Submitted:
15 October 2024
Posted:
15 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantification of Silymarin Components in the Total Methanolic Extract of Anastatica hierochuntica
2.2.1. Stock Preparation of the Total Methanolic Extract and Silymarin Components
2.2.2. Instrument and Chromatographic Parameters
2.3. Fabrication of Anastatica hierochuntica Extract-Loaded TPU-Based Nanofibers
2.3.1. Preparation of the Spinning Solution
2.3.2. The Electrospinning Process
2.4. Characterization of the Fabricated Nanofibers
2.4.1. Evaluation of Nanofibers Morphology and Surface Roughness
2.4.2. Static Water Contact Angle (WCA)
2.4.3. Mechanical Properties
2.4.4. Percentage Water Uptake (%Wu)
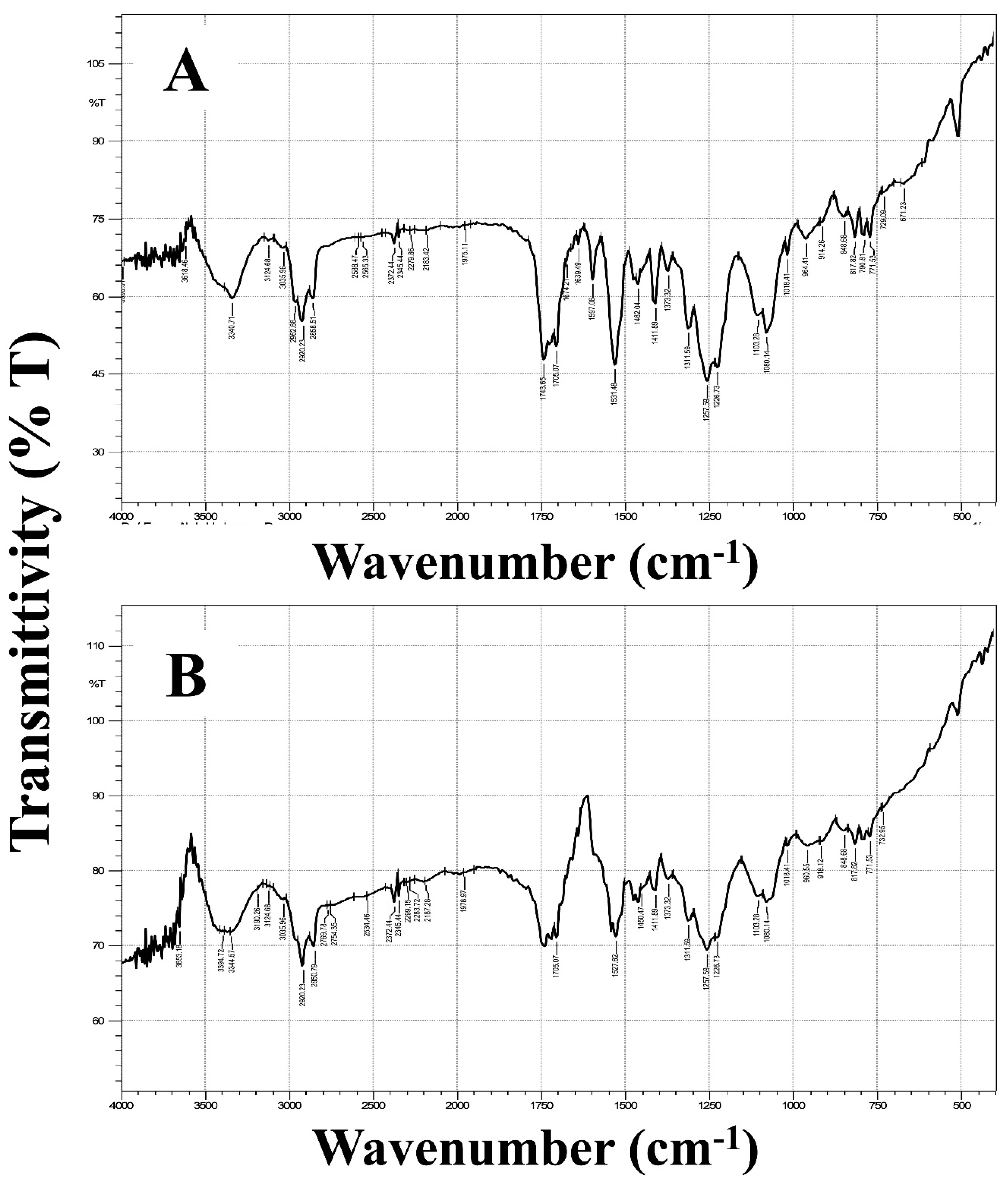
2.4.5. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.4.6. Inspection of In-Vitro Release Behavior
2.5. Microbiological Assay
2.5.1. Selection of Bacterial and Fungal Strains
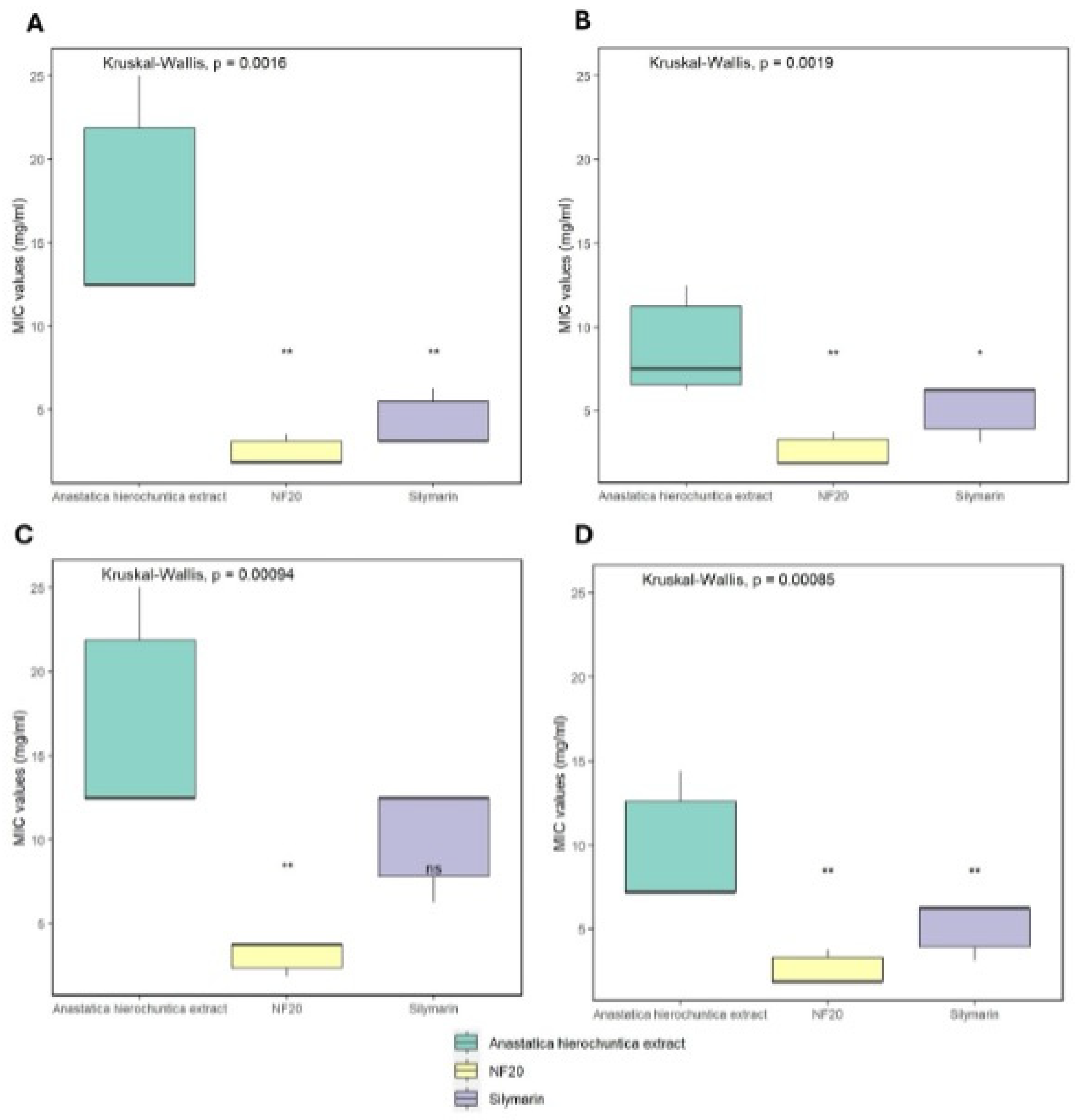
2.5.2. Determination of Minimum Inhibitory Concentration (MIC)
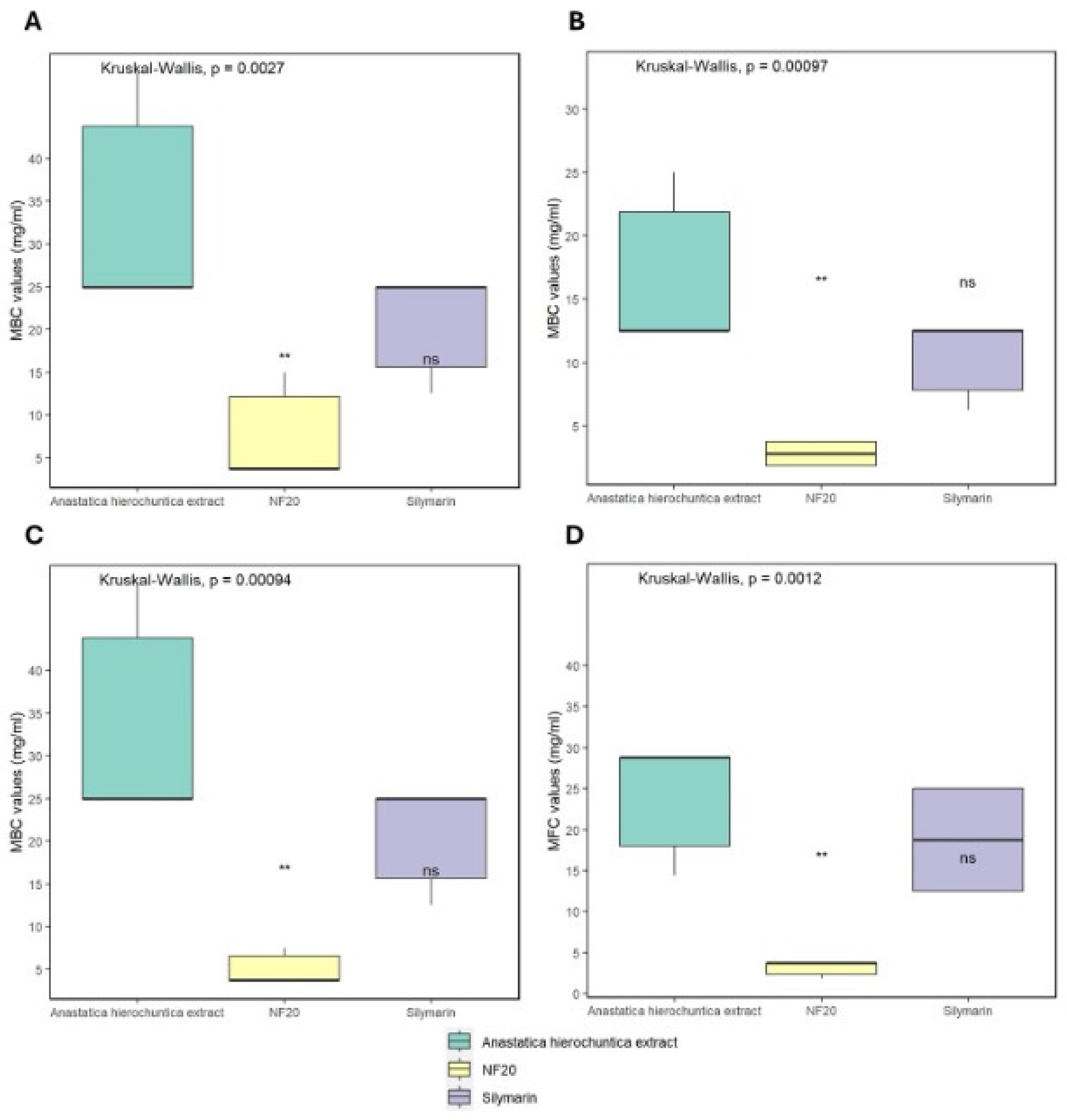
2.5.3. Determination of Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration (MFC)
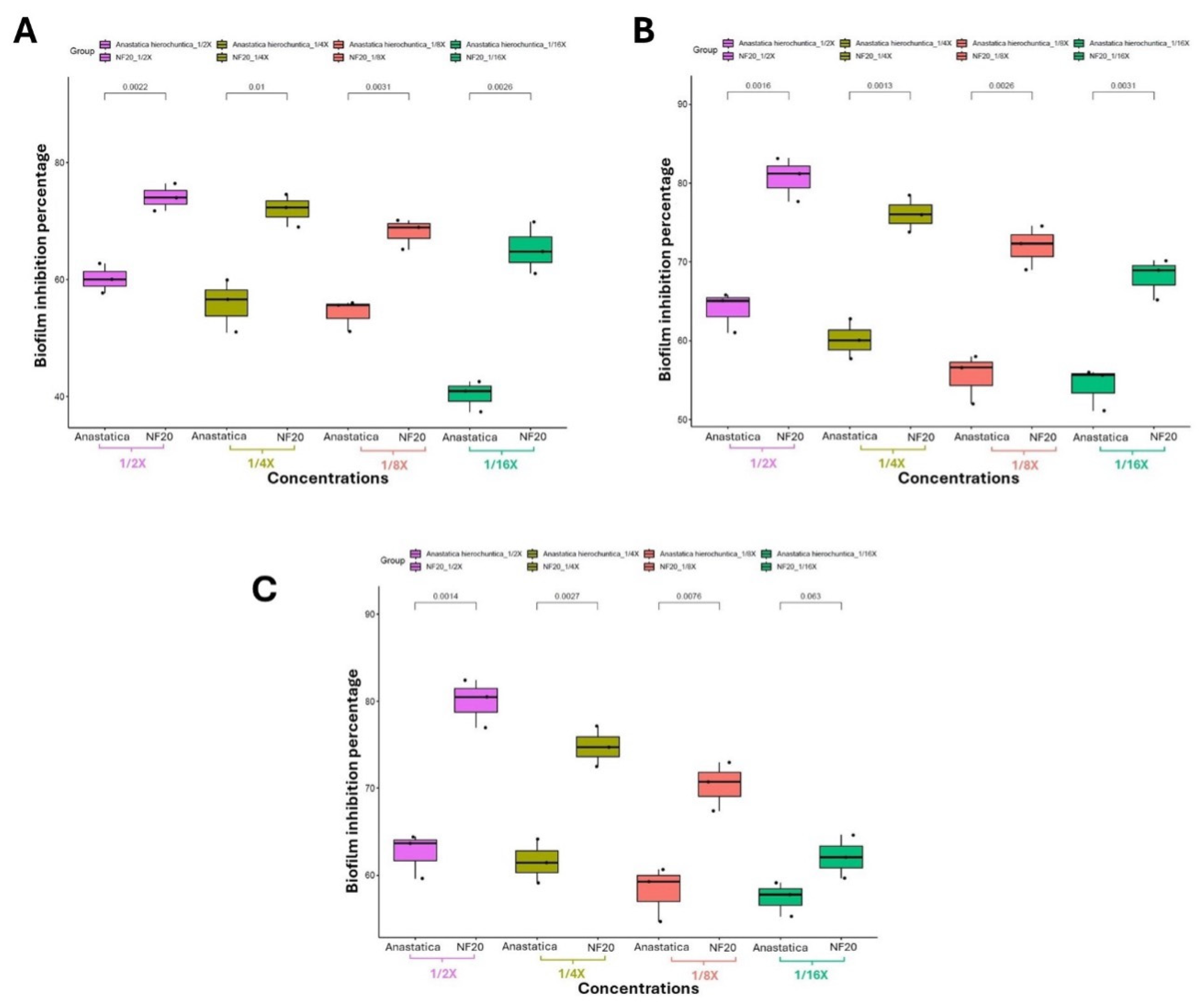
2.5.4. Investigating the Biofilm Inhibitory Effect via Crystal Violet Technique
2.6. Cell-line Studies
2.6.1. Cell-Line Preparation
2.6.2. Wound Healing Assay (Scratch Assay)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Silymarin Components Quantification
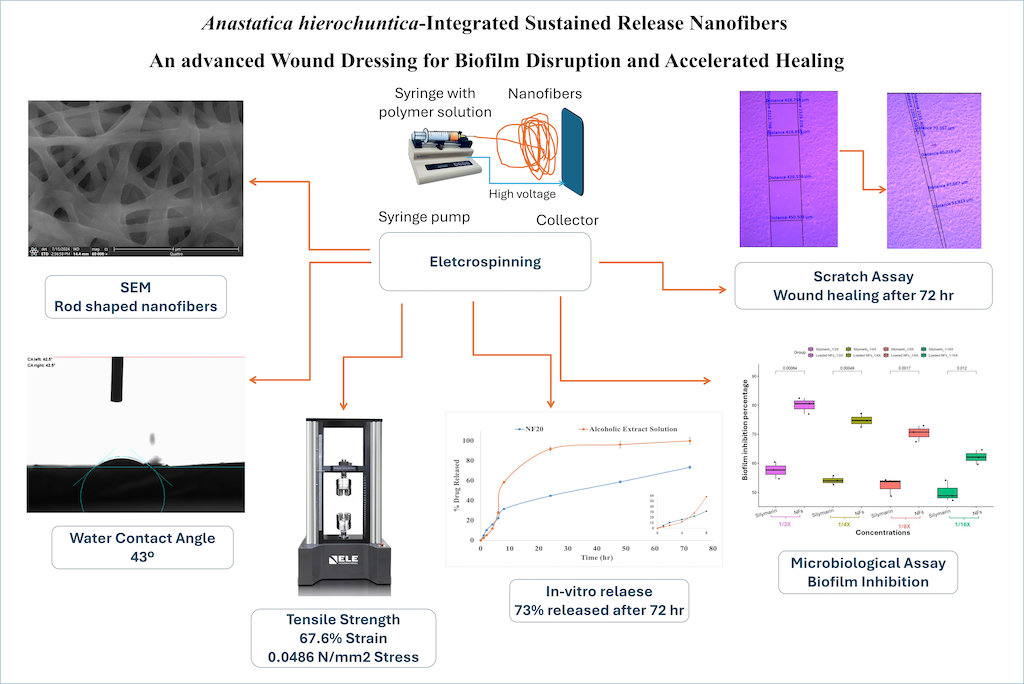
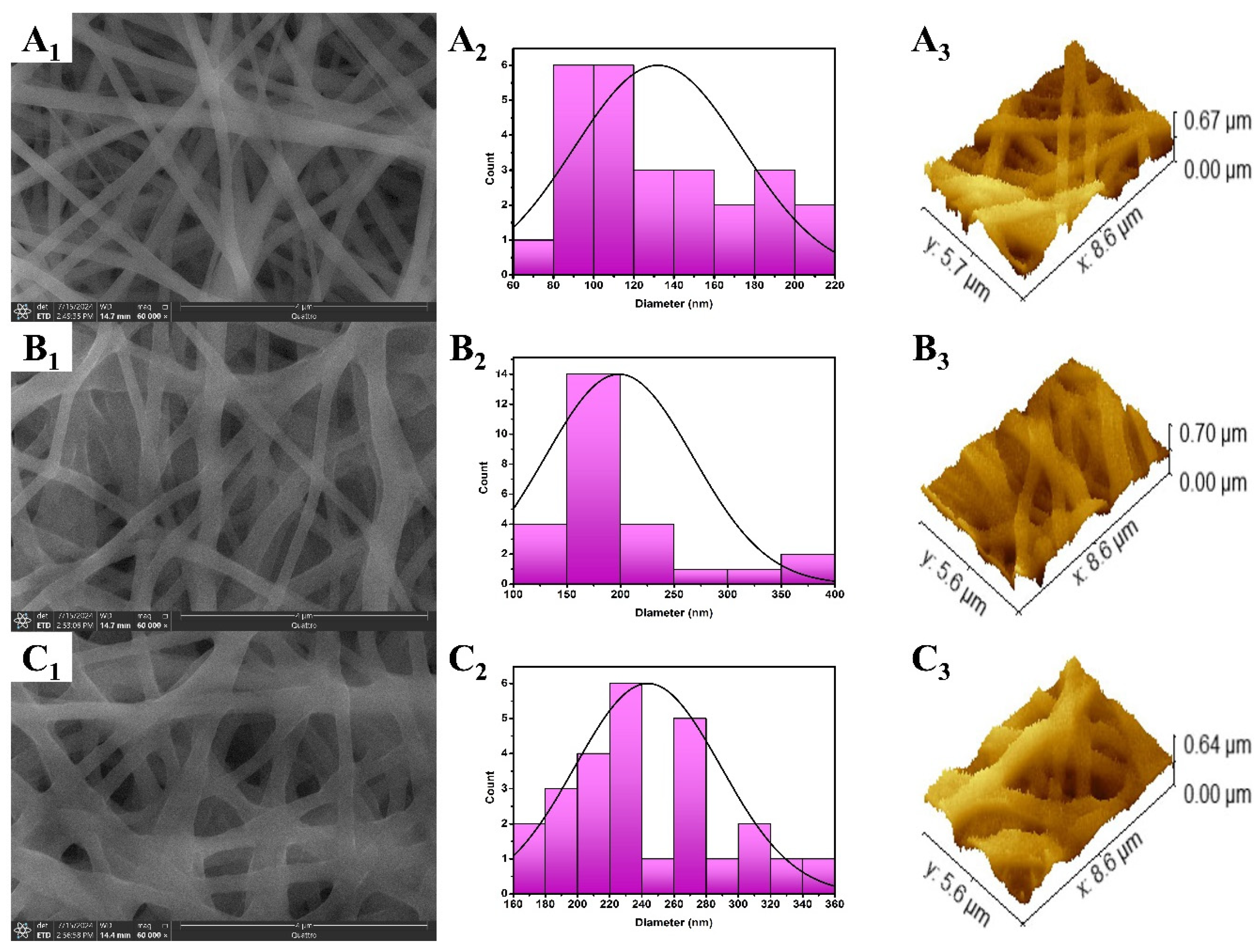
3.2. Characterization of the Fabricated Nanofibers
3.2.1. Evaluation of Nanofibers Morphology and Surface Roughness
3.2.2. Static Water Contact Angle (WCA)
3.2.3. Mechanical Properties
3.2.4. Percentage Water Uptake (%Wu)
3.2.5. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.2.6. Inspection of In-Vitro Release Behavior
3.3. Microbiological Assay
3.3.1. Determination of Minimum Inhibitory Concentration (MIC)
3.3.2. Determination of Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration (MFC)
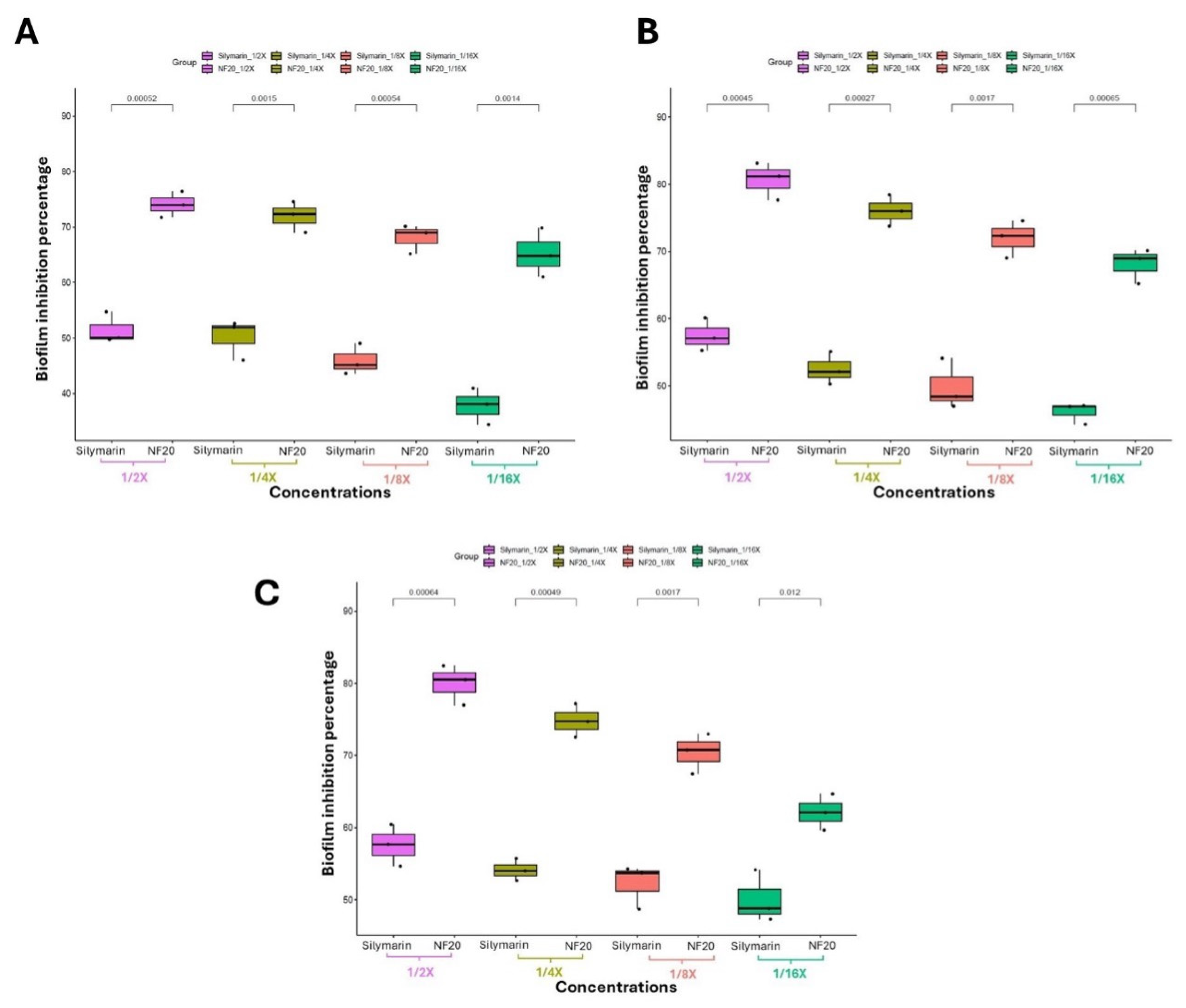
3.3.3. Investigating the Biofilm Inhibitory Effect via Crystal Violet Technique
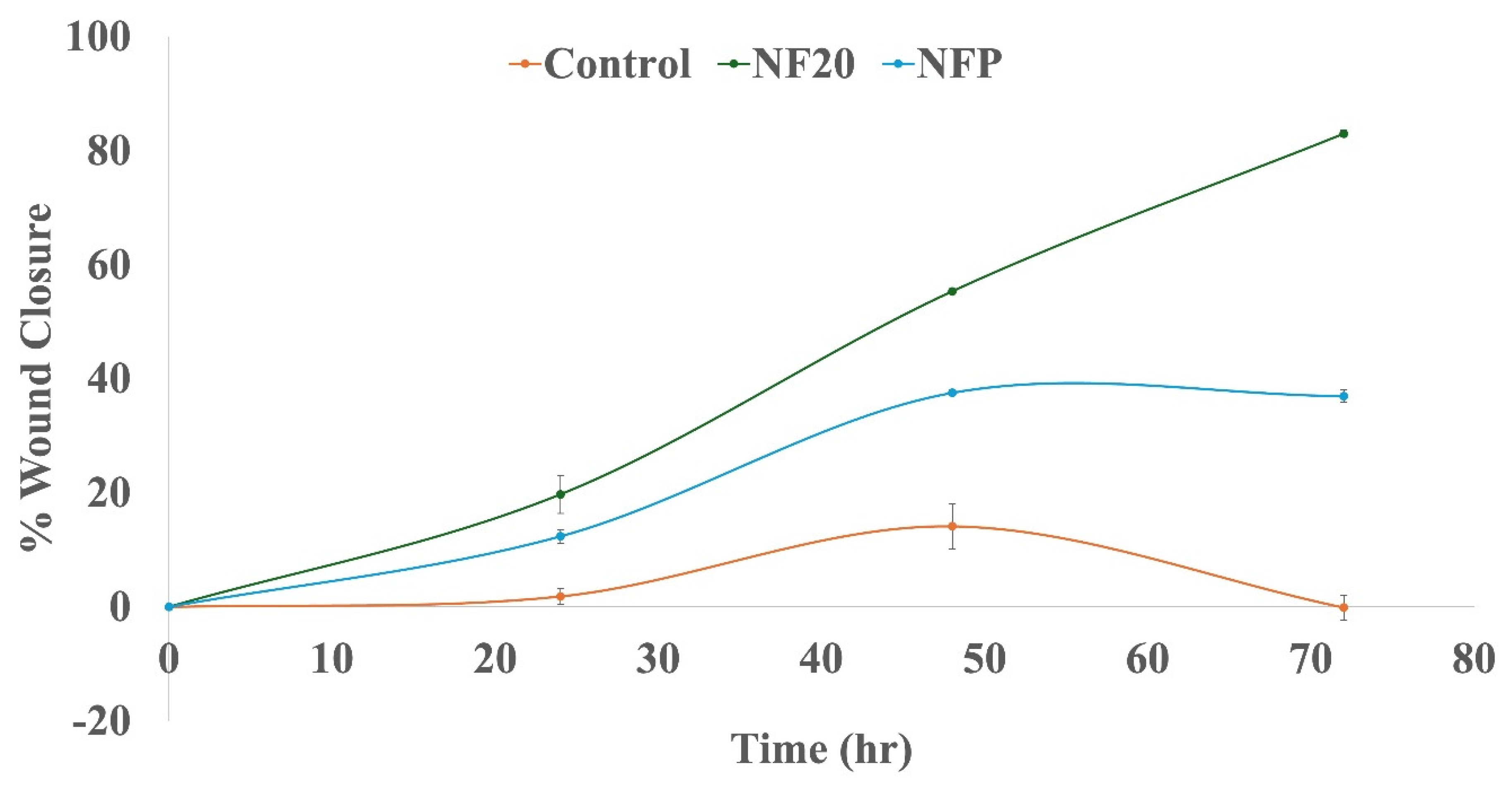
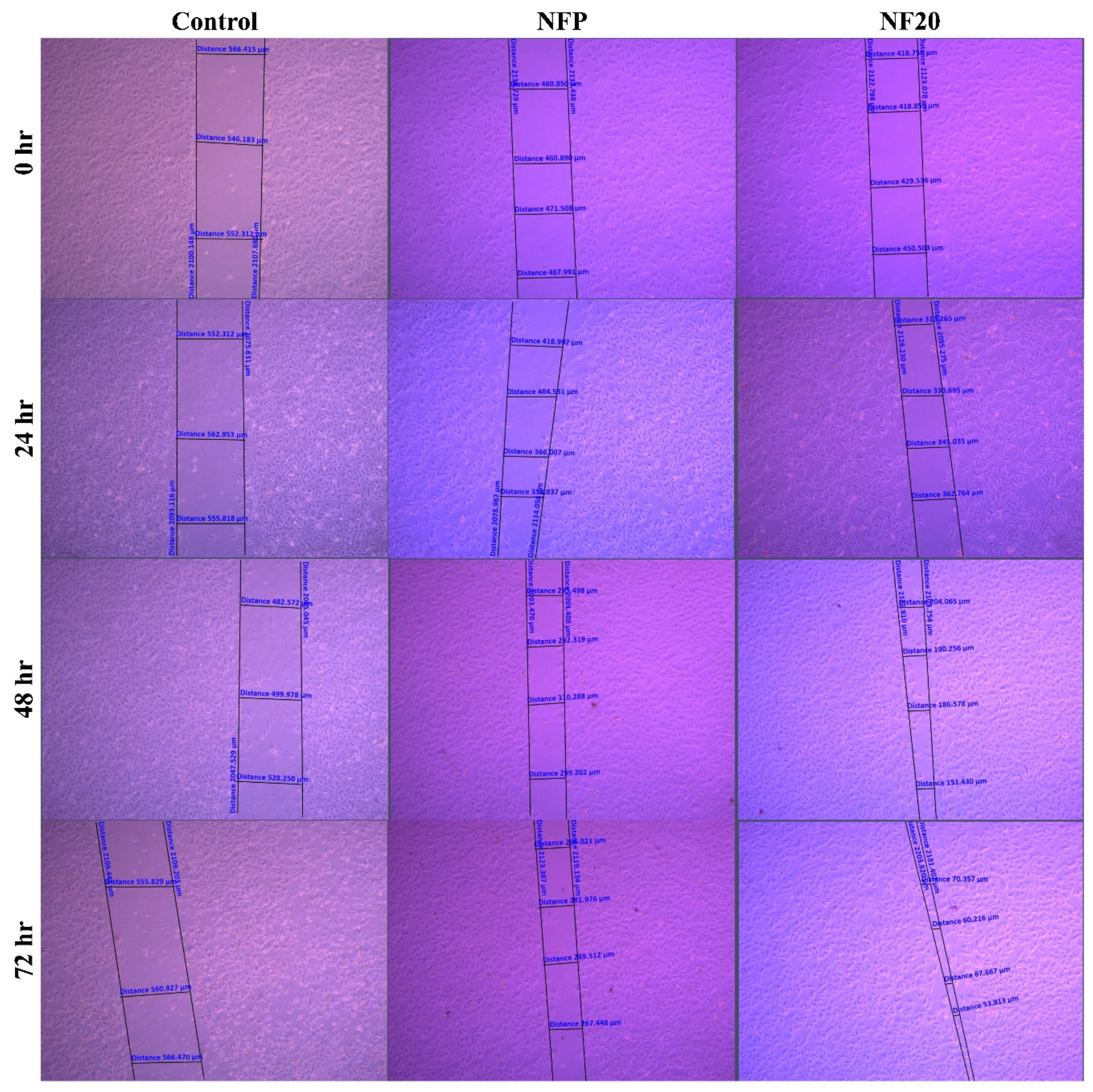
3.4. Cell-line Studies; Wound Healing Assay
4. Conclusion
Author contributions
Funding
Acknowledgement
Conflict of Interest
References
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Chapter 1 - Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, 2016; pp. 1–14; ISBN 978-0-12-802926-8. [Google Scholar]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial Biomaterials for Skin Wound Dressing. Asian J Pharm Sci 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Gao, Z.; Mao, X.; Cheng, J.; Huang, L.; Tang, J. Advances in Wound Dressing Based on Electrospinning Nanofibers. J Appl Polym Sci 2024, 141, e54746. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. Journal of International Medical Research 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J Dent Res 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Leaper, D.J. Basic Science of Wound Healing. Surgery (Oxford) 2008, 26, 31–37. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Holloway, S.; Harding, K.G. Wound Dressings. Surgery - Oxford International Edition 2022, 40, 25–32. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J Adv Res 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Kenry; Lim, C. T. Nanofiber Technology: Current Status and Emerging Developments. Prog Polym Sci 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Yan, X.; Xiao, X.; Au, C.; Mathur, S.; Huang, L.; Wang, Y.; Zhang, Z.; Zhu, Z.; Kipper, M.J.; Tang, J.; et al. Electrospinning Nanofibers and Nanomembranes for Oil/Water Separation. J. Mater. Chem. A 2021, 9, 21659–21684. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun Fibers and Their Application in Drug Controlled Release, Biological Dressings, Tissue Repair, and Enzyme Immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Abdullah, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl Bio Mater 2019, 2, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun Cellulose Acetate/Gelatin Nanofibrous Wound Dressing Containing Berberine for Diabetic Foot Ulcer Healing: In Vitro and in Vivo Studies. Sci Rep 2020, 10, 8312. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, H.; Tian, X.; Sun, Y.; Shi, B.; Zhou, Y.; Sheng, D.; Liu, X.; Yang, Y. Hard, Tough and Fast Self-Healing Thermoplastic Polyurethane. Prog Org Coat 2021, 159, 106409. [Google Scholar] [CrossRef]
- Liang, W.; Ni, N.; Huang, Y.; Lin, C. An Advanced Review: Polyurethane-Related Dressings for Skin Wound Repair. Polymers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Yusof, J.; Mahdy, Z.A.; Noor, R.M. Use of Complementary and Alternative Medicine in Pregnancy and Its Impact on Obstetric Outcome. Complement Ther Clin Pract 2016, 25, 155–163. [Google Scholar] [CrossRef]
- Zin, S.R.M.; Kassim, N.M.; Alshawsh, M.A.; Hashim, N.E.; Mohamed, Z. Biological Activities of Anastatica Hierochuntica L.: A Systematic Review. Biomedicine & Pharmacotherapy 2017, 91, 611–620. [Google Scholar] [CrossRef]
- Sobhy, E.; Tailang, M.; Benyounes, S.; Karunakaran, G. Antimalarial and Hepatoprotective Effects of Entire Plants of Anastatic Hierochuntica. 2011, 1, 24–27.
- abou elella, F.; Ahmed, E.; Gavamukulya, Y. Determination of Antioxidant and Anti-Inflammatory Activities, as Well as in Vitro Cytotoxic Activities of Extracts of Anastatica Hierochuntica (Kaff Maryam) against HeLa Cell Lines. J Med Plant Res 2016, 10, 77–87. [Google Scholar] [CrossRef]
- AL-Saeed, A.; Jaber, N. Chemical Content and Antibacterial Activity of Some Extracts of Anastatica Hierochuntica Leaves. 2013, 4.
- Alatshan, A.; Qnais, E.; Wedyan, M.; Bseiso, Y.; Alzyoud, E.; Banat, R.; Alkhateeb, H. Antinociceptive and Antiinflammatory Activities of Anastatica Hierochuntica and Possible Mechanism of Action. Indian J Pharm Sci 2018, 80. [Google Scholar] [CrossRef]
- Daur, I. Chemical Properties of the Medicinal Herb Kaff Maryam (Anastatica Hierochuntica L.) and Its Relation to Folk Medicine Use. Afr J Microbiol Res 2012, 6, 5048–5051. [Google Scholar] [CrossRef]
- Alqudah, A.; AbuDalo, R.; Qnais, E.; Wedyan, M.; Qudah, T.; Oqal, M. Potential Anti-Inflammatory Activity of the Anastatica Hierochuntica Essential Oil. Journal of Essential Oil Research 2023, 35, 1–10. [Google Scholar] [CrossRef]
- Zin, S.R.M.; Kassim, N.M.; Alshawsh, M.A.; Hashim, N.E.; Mohamed, Z. Biological Activities of Anastatica Hierochuntica L.: A Systematic Review. Biomedicine & Pharmacotherapy 2017, 91, 611–620. [Google Scholar] [CrossRef]
- Amin Mohamed, A.; Khalil, A.; El-Beltagi, H. Antioxidant and Antimicrobial Properties of Kaff Maryam(Anastatica Hierochuntica) and Doum Palm (Hyphaene Thebaica). Grasas y Aceites 2010, 61, 67–75. [Google Scholar] [CrossRef]
- Petrásková, L.; Káňová, K.; Biedermann, D.; Křen, V.; Valentová, K. Simple and Rapid HPLC Separation and Quantification of Flavonoid, Flavonolignans, and 2,3-Dehydroflavonolignans in Silymarin. Foods 2020, 9. [Google Scholar] [CrossRef]
- Adel, I.M.; ElMeligy, M.F.; Elkasabgy, N.A. Conventional and Recent Trends of Scaffolds Fabrication: A Superior Mode for Tissue Engineering. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Salam, A.; Khan, M.Q.; Hassan, T.; Hassan, N.; Nazir, A.; Hussain, T.; Azeem, M.; Kim, I.S. In-Vitro Assessment of Appropriate Hydrophilic Scaffolds by Co-Electrospinning of Poly(1,4 Cyclohexane Isosorbide Terephthalate)/Polyvinyl Alcohol. Sci Rep 2020, 10, 19751. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Amer, M.S.; Elkasabgy, N.A. 3D Nanocomposite Alginate Hydrogel Loaded with Pitavastatin Nanovesicles as a Functional Wound Dressing with Controlled Drug Release; Preparation, in-Vitro and in-Vivo Evaluation. J Drug Deliv Sci Technol 2022, 71, 103292. [Google Scholar] [CrossRef]
- Samy, M.; Ekram, B.; Abd El-Hady, B.M.; Ayoub, M.M.H. In Vitro Release Study of Electrospun Poly(ε-Caprolactone)/Gelatin Nanofiber Mats Loaded with 5-Fluorouracil. Polymer Bulletin 2024, 81, 3953–3972. [Google Scholar] [CrossRef]
- Adel, I.M.; ElMeligy, M.F.; Abdelkhalek, A.A.; Elkasabgy, N.A. Design and Characterization of Highly Porous Curcumin Loaded Freeze-Dried Wafers for Wound Healing. European Journal of Pharmaceutical Sciences 2021, 164, 105888. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-Based 96-Well Plate Microdilution Method for the Determination of Minimum Inhibitory Concentration of Biosurfactants. Biotechnol Lett 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.; Trimble, M.; Hancock, R. Microtiter Plate Assays to Assess Antibiofilm Activity against Bacteria. Nat Protoc 2021, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. JoVE 2011, e2437. [Google Scholar] [CrossRef]
- Racine, J. RStudio: A Platform-Independent IDE for R and Sweave. Journal of Applied Econometrics 2012, 27. [Google Scholar] [CrossRef]
- Kauanova, S.; Urazbayev, A.; Vorobjev, I. The Frequent Sampling of Wound Scratch Assay Reveals the “Opportunity” Window for Quantitative Evaluation of Cell Motility-Impeding Drugs. Front Cell Dev Biol 2021, 9. [Google Scholar] [CrossRef]
- Hussein, J.; ELBana, M.; Abdel Latif, Y.; Saleh, S.; Tolba, E. Wound Healing Activity of Cotton Fabrics Loaded with Silver Nanoparticles in Experimental Model of Diabetes. Biomedical and Pharmacology Journal 2023, 16, 53–65. [Google Scholar] [CrossRef]
- Meenatchi, V.; Bhaskar, R.; Sood, A.; Han, S.S. Preparation of β-Cyclodextrin Derivatives/Metamizole Inclusion Complex Nanofibers: Characterization, Drug Release, and Wound Scratch Assay. J Drug Deliv Sci Technol 2024, 97, 105752. [Google Scholar] [CrossRef]
- Morilla-Herrera, J.C.; Morales-Asencio, J.M.; Gómez-González, A.J.; Díez-De Los Ríos, A.; Lupiáñez-Pérez, I.; Acosta-Andrade, C.; Aranda-Gallardo, M.; Moya-Suárez, A.B.; Kaknani-Uttumchandani, S.; García-Mayor, S. Effectiveness of a Hydrophobic Dressing for Microorganisms’ Colonization of Vascular Ulcers: Protocol for a Randomized Controlled Trial (CUCO-UV Study). J Adv Nurs 2020, 76, 2191–2197. [Google Scholar] [CrossRef]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J Funct Biomater 2021, 12. [Google Scholar] [CrossRef]
- Yang, S.; Lan, L.; Gong, M.; Yang, K.; Li, X. An Asymmetric Wettable PCL/Chitosan Composite Scaffold Loaded with IGF-2 for Wound Dressing. J Biomater Appl 2022, 37, 577–587. [Google Scholar] [CrossRef]
- Liu, L.; Sun, H.; Zhang, J.; Xu, B.; Gao, Y.; Qi, D.; Mao, Z.; Wu, J. Trilayered Fibrous Dressing with Wettability Gradient for Spontaneous and Directional Transport of Massive Exudate and Wound Healing Promotion. Advanced Fiber Materials 2023, 5, 574–587. [Google Scholar] [CrossRef]
- Daur, I. Chemical Properties of the Medicinal Herb Kaff Maryam (Anastatica Hierochuntica L.) and Its Relation to Folk Medicine Use. Afr J Microbiol Res 2012, 6, 5048–5051. [Google Scholar] [CrossRef]
- Kalra, A.; Lowe, A. Mechanical Behaviour of Skin: A Review. Journal of Material Science & Engineering. [CrossRef]
- Taha, E.; Nour, S.A.; Mamdouh, W.; Naguib, M.J. Investigating the Potential of Highly Porous Zopiclone-Loaded 3D Electrospun Nanofibers for Brain Targeting via the Intranasal Route. Int J Pharm 2024, 660, 124230. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Newton, M.A.A.; Xin, B.; Li, T. Preparation and Characterization of Unidirectional Water-Transported Bilayer PLA/ZnO-PAN/SPA Nanofibrous Membrane for Wound Healing. Colloids Surf A Physicochem Eng Asp 2023, 676, 132308. [Google Scholar] [CrossRef]
- Suryamathi, M.; Ruba, C.; Viswanathamurthi, P.; Balasubramanian, V.; Perumal, P. Tridax Procumbens Extract Loaded Electrospun PCL Nanofibers: A Novel Wound Dressing Material. Macromol Res 2019, 27, 55–60. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kang, B.-K.; Kim, H.-D.; Yoo, H.-J.; Kim, J.-S.; Huh, J.-H.; Jung, Y.-J.; Lee, D.-J. Effect of Hot Pressing/Melt Mixing on the Properties of Thermoplastic Polyurethane. Macromol Res 2009, 17, 616–622. [Google Scholar] [CrossRef]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsad, B.; Bonakdar, S. Manufacturing of Biodegradable Polyurethane Scaffolds Based on Polycaprolactone Using a Phase Separation Method: Physical Properties and in Vitro Assay. Int J Nanomedicine 2011, 6, 2375–2384. [Google Scholar] [CrossRef]
- P. , B. Wound Healing and the Role of Fibroblasts. J Wound Care 2013, 22, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Naeini, A.; Moshiri, A.; Mohammadalipour, A.; Tabandeh, M. reza Modulation of Cutaneous Wound Healing by Silymarin in Rats. J Wound Care 2012, 21, 457–464. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
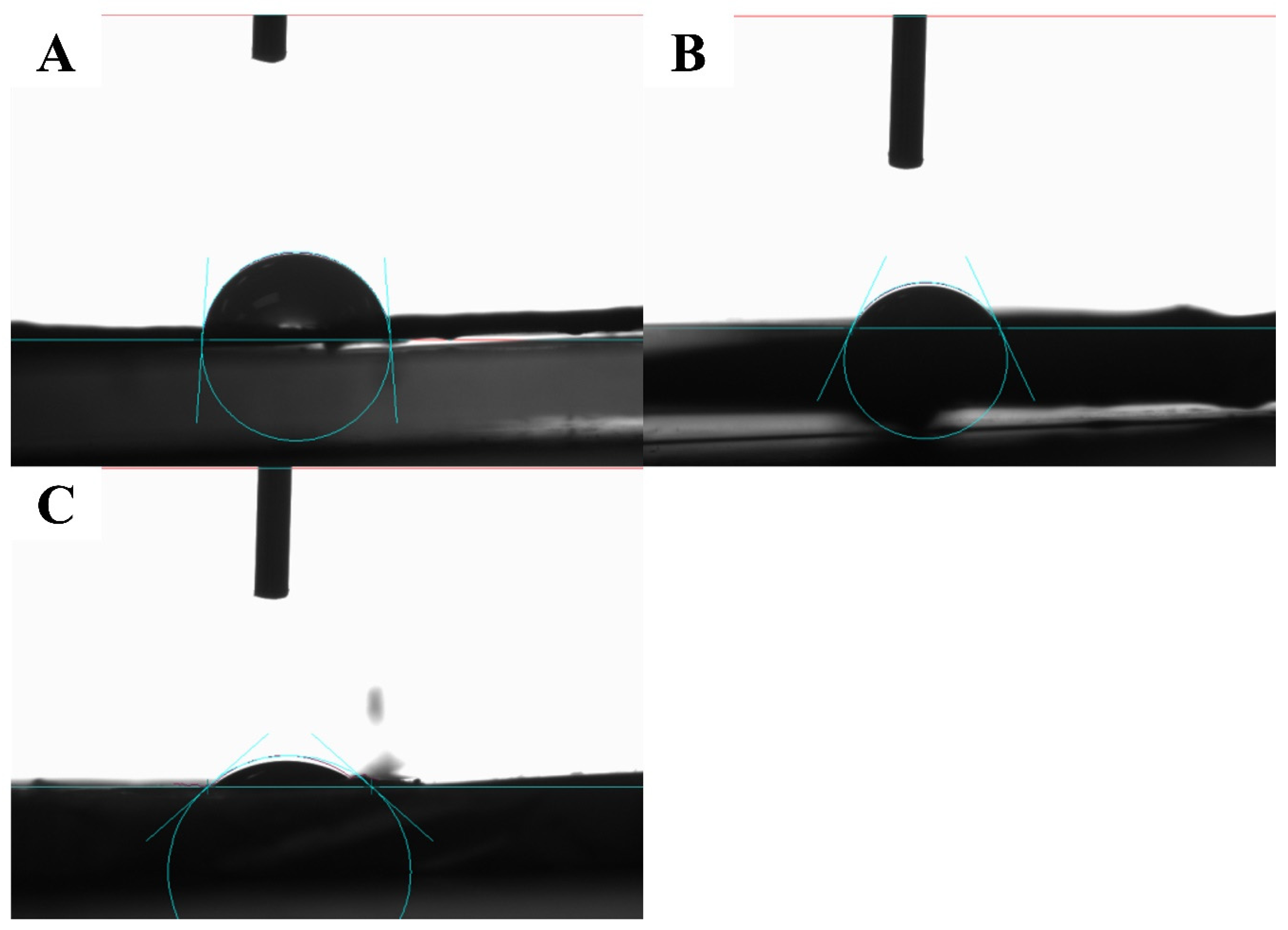

| Formulation | Characterization | ||||
|---|---|---|---|---|---|
| Tensile Strength | Maximum %Wu | ||||
| Strain (%) | Stress (N/mm2) |
Young’s modulus (kPa) |
|||
| NFP | 15.1014 | 0.01204 | 79.7277 | 2.36 | |
| NF10 | 45.6111 | 0.03964 | 86.9087 | 122.53.54 | |
| NF20 | 67.5833 | 0.04859 | 71.8965 | 2.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





