3.1. Characterization of Nano Filler
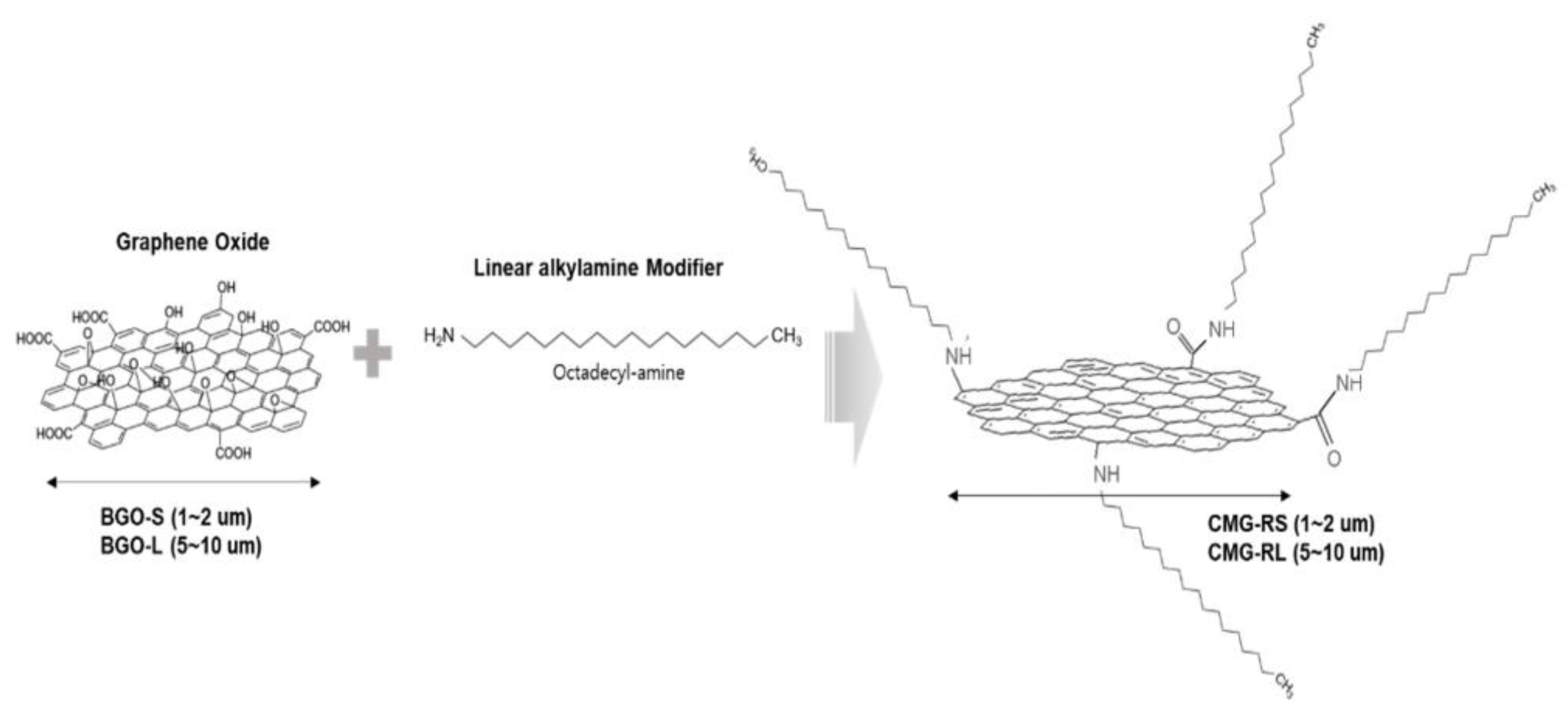
Figure 1 illustrates a schematic diagram of the functionalization of graphene oxide through a chemical modification reaction with alkylamine. In this process, graphene oxide reacts with alkylamine, resulting in the attachment of linear alkyl hydrocarbon chains to the graphene oxide structure. This functionalization enhances the properties of graphene oxide, making it suitable for various applications in materials science and nanotechnology. In the described synthesis, BGO-S (small graphene oxide) has a lateral size of 1 to 2
while BGO-L (large graphene oxide) has a lateral size of 5 to 10
. As a result, CMG-RS (chemically modified graphene- by alkylation – small size) of 1 to 2
and CMG-RL (chemically modified graphene- by alkylation - large size) of 5 to 10
can be created. The alkyl chains in the structure of CMG-RS and CMG-RL are expected to exhibit high compatibility with the main hydrocarbon chains of PBT and ASA. Furthermore, the -C-NH- and -CONH- groups formed at the connection points between the graphene surface and the alkyl chains in CMG-RS and CMG-RL are likely to form hydrogen bonds with the -COO- groups in PBT and the -COO- and -CN groups in ASA. This suggests that CMG-RS and CMG-RL will have excellent compatibility with the PBT/ASA matrix.
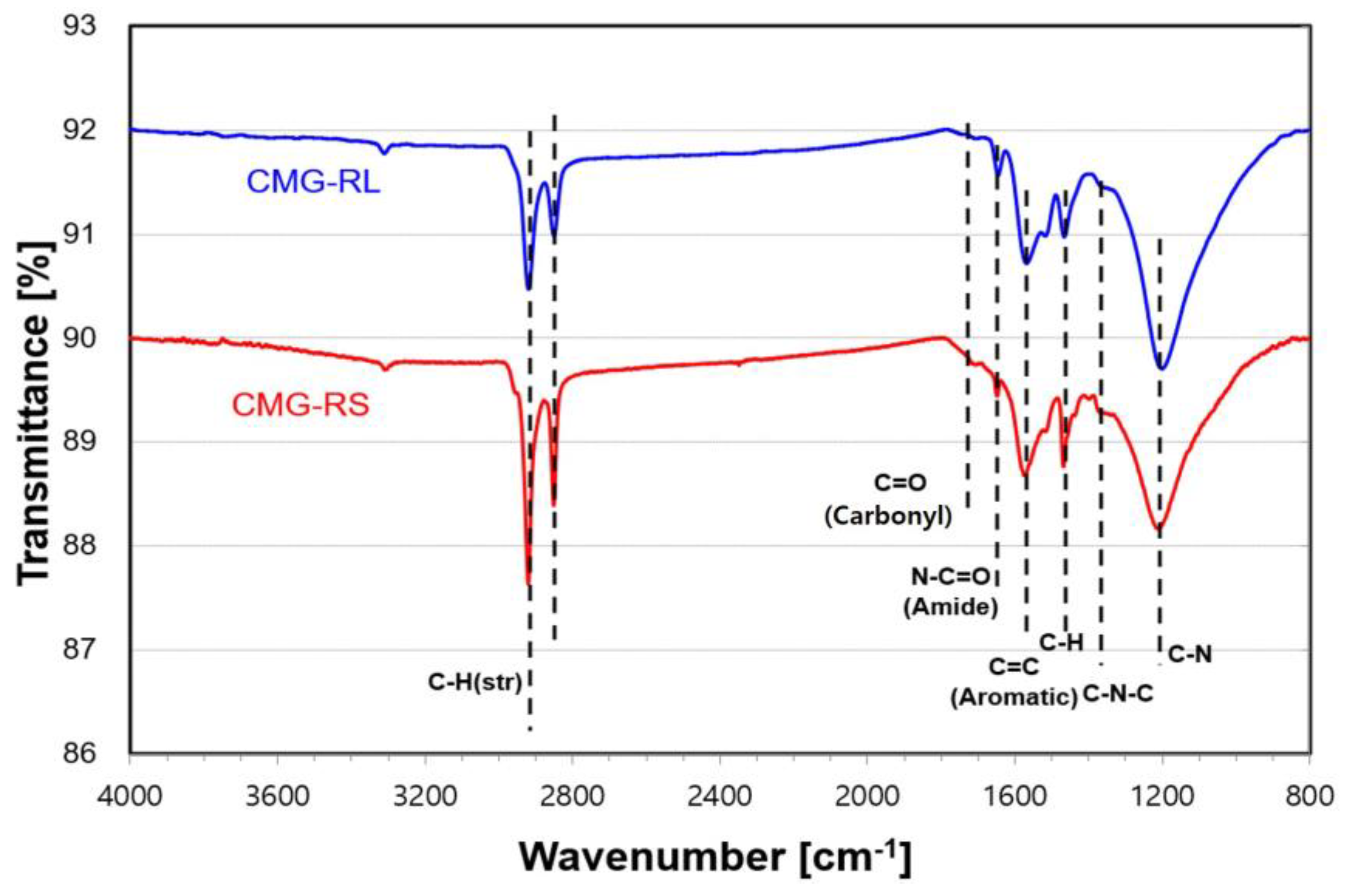
Figure 2 presents the FT-IR results for CMG-RS and CMG-RL, showing their transmittance spectra over the wavenumber range of 4000 to 800 cm⁻¹. The successful functionalization reaction, which involved reduction, is evidenced by a significant reduction in the intensity of peaks related to the C=O (carbonyl group, 1725 cm⁻¹) and C–O–C (epoxide group, 1055 cm⁻¹) of graphene oxide (GO). Additionally, two peaks between 2850 and 2920 cm⁻¹ and a strong peak at 1486 cm⁻¹ were observed, corresponding to the linear hydrocarbon (–CH) of the alkyl group. Furthermore, peaks at 1080 cm⁻¹ (attributed to C–N) and 1570 cm⁻¹ (attributed to N–H) confirm that alkylation was successfully achieved [
19]. The FT-IR analysis conclusively demonstrates the successful functionalization of graphene oxide with alkylamine, resulting in the incorporation of various functional groups such as carbonyl, amide, and aromatic groups. The similarity in peak positions between CMG-RS and CMG-RL suggests that the functionalization process is uniformly effective across different lateral sizes. However, the variations in peak intensities highlight differences in the degree of functionalization or the accessibility of these groups, likely influenced by the lateral size of the graphene sheets. Notably, the C-H (aliphatic) and C=C (aromatic) peak intensities are greater in CMG-RL than in CMG-RS, which can be attributed to the influence of the larger lateral size of CMG-RL.
The structural characteristics of the as-synthesized CMG-RS and CMG-RL were identified through their Raman spectra.
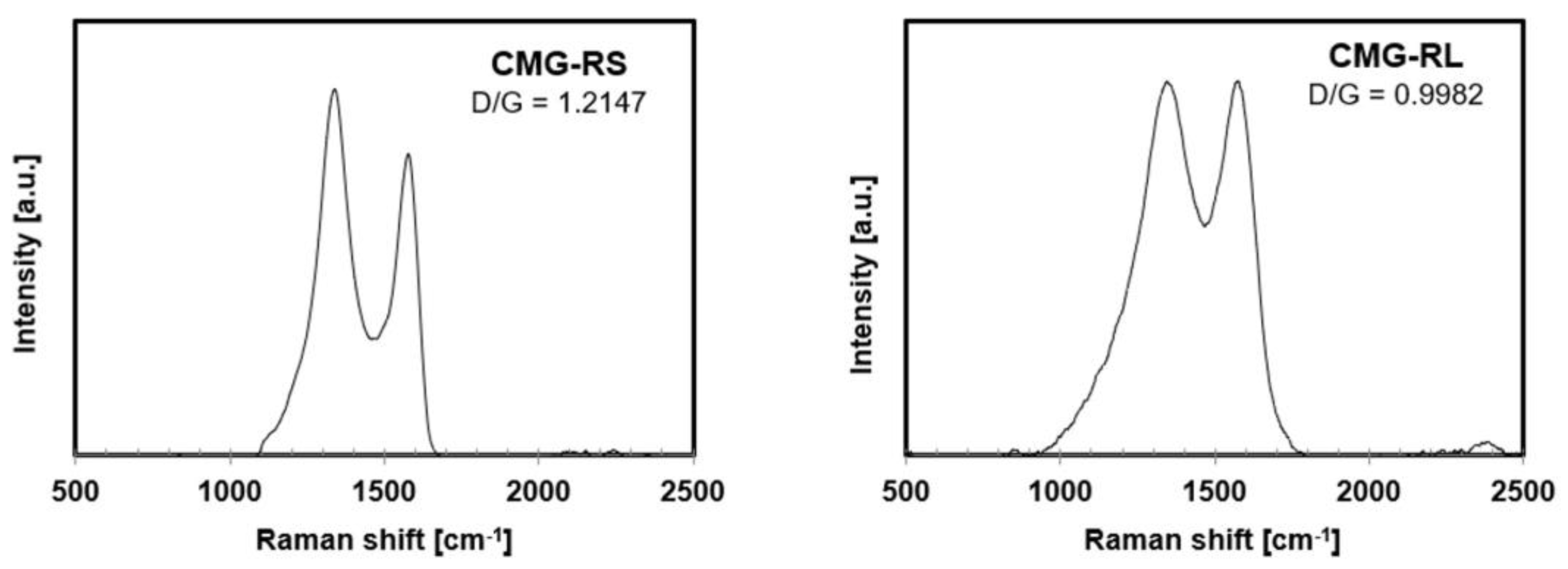
Figure 3 shows the Raman spectra of CMG-RS and CMG-RL produced by the chemically surface modification, wherein the characteristic D band (near 1360 cm
-1) and G band (near 1580 cm
-1) peaks of graphene can be identified [
18]. In the spectrum of CMG, the D/G ratio for CMG-RS was found to be 1.2147, while for CMG-RL, it was 0.9982. The higher D/G ratio for CMG-RS is due to its smaller lateral size, which results in more detectable edges compared to CMG-RL.
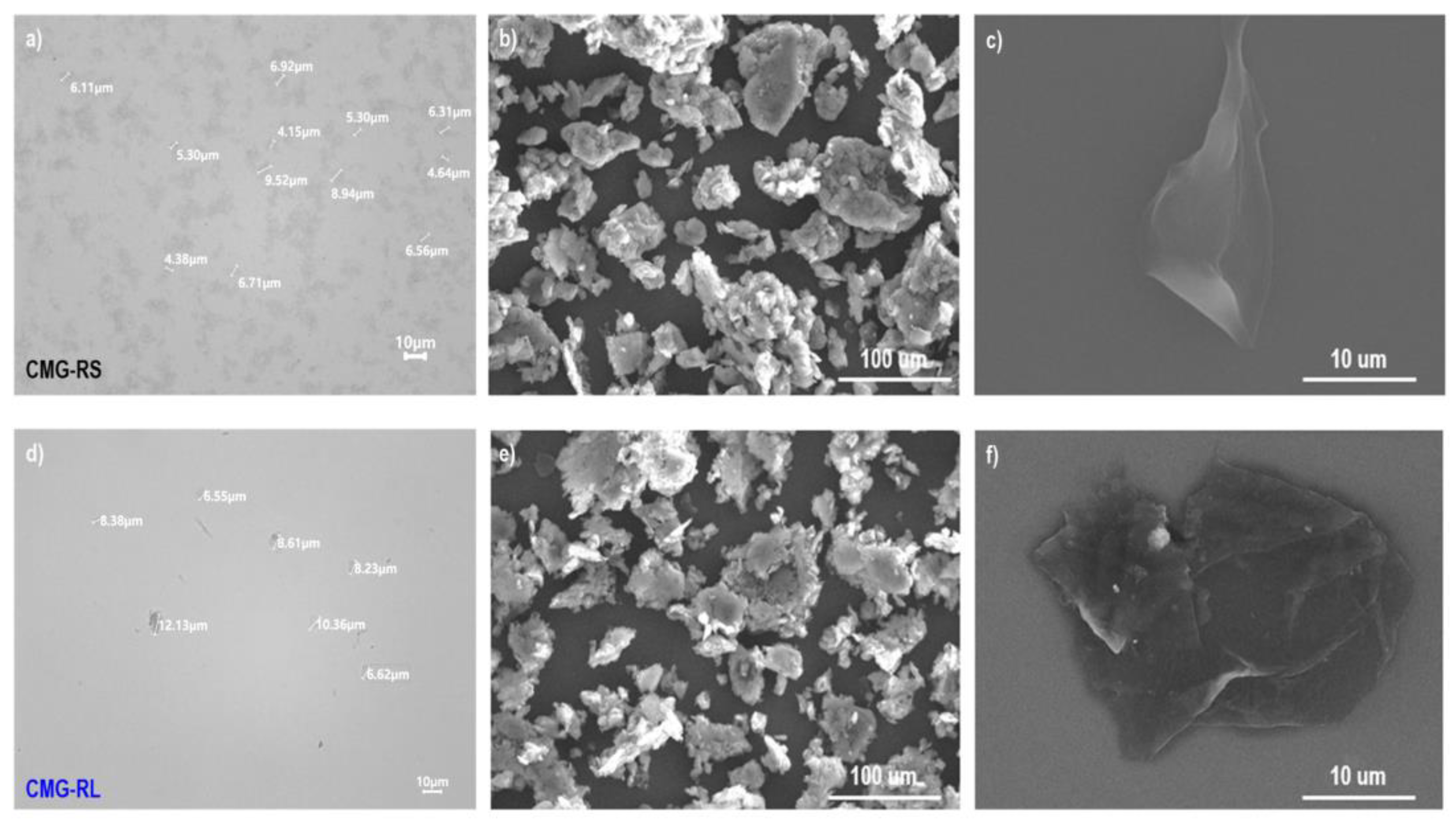
Figure 4 (a) and (d) show optical microscope images in transmission mode for CMG-RS and CMG-RL, respectively, while (b, c) and (e, f) present their corresponding SEM images. Generally, in the production of reduced graphene oxide (RGO) powder, freeze-drying is used to prevent reagglomeration. However, freeze-drying is not suitable for large-scale production and produces RGO powder with a very high specific surface area, significantly reducing workability during polymer compounding processes.
Figure 4 (b) and (e) display SEM images of dried CMG-RS and -RL powders at low magnification, revealing granulated flakes with sizes ranging from 20 to 100 μm. The granulated structure of CMG-RS and -RL enhances workability during the polymer compounding process, improving the weighing, feeding, and premixing steps. Additionally, the alkyl chains inserted between graphene sheets prevent strong stacking caused by van der Waals forces and π-π interactions, allowing for easy separation into individual sheets. To test dispersion, the dried CMG-RS and -RL granules were mixed with xylene at 0.05 wt.% and shaken thoroughly. After applying 1 drop to a slide glass and drying, optical microscopy and SEM observations confirmed that the granules easily separated into individual particles without aggregation, showing good dispersion.
3.2. Characterization of Nanocomposites
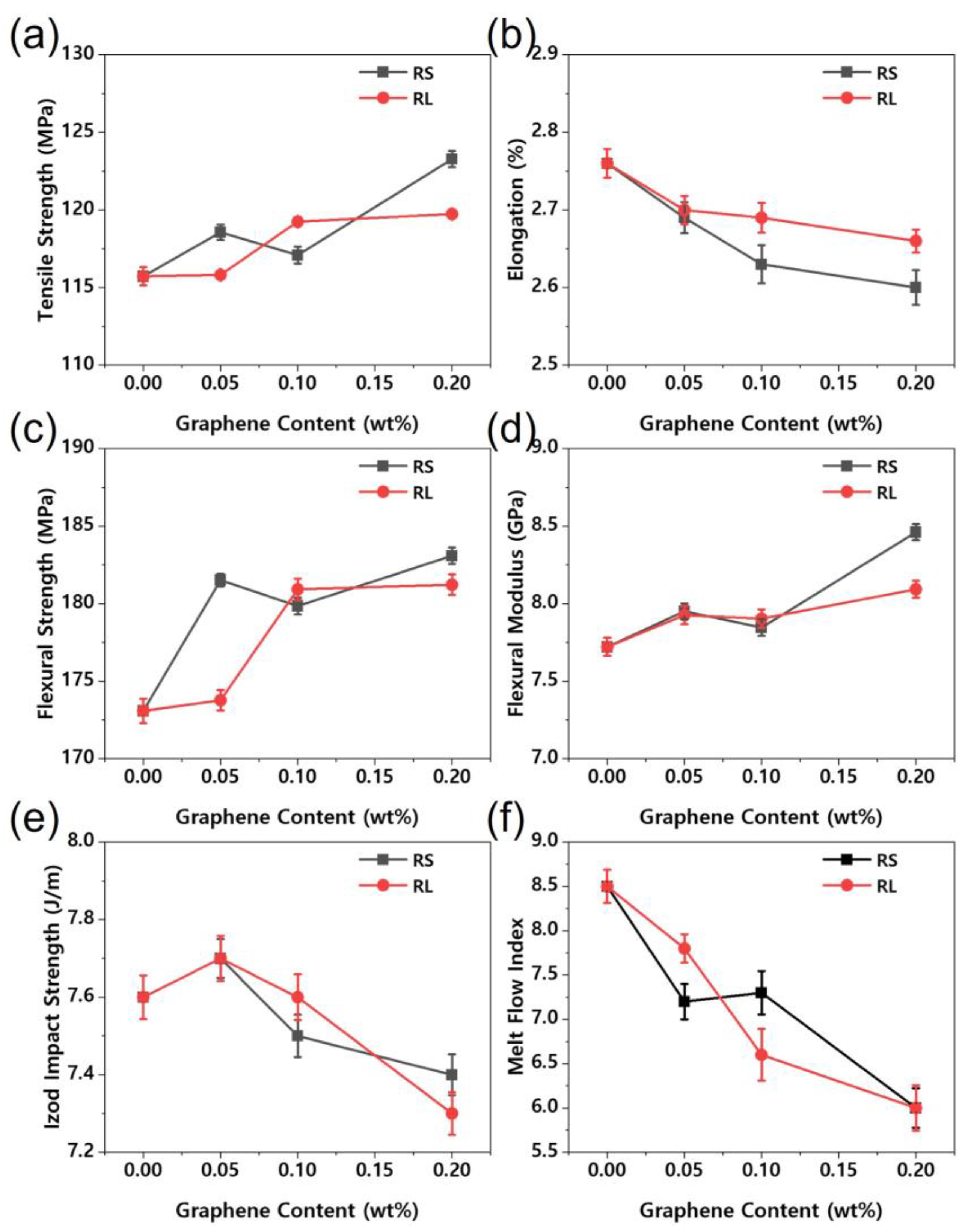
Figure 5 illustrates the mechanical properties of the nanocomposites. The mechanical properties of nanocomposites are crucial for a wide range of applications. As shown in
Figure 5, the tensile strength, flexural strength, and flexural modulus of the PBT/ASA-graphene nanocomposites were improved, likely due to enhanced compatibility between the nanofiller and the PBT/ASA matrix. In general, the mechanical properties of polymer nanocomposites are influenced by factors such as nanoparticle size, degree of dispersion, polymer-filler interface characteristics, and filler content [
9]. The infiltration of nanofillers significantly affects the viscosity and melt flow behavior of the composites. At very low graphene content, the Izod impact strength was enhanced due to the effective infiltration of graphene into the PBT/ASA matrix. However, as the graphene content increased, the Izod impact strength showed a negative trend. Additionally, both the elongation at break and the melt flow index of the nanocomposites were found to decrease with increasing graphene content.
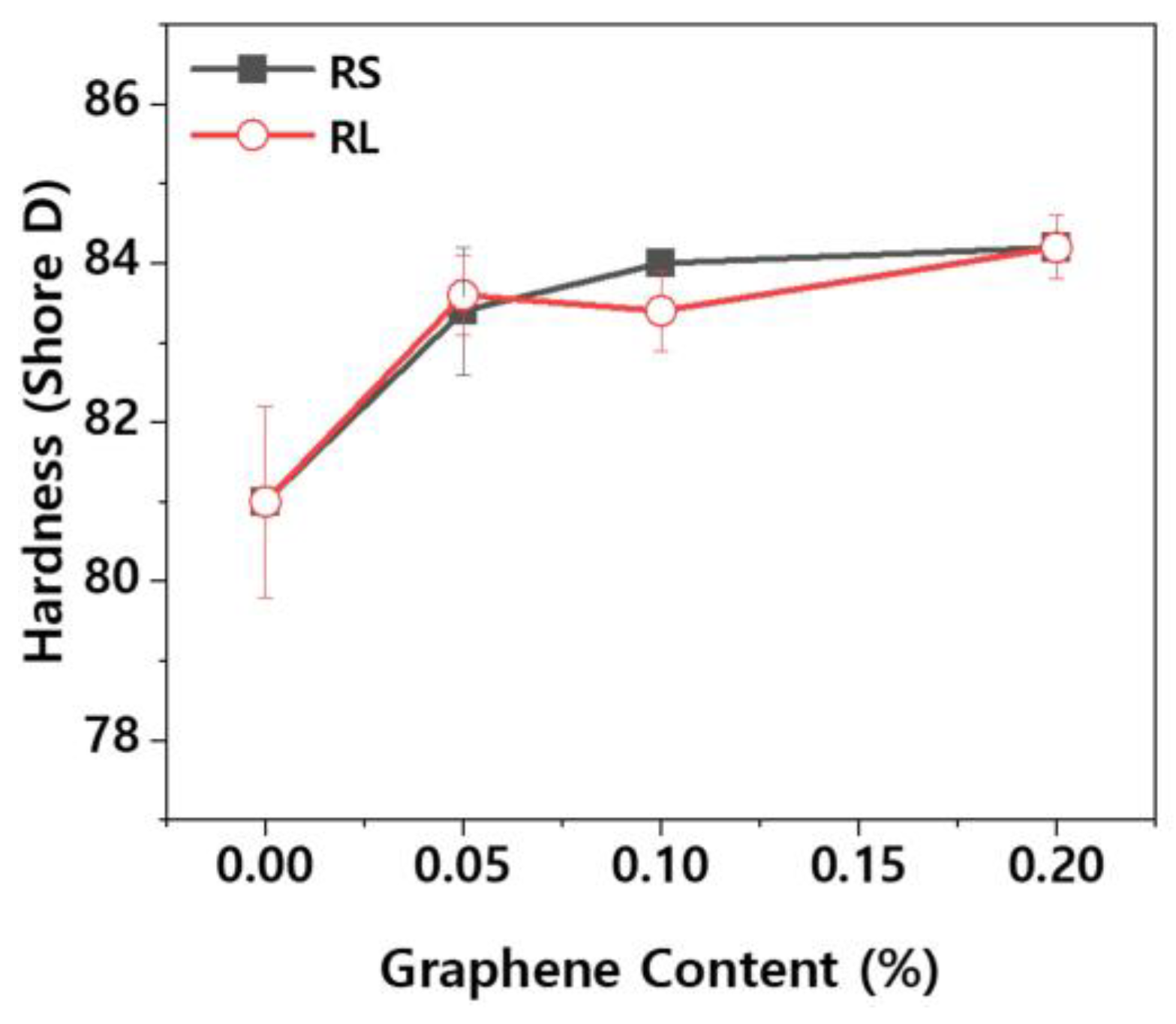
The lens holder of the projection module within a headlamp requires not only mechanical strength but also excellent tribological properties, as friction and wear are critical factors during operation. Wear particles generated from plastic components can contaminate the interior of the headlamp. One of the key properties influencing the wear characteristics of plastic materials is hardness.
Figure 6 shows the Shore D hardness values for PBT/ASA nanocomposites. The hardness of plastics is measured using Shore A for softer materials and Shore D for harder materials. While the PBT/ASA-GF composite exhibited a hardness of 81.0, an increase in graphene content resulted in a corresponding rise in surface hardness. However, the difference in surface hardness between different types of graphene was negligible.
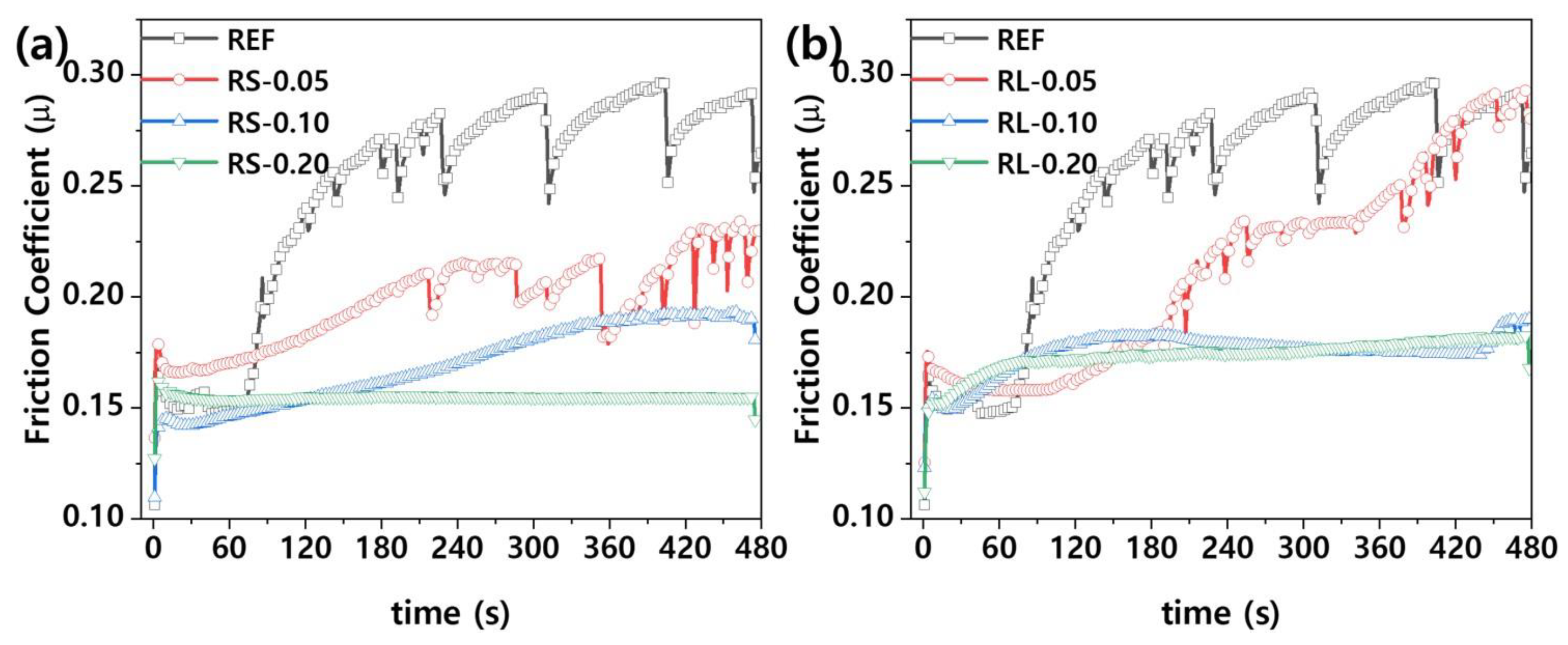
Figure 7 presents the friction coefficient graph of nanocomposites based on the type and content of graphene. As shown in
Figure 7, the friction behavior of polymer nanocomposites shows two distinct trends. Initially, there is a significant increase in the friction coefficient due to the strong frictional force between the steel ball and the sample. As friction continues, the coefficient gradually decreases and stabilizes, which can be attributed to the smoothing of the contact surfaces. This behavior is common in composite materials [
18,
20]. The friction and wear phenomena observed are a combination of abrasive and adhesive wear, where polishing and smoothing are the primary damage mechanisms during friction. These mechanisms typically occur when a smooth and hard surface is in contact with another, causing polymer material to transfer to the harder surface and then be removed as wear debris [
21,
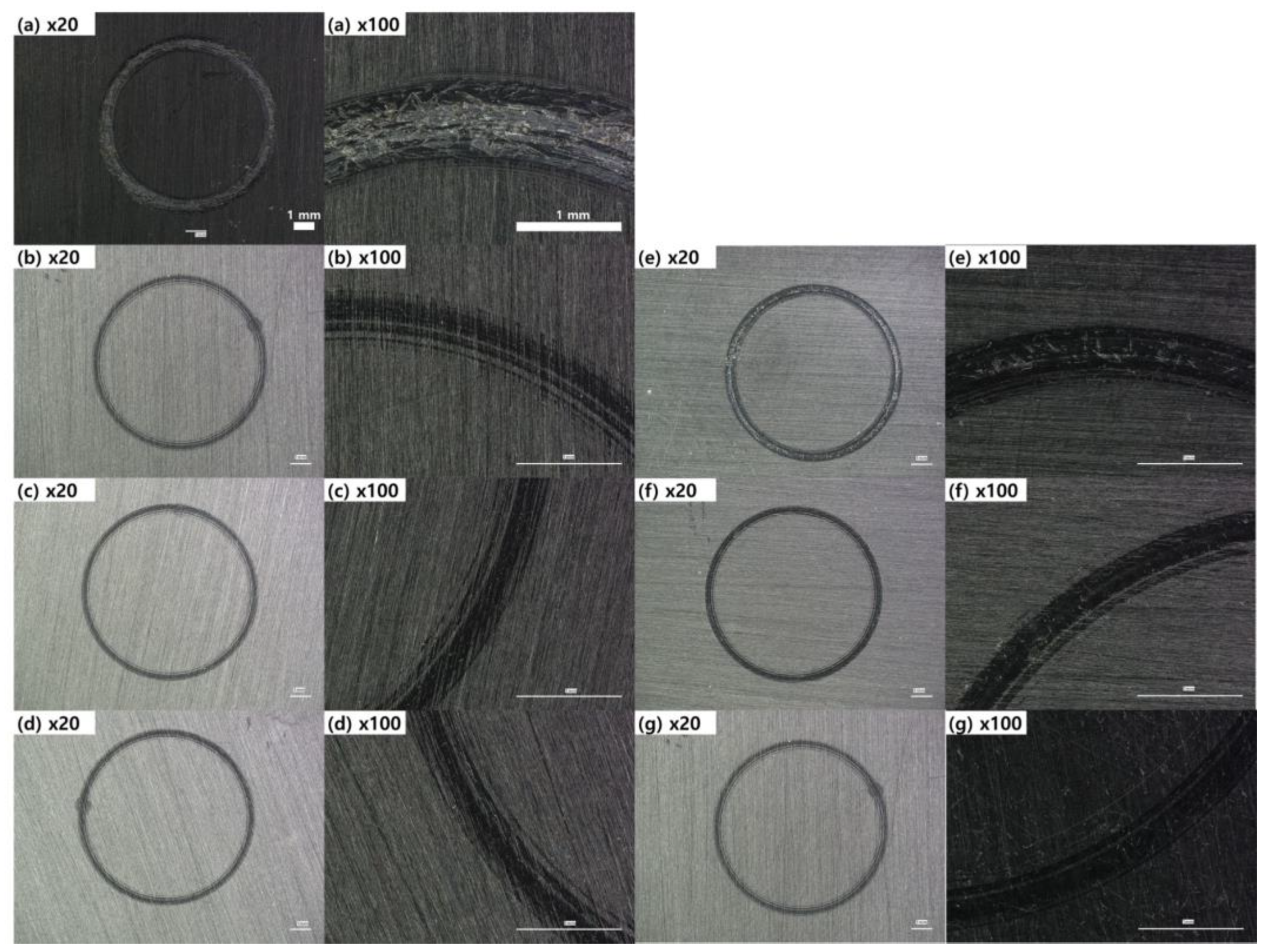
22]. For the PBT/ASA-GF reference sample, the friction coefficient initially increases and stabilizes until about 90 sec, after which it sharply rises. The friction coefficient also fluctuates, showing periodic decreases followed by increases. The cause of this behavior can be inferred from the optical microscope images in
Fig 8, which show the friction surface of the PBT/ASA nanocomposite. In
Figure 8(a), the friction area appears very rough. This instability in friction behavior is likely due to the removal of polymer material during initial friction, exposing the underlying glass fibers, which leads to uneven wear and higher friction. The graph in
Figure 7 shows that when the graphene content is low, the solid lubrication properties of graphene result in a lower friction coefficient compared to the reference. However, as the friction time increases, the friction behavior becomes unstable, likely due to insufficient lubrication from the low graphene content. For example, as seen in
Figure 8(e), the RS-0.05 sample exhibits a rougher surface than the RS-0.05 sample in
Figure 8(b), correlating with its unstable friction behavior. When graphene content is sufficient, the solid lubrication effect is enhanced, leading to more stable friction behavior and a consistently lower friction coefficient.
Even though the friction coefficient is low, wear behavior cannot be fully explained by the friction coefficient alone, as wear rate can still be high [
23]. Therefore, it is also necessary to examine the wear rate.
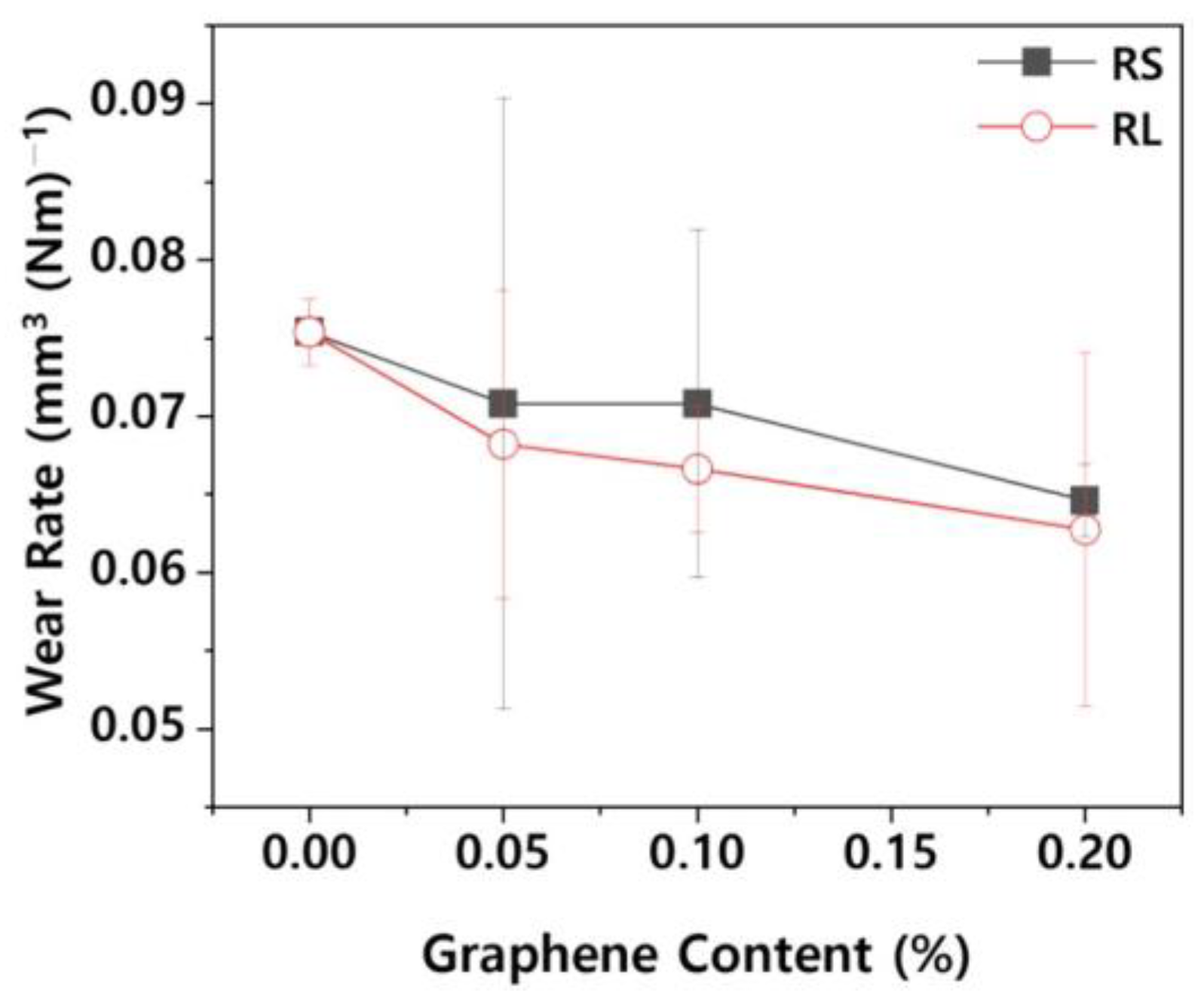
Figure 9 shows the wear rate of PBT/ASA. The wear rate is influenced by the shape of the particles [
24] and is closely related to surface hardness. As surface hardness increases, the wear rate tends to decrease. Specifically, as surface hardness improves, a corresponding reduction in wear rate is observed. For the PBT/ASA-GF reference sample, the wear rate is 7.54 x 10⁻² mm³ N⁻¹ m⁻¹. However, the RS-0.20 sample showed a 14.3 % reduction in wear rate, and the RL-0.20 sample demonstrated a 16.8 % reduction. Based on the results of both the friction coefficient and wear rate, it can be concluded that the PBT/ASA nanocomposites exhibit improved wear resistance.
Since PBT/ASA nanocomposites absorb moisture and release it depending on their use environment, the hygroscopic behavior of polymer composites used in components is crucial.
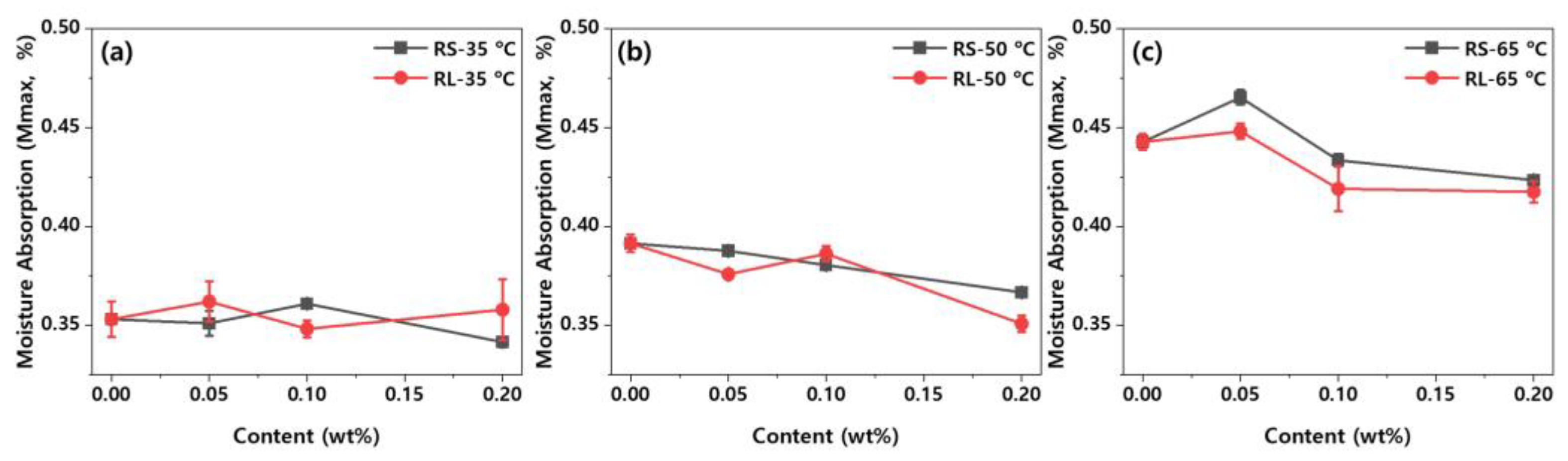
Figure 10 shows the maximum moisture absorption as a function of graphene content and type at different ambient temperatures. As shown in
Figure 10, the maximum moisture absorption tends to increase as the ambient temperature rises. At lower temperatures, the differences in maximum moisture absorption between the various types and contents of graphene are minimal. However, at higher ambient temperatures, an increase in graphene content results in a noticeable decrease in maximum moisture absorption. This indicates that graphene effectively blocks moisture diffusion pathways, demonstrating its efficacy in reducing moisture absorption.
, (b) 50 and (c) 65 .
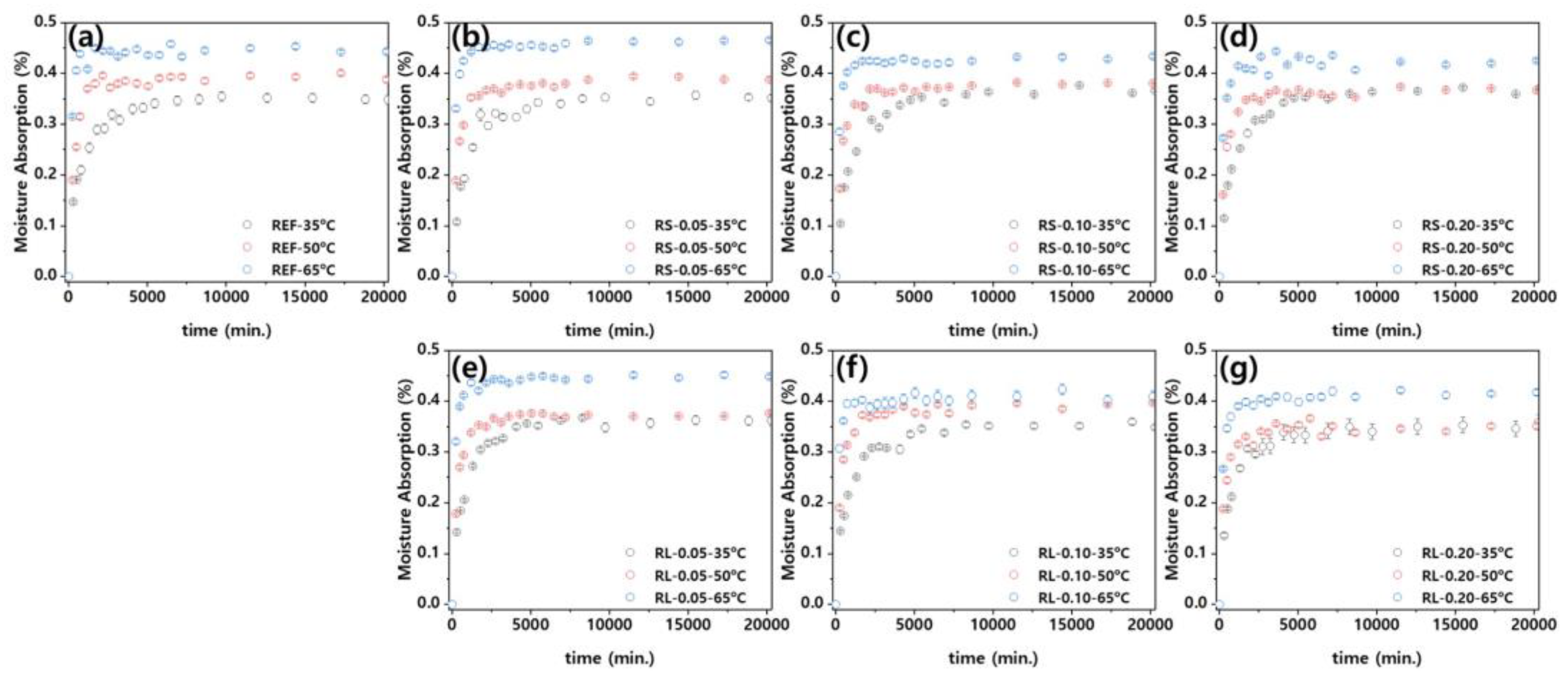
Figure 11 presents a graph showing the moisture absorption rate of the nanocomposites over time, calculated according to Eq. (3). Studies on the moisture absorption behavior of polymers often use diffusion-controlled equations, such as Fick's 2nd law, to model various aspects of the process [
11,
12,
13,
14,
15]. Moisture absorption is described as the cumulative uptake of moisture in the material as a function of time, represented by the diffusion coefficient (D). Crank
et al. [
25] developed a simplified mathematical solution to Fick’s 2nd law based on trigonometric functions, which applies to a large flat plate with thickness
h. The amount of absorbed water at any given time
t, Mt (%), can be calculated using Eq. (3).
Where
represents the maximum moisture absorption (%), D is the diffusion coefficient (m² min⁻¹), and h is the thickness of the sample (m). As shown in
Figure 11, the moisture absorption of the nanocomposites increases over time until it reaches an equilibrium state after absorbing a certain amount of moisture. When considering the influence of ambient temperature, the time required to reach equilibrium decreases as the ambient temperature increases. Figure S1–S3 present the moisture absorption graphs of PBT/ASA nanocomposites at different ambient temperatures, plotted using Eq. (3) for curve fitting. As described earlier, each nanocomposite exhibits moisture absorption behavior consistent with the diffusion-controlled equation. Additionally, the adjusted coefficient of determination (adj.
) for the curve fitting method ranges from 0.90 to 0.99, indicating a very high level of accuracy. This fitting allows for the extraction of the diffusion coefficients.양식의 맨 아래
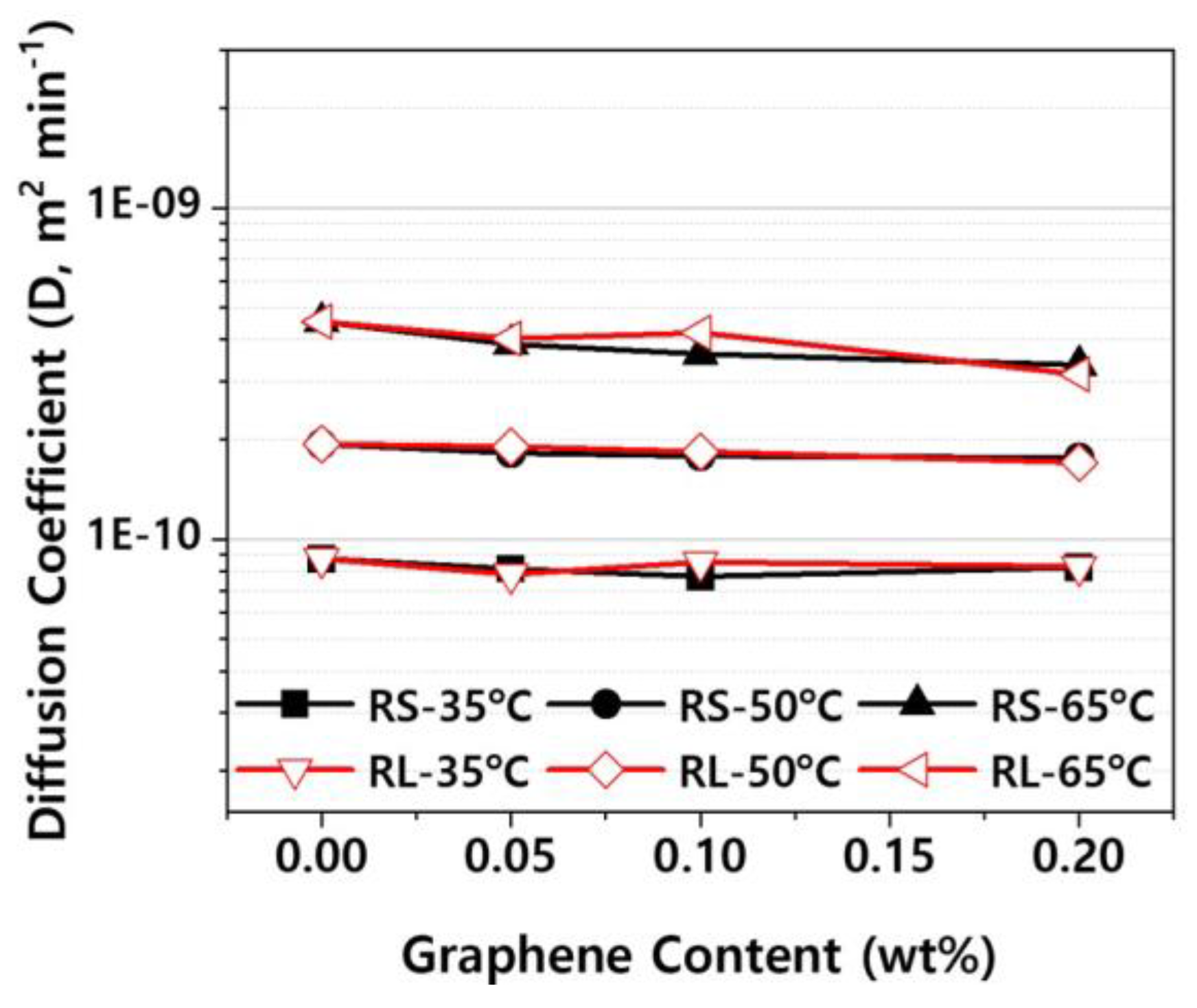
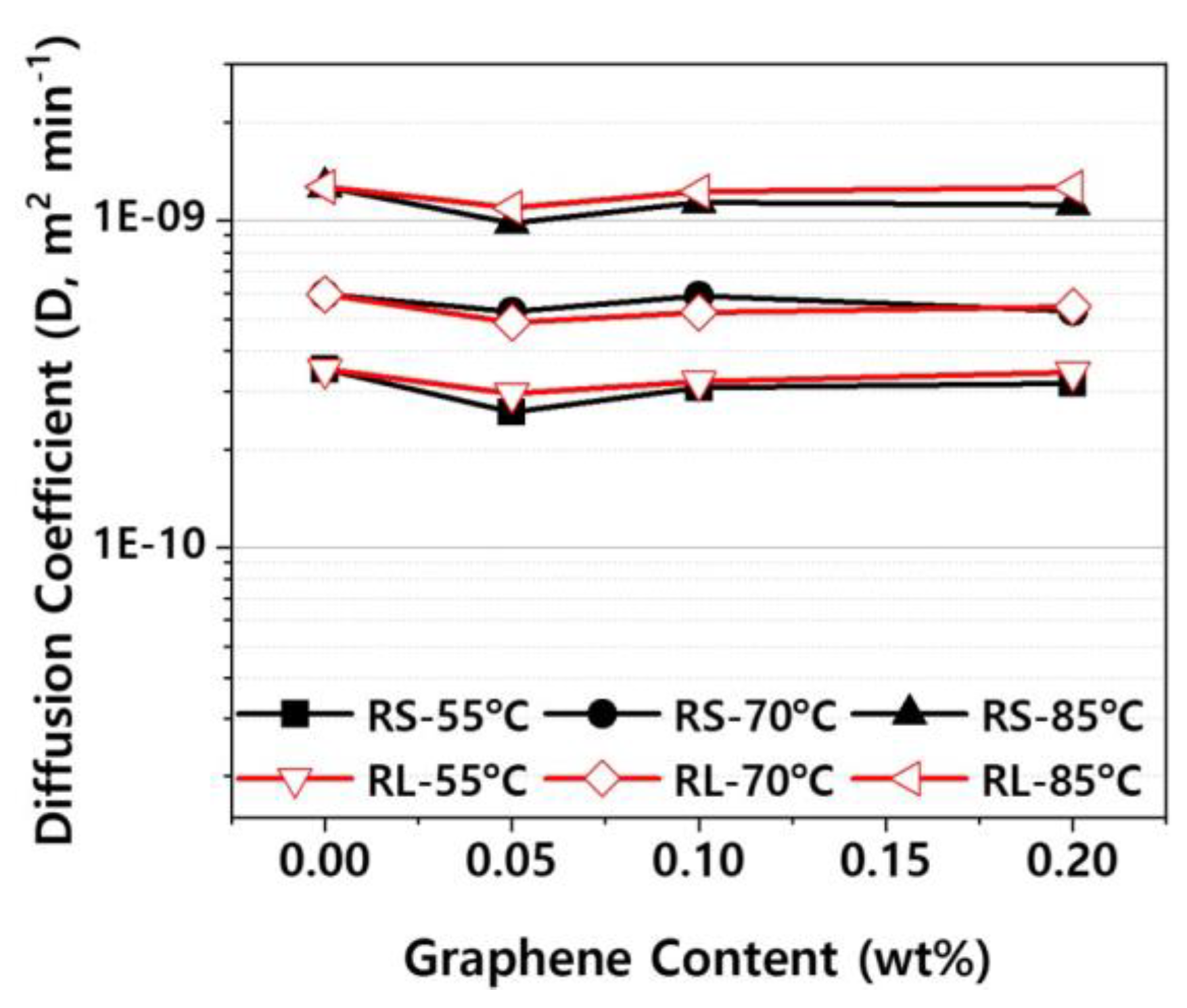
Figure 12 presents the diffusion coefficients derived from the fitting curves in Figure S1–S3. As shown in Figure 12, the diffusion coefficient decreases slightly as the graphene content increases. This is likely due to the increased amount of graphene sheets, which act as barriers, reducing the rate of moisture absorption [
9,
10]. Additionally, the diffusion coefficient increases with rising ambient temperature, indicating that the temperature has a much larger effect on the diffusion coefficient than the graphene additive. This is because the diffusion of moisture in plastics is primarily an energy activation process, and the diffusion coefficient follows an Arrhenius equation, which exhibits exponential temperature dependence alongside activation energy [
11,
12,
13,
14].
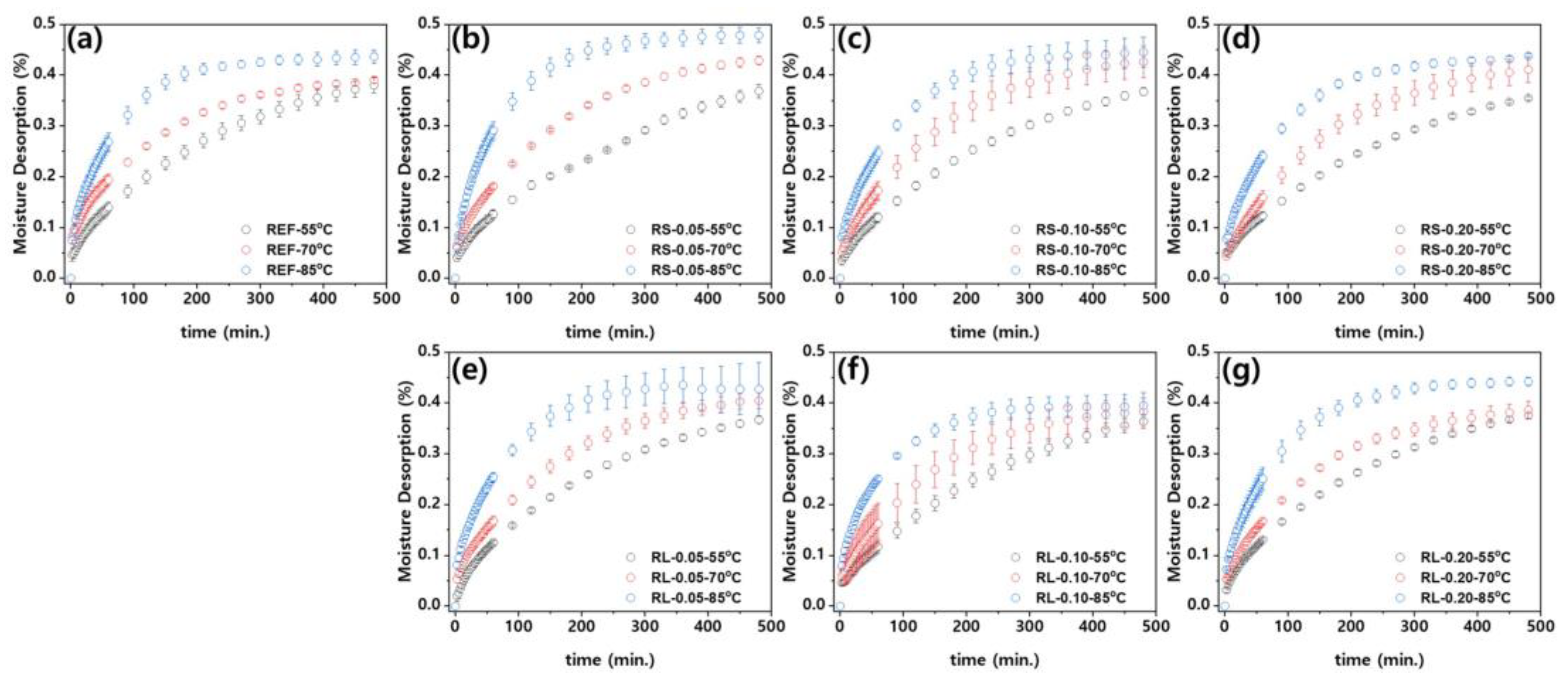
Figure 13 presents the moisture desorption of the nanocomposites over time, calculated according to Eq. (2). Similar to the moisture absorption behavior, the desorption process also follows a diffusion-governing equation. As shown in Figure 13, the desorption of the nanocomposites increases over time until all the moisture inside the polymer is released, eventually reaching an equilibrium state. When examining the influence of ambient temperature, it becomes clear that the time required to reach equilibrium decreases as the ambient temperature increases. Figure S4–S6 show the moisture desorption curves of PBT/ASA nanocomposites at different ambient temperatures, plotted using Eq. (2) for curve fitting. As with the hygroscopic behavior, it can be seen that each nanocomposite material exhibits a hygroscopic behavior according to the diffusion-governing equation. Furthermore, the curve fitting method shows a very high level of accuracy, with adjusted coefficients of determination (adj. R
2) ranging from 0.90 to 0.99. This high accuracy allows for the calculation of the diffusion coefficients.
When comparing
Figure 12 and
Figure 14, it is evident that the diffusion coefficient during the desorption process is higher than that during the absorption process. In a closed system where no external moisture is supplied, polymers undergo a reversible reaction, simultaneously absorbing and releasing moisture. If the diffusion coefficient for desorption is higher than that for absorption, desorption becomes the dominant process. Therefore, in sealed environments like headlamps, the internal absolute humidity gradually increases, leading to condensation on the inner surface of the outer lens due to temperature differences with the outside. The variation in the difference between absorption and desorption diffusion coefficients with respect to the nanofiller content was found to be negligible.
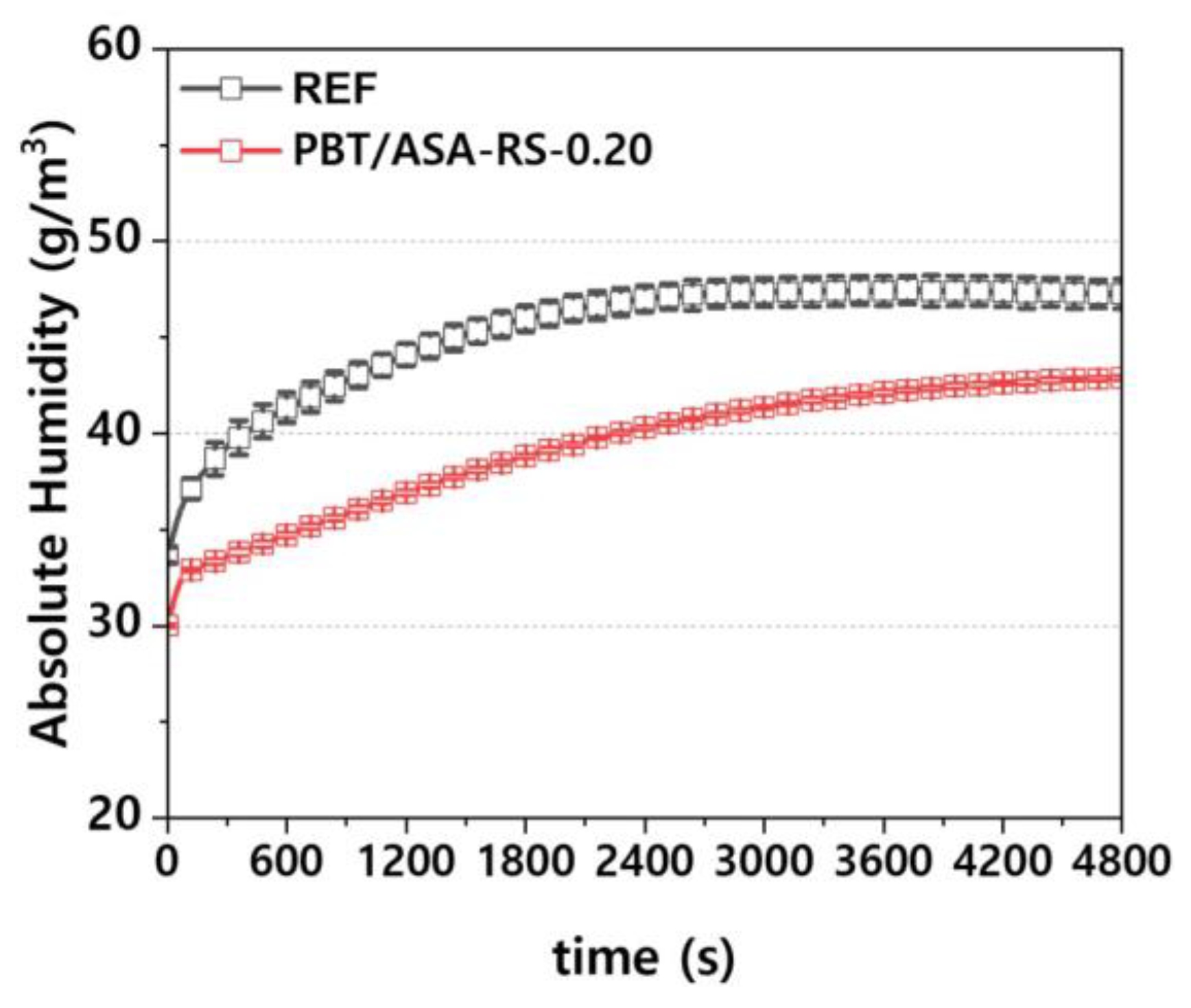
To verify the moisture reduction effect of the PBT/ASA nanocomposites, headlamps were manufactured and the internal absolute humidity was measured. Measurements were taken at five different points, and the average values are presented. As shown in
Figure 15, the error at each measurement point is minimal. It can be observed from the figure that as the lamp operation time increases, the absolute humidity increases because of moisture released from within the polymer. However, the nanocomposite’s barrier properties cause the rate of moisture release to be lower compared to conventional composites. Additionally, during headlamp preconditioning, the nanocomposites absorbed less external moisture than conventional composites, resulting in a reduced amount of released moisture during desorption.
As previously discussed, the moisture absorption and desorption behavior of polymer materials used in sealed components, such as headlamps, can lead to issues like fogging. After injection molding, polymer parts undergo cooling, storage, transportation, pre-assembly staging, and assembly, during which they absorb moisture from the air. While thorough drying before assembly can minimize moisture content, completely eliminating it is challenging and would lead to increased time and costs, making it impractical for real-world production lines. Therefore, understanding the moisture absorption and desorption characteristics of the materials used is essential to address these issues effectively through optimized part design.