Submitted:
10 May 2025
Posted:
12 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Gold Nanoparticles
2.2. Functionalization of Gold Nanoparticles with Colon Cancer Targeting Molecule
2.3. In Vitro Studies - Cell Suspension Preparation and Maintenance
2.3.1. Thawing Procedure:
2.3.2. Maintenance Procedure:
2.4. In Vitro Exposure of the Cell Suspension to the Vaccine Nanoconstruct
2.5. Evaluation of Antigen Trafficking in the Exposed Cell Suspension
3. Results
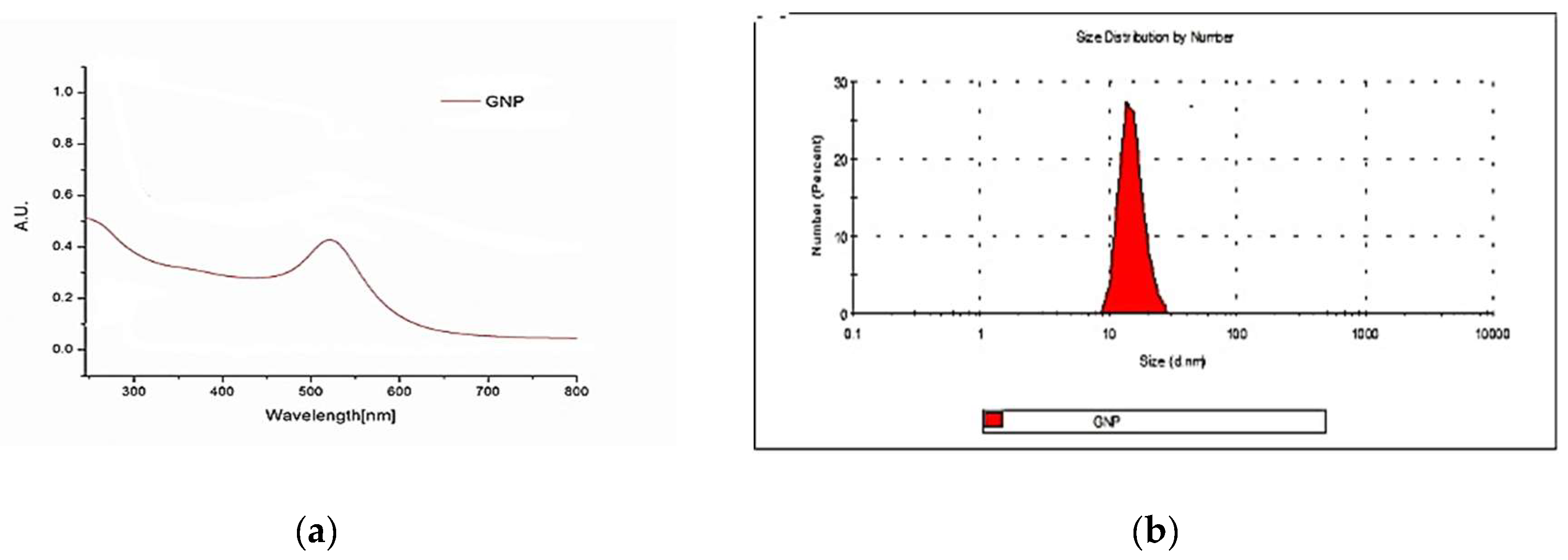
3.1. Synthesis and Characterization of Gold Nanoparticles
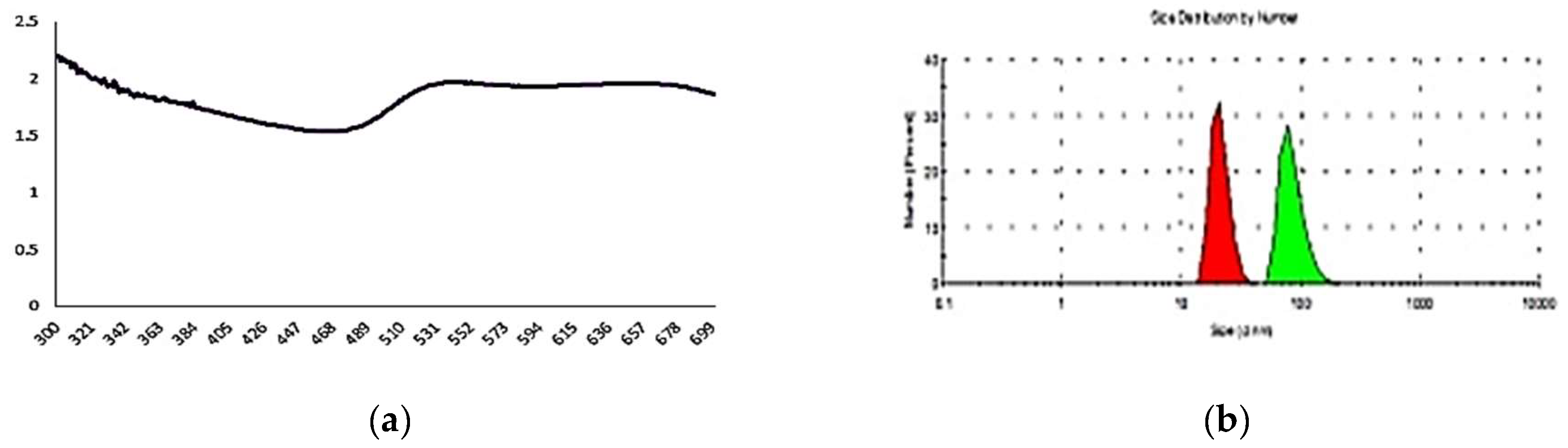
3.2. Functionalization. Physical and chemical characterization of the CEA-AuNP
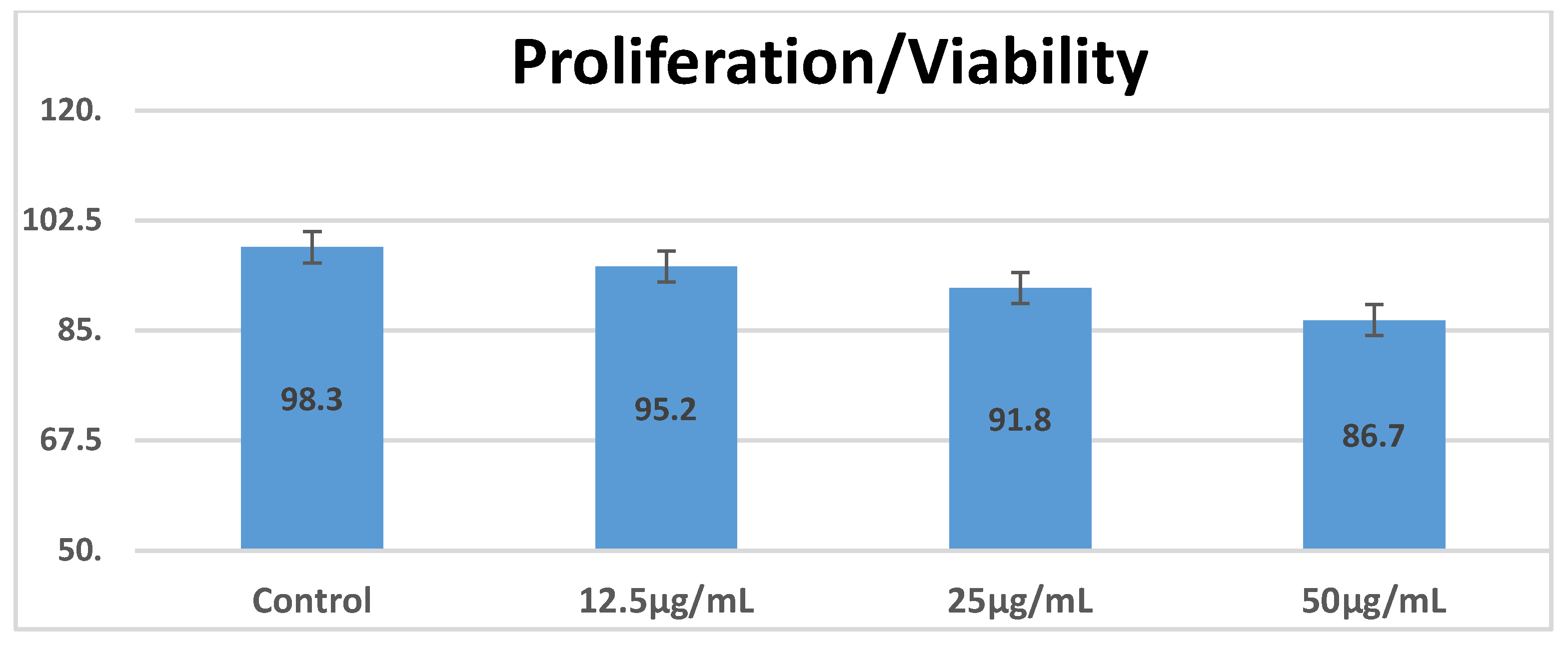
3.3. In Vitro Exposure of the Cell Suspension to the Vaccine Nanoconstruct
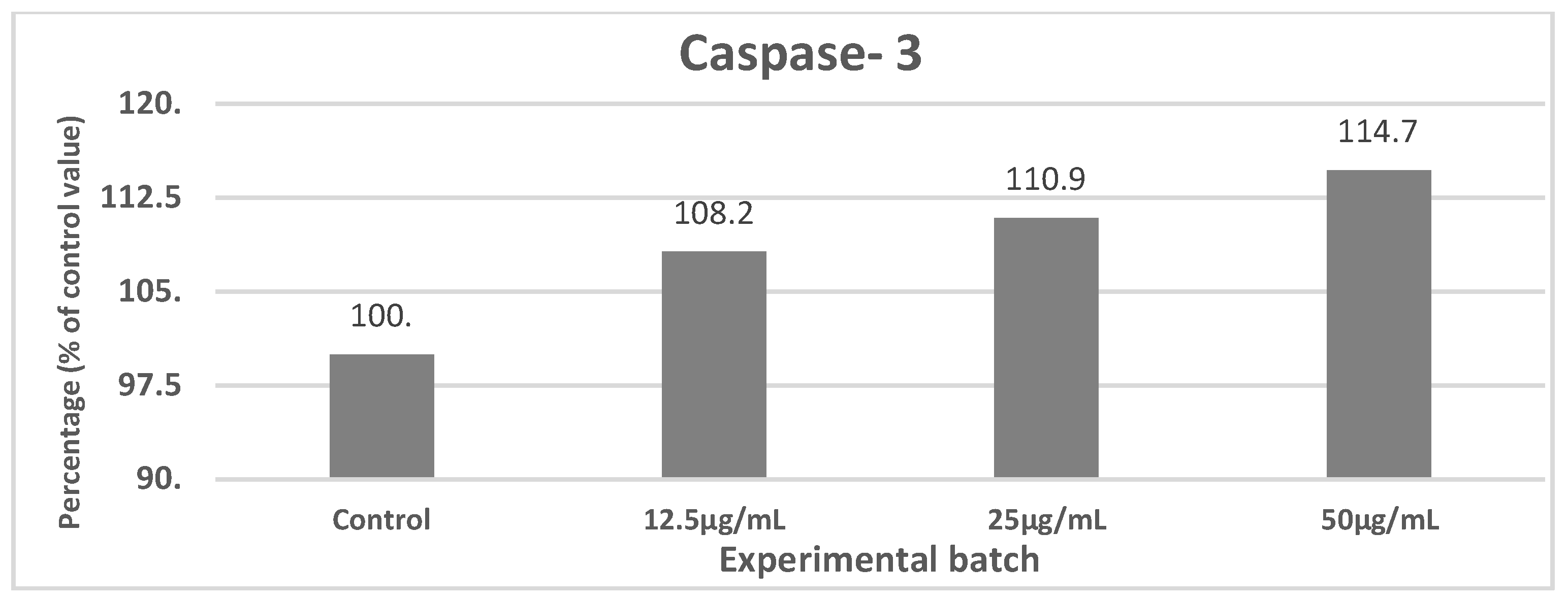
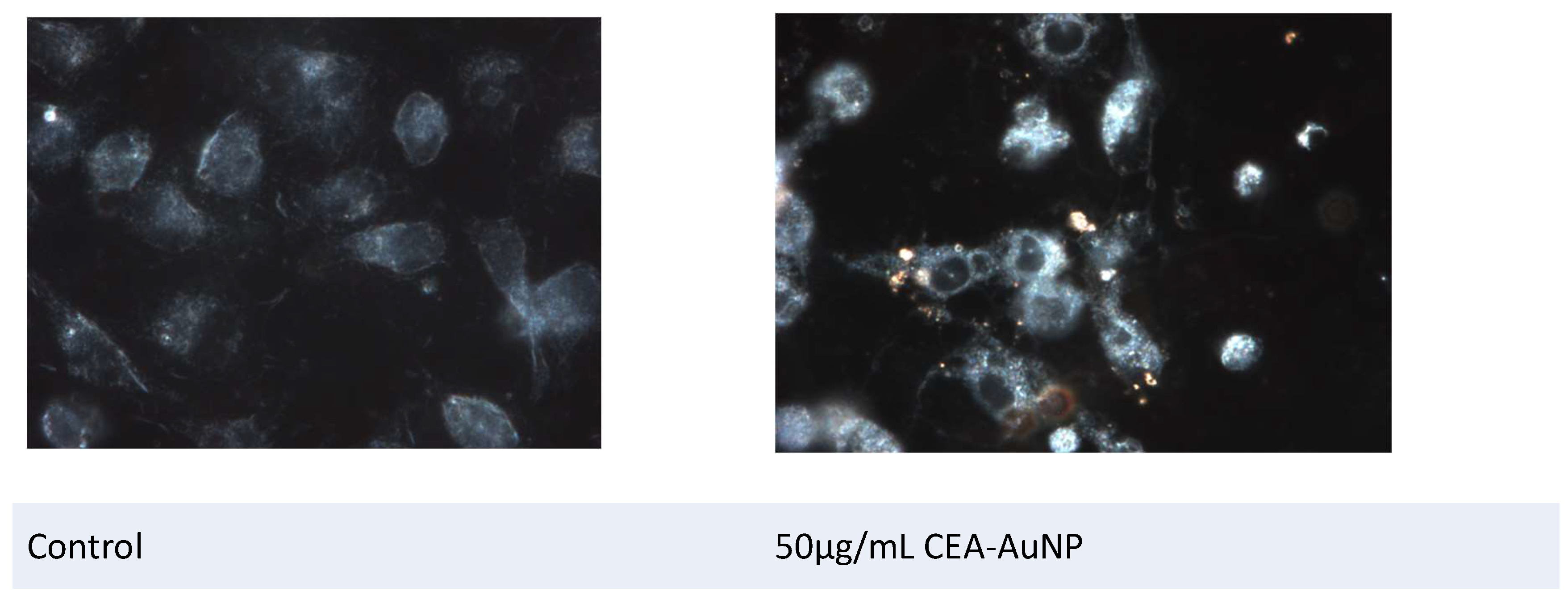
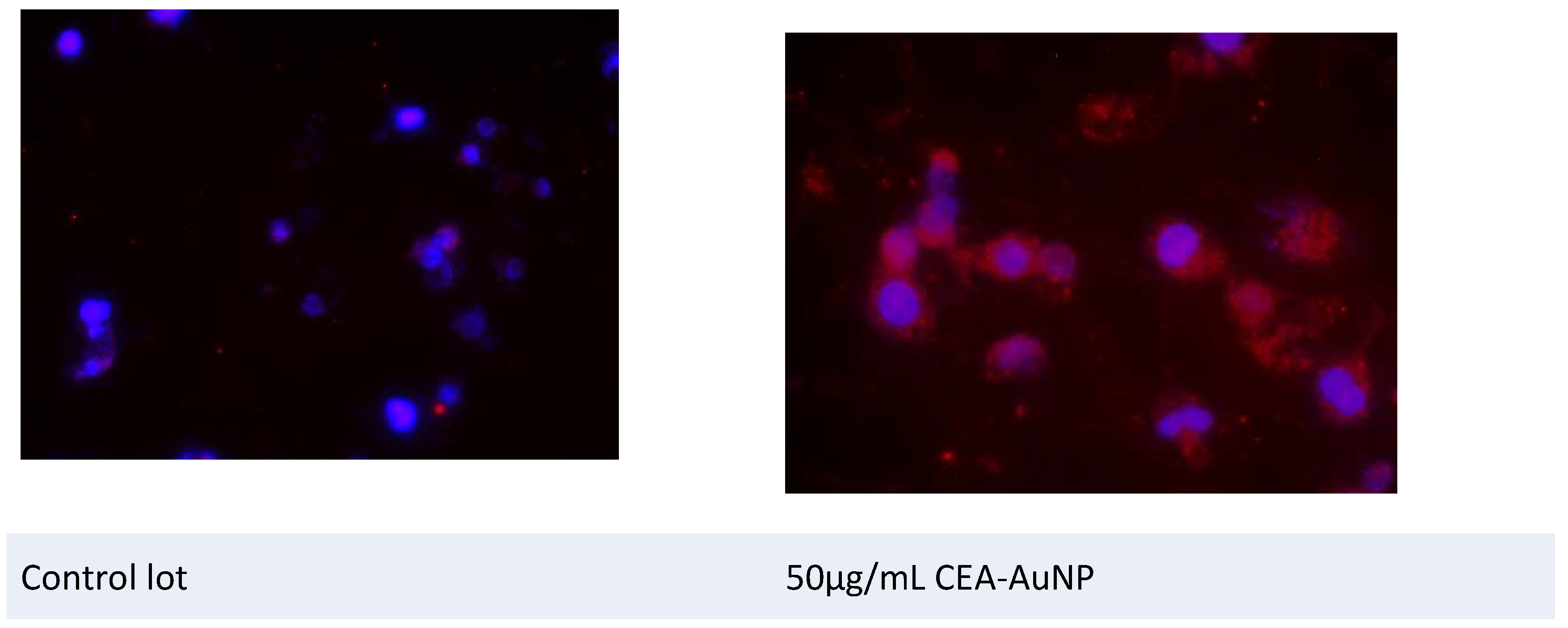
3.4. Assessment of Antigen Processing by Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNP | Gold nanoparticle |
| CEA | Carcinoembrionic Antigen |
| CEA-AuNP | CEA functionalized gold nanoparticles |
| MHC | Major histocompatibility complex |
References
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2024;74(3):229–63. [CrossRef]
- Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2(2):141–60.
- Fan T, Zhang M, Yang J, Zhu Z, Cao W, Dong C. Therapeutic cancer vaccines: advancements, challenges and prospects. Signal Transduction and Targeted Therapy. 2023;8(1):450.
- Kaczmarek M, Poznańska J, Fechner F, Michalska N, Paszkowska S, Napierała A, et al. Cancer Vaccine Therapeutics: Limitations and Effectiveness-A Literature Review. Cells. 2023;12(17). [CrossRef]
- Sobhani N, Scaggiante B, Morris R, Chai D, Catalano M, Tardiel-Cyril DR, et al. Therapeutic cancer vaccines: From biological mechanisms and engineering to ongoing clinical trials. Cancer Treat Rev. 2022;109:102429. [CrossRef]
- Kankanala VL ZM, Mukkamalla SKR. Carcinoembryonic Antigen. [Updated 2024 Dec 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK578172/.
- Hall C, Clarke L, Pal A, Buchwald P, Eglinton T, Wakeman C, et al. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann Coloproctol. 2019;35(6):294–305. [CrossRef]
- Choi SH, Yang SY, Han YD, Cho MS, Hur H, Lee KY, et al. Carcinoembryonic antigen levels of tumor-draining venous blood as a prognostic marker in colon cancer. Korean J Clin Oncol. 2017;13(2):68–74. [CrossRef]
- Bhagat A, Lyerly HK, Morse MA, Hartman ZC. CEA vaccines. Hum Vaccin Immunother. 2023;19(3):2291857. [CrossRef]
- Wen R, Umeano AC, Kou Y, Xu J, Farooqi AA. Nanoparticle systems for cancer vaccine. Nanomedicine (Lond). 2019;14(5):627–48. [CrossRef]
- Almeida JP, Figueroa ER, Drezek RA. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine. 2014;10(3):503–14. [CrossRef]
- Almeida JPM, Lin AY, Figueroa ER, Foster AE, Drezek RA. In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small. 2015;11(12):1453–9. [CrossRef]
- Cao-Milan R, Liz-Marzan LM. Gold nanoparticle conjugates: recent advances toward clinical applications. Expert Opin Drug Deliv. 2014;11(5):741–52. [CrossRef]
- Liu M, Li Q, Liang L, Li J, Wang K, Li J, et al. Real-time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging. Nature Communications. 2017;8(1):15646. [CrossRef]
- Mocan T, Matea C, Tabaran F, Iancu C, Orasan R, Mocan L. In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. J Cancer. 2015;6(6):583–92. [CrossRef]
- Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110(32):15700–7. [CrossRef]
- Ghasemi M, Turnbull T, Sebastian S, Kempson I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int J Mol Sci. 2021;22(23). [CrossRef]
- Zdrehus R, Delcea C, Mocan L. Role of Biofunctionalized Nanoparticles in Digestive Cancer Vaccine Development. Pharmaceutics. 2024;16(3). [CrossRef]
- Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussions of the Faraday Society. 1951;11(0):55–75. [CrossRef]
- Daniel M-C, Astruc D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chemical Reviews. 2004;104(1):293–346. [CrossRef]
- Humbert C, Pluchery O, Lacaze E, Tadjeddine A, Busson B. Optical spectroscopy of functionalized gold nanoparticles assemblies as a function of the surface coverage. Gold Bulletin. 2013;46:299–309. [CrossRef]
- Thambiraj S, Hema S, Shankaran DR. Functionalized gold nanoparticles for drug delivery applications. Materials Today: Proceedings. 2018;5(8):16763–73. [CrossRef]
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2007;2(5):681–93. [CrossRef]
- Saenmuangchin R, Siripinyanond A. Flow field-flow fractionation for hydrodynamic diameter estimation of gold nanoparticles with various types of surface coatings. Analytical and bioanalytical chemistry. 2018;410(26):6845–59. [CrossRef]
- Kang S, Ahn S, Lee J, Kim JY, Choi M, Gujrati V, et al. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J Control Release. 2017;256:56–67. [CrossRef]
- Nguyen B, Tolia NH. Protein-based antigen presentation platforms for nanoparticle vaccines. npj Vaccines. 2021;6(1):70. [CrossRef]
- Wei Y, Quan L, Zhou C, Zhan Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine. 2018;13(12):1495–512. [CrossRef]
- Modena MM, Rühle B, Burg TP, Wuttke S. Nanoparticle characterization: what to measure? Advanced Materials. 2019;31(32):1901556.
- Narayan R, Gadag S, Garg S, Nayak UY. Understanding the effect of functionalization on loading capacity and release of drug from mesoporous silica nanoparticles: a computationally driven study. ACS omega. 2022;7(10):8229–45. [CrossRef]
- Baharara J, Ramezani T, Divsalar A, Mousavi M, Seyedarabi A. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna journal of medical biotechnology. 2016;8(2):75.
- McNamara K, Tofail SA. Nanoparticles in biomedical applications. Advances in Physics: X. 2017;2(1):54–88.
- Bharathala S, Sharma P. Biomedical applications of nanoparticles. Nanotechnology in modern animal biotechnology: Elsevier; 2019. p. 113–32.
- Johnson S, Nguyen V, Coder D. Assessment of cell viability. Current protocols in cytometry. 2013;64(1):9.2. 1–9.2. 26.
- Conners CM, Bhethanabotla VR, Gupta VK. Concentration-dependent effects of alendronate and pamidronate functionalized gold nanoparticles on osteoclast and osteoblast viability. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2017;105(1):21–9. [CrossRef]
- Grippin AJ, Sayour EJ, Mitchell DA. Translational nanoparticle engineering for cancer vaccines. Oncoimmunology. 2017;6(10):e1290036. [CrossRef]
- Ma W, Jing L, Valladares A, Mehta SL, Wang Z, Li PA, et al. Silver nanoparticle exposure induced mitochondrial stress, caspase-3 activation and cell death: amelioration by sodium selenite. International journal of biological sciences. 2015;11(8):860. [CrossRef]
- Asadi M, Taghizadeh S, Kaviani E, Vakili O, Taheri-Anganeh M, Tahamtan M, et al. Caspase-3: structure, function, and biotechnological aspects. Biotechnology and Applied Biochemistry. 2022;69(4):1633–45.
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Developmental cell. 2001;1(4):515–25. [CrossRef]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–9. [CrossRef]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicologic pathology. 2007;35(4):495–516. [CrossRef]
- Mohammadinejad R, Moosavi MA, Tavakol S, Vardar DÖ, Hosseini A, Rahmati M, et al. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy. 2019;15(1):4–33. [CrossRef]
- Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunological reviews. 2003;193(1):10–21. [CrossRef]
- Gogolák P, Réthi B, Hajas G, Rajnavölgyi É. Targeting dendritic cells for priming cellular immune responses. Journal of Molecular Recognition. 2003;16(5):299–317. [CrossRef]
- Li P, Gregg JL, Wang N, Zhou D, O'Donnell P, Blum JS, et al. Compartmentalization of class II antigen presentation: contribution of cytoplasmic and endosomal processing. Immunological reviews. 2005;207(1):206–17. [CrossRef]
- Tonigold M, Mailänder V. Endocytosis and intracellular processing of nanoparticles in dendritic cells: routes to effective immunonanomedicines. Taylor & Francis; 2016. p. 2625–30. [CrossRef]
- Boraschi D, Costantino L, Italiani P. Interaction of nanoparticles with immunocompetent cells: nanosafety considerations. Nanomedicine. 2012;7(1):121–31. [CrossRef]
- Li L, Yan X, Xia M, Shen B, Cao Y, Wu X, et al. Nanoparticle/nanocarrier formulation as an antigen: the immunogenicity and antigenicity of itself. Molecular Pharmaceutics. 2021;19(1):148–59. [CrossRef]
- Baljon JJ, Wilson JT. Bioinspired vaccines to enhance MHC class-I antigen cross-presentation. Current opinion in immunology. 2022;77:102215.
- Zhang W, Wang L, Liu Y, Chen X, Liu Q, Jia J, et al. Immune responses to vaccines involving a combined antigen–nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials. 2014;35(23):6086–97. [CrossRef]
- Liang J, Yao L, Liu Z, Chen Y, Lin Y, Tian T. Nanoparticles in Subunit Vaccines: Immunological Foundations, Categories, and Applications. Small. 2025;21(1):2407649. [CrossRef]
- Mundekkad D, Cho WC. Nanoparticles in clinical translation for cancer therapy. International journal of molecular sciences. 2022;23(3):1685. [CrossRef]
- Lee IH, Kwon HK, An S, Kim D, Kim S, Yu MK, et al. Imageable antigen-presenting gold nanoparticle vaccines for effective cancer immunotherapy in vivo. Angewandte Chemie-International Edition. 2012;51(35):8800. [CrossRef]
- Silva JM, Gaëlle V, G. OV, N. PS, Catarina R, Ana S, et al. Development of Functionalized Nanoparticles for Vaccine Delivery to Dendritic Cells: A Mechanistic Approach. Nanomedicine. 2014;9(17):2639–56.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




