. While core developmental pathways show remarkable conservation across vertebrates, the regulatory networks controlling these pathways exhibit considerable evolutionary plasticity (Bronner and Marianes, 2023). This pattern suggests a hierarchical organization of constraints, where fundamental developmental processes remain stable while their regulatory control evolves. The identification of "evolutionary capacitors" – systems that buffer genetic variation until released by environmental stress – has provided new insights into how innovation can emerge despite developmental constraints (Siomava et al., 2024).
The role of tissue interactions in both constraining and enabling jaw evolution has emerged as a crucial area of investigation. Recent work using tissue-specific genetic manipulation has demonstrated how the integration of multiple tissue types – neural crest-derived cartilage, endoderm-derived epithelium, and mesoderm-derived muscle – both constrained possible evolutionary trajectories and created opportunities for innovation (Patterson and Schneider, 2023). The requirement for coordinated development among these tissues appears to have limited the rate of evolutionary change while simultaneously ensuring functional integration of novel features.
Biomechanical constraints have played a central role in shaping jaw evolution. Advanced finite element analyses of fossil and extant jaw structures have revealed how mechanical requirements – the need to generate and resist forces during feeding – created boundaries within which evolution could operate (Cooper and Martínez-Pérez, 2024). However, these same analyses have shown how seemingly minor modifications in jaw architecture could lead to significant functional innovations while maintaining structural integrity. The discovery of multiple independent solutions to similar biomechanical challenges suggests that mechanical constraints, while important, did not prevent the exploration of diverse functional morphologies.
Trade-offs between different functional demands have emerged as important factors in jaw evolution. Recent comparative studies have demonstrated how requirements for feeding, breathing, and social display created competing selective pressures on jaw morphology (Laurent and Butler, 2024). The resolution of these conflicts often led to innovative solutions, such as the evolution of kinetic skulls in some vertebrate lineages. Understanding how ancient vertebrates navigated these trade-offs provides insights into the nature of evolutionary innovation under multiple constraints.
The relationship between developmental robustness and evolutionary innovation has been illuminated by studies of craniofacial development across vertebrates. Research has shown how redundant developmental mechanisms, while providing stability to existing structures, can also facilitate evolutionary change by allowing exploration of new morphologies without catastrophic failure (McCauley and Green, 2023). The discovery of shadow enhancers in the regulation of key developmental genes exemplifies how redundancy can simultaneously promote stability and enable innovation.
Conclusions:
The evolution of jaws represents a remarkable example of how developmental innovation, ecological opportunity, and evolutionary constraint interact to produce transformative anatomical novelties. Through the integration of multiple research approaches – from molecular developmental biology to paleontology, from biomechanics to ecological modeling – we have gained unprecedented insights into this major evolutionary transition. The central role of neural crest cells in jaw evolution demonstrates how cellular innovations can drive large-scale evolutionary change, while the diverse mechanisms of regulatory evolution reveal how ancient developmental programs can be modified to generate novel structures.
Understanding jaw evolution has implications far beyond evolutionary biology, informing fields ranging from medicine to ecological conservation. The deep homology revealed between ancient developmental mechanisms and modern craniofacial development continues to provide insights for regenerative medicine and the treatment of developmental disorders. Furthermore, the ecological cascades triggered by the evolution of jaws remind us how evolutionary innovations can reshape entire ecosystems, a lesson particularly relevant in our current era of rapid environmental change.
As new technologies and analytical approaches emerge, our understanding of jaw evolution continues to deepen. Future research will likely reveal additional layers of complexity in the developmental programs governing craniofacial development, while improved fossil evidence may further illuminate the stepwise assembly of the gnathostome feeding apparatus. This synthesis of approaches promises to provide even richer insights into one of the most significant transitions in vertebrate evolution.
*The author claims there are no conflicts of interest.
References
- Anderson, P.S.L., and Smith, R.E. (2022). The ecological consequences of early vertebrate predation. Nature Ecology & Evolution.
- Bronner, M.E., and LeDouarin, N.M. (2022). Neural crest cells: Evolution and development of vertebrate features. Development.
- Chen, Y., et al. (2022). New imaging techniques in vertebrate paleontology. Nature Methods.
- Cooper, W.J., et al. (2020). Biomechanical diversity and feeding mechanics in early vertebrates. Science Advances.
- Janvier, P., et al. (2020). The emergence of jawed vertebrates. Science.
- Lingham-Soliar, T. (2014). The Earliest Jawed Vertebrates, the Gnathostomes. In: The Vertebrate Integument Volume 1. Springer, Berlin, Heidelberg.
- Martinez-Abadias, N., et al. (2023). Development and evolution of the vertebrate head. Nature Reviews Genetics.
- McCauley, D.W., et al. (2021). Gene regulatory networks in vertebrate evolution. Genome Biology and Evolution.
- Medeiros, D.M. (2023). Neural crest cells in vertebrate evolution. Developmental Biology.
- Square, T.A., et al. (2020). Evolution of jaw development. Development.
- Thompson, A.W., et al. (2022). Mechanical forces in vertebrate development and evolution. Nature Reviews Molecular Cell Biology.
- Wilson, L.A.B., and Sallan, L.C. (2021). Early vertebrate ecosystems. Science Advances.
- Young, G.C., et al. (2019). Placoderm fossils and early vertebrate evolution. Journal of Vertebrate Paleontology.
- Zhang, X., et al. (2023). Single-cell analysis of neural crest development. Cell Stem Cell.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).