Submitted:
23 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
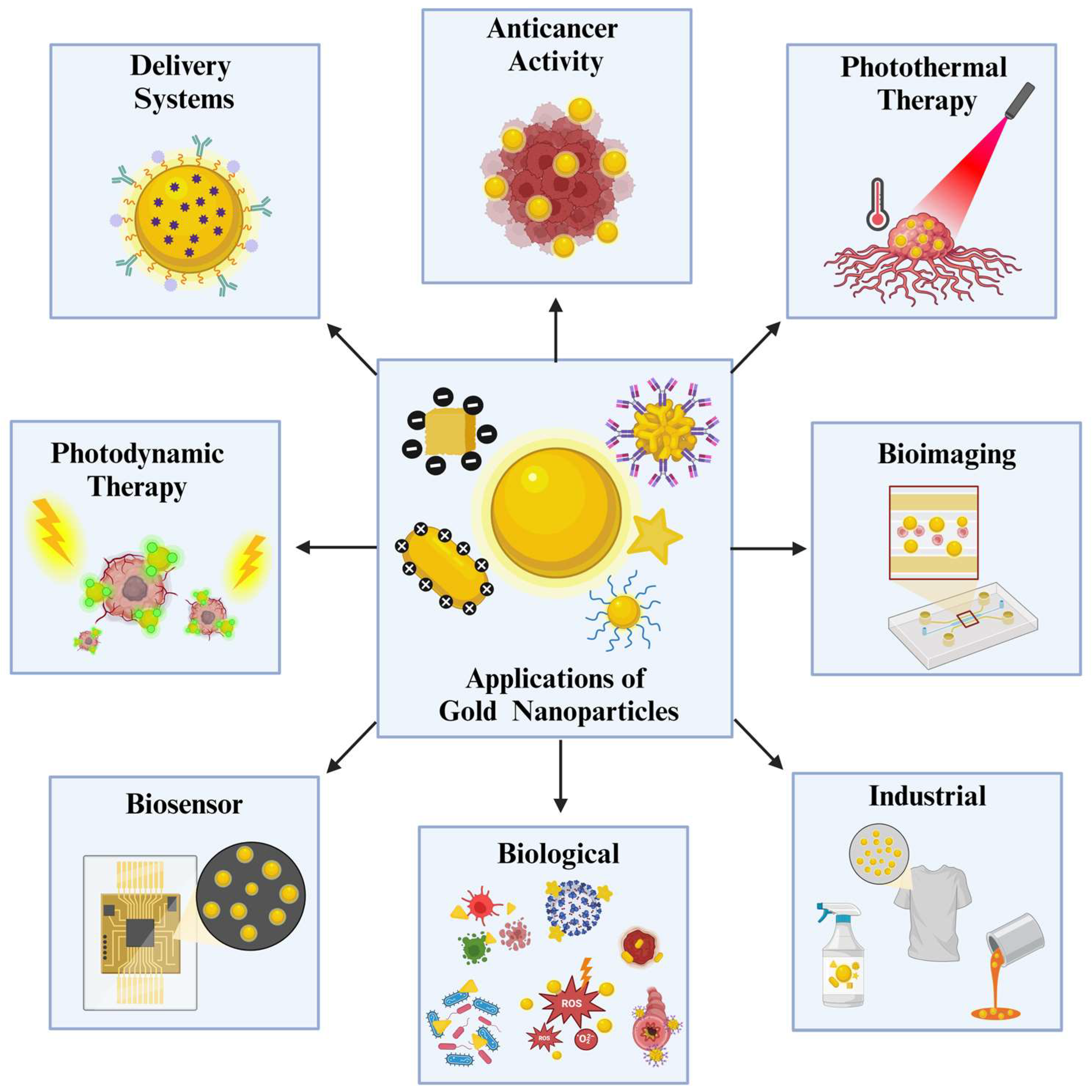
2. Applications of Gold Nanoparticles
2.1. Delivery systems
| Application | Synthesis Method | Properties | Results | Reference |
|---|---|---|---|---|
| In vitro cervical cancer treatment with curcumin conjugation | Chemical synthesis | Average size of 7 nm ± 2.29 nm Spherical morphology SPR peaks at 525 nm |
-Enhanced bioavailability of curcumin against HeLa cells. -Insignificant toxicity in the zebrafish embryo model. -Significant radiosensitizing activity. -Enhanced intracellular reactive oxygen species (ROS) generation, apoptotic signals and DNA damage. |
[16] |
| Co-delivery with miRNA-33a to MCF-7 breast cancer cells | Purchased from Nanosany with >95% purity. . |
Size ranges from 50 to 100 nm Spherical morphology (properties of modified gold NPs) |
-Enhanced gene expression in co-delivery. -Enhanced inhibitory activity against breast cancer cells. -Potential synergistic effect through modulation of signaling pathways -Increased apoptosis rate. |
[17] |
| Enhanced delivery of bleomycin in electrochemotherapy | Chemical synthesis | 13 nm size Spherical morphology LSPR peak at 521 nm |
-Enhanced cell permeabilization by 40%. -The electric field of 0.9 kV/cm and 1–100 kHz protocols demonstrated significant cytotoxicity by gold NP involvement. |
[18] |
| Delivery of chlorpromazine | Chemical synthesis | Average size of 15 nm and 55 nm Quasi-spherical morphology |
-Enhanced activity of chlorpromazine in cytotoxicity assays against human (COLO 679) and murine (B16-F0) melanoma cells. -Inhibition of mitochondrial activity and disruption of the cell membrane. |
[19] |
| Delivery of colistin | Chemical synthesis | Average size of 44.34 ± 1.02 Absorbance peaks at 300 ± 0.2 and 515 ± 0.3 nm (values from chitosan capped particles) |
-Average drug loading efficacy by 76.4%. -A consistent drug release profile. -A developed metered-dose inhaler efficiently destroys bacteria over a 12 hour period. |
[20] |
| Delivery of phosphazene Delivery of yeast RNA |
Chemical synthesis | Spherical Morphology | -Efficient, controlled and long-term release profiles for both drug and RNA delivery. -Antibacterial and antifungal activity. |
[21] |
| Nucleic acid DNA RNA | ||||
| Delivery of anti Glut1 SiRNA | Chemical synthesis | Approximate size of 14 nm Uniform Morphology LSPR peak at 520 nm (red-shifted to 528 nm) (values from SiRNA containing particles) |
-Significant reduction in Glut1 expression. -Promotion of apoptosis through glucose starvation and ROS cascade signaling. -Inhibition of cancer cell proliferation and tumor growth. (in vivo) -Induction of apoptosis. |
[22] |
| Delivery of Fluc mRNA | Chemical synthesis | Size between 11.52 nm - 12.97 nm Spherical Morphology Absorption peak at 520 nm |
-Significant expression of the luciferase gene compared to naked mRNA delivery. | [23] |
| SiRNA delivery | Commercially purchased | Size ranging between 20 - 30 nm Spherical Morphology SPR peak at 520 nm |
-At pH 5.5, green fluorescent protein knockdown levels decreased to 65% in HeLa cells. | [24] |
| Delivery of Fluc-zetagreen reporter genes Delivery of plasmid DNA and synthetic mRNA of SARS-CoV-2 S protein |
Chemical synthesis | Mean size of 53 nm Nanostar Morphology Absorbance peak at 630 nm |
-Enhanced transfection efficiency of Fluc gene delivery by increased gold NP-included nanocomposite concentrations in several cell lines. -Significant transfection efficiency of mRNA (at 1000 ng) and DNA (at 250 ng) of S protein by gold NPs. |
[25] |
| Protein | ||||
| Delivery of SARS-CoV-2 spike protein | Chemical synthesis | Size of 50 nm Spherical Morphology SPR peak at 529 nm |
-Induced IgG levels. -Induced neutralizing antibody levels. -Significant levels of IFN-γ. (All levels observed in mice) |
[26] |
| Delivery of atrial natriuretic peptide | Chemical synthesis | Size of 22.34 ± 0.54 | -Significant reduction of tumor formation capacity of retinoblastoma cells from >75% to <50% and <25%. -Significant reduction of tumor formation capacity in mice when the particles are topically administered. |
[27] |
| Antimicrobial peptide delivery | Chemical synthesis | Size of 10 nm SPR band at 518.5 nm |
-Significant reduction in MIC and MBC values by average of 200-fold. -20-fold faster killing capability in bacterial killing kinetics. -17-fold lower minimum biofilm eradication concentration (MBEC). |
[28] |
| Antibiotic | ||||
| Delivery of ciprofloxacin | Chemical synthesis | Approximate size of 13 nm Spherical Morphology Absorption peak at 520 nm |
-Significant reduction in MIC values when the ciprofloxacin administered with gold NPs (2 to 4 time reduction) -Increased zone of inhibition by approximately 4 mm. -Significant biofilm inhibition (from 36.30 nm to 12 nm in analysis on nebulizer masks) and antioxidant capacity (84.66%). |
[29] |
| Conjugation of amikacin for contact lens preservation | Chemical synthesis | Average size of 21 nm Spherical Morphology Absorption peak at 520 nm |
-Significant reduction of MIC with conjugation on NPs (2 to 4 time reduction). -More than 50% increase in inhibitory zones. -Efficient prevention of biofilm formation in contact lenses (approximate 2-4 time reduction). -Antioxidant capacity by 87.40 at the highest concentration, 100 µg/mL. |
[30] |
| Enhanced antimicrobial activity of berberine | Chemical synthesis | Average size of 49.38 nm Spherical Morphology Absorption peak at 520 nm |
-72% maximum release at 72 hours. -Nearly 50% decrease in MIC values. -Enhanced bactericidal activity through ROS-mediated membrane disruption and DNA damage. -Approximately 2-fold higher antibiofilm activity by 73.35%. -Significant enhancement of wound healing percentage in mice, with only 2.7 % bacteria survival rate. |
[31] |
2.1.1 Delivery for Cancer Treatment
2.1.2. Nucleic acid delivery
DNA
RNA
2.1.3. Protein Delivery
2.1.4. Antibiotic Delivery
2.2. Anticancer
| Application | Synthesis Method | Properties | Results | Reference |
|---|---|---|---|---|
| Anticancer Activity Against Osteosarcoma | Green-synthesis using Phormidesmis communis Strain AB_11_10 | Average size of 9.6 ± 4.3 nm Size between 4 – 20 nm (chemically synthesized) Quasi-spherical and Triangular morphology SPR peak at 524.5 nm |
-Significant cytotoxicity against MG-63 and SAOS-2 cell lines with 50% inhibition at concentration 297.5 and 15.5 µg/mL, respectively. -Chemically synthesized gold NPs inhibited 50% of cells at concentrations of 72 and 62 µg/mL, respectively. -Green-synthesized particles showed significant specificity against SAOS-2 cells. |
[80] |
| Anticancer Activity Against Pancreatic Cell Lines | Chemical Synthesis | Mean sizes of 83 ± 20 nm (coated with hyaluronic and oleic acids) 49 ± 12 nm (coated with bombesin peptides) Spherical Morphology |
-Significant anticancer activity against BxPC-3 tumor cells with combined treatment of radiation therapy. | [81] |
| Determination of anticancer and antioxidant properties of green-synthesized NPs. | Green synthesis from Coleus scutellarioides (L.) Benth leaves | Average size of 40.10 nm Spherical Morphology SPR band at 532 nm |
-At maximum concentration (120 µg/ml), DPPH scavenging activity is determined by 38.07 %. -Significant cytotoxicity against MDA-MB-231 cell line (IC50 at 36.10 µg/ml). |
[82] |
| Anticancer Effect on Hepatic Carcinoma Through Immunoregulation | Green synthesis from polygahatous polysaccharides | Average sizes of 10-14 nm (green-NP) and 30 - 34 nm (NP) Spherical Morphology |
-Induction of TNF-α, and IL-12p70 levels (in vitro) -Increased body weights of mice (decreased when NPs administered with adriamycin). -Increased serum TNF-α levels and CD4+/CD8+ lymphocyte ratios and decreased serum IL-10 levels (in vivo) -Increased tumor inhibition rate and decreased tumor growth. |
[83] |
| Determination of Anticancer Property | Green-synthesis using the seed extracts of Momordica cymbalaria. | Average size of 38 nm Spherical Morphology |
-Decreased cell viability of lung cancer lines to nearly 20% levels, through ROS synthesis and apoptosis induction. -Up to 58% inhibition of ROS synthesis. -Antibacterial activity against Klebsiella pneumoniae. -Capability to protect proteins and DNA from oxidative damage. |
[84] |
| Anticancer And Anti-plasmodial Activity | Green-synthesis from multiple types of leaf extracts | Size between 13.8 - 25.1 nm (depending on the extract) Polydisperse and spherical morphology |
-Significant inhibition of cancer growth up to 96% at the highest concentration, 50 μg/mL (IC 50 at 8.253 μg/mL). -Effective antiplasmodial activity with high selectivity. |
[85] |
| Determination of Anticancer Property | Green-synthesis from Chrysothemis pulchella leaf extracts | Average size of 14.7 nm Spherical morphology Absorption band at 527 nm |
-Significant cytotoxicity against HEK 293 and HeLa cells with IC50 values of 34.5 and 54.05 µg, respectively. -Strong antimicrobial activity. |
[86] |
| Anticarcinogenic Activity | Chemical Synthesis | Average size of 14 nm Spherical morphology Absorbance peak at 520 nm |
-Significant reduction in cell viabilities of MCF7 and A549 cancer cell lines to 31.25% and 28.13% at the highest concentration 100 µM, respectively. -Significant increase in TNF-α levels and apoptosis levels. |
[87] |
| Anticancer Activity Against Lymphoma Cells | Green synthesis from Moringa Oleifera leaf Extract | Size ranging from 6 - 18 nm Spherical, trigonal and hexagonal morphologies |
-Significant reduction in cell viability of Dalton’s lymphoma to approximately 30% at the highest concentration 150 μg/mL (IC50 at 75 ± 2.31 μg/mL). -Induction of apoptosis through nuclear fragmentation, diffuse chromatin condensation and increased apoptosis protein expression. -Increased ROS levels by 68.41% at the highest concentration. -Loss of mitochondrial membrane potential by 50.21%. - Cell cycle arrest at G2/M phase by increase of 35.66%. |
[88] |
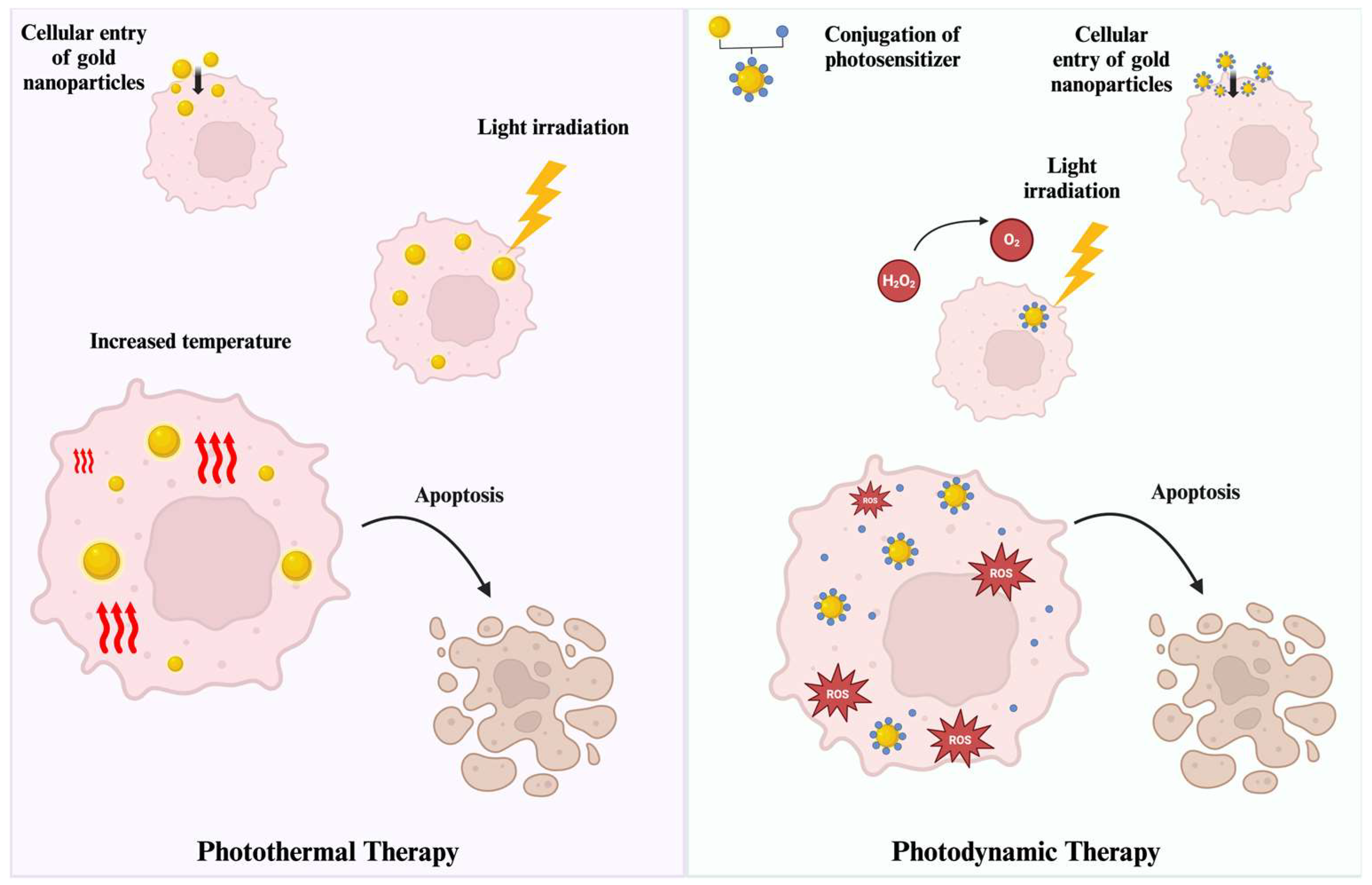
2.3. Photothermal Therapy Applications
| Application | Synthesis Method | Properties | Results | Reference |
|---|---|---|---|---|
| Selective Destruction of Cancer Cells | Chemical synthesis | Average size of 204 nm Nanostar morphology |
-Selective, site-specific destruction of HeLa, HEK-293, and SAOS-2 cell lines through laser irradiation. | [97] |
| Surface-Enhanced Raman Scattering (SERS) Image-guided Tumor PTT | Chemical synthesis | Average size of 35 nm Spherical morphology Peak at 521 nm (red-shifted to 548 nm with coating) |
-Significant PTT activity with 808-nm laser irradiation in both in vitro and in vivo. -Enhanced therapeutic efficacy, achieving complete cure in mice by day 30. |
[98] |
| Synergistic Ionidamine Release With PTT for Anticancer Activity | Chemical synthesis | Size of 5–30 nm Spherical morphology |
-Enhanced cytotoxicity through the release of lonidamine and 808 nm laser irradiation. Nanoparticle aggregation at tumor sites in mice, with enhanced PTT effects. |
[99] |
| PTT Against Drug-Resistant Cancer Cells | Green synthesis by fabrication with histidine and carboxylated chitosan | Approximate size of 6.37 nm SPR peak at 535 nm |
-27.8% photothermal efficiency under 660 nm laser irradiation. -Significant reduction in cell viability through PTT, down to 10%, at concentrations above 0.5 mg/mL. -90% reduction in tumor volume in mice treated with PTT. |
[100] |
| PTT for Cancer Treatment With Nucleic Acid Functionalization | Chemical synthesis | Approximate size of 13.7 nm Spherical morphology Absorption peak at 520 nm |
-PTT induction with 808 nm NIR laser irradiation in the presence of intracellular mRNAs. -Significant reduction in tumor growth in animal models. |
[101] |
| PTT for Cancer Treatment with 2D Self-Assembled Amphiphilic Peptide Modification | Chemical synthesis | Average size of 12.71 nm Ellipsoid-like morphology SPR peak at 520 nm (slight red-shift to 530 nm) |
-Enhanced photothermal conversion efficiency from 19.79% to 27.42%. -High biocompatibility and low toxicity. -79% death rate of MCF-7 cells under 808 nm laser irradiation. |
[102] |
| Plasmonic PTT Through Synergistic Drug Release With PLGA NPs | Chemical synthesis | Spherical and nanostar morphology | -Concentration and dose dependent cytotoxicity against neuroblastoma cells. -Off/On based triggered drug release for targeted delivery. -Enhanced cytotoxicity with 808 nm NIR laser irradiation. -Significant induction of ROS. |
[103] |
| PTT-mediated Multi-wavelength Photomagnetic Imaging (PMI) | Chemical synthesis | Size of 10 nm Nanorod morphology Peak absorption at 850 nm |
-Potential new model to make precise determination of the concentration of gold NPs in tumors. -Determination of PTT parameters, such as illumination power, duration and wavelength can be possible with PMI. |
[104] |
| Combination of PTT and Radiotherapy For Breast Cancer Treatment | Green-synthesis by using dopamine (DA)-conjugated alginate as a reducing and stabilizing agent | Mean size of 8.7 ± 1.3 nm Spherical and monodisperse morphology SPR peak at 540 nm |
-High biocompatible. -Simultaneous treatment of PTT (NIR) and radiotherapy (X-ray) enhanced cell viability reduction up to 35%. -Lowest rate of colony formation was observed in combined therapy (~ 0.37). -Increased ROS levels. -Significant reduction in tumor growth and volumes in vivo. |
[105] |
| Combined Antibacterial Activity In Dental resin Delivery With PTT | Purchased | Approximate size of 20 nm Spherical norphology (Shell) Peak absorbance at 660 nm |
-MIC of gold NPs against S.mutants was determined as 100 μg/mL. -Significant reduction in OD values of S.mutants with light irradiation. |
[106] |
| PTT With Methotrexate Delivery Through Dual-targeted NPs For Colorectal Cancer | Chemical synthesis | Size of 51.33 ± 5.70 nm Spherical Morphology (hollow) SPR peaks at 690 nm and between 800–820 nm |
-Negligible cytotoxicity. -Stabile drug release profile. -Significant reduction in tumor growths in both PTT (most significant) and non-PTT mice groups. -Larger necrotic region in tumor tissue of PTT-treated mice group. |
[107] |
Gold NP-based PTT for Anticancer Application
Gold NP-based PTT with CRISPR-Cas9 System
Gold NP-based PTT Combined With Immunotherapy
2.4. Photodynamic Therapy Applications
| Application | Synthesis Methods | Properties | Results | Reference |
|---|---|---|---|---|
| Photo-Eradication of Methicillin-Resistant Staphylococcus aureus Biofilm | Green synthesis using the cell-free filtrate obtained from Trichoderma koningii | Two size averagely 15 ± 3 nm and 20 ± 3 nm Spherical morphology |
-Enhanced photodestruction efficiency against biofilms. -Increased ROS production. -Approximately 100% destruction of biofilms. |
[129] |
| PDT-based Anticancer Therapy | Chemical synthesis | Size of 120 nm Star-like morphology |
-Enhanced anticancer activity with light treatment. -Increased ROS synthesis under 660 nm light irradiation. |
[130] |
| PDT against Staphylococcus aureus | Chemical synthesis | Size of length 53.2 nm±1.8 nm and width 23.6 nm±1.3 nm Nanorod morphology Transversal and longitudinal peaks at 520 nm and 660 nm |
-Significant bactericidal activity through 525 (superior) and 660 nm light irradiation (near to 100% reduction) -Agglomeration of NPs in bacteria surface. |
[131] |
| PDT for Hypoxic Tumor | Chemical synthesis | Mean size of 3 nm Nanocluster morphology Absorption peak at 385 nm |
-Strong photosensitizing property. -Modified NPs selectively target cancer cells. -Significant cytotoxicity (decreased up to 40%) through ROS generation with 532 nm light irradiation. |
[132] |
| SERS Imaging Integrated PTT/PDT | Chemical synthesis | Size of 40 nm and 17 nm in width Nanorod morphology |
-52.38% photothermal conversion efficiency -Significant ROS generation under 808 nm laser irradiation. -Successful targeting and imaging of 4T1 cells both in vitro and in vivo. -Combined therapy reduced cell viability to less than 15% and demonstrated 86.2% apoptosis rate. |
[133] |
| PDT against resistant bacteria | Chemical synthesis | Average size of 11.38 ± 4.38 nm Spherical morphology (properties of bismuth-gold NP hybrid) |
-Significant bacterial reduction by PDT up to 46.57% (almost 2x higher than non-PDT treatment). | [134] |
| Combined Therapy with PTT Against Breast Cancer | Chemical synthesis | Size between 30 - 40 nm Spherical morphology SPR peak at 530 nm |
-Reduced cytotoxicity of MB by conjugation into NPs. -Significant cytotoxicity levels (to 10%) with combined therapy. -Strong cytotoxicity, including at low concentrations. |
[135] |
| PDT-based Anticancer Activity Through Nanocomplex Against Melanoma | Chemical synthesis | Size of 13.58 nm Spherical morphology Absorption peak at 535 nm |
-Reduction in cell survival rate down to less than 20%. -Increased levels of lactate dehydrogenase (LDH) and caspase-3 and decreased levels of ATP and mitochondrial membrane potential. |
[136] |
Gold Nanoparticles-based PDT in Antimicrobial Applications
Gold Nanoparticles-based PDT in Cancer Applications
2.5. Bioimaging and Biosensor Applications
| Application | Synthesis Methods | Properties | Results | Reference |
|---|---|---|---|---|
| Morphine Quantification | Chemical synthesis | Approximately 4.13 nm sized particles. LSPR peaks at 532 nm. Negative surface charge. |
-The linear range for detecting increased morphine concentration was determined to be between 0.01-1.0 µg/mL -The limit of detection was determined to be 0.006 µg/mL. -Recovery range between 96.4 - 101.6% in real samples. -High specificity. |
[151] |
| Development of highly sensitive label-free optical biosensor | Chemical synthesis | Average size of 10.1 ± 1.7 nm Absorbance peak at 524 nm |
-Enhancement in performance with involvement of thin glass substrates (1 mm). -The limit of detection for streptavidin was determined to be 3.2 × 10-10 M. -Limit of detection for dinitrophenyl antibodies determined as 5.8 × 10-11 M. |
[152] |
| Detection of Interleukin-6 | Chemical synthesis | Size of 32.8 nm Spherical morphology (shell) Absorbance peak at 779 nm |
-Colorimetric detection of interleukin-6 with a limit of 5 ng/mL. -Photothermal quantitative detection of interleukin-6 with a limit of detection of 0.3 ng/mL (20-time lower than naked eye detection). -Rapid and specific detection. |
[153] |
| SERS | ||||
| Detection of Serum Dopamine | Chemical synthesis | Approximately 25 nm size Spherical morphology (nanoshell) (Size considered by the increased nm after coating) |
-Successful dopamine detection but non-specific in a label-free SERS system. -A direct and sensitive detection of dopamine was observed under the Azo reaction. -Significant linear range 10-3 – 10-12 mol/L and low limit of detection 10-12 mol/L in serum sample. |
[154] |
| Detection of Biothiols | Chemical synthesis | Approximate size of 25 ± 2.3 nm (Nanocomposite) Spherical morphology Extinction peak at 530 nm (redshifted peak at 545 nm) |
-Enhanced detection of biothiols with SERS-based dye-conjugated gold NPs. -The limit of detection ranges between 10-12 – 10-15. -Enhanced cellular imaging through discrimination of cancer cells based on biothiol concentration. |
[155] |
| Biosensor Development Through Freeze-Driven Synthesis | Chemical synthesis | Predominant sizes of 20, 40 and 80 nm Absorption peak at 520 nm (red-shifted to ∼650 nm) |
-The successful development of DNA hairpin-conjugated gold nanoparticles enabled dual-mode detection using novel methods. | [156] |
| Others | ||||
| Visualization of Tissue-specific Distribution Patterns of Functional Metabolites | Chemical synthesis | Approximately 27 nm size Spherical morphology 355 nm UV–VIS absorption (Synthesis based on cited references in the paper) |
-Wide-ranging detection of pesticides. -Visualization of primary and secondary metabolites and mechanical damages between healthy and infected citrus leaves. |
[157] |
| Detection of miRNA Levels in Raw Milk Samples | Chemical synthesis | Average size of 16 ± 1 nm Spherical morphology |
-Sensitive and rapid miRNA detection from four different milk samples through color change. | [158] |
| Detection of Sesame DNA in Food | Chemical synthesis | Average size of 13.6 ± 1.6 and 15.2 ± 1.2 nm (15 nm used) Spherical morphology Maximum absorbance ~527 nm (541 nm in non-sesame samples) |
-High specific, significant detection of sesame DNA in various food samples. -Easy determination by clear color changes. |
[159] |
| Detection of Hepatitis Virus | Purchased | 20 nm in size Spherical morphology Maximum absorbance at 520 nm (red-shifted to 550 nm) |
-Colorimetric response of gold NP-DNA Walkers in presence of hepatitis A virus target sequences. -Specific detection of target sequence. -Approximate limit of detection by 200 copies/mL. |
[160] |
| Detection of Candida albicans | Chemical synthesis | 40 nm in size |
-Colorimetric detection of Candida albicans β-1,3-D-glucans aptamers. -Significant stability and non-aggregative behavior of gold NPs. |
[161] |
Gold NP-included SERS Sensors
Gold NP-based LSPR Sensors
2.6. Other Biological Applications
Antimicrobial activity
Wound Healing
Anti-inflammatory
Anti-diabetic Activity
| Application | Synthesis Methods | Properties | Results | Reference |
|---|---|---|---|---|
| Antibacterial | ||||
| Metabolomic and Docking Study of Gold NP’s Antimicrobial Activity | Green-synthesis using using Arthrospira platensis extract | Mean size of 134.8 nm Rod shaped morphology |
-Antibacterial activity against Streptococcus pneumoniae was shown with 12 μg/mL MIC value. -The molecular docking study showed the docking score of A. platensis derived compound by −6.84 kcal/mol against many residues. |
[210] |
| Antibacterial Activity Against Bovine Locomotion Disorders | Commercially purchased, synthesized with physical methods | 5-40 nm size range Spherical morphology |
-6.25 mg/L concentration of gold NP treatment reduced bacterial cell viability between 40–50%. -Higher concentrations up to 50 mg/L increased the reduction to approximately 90%. |
[211] |
| Antibacterial activity against both Gram-positive and Gram-negative bacteria | Green-synthesis from Lannea discolor. | Size between 30 - 97 nm Flower-shaped SPR peak at 316 nm |
-Antibacterial activity against E. coli, S. aureus, K. pneumoniae, B. subtilis, P. aeruginosa, with MIC values down to 7.81 µg/mL for B. subtilis, K. pneumoniae, P. aeruginosa. | [212] |
| Antibacterial activity against Salmonella typhimurium (S. typhimurium), one of the most important food pathogens | Green-synthesis from Jatropha curcas | Average size of 17 nm Predominantly spherical SPR peak at 526 nm |
-Efficient bactericidal activity, in comparison to the plant extract alone, was observed against S. typhimurium -Reduction in bacterial growth was observed at 18 μg/mL of gold NPs. |
[213] |
| Antifungal | ||||
| Determination of Antifungal Activity | Green-synthesis from aqueous extract of Ricinus cummunis leaves | Size between 15 - 20 nm Predominantly spherical, and some triangular morphology SPR peak at 550 nm |
-Against Aspergillus fumigatus and Candida albicans IC50 values of gold NPs were determined as 88.90 and 58.31 μg/mL, respectively. -Antibacterial and dye degeneration studies were also conducted. |
[214] |
| Determination of Antifungal Activity | Green-synthesis from Callistemon viminalis extracts | 100 nm size Spherical morphology Absorption peak at 525 nm |
-The antifungal activity against multiple types of fungal strains with inhibition zones ranging from 8.5 - 10.5 mm. -Antibacterial activity was also evaluated. |
[215] |
| Antifungal activity against multidrug-resistant fungus | Tricyclic microwave-assisted chemical synthesis | Size ranging 9 - 55 nm Spherical morphology Absorption peak at 506 nm (from three variants) |
-Zone of inhibition determined between 1.2 and 1.7 cm. -MIC values ranged from 21-52, 24-46 and 44-102 µg/mL, depending on the gold NP formulation. |
[216] |
| Determination of Antifungal Activity of Functionalized Gold NPs | Chemical synthesis | Near size of 7 nm Spherical morphology SPR peak at 515 nm |
-Conjugation of vancomycin onto gold NPs reduced the MIC value from 37.5 to 4.68 µg/mL and 75.0 to 1.5 µg/mL (difference from fungal strains). -Germination inhibition of fungal strains by 93% and 35%. -Increased ROS levels. |
[217] |
| Antiviral | ||||
| Antiviral activity against human adenovirus serotype 5 (HAdV-5). | Green-synthesis using fodder yeast | Size ranging 10 - 23 nm Irregular and spherical SPR peak at 540 nm |
Virucidal activity against HAdV-5 was observed with a reduction of -2.25 log10 50% tissue culture infection dose (TCID50). | [218] |
| Antiviral activity against herpes simplex virus-2 (HSV-2) by the use of gold NPs coated with poly(styrene sulfonate | Brust-Schriffin method | Size - Spherical morphology |
-Antiviral activity against HSV-2 was observed with an IC50 of 89.91 ng/mL | [219] |
| Antiviral activity against White spot syndrome virus (WSSV) | Green-synthesis from Brevibacterium casei (SOSIST-06) | Size ranging 9.5 to 52.3 nm Spherical and triangular morphologies |
-Dose-dependent antiviral activity was demonstrated against WSSV. -At a concentration of 320 μg/mL, NPs reduced the expression of viral gene VP-28. |
[220] |
| Wound Healing | ||||
| Diabetes-induced Wound Healing Activity With Hydrogels | Chemical synthesis | Size of 14.15±1.02 nm Spherical morphology Absorbance peak at 520 nm |
-Significant antibacterial activity against wound infections with >15mm inhibition zone and colony reduction near to 0%.. -Significant reduction in wound area by day 6 compared to the control group, ranging from 80.5% - 50.7% and 92.8%, respectively (in vivo). -Enhanced wound healing through immunoregulation and induction of angiogenesis. (results demonstrate difference based on the loaded gold NP ratio, from 5% to 50%) |
[221] |
| Determination of Wound Healing Potential of Collagen-I Coated gold NPs | Chemical synthesis | Average size of ∼19 ± 0.2 nm Spherical morphology Absorption peak at 524 nm |
-Coated gold NPs induced the proliferation of human skin fibroblast (HSF) cells, ranging from 135.21 ± 6.69% to 154.74 ± 9.72%. -Uncoated particles did not induce visible toxicity or alterations in HSF proliferation. -Wound closure rates of ∼57.16 ± 1.31% and ∼67.67 ± 1.67% for uncoated and coated particles, respectively, while the control group showed only 22.12 ± 1.22% wound closure at 24 hours. -After 48 hours, the wound closure rates increased to 91.01 ± 2.71%, 95.19 ± 1.67%, and 59.96 ± 2.66% for coated particles, uncoated particles, and the control group, respectively. |
[222] |
| Determination of wound healing activity | Green-synthesis using Bulbine frutescens (L.) Willd | Various size between 51.82 ± 33.76 nm and 289.3 ± 88.68 Round, hexagonal and triangular morphologies Absorption peak at 550 nm (considered differences among 4 types of extracts) |
-Up to 31.40 ± 0.88% wound closure ratio compared to control group 9.63 ± 0.22% -Increased cell viability to 116.40 ± 9.35%. |
[223] |
| Anti-inflammatory | ||||
| Therapeutic effects by gold NPs on asthma treatment | Green-synthesis from Descurainia sophia extract | Size ranging 10 - 38 nm Spherical morphology Absorption peak at 537 nm |
-Gold NPs showed anti-inflammatory effects by reducing inflammatory markers IgE, PLA2 and total protein levels -Following treatment with gold NPs, improved lung pathology was observed in sensitized rats |
[224] |
| Determination of anti-inflammatory activity with ginsenoside compound K (CK) loading | Green synthesis using probiotic bacteria, Bifidobacterium animalis subsp. lactis. | Size ranging 10 - 25 nm Spherical morphology |
-Inhibition of ROS activation, reduction in the expression of iNOS, COX-2 and pro-inflammatory cytokines, IL-1β, IL-6, and TNF-α, were observed in RAW 264.7 cells. -Reduction in inflammation was observed in kidney, lung and liver tissues of mice, without toxicity, following administration of gold NPs. |
[225] |
| Antioxidant | ||||
| Determination of antioxidant activity | Green synthesis from Allium cepa L. peel aqueous extract | Size ranging 6.08 and 54.20 nm Spherical morphology SPR peak at 561.11 nm |
-The antioxidant activity was evaluated with 4 different assays, with IC50 values ranging between 165.3 ± 1.93 and 193.7 ± 0.36 µg/mL. -Antipathogenic and enzyme inhibition activities were also observed. |
[226] |
| Antioxidant activity with carrying extran-graft-polyacrylamide polymer | Chemical synthesis | Size of 5.5 ± 2.0 nm Spherical morphology |
-At the highest concentration (50 mg/L), 64.66% radical scavenging activity was observed. -Antimicrobial and antibiofilm activity were also demonstrated. |
[227] |
| Antidiabetic | ||||
| Determination of therapeutics effects of nature-friendly synthesized gold NPs | Green-synthesis using Nepeta bodeana Bunge leaf extract | Size ranging 20 - 30 nm Spherical morphology SPR peak at 547 nm |
Antidiabetic activity was demonstrated with 52% inhibition of α-amylase and 62% glucose uptake activity in yeast cells, at 300 µg/mL of gold NPs. | [228] |
| Demonstration of antidiabetic activity of gold NPs | Green-synthesis using seaweed extracts (Ulva linza, Ulva fasciata, Ulva intestinalis, Petalonia fascia, and Corallina officinalis | Average diameter of 9.02 ±1.7 nm Spherical morphology SPR peak at 540 nm |
-Gold NPs achieved maximum α-glucosidase inhibition of 90.6%, at a concentration of 100 mg/mL, with IC50 value of 0.078 ± 0.003 mg/mL. -In the same tested concentrations, NPs exhibited maximum inhibition of 87.4% against α-amylase, with IC50 of 0.312 ± 0.014 mg/mL. |
[229] |
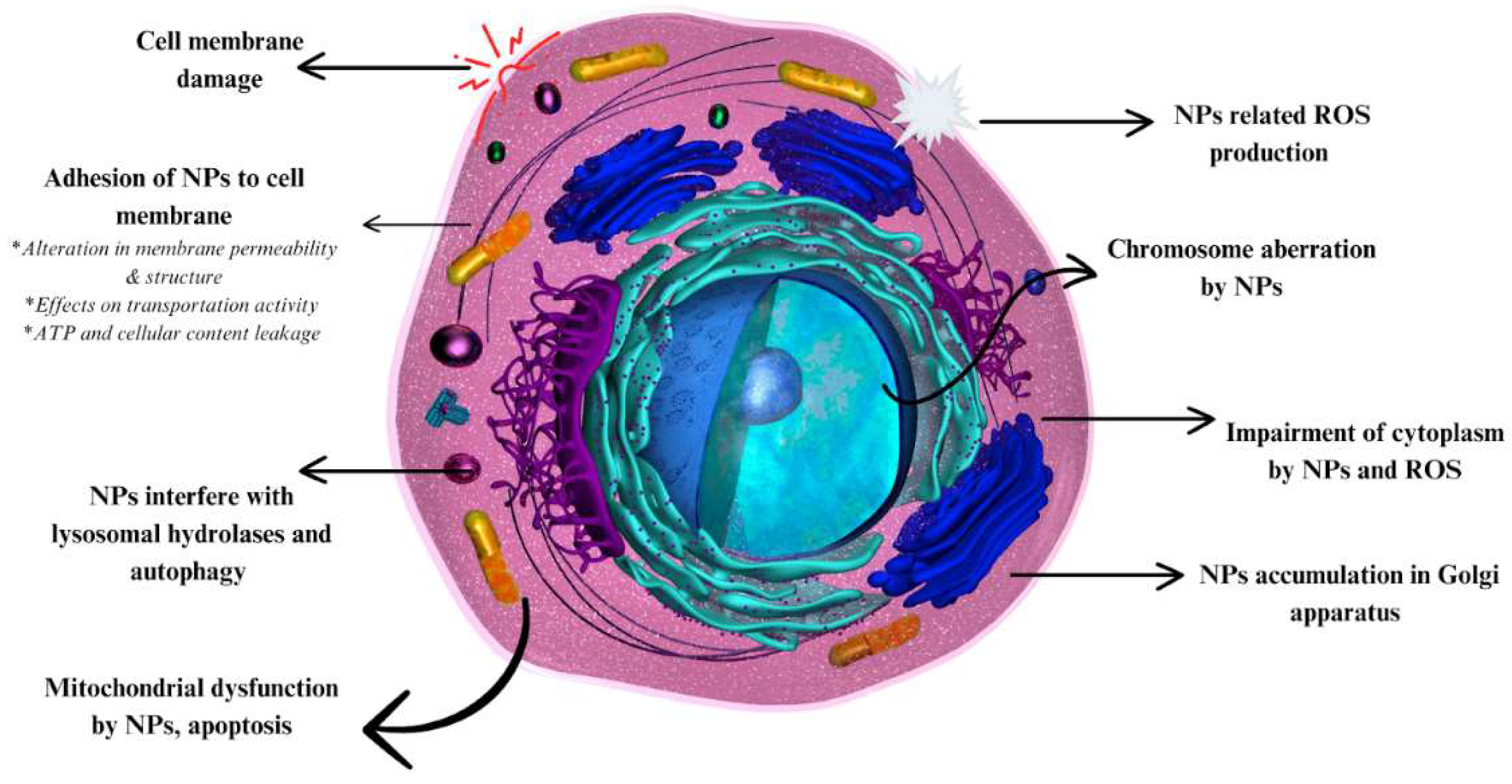
4. Toxicity
5. Future Trends
6. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J Nanobiotechnol 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of Gold Nanoparticles in Advanced Biomedical Applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on Gold Nanoparticles and Their Applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud Univ. - Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold Nanoparticles for Applications in Cancer Radiotherapy: Mechanisms and Recent Advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. IJN 2020, Volume 15, 9407–9430. [Google Scholar] [CrossRef]
- Document Search - Web of Science Core Collection. Available online: Https://Www.Webofscience.Com/Wos/Woscc/Basic-Search (accessed on day month year).
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles in Therapeutics and Beyond: A Review of Mechanism Insights and Applications. Nanomaterials 2024, 14, 1618. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.; Rotello, V. Gold Nanoparticles in Delivery Applications☆. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, X.; Liang, X.-J. Gold Nanoparticles: Emerging Paradigm for Targeted Drug Delivery System. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. IJN 2020, Volume 15, 9823–9857. [Google Scholar] [CrossRef]
- Yadav, P.; Bandyopadhyay, A.; Sarkar, K. Enhancement of Gold-Curcumin Nanoparticle Mediated Radiation Response for Improved Therapy in Cervical Cancer: A Computational Approach and Predictive Pathway Analysis. Discov. Nano 2024, 19, 153. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Seyedabadi, M.; Ebrahimnejad, P.; Abasi, M.; Nokhodchi, A. Efficient Delivery of Gold Nanoparticles and miRNA-33a Via Cationic PEGylated Niosomal Formulation to MCF-7 Breast Cancer Cells. AAPS PharmSciTech 2024, 25, 213. [Google Scholar] [CrossRef]
- Lekešytė, B.; Mickevičiūtė, E.; Malakauskaitė, P.; Szewczyk, A.; Radzevičiūtė-Valčiukė, E.; Malyško-Ptašinskė, V.; Želvys, A.; German, N.; Ramanavičienė, A.; Kulbacka, J.; et al. Application of Gold Nanoparticles for Improvement of Electroporation-Assisted Drug Delivery and Bleomycin Electrochemotherapy. Pharmaceutics 2024, 16, 1278. [Google Scholar] [CrossRef]
- Oćwieja, M.; Barbasz, A.; Kowalska, O.; Maciejewska-Prończuk, J.; Lada, A. The Adsorption of Chlorpromazine on the Surface of Gold Nanoparticles and Its Effect on the Toxicity to Selected Mammalian Cells. Materials 2024, 17, 4774. [Google Scholar] [CrossRef]
- Changsan, N.; Atipairin, A.; Muenraya, P.; Sritharadol, R.; Srichana, T.; Balekar, N.; Sawatdee, S. In Vitro Evaluation of Colistin Conjugated with Chitosan-Capped Gold Nanoparticles as a Possible Formulation Applied in a Metered-Dose Inhaler. Antibiotics 2024, 13, 630. [Google Scholar] [CrossRef]
- Ozsoy, F.; Ozay, O. Phosphazene-Based Nanostructures Modified with Gold Nanoparticles as Drug and Gene Carrier Materials with Antibacterial and Antifungal Properties. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 383–394. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Fang, Q.; Du, Y.; Zhang, X. Gold Nanoparticle Delivery of Glut1 SiRNA Facilitates Glucose Starvation Therapy in Lung Cancer. ChemBioChem 2024, 25, e202400239. [Google Scholar] [CrossRef] [PubMed]
- Venkatas, J.; Singh, M. Chemical and Green Synthesis of Gold Nanoparticles for mRNA Delivery in Vitro. Adv. Nat. Sci: Nanosci. Nanotechnol. 2024, 15, 015009. [Google Scholar] [CrossRef]
- Martinez Júnior, A.M.; Aparecida De Oliveira Tiera, V.; José Tiera, M. Tuning the Surface Charge and Colloidal Stability of Hybrid Gold-Chitosan Derivative Nanoparticles for siRNA Delivery. J. Drug Deliv. Sci. Technol. 2024, 101, 106167. [Google Scholar] [CrossRef]
- Tabesh, F.; Haghverdi, G.; Devarakonda, K.P.; Massoud, T.F.; Paulmurugan, R. Synthesis, Characterization, and Application of a Biocompatible Gene Delivery Nanocarrier Constructed from Gold Nanostars and a Chitosan–Cyclodextrin–Poly(Ethylene Imine) Graft Polymer. Mater. Adv. 1039. [Google Scholar] [CrossRef]
- Salazar, V.A.; Comenge, J.; Suárez-López, R.; Burger, J.A.; Sanders, R.W.; Bastús, N.G.; Jaime, C.; Joseph-Munne, J.; Puntes, V. Gold Nanoparticle Virus-like Particles Presenting SARS-CoV-2 Spike Protein: Synthesis, Biophysical Properties and Immunogenicity in BALB/c Mice. Vaccines 2024, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.; Miroschnikov, N.; Klein, S.; Doege, A.; Dünker, N.; Van Meenen, D.; Junker, A.; Göpferich, A.; Apaolaza, P.S.; Busch, M.A. New Retinoblastoma ( RB ) Drug Delivery Approaches: Anti-tumor Effect of Atrial Natriuretic Peptide ( ANP )-conjugated Hyaluronic-acid-coated Gold Nanoparticles for Intraocular Treatment of Chemoresistant RB. Mol. Oncol. 2024, 18, 832–849. [Google Scholar] [CrossRef]
- Rajchakit, U.; Lamba, S.; Wang, K.; Lyons, N.; Lu, J.; Swift, S.; Pletzer, D.; Sarojini, V. Size-Controlled Synthesis of Gold Nanoparticles Tethering Antimicrobial Peptides with Potent Broad-Spectrum Antimicrobial and Antibiofilm Activities. Mol. Pharm. 2024, 21, 596–608. [Google Scholar] [CrossRef]
- Hasoon, B.A.; Jawad, K.H.; Abdulsahib, S.S. Synthesis of Ciprofloxacin-Conjugated Gold Nanoparticles and Their Study Antibacterial Effects on Growth Biofilm Formation Through Nebulizer Mask Against Respiratory Infection. Plasmonics 2024, 19, 1875–1889. [Google Scholar] [CrossRef]
- Jawad, K.H.; Jamagh, F.K.; Sulaiman, G.M.; Hasoon, B.A.; Albukhaty, S.; Mohammed, H.A.; Abomughaid, M.M. Antibacterial and Antibiofilm Activities of Amikacin-Conjugated Gold Nanoparticles: A Promising Formulation for Contact Lens Preservation. Inorg. Chem. Commun. 2024, 162, 112286. [Google Scholar] [CrossRef]
- Sadeghi, S.; Agharazi, F.; Hosseinzadeh, S.A.; Mashayekhi, M.; Saffari, Z.; Shafiei, M.; Nader Shahrokhi; Ebrahimi-Rad, M. ; Sadeghi, M. Gold Nanoparticle Conjugation Enhances Berberine’s Antibacterial Activity against Methicillin-Resistant Staphylococcus Aureus (MRSA). Talanta 2024, 268, 125358. [Google Scholar] [CrossRef]
- Chandran, P.R.; Thomas, R.T. Gold Nanoparticles in Cancer Drug Delivery. In Nanotechnology Applications for Tissue Engineering; Elsevier, 2015; pp. 221–237 ISBN 978-0-323-32889-0.
- Pooja, D.; Panyaram, S.; Kulhari, H.; Reddy, B.; Rachamalla, S.S.; Sistla, R. Natural Polysaccharide Functionalized Gold Nanoparticles as Biocompatible Drug Delivery Carrier. Int. J. Biol. Macromol. 2015, 80, 48–56. [Google Scholar] [CrossRef]
- Ajnai, G.; Chiu, A.; Kan, T.; Cheng, C.-C.; Tsai, T.-H.; Chang, J. Trends of Gold Nanoparticle-Based Drug Delivery System in Cancer Therapy. J. Exp. Clin. Med. 2014, 6, 172–178. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of Gold Nanoparticles Shape on Their Cytotoxicity against Human Osteoblast and Osteosarcoma in in Vitro Model. Evaluation of the Safety of Use and Anti-Cancer Potential. J Mater Sci: Mater Med 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ardjmand, M.; Rad, A.S.; Esfahani, M.R. Gelatin-Coated Gold Nanoparticles as an Effective pH-Sensitive Methotrexate Drug Delivery System for Breast Cancer Treatment. Mater. Today Chem. 2021, 20, 100474. [Google Scholar] [CrossRef]
- Lim, D.-J.; Park, H. Near-Infrared Light for on-Demand Drug Delivery. J. Biomater. Sci. Polym. Ed. 2018, 29, 750–761. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold Nanoparticle-mediated Photothermal Therapy: Applications and Opportunities for Multimodal Cancer Treatment. WIREs Nanomed Nanobiotechnol 2017, 9, e1449. [Google Scholar] [CrossRef]
- Niikura, K.; Iyo, N.; Matsuo, Y.; Mitomo, H.; Ijiro, K. Sub-100 Nm Gold Nanoparticle Vesicles as a Drug Delivery Carrier Enabling Rapid Drug Release upon Light Irradiation. ACS Appl. Mater. Interfaces 2013, 5, 3900–3907. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Aghanejad, A.; Safari, B.; Barar, J.; Rasta, S.H.; Davaran, S. Aptamer-Conjugated Gold Nanoparticles for Targeted Paclitaxel Delivery and Photothermal Therapy in Breast Cancer. J. Drug Deliv. Sci. Technol. 2022, 67, 102954. [Google Scholar] [CrossRef]
- Pissuwan, D.; Niidome, T.; Cortie, M.B. The Forthcoming Applications of Gold Nanoparticles in Drug and Gene Delivery Systems. J. Control. Release 2011, 149, 65–71. [Google Scholar] [CrossRef]
- Mendes, R.; Fernandes, A.; Baptista, P. Gold Nanoparticle Approach to the Selective Delivery of Gene Silencing in Cancer—The Case for Combined Delivery? Genes 2017, 8, 94. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold Nanoparticles for Nucleic Acid Delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Fontinha, D.; Martins, C.; Pires, D.; Fernandes, A.R.; Baptista, P.V. Gold Nanoparticles for Vectorization of Nucleic Acids for Cancer Therapeutics. Molecules 2020, 25, 3489. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Gold Nanoparticles and Gold Nanoparticle-Conjugates for Delivery of Therapeutic Molecules. Progress and Challenges. J. Mater. Chem. B 2014, 2, 4204–4220. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, S.; Li, H.; Chang, H. Biomedical Applications of DNA-Conjugated Gold Nanoparticles. ChemBioChem 2016, 17, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, X.; Luo, G.; Jiao, J. DNA-Functionalized Gold Nanoparticles: Modification, Characterization, and Biomedical Applications. Front. Chem. 2022, 10, 1095488. [Google Scholar] [CrossRef]
- Encabo-Berzosa, M.M.; Sancho-Albero, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Santamaria, J.; Martín Duque, P. Polymer Functionalized Gold Nanoparticles as Nonviral Gene Delivery Reagents. J Gene Med 2017, 19, e2964. [Google Scholar] [CrossRef]
- Bishop, C.J.; Tzeng, S.Y.; Green, J.J. Degradable Polymer-Coated Gold Nanoparticles for Co-Delivery of DNA and siRNA. Acta Biomater. 2015, 11, 393–403. [Google Scholar] [CrossRef]
- Lee, K.; Conboy, M.; Park, H.M.; Jiang, F.; Kim, H.J.; Dewitt, M.A.; Mackley, V.A.; Chang, K.; Rao, A.; Skinner, C.; et al. Nanoparticle Delivery of Cas9 Ribonucleoprotein and Donor DNA in Vivo Induces Homology-Directed DNA Repair. Nat Biomed Eng 2017, 1, 889–901. [Google Scholar] [CrossRef]
- Graczyk, A.; Pawlowska, R.; Jedrzejczyk, D.; Chworos, A. Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules 2020, 25, 204. [Google Scholar] [CrossRef]
- Artiga, Á.; Serrano-Sevilla, I.; De Matteis, L.; Mitchell, S.G.; De La Fuente, J.M. Current Status and Future Perspectives of Gold Nanoparticle Vectors for siRNA Delivery. J. Mater. Chem. B 2019, 7, 876–896. [Google Scholar] [CrossRef]
- Paul, A.M.; Shi, Y.; Acharya, D.; Douglas, J.R.; Cooley, A.; Anderson, J.F.; Huang, F.; Bai, F. Delivery of Antiviral Small Interfering RNA with Gold Nanoparticles Inhibits Dengue Virus Infection in Vitro. J. Gen. Virol. 2014, 95, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Ambrosone, A.; Sanz, V.; Hernandez, Y.; Marchesano, V.; Tian, F.; Child, H.; Berry, C.C.; Ibarra, M.R.; Baptista, P.V.; et al. Design of Multifunctional Gold Nanoparticles for In Vitro and In Vivo Gene Silencing. ACS Nano 2012, 6, 8316–8324. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S.; et al. A Light-Driven Therapy of Pancreatic Adenocarcinoma Using Gold Nanorods-Based Nanocarriers for Co-Delivery of Doxorubicin and siRNA. Theranostics 2015, 5, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, W.; Shen, Y.; Huang, Q.; Zhou, D.; Guo, S. Efficient RNA Delivery by Integrin-Targeted Glutathione Responsive Polyethyleneimine Capped Gold Nanorods. Acta Biomater. 2015, 23, 136–146. [Google Scholar] [CrossRef]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer Coated Gold Nanoparticles for Delivery Applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef]
- Khandelia, R.; Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. Gold Nanoparticle–Protein Agglomerates as Versatile Nanocarriers for Drug Delivery. Small 2013, 9, 3494–3505. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, P.; Sharma, S.; Dhiman, K.; Sharma, D.; Verma, A. Gold Nanoparticle-Mediated Delivery of Therapeutic Enzymes for Biomedical Applications. In Nanoscience in Medicine Vol. 1; Daima, H.K., Pn, N., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, 2020; ISBN 978-3-030-29206-5. [Google Scholar]
- Ghosh, P.; Yang, X.; Arvizo, R.; Zhu, Z.-J.; Agasti, S.S.; Mo, Z.; Rotello, V.M. Intracellular Delivery of a Membrane-Impermeable Enzyme in Active Form Using Functionalized Gold Nanoparticles. J. Am. Chem. Soc. 2010, 132, 2642–2645. [Google Scholar] [CrossRef]
- Ryou, S.-M.; Yeom, J.-H.; Kang, H.J.; Won, M.; Kim, J.-S.; Lee, B.; Seong, M.-J.; Ha, N.-C.; Bae, J.; Lee, K. Gold Nanoparticle–DNA Aptamer Composites as a Universal Carrier for in Vivo Delivery of Biologically Functional Proteins. J. Control. Release 2014, 196, 287–294. [Google Scholar] [CrossRef]
- Pereira, M.; Rodrigues, A.R.O.; Amaral, L.; Côrte-Real, M.; Santos-Pereira, C.; Castanheira, E.M.S. Bovine Lactoferrin-Loaded Plasmonic Magnetoliposomes for Antifungal Therapeutic Applications. Pharmaceutics 2023, 15, 2162. [Google Scholar] [CrossRef]
- Eker, F.; Akdaşçi, E.; Duman, H.; Yalçıntaş, Y.M.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. [Google Scholar] [CrossRef]
- Eker, F.; Bolat, E.; Pekdemir, B.; Duman, H.; Karav, S. Lactoferrin: Neuroprotection against Parkinson’s Disease and Secondary Molecule for Potential Treatment. Front. Aging Neurosci. 2023, 15, 1204149. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, M.N.; Qureshi, T.S.; Krishna, C.M.; Pansare, K.; Gadewal, N.; Hole, A.; Dongre, P.M. β-Lactoglobulin-Gold Nanoparticles Interface and Its Interaction with Some Anticancer Drugs – an Approach for Targeted Drug Delivery. J. Biomol. Struct. Dyn. 2022, 40, 6193–6210. [Google Scholar] [CrossRef]
- Waghmare, M.N.; Qureshi, T.S.; Shaikh, A.N.; Khade, B.S.; Murali Krishna, C.; Dongre, P.M. Functionalized Alpha-lactalbumin Conjugated with Gold Nanoparticle for Targeted Drug Delivery. ChemistrySelect 2020, 5, 2035–2049. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; Lin, A.Y.; Figueroa, E.R.; Foster, A.E.; Drezek, R.A. In Vivo Gold Nanoparticle Delivery of Peptide Vaccine Induces Anti-Tumor Immune Response in Prophylactic and Therapeutic Tumor Models. Small 2015, 11, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Chu, Y.; Huang, Y.-F.; Chong, Y.-S.; Jiang, Z.-H.; Mao, Z.-W.; Peng, L.-H.; Gao, J.-Q. Transdermal Gene Delivery by Functional Peptide-Conjugated Cationic Gold Nanoparticle Reverses the Progression and Metastasis of Cutaneous Melanoma. ACS Appl. Mater. Interfaces 2017, 9, 9388–9401. [Google Scholar] [CrossRef]
- Sarma, P.P.; Rai, A.; Baruah, P.K. Recent Advances in the Development of Antibiotics-Coated Gold Nanoparticles to Combat Antimicrobial Resistance. Antibiotics 2024, 13, 124. [Google Scholar] [CrossRef]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the Next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Rastogi, L.; Kora, A.J.; J. , A. Highly Stable, Protein Capped Gold Nanoparticles as Effective Drug Delivery Vehicles for Amino-Glycosidic Antibiotics. Mater. Sci. Eng. : C 2012, 32, 1571–1577. [Google Scholar] [CrossRef]
- Pradeepa; Vidya, S. M.; Mutalik, S.; Udaya Bhat, K.; Huilgol, P.; Avadhani, K. Preparation of Gold Nanoparticles by Novel Bacterial Exopolysaccharide for Antibiotic Delivery. Life Sci. 2016, 153, 171–179. [Google Scholar] [CrossRef]
- Meeker, D.G.; Jenkins, S.V.; Miller, E.K.; Beenken, K.E.; Loughran, A.J.; Powless, A.; Muldoon, T.J.; Galanzha, E.I.; Zharov, V.P.; Smeltzer, M.S.; et al. Synergistic Photothermal and Antibiotic Killing of Biofilm-Associated Staphylococcus Aureus Using Targeted Antibiotic-Loaded Gold Nanoconstructs. ACS Infect. Dis. 2016, 2, 241–250. [Google Scholar] [CrossRef]
- Shaker, M.A.; Shaaban, M.I. Formulation of Carbapenems Loaded Gold Nanoparticles to Combat Multi-Antibiotic Bacterial Resistance: In Vitro Antibacterial Study. Int. J. Pharm. 2017, 525, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Majhi, R.K.; Singh, A.; Mishra, M.; Tiwari, A.; Chawla, S.; Guha, P.; Satpati, B.; Mohapatra, H.; Goswami, L.; et al. Carbohydrate-Coated Gold–Silver Nanoparticles for Efficient Elimination of Multidrug Resistant Bacteria and in Vivo Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 42998–43017. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Kim, G.-D. Eco-Friendly Approach for Nanoparticles Synthesis and Mechanism behind Antibacterial Activity of Silver and Anticancer Activity of Gold Nanoparticles. Appl Microbiol Biotechnol 2017, 101, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S. Anticancer Activity of Eco-Friendly Gold Nanoparticles against Lung and Liver Cancer Cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Hamida, R.S.; AlMotwaa, S.M.; Al-Otaibi, W.A.; Alqhtani, H.A.; Ali, M.A.; Bin-Meferij, M.M. Apoptotic Induction by Biosynthesized Gold Nanoparticles Using Phormidesmis Communis Strain AB_11_10 against Osteosarcoma Cancer. Biomedicines 2024, 12, 1570. [Google Scholar] [CrossRef]
- Martins, A.; Ferreira, B.C.; Gaspar, M.M.; Vieira, S.; Lopes, J.; Viana, A.S.; Paulo, A.; Mendes, F.; Campello, M.P.C.; Martins, R.; et al. Enhanced Cytotoxicity against a Pancreatic Cancer Cell Line Combining Radiation and Gold Nanoparticles. Pharmaceutics 2024, 16, 900. [Google Scholar] [CrossRef]
- Al-Mafarjy, S.S.; Suardi, N.; Ahmed, N.M.; Kernain, D.; Hisham Alkatib, H.; Dheyab, M.A. Green Synthesis of Gold Nanoparticles from Coleus Scutellarioides (L.) Benth Leaves and Assessment of Anticancer and Antioxidant Properties. Inorg. Chem. Commun. 2024, 161, 112052. [Google Scholar] [CrossRef]
- Leng, M.; Jiang, H.; Zhang, S.; Bao, Y. Green Synthesis of Gold Nanoparticles from Polygahatous Polysaccharides and Their Anticancer Effect on Hepatic Carcinoma through Immunoregulation. ACS Omega 2024, 9, 21144–21151. [Google Scholar] [CrossRef]
- Mathimaran, A.; Pandian, C.J.; Sappanimuthu, P.; Kirshnakumar, H.; Amala, M.; Veerapandiyan, M.; Kingsly, J.; Solomon, A.; Sonamuthu, J.; Jeyaraman, J. Synthesis of Multifunctional Silver Oxide, Zinc Oxide, Copper Oxide and Gold Nanoparticles for Enhanced Antibacterial Activity against ESKAPE Pathogens and Antioxidant, Anticancer Activities Using Momordica Cymbalaria Seed Extract. Mater. Today Commun. 2024, 39, 108838. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Upadhyay, P.; Sahal, D.; Kamaraj, C.; Thirugnanasambandam, R.; Siva, D.; Saravanan, D.; Regina Mary, R. Biosynthesis of Gold Nanoparticles Mediated by Medicinal Phytometabolites: An Effective Tool against Plasmodium Falciparum and Human Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2024, 95, 105520. [Google Scholar] [CrossRef]
- Ogwuche, C.E.; Elemike, E.E.; Oju, D.; Onwudiwe, D.C.; Singh, M.; Akpeji, B.H. Synthesis, Characterization, Anticancer and Antimicrobial Potentials of Chrysothemis Pulchella Leaf Extract Mediated Gold Nanoparticles. J Inorg Organomet Polym 2024, 34, 944–951. [Google Scholar] [CrossRef]
- Yeşildağ, A.; Kızıloğlu, H.T.; Dirican, E.; Erbaş, E.; Gelen, V.; Kara, A. Anticarcinogenic Effects of Gold Nanoparticles and Metformin Against MCF-7 and A549 Cells. Biol Trace Elem Res 2024, 202, 4494–4507. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, A.; Singh, S.P.; Singh, R.K.; Patel, A.K.; Verma, P.K.; Kumar, S.; Kumar, N.; Singh, V.; Wasnik, K.; et al. Synthesized Gold Nanoparticles with Moringa Oleifera Leaf Extract Induce Mitotic Arrest (G2/M Phase) and Apoptosis in Dalton’s Lymphoma Cells. Cell Biochem Biophys 2024, 82, 1043–1059. [Google Scholar] [CrossRef]
- Sun, B.; Hu, N.; Han, L.; Pi, Y.; Gao, Y.; Chen, K. Anticancer Activity of Green Synthesised Gold Nanoparticles from Marsdenia Tenacissima Inhibits A549 Cell Proliferation through the Apoptotic Pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4012–4019. [Google Scholar] [CrossRef]
- Huang, H.; Liu, R.; Yang, J.; Dai, J.; Fan, S.; Pi, J.; Wei, Y.; Guo, X. Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1868. [Google Scholar] [CrossRef]
- Ong, C.; Cha, B.G.; Kim, J. Mesoporous Silica Nanoparticles Doped with Gold Nanoparticles for Combined Cancer Immunotherapy and Photothermal Therapy. ACS Appl. Bio Mater. 2019, 2, 3630–3638. [Google Scholar] [CrossRef]
- Lin, A.Y.; Mattos Almeida, J.P.; Bear, A.; Liu, N.; Luo, L.; Foster, A.E.; Drezek, R.A. Gold Nanoparticle Delivery of Modified CpG Stimulates Macrophages and Inhibits Tumor Growth for Enhanced Immunotherapy. PLoS ONE 2013, 8, e63550. [Google Scholar] [CrossRef]
- He, X.; Zhang, S.; Tian, Y.; Cheng, W.; Jing, H. Research Progress of Nanomedicine-Based Mild Photothermal Therapy in Tumor. IJN 2023, Volume 18, 1433–1468. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Rahman, A.A.; Braim, F.S.; Mehrdel, B. Gold Nanoparticles-Based Photothermal Therapy for Breast Cancer. Photodiagnosis Photodyn. Ther. 2023, 42, 103312. [Google Scholar] [CrossRef]
- Bhatia, P.; Verma, S.S. Enhancement of LSPR Properties of Temperature-Dependent Gold Nanoparticles. Mater. Today: Proc. 2023, 78, 871–876. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Inaam, R.; Okamoto, S.; Shibata, T.; Santra, T.S.; Nagai, M. Visible Pulsed Laser-Assisted Selective Killing of Cancer Cells with PVP-Capped Plasmonic Gold Nanostars. Micromachines 2023, 14, 1173. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, S.; Sun, J.; Huang, T.; Lin, H.; Li, Z.; Xiao, Z.; Lu, W. Au@CuS Nanoshells for Surface-Enhanced Raman Scattering Image-Guided Tumor Photothermal Therapy with Accelerated Hepatobiliary Excretion. Pharmaceutics 2024, 16, 1089. [Google Scholar] [CrossRef]
- Yan, X.; Li, K.; Xie, T.; Jin, X.; Zhang, C.; Li, Q.; Feng, J.; Liu, C.; Zhang, X. Bioorthogonal “Click and Release” Reaction-Triggered Aggregation of Gold Nanoparticles Combined with Released Lonidamine for Enhanced Cancer Photothermal Therapy. Angew Chem Int Ed 2024, 63, e202318539. [Google Scholar] [CrossRef]
- Wang, R.; Xue, L.; Dong, X.; Yan, W.; Li, Y. Chitosan-Initiated Gold Nanoparticles with Enhanced Fluorescence for Unique Fe3+/PPi Sensing and Photothermal Therapy. Talanta 2024, 271, 125719. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Bao, Y.; Shen, H.; Fan, M.; Wang, Y.; Xiang, S.; Ran, X. Nucleic Acid-Functionalized Gold Nanoparticles as Intelligent Photothermal Therapy Agents for Precise Cancer Treatment. Nanotechnology 2024, 35, 465101. [Google Scholar] [CrossRef]
- Xu, Y.; He, P.; Gu, G.; Zhu, D.; Luan, X.; Mu, R.; Wei, G. Gold Nanoparticles-Modified 2D Self-Assembled Amphiphilic Peptide Nanosheets with High Biocompatibility and Photothermal Therapy Efficiency. Macromol. Rapid Commun. 2024, 2400386. [Google Scholar] [CrossRef]
- Erdoğan, H.; Bacanlı, M.G.; Karayavuz, B.; Eşim, Ö.; Sarper, M.; Erdem, O.; Özkan, Y. Plasmonic Photothermal Therapy Based Synergistic Drug Release Application through Gold Nanoparticles Coated and Embedded PLGA Nanoparticles. J. Drug Deliv. Sci. Technol. 2024, 97, 105794. [Google Scholar] [CrossRef]
- Nouizi, F.; Algarawi, M.; Erkol, H.; Gulsen, G. Gold Nanoparticle-Mediated Photothermal Therapy Guidance with Multi-Wavelength Photomagnetic Imaging. Photodiagnosis Photodyn. Ther. 2024, 45, 103956. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; Rashidzadeh, H.; Mohammadi, A.; Mousazadeh, N.; Barsbay, M.; Sharafi, A.; Gharbavi, M.; Danafar, H.; Javani, S. Photothermal and Radiotherapy with Alginate-Coated Gold Nanoparticles for Breast Cancer Treatment. Sci Rep 2024, 14, 13299. [Google Scholar] [CrossRef] [PubMed]
- Darvish, S.; Budala, D.-G.; Goriuc, A. Antibacterial Properties of an Experimental Dental Resin Loaded with Gold Nanoshells for Photothermal Therapy Applications. JFB 2024, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Hassibian, S.; Taghdisi, S.M.; Jamshidi, Z.; Samie, A.; Alinezhad Nameghi, M.; Shayan, M.; Farrokhi, N.; Alibolandi, M.; Ramezani, M.; Dehnavi, S.M.; et al. Surface Modification of Hollow Gold Nanoparticles Conducted by Incorporating Cancer Cell Membrane and AS1411 Aptamer, Aiming to Achieve a Dual-Targeted Therapy for Colorectal Cancer. Int. J. Pharm. 2024, 655, 124036. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold Nanoparticle Based Photothermal Therapy: Development and Application for Effective Cancer Treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape Effects of Gold Nanoparticles in Photothermal Cancer Therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Depciuch, J.; Stec, M.; Maximienko, A.; Baran, J.; Parlinska-Wojtan, M. Size-Dependent Theoretical and Experimental Photothermal Conversion Efficiency of Spherical Gold Nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 39, 102979. [Google Scholar] [CrossRef]
- García Calavia, P.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-Gold Nanoparticle Conjugates for Photodynamic Therapy of Cancer. Photochem Photobiol Sci 2018, 17, 1534–1552. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Zheng, W.; Cong, L.; Guo, Z.; Xie, Y.; Wang, L.; Tang, R.; Feng, Q.; Hamada, Y.; et al. Thermo-triggered Release of CRISPR-Cas9 System by Lipid-Encapsulated Gold Nanoparticles for Tumor Therapy. Angew Chem Int Ed 2018, 57, 1491–1496. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, W.; Xiang, H.; Chen, Y.; Xu, H. CRISPR/Cas9-Based Genome Editing for Multimodal Synergistic Cancer Nanotherapy. Nano Today 2023, 48, 101734. [Google Scholar] [CrossRef]
- Tao, W.; Cheng, X.; Sun, D.; Guo, Y.; Wang, N.; Ruan, J.; Hu, Y.; Zhao, M.; Zhao, T.; Feng, H.; et al. Synthesis of Multi-Branched Au Nanocomposites with Distinct Plasmon Resonance in NIR-II Window and Controlled CRISPR-Cas9 Delivery for Synergistic Gene-Photothermal Therapy. Biomaterials 2022, 287, 121621. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, M.; Abbas, G.; Li, C.; Cui, M.; Zhang, X.; Wang, D. A Cancer Cell Membrane-Derived Biomimetic Nanocarrier for Synergistic Photothermal/Gene Therapy by Efficient Delivery of CRISPR/Cas9 and Gold Nanorods. Adv Healthc. Mater. 2022, 11, 2201038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, Y.; Wu, Q.; Gong, C. Nanotechnology-based CRISPR/Cas9 Delivery System for Genome Editing in Cancer Treatment. MedComm – Biomaterials and Applications 2024, 3, e70. [Google Scholar] [CrossRef]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold Nanoparticle-Mediated Photothermal Therapy: Current Status and Future Perspective. Nanomed. (Lond.) 2014, 9, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. The Mechanisms Tumor Cells Utilize to Evade the Host’s Immune System. Maturitas 2017, 105, 8–15. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, T.; Qin, X.; Qiao, Q.; Shang, L.; Song, Q.; Yang, C.; Zhang, Z. Intracellularly Generated Immunological Gold Nanoparticles for Combinatorial Photothermal Therapy and Immunotherapy against Tumor. Nano Lett. 2019, 19, 6635–6646. [Google Scholar] [CrossRef]
- Kim, H.; Baek, Y.; Ha, T.; Choi, D.; Lee, W.J.; Cho, Y.; Park, J.; Kim, S.; Doh, J. Gold Nanoparticle-Carrying T Cells for the Combined Immuno-Photothermal Therapy. Small 2023, 19, 2301377. [Google Scholar] [CrossRef]
- Hsieh, H.-H.; Chen, C.-L.; Chan, H.-W.; Chi, K.-H.; Wu, C.-Y. Enhanced Antitumour Response of Gold Nanostar-Mediated Photothermal Therapy in Combination with Immunotherapy in a Mouse Model of Colon Carcinoma. Br J Cancer 2024, 130, 406–416. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal Therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Tarighatnia, A.; Barar, J.; Aghanejad, A.; Davaran, S. Recent Advances and Trends in Nanoparticles Based Photothermal and Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2022, 37, 102697. [Google Scholar] [CrossRef]
- Didamson, O.C.; Chandran, R.; Abrahamse, H. A Gold Nanoparticle Bioconjugate Delivery System for Active Targeted Photodynamic Therapy of Cancer and Cancer Stem Cells. Cancers 2022, 14, 4558. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J. Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment. J. Nanosci. Nanotech. 2006, 6, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Prokopovich, P.; Pratten, J.; Parkin, I.P.; Wilson, M. Nanoparticles: Their Potential Use in Antibacterial Photodynamic Therapy. Photochem Photobiol Sci 2011, 10, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Dediu, V.; Ghitman, J.; Gradisteanu Pircalabioru, G.; Chan, K.H.; Iliescu, F.S.; Iliescu, C. Trends in Photothermal Nanostructures for Antimicrobial Applications. IJMS 2023, 24, 9375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-Based Photothermal Therapy and Its Potentials in Antibacterial Treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Maliszewska, I.; Zdubek, A. On the Photo-Eradication of Methicillin-Resistant Staphylococcus Aureus Biofilm Using Methylene Blue. IJMS 2023, 24, 791. [Google Scholar] [CrossRef]
- Yeshchenko, O.; Khort, P.; Fedotov, O.; Chumachenko, V.; Virych, P.; Warren, H.S.; Booth, B.W.; Bliznyuk, V.; Kutsevol, N. Third-Generation Anticancer Photodynamic Therapy Systems Based on Star-like Anionic Polyacrylamide Polymer, Gold Nanoparticles, and Temoporfin Photosensitizer. Molecules 2024, 29, 2224. [Google Scholar] [CrossRef]
- Ziental, D.; Błaszkiewicz, P.; Długaszewska, J.; Güzel, E.; Dudkowiak, A.; Sobotta, L. Modified Gold Nanoparticles Modulated Fluorescence and Singlet Oxygen Generation of Pheophorbide a as an Effective Platform for Photodynamic Therapy against Staphylococcus Aureus. Eur J Inorg Chem 2024, 27, e202300668. [Google Scholar] [CrossRef]
- A․N․, R.; S․, S.; C․R․, R.; Papasouli, E.; Kunnumpurathu, J.; Praveen, C.S.; Koukaras, E.N.; Rerat, M.; Karamanis, P.; Jayasree, R.S. Nanoarchitectonics of Fluorescent Gold Nanoclusters: A Platform for Image Guided Photodynamic Therapy of Hypoxic Tumor. Appl. Mater. Today 2024, 39, 102273. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C.; Xu, P.; Li, Y.; Yan, X.; Guo, X.; Wen, C.; Shen, X. Biomimetic Gold Nanorods-Manganese Porphyrins with Surface-Enhanced Raman Scattering Effect for Photoacoustic Imaging-Guided Photothermal/Photodynamic Therapy. Small 2024, 2401117. [Google Scholar] [CrossRef]
- Nomani, A.; Nosrati, H.; Faraji, N.; Charmi, J.; Javani, S. Bismuth-Gold Nanohybrid Based Nano Photosensitizer to Combat Antimicrobial Resistance. Sci Rep 2024, 14, 22598. [Google Scholar] [CrossRef]
- Sangaletti, P.; Bergmann, E.V.; Vieira, G.N.; Horn Jr, A.; Malacarne, L.C.; Zanotto-Filho, A.; Gerola, A.P. N-(2-Hydroxyl)Propyl-3-Trimethyl Ammonium Chitosan-Coated Gold Nanoparticle-Based Platform for Photothermal/Chemo-Photodynamic Therapy. J. Mol. Liq. 2024, 399, 124358. [Google Scholar] [CrossRef]
- Nene, L.C.; Nkune, N.W.; Abrahamse, H. Anticancer Photodynamic Activities of Triphenylphosphine-Labelled Phthalocyanines and Their Bovine Serum Albumin-Gold Nanoparticles- Complexes on Melanoma A375 Cell Lines in Vitro. J. Inorg. Biochem. 2024, 256, 112570. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-J. Methylene Blue-Based Nano and Microparticles: Fabrication and Applications in Photodynamic Therapy. Polymers 2021, 13, 3955. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, I.; Wanarska, E.; Thompson, A.C.; Samuel, I.D.W.; Matczyszyn, K. Biogenic Gold Nanoparticles Decrease Methylene Blue Photobleaching and Enhance Antimicrobial Photodynamic Therapy. Molecules 2021, 26, 623. [Google Scholar] [CrossRef]
- Lavaee, F.; Motamedifar, M.; Rafiee, G. The Effect of Photodynamic Therapy by Gold Nanoparticles on Streptococcus Mutans and Biofilm Formation: An in Vitro Study. Lasers Med Sci 2021, 37, 1717–1725. [Google Scholar] [CrossRef]
- Braga, T.L.; Conrado, P.C.V.; Silva, L.G.Z.; Bergmann, E.V.; Da Silva, A.C.P.; De Souza Bonfim De Mendonça, P.; Mikcha, J.M.G.; Herculano, L.S.; Malacarne, L.C.; I Muniz, E.C.; et al. Pluronic® P-123 as a Reductant and Stabilizing Agent for Gold Nanoparticles (AuNPs) Combined with Methylene Blue for Photodynamic and Photothermal Therapy. J. Mol. Liq. 2023, 383, 122111. [Google Scholar] [CrossRef]
- Khan, A. ; Khan; Azam; Alam Gold Nanoparticles Enhance Methylene Blue– Induced Photodynamic Therapy: A Novel Therapeutic Approach to Inhibit Candida Albicans Biofilm. IJN 2012, 3245. [Google Scholar] [CrossRef]
- Maliszewska, I.; Lisiak, B.; Popko, K.; Matczyszyn, K. Enhancement of the Efficacy of Photodynamic Inactivation of Candida Albicans with the Use of Biogenic Gold Nanoparticles. Photochem Photobiol. 2017, 93, 1081–1090. [Google Scholar] [CrossRef]
- Soares, J.C.M.; Luiz, M.T.; Oshiro Junior, J.A.; Besegato, J.F.; De Melo, P.B.G.; Rastelli, A.N.D.S.; Chorilli, M. Antimicrobial Photodynamic Therapy Mediated by Methylene Blue-Loaded Polymeric Micelles against Streptococcus Mutans and Candida Albicans Biofilms. Photodiagnosis Photodyn. Ther. 2023, 41, 103285. [Google Scholar] [CrossRef]
- Rodrigues, C.H.; Silva, B.P.; Silva, M.L.R.; Gouveia, D.C.; Fontes, A.; Macêdo, D.P.C.; Santos, B.S. Methylene Blue@silver Nanoprisms Conjugates as a Strategy against Candida Albicans Isolated from Balanoposthitis Using Photodynamic Inactivation. Photodiagnosis Photodyn. Ther. 2024, 46, 104066. [Google Scholar] [CrossRef]
- Mokoena, D.; George, B.P.; Abrahamse, H. Conjugation of Hypericin to Gold Nanoparticles for Enhancement of Photodynamic Therapy in MCF-7 Breast Cancer Cells. Pharmaceutics 2022, 14, 2212. [Google Scholar] [CrossRef] [PubMed]
- Saw, W.S.; Anasamy, T.; Do, T.T.A.; Lee, H.B.; Chee, C.F.; Isci, U.; Misran, M.; Dumoulin, F.; Chong, W.Y.; Kiew, L.V.; et al. Nanoscaled PAMAM Dendrimer Spacer Improved the Photothermal‒Photodynamic Treatment Efficiency of Photosensitizer-Decorated Confeito-Like Gold Nanoparticles for Cancer Therapy. Macromol. Biosci. 2022, 22, 2200130. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Xiong, G.; Li, Z.; Jiang, Q.; Yin, J.; Yin, T.; Zheng, H. Gold Nanoparticle-Based Nanoprobes with Enhanced Tumor Targeting and Photothermal/Photodynamic Response for Therapy of Osteosarcoma. Nanotechnology 2021, 32, 155102. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Gold Nanoparticles: Synthesis Methods, Functionalization and Biological Applications. J Clust Sci 2023, 34, 705–725. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold Nanoparticle-Based Biosensors. Gold Bull 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Cao, X.; Ye, Y.; Liu, S. Gold Nanoparticle-Based Signal Amplification for Biosensing. Anal. Biochem. 2011, 417, 1–16. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Jouyban, A.; Khoubnasabjafari, M.; Jouyban-Gharamaleki, V.; Rahimpour, E. Quantification of Morphine in Exhaled Breath Condensate Using a Double Network Polymeric Hybrid Hydrogel Functionalized with AuNPs. BMC Chem. 2024, 18, 175. [Google Scholar] [CrossRef]
- Hsu, W.-T.; Lin, Y.-C.; Yang, H.-C.; Barshilia, D.; Chen, P.-L.; Huang, F.-C.; Chau, L.-K.; Hsieh, W.-H.; Chang, G.-E. Label-Free Biosensor Based on Particle Plasmon Resonance Coupled with Diffraction Grating Waveguide. Sensors 2024, 24, 5536. [Google Scholar] [CrossRef]
- Wen, C.; Dou, Y.; Liu, Y.; Jiang, X.; Tu, X.; Zhang, R. Au Nanoshell-Based Lateral Flow Immunoassay for Colorimetric and Photothermal Dual-Mode Detection of Interleukin-6. Molecules 2024, 29, 3683. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, G.; Cui, J.; Jiang, Y.; Li, X.; Wang, Z.; Zhou, X. The Efficient and Sensitive Detection of Serum Dopamine Based on a MOF-199/Ag@Au Composite SERS Sensing Structure. Chemosensors 2024, 12, 187. [Google Scholar] [CrossRef]
- Kumar, P.P.P. A Multimode Detection Platform for Biothiols Using BODIPY Dye-Conjugated Gold Nanoparticles. Colorants 2024, 3, 214–228. [Google Scholar] [CrossRef]
- San Juan, A.M.; Jaitpal, S.; Ng, K.W.; Martinez, C.; Tripathy, S.; Phillips, C.; Coté, G.L.; Mabbott, S. Freeze-Driven Synthesis of DNA Hairpin-Conjugated Gold Nanoparticle Biosensors for Dual-Mode Detection. ACS Appl. Bio Mater. 2024, 7, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, D.; Chen, X.; Wu, X. Stress Response of Citrus Leaves under Mechanical Damage and Huanglongbing Disease Infection Using Plasmonic TiO2 Nanotube Substrate-Based Imprinting Mass Spectrometry Imaging. Agronomy 2024, 14, 1797. [Google Scholar] [CrossRef]
- Lopez-Benitez, K.; Alcazar-Gonzalez, P.; El Qassim, L.A.; Fernandez-Argüelles, M.T.; Vicente, F.; Royo, L.J.; Menendez-Miranda, M. Development of a Gold Nanoparticle-Based Sensor for Authentication of Organic Milk Based on Differential Levels of miRNA. Nanomaterials 2024, 14, 1364. [Google Scholar] [CrossRef]
- Llano-Suárez, P.; Sánchez-Visedo, A.; Ortiz-Gómez, I.; Fernández-Argüelles, M.T.; Prado, M.; Costa-Fernández, J.M.; Soldado, A. Sesame Detection in Food Using DNA-Functionalized Gold Nanoparticles: A Sensitive, Rapid, and Cost-Effective Colorimetric Approach. Biosensors 2024, 14, 377. [Google Scholar] [CrossRef]
- Sun, B.; Zheng, C.; Pan, D.; Shen, L.; Zhang, W.; Chen, X.; Wen, Y.; Shi, Y. Using AuNPs-DNA Walker with Fluorophores Detects the Hepatitis Virus Rapidly. Biosensors 2024, 14, 370. [Google Scholar] [CrossRef]
- Clack, K.; Sallam, M.; Matheson, C.; Muyldermans, S.; Nguyen, N.-T. Towards a Wearable Feminine Hygiene Platform for Detection of Invasive Fungal Pathogens via Gold Nanoparticle Aggregation. Micromachines 2024, 15, 899. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, W.; Chen, X.; Li, J.; Ye, H.; Li, C.; Yin, X.; Zhou, X.; Qiao, X.; Xue, Z.; et al. Gold Nanoparticle-Bridge Array to Improve DNA Hybridization Efficiency of SERS Sensors. J. Am. Chem. Soc. 2022, 144, 17533–17539. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A. Gold Nanostars-Diagnosis, Bioimaging and Biomedical Applications. Drug Metab. Rev. 2020, 52, 299–318. [Google Scholar] [CrossRef]
- Meng, X.; Dyer, J.; Huo, Y.; Jiang, C. Greater SERS Activity of Ligand-Stabilized Gold Nanostars with Sharp Branches. Langmuir 2020, 36, 3558–3564. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Dong, X.; Huang, Q. A Review: Research Progress of SERS-Based Sensors for Agricultural Applications. Trends Food Sci. Technol. 2022, 128, 90–101. [Google Scholar] [CrossRef]
- Duan, L.; Liu, X.; Meng, X.; Qu, L. Highly Sensitive SERS Detection of Pesticide Residues Based on Multi-Hotspot Buckypaper Modified with Gold Nanoparticles. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2024, 308, 123665. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, Y.; Zhang, S.; Wei, S.; Ming, A.; Mao, C. Hydrophobic Wafer-Scale High-Reproducibility SERS Sensor Based on Silicon Nanorods Arrays Decorated with Au Nanoparticles for Pesticide Residue Detection. Biosensors 2022, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Han, E.; Yin, L.; Xu, Q.; Zou, C.; Bai, J.; Wu, W.; Cai, J. Simultaneous Detection of Mixed Pesticide Residues Based on Portable Raman Spectrometer and Au@Ag Nanoparticles SERS Substrate. Food Control 2023, 153, 109951. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Xu, C.; Liu, D. SERS Tags for Biomedical Detection and Bioimaging. Theranostics 2022, 12, 1870–1903. [Google Scholar] [CrossRef]
- You, S.-M.; Luo, K.; Jung, J.-Y.; Jeong, K.-B.; Lee, E.-S.; Oh, M.-H.; Kim, Y.-R. Gold Nanoparticle-Coated Starch Magnetic Beads for the Separation, Concentration, and SERS-Based Detection of E. Coli O157:H7. ACS Appl. Mater. Interfaces 2020, 12, 18292–18300. [Google Scholar] [CrossRef]
- Nam, W.; Kim, W.; Zhou, W.; You, E.-A. A Digital SERS Sensing Platform Using 3D Nanolaminate Plasmonic Crystals Coupled with Au Nanoparticles for Accurate Quantitative Detection of Dopamine. Nanoscale 2021, 13, 17340–17349. [Google Scholar] [CrossRef]
- Anh, N.H.; Doan, M.Q.; Dinh, N.X.; Huy, T.Q.; Tri, D.Q.; Ngoc Loan, L.T.; Van Hao, B.; Le, A.-T. Gold Nanoparticle-Based Optical Nanosensors for Food and Health Safety Monitoring: Recent Advances and Future Perspectives. RSC Adv. 2022, 12, 10950–10988. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Pellas, V.; Hu, D.; Mazouzi, Y.; Mimoun, Y.; Blanchard, J.; Guibert, C.; Salmain, M.; Boujday, S. Gold Nanorods for LSPR Biosensing: Synthesis, Coating by Silica, and Bioanalytical Applications. Biosensors 2020, 10, 146. [Google Scholar] [CrossRef]
- Gao, J.; Yang, W.; Liu, R.; Feng, J.; Li, Y.; Jiang, M.; Jiang, S. A Reliable Gold Nanoparticle/Cu-TCPP 2D MOF/Gold/D-Shaped Fiber Sensor Based on SPR and LSPR Coupling for Dopamine Detection. Appl. Surf. Sci. 2024, 655, 159523. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Yang, S.; Xie, Q.; Danzeng, Q.; Liu, C.; Zhou, C.-H. Integrating Carbon Dots and Gold/Silver Core-Shell Nanoparticles to Achieve Sensitive Detection of Dopamine with Fluorometric/Colorimetric Dual Signal. Anal Bioanal Chem 2024, 416, 4951–4960. [Google Scholar] [CrossRef] [PubMed]
- Do, P.Q.T.; Huong, V.T.; Phuong, N.T.T.; Nguyen, T.-H.; Ta, H.K.T.; Ju, H.; Phan, T.B.; Phung, V.-D.; Trinh, K.T.L.; Tran, N.H.T. The Highly Sensitive Determination of Serotonin by Using Gold Nanoparticles (Au NPs) with a Localized Surface Plasmon Resonance (LSPR) Absorption Wavelength in the Visible Region. RSC Adv. 2020, 10, 30858–30869. [Google Scholar] [CrossRef] [PubMed]
- Mirshekari, H.; Dabirmanesh, B.; Daneshjou, S.; Khajeh, K. Fabrication and Evaluation of a Plasmonic Biosensor Based on Silica-Coated Gold Nanorods for Highly-Sensitive Detection of Anti-Müllerian Hormone. Colloid Interface Sci. Commun. 2024, 61, 100795. [Google Scholar] [CrossRef]
- Farooq, S.; Wali, F.; Zezell, D.M.; De Araujo, R.E.; Rativa, D. Optimizing and Quantifying Gold Nanospheres Based on LSPR Label-Free Biosensor for Dengue Diagnosis. Polymers 2022, 14, 1592. [Google Scholar] [CrossRef]
- Apaydın, B.B.; Çamoğlu, T.; Canbek Özdil, Z.C.; Gezen-Ak, D.; Ege, D.; Gülsoy, M. Chitosan-enhanced Sensitivity of Mercaptoundecanoic Acid (MUA)- Capped Gold Nanorod Based Localized Surface Plasmon Resonance (LSPR) Biosensor for Detection of Alpha-synuclein Oligomer Biomarker in Parkinson’s Disease. Biotech App Biochem, 2653. [Google Scholar] [CrossRef]
- Mutalik, C.; Saukani, M.; Khafid, M.; Krisnawati, D.I.; Widodo; Darmayanti, R. ; Puspitasari, B.; Cheng, T.-M.; Kuo, T.-R. Gold-Based Nanostructures for Antibacterial Application. IJMS 2023, 24, 10006. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and Antibacterial Properties of Gold Nanoparticles: A Review. Env. Chem Lett 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Ge, P.; Zhang, J.; Ding, T.; Xianyu, Y. Surface Chemistry of Gold Nanoparticles for Bacterial Detection and Antimicrobial Applications. ACS Mater. Lett. 2023, 5, 638–655. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Olugbeko, S.C.; Folorunso, A.S. A Review on Synthesis, Optimization, Characterization and Antibacterial Application of Gold Nanoparticles Synthesized from Plants. Int Nano Lett 2020, 10, 237–248. [Google Scholar] [CrossRef]
- Tian, E.-K.; Wang, Y.; Ren, R.; Zheng, W.; Liao, W. Gold Nanoparticle: Recent Progress on Its Antibacterial Applications and Mechanisms. J. Nanomater. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Oladzadabbasabadi, N.; Alsaedi, A.; Braim, F.S.; Jameel, M.S.; Ramizy, A.; Alrosan, M.; Almajwal, A.M. Comparative Analysis of Stable Gold Nanoparticles Synthesized Using Sonochemical and Reduction Methods for Antibacterial Activity. Molecules 2023, 28, 3931. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi-Ahodashti, M.; Mizwari, Z.M.; Mohammadi-Aghdam, S.; Ahmadi, S.; Ali Ebrahimzadeh, M.; Mortazavi-Derazkola, S. Optimization and Evaluation of Anticancer, Antifungal, Catalytic, and Antibacterial Activities: Biosynthesis of Spherical-Shaped Gold Nanoparticles Using Pistacia Vera Hull Extract (AuNPs@PV). Arab. J. Chem. 2023, 16, 104423. [Google Scholar] [CrossRef]
- Soliman, M.K.Y.; Salem, S.S.; Abu-Elghait, M.; Azab, M.S. Biosynthesis of Silver and Gold Nanoparticles and Their Efficacy Towards Antibacterial, Antibiofilm, Cytotoxicity, and Antioxidant Activities. Appl Biochem Biotechnol 2023, 195, 1158–1183. [Google Scholar] [CrossRef]
- Obaid, A.S.; Hassan, K.T.; Hassan, O.M.; Ali, H.H.; Ibraheem, I.J.; Salih, T.A.; Adil, B.H.; Almoneef, M.M. In-Vitro Antibacterial, Cytotoxicity, and Anti-Prostate Cancer Effects of Gold Nanoparticles Synthesized Using Extract of Desert Truffles (Tirmania Nivea). Mater. Chem. Phys. 2023, 301, 127673. [Google Scholar] [CrossRef]
- Umamaheswari, K.; Abirami, M. Assessment of Antifungal Action Mechanism of Green Synthesized Gold Nanoparticles (AuNPs) Using Allium Sativum on Candida Species. Mater. Lett. 2023, 333, 133616. [Google Scholar] [CrossRef]
- Alqurashi, Y.E.; Almalki, S.G.; Ibrahim, I.M.; Mohammed, A.O.; Abd El Hady, A.E.; Kamal, M.; Fatima, F.; Iqbal, D. Biological Synthesis, Characterization, and Therapeutic Potential of S. Commune-Mediated Gold Nanoparticles. Biomolecules 2023, 13, 1785. [Google Scholar] [CrossRef]
- Babaei, A.; Mousavi, S.M.; Ghasemi, M.; Pirbonyeh, N.; Soleimani, M.; Moattari, A. Gold Nanoparticles Show Potential in Vitro Antiviral and Anticancer Activity. Life Sci. 2021, 284, 119652. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral Activity of Algae Biosynthesized Silver and Gold Nanoparticles against Herps Simplex (HSV-1) Virus in Vitro Using Cell-Line Culture Technique. Int. J. Environ. Health Res. 2022, 32, 616–627. [Google Scholar] [CrossRef]
- Paradowska, E.; Studzińska, M.; Jabłońska, A.; Lozovski, V.; Rusinchuk, N.; Mukha, I.; Vitiuk, N.; Leśnikowski, Z.J. Antiviral Effect of Nonfunctionalized Gold Nanoparticles against Herpes Simplex Virus Type-1 (HSV-1) and Possible Contribution of Near-Field Interaction Mechanism. Molecules 2021, 26, 5960. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Fayaz, M.; Hosseini-Doabi, F.; Rezaei, P. The Application of Green Synthesis Nanoparticles in Wound Healing: A Review. Mater. Today Sustain. 2023, 21, 100272. [Google Scholar] [CrossRef]
- Gowda, B.H.J.; Mohanto, S.; Singh, A.; Bhunia, A.; Abdelgawad, M.A.; Ghosh, S.; Ansari, M.J.; Pramanik, S. Nanoparticle-Based Therapeutic Approaches for Wound Healing: A Review of the State-of-the-Art. Mater. Today Chem. 2023, 27, 101319. [Google Scholar] [CrossRef]
- Ovais, M.; Ahmad, I.; Khalil, A.T.; Mukherjee, S.; Javed, R.; Ayaz, M.; Raza, A.; Shinwari, Z.K. Wound Healing Applications of Biogenic Colloidal Silver and Gold Nanoparticles: Recent Trends and Future Prospects. Appl Microbiol Biotechnol 2018, 102, 4305–4318. [Google Scholar] [CrossRef] [PubMed]
- Subbulakshmi, A.; Durgadevi, S.; Anitha, S.; Govarthanan, M.; Biruntha, M.; Rameshthangam, P.; Kumar, P. Biogenic Gold Nanoparticles from Gelidiella Acerosa: Bactericidal and Photocatalytic Degradation of Two Commercial Dyes. Appl Nanosci 2023, 13, 4033–4042. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound Healing and Antimicrobial Effect of Active Secondary Metabolites in Chitosan-Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Fadel, B.A.; Elwakil, B.H.; Fawzy, E.E.; Shaaban, M.M.; Olama, Z.A. Nanoemulsion of Lavandula Angustifolia Essential Oil/Gold Nanoparticles: Antibacterial Effect against Multidrug-Resistant Wound-Causing Bacteria. Molecules 2023, 28, 6988. [Google Scholar] [CrossRef]
- Das, G.; Seo, S.; Yang, I.-J.; Nguyen, L.T.H.; Shin, H.-S.; Patra, J.K. Synthesis of Biogenic Gold Nanoparticles by Using Sericin Protein from Bombyx Mori Silk Cocoon and Investigation of Its Wound Healing, Antioxidant, and Antibacterial Potentials. IJN 2023, Volume 18, 17–34. [Google Scholar] [CrossRef]
- Aili, M.; Zhou, K.; Zhan, J.; Zheng, H.; Luo, F. Anti-Inflammatory Role of Gold Nanoparticles in the Prevention and Treatment of Alzheimer’s Disease. J. Mater. Chem. B 2023, 11, 8605–8621. [Google Scholar] [CrossRef]
- Hornos Carneiro, M.F.; Barbosa, F. Gold Nanoparticles: A Critical Review of Therapeutic Applications and Toxicological Aspects. J. Toxicol. Environ. Health Part B 2016, 19, 129–148. [Google Scholar] [CrossRef]
- Gao, L.; Mei, S.; Ma, H.; Chen, X. Ultrasound-Assisted Green Synthesis of Gold Nanoparticles Using Citrus Peel Extract and Their Enhanced Anti-Inflammatory Activity. Ultrason. Sonochemistry 2022, 83, 105940. [Google Scholar] [CrossRef]
- Dhandapani, S.; Wang, R.; Cheol Hwang, K.; Kim, H.; Kim, Y.-J. Enhanced Skin Anti-Inflammatory and Moisturizing Action of Gold Nanoparticles Produced Utilizing Diospyros Kaki Fruit Extracts. Arab. J. Chem. 2023, 16, 104551. [Google Scholar] [CrossRef]
- Sekar, V.; Al-Ansari, M.M.; Narenkumar, J.; Al-Humaid, L.; Arunkumar, P.; Santhanam, A. Synthesis of Gold Nanoparticles (AuNPs) with Improved Anti-Diabetic, Antioxidant and Anti-Microbial Activity from Physalis Minima. J. King Saud Univ. - Sci. 2022, 34, 102197. [Google Scholar] [CrossRef]
- Kiran, M.S.; Rajith Kumar, C.R.; Shwetha, U.R.; Onkarappa, H.S.; Betageri, V.S.; Latha, M.S. Green Synthesis and Characterization of Gold Nanoparticles from Moringa Oleifera Leaves and Assessment of Antioxidant, Antidiabetic and Anticancer Properties. Chem. Data Collect. 2021, 33, 100714. [Google Scholar] [CrossRef]
- Lin, Q.; Qiu, C.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Tian, Y.; Jin, Z. The Inhibitory Mechanism of Amylase Inhibitors and Research Progress in Nanoparticle-Based Inhibitors. Crit. Rev. Food Sci. Nutr. 2023, 63, 12126–12135. [Google Scholar] [CrossRef]
- Azmy, L.; Al-Olayan, E.; Abdelhamid, M.A.A.; Zayed, A.; Gheda, S.F.; Youssif, K.A.; Abou-Zied, H.A.; Abdelmohsen, U.R.; Ibraheem, I.B.M.; Pack, S.P.; et al. Antimicrobial Activity of Arthrospira Platensis-Mediated Gold Nanoparticles against Streptococcus Pneumoniae: A Metabolomic and Docking Study. IJMS 2024, 25, 10090. [Google Scholar] [CrossRef]
- Kalińska, A.; Wawryło, C.; Tlatlik, W.; Gołębiewski, M.; Kot, M.; Lange, A.; Jaworski, S. Preliminary In Vitro Evaluation of Silver, Copper and Gold Nanoparticles as New Antimicrobials for Pathogens That Induce Bovine Locomotion Disorders. IJMS 2024, 25, 9494. [Google Scholar] [CrossRef]
- Rambau, U.; Masevhe, N.A.; Samie, A. Green Synthesis of Gold and Copper Nanoparticles by Lannea Discolor: Characterization and Antibacterial Activity. Inorganics 2024, 12, 36. [Google Scholar] [CrossRef]
- Gómez-Gómez, A.L.; Martínez-Ayala, A.L.; Corea-Ventura, P.; Stasiewicz, M.J.; De Mejia, E.G.; Dávila-Ortiz, G. Green Synthesis of Gold Nanoparticles by Curcin from Jatropha Curcas: Characterization and Antibacterial Activity. MRS Adv. 2024, 9, 254–258. [Google Scholar] [CrossRef]
- Parmar, M.; Sanyal, M. Biosynthesis of Gold Nanoparticles Using Aqueous Extract of Ricinus Cummunis Leaves to Augment Catalytic Degradation of Organic Dyes and Study of Its Antifungal and Antibacterial Activities. Particuology 2024, 87, 87–98. [Google Scholar] [CrossRef]
- Khan, S.; Rauf, A.; Aljohani, A.S.M.; Al-Awthan, Y.S.; Ahmad, Z.; Bahattab, O.S.; Khan, S.; Saadiq, M.; Khan, S.A.; Thiruvengadam, R.; et al. Green Synthesis of Silver and Gold Nanoparticles in Callistemon Viminalis Extracts and Their Antimicrobial Activities. Bioprocess Biosyst Eng 2024, 47, 1197–1211. [Google Scholar] [CrossRef]
- Al Azzam, S.; Ullah, Z.; Azmi, S.; Islam, M.; Ahmad, I.; Hussain, M.K. Tricyclic Microwave-Assisted Synthesis of Gold Nanoparticles for Biomedical Applications: Combatting Multidrug-Resistant Bacteria and Fungus. Beni-Suef Univ J Basic Appl Sci 2024, 13, 53. [Google Scholar] [CrossRef]
- Nikhil, A.; Tiwari, A.K.; Tilak, R.; Kumar, S.; Bharti, P.S.; Pandey, P.C.; Narayan, R.J.; Gupta, M.K. Vancomycin-Conjugated Polyethyleneimine-Stabilized Gold Nanoparticles Attenuate Germination and Show Potent Antifungal Activity against Aspergillus Spp. Appl. Sci. 2024, 14, 6926. [Google Scholar] [CrossRef]
- Khedr, W.E.; Shaheen, M.N.F.; Elmahdy, E.M.; El-Bendary, M.A.; Hamed, A.A.; Mohamedin, A.H. Silver and Gold Nanoparticles: Eco-Friendly Synthesis, Antibiofilm, Antiviral, and Anticancer Bioactivities. Prep. Biochem. Biotechnol. 2024, 54, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Bhebhe, L.M.; Kim, J.; Jones, L.M.; Super, E.H.; Jones, S.T. Antiviral Mechanism Change of Poly(Styrene Sulfonate) through Gold Nanoparticle Coating. Polym. Chem. 2024, 15, 945–951. [Google Scholar] [CrossRef]
- Namitha, R.; Abirami, B.; Anoop, B.S.; Dominic, D.V.D.; Ameer, A.; Manigundan, K.; Radhakrishnan, M.; Santhiya, P.; Bhaskar, P.V.; Govindaraju, K.; et al. Synthesis and Characterization of Gold Nanoparticles Using Brevibacterium Casei (SOSIST-06) Isolated from Southern Ocean Water Samples and Their in Vitro and in Silico Anti-WSSV Activity. Aquaculture 2024, 579, 740205. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, Y.; Cai, H.; You, D.; Wang, Y.; Wu, S.; Wang, Y.; Guo, W.; Qu, W. Hydrogels Containing Chitosan-Modified Gold Nanoparticles Show Significant Efficacy in Healing Diabetic Wounds Infected with Antibiotic-Resistant Bacteria. IJN 2024, Volume 19, 1539–1556. [Google Scholar] [CrossRef]
- Poomrattanangoon, S.; Pissuwan, D. Gold Nanoparticles Coated with Collagen-I and Their Wound Healing Activity in Human Skin Fibroblast Cells. Heliyon 2024, 10, e33302. [Google Scholar] [CrossRef]
- Cuyler, M.; Twilley, D.; Thipe, V.; Mandiwana, V.; Kalombo, M.; Ray, S.; Rikhotso-Mbungela, R.; Janse Van Vuuren, A.; Coetsee, W.; Katti, K.; et al. Antihistamine and Wound Healing Potential of Gold Nanoparticles Synthesized Using Bulbine Frutescens (L.) Willd. NSA, 17. [CrossRef]
- An, X.; Gao, Y.; Liu, X.; Yin, Q.; Meng, L.; Wei, L. Gold Nanoparticles Green-Mediated by Descurainia Sophia Extract for the Treatment of Ovalbumin-Induced Asthma in Rats. Inorg. Chem. Commun. 2024, 161, 112011. [Google Scholar] [CrossRef]
- Kim, S.; Wang, R.; Dhandapani, S.; Kang, K.; Cho, I.-H.; Kim, Y.-J. Novel Modified Probiotic Gold Nanoparticles Loaded with Ginsenoside CK Exerts an Anti-Inflammation Effect via NF-κB/MAPK Signaling Pathways. Arab. J. Chem. 2024, 17, 105650. [Google Scholar] [CrossRef]
- İpek, P.; Baran, M.F.; Baran, A.; Hatipoğlu, A.; Keskin, C.; Yildiztekin, M.; Küçükaydin, S.; Becerekli, H.; Kurt, K.; Eftekhari, A.; et al. Green Synthesis and Evaluation of Antipathogenic, Antioxidant, and Anticholinesterase Activities of Gold Nanoparticles (Au NPs) from Allium Cepa L. Peel Aqueous Extract. Biomass Conv. Bioref. 2024, 14, 10661–10670. [Google Scholar] [CrossRef]
- Tkachenko, A.; Özdemir, S.; Tollu, G.; Dizge, N.; Ocakoglu, K.; Prokopiuk, V.; Onishchenko, A.; Сhumachenko, V.; Virych, P.; Pavlenko, V.; et al. Antibacterial and Antioxidant Activity of Gold and Silver Nanoparticles in Dextran–Polyacrylamide Copolymers. Biometals 2024, 37, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Kishore Mohanta, Y.; Pohl, P.; Nayak, D.; Messaoudi, M. Facile Phytosynthesis of Gold Nanoparticles Using Nepeta Bodeana Bunge: Evaluation of Its Therapeutics and Potential Catalytic Activities. J. Photochem. Photobiol. A: Chem. 2024, 446, 115150. [Google Scholar] [CrossRef]
- Badr, H.A.; Abd ElMohsen E Nasr, S.; Obiedallah, M. Rapid Synthesis of Gold Nanoparticles from Some Egyptian Seaweed: Characterization and Antidiabetic Potential. Egypt. J. Phycol. 2024, 25, 46–56. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In Vivo Evaluation of Safety, Biodistribution and Pharmacokinetics of Laser-Synthesized Gold Nanoparticles. Sci Rep 2019, 9, 12890. [Google Scholar] [CrossRef]
- Van Pomeren, M.; Peijnenburg, W.J.G.M.; Vlieg, R.C.; Van Noort, S.J.T.; Vijver, M.G. The Biodistribution and Immuno-Responses of Differently Shaped Non-Modified Gold Particles in Zebrafish Embryos. Nanotoxicology 2019, 13, 558–571. [Google Scholar] [CrossRef]
- Donaldson, K.; Borm, P.J.; Castranova, V.; Gulumian, M. The Limits of Testing Particle-Mediated Oxidative Stress in Vitro in Predicting Diverse Pathologies; Relevance for Testing of Nanoparticles. Part Fibre Toxicol 2009, 6, 13. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. IJN 2020, Volume 15, 275–300. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold Nanoparticles: Distribution, Bioaccumulation and Toxicity. In Vitro and in Vivo Studies. Nanomed. : Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Schmid, G.; Kreyling, W.G.; Simon, U. Toxic Effects and Biodistribution of Ultrasmall Gold Nanoparticles. Arch Toxicol 2017, 91, 3011–3037. [Google Scholar] [CrossRef]
- Lipka, J.; Semmler-Behnke, M.; Sperling, R.A.; Wenk, A.; Takenaka, S.; Schleh, C.; Kissel, T.; Parak, W.J.; Kreyling, W.G. Biodistribution of PEG-Modified Gold Nanoparticles Following Intratracheal Instillation and Intravenous Injection. Biomaterials 2010, 31, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Z.; Ma, J.; Wang, X.; Zhang, Y.; Wang, W.; Yuan, Z. The Systematic Evaluation of Size-Dependent Toxicity and Multi-Time Biodistribution of Gold Nanoparticles. Colloids Surf. B: Biointerfaces 2018, 167, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, R.J.; Karsh, E.H.; Taqi, Z.J.; Jabir, M.S. Biocompatibility of Gold Nanoparticles: In-Vitro and In-Vivo Study. Mater. Today: Proc. 2021, 42, 3041–3045. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.A.; Olmedo, I.; Clos, A.; Sadagopa Ramanujam, V.M.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and Toxicity of Gold Nanoparticles after Repeated Administration in Mice. Biochem. Biophys. Res. Commun. 2010, 393, 649–655. [Google Scholar] [CrossRef]
- Chuang, S.-M.; Lee, Y.-H.; Liang, R.-Y.; Roam, G.-D.; Zeng, Z.-M.; Tu, H.-F.; Wang, S.-K.; Chueh, P.J. Extensive Evaluations of the Cytotoxic Effects of Gold Nanoparticles. Biochim. Et Biophys. Acta (BBA) - Gen. Subj. 2013, 1830, 4960–4973. [Google Scholar] [CrossRef]
- Ng, C.-T.; Li, J.J.; Gurung, R.L.; Hande, M.P.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Toxicological Profile of Small Airway Epithelial Cells Exposed to Gold Nanoparticles. Exp Biol Med (Maywood) 2013, 238, 1355–1361. [Google Scholar] [CrossRef]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in Vitro and in Vivo Toxicity of Gold Nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Google Patents Available Online:. Available online: Https://Patents.Google.Com/ (accessed on day month year).
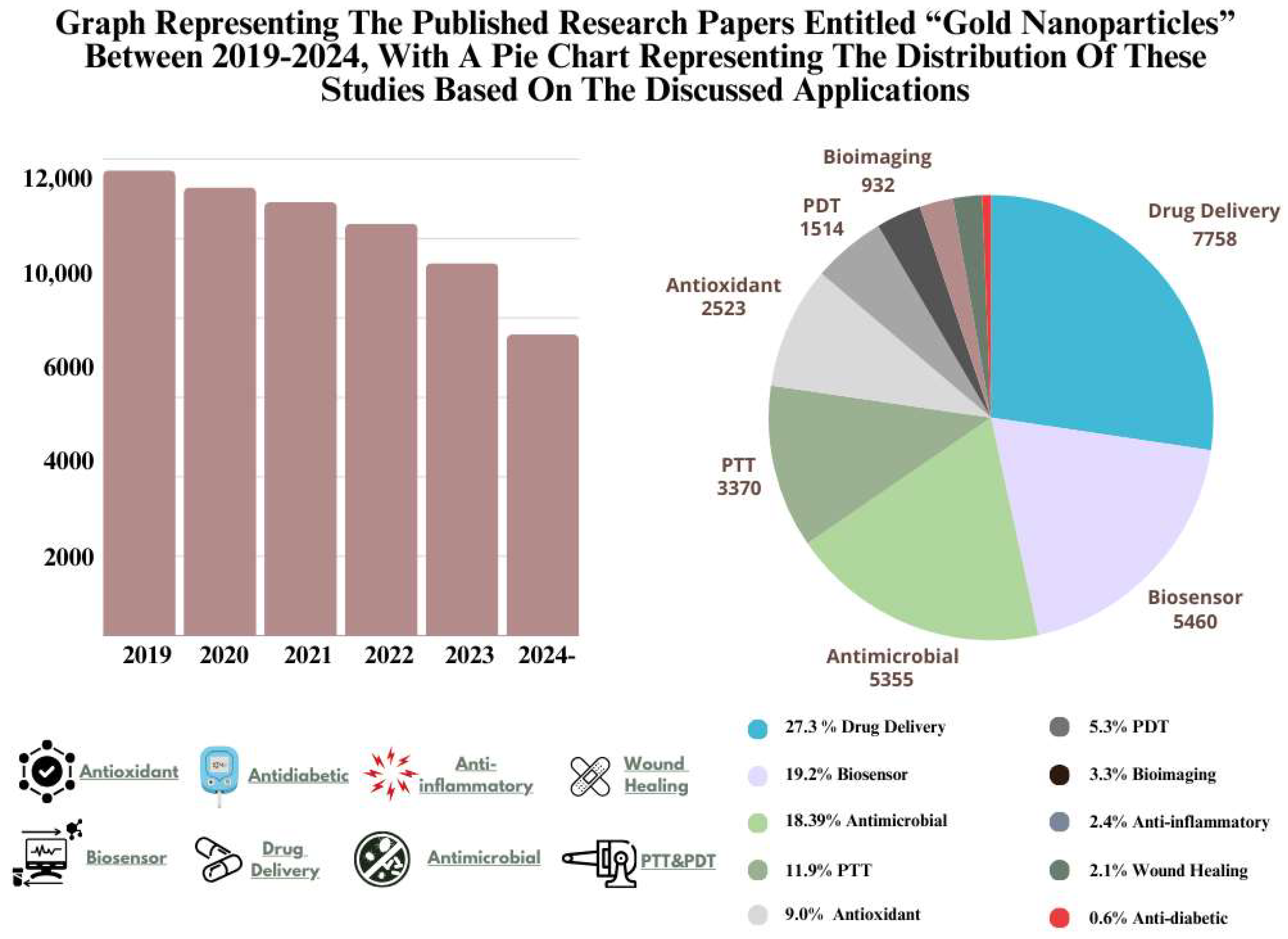
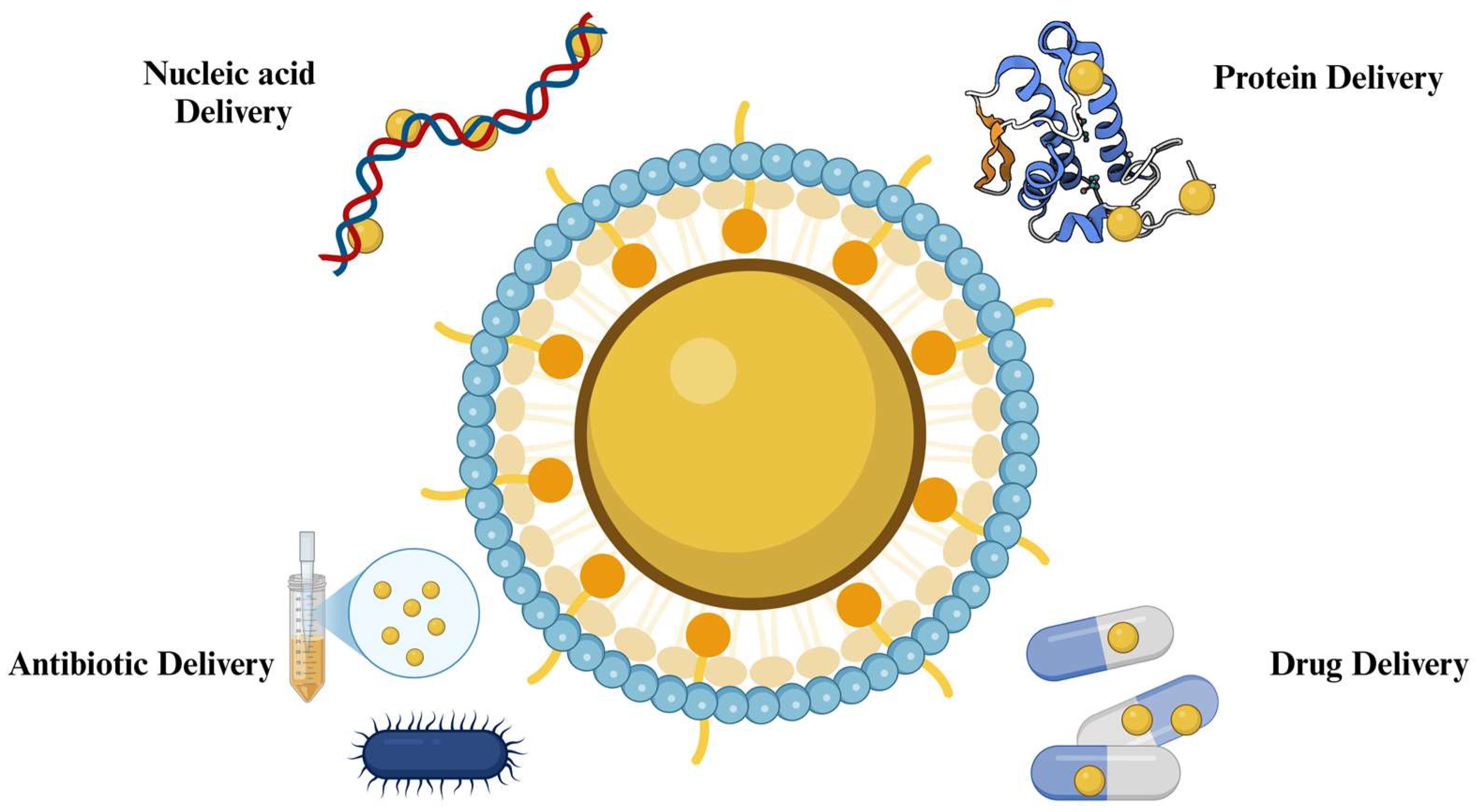
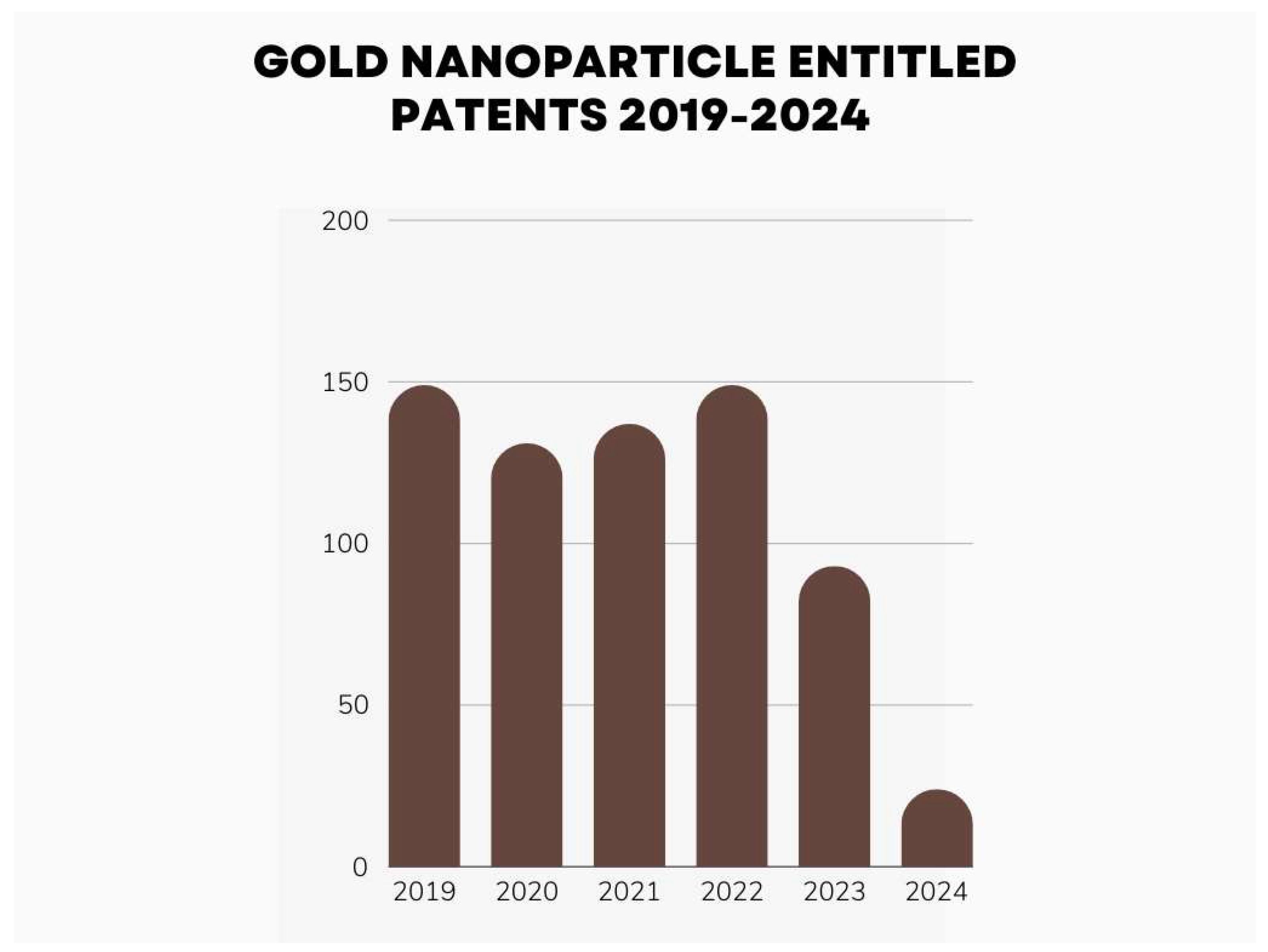
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).