Submitted:
14 October 2024
Posted:
15 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Highlights
- Xenobiotic defence mechanisms in plants: the study emphasizes how plants are equipped with intricate enzymatic (e.g., cytochrome P450, peroxidases) and non-enzymatic (e.g., flavonoids, vitamins C and E) antioxidant systems to defend against oxidative stress induced by xenobiotics like pollutants, pesticides, and heavy metals.
- Oxidative stress pathways: xenobiotic exposure elevates ROS such as hydrogen peroxide, superoxide anions, and hydroxyl radicals, which damage cellular components. Plants counteract this with antioxidants like catalase, superoxide dismutase, and glutathione.
- Therapeutic potential: the metabolic pathways in plants offer valuable insights into developing plant-based detoxification strategies that can mitigate the impact of environmental pollution and improve human health by harnessing plant metabolites.
1.2. Significance of the Study
- Environmental health insights: this research contributes to understanding how plants can manage the adverse effects of xenobiotics, providing strategies to mitigate the impacts of environmental pollution on plant and human health.
- Therapeutic applications: by exploring the xenobiotic metabolism in plants, the study opens up possibilities for developing plant-based remedies or enhancing the dietary use of plants to boost human health through their detoxification and antioxidant properties.
- Redox homeostasis and disease prevention: understanding how plants maintain redox homeostasis offers insights into preventing diseases caused by oxidative stress, such as cancer, highlighting the role of plant-based compounds in counteracting chemically induced toxicity.
- Model for risk assessment: the dual focus on plant pharmacokinetics and pharmacodynamics enhances the ability to model and assess risks posed by environmental contaminants and develop new plant-based strategies for detoxification.
2. Oxidative Processes and Xenobiotic Metabolism by Plants
2.1. Environmental Challenges and Oxidative Stress in Plants
2.2. Xenobiotic Metabolism in Plants
3. The Effect of Oxidative Imbalance on Environmental Pollutants
3.1. Environmental Pollutants and Their Impact on Oxidative Stress
4. Phytochemicals as Free Radical Neutralizers in Combating Chemical-Induced Toxicity
4.1. The Impact of Reactive Oxygen Species
4.2. The Role of Boerhavia diffusa in Counteracting Toxic Effects in Drosophila
4.3. Additional Phytochemicals and Their Impacts
4.4. Mitigating Ovarian and Uterine Toxicity
4.5. Further Botanical Studies and Their Findings
4.6. Phytochemicals in Renal Protection
4.7. Neuroprotection and Environmental Toxins
4.8. Variability in Plant Metabolite-Xenobiotic Interactions
4.9. Differential Plant Responses to Xenobiotics
5. Conclusions
Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Malchi, T.; Eyal, S.; Czosnek, H.; Shenker, M.; Chefetz, B. Plant pharmacology: Insights into in-planta kinetic and dynamic processes of xenobiotics. Critical Reviews in Environmental Science and Technology 2021, 52(19), 3525–3546. [Google Scholar] [CrossRef]
- Kaushik, B.; Sharma, J.; Yadav, K.; Kumar, P.; Shourie, A. Phytochemical Properties and Pharmacological Role of Plants: Secondary Metabolites. Biosciences Biotechnology Research Asia 2021, 18(1), 23–35. [Google Scholar] [CrossRef]
- Fik-Jaskółka, M.; Mittova, V.; Motsonelidze, C.; Vakhania, M.; Vicidomini, C.; Roviello, G.N. Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics. Antibiotics 2024, 13(6), 487. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Gilhen-Baker, M.; Vicidomini, C.; Roviello, G.N. The Healing Power of Clean Rivers: In Silico Evaluation of the Antipsoriatic Potential of Apiin and Hyperoside Plant Metabolites Contained in River Waters. International Journal of Environmental Research and Public Health 2022, 19(5), 2502. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Gilhen-Baker, M.; Roviello, G.N.; Lichtfouse, E. River therapy. Environmental Chemistry Letters 2022, 20(5), 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Prasad, S.M.; Singh, R.P. 2016.
- Sauvêtre, A.; Eichhorn, P.; Pérez, S. Sauvêtre, A.; Eichhorn, P.; Pérez, S. Metabolism of Pharmaceuticals in Plants and Their Associated Microbiota. 2020, 103, 221–264.
- Wu, J.-C.; Lai, C.-S.; Tsai, M.-L.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Chemopreventive effect of natural dietary compounds on xenobiotic-induced toxicity. Journal of Food and Drug Analysis 2017, 25(1), 176–186. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in plants– maintenance of structural individuality and functional blend. Advances in Redox Research 2022, 5, 100039. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxidative Medicine and Cellular Longevity 2020, 2020, 1–32. [Google Scholar] [CrossRef]
- Goncharov, N.; Avdonin, P.; Nadeev, A.; Zharkikh, I.; Jenkins, R. Reactive Oxygen Species in Pathogenesis of Atherosclerosis. Current Pharmaceutical Design 2015, 21(9), 1134–1146. [Google Scholar] [CrossRef]
- Chen, H.; Ma, A.; Qi, S. Antioxidant Therapy for Prevention of Inflammation, Ischemic Reperfusion Injuries and Allograft Rejection. Cardiovascular & Hematological Agents in Medicinal Chemistry 2008, 6(1), 20–43. [Google Scholar]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiological Reviews 2007, 87(1), 245–313. [Google Scholar] [CrossRef] [PubMed]
- Giulia Battelli, M.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase in Drug Metabolism: Beyond a Role as a Detoxifying Enzyme. Current Medicinal Chemistry 2016, 23(35), 4027–4036. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.; Moorthy, B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Current Opinion in Toxicology 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Rosen, G.M.; Tsai, P.; Pou, S. Mechanism of Free-Radical Generation by Nitric Oxide Synthase. Chemical Reviews 2002, 102(4), 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-J.; Seo, J.-M.; Kim, J.-H. Bioactive Lipoxygenase Metabolites Stimulation of NADPH Oxidases and Reactive Oxygen Species. Molecules and Cells 2011, 32(1), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kumar, P. Environmental threats posed by xenobiotics. 2024, 183–201.
- Wang, X.; Sial, M.U.; Bashir, M.A.; Bilal, M.; Raza, Q.-U.-A.; Ali Raza, H.M.; Rehim, A.; Geng, Y. Pesticides Xenobiotics in Soil Ecosystem and Their Remediation Approaches. Sustainability 2022, 14(6), 3353. [Google Scholar] [CrossRef]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants. International Journal of Molecular Sciences 2022, 23(4), 1995. [Google Scholar] [CrossRef]
- Labudda, M.; Dziurka, K.; Fidler, J.; Gietler, M.; Rybarczyk-Płońska, A.; Nykiel, M.; Prabucka, B.; Morkunas, I.; Muszyńska, E. The Alleviation of Metal Stress Nuisance for Plants—A Review of Promising Solutions in the Face of Environmental Challenges. Plants 2022, 11(19), 2544. [Google Scholar] [CrossRef]
- Tiwari, S.; Tiwari, S.; Singh, M.; Singh, A.; Prasad, S.M. Generation Mechanisms of Reactive Oxygen Species in the Plant Cell. 2017, 1–22.
- Gill, S.S.; Peter Singh, L.; Gill, R.; Tuteja, N. Generation and Scavenging of Reactive Oxygen Species in Plants under Stress. 2012, 49–70.
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. 2013, 209–250.
- Marwicka, J.; Zięba, A. Antioxidants as a defence against reactive oxygen species. Aesthetic Cosmetology and Medicine 2021, 10(6), 271–276. [Google Scholar] [CrossRef]
- Fan, S.; Cong, Z. Emerging Strategies for Modifying Cytochrome P450 Monooxygenases into Peroxizymes. Accounts of Chemical Research 2024. [Google Scholar] [CrossRef]
- Döring, B.; Petzinger, E. Phase 0 and phase III transport in various organs: Combined concept of phases in xenobiotic transport and metabolism. Drug Metabolism Reviews 2014, 46(3), 261–282. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Biswas, A.; Dey, S.; Bhattacharjee, T.; Chakrabarty, S. Cytochrome P450 Gene Families: Role in Plant Secondary Metabolites Production and Plant Defense. Journal of Xenobiotics 2023, 13(3), 402–423. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Mittova, V.; Tsetskhladze, Z.R.; Palumbo, R.; Pastore, R.; Roviello, G.N. Georgian Medicinal Plants as Rich Natural Sources of Antioxidant Derivatives: A Review on the Current Knowledge and Future Perspectives. Current Medicinal Chemistry 2024, 31(28), 4407–4424. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Cho, W.C.; Upadhyay, G. Drug-Induced Liver Toxicity and Prevention by Herbal Antioxidants: An Overview. Frontiers in Physiology 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A. Health Alternatives. Morton Grove, IL: Megasystems 1995.
- Markham, K.R.; Mitchell, K.A. The mis-identification of the major antioxidant flavonoids in young barley (Hordeum vulgare) leaves. Zeitschrift für Naturforschung C 2003, 58(1–2), 53–56. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Caballero, B.; Cousins, R.J.; Tucker, K.L. Modern nutrition in health and disease. Jones & Bartlett Learning: 2020.
- Bol’shakova, I.; Lozovskaia, E.; Sapezhinskiĭ, I. Antioxidant properties of a series of extracts from medicinal plants. Biofizika 1997, 42(2), 480–483. [Google Scholar]
- Marderosion, A. The Review of Natural Products, Facts and Comparisons. St. Louis, MI, USA 2001, 630–632.s.
- Yu, C.; Kim, S.; Lim, J.; Kim, M.; Chung, I. Intraspecific relationship analysis by DNA markers and in vitro cytotoxic and antioxidant activity in Eleutherococcus senticosus. Toxicology in vitro 2003, 17(2), 229–236. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F. A systematic screening of total antioxidants in dietary plants. The Journal of nutrition 2002, 132(3), 461–471. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, D.-S.; Oppel, M.N. Shogaols from Zingiber officinale protect IMR32 human neuroblastoma and normal human umbilical vein endothelial cells from β-amyloid (25–35) insult. Planta medica 2002, 68(04), 375–376. [Google Scholar] [CrossRef]
- Chen, J.-W.; Chen, Y.-H.; Lin, F.-Y.; Chen, Y.-L.; Lin, S.-J. Ginkgo biloba extract inhibits tumor necrosis factor-α–induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arteriosclerosis, thrombosis, and vascular biology 2003, 23(9), 1559–1566. [Google Scholar] [CrossRef]
- DeFeudis, F.V.; Papadopoulos, V.; Drieu, K. Ginkgo biloba extracts and cancer: A research area in its infancy. Fundamental & clinical pharmacology 2003, 17((4), 405–417. [Google Scholar]
- Guerra, M.; Speroni, E.; Broccoli, M.; Cangini, M.; Pasini, P.; Minghetti, A.; Crespi-Perellino, N.; Mirasoli, M.; Cantelli-Forti, G.; Paolini, M. Comparison between Chinese medical herb Pueraria lobata crude extract and its main isoflavone puerarin: Antioxidant properties and effects on rat liver CYP-catalysed drug metabolism. Life Sciences 2000, 67(24), 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Gan, Q.; Matthews, R.; Johnson, P.A. Comparison of optimised isotherm models for basic dye adsorption by kudzu. Bioresource technology 2003, 88(2), 143–152. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, A.H.; Overstreet, D.H.; Perfumi, M.; Massi, M. Plant derivatives in the treatment of alcohol dependency. Pharmacology Biochemistry and Behavior 2003, 75(3), 593–606. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Yatziv, S.; Sela, M.; Greenberg, S.; Rachmilevich, B.; Shwarzman, M.; Weshler, Z. e. Effect of natural b-carotene supplementation in children exposed to radiation from the Chernobyl accident. Radiation and Environmental Biophysics 1998, 37, 187–193. [Google Scholar] [CrossRef]
- Shimizu, I. Antifibrogenic therapies in chronic HCV infection. Current Drug Targets-Infectious Disorders 2001, 1, 227–240. [Google Scholar] [CrossRef]
- Kvasnička, F.; Bıba, B.; Ševčı́k, R.; Voldřich, M.; Kratka, J. Analysis of the active components of silymarin. Journal of Chromatography A 2003, 990(1–2), 239–245. [Google Scholar] [CrossRef]
- Upadhyay, G.; Kumar, A.; Singh, M.P. Effect of silymarin on pyrogallol-and rifampicin-induced hepatotoxicity in mouse. European journal of pharmacology 2007, 565(1–3), 190–201. [Google Scholar] [CrossRef]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. PDR for herbal medicines. Thomson, Reuters: 2007.
- Lu, H.; Liu, G.-T. Anti-oxidant activity of dibenzocyclooctene lignans isolated from Schisandraceae. Planta Medica 1992, 58(04), 311–313. [Google Scholar] [CrossRef]
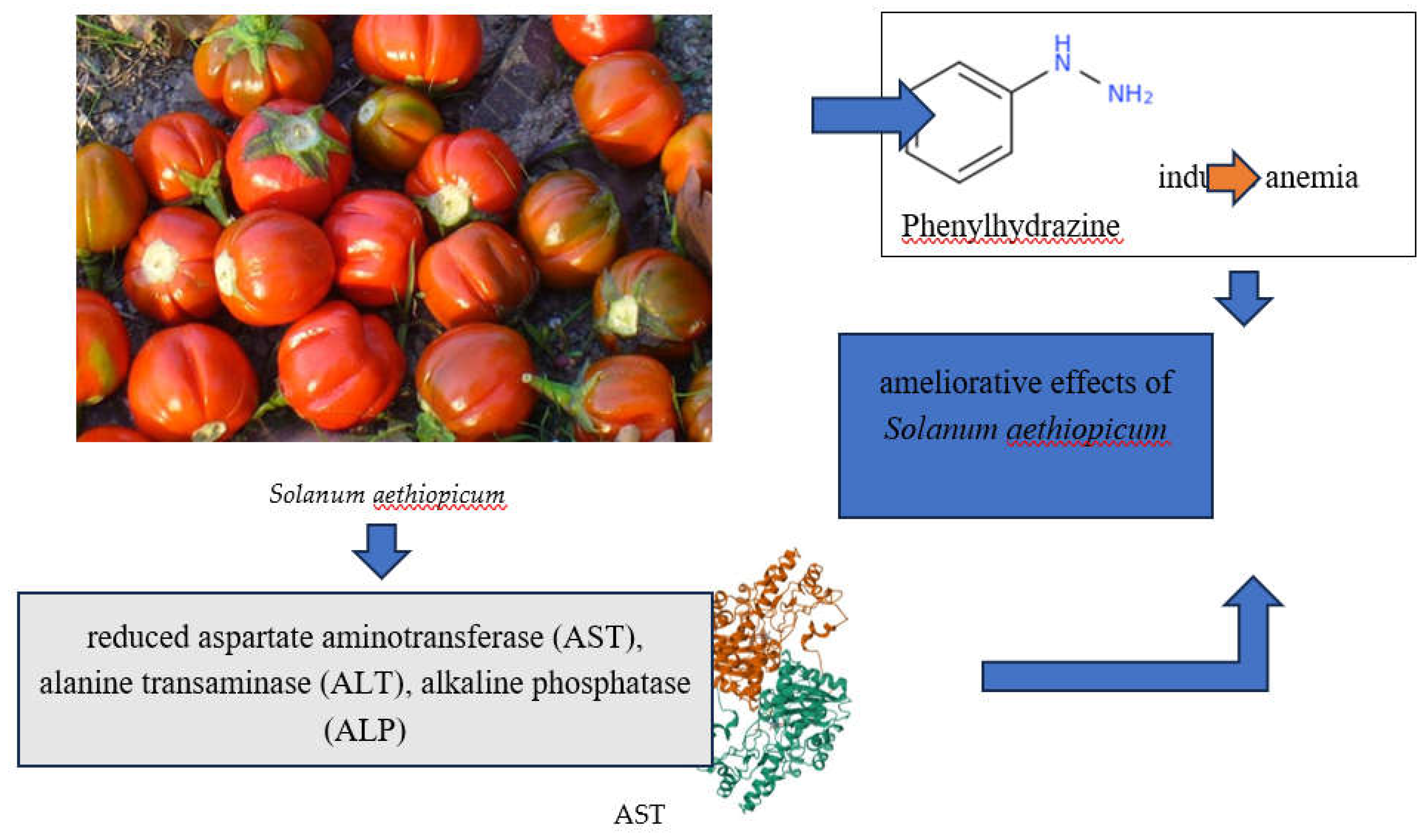
- Ekweogu, C.N.; Ude, V.C.; Nwankpa, P.; Emmanuel, O.; Ugbogu, E.A. Ameliorative effect of aqueous leaf extract of Solanum aethiopicum on phenylhydrazine-induced anaemia and toxicity in rats. Toxicological Research 2019, 36(3), 227–238. [Google Scholar] [CrossRef]
- Adelakun, S.A.; Ukwenya, V.O.; Ojewale, A.O.; Aniah, J.A.; Kolawole, B.P. Excessive exposure to sodium fluoride impaired spermatogenesis, induced hormonal and biochemical imbalance and testicular atrophy: Ameliorating potential of bioactive component of Solanum aethiopicum supplementation. Phytomedicine Plus 2023, 3(3), 100458. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U. Modification of enzymatic and non-enzymatic in vitro oxidative defence system by bioaccessible phytonutrients of selected spices. LWT - Food Science and Technology 2014, 57(1), 434–441. [Google Scholar] [CrossRef]
- Piedrafita, G.; Keller, M.; Ralser, M. The Impact of Non-Enzymatic Reactions and Enzyme Promiscuity on Cellular Metabolism during (Oxidative) Stress Conditions. Biomolecules 2015, 5(3), 2101–2122. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.; Larondelle, Y.; Evers, D. Dietary Antioxidants and Oxidative Stress from a Human and Plant Perspective: A Review. Current Nutrition & Food Science 2010, 6(1), 2–12. [Google Scholar]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Medicine and Cellular Longevity 2016, 2016, 1–44. [Google Scholar] [CrossRef]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.-C.; Trus, C.; Ciobica, A.; Timofte, D.; Szewczyk-Golec, K. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxidative Medicine and Cellular Longevity 2021, 2021, 1–25. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Communication and Signaling 2022, 20(1). [Google Scholar] [CrossRef]
- Safaroghli-Azar, A.; Sanaei, M.-J.; Pourbagheri-Sigaroodi, A.; Bashash, D. Phosphoinositide 3-kinase (PI3K) classes: From cell signaling to endocytic recycling and autophagy. European Journal of Pharmacology 2023, 953, 175827. [Google Scholar] [CrossRef]
- Dwivedi, S.; Kushalan, S.; Paithankar, J.G.; D’Souza, L.C.; Hegde, S.; Sharma, A. Environmental toxicants, oxidative stress and health adversities: Interventions of phytochemicals. Journal of Pharmacy and Pharmacology 2022, 74(4), 516–536. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10(4), 595. [Google Scholar] [CrossRef]
- Carmo de Carvalho e Martins, M.d.; Martins; da Silva Santos Oliveira, A.S.; da Silva, L.A.A.; Primo, M.G.S.; de Carvalho Lira, V.B. Biological Indicators of Oxidative Stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and Their Application in Nutrition. 2022, 1–25.
- Pagano, J.S.; Dworzański, J.; Strycharz-Dudziak, M.; Kliszczewska, E.; Kiełczykowska, M.; Dworzańska, A.; Drop, B.; Polz-Dacewicz, M. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS ONE 2020, 15(3), e0230374. [Google Scholar]
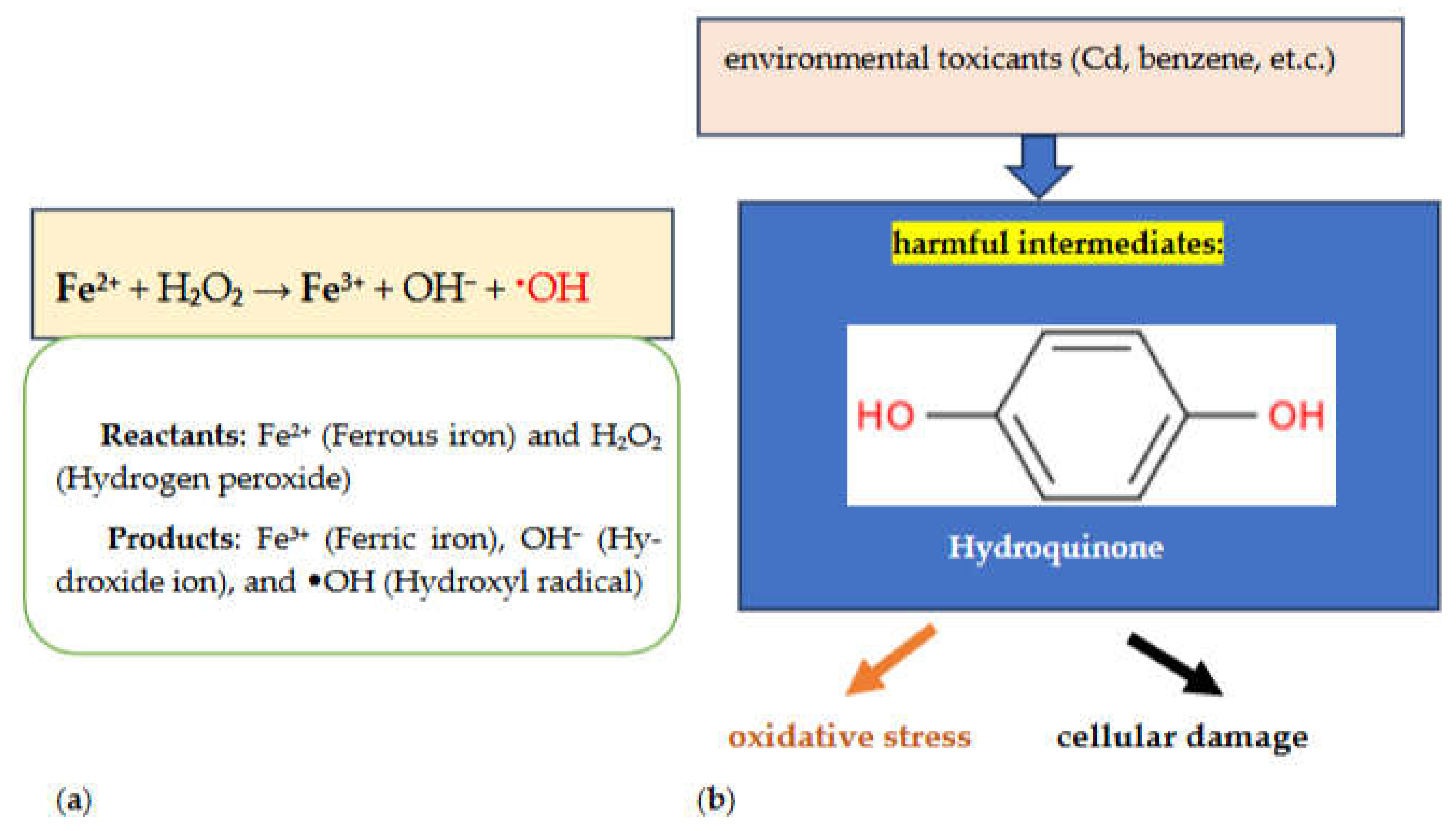
- Zhang, S.; Sun, M.; Hedtke, T.; Deshmukh, A.; Zhou, X.; Weon, S.; Elimelech, M.; Kim, J.-H. Mechanism of Heterogeneous Fenton Reaction Kinetics Enhancement under Nanoscale Spatial Confinement. Environmental Science & Technology 2020, 54(17), 10868–10875. [Google Scholar]
- Chen, S.; Zhu, M.; Guo, X.; Yang, B.; Zhuo, R. Coupling of Fenton reaction and white rot fungi for the degradation of organic pollutants. Ecotoxicology and Environmental Safety 2023, 254, 114697. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, X.; Liu, M.; Cai, B.; He, N.; Wang, Z. Fenton reaction-based nanomedicine in cancer chemodynamic and synergistic therapy. Applied Materials Today 2020, 21, 100864. [Google Scholar] [CrossRef]
- Vedi, M.; Rasool, M.; Sabina, E.P. Protective effect of administration of Withania somifera against bromobenzene induced nephrotoxicity and mitochondrial oxidative stress in rats. Renal failure 2014, 36(7), 1095–1103. [Google Scholar] [CrossRef]
- Cai, S.; Liu, J.; Shi, X.; Hu, S.; Zhao, L. Allicin alleviated learning and memory deficits caused by lead exposure at developmental stage. Life sciences 2019, 231, 116532. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Li, Y.; Ma, F.; Fang, Y.; Ni, C.; Wu, K.; Pan, P.; Ge, R.-S. Taxifolin attenuates the developmental testicular toxicity induced by di-n-butyl phthalate in fetal male rats. Food and Chemical Toxicology 2020, 142, 111482. [Google Scholar] [CrossRef]
- Fernandes, P.; Monteiro, S.M.; Venâncio, C.; Félix, L. 24-Epibrassinolide protects against ethanol-induced behavioural teratogenesis in zebrafish embryo. Chemico-Biological Interactions 2020, 328, 109193. [Google Scholar] [CrossRef]
- Faddah, L.M.; Baky, N.A.A.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Fatani, A.J.; Atteya, M. Role of quercetin and arginine in ameliorating nano zinc oxide-induced nephrotoxicity in rats. BMC complementary and alternative medicine 2012, 12, 1–14. [Google Scholar] [CrossRef]
- Tu, P.; Xue, J.; Bian, X.; Chi, L.; Gao, B.; Leng, J.; Ru, H.; Knobloch, T.J.; Weghorst, C.M.; Lu, K. Dietary administration of black raspberries modulates arsenic biotransformation and reduces urinary 8-oxo-2′-deoxyguanosine in mice. Toxicology and Applied Pharmacology 2019, 377, 114633. [Google Scholar] [CrossRef]
- Sener, U.; Uygur, R.; Aktas, C.; Uygur, E.; Erboga, M.; Balkas, G.; Caglar, V.; Kumral, B.; Gurel, A.; Erdogan, H. Protective effects of thymoquinone against apoptosis and oxidative stress by arsenic in rat kidney. Renal Failure 2016, 38(1), 117–123. [Google Scholar] [CrossRef] [PubMed]
- Guvvala, P.R.; Ravindra, J.P.; Selvaraju, S.; Arangasamy, A.; Venkata, K.M. Ellagic and ferulic acids protect arsenic-induced male reproductive toxicity via regulating Nfe2l2, Ppargc1a and StAR expressions in testis. Toxicology 2019, 413, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.; Raveendran, R.; Madhusoodanan, S.; Sundar, M.; Shankar, S.S.; Sharma, S.; Sundararajan, V.; Dan, P.; Mohideen, S.S. Developmental and behavioural toxicity induced by acrylamide exposure and amelioration using phytochemicals in Drosophila melanogaster. Journal of hazardous materials 2020, 394, 122533. [Google Scholar] [CrossRef] [PubMed]
- Crown, O.; Ogundele, O.; Akinmoladun, A.; Famusiwa, C.; Josiah, S.; Olaleye, M.; Akindahunsi, A. Effects of catechin, quercetin and taxifolin on redox parameters and metabolites linked with renal health in rotenone-toxified rats. Nigerian Journal of Physiological Sciences 2019, 34(1), 1–10. [Google Scholar] [PubMed]
- Erboga, M.; Kanter, M.; Aktas, C.; Sener, U.; Fidanol Erboga, Z.; Bozdemir Donmez, Y.; Gurel, A. Thymoquinone ameliorates cadmium-induced nephrotoxicity, apoptosis, and oxidative stress in rats is based on its anti-apoptotic and anti-oxidant properties. Biological trace element research 2016, 170, 165–172. [Google Scholar] [CrossRef]
- Micali, A.; Pallio, G.; Irrera, N.; Marini, H.; Trichilo, V.; Puzzolo, D.; Pisani, A.; Malta, C.; Santoro, G.; Laurà, R. Flavocoxid, a Natural Antioxidant, Protects Mouse Kidney from Cadmium-Induced Toxicity. Oxidative medicine and cellular longevity 2018, 2018(1), 9162946. [Google Scholar] [CrossRef]
- Saad, A.B.; Rjeibi, I.; Brahmi, N.; Elaloui, E.; Zouari, N. Nicotine-induced oxidative stress, testis injury, AChE inhibition and brain damage alleviated by Mentha spicata. Inflammopharmacology 2020, 28, 939–948. [Google Scholar] [CrossRef]
- Han, Y.; Ishibashi, S.; Iglesias-Gonzalez, J.; Chen, Y.; Love, N.R.; Amaya, E. Ca2+-Induced Mitochondrial ROS Regulate the Early Embryonic Cell Cycle. Cell Reports 2018, 22(1), 218–231. [Google Scholar] [CrossRef]
- Hunter, M.V.; Willoughby, P.M.; Bruce, A.E.E.; Fernandez-Gonzalez, R. Oxidative Stress Orchestrates Cell Polarity to Promote Embryonic Wound Healing. Developmental Cell 2018, 47(3), 377–387.e4. [Google Scholar] [CrossRef]
- Pb, B.; Rani, S.; Kim, Y.O.; Ahmed Al-Ghamdi, A.; Elshikh, M.S.; Al-Dosary, M.A.; Hatamleh, A.A.; Arokiyaraj, S.; Kim, H.-J. Prophylactic efficacy of Boerhavia diffusa L. aqueous extract in toluene induced reproductive and developmental toxicity in Drosophila melanogaster. Journal of Infection and Public Health 2020, 13(2), 177–185. [Google Scholar] [CrossRef]
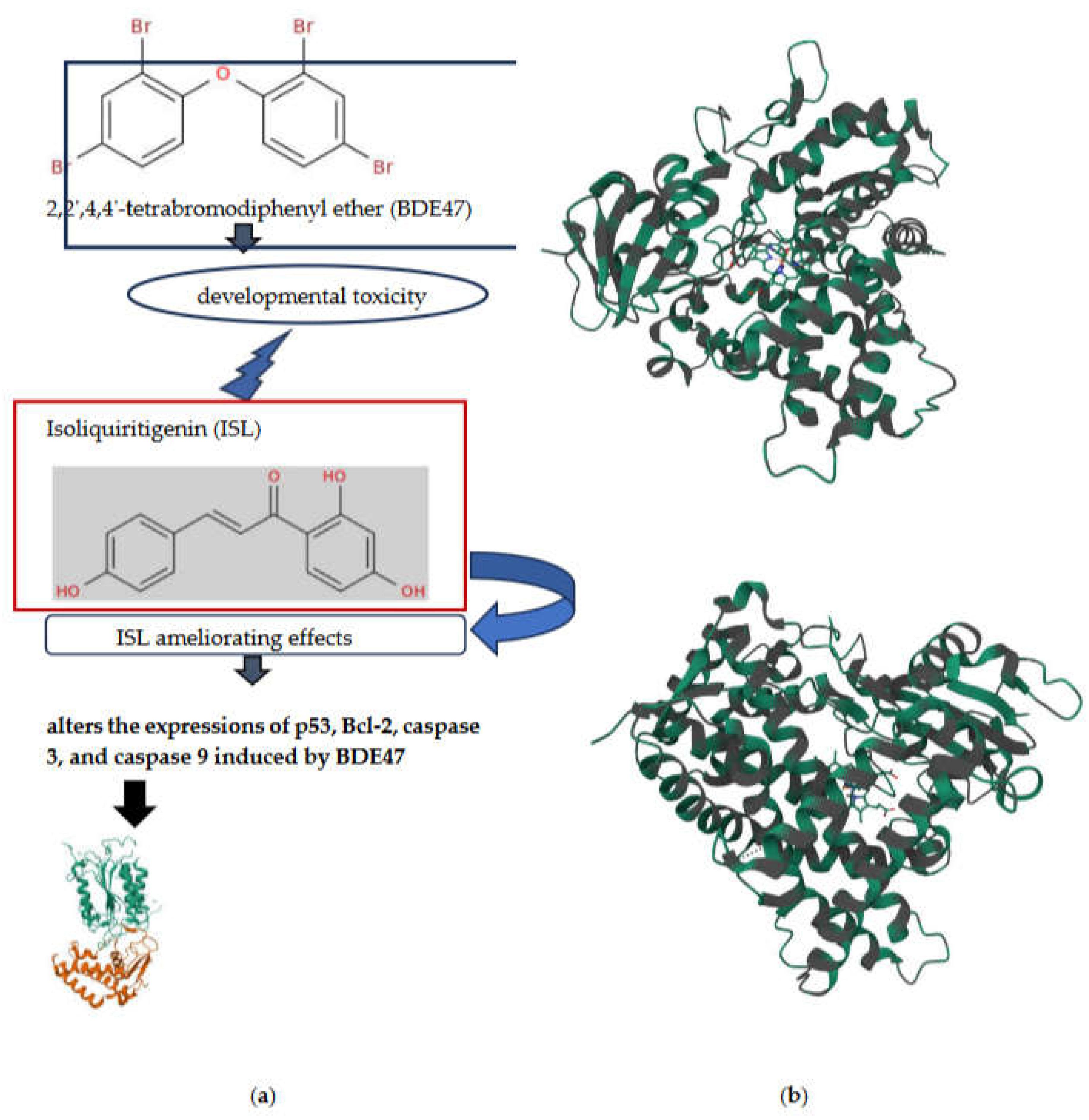
- Wang, C.; Yang, L.; Hu, Y.; Zhu, J.; Xia, R.; Yu, Y.; Shen, J.; Zhang, Z.; Wang, S.-L. Isoliquiritigenin as an antioxidant phytochemical ameliorates the developmental anomalies of zebrafish induced by 2,2′,4,4′-tetrabromodiphenyl ether. Science of The Total Environment 2019, 666, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Yu, Y.-C.; Hsia, S.-M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13(1), 115. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Zebrafish (Danio rerio): A newcomer with great promise in behavioral neuroscience. Neuroscience & Biobehavioral Reviews 2023, 144, 104978. [Google Scholar]
- Adefisan, A.O.; Madu, J.C.; Owumi, S.E.; Adaramoye, O.A. Calliandra portoricensisameliorates ovarian and uterine oxido-inflammatory responses inN-methyl-N-nitrosourea and benzo[a]pyrene-treated rats. Experimental Biology and Medicine 2020, 245(16), 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Ijaz, F.; Baig, M.W.; Nasir, B.; Khan, M.R.; Haq, I.-u. Scientific Validation of Ethnomedicinal Use ofIpomoea batatasL. Lam. as Aphrodisiac and Gonadoprotective Agent against Bisphenol A Induced Testicular Toxicity in Male Sprague Dawley Rats. BioMed Research International 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods 2022, 11(7), 1058. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Ganguly, R.; Singh, A.K.; Rana, H.K.; Pandey, A.K. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicology Reports 2021, 8, 44–52. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Abd El-Twab, S.M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environmental Science and Pollution Research 2019, 27(8), 7910–7921. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, K.; Tang, X.; Liu, H.; Zhang, H.; Yang, X.; Nie, X.; Luo, H. Chronic exposure to di-n-butyl phthalate causes reproductive toxicity in zebrafish. Journal of Applied Toxicology 2020, 40(12), 1694–1703. [Google Scholar] [CrossRef]
- Gupta, P.; Seth, C.S. 24-Epibrassinolide Regulates Functional Components of Nitric Oxide Signalling and Antioxidant Defense Pathways to Alleviate Salinity Stress in Brassica juncea L. cv. Varuna. Journal of Plant Growth Regulation 2022, 42(7), 4207–4222. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.I.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides (Review). Experimental and Therapeutic Medicine 2019. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.T.B.; Majid, M.; Maryam, S.; Rahat, A.; Ahmed, M.; Khan, M.R.; Haq, I.u. Quercus dilatata Lindl. ex Royle ameliorates BPA induced hepatotoxicity in Sprague Dawley rats. Biomedicine & Pharmacotherapy 2018, 102, 728–738. [Google Scholar]
- Das, M.; Basu, S.; Banerjee, B.; Sen, A.; Jana, K.; Datta, G. Hepatoprotective effects of green Capsicum annum against ethanol induced oxidative stress, inflammation and apoptosis in rats. Journal of Ethnopharmacology 2018, 227, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Andersen, Ø.M. Anthocyanins from tubers and shoots of the purple potato, Solanum tuberosum. The Journal of Horticultural Science and Biotechnology 2015, 75(3), 360–363. [Google Scholar] [CrossRef]
- Fareed, M.M.; Khalid, H.; Khalid, S.; Shityakov, S. Deciphering Molecular Mechanisms of Carbon Tetrachloride-Induced Hepatotoxicity: A Brief Systematic Review. Current Molecular Medicine 2024, 24(9), 1124–1134. [Google Scholar] [CrossRef]
- Jeong, T.B.; Kwon, D.; Son, S.W.; Kim, S.H.; Lee, Y.-H.; Seo, M.-S.; Kim, K.S.; Jung, Y.-S. Weaning Mice and Adult Mice Exhibit Differential Carbon Tetrachloride-Induced Acute Hepatotoxicity. Antioxidants 2020, 9(3), 201. [Google Scholar] [CrossRef]
- Unsal, V.; Cicek, M.; Sabancilar, İ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Reviews on Environmental Health 2021, 36(2), 279–295. [Google Scholar] [CrossRef]
- Arbab, A.H.; Parvez, M.K.; Al-Dosari, M.S.; Al-Rehaily, A.J.; Ibrahim, K.E.; Alam, P.; Alsaid, M.S.; Rafatullah, S. Therapeutic efficacy of ethanolic extract ofAerva javanicaaerial parts in the amelioration of CCl4-induced hepatotoxicity and oxidative damage in rats. Food & Nutrition Research 2016, 60(1), 30864. [Google Scholar]
- Ben Hsouna, A.; Gargouri, M.; Dhifi, W.; Saibi, W. Antioxidant and hepato-preventive effect ofCitrus aurantiumextract against carbon tetrachloride-induced hepatotoxicity in rats and characterisation of its bioactive compounds by HPLC-MS. Archives of Physiology and Biochemistry 2018, 125(4), 332–343. [Google Scholar] [CrossRef]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food and Chemical Toxicology 2018, 113, 145–153. [Google Scholar] [CrossRef]
- Mohamed, M.R.; Emam, M.A.; Hassan, N.S.; Mogadem, A.I. Umbelliferone and daphnetin ameliorate carbon tetrachloride-induced hepatotoxicity in rats via nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1 expression. Environmental Toxicology and Pharmacology 2014, 38(2), 531–541. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; D’Souza, U.J.A.; Iqbal, M. The potential protective effect of Commelina nudiflora L. against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats, mediated by suppression of oxidative stress and inflammation. Environmental Health and Preventive Medicine 2017, 22(1). [Google Scholar] [CrossRef] [PubMed]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytotherapy Research 2000, 14(5), 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, Y.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. A comprehensive review of the physicochemical, quality and nutritional properties of Nigella sativa oil. Food Reviews International 2019, 35(4), 342–362. [Google Scholar] [CrossRef]
- Najmi, A.; Nasiruddin, M.; Khan, R.; Haque, S. Effect of <i> Nigella sativa</i> oil on various clinical and biochemical parameters of insulin resistance syndrome. International Journal of Diabetes in Developing Countries 2008, 28(1), 11. [Google Scholar]
- Imafidon, C.E.; Olukiran, O.S.; Ogundipe, D.J.; Eluwole, A.O.; Adekunle, I.A.; Oke, G.O. Acetonic extract of Vernonia amygdalina (Del.) attenuates Cd-induced liver injury: Potential application in adjuvant heavy metal therapy. Toxicology Reports 2018, 5, 324–332. [Google Scholar] [CrossRef]
- Ibiam, A.U.; Ugwuja, E.I.; Ejeogo, C.; Ugwu, O. Cadmium-induced toxicity and the hepatoprotective potentials of aqueous extract of jessiaea nervosa leaf. Adv Pharm Bull 2013, 3(2), 309–313. [Google Scholar]
- Bhattacharjee, B.; Pal, P.K.; Ghosh, A.K.; Mishra, S.; Chattopadhyay, A.; Bandyopadhyay, D. Aqueous bark extract of Terminalia arjuna protects against cadmium-induced hepatic and cardiac injuries in male Wistar rats through antioxidative mechanisms. Food and Chemical Toxicology 2019, 124, 249–264. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13(6), 1041. [Google Scholar] [CrossRef]
- Hfaiedh, M.; Brahmi, D.; Zourgui, M.N.; Zourgui, L. Phytochemical analysis and nephroprotective effect of cactus (Opuntia ficus-indica) cladodes on sodium dichromate-induced kidney injury in rats. Applied Physiology, Nutrition, and Metabolism 2019, 44(3), 239–247. [Google Scholar] [CrossRef]
- Ncibi, S.; Ben Othman, M.; Akacha, A.; Krifi, M.N.; Zourgui, L. Opuntia ficus indica extract protects against chlorpyrifos-induced damage on mice liver. Food and Chemical Toxicology 2008, 46(2), 797–802. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.b.; Rjeibi, I.; Ncib, S.; Zouari, N.; Zourgui, L. Ameliorative Effect of Cactus(Opuntia ficus indica)Extract on Lithium-Induced Nephrocardiotoxicity: A Biochemical and Histopathological Study. BioMed Research International 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.B.; Song, C.H.; Kim, W.H.; Kim, Y.K. Effects of Juglans sinensis Dode extract and antioxidant on mercury chloride-induced acute renal failure in rabbits. Journal of Ethnopharmacology 2002, 82(1), 45–49. [Google Scholar] [CrossRef] [PubMed]
- Sarwar Alam, M.; Kaur, G.; Jabbar, Z.; Javed, K.; Athar, M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food and Chemical Toxicology 2007, 45(6), 910–920. [Google Scholar] [CrossRef]
- Ruiz, J.; Mahmud, M.; Modasshir, M.; Shamim Kaiser, M.; Alzheimer’s Disease Neuroimaging In, f. t. 3D DenseNet Ensemble in 4-Way Classification of Alzheimer’s Disease. 2020, 12241, 85–96.
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA neurology 2014, 71(4), 505–508. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Rolston, R.K.; Smith, M.A. Alzheimer Disease. Disease-a-Month 2010, 56(9), 484–546. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Klug, A.; Crowther, R.A. Tau protein, the paired helical filament and Alzheimer’s disease. Journal of Alzheimer’s Disease 2006, 9(s3), 195–207. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, Z.; Jiang, Y. Clinical trials of amyloid-based immunotherapy for Alzheimer’s disease: End of beginning or beginning of end? Expert opinion on biological therapy 2013, 13(11), 1515–1522. [Google Scholar] [CrossRef]
- Cardoso, F.; Goetz, C.G.; Mestre, T.A.; Sampaio, C.; Adler, C.H.; Berg, D.; Bloem, B.R.; Burn, D.J.; Fitts, M.S.; Gasser, T.; Klein, C.; de Tijssen, M.A.J.; Lang, A.E.; Lim, S.Y.; Litvan, I.; Meissner, W.G.; Mollenhauer, B.; Okubadejo, N.; Okun, M.S.; Postuma, R.B.; Svenningsson, P.; Tan, L.C.S.; Tsunemi, T.; Wahlstrom-Helgren, S.; Gershanik, O.S.; Fung, V.S.C.; Trenkwalder, C. A Statement of the MDS on Biological Definition, Staging, and Classification of Parkinson’s Disease. Movement Disorders 2023, 39(2), 259–266. [Google Scholar] [CrossRef]
- Irwin, K.E.; Sheth, U.; Wong, P.C.; Gendron, T.F. Fluid biomarkers for amyotrophic lateral sclerosis: A review. Molecular Neurodegeneration 2024, 19(1). [Google Scholar] [CrossRef]
- Garofalo, M.; Pandini, C.; Bordoni, M.; Pansarasa, O.; Rey, F.; Costa, A.; Minafra, B.; Diamanti, L.; Zucca, S.; Carelli, S.; Cereda, C.; Gagliardi, S. Alzheimer’s, Parkinson’s Disease and Amyotrophic Lateral Sclerosis Gene Expression Patterns Divergence Reveals Different Grade of RNA Metabolism Involvement. International Journal of Molecular Sciences 2020, 21(24), 9500. [Google Scholar] [CrossRef] [PubMed]
- Zahra, W.; Rai, S.N.; Birla, H.; Singh, S.S.; Dilnashin, H.; Rathore, A.S.; Singh, S.P. The Global Economic Impact of Neurodegenerative Diseases: Opportunities and Challenges. 2020, 333–345.
- Pathak, N.; Vimal, S.K.; Tandon, I.; Agrawal, L.; Hongyi, C.; Bhattacharyya, S. Neurodegenerative Disorders of Alzheimer, Parkinsonism, Amyotrophic Lateral Sclerosis and Multiple Sclerosis: An Early Diagnostic Approach for Precision Treatment. Metabolic Brain Disease 2021, 37(1), 67–104. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Advances in Therapy 2019, 37(1), 113–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, M.; Kumar, M.; Ngo, F.Y.; Li, Y.; Lao, L.; Rong, J. A Pharmacological Appraisal of Neuroprotective and Neurorestorative Flavonoids Against Neurodegenerative Diseases. CNS & Neurological Disorders - Drug Targets 2019, 18(2), 103–114. [Google Scholar]
- Akter, R.; Chowdhury, M.A.R.; Rahman, M.H. Flavonoids and Polyphenolic Compounds as Potential Talented Agents for the Treatment of Alzheimer’s Disease and their Antioxidant Activities. Current Pharmaceutical Design 2021, 27(3), 345–356. [Google Scholar] [CrossRef]
- Olajide, O.J.; Enaibe, B.U.; Bankole, O.O.; Akinola, O.B.; Laoye, B.J.; Ogundele, O.M. Kolaviron was protective against sodium azide (NaN3) induced oxidative stress in the prefrontal cortex. Metabolic Brain Disease 2015, 31(1), 25–35. [Google Scholar] [CrossRef]
- Saad, A.B.; Rjeibi, I.; Brahmi, N.; Elaloui, E.; Zouari, N. Nicotine-induced oxidative stress, testis injury, AChE inhibition and brain damage alleviated by Mentha spicata. Inflammopharmacology 2019, 28(4), 939–948. [Google Scholar] [CrossRef]
- Rebai, O.; Belkhir, M.; Boujelben, A.; Fattouch, S.; Amri, M. Morus alba leaf extract mediates neuroprotection against glyphosate-induced toxicity and biochemical alterations in the brain. Environmental Science and Pollution Research 2017, 24(10), 9605–9613. [Google Scholar] [CrossRef]
- Abdul-Aziz Ahmed, K.; Jabbar, A.A.J.; Abdulla, M.A.; Zuhair Alamri, Z.; Ain Salehen, N.; Abdel Aziz Ibrahim, I.; Almaimani, G.; Bamagous, G.A.; Almaimani, R.A.; Almasmoum, H.A.; Ghaith, M.M.; Farrash, W.F. Mangiferin (mango) attenuates AOM-induced colorectal cancer in rat’s colon by augmentation of apoptotic proteins and antioxidant mechanisms. Scientific Reports 2024, 14(1). [Google Scholar] [CrossRef]
- Plavec, J. Quadruplex targets in neurodegenerative diseases. 2020, 54, 441–483.
- Wink, M. Compartmentation of Secondary Metabolites and Xenobiotics in Plant Vacuoles. In The Plant Vacuole, 1997; pp 141–169.
- Kolb, M.; Harms, H. Metabolism of fluoranthene in different plant cell cultures and intact plants. Environmental Toxicology and Chemistry 2009, 19(5), 1304–1310. [Google Scholar] [CrossRef]
- Sikandar, A.; Shehzadi, K.; Arshad, Q.; Munir, K. Phytoremediation: An analytical technique for the assessment of biodegradation of organic xenobiotic pollutants: A review. Int. J. Sci. Res 2013, 4(2), 2250–2253. [Google Scholar]
- Sun, C.; Dudley, S.; McGinnis, M.; Trumble, J.; Gan, J. Acetaminophen detoxification in cucumber plants via induction of glutathione S-transferases. Science of The Total Environment 2019, 649, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicology and Environmental Safety 2020, 195. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, S.; Mohri, S.; Ono, Y. Plant response to heavy metal toxicity: Comparative study between the hyperaccumulator Thlaspi caerulescens (ecotype Ganges) and nonaccumulator plants: Lettuce, radish, and alfalfa. Environmental Toxicology 2008, 23(5), 607–616. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Yang, B.; Fang, J.; Liu, Z. Transcriptomic responses to different doses of cycloxaprid involved in detoxification and stress response in the whitebacked planthopper, Sogatella furcifera. Entomologia Experimentalis et Applicata 2016, 158(3), 248–257. [Google Scholar] [CrossRef]
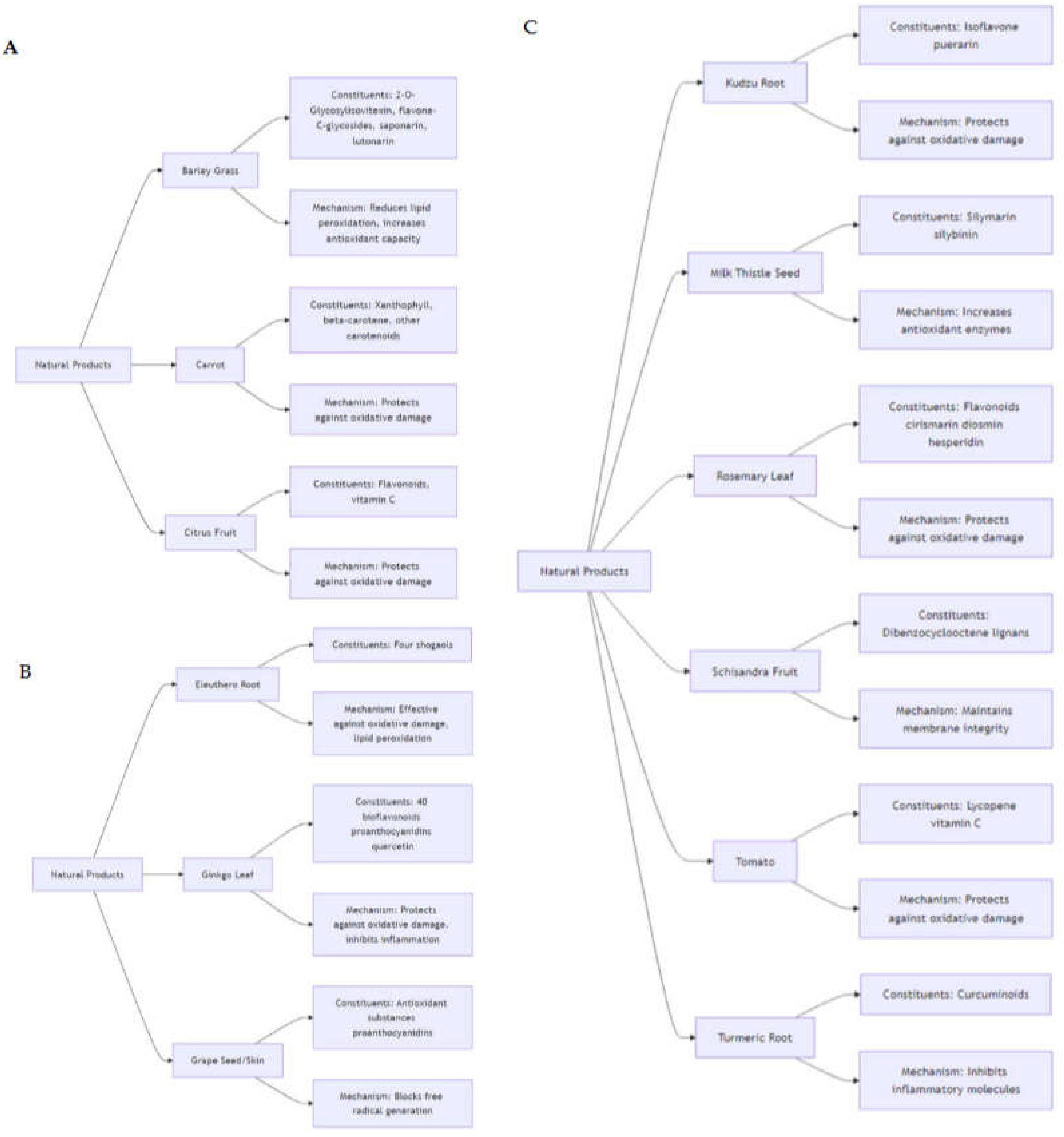


| Natural Product | Major Constituents | Effects | Ref. |
|---|---|---|---|
| Barley grass (Hordeum leporinum) | 2-O-glycosylisovitexin (2-O-GIV; C27H30O15), flavone-C-glycosides, saponarin (C27H30O15), and lutonarin (C27H30O16) | Decreased lipid peroxidation and enhanced antioxidant capacity. | [31,32] |
| Carrot (Daucus Carota) | Xanthophyll (C40H56O2), beta-carotene (C40H56), and other antioxidant carotenoids | Prevention of oxidative damage through strong antioxidant capacity. | [31] |
| Citrus fruit | Flavonoids and vitamin C (C6H8O6) | Highly effective in protecting against oxidative damage | [33] |
| Eleuthero root (Eleutherococcus senticosus) | Four shogaols (bioactive compounds derived from ginger) with antioxidant properties | Provides strong protection against oxidative damage and lipid peroxidation | [34,35,36] |
| Ginger root (Zingiber officinale) | About 40 bioflavonoids, including proanthocyanidins and quercetin (C15H10O7) | Offers protection against oxidative damage and lipid peroxidation | [35,37,38] |
| Ginkgo leaf (Ginkgo biloba) | About 40 bioflavonoids, including proanthocyanidins and quercetin (C15H10O7) | Provides protection against oxidative damage while inhibiting inflammation and atherosclerosis. | [33,35,39,40] |
| Grape (vitis sp.) seed/skin | Antioxidant substances, over 90% proanthocyanidins | Efficient at preventing free radical formation and decreasing oxidative stress. | [35] |
| Kudzu root (Pueraria lobata) | Isoflavone puerarin (C21H20O9, crude form more efficient) | Active against oxidative damage due to elevated antioxidant capacity. | [41,42,43] |
| Milk thistle seed (Silybum marianum) | Silymarin (C25H22O10, major constituent silybinin) | Provides protection against oxidative damage and lipid peroxidation; enhances antioxidant enzyme activity. | [44,45,46,47] |
| Rosemary leaf (Rosmarinus officinalis) | Flavonoids such as cirsimarin (C23H24O11), diosmin (C28H32O15), hesperidin (C28H34O15), and homoplantaginin (C22H22O11) | Effectively protects against oxidative damage and also possesses analgesic properties. | [31,48] |
| Schisandra fruit (Schisandra chinensis) | At least 9 dibenzocyclooctene lignans | Provides protection against oxidative damage and helps preserve membrane integrity. | [49] |
| Tomato (Solanum lycopersicum) | Lycopene (C15H10O7) and vitamin C (C6H8O6) | Offers strong protection against oxidative damage due to its high antioxidant content. | [33] |
| Turmeric root (Curcuma longa) | Curcuminoids | Protects against oxidative damage and inflammation; inhibits various inflammatory molecules | [35] |
| Organ toxicity | Environmental Toxicants | Oxidative stress Parameters | Effects | Natural Products | Ref. |
|---|---|---|---|---|---|
| nephrotoxicity | bromobenzene C₆H₅Br | increased LPO, reduced antioxidant enzyme activity | inflammation and irregularities in kidney function | withaferin A | [66] |
| reproductive and developmental toxicity | lead Pb | redox imbalance | learning and memory impairments in young rats | allicin | [67] |
| ” | di-n-butyl phthalate C₁6H₂₂O₄ |
increased MDA, decreased SOD and GPx levels | multinucleated gonocytes and reduced serum testosterone levels | taxifolin | [68] |
| ” | Ethanol C2H5OH | altered levels of reduced and oxidized glutathione | malformations and changes in embryonic behavior | 24-epibrassinolide | [69] |
| nephrotoxicity | zinc oxide (ZnO) nanoparticle | reduced non-enzymatic antioxidant levels | reduced renal GSH, elevated serum inflammatory markers, and glucose levels | quercetin and arginine | [70] |
| ” | Arsenic As | formation of 8-OxodG | raised methylation levels, urinary excretion | black raspberries | [71] |
| ” | ” | reduced antioxidant enzyme activity, increased MDA | renal damage causing cellular apoptosis | thymoquinone | [72] |
| reproductive and developmental toxicity | ” | regulation of Nfe2l2, StAR, Ppargc1a expressions | abnormalities in sperm and testis structure and function | ellagic and ferulic acid | [73] |
|
” |
acrylamide C3H5NO |
elevated ROS | mobility problems, egg development deficiencies | thymoquinone and curcumin | [74] |
| nephrotoxicity | rotenone C23H22O6 |
redox imbalance | renal dysfunction | catechin, quercetin, taxifolin | [75] |
| ” | cadmium Cd |
decreased antioxidant enzyme activity, increased LPO | injury to proximal tubules, dysfunction of the glomerulus | thymoquinone | [76] |
| ” | ” | decreased antioxidant enzyme activity, increased iNOS, MMP-9, pERK 1/2 | alterations in tubular epithelium and glomerular structure | flavocoxid | [77] |
| reproductive and developmental toxicity | nicotine C10H14N2 |
reduced antioxidant enzyme levels | structural and functional impairment in the testis and brain | Mentha spicata | [78] |
| Phytochemical | Content (Dry Extract) | ± |
|---|---|---|
| Total Phenolic Content (TPC) | 304.32 μg gallic acid equivalents/mg | 7.20 |
| Total Flavonoid Content (TFC) | 214.77 μg quercetin equivalents/mg | 4.09 |
| Rutin Content | 7.3 μg/mg | 0.12 |
| Myricetin Content | 2.7 μg/mg | 0.14 |
| Caffeic Acid Content (ethyl acetate extract) | 4.05 μg/mg | 0.22 |
| Caffeic Acid Content (methanol extract) | 1.92 μg/mg | 0.17 |
| Natural product | Experimental Model | Experimental Details & Results | Protective Effects & Mechanism | Ref. |
|---|---|---|---|---|
| Boerhavia diffusa | Drosophila larvae | B. diffusa aqueous extract (BDAE) was tested at four concentrations: 25, 50, 100, and 200 mg/ml. Results: BDAE mitigated toluene-induced developmental and reproductive toxicity, significantly improved reproductive and developmental parameters, and increased antioxidant enzyme levels. | Effectiveness: Reduced developmental and reproductive toxicity induced by toluene. Mechanism: Antioxidant activity, elevated antioxidant enzyme levels (catalase, glutathione-S-transferase, superoxide dismutase). | [81] |
| Isoliquiritigenin (ISL) | Zebrafish embryos | ISL was used at 4 μM with exposure to BDE47 (1 and 10 μM) for up to 120 hours post-fertilization. Results: ISL mitigated BDE47-induced developmental abnormalities, excessive ROS accumulation, and altered apoptosis-related gene expressions. | Effectiveness: Ameliorated developmental toxicity caused by BDE47. Mechanism: Enhanced antioxidant enzyme activities (SOD and CAT), modulated apoptosis-related gene expressions (p53, Bcl-2, caspase 3, caspase 9). | [82] |
| Calliandra portoricensis (CP) | Rats | CP was evaluated for its effects against ovarian and uterine toxicity induced by benzo[a]pyrene (BaP) and N-methyl nitrosourea (NMU). Results: CP reduced toxic responses from NMU and BaP, alleviating oxidative damage and inflammation. | Effectiveness: Mitigated ovarian and uterine toxicity from carcinogens. Mechanism: Antioxidant and anti-inflammatory properties. | [85] |
| Ipomoea batatas | Male Sprague Dawley rats | Ipomoea batatas extracts were tested for protective effects against BPA-induced gonadotoxicity at 300 mg/kg. Results: Enhanced sexual behavior, improved semen quality, increased levels of testosterone, FSH, LH, and estradiol, and restored antioxidant enzyme levels. | Effectiveness: Improved reproductive health and mitigated BPA-induced toxicity. Mechanism: Antioxidant activity, restoration of hormonal balance, improved semen quality. | [85] |
| Beetroot (Beta vulgaris L.) | Mice | Beetroot was evaluated for its protective effects against chlorpyrifos (CPF) nephrotoxicity. Results: Restored antioxidant levels and reduced oxidative stress markers. | Effectiveness: Mitigated CPF-induced nephrotoxicity. Mechanism: Enhanced antioxidant levels, reduced oxidative stress. Beetroot extracts enhance nuclear factor erythroid 2-related factor 2 (Nrf2), which is essential for promoting the expression of defensive enzymes. | [85] |
| Opuntia ficus indica | Mice | Opuntia cladodes extract was tested for its effects on liver damage caused by chlorpyrifos. Results: Improved liver function, reduced oxidative stress, and normalized biochemical parameters. | Effectiveness: Protected against liver damage from CPF. Mechanism: Restored antioxidant levels, reduced oxidative stress and liver damage. | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





