1. Introduction
Biopesticides, which are safer alternatives to conventional pest control chemicals, are primarily derived from natural sources. These include microbial and biochemical products, as well as plant-incorporated protectants (PIPs). Together, they currently account for 5% of the global pesticide market [
1,
2]. Chalcones and their derivatives have garnered significant attention due to their wide range of nutritional and biological activities, such as anti-inflammatory, antitumor, antibacterial, antifungal, antimalarial, antitubercular, and anti-pigmentation effects, often demonstrating remarkable efficacy [
3]. They are also recognized for their role as bioprotectants [
4,
5] or herbicidal agents [
6,
7]. Dihydrochalcones, naturally biosynthesized through the well-understood phenylpropanoid pathway in plants, were initially believed to be limited to around 30 plant families. However, along with more advanced analytical techniques development, it has been discovered that dihydrochalcones are more widely distributed in nature. Apples are considered as the richest dietary source of these compounds, which are also found in leaves and roots of apple and hairy roots obtained through genetic transformation [
8]. In addition, dihydrochalcones were also found in other different plant families, including
Asteraceae, Lauraceae, and
Salicaceae [
9,
10].
Pinocembrin dihydrochalcone has demonstrated effectiveness against a wide range of target pathogens, including fungi such as
Phytophthora,
Pythium, downy mildew (
Pseudoperonospora cubensis, Plasmopara),
Colletotrichum, Rhizoctonia, and rust (
Puccinia triticina), as well as bacteria like
Xanthomonas and
Pseudomonas. These compounds have been tested on various host plants, including vegetables, leafy greens, fruits, and wheat [
11,
12]. Werner et al. (2020) demonstrated that 2’,4’-dihydroxyhydrochalcone and its synthetic analogs show great promise as potential anti-
Saprolegnia agents, largely due to their high lipophilicity, particularly in molecules with alkyl chains longer than 10 carbon atoms. These compounds are considered promising scaffolds for the development of new, potent anti-oomycete agents [
13].
Therefore, in light of the proven fungicidal and bactericidal properties of pinocembrin dihydrochalcone, antioxidant properties were evaluated, because very often they occur parallelly. Dihydrochalcones are known as potent antioxidants, with their other bioactivities linked to their antioxidant properties [
14]. Thus, determination of the antioxidant activity is crucial for evaluating their biodegradability in the environment. Antioxidants can impact decomposition processes by influencing radicals and redox reactions, which directly supports the rationale behind our experiments [
15].
There are many various methods for the evaluation of the antioxidant capacity but for our needs DPPH and FRAP tests were employed. It should be emphasized that antioxidant activity must not be tested on the basis of only one particular assay. Ideally, several methods should be performed in order to assess the antioxidant capacity for the chosen analyte.
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay is a widely used, quick, simple, and cost-effective method for evaluating antioxidant activity by using free radicals. This assay measures the ability of compounds to act as hydrogen donors or free radical scavengers (FRS). In the DPPH test, a stable free radical, DPPH, is reduced in the presence of an antioxidant, leading to a change in its distinctive purple color, which has a strong absorbance peak at 517 nm. When an FRS antioxidant reacts with DPPH, it forms DPPH-H, resulting in decreased absorbance and a color shift from purple to yellow as hydrogen atoms are donated. This color change indicates a reduction in radical concentration, directly reflecting the antioxidant’s capacity to neutralize free radicals. As the DPPH solution interacts with a hydrogen-donating compound, it trans-forms into diphenylpicrylhydrazine, losing its violet color, which signifies the antioxidant's electron-donating power [
16,
17]. The FRAP test is based on the reducing power which is determined by the reduction of Fe (III) to Fe (II) in the presence of the tested compound. The concentration of Fe (II) can be monitored by measuring the formation of Prussian blue at 700 nm. The higher dye concentration and therefore the higher absorbance, the greater reducing power and enhanced antioxidant activity of the tested compound. The advantages of this method are simplicity, low cost and time, together with no need of the specialized equipment [
18].
Information on the degradability of organic chemicals is valuable for both hazard and risk assessments. Aquatic hazard classification and general hazard assessments typically rely on standardized tests for ready biodegradability. However, data from tests simulating biodegradation in environments such as water, aquatic sediment, and soil can also contribute to these samples’ assessment [
19]. The persistence and dissipation of active substances are measured using DT (dissipation time), especially DT50 and DT90 values, which represent the time required for 50% and 90% of a substance, respectively, to disappear. “Disappearance” encompasses all processes leading to the substance’s transformation, degradation, and eventual mineralization, including microbial degradation, chemical hydrolysis, photochemical reactions, and other mechanisms like leaching, volatilization, and plant uptake. In soil, laboratory experiments generally consider a DT50 of less than 60 days at 20°C acceptable (or less than 90 days at 10°C for applications in colder regions). In field conditions, acceptable thresholds are a DT50 of less than 3 months and a DT90 of less than 1 year. It should be noted that DT50 and DT90 indicators do not differentiate between degradation processes—such as mineralization, microbial degradation, hydrolysis, or photochemical reactions—and loss through leaching, volatilization, or plant uptake. Instead, these values provide a holistic assessment of a substance’s dissipation, encompassing all contributing processes [
20]. In terms of adsorption - it depends on two main factors - pesticide proper-ties and soil properties showing the behavior of pesticide in soil. Molecular properties of pesticides like solubility, ionisability and hydrophilicity and lipophilicity are crucial [
21].
The organic carbon partition coefficient Koc was used as a key parameter to assess the environmental risk of pinocembrin dihydrochalcone. The Koc value indicates the compound’s binding capacity and its potential for sorption in soil [
22].
Various analytical methods are available for determining chalcone concentrations across different matrices, including chromatography with a range of detectors, MALDI, and HNMR techniques [
3]. Pompermaier et al. employed an UHPLC-DAD method to identify six primary dihydrochalcone derivatives [
23]. In this experiment, UHPLC-ESI-MS/MS was used to determine the DT50 and DT90 values as well as to assess the pinocembrin dihydrochalcone concentration after sorption experiment.
The aim of the study was to evaluate the attributes of pinocembrin dihydrochalcone, including its antioxidant properties and environment behavior as potential biopesticide. This could be assessed through the soil dissipation study experiment as well as sorption affinity to soil.
2. Materials and Methods
2.1. Material
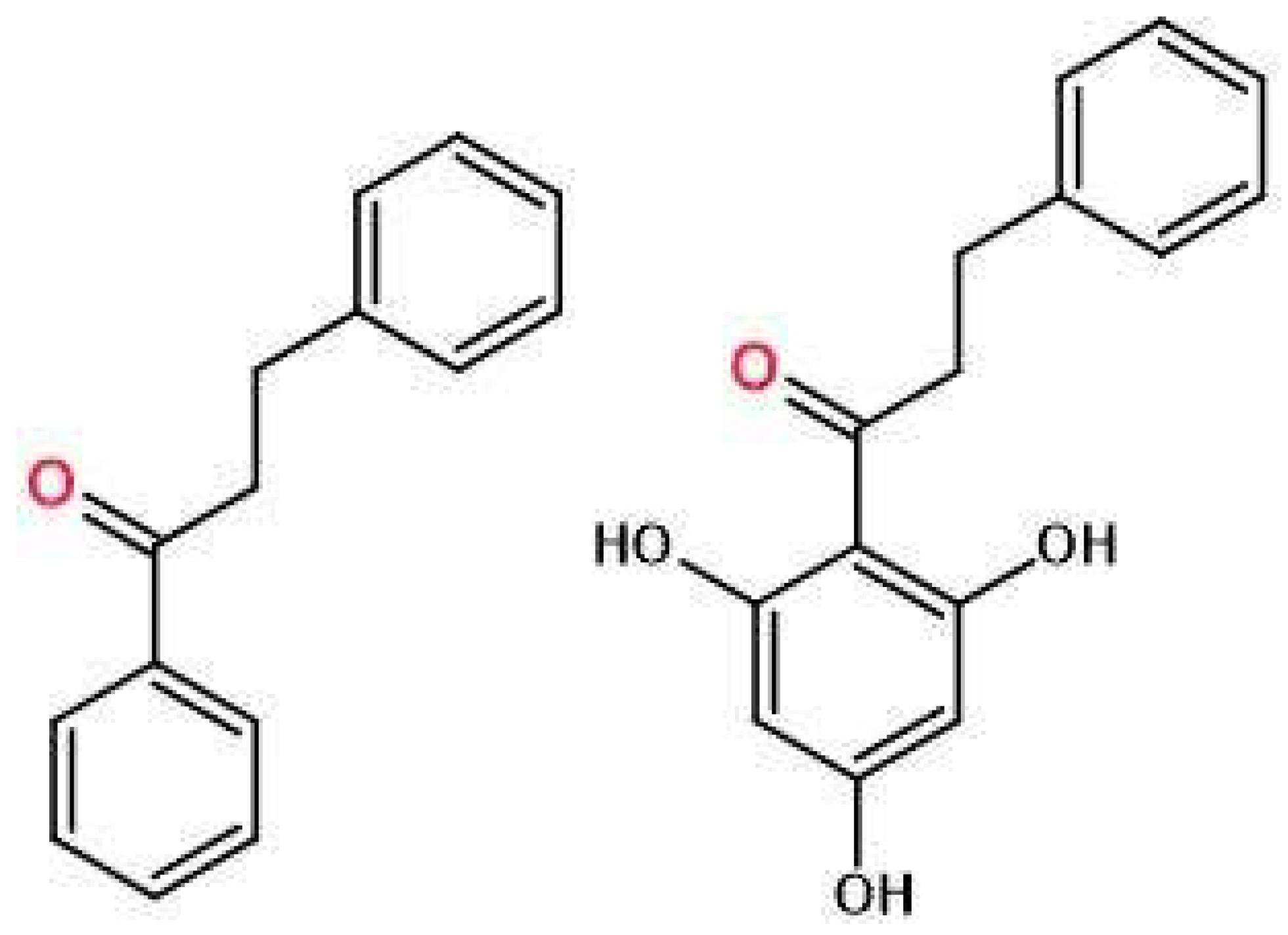
The tested material, pinocembrin dihydrochalcone, was supplied by Methabolic Insight Ltd. (Ness Ziona, Izrael). Pinocembrin dihydrochalcone (3-phenyl-1-(2,4,6-trihydroxyphenyl)-1-propanone) is a white, amorphous powder, produced synthetically with 99,5% purity.
2.2. Soil Dissipation Studies
The dissipation study of dihydrochalcone was done under controlled laboratory conditions using growth chambers and followed the OECD 307 protocol with slight modifications [
24]. Soil samples were collected from the upper layer (0-15 cm) of two soil types differ in the physicochemical properties: Laskowice and Biostrateg, both free of dihydrochalcone residues. After passing the soil through a 2-mm sieve, it was stored in covered trays in a greenhouse for 10 days with regular mixing. Soil moisture content was measured by drying samples at 105°C for 24 hours and calculating the weight difference. To standardize conditions, soil moisture was maintained at 60% of field capacity, monitored regularly, and adjusted with distilled water as needed. Soil samples were placed in 60 mm diameter pots (height: 55 mm), with three replicates per variant. These pots were then transferred to growth chambers set to a day/night temperature cycle of 20°C, with light intensity at 250 ±10 µmol·m
-2·s
-1 photosynthetic photon flux. Two days after setting up the pots in the chambers, dihydrochalcone was applied using a stationary chamber sprayer equipped with a TeeJet XR 11003-VS mobile nozzle. The application rate matched field conditions (200 L/ha). Soil samples (one pot, approximately 150 g, per sample/replicate) were collected at 1 hour (initial concentration) and at 1, 3, 6, 12, 21, and 36 days after treatment (DAT). For analysis, soil samples were extracted with methanol using a high-pressure, high-temperature extractor. The methanolic extract was then centrifuged at 7500 rpm, and the supernatant was used. The quantitative analysis of pinocembrin dihydrochalcones was performed using ultrafast liquid chromatography coupled with a triple quadrupole mass spectrometer (UHPLC-MS/MS).
2.3. Basic Physicochemical Properties of the Soil
Key physicochemical parameters of the two soil types - Laskowice and Biostrateg - were analyzed, including pH, phosphorus, and potassium content, as well as granulometric composition. Soil pH was measured potentiometrically using a 1 M KCl solution, and phosphorus and potassium content was determined using the Egner-Riehm method. Soil texture analysis was performed with the modified Casagrande method [
25,
26]. The results are presented in
Table 1.
2.4. Partition Coefficient
The partition coefficient of dihydrochalcone was determined experimentally based on its sorption into the soil. A silt loam soil, representative of agriculturally used soils in Poland, was selected for the study. The soil composition included 35% sand, 63% silt, and 2% clay, with the following properties: pH (KCl) = 7 .4, total carbon (TC) = 9 .02 g kg
-1, total nitrogen (TN) = 0.97 g kg
-1, and total organic carbon (TOC) = 7.74 g kg
-1. It was free from contamination by polycyclic aromatic hydrocarbons, pesticides, and heavy metals, ensuring reliable results by minimizing interference with the sorption process. The distribution coefficient (K
oc) was determined by plotting a logarithmic curve fitted to the experimental data. Sorption studies were conducted using test solutions with varying dihydrochalcone concentrations (0.1 mg kg
-1, 1 mg kg
-1, 2 mg kg
-1, 3, mg kg
-1, 5 mg kg
-1, and 10 mg kg
-1), with each concentration measured in duplicate. The relative standard deviation (RSD) was < 2%. A matrix blank and a surrogate standard were included in the analysis to assess compound stability during the sorption period. Sorption was carried out over 24h at a constant temperature of 20
o C, with 5 ml of the test solution and 1 g of soil shaken on a rotary shaker at 120 rpm. The concentration of dihydrochalcone in the solution was determined using LC-ESI-MS/MS method described in the
Section 2.5.
2.5. Identification and Quantification of Pinocembrin Dihydrochalcone
The identification and quantification of pinocembrin dihydrochalcone after soil dissipation studies and partition coefficient experiment was conducted using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) with a triple quadrupole analyzer operating in negative ionization mode. Chromatographic separation was achieved using a Shimadzu Prominence UFLC system (Shimadzu, Kyoto, Japan), equipped with an LC-30ADXR binary solvent manager, DGU-20A3 degasser, CTO-10ASVP column oven, Kinetex C18 column (2.6 μm particle size, 50 × 3.0 mm, Phenomenex, Torrance, CA, USA), SIL-20AXR autosampler, and CBM-20A system controller. Separation was performed under reversed-phase conditions, with a mobile phase of water (A) and acetonitrile (B) in a binary gradient, containing 32% water. The total analysis time was 10 minutes, with a flow rate of 0.40 mL/min, a column temperature of 35°C, and a sample injection volume of 10 µL. Quantitative data were processed using LabSolution Ver. 5.6 (Shimadzu, Kyoto, Japan) software. Pinocembrin dihydrochalcone identification and quantification was conducted in selected reaction monitoring (SRM) mode. Negative ionization mode was used for the analysis, with the following transitions selected for dihydrochalcone identification and quantification: SRM 1: 257.26 > 213.15, dwell time 100 ms, collision energy (CE) = 19; SRM 2: 257.26 > 81.00, dwell time 100 ms, CE = 29; SRM 3: 257.26 > 173, dwell time 100 ms, CE = 35.
2.6. Antioxidant Capacity Assays
2.6.1. DPPH Assay
The DPPH radical scavenging assay was conducted using a modified version of the method developed by Brand-Williams et al. (1995) [
17]. Pinocembrin dihydrochalcone was initially dissolved in methanol at a concentration of 1.0 mg mL
-1, then further diluted in water to final concentrations of 100, 50, 30, 20, 10, 5, 3, 2, 1, 0.1, 0.01 mg L
-1. Each concentration, was transferred to a 96-well plate in eight replicates. An equal volume of 0.2 mM methanolic DPPH• solution was added to each well. Absorbance was measured immediately at 517 nm using a BioTeq μQuant™ Microplate Spectrophotometer, and then recorded every 20 minutes over a 3-hour period during which samples were incubated in the dark at room temperature. Pure water was used as a control.
The scavenging effect percentage was calculated with the following formula:
where:
AbsDPPH is the absorbance of water (control) with DPPH•
Abssample is the absorbance of the sample with DPPH•
Abscontrol is the absorbance of water without DPPH•
From the obtained results, the EC50 value was calculated after 3-hour reaction using AAT Bioquest, Inc. (2024); [
27].
2.6.2. FRAP Test
The antioxidant activity of dihydrochalcone was evaluated using FRAP test following a method described by Oyaizu (1986) [
28] and then slightly modified by Oleszek and Kozachok (2018) [
29]. Briefly, 1 ml aliquots of dihydrochalcone, with concentrations ranging from 0 to 250 μg ml-1, were mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium (III) ferricyanide. The mixture was incubated at 50°C for 30 min. Next, 2.5 ml of 10% trichloroacetic acid (TCA) was added, and the samples were centrifuged at 6000 rpm for 10 min. The resulting supernatant (2.5 ml) was diluted with 2.5 ml of deionized water and 0.5 ml of 0.1% ferric (III) chloride. The absorbance was measured at 700 nm. The results were expressed as the effective concentration (EC
50), which represents the concentration corresponding to an absorbance of 0.5. Ascorbic acid was used as reference sample. It is strong antioxidant, typically used in FRAP assay as a standard, to allow easily assess the antioxidant activity of the test sample by comparison [
18].
3. Results
3.1. Dissipation study results
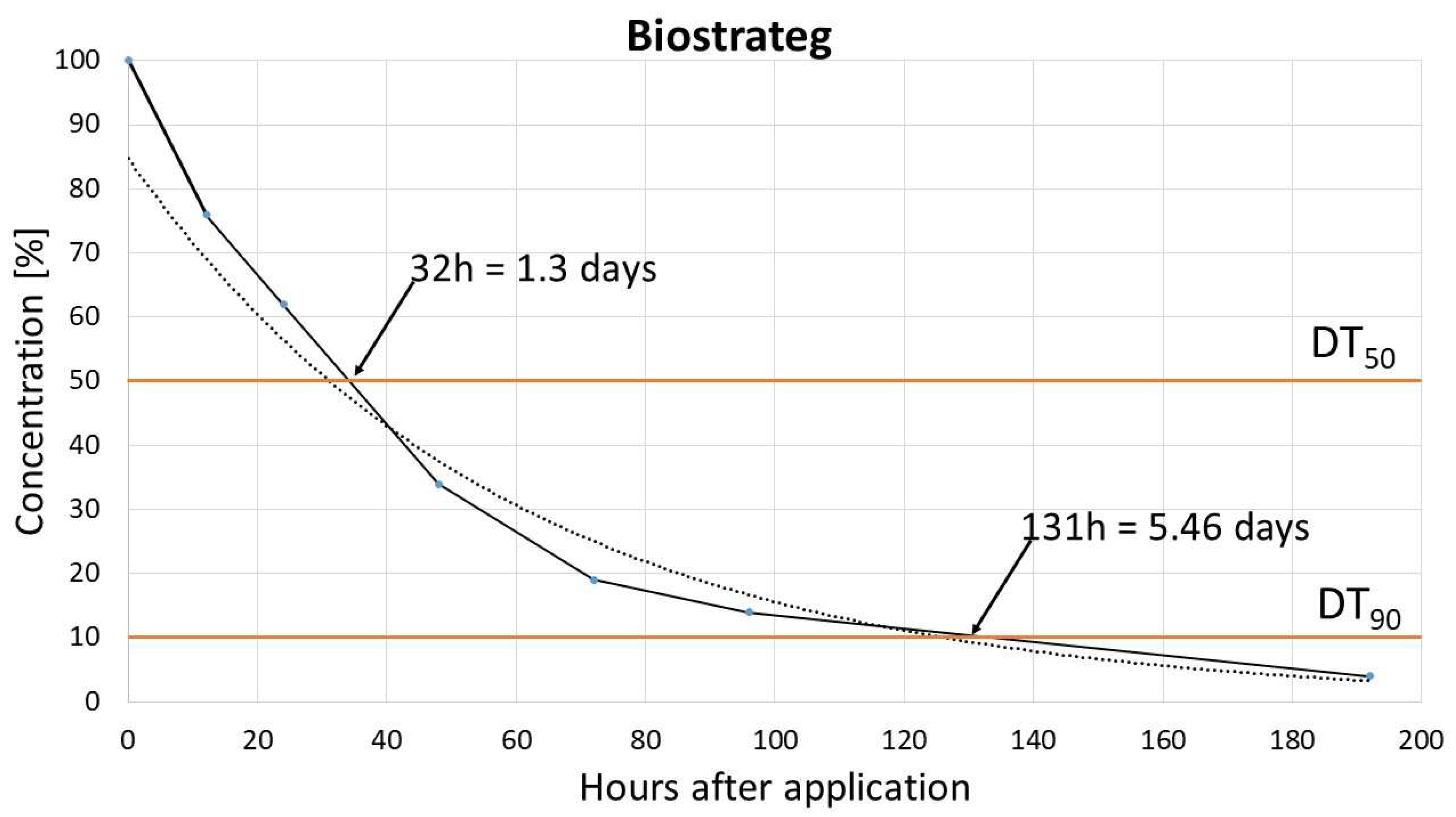
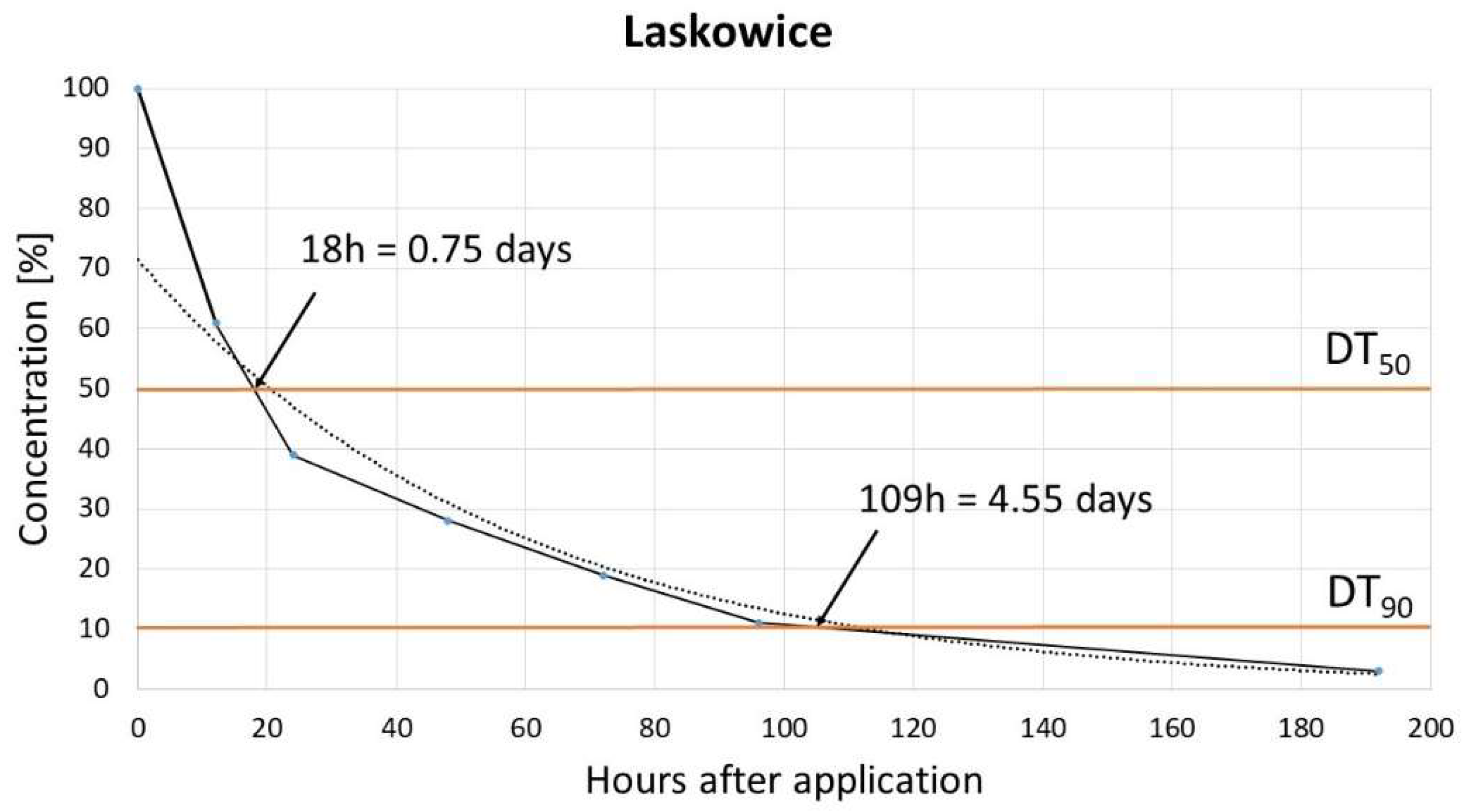
The dissipation study aimed to determine the DT50 (time for concentration to reduce to 50% of its initial value, half life time) and DT90 (time for concentration to reduce to 10% of its initial value) for pinocembrin dihydrochalcone. Results obtained in two different soils: Biostrateg and Laskowice, indicated that dissipation of dihydrochalcone follows single first-order (SFO) kinetic model, with very rapid degradation. For Biostrateg model DT50 and DT90 reached 1.3 and 5.46 days, respectively, while in the case of Laskowice soil relevant values were 0.75 and 4.55 days. [
Figure 2 and
Figure 3].
These findings align with those of Pedrinho et al. who reported a dihydrochalcone dissipation rate of 2.3 to 9.85 days (for DT50 and DT90) across three soil types, also described by the SFO model [
11].
3.2. Partition Coefficient Results
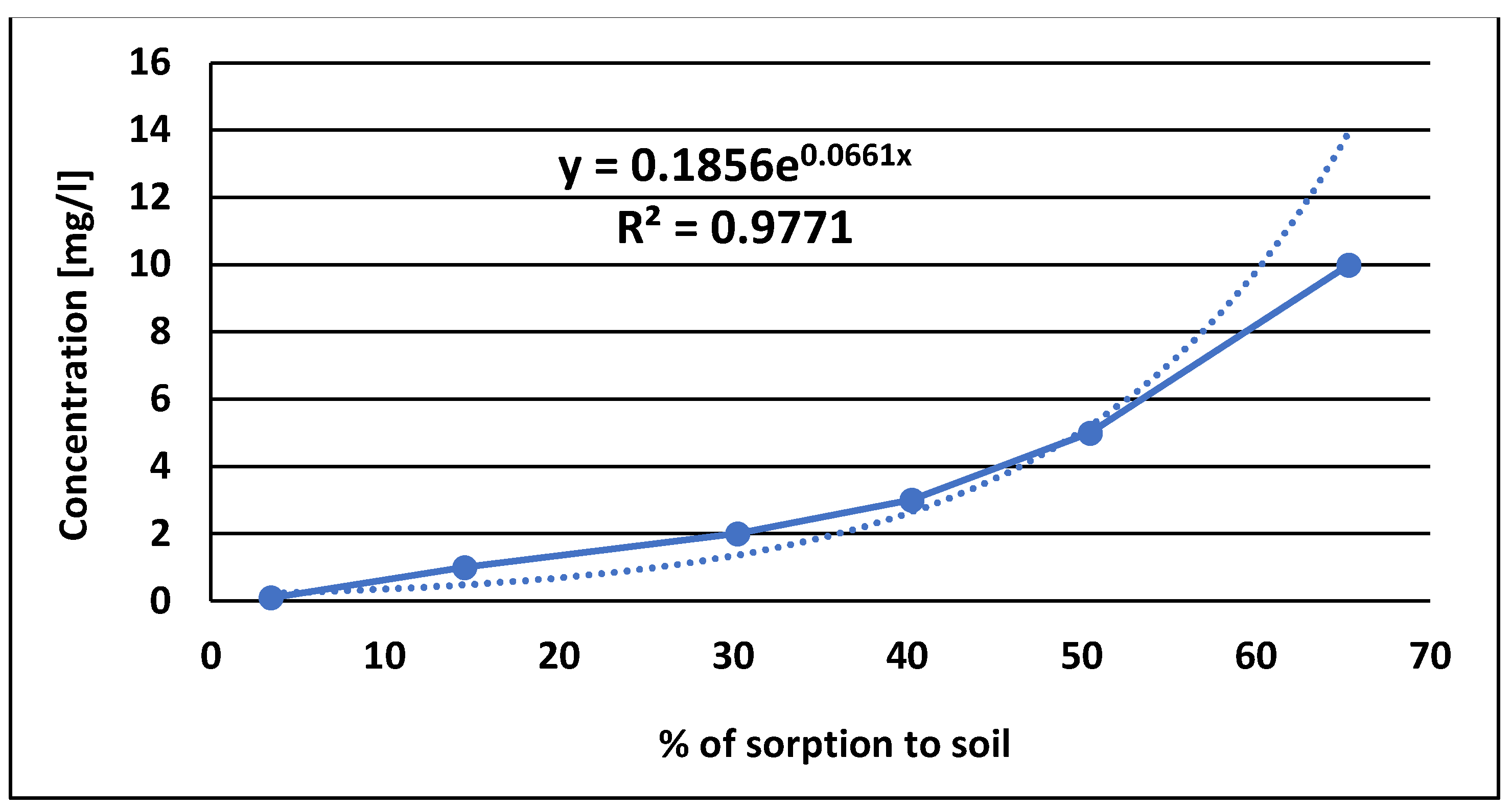
Accumulation of pesticides belonging to organic compounds in soils is the result of complex processes such as partitioning, sorption, desorption and diffusion, which involve both the mineral and organic fractions of the soil. These processes determine the persistence and residence of these compounds in the soil as well as their potential for degradation. Partition coefficient-K
oc is used to describe the binding capacity and release of organic compounds in soil [
22]. In our study, the sorption of pinocembrin dihydrochalcone exhibited the character of a Freundlich curve model which indicates the nonlinear nature of the binding processes in the soil, involving both absorption and adsorption [
Figure 4]. This model suggests that contaminant molecules are influenced by active sites (functional groups on soil components) that vary in both quantity and quality, which in turn affects the strength of pinocembrin dihydrochalcone adsorption to the soil.
Pesticides with a high value of the partition coefficient typically exhibit a pronounced tendency to sorb in the soil and a limited desorption capacity. On the other hand, pesticides with low value may pose a risk to aquatic organisms, if they easily enter groundwater through rapid leaching, which indicates its low affinity for soil and resistance to aging processes. The result of partitioning is sorption, or the process of binding pesticides to the solid phase. Observed sorption processes in studied soil occur probably between the soil solution in which these compounds are dissolved and organic/mineral matter. This is due to the high affinity of these compounds mainly to nonpolar soil components characterized by a high carbon-to-oxygen ratio – such as organic matter [
30,
31]. The binding of pesticides can take place inside the three-dimensional structures of organic matter (absorption) and/or on the surface of various components as a result of physical (physical adsorption) and chemical (chemical adsorption) processes. The rate of these sorption processes occurs mainly limited by the rate of diffusion of contaminants in the soil matrix [
32].
3.3. Antioxidant Capacity Assays
3.3.1. DPPH Assay
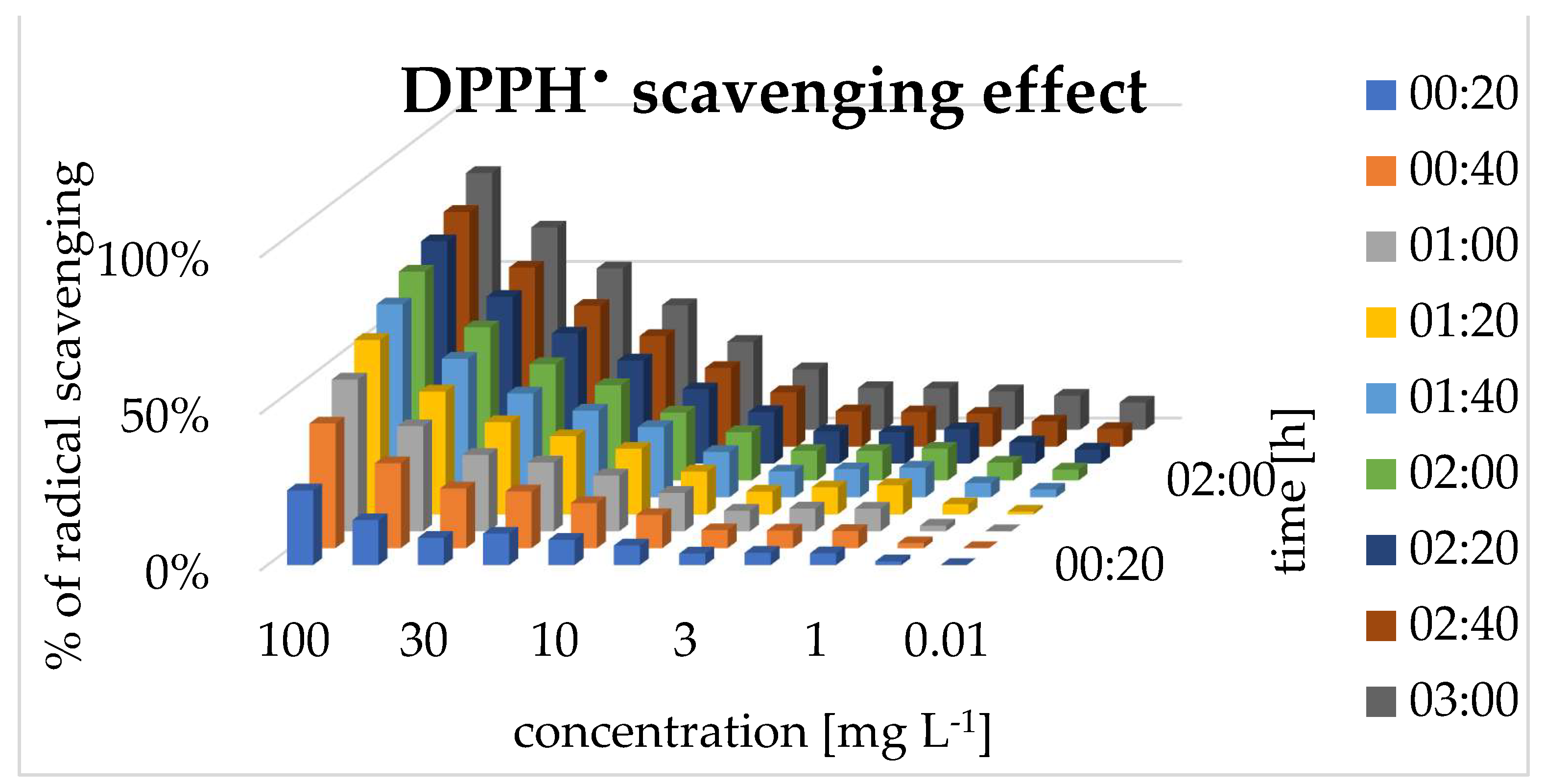
Anti-radical activity (DPPH scavenging) was observed in the water solution, within the tested concentrations. The scavenging effect depended on time and the concentration and the strongest effect was observed at the higher concentration of 0.1 mg mL
-1, reaching a plateau after 1.5 hours. As the concentration decreased, the time required to achieve peak activity increased correspondingly [
Figure 5]. Referring to obtained results the half maximal effective concentration (EC50) of pinocembrin dihydrochalcone was calculated to be 0.041 mg mL
-1. This value is lower than values of the typically used range in agricultural applications 0.3-0.6 mg mL
-1.
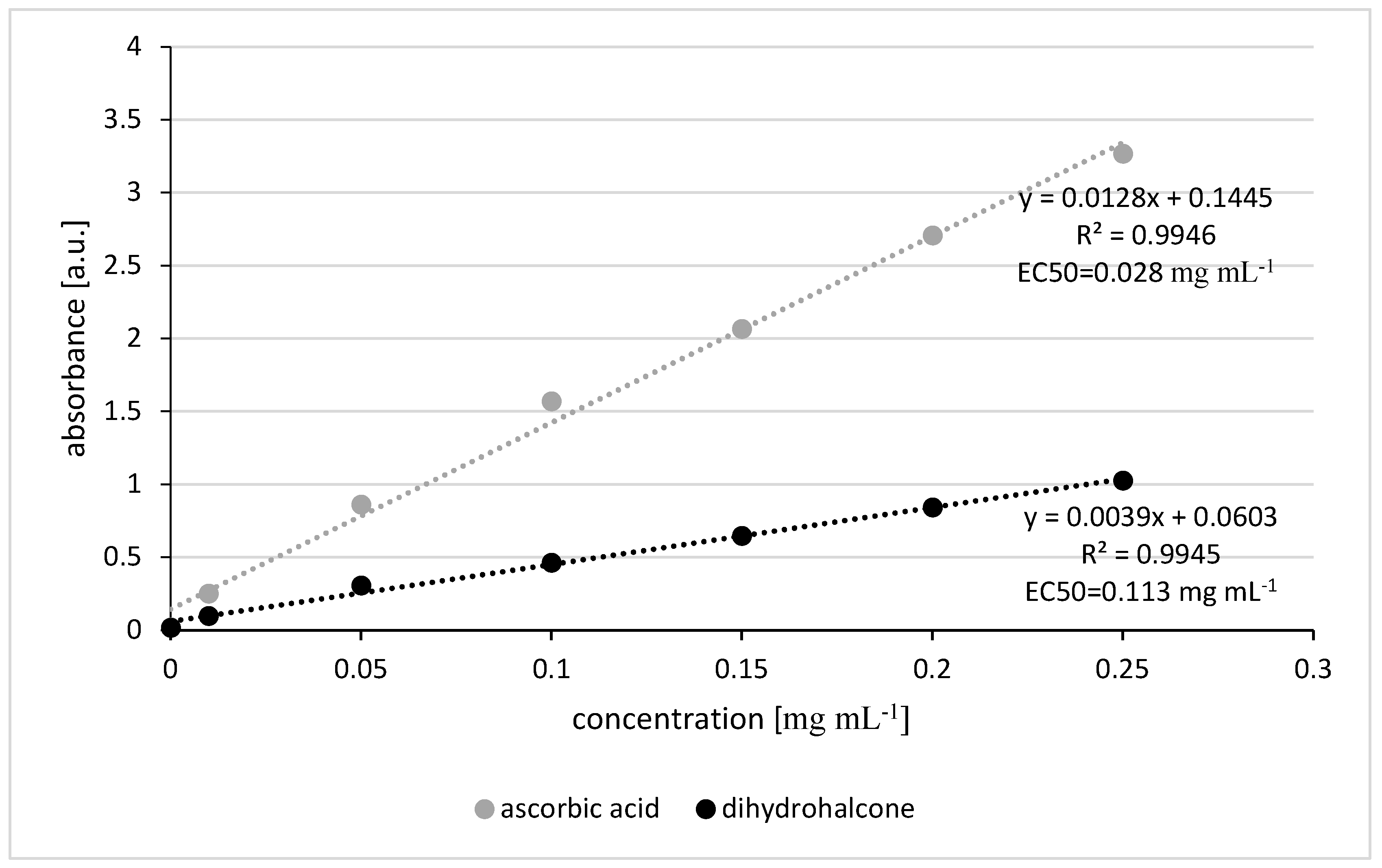
3.3.2. FRAP Assay
According to the FRAP test, the reducing power of dihydrochalcone was lower than that of ascorbic acid, which serves as the reference sample. The EC
50 values for dihydrochalcone and ascorbic acid were 0.113 and 0.028 mg mL
-1, respectively [
Figure 6]. Antioxidant activity increased proportionally with concentration within the tested range. The EC50 value in this test is also much lower than recommended concentration for use as fungicide (0.3 – 0.6 mg mL
-1). It proves that pinocembrin dihydrochalcone will have strong antioxidant activity when used in the field.
4. Discussion
Among the widely used methods, the FRAP and DPPH radical scavenging assays are prominent. Although both assays aim to evaluate antioxidant potential, they operate via distinct mechanisms. This study compares the fundamental principles, reaction mechanisms, and applications of these two assays.
The FRAP assay is based on the principle of electron transfer, where antioxidants reduce ferric ion (Fe³⁺) to ferrous ion (Fe²⁺). The reaction involves the ferrocyanide, which, upon reduction, forms a Prussian blue complex. This assay is particularly effective in assessing reducing power rather than radical scavenging capacity. The reducing power of pinocembrin dihydrochalcone was found to be lower than that of ascorbic acid, with EC50 values of 0.113 mg mL-1 for pinocembrin dihydrochalcone and 0.028 mg mL-1 for ascorbic acid. The observed linear increase in antioxidant activity with concentration suggests that pinocembrin dihydrochalcone could be even stronger antioxidant at higher concentrations. First of all, it may be expected that pinocembrin dihydrochalcone will exhibit strong antioxidant activity when used as a fungicide at recommended dose of 0.3 – 0.6 mg mL-1.
These findings are consistent with previous studies, such as those by Chen et al. [
33], who reported comparable reducing power between phloridzin (a dihydrochalcone) and ascorbic acid within a concentration range of 0.1-0.3 mg mL
-1.
In turn, the DPPH assay evaluates the ability of antioxidants to scavenge free radicals by donating hydrogen or electrons. Unlike the FRAP assay, the DPPH assay operates in a neutral or slightly acidic organic solvent (typically methanol or ethanol). It is widely used for assessing both electron-donating and hydrogen-donating antioxidants.
In this matter, regarding the structural activity relationship (SAR), the pinocembrin dihydrochalcone possess antioxidant activity thanks to three hydroxyl groups, which allow to electron transfer (ET), hydrogen atom transfer (HAT) and radical adduct formation (RAF) during the contact with free radicals [
14]. The number of phenolic hydroxyl groups within the pinocembrin dihydrochalcone structure may enhance antioxidant activity, while glycosylation could reduce it [
14,
34].
Moreover, the antioxidant activity is enhanced also by formation of the intramolecular hydrogen bond (IHB) between 2’-OH (or 6’-OH) and adjacent keto group.
In general, the FRAP and DPPH assays provided comprehensive evaluation of antioxidant properties of pinocembrin dihydrochalcone. Our results showed differences in the direction of activity of the tested compound. It should be emphasized that the choice of assay depends on the nature of antioxidants being tested and the desired analytical outcome. Therefore, in many studies, both assays are used together to provide a complex insight into the mechanisms of action [
16,
35], while others have successfully incorporated only one method for antioxidative assessment of chalcones and phenolic compounds [
36].
The dissipation study, which aimed to determine the degradation rates of pinocembrin dihydrochalcone, revealed that it follows a single first-order (SFO) kinetic model. Rapid degradation of pinocembrin dihydrochalcone was observed, with a DT50 ranging from 0.85 to 1.7 days and a DT90 (time for concentration to decrease to 10%) between 4.55 and 5.26 days. These findings align with the results reported by Pedrinho et al. [
11], who also observed rapid dissipation of dihydrochalcone across different soil types, suggesting that dihydrochalcone breaks down quickly in the environment, reducing its persistence and potential for accumulation. This rapid degradation may be advantageous for minimizing environmental impact, but it also implies that repeated or continuous applications may be necessary for sustained effectiveness in agricultural settings. Soil degradation rates can be influenced by various factors, including the soil's physicochemical properties (such as pH and organic carbon content), biological properties (such as microbial activity and distribution), and environmental conditions, like temperature and moisture. The soil chemical properties also play a role in determining both the degradation route and rate. Degradation rates may also vary, with multiple studies confirming significant field-to-field differences in pesticide dissipation [
34,
37]. The rate of degradation in soil depends also on the properties of a substance, such as its antioxidant activity [
20]. Nonetheless, the results of our study have shown that antioxidant properties of pinocembrin dihydrochalcone did not affect its rapid degradation in soil.
The sorption characteristics of pinocembrin dihydrochalcone were also studied, and the results indicated that its sorption to soil followed a Freundlich isotherm, which is indicative of a nonlinear adsorption process involving both absorption and adsorption mechanisms. The Freundlich model suggests that pinocembrin dihydrochalcone interacts with soil through active sites that vary in both quantity and quality, influencing the strength of adsorption. The measured Koc value of 0.0661 suggests a low affinity for soil, indicating that pinocembrin dihydrochalcone is less likely to be strongly bound to soil particles due to chemical or physical interactions. This result implies that pinocembrin dihydrochalcone may be subject to quicker leaching from the soil, reducing its potential for long-term soil residence. Furthermore, the low Koc value suggests that pinocembrin dihydrochalcone is less likely to undergo aging processes that would otherwise increase its persistence in the soil. A higher Koc values for pesticides is generally beneficial for environmental persistence, as it reduces the likelihood of leaching or surface runoff, thereby minimizing groundwater contamination. However, low hydrophobicity and high water solubility creates a risk of uptake by plants and uptake by soil organisms in the first period after its deposition in the soil. Conversely, an excessively high Koc value (log Koc>4.5) may lead to adverse effects on terrestrial organisms, such as earthworms, due to prolonged soil retention and potential bioaccumulation.
Overall, the findings of this study highlight the promising antioxidant potential of dihydrochalcone, while also underscoring the influence of environmental factors such as solvent type, concentration, and soil interactions on its bioactivity. While pinocembrin dihydrochalcone exhibits antioxidant effect and rapid degradation in the soil, its low affinity for soil and rapid dissipation suggest that it may be more effective in short-term applications, with careful consideration needed for its persistence and long-term efficacy in agricultural settings.
5. Conclusions
The DPPH and FRAP assays indicated a potential antioxidant activity of pinocembrin dihydrochalcone. Its effective concentrations (EC50) were assessed at 0.041 mg mL-1 and 0.113 mg mL-1, respectively. It suggests that the compound activity may be even higher at the concentration of 0.3-0.6 mg mL-1 - the recommended concentration for agricultural applications.
Biodegradation in soils involves free radicals, raising questions about the role of antioxidant properties in this process. Polyphenolic compounds, known for their antioxidant activity, are generally considered resistant to degradation due to their ability to neutralize free radicals. However, dissipation studies have shown that, despite its strong antioxidant and antiradical properties, pinocembrin dihydrochalcone degrades rapidly in soil, following a single first-order (SFO) kinetic model.
The DT50 and DT90 values were within a narrow range, indicating the compound’s rapid breakdown in soil. This fast dissipation suggests that pinocembrin dihydrochalcone has low persistence in soil, potentially minimizing its environmental impact but also limiting its effectiveness as a long-term agricultural additive. Its low organic carbon partition coefficient (Koc) suggests minimal risk to soil environment, but raises concerns about increased leaching into groundwater, posing a higher risk to aquatic organisms. The compound's limited longevity may further reduce its efficacy over time. Future research should explore strategies to enhance its stability and effectiveness in both soil and agricultural applications.
Author Contributions
Conceptualization, M.D.-B.; methodology, M.D.-B, M.O. ,A.U.-J.,W.K..; software, M.D.-B., M.K.; validation, M.D.-B. and W.K..; formal analysis, M.D.-B, M.O., W.K. .; investigation, M.D.-B., M.O.; data curation, M.D.-B., M.O, M.K.,W.K., S.Z.; writing—original draft preparation, M.D.-B, M.O., A.U.-J., S.Z., ; writing—review and editing, M.D.-B., M.O., S.Z., M.B.; visualization, M.D.-B., M.O.; supervision, M.D.-B. and S.Z. ; project administration, M.D.-B..; funding acquisition, M.D.-B., M.O., M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the European Union’s Horizon Europe research and innovation programme under the grant agreement No 101084163 project RATION.
Institutional Review Board Statement
“Not applicable”
Informed Consent Statement
“Not applicable.”
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants (Basel) 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in Sustainable agriculture: a critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Dziągwa-Becker, M.; Oleszek, M.; Zielińska, S.; Oleszek, W. Chalcones—Features, Identification Techniques, Attributes, and Application in Agriculture. Molecules 2024, 29, 2247. [Google Scholar] [CrossRef]
- Szparaga, A. From biostimulant to possible plant bioprotectant agents. Sciendo 2023, 27, 87–98. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry 2006, 661, 23–67. [Google Scholar]
- Garrido, R.M.; Dayan, F.E.; Ozanique, P.R.; Regasini, L.O.; Kolb, R.M. Hydroxychalcones as Herbicides. Agroomy 2025, 15, 572. [Google Scholar] [CrossRef]
- Garrido, R.M. , Dayan F.E., Ozanique P.R., Regasini L.O., and Kolb R.M.; Methoxychalcones and Cinnamaldehyde as Herbicidal Compounds; ACS Agricultural Science & Technology 2025. [CrossRef]
- Stompor, M.; Broda, D.; Bajek-Bil, A. Dihydrochalcones: methods of acquisition and pharmacological properties—a first systematic review. Molecules 2019, 24, 4468. [Google Scholar] [CrossRef]
- Safety Data Sheet – Dihydrochalcone, company -Metabolic Insights Ltd., Ness Ziona 7414002, Izrael.
- Pobłocka-Olech, L. Zastosowanie Metod Chromatograficznych w Badaniach Składu Chemicznego Kory Niektórych Gatunków i Klonów Wierzby. Ph.D. Dissertation, Medical University of Gdańsk, Gdańsk, Poland, 2006. [Google Scholar]
- Pedrinho, A.; Karas, P.A; Kanellopoulos, A.; Feray, E.; Korman, I.; Wittenberg, G.; Ramot, O.; Karpouzas, D.G. The effect of natural products used as pesticides on the soil microbiota: OECD 216 nitrogen transformation test fails to identify effects that were detected via q-PCR microbial abundance measurement. Pest Manag Sci. 2024, 80, 2563–2576. [Google Scholar] [CrossRef]
- Santra, H.K.; Banerjee, D. Natural products as fungicide and their role in crop protection. Natural Bioactive Products in Sustainable Agriculture 2020, 12, 131–219. [Google Scholar] [CrossRef]
- Werner, E.; Montenegro, I.; Said, B.; Godoy, P.; Besoain, X.; Caro, N.; Madrid, A. Synthesis and anti-saprolegnia activity of new 2',4'-dihydroxydihydrochalcone derivatives. Antibiotics (Basel) 2020, 9, 317. [Google Scholar] [CrossRef]
- Li, X. ; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant Structure–Activity Relationship Analysis of Five Dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef]
- Rimmer, D. L. Free radicals, antioxidants, and soil organic matter recalcitrance. European journal of Soil Science 2006, 57, 91–94. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M. E.; and Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. u. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hsieh, C. , & Rajashekaraiah, V. (2021). Ferric reducing ability of plasma: a potential oxidative stress marker in stored plasma. 2021, 52, 61–67. [Google Scholar]
- OECD (2006), Revised Introduction to the OECD Guidelines for Testing of Chemicals, Section 3, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris. [CrossRef]
- Berger, M.; Sonderegger, T.; Alvarenga, R.; Bach, V.; Cimprich, A.; Dewulf, J.; Frischknecht, R.; Guinée, J.; Helbig, C.; Huppertz, T.; Jolliet, O.; Motoshita, M.; Northey, S.; Peña, C.A.; Rugani, B.; Sahnoune, A.; Schrijvers, D.; Schulze, R.; Sonnemann, Guido. ; Valero, A.; Weidema, B.P.; Young, S.B. Mineral resources in life cycle impact assessment: part II –recommendations on application-dependent use of existing methods and on future method development needs. Int. J. Life Cycle Assess. 2020, 25, 798–813. [Google Scholar] [CrossRef]
- Saheem Rasool, Tanveer Rasool, Khalid Muzamil Gani, A review of interactions of pesticides within various interfaces of intrinsic and organic residue amended soil environment. Chemical Engineering Journal Advances 2022, 11, 100301. [CrossRef]
- Zhang, J.; Zhu, T.; Cai, Z.; Müller, C. Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil 2011, 342, 419–432. [Google Scholar] [CrossRef]
- Pompermaier, L.; Schwaiger, S.; Mawunu, M.; Lautenschlaeger, T.; Stuppner, H. Development and validation of a UHPLC-DAD method for the quantitative analysis of major dihydrochalcone glucosides from Thonningia sanguinea VAHL. Planta Med. 2019, 85, 911–916. [Google Scholar] [CrossRef]
- OECD (2002), Test No. 307, Aerobic and Anaerobic Transformation in Soil, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris. [CrossRef]
- Drozd, J.; Licznar, M.; Licznar, S.E.; Weber, J. Gleboznawstwo z elementami mineralogii i petrografii. Wydawnictwo Akademii Rolniczej we Wrocławiu, wydanie II, 210. 1998.
- Kabała, C.; Karczewska, A. Metodyka analiz laboratoryjnych gleb i roślin. Wydanie 4, 2008, http://www.ar.wroc.pl/~kabala, pp. 37–41.
- AAT Bioquest, Inc. Quest Graph™ IC50 Calculator. AAT Bioquest. 2021. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 15 April 2024).
- Oyaizu, M. Studies on products of browning reaction – Antioxidative activities of products of browning reaction prepared from glucosamine. Japanese Journal of Nutrition 1986, 44, 307–315. [Google Scholar]
- Oleszek, M. , Kozachok, S. Antioxidant activity of plant extracts and their effect on methane fermentation in bioreactors. International Agrophysics 2018, 32. [Google Scholar]
- Cornelissen, G.; Gustafsson, Ö.; Bucheli, T.D.; Jonker, M.T. O.; Koelmans, A.A. van Noort, P.C. M. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and Consequences for Distribution, Bioaccumulation, and Biodegradation. Envir. Sci. Tech. 2005, 39, 6881–6895. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J. Dynamic interactions of natural organic matter and organic compounds. J. Soil Sediments 2012, 12, 1241–1256. [Google Scholar] [CrossRef]
- Ehlers, G.; Loibner, A. Linking organic pollutant (bio)availability with geosorbent properties and biomimetic methodology: A review of geosorbent characterization and (bio)availability prediction. Environ. Pollut. 2006, 141, 494–512. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, L.Z.; Zhao, L.; Shu, G.; Yuan, Z.X.; Fu, H. L.; Cheng, L.; Lin, J. C. Optimization of the ultrasound-assisted extraction of antioxidant phloridzin from Lithocarpus polystachyus Rehd. using response surface methodology. J. Sep. Sci., 2017, 40, 4329–4337. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Brown, C.D. Factors influencing degradation of pesticides in soil. J Agric Food Chem. 2007, 55, 4487–92. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Shang YF, Oidovsambuu S, Jeon JS, Nho CW, Um BH. Chalcones from the flowers of Coreopsis lanceolata and their in vitro antioxidative activity. Planta Med. 2013 Mar;79(3-4):295-300. [CrossRef]
- Farha, W.; Abd El-Aty, A.M.; Rahman, M.M.; Shin, H.C.; Shim, J.H. An overview on common aspects influencing the dissipation pattern of pesticides: a review. Environ Monit Assess. 2016 188, 693. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).