1. Introduction
Tomato (
Solanum lycopersicum L.) is one of the most important vegetables for humans. It is the source of nutrients such as phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and iron (Fe) [
1], and secondary metabolites including vitamin C [
2], vitamin K1 [
3], vitamin B9 [
4]. Moreover, tomatoes contain a high amount of lycopene [
5], a potential antioxidant with anticarcinogenic properties [
6]. Fresh tomatoes have a relatively high K content, approximately 290 mg 100 g/fresh weight (FW) [
7]. Therefore, people on dialysis may need to limit K intake in their diet to less than 200 mg of K per serving or 2,000 mg per day [
8,
9]. Plant-based foods (fruits and vegetables) vary in fiber, vitamins, and minerals, which help support a healthy kidney disorder patient body [
10]. Consequently, low K foods are necessary for patients who suffer from kidney disease [
11].
Potassium (K) is an essential element for plant growth, and it is a high requirement for tomatoes. K contributes to plant photosynthesis, enzyme activation, protein synthesis, and the role of biochemical and physiological substance in plant cell metabolism, such as protoplasmic structure maintenance, pH level balance, and plant osmatic [
12,
13,
14,
15]. K deficiency directly disrupts the biochemical activities of plants, leading to a reduced growth rate and accelerated leaf senescence, which ultimately results in irreversible wilting [
16]. In the other hand, excessed K application leads to lower K use efficiency [
17,
18], and induces antagonistic effects with Mg and Ca due to K could compete for the same absorption sites [
19]. This result is leading to decrease in fruit qualities by enhancing incidences of blossom-end rot [
20]. Tomato plants require a higher level of K compared to other nutrients [
21]. K absorption in tomato plants increased dramatically during the fruit development stage [
22,
23]. K plays an important role in fruit quality and color [
24]. On the contrary, when K uptake is insufficient, several physiological mechanisms were changed including the mobilization of K from foliar reserves to support critical processes of fruit development resulting to uneven ripening, blotchy ripening, high levels of internal white tissue, yellow shoulder, decreased lycopene (a pigment responsible for red color), irregular shape, and hollow fruit [
25,
26,
27,
28]. However, the limited amount of K-level management has a significant effect on the productivity and fruit quality parameters of tomatoes [
29]. Nowadays more researchers in Japan [
11,
30,
31,
32,
33,
34,
35,
36,
37,
38], China [
29], South Korea [
39], and Italy [
40] have made notable progress in developing methods for producing low K fruits and vegetables, which are particularly beneficial for patients with chronic kidney disease (CKD). These methods aim to address the dietary restrictions of CKD patients, who need to limit their K intake to prevent hyperkalemia. Several factors have been identified that affect the K content in plants and the quality of the resulting fruits and vegetables.
This research concentrated on optimizing nutrient solution management within hydroponic systems, with the specific goal of applying a nutrient solution that maintains a low potassium nitrate concentration during the growth of cherry tomatoes. The objective was to ensure normal plant growth, yield, and high fruit quality while reducing K accumulation in the fruits. The findings from this study suggest that cultivating cherry tomatoes with lower K levels could provide a viable option for consumers who need to avoid high K foods, such as patients with CKD. Conducting a comprehensive nutritional analysis of low K cherry tomatoes is crucial to confirm that they meet dietary requirements, particularly for patients with CKD. This analysis should encompass the evaluation of various nutritional components, including phytochemicals like lycopene and beta-carotene, as well as the assessment of antioxidant activity (measured by DPPH and FRAP assays), which are known to contribute to overall health and potentially reduce inflammation in patients.
2. Materials and Methods
2.1. Plant Materials and Experimental Design
The experiment was conducted in a greenhouse at the Faculty of Agriculture, Khon Kaen University, Thailand (latitude 16°28′31.5″N, longitude 102°49′14.7″E). Two Thai cherry tomato varieties were utilized: ‘Tabtim Deang T2021′ (red color) from KNOWN-YOU SEED Co., Ltd., Chiangmai, Thailand, and ‘Sweet Boy 1’ (yellow color) from CHIA TAI Co., Ltd., Bangkok, Thailand. Both varieties are F1 hybrids characterized by small fruit size, cylindrical shape, and smooth surface.
Seed of each variety was geminated on the moisture towel and kept in the dark for 2 weeks. After germination, the seedlings were pre-cultured in the solution for 20 – 30 days. The culture solution was contained following macronutrients [
11]: 3.0 x 10
-3 M KNO
3, 2.0 x 10
-3 Ca(NO
3)
2 4H
2O, 5.0 x 10
-4 M NH
4H
2PO
4, 1.0 x 10
-3 MgSO
4 7H
2O, and micronutrients: 2.6 x 10
-5 M C
10H
12FeN
2NaO
8 3H
2O (FeNa-EDTA), 4.6 x 10
-6 M MnCl
2 4H
2O, 2.4 x 10
-5 M H
3BO
3, 3.8 x 10
-7 M ZnSO
4 7H
2O, 1.6 x 10
-7 M CuSO
4 5H
2O and 1.5 x 10
-8 M (NH
4)
6Mo
7O
2 4H
2O, respectively. Then, seedling with 3 – 5 leaves were transferred to 45 L plastic containers (54.0 x 39.0 x 28.0 cm; length x width x depth) with the same culture solutions with different KNO
3 contents. The experiment was conducted using a randomized complete block design with four replications, utilizing four plants per pot. Five treatments were applied: 3.0 mM KNO₃ as the control (K1), 1.5 mM KNO₃ (K2), 0.75 mM KNO₃ (K3), 0.50 mM KNO₃ (K4), and 0.375 mM KNO₃ (K5). The study was carried out over 150 days under natural sunlight. The nutrient solution was replenished every 14 days, with the pH maintained between 6.50±0.50 using 0.5 N HNO₃ and 0.5 N NaOH, measured by a pH meter (Model: pH-22, LAQUA twin, HORIBA Ltd., Kyoto, Japan). The electrical conductivity (EC) of the solution was controlled within the range of 0.8 to 4.2 dS/m.
2.2. Data Collection
2.2.1. The Yields and Physical Quality of Fruits
Tomatoes were harvested at 90% maturity, indicated by a color change to light red after 110 days of cultivation. Post-harvest, the physical characteristics of the cherry tomatoes including yield, fruit weight, diameter, and length, were recorded for each treatment from the net pots.
2.2.2. The Quality of Fruit Parameters
The total soluble solids (TSS) were measured with a digital refractometer (Model: MSDR-P2-102, IMS Euro Ltd. Stockport, England). Titratable acidity (TA) was determined by titration against 0.1 N NaOH solution and expressed in form of citric acid (%).
Ascorbic acid (AA) was determined by the 2,4-dinitrophenylhydrazine (DNP) colorimetry. Tomato juice (0.5 ml) was transferred into a 75 ml test tube and 0.5 ml of 10% metaphosphoric acid solution was added following by method of [
31]. In brief, the solution contained 1 ml of distilled water, 1 ml of 0.03% 2,6-dichlorophenol-indophenol (DCP), 2 ml of thiourea, and 1 ml of 2,4-DNP were added for each tube. The samples were kept at 37°C for 3 hours. Subsequently, the samples were cooled down on with ice and 5 ml of 85% H
2SO
4 was added to end the reaction. The ascorbic acid content was determined by using a UV-VIS spectrophotometer at 520 nm (Model; Spectro UV-2550, LABOMED Inc., Los Angeles, United States of America).
Lycopene and beta-carotene were analyzed following the method described by [
41]. Briefly, homogenates of fresh tomato fruits (0.40 g) were prepared in 50 ml centrifuge tubes with 5 ml of a mixed solution of hexane, acetone, and ethanol in a ratio of 2:1:1 (v/v/v). The mixture was thoroughly mixed and incubated in the dark for 10 minutes. After incubation, 10 ml of distilled water was added, and the solution was left to stand for 10 minutes to allow phase separation and for air bubbles to dissipate. Lycopene and beta-carotene concentrations were measured using a UV-VIS spectrophotometer at wavelengths of 503 nm and 444 nm, respectively.
2.2.3. The Mineral Content Analysis
Tomato fruits were dried at 70°C for 72 hours with hot oven (Models: 30 -1060, UF1060 MEMMERT UNIVERSAL OVENS, Memmert, United States of America). The samples were ground and homogenized into the powder that was used for mineral analysis. Nitrogen content was determined using the Kjeldahl method [
42]. The other nutrients were analyzed by using ICP-OES after preparing sample by dry ashing method. Weight 0.2500 g samples powder into ashing crucible. Plant the ashing crucible into the muffle furnace (Models: 550-126, Fisher Scientific, United States of America) and the ashed at 550°C for 6 hours. Remove the ashing crucible from the muffle furnace, add 10 ml of 1 N HCl and leave for 1 hour. And then add 10 ml of DI water and leave overnight. The ashed were then filled through Whatman No. 5 filter paper [
43].
2.2.4. Determination of the Antioxidant Activity
An aliquot for antioxidant analysis was extracted as following steps: 1.0 g of homogenates of dry sample were placed in a 25 ml of Erlenmeyer flask then 5 ml of mixed solution (ethyl acetate: ethyl alcohol at a ratio of 2:3 (v/v)) was added. The sample was incubated at 40°C for overnight. Subsequently, the solution was flitted using filter papers (Whatman No. 1). The extraction process was repeated two times and then an aliquot was evaporated until approximately 1 ml of liquid was left. The obtained sample was kept at 4°C for further analysis.
The DHHP (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging activity was determined using the method described by [
44]. Briefly, 20 µl of an aliquot was added to well plate, and then 180 µl DPPH methanolic solution was added to initial the reaction. After 30 minutes, the absorbance was measured at 517 nm using a UV-VIS spectrophotometer (SPECTROstar Nano, BMG LABTHEH, Ortenberg, Germany). The standard curves were prepared using different concentrations of ascorbic acid. The results were expressed as EC
50 (milligrams of sample that bleached 50% of the DPPH methanolic solution) by the construction of a kinetic curve for each sample. Analyses were performed in 3 replications. The scavenging activity was calculated using the following equation:
A0 is the absorbance of the DPPH solution (Blank)
Ae is the absorbance of the sample solution.
Abg is the absorbance of the background.
The total antioxidant potential was determined using FRAP assay adapted of [
45]. The FRAP reagent was prepared by mixing solution of acetate buffer 250 mM (pH 3.6), a solution of 10 mM TPTZ (2,4,6-trispyridyltriazine), and 20 mM FeCl
3 at a ratio 10:1:1 (v/v/v). The obtained FRAP reagent (182 µL) were added to each well plate and followed by 18 µL of sample solution then incubated at 37°C for 30 min. The reactions were measured at OD 593 nm using a UV-VIS spectrophotometer. The calibration curve was prepared using different concentrations of ascorbic acid. The results were expressed as mg ascorbic acid equivalent per one-gram dry weight (mg ascorbic acid equivalent/1 g DW). The reducing power was calculated using the following equation:
y is the absorbance of the test - the absorbance of the blank control (containing all reagents except the extract solution)
a is slop of equation and x is an ascorbic acid concentration in unit µg/mL
b is intercept.
2.3. Data Analysis
The obtained data were subjected to analysis of variance for assessment the effects of each treatment. Experiment data was performed in 3 replications. Significance of differences between means were analyzed by using Tukey’s multiple range test (p < 0.05). The relationships between K concentration, fruit’s yield, quality of fruits, and the antioxidant activity were tested as Pearson correlations and statistical significance of the correlation at p < 0.05 was examined. All statistical analyses were computed using the SPSS program software version 28.0.1.0(142).
3. Results
3.1. The Effect of Different K Levels on Fruit Yield and Physical Qualities of Cherry Tomatoes
Compared to yield of the control (K1), ‘Tabtim Deang T2021’ yield was significantly decreased at K5 (1.50 kg/plant) as well as ‘Sweet Boy 1’ yield was significantly decreased at K4 (1.70 kg/plant) and K5 (1.65 kg/plant), respectively. The highest yield was recorded at K2 (2.24 kg/plant) for ‘Tabtim Deang T2021’. However, the result was not significantly different from K1. Meanwhile, the highest yield of ‘Sweet Boy 1’ was recorded in K1 (2.43 kg/plant) followed by K2 (2.17 kg/plant) and K3 (1.97 kg/plant), respectively (
Table 1). Similarly to the tomato yield, the lowest significant of fruit weight and diameter were observed in K5 (23.83 mm) for ‘Tabtim Deang T2021’, K4 (24.72 mm) and K5 (23.94 mm) for ‘Sweet Boy 1’. Meanwhile, the significantly highest fruit weight and diameter were observed in K1 the same as fruit yield. However, there were no significant differences in fruits weight between K1 in both varieties.
3.2. Nutrient Contents in Cherry Tomato Fruits
3.2.1. Macronutrients
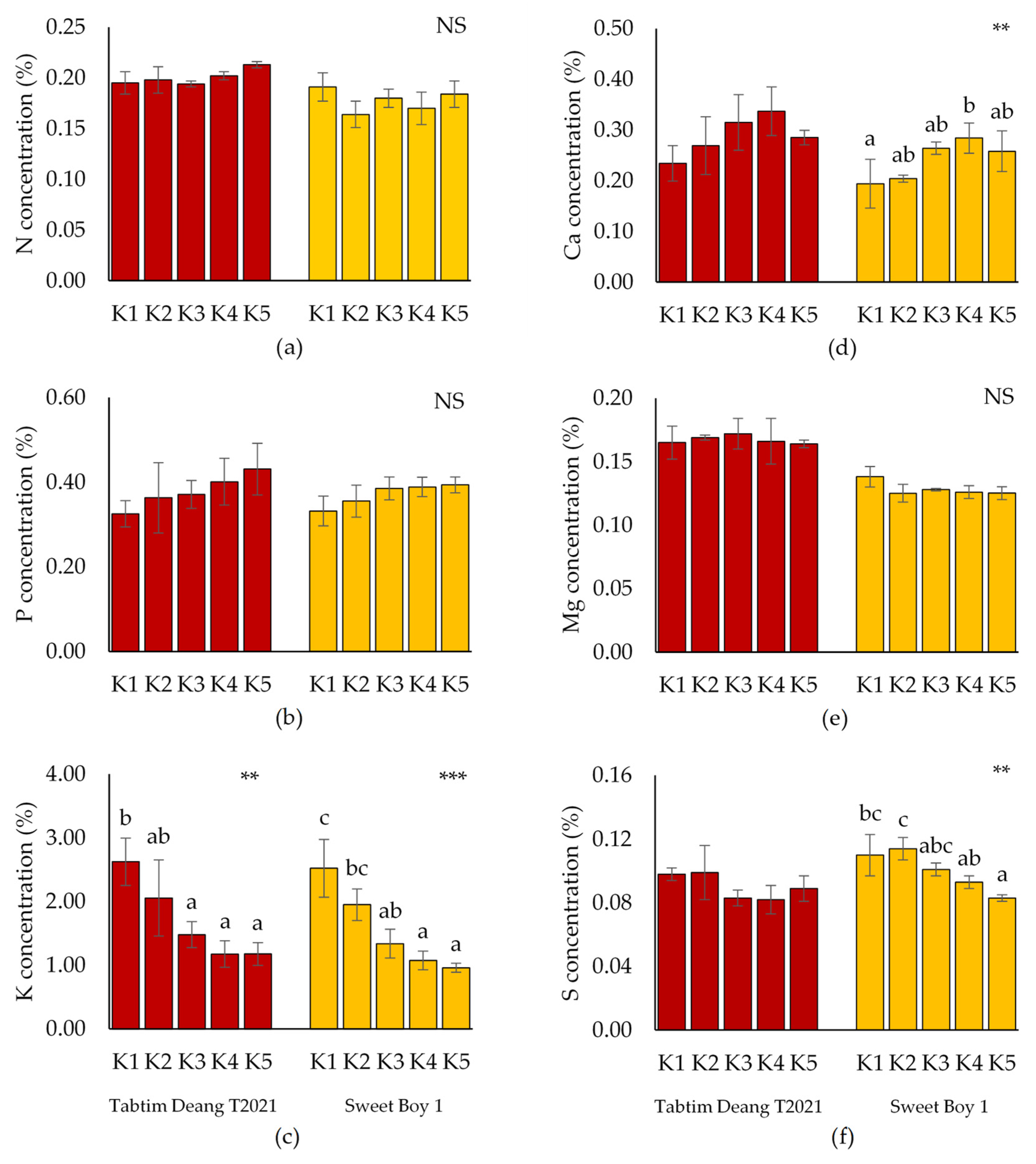
Compared to the control (K1), the concentrations of N, P, K, and Mg did not significantly differ across the K treatments for both varieties (
Figure 1). However, P concentration gradually increased from K1 to K5 in both varieties. The results also showed that K content in ‘Tabtim Deang T2021’ and ‘Sweet Boy 1’ significantly decreased from K1 to K5. Additionally, Ca content gradually increased in ‘Tabtim Deang T2021’, except for K5, where it decreased to 0.29%. In contrast, the Ca content in ‘Sweet Boy 1’ significantly increased, with the highest value observed at K4 (0.28%). The sulfur (S) concentration in ‘Sweet Boy 1’ also showed significant differences compared to K1, with a marked decrease in S concentration as the KNO₃ content in the culture solution increased. The lowest value was observed at K5 (0.084%).
3.2.2. Micronutrients
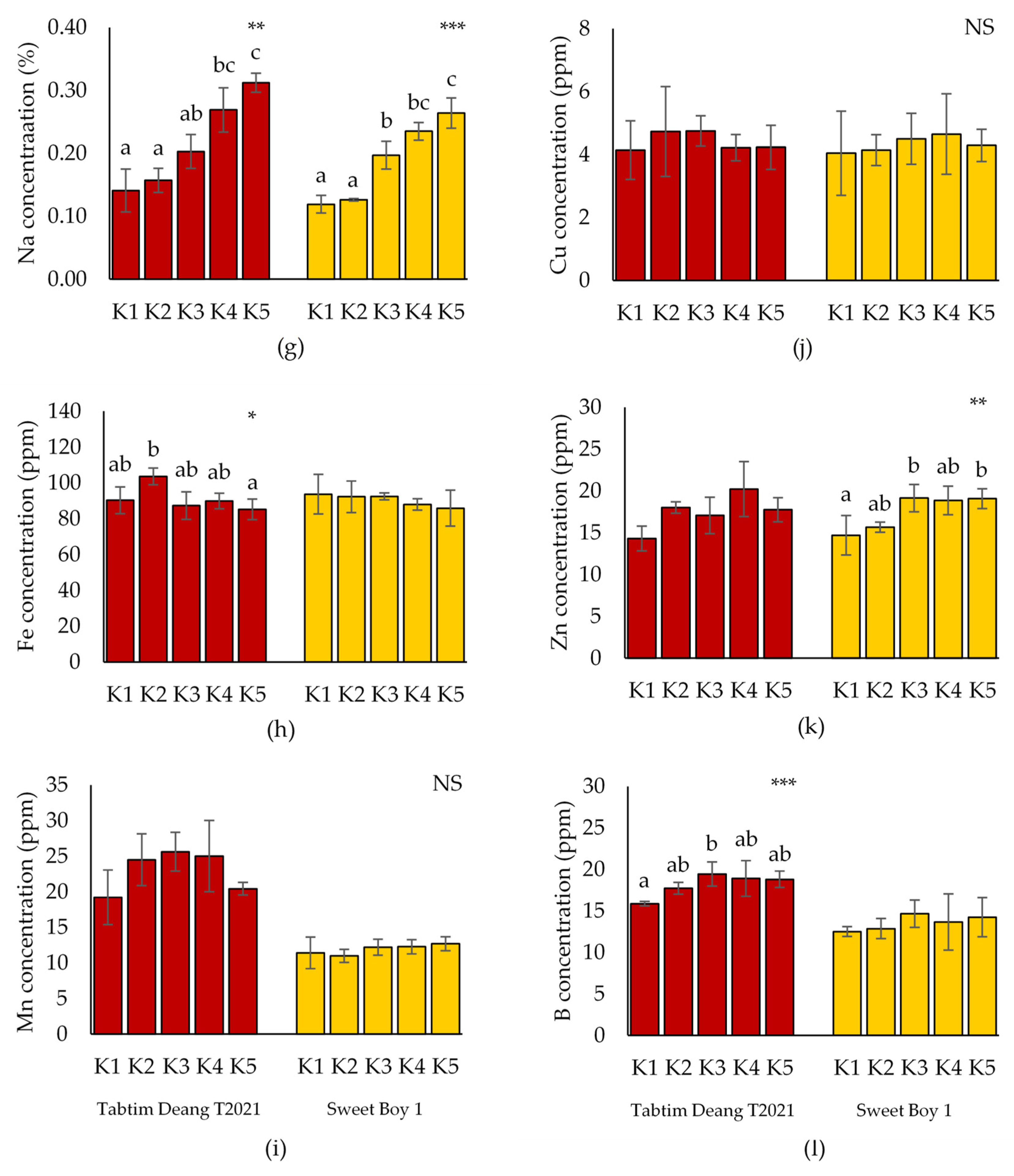
The micronutrient content in ‘Tabtim Deang T2021’ and ‘Sweet Boy 1’ is presented in
Figure 2. The results indicated that Na content in tomato fruits significantly increased from K1 to K5 for both varieties. The concentrations of Fe and B in ‘Tabtim Deang T2021’ were significantly different from the control, with Fe concentration being significantly higher under K2 treatment (103.6 ppm) compared to K1. However, Fe concentration under K5 treatment (85.18 ppm) was significantly lower than K1. Additionally, B concentration in the fruit gradually increased as K content in the culture solution decreased, with the highest significant B concentration observed under K3 treatment (19.44 ppm). In contrast, there were no significant differences in Fe and B concentrations between K1 and other treatments in the ‘Sweet Boy 1’ variety. Zn concentration in ‘Sweet Boy 1’ was significantly different from K1, with higher concentrations observed under K2, K3, K4, and K5 treatments. The highest significant Zn concentrations were recorded under K3 (19.14 ppm) and K5 (19.07 ppm), respectively. However, no significant differences in Zn concentration were observed in ‘Tabtim Deang T2021’. Furthermore, Mn and Cu concentrations showed no significant differences compared to K1 for both varieties.
3.3. Biological Properties and Antioxidant Activity
The biological properties are presented in
Table 2. The results indicated that the pH values of tomato juice, as well as citric acid and ascorbic acid contents, slightly decreased with KNO₃ treatment in ‘Tabtim Deang T2021’. Meanwhile, total soluble solids (TSS) significantly decreased in K3 (4.43 %Brix), K5 (4.29 %Brix), and K4 (4.18 %Brix), except for K2 (5.31 %Brix), where no significant difference was observed between K1 and K2. The pH, TSS, citric acid, and ascorbic acid contents in ‘Sweet Boy 1’ differed from those in ‘Tabtim Deang T2021’, showing significant decreases with KNO₃ treatment. The lowest significant values for pH (4.15), TSS (5.99 %Brix), citric acid (0.61%), and ascorbic acid (0.42 mg/g FW) were observed in K5. Additionally, the ascorbic acid content in K2 (0.45 mg/g FW) showed no significant difference compared to K1. Beta-carotene content in ‘Tabtim Deang T2021’ and ‘Sweet Boy 1’ was difference by non-statistically significant in K1.
The beta-carotene content slightly decreased with KNO₃ treatment in both varieties, ranging from 17.90 to 18.69 µg/g FW in ‘Tabtim Deang T2021’ and from 7.71 to 9.09 µg/g FW in ‘Sweet Boy 1’. Lycopene content in ‘Tabtim Deang T2021’ gradually decreased with KNO₃ treatment; however, no significant differences were observed compared to K1, with lycopene content ranging from 36.00 to 43.05 µg/g FW. This value was slightly lower than the typical range for cherry tomatoes, which contain between 48.9 and 116.7 µg of lycopene per gram of fresh weight [
46]. In contrast, lycopene content could not be determined in ‘Sweet Boy 1’, as only red tomatoes contain lycopene pigment.
The antioxidant activities were shown in
Table 3. The FRAP activities of ‘Tabtim Deang T2021’ and ‘Sweet Boy 1’ showed no significant differences with K1. As with the FRAP activity, the DPPH activity in ‘Sweet Boy 1’ shows no significant differences with K1. Meanwhile, the significantly increased DPPH activity was determined in ‘Tabtim Deang T2021’. The highest significance was the activities of DPPH determined in K3 (0.082 EC
50 mg ascorbic acid equivalent/1 g DW) and K5 (0.082 EC
50 mg ascorbic acid equivalent/1 g DW), respectively.
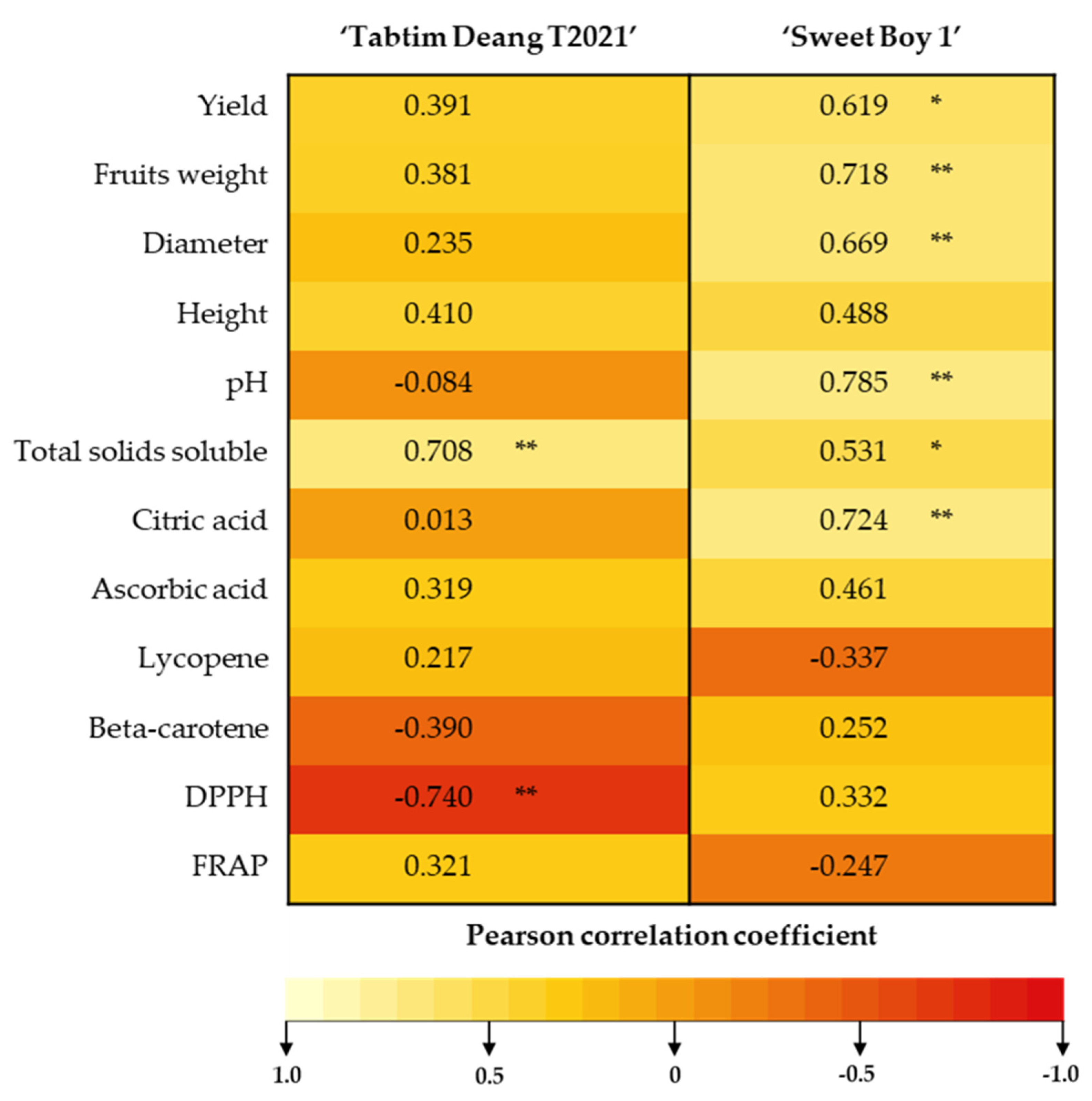
3.4. Correlation between Potassium Content, Yield, Quality of Fruit and the Antioxidant Activity
The correlation analysis was presented in
Table 3. The cherry tomato varieties under study exhibited different responses. In the ‘Sweet Boy 1’ variety, yield, fruit weight, and diameter were significantly positively correlated with K content (r = 0.619), whereas no such correlation was observed in the ‘Tabtim Deang T2021’ variety. Plant height in both cherry tomato varieties was not significantly correlated with KNO₃ content. The pH value showed a significant positive correlation with K content in ‘Sweet Boy 1’ (r = 0.785), but this was not the case in ‘Tabtim Deang T2021’. Total soluble solids in both varieties were significantly positively correlated with K content in ‘Tabtim Deang T2021’ (r = 0.708) and ‘Sweet Boy 1’ (r = 0.531). Additionally, citric acid content was positively correlated with K content in ‘Sweet Boy 1’ (r = 0.724). There were no significant correlations between K content and the levels of lycopene or beta-carotene in both varieties. However, antioxidant activity was generally not significantly correlated with K content, except for DPPH activity in the ‘Tabtim Deang T2021’ variety, which exhibited a significant negative correlation with K content (r = -0.740).
Figure 3.
Correlation between potassium content, yield, quality of fruit and the antioxidant activity. *, ** Pearson’s rank test with two levels of significance (p < 0.05 and p < 0.01).
Figure 3.
Correlation between potassium content, yield, quality of fruit and the antioxidant activity. *, ** Pearson’s rank test with two levels of significance (p < 0.05 and p < 0.01).
4. Discussion
The results indicate that reducing KNO₃ in the culture solution had a significant impact on yield, fruit weight, and fruit size, primarily due to potassium’s critical role in various physiological processes within plants. K is a vital element for photosynthesis, a process essential for plant growth and energy production [
47,
48]. Additionally, K is integral to physiological processes that involve water, such as stomatal regulation, the translocation of photoassimilates, enzyme activation, and heliotropic leaf movements [
49]. K deficiency can severely suppress the biosynthesis and activity of Rubisco, a crucial enzyme for carbon dioxide fixation during photosynthesis. This deficiency directly affects plant growth and yield [
50,
51]. The research by Tsukagoshi et al. [
38] observed that K deficiency in the cherry-type tomato (
Solanum lycopersicum L.) ‘Carol 10’ led to reduced dry weight of leaves and stems, decreased yield, and a lower number of fruits, ultimately resulting in diminished overall plant growth.
The results demonstrate that reducing KNO₃ in the culture solution significantly influenced plant nutrient uptake, particularly affecting Ca and Na levels in tomato fruits. K the most abundant cation in plants, plays a critical role in maintaining the osmotic potential of plant cells, which is essential for mitigating stress. Low K levels can trigger stress responses, leading to alterations in ion transport and accumulation. Elevated Ca levels may function as secondary messengers in stress signaling pathways [
52], while increased Na levels could represent a non-specific response to ionic imbalances [
53]. These mechanisms are vital for managing nutrient imbalances in crops and refining plant nutrition strategies. However, K uptake is also influenced by plant-specific factors, including genetic makeup and developmental stages, such as the vegetative and reproductive phases [
54].
Specifically, in the ‘Tabtim Deang T2021’ cultivar, a decrease in KNO₃ led to lower Fe and higher B content. The uptake of Ca and Na can interfere with Fe absorption. These elements may compete with Fe for uptake or affect root exudates that solubilize Fe [
55,
56]. In the cultivar ‘Sweet Boy 1’ the reduction in KNO₃ in the culture solution led to a decrease in S content and an increase in Zn content, as observed in
Figure 2. S and N are essential nutrients interconnected in plant metabolic pathways [
57,
58]. S is critical for the synthesis of specific amino acids and vitamins and allowing plants to absorb increasing the Zn content in plant tissues. [
59]. Zn and K also exhibited a significant synergetic relation in soil and plants by influencing the uptake of both nutrients. The interaction between K and Zn has a synergistic effect on the uptake of different nutrients by plants [
60]. However, decreasing KNO₃ also reduces N and P availability, which can impact sulfur metabolism in the plant. While reduced K levels can enhance Zn uptake, it may also have implications for the nutrient balance in the plant. [
61,
62].
These results indicate that K is a key activator of numerous vital enzymes involved in processes such as protein synthesis, sugar transport, N and carbon (C) metabolism, and photosynthesis. It plays a significant role in yield formation and quality enhancement [
28,
63]. Hermans et al. (2006) [
64] demonstrated that inorganic compounds such as sugars and amino acids accumulate in plants with low K levels. The research by Al-Moshileh et al. [
65] highlighted potassium’s involvement in the regulation of stomatal conductance through the opening and closing of guard cells, which also influences photosynthesis. These functions can indirectly impact sugar production and accumulation in fruits. Takahashi et al. [
37] further reported that K restriction for less than ten weeks in pot culture with fertigation did not significantly affect mean fruit weight, soluble sugar content, and titratable acidity in blueberry fruits, indicating that low K conditions can be managed without compromising these fruit quality parameters.
However, low K levels adversely affect the synthesis and accumulation of lycopene and beta-carotene in tomato fruits due to potassium’s critical role in activating enzymes involved in photosynthesis, protein synthesis, and carbohydrate metabolism [
66,
67]. Efficient enzyme activity is essential for the synthesis of secondary metabolites, such as carotenoids. K also influences chloroplast development and function, which are key sites for carotenoid biosynthesis, including beta-carotene, an important compound for photosynthesis and photoprotection [
68,
69]. Moreover, K may play a role in the synthesis of lycopene, as it potentially acts as an activator of enzymes responsible for converting beta-carotene to lycopene in tomato fruits [
27,
70,
71]
K affects antioxidant activity in plants by influencing factors such as phenolic composition, flavonoid content, and plant health. The research by [
72] the foliar (potassium sulphate) spray influenced the biochemical profile of total fig fruit and achenes by increasing the expression of phenolic compounds which was correlated with the strong antioxidant potential in fig. Marlin et al. [
73]. These results indicated that fertilization application of N and K improved polyphenol content, and antioxidant activities such as DPPH radical scavenging activity and Ferric reducing antioxidant power in
Eleutherine palmifolia. However, our study could be useful to improve kidney patient health by maximizing the quality of diet for CKD consumption.
5. Conclusions
The results demonstrated that both ‘Tabtim Deang T2021’ and ‘Sweet Boy 1’ cherry tomato plants cultivated with a 1.5 mM KNO₃ nutrient solution exhibited a successful reduction in K content in the fruits without inducing significant K deficiency symptoms. This reduction was achieved without adversely affecting the fresh weight, fruit quality, and antioxidant activity. The successful decrease in K levels observed in both cherry tomato varieties has significant implications for dietary management, particularly for patients with kidney disease, offering potential health benefits by providing low K fruits. Ever those people without problems with health can consume it without becoming less nutrients.
Author Contributions
Conceptualization, K.M., A.S., R.K., S.A and P.K.; validation, K.M., A.S. and R.K.; formal analysis, K.M., A.S. and R.K.; investigation, K.M., A.S. and R.K.; data curation, K.M.; writing—original draft preparation, K.M.; writing—review and editing, K.M., A.S., R.K., S.A and P.K.; visualization, K.M.; supervision, A.S. and R.K; funding acquisition, A.S. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Khon Kaen University under the Chronic Kidney Disease Northeast Thailand Research Project (CKDNET. 2018).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data Availability Statement: The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to extend our sincere thanks to the Faculty of Agriculture, Khon Kaen University, for providing access to laboratory facilities, equipment, and the greenhouse for research. Your support was invaluable to the success of this research. The authors would like to specially thank the AMS Medical Laboratory, Faculty of Associated Medical Sciences, Khon Kaen University, for providing the facilities and support necessary to conduct the FRAP and DPPH assay analyses. This research was financially supported by Khon Kaen University through the Chronic Kidney Disease Northeast Thailand Research Project (CKDNET, 2018). We gratefully acknowledge their funding and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. 2016. Tomato As a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers (Basel) 2016, 8, 58–86. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the Health-Promoting Effects of Tomato Fruit for Biofortified Food. Mediat Inflamm. 2014, 1, 139873. [Google Scholar]
- Bügel, S. Vitamin K and Bone Health. Proc. Nutr. Soc. 2003. 62, 839 – 843.
- Fekete, K.; Berti, C.; Trovato, M.; Lohner, S.; Dullemeijer, C.; Souverein, O.W.; Cetin I.; Decsi. T. Effect of Folate Intake on Health Outcomes in Pregnancy: A Systematic Review and Meta-analysis on Birth Weight, Placental Weight, and Length of Gestation. Nutr. J. 2012, 11, 75. [CrossRef]
- Story, E. N., R. E. Kopec, S. J. Schwartz and G. K. Harris. An Update on The Health Effects of Tomato Lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189 - 210.
- Burton-Freeman, B; Reimars, K. Tomato Consumption and Health: Emerging Benefits. Am. J. Lifestyle. Med. 2011, 5, 182-191.
- Tomatoes, Red, Ripe, Raw, Year-Round Average. U.S. Department of Agriculture. USDA. 2019, Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170457/nutrients. (accessed on 11 October 2023.
- St-Jules, D.E.; Goldfarb, D.S.; Sevick. M.A. Nutrient Non-Equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients. J. Ren. Nutr. 2016, 26, 282–287. [CrossRef]
- Yamada, S; Inaba. M. Potassium Metabolism and Management in Patients with CKD. Nutr. 2021, 13, 1751.
- Ceccanti, C.; Guidi, L; D’Alessandro, C.; Cupisti. A. Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from A Static in Vitro Digestion Methodology. Toxins (Basel). 2022. 14: 668.
- Ogawa, A.; Taguchi, S.; Kawashima, C. A Cultivation Method of Spinach with A Low Potassium Content for Patients on Dialysis (In Japanese with English Abstract). Jpn. J. Crop. Sci. 2007, 76, 232–237. [Google Scholar] [CrossRef]
- Amtmann, A.; Hammond, J.P.; Armengaud, P.; White, P.J. Nutrient Sensing and Signaling in Plants: Potassium and Phosphorus. Adv. Bot. Res. 2005, 43, 209–257. [Google Scholar]
- Ogawa, A.; Yamauchi, A. Root Osmotic Adjustment Under Osmotic Stress in Maize Seedlings 2. Mode of Accumulation of Several Solutes for Osmotic Adjustment in Root. Plant. Prod. Sci. 2006, 9, 39–46. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Jones, C.A. Growth and Mineral Nutrient of Field Crops. 3rd ed., CRC Press, New York. 2011, pp 199-200.
- White, P.J.; Karley, A.J. Potassium Cell Biology of Metals and Nutrients. Berlin: Springer, 2010, 199-224.
- Pujos, A.; Morard, P. Effects of Potassium Deficiency on Tomato Growth and Mineral Nutrition at The Early Production Stage. Plant. Soil. 1997, 189, 189–196. [Google Scholar] [CrossRef]
- Chen, S.; Yan, Z.; Chen, Q. Estimating the Potential to Reduce Potassium Surplus in Intensive Vegetable Fields of China. Nutr. Cycl. Agroecosys. 2017, 107, 265–277. [Google Scholar]
- Zhu, Q.; Ozores-Hampton, M.; Li, Y.; Morgan, K.; Lu, Y. Potassium Rages Affected Potassium Uptake and Use Efficiency in Drip-Irrigated Tomato. J. Agron. 2017, 109, 2945–2956. [Google Scholar] [CrossRef]
- Jakobsen, S. Interaction Between Plant Nutrients, III. Antagonism Between Potassium, Magnesium, and Calcium. Acta Agric. Scand. B - Soil Plant Sci. 1992, 43, 1–5. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, T.; Tan, C.; Astatkie, T. Responses of Fruit Yield and Quality of Processing Tomato to Drip-Irrigation and Fertilizers Phosphorus and Potassium. Agron. J. 2011, 103, 1339–1345. [Google Scholar] [CrossRef]
- Voogt, W.; Sonneveld, C. Nutrient Management in Closed Growing Systems for Greenhouse Production. In: Plant Production in Closed Ecosystems Automation, Culture and Environment. Kluwer Academic Press Dordrecht. 1996, 83 - 102.
- Huett, D.O.; Dettmann, E.B. Effect of Nitrogen on Growth, Fruit Quality and Nutrient Uptake of Tomatoes Grown in Sand Culture. Aust. J. Exp. Agric. 1988, 28, 391–399. [Google Scholar] [CrossRef]
- Chapagain, B.P.; Wiesman, Z. Effect of Nutri-Vant-Pea K Foliar Spray on Plant Development, Yield, and Fruit Quality in Greenhouse Tomatoes. Sci. Horti. 2004, 102, 177–188. [Google Scholar] [CrossRef]
- Hartz, T.K.; Johnstone, P.R.; Francis, D.M.; Miyao, E.M. Processing Tomato Yield and Fruit Quality Improved with Potassium Fertigation. Hort. Sci. 2005, 40, 1862–1867. [Google Scholar] [CrossRef]
- Besford, R.T.; Maw, G.A. Effect of Potassium Nutrition on Some Enzymes of The Tomato Plant. Ann. Bot. 1976, 40, 461–471. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition. International Potash Institute. Berne. 1987, pp 588-594.
- Madakadze, R.M.; Kwaramba, J. Effect of Pre-Harvest Factors on Quality of Vegetables Produced in The Tropics; Vegetables: Growing Environment and Quality of Produce. 1 - 36. In: Dris, R. and Jain, S.M. Eds. Production Practices and Quality Assessment of Food Crops, Vol. 1. Preharvest Practice. Kluwer Academic Publishers, The Netherlands. 2004.
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants. Cambridge, MA: Academic Press. 2012,.
- Liu, X. Effect of Low-Potassium Nutrition on The Quality and Potassium Content of Leafy Vegetables. Master’s Thesis, South China Agricultural University, Guangzhou, China. 2016.
- Ogawa, A.; Eguchi, T.; Toyofuku, K. Cultivation Methods for Leafy Vegetables and Tomatoes with Low Potassium Content for Dialysis Patients. Environ. Control. Biol. 2012, 50, 407–414. [Google Scholar] [CrossRef]
- Asao, T.; Asaduzzaman, M.; Mondal, M. F.; Tokura, M.; Adachi, F.; Ueno, M.; Kawaguchi, M.; Yano, S.; Ban, T. Impact of Reduced Potassium Nitrate Concentration in Nutrient Solution on The Growth, Yield Fruit Quality of Melon in Hydroponics. Sci. Hortic. 2013, 164, 221–231. [Google Scholar] [CrossRef]
- Ogawa, A.; Fujita, S.; Toyofuku, K. A Cultivation Method for Lettuce and Spinach with High Levels of Vitamin C Using Potassium Restriction. Environ. Control Biol. 2014, 52, 95–99. [Google Scholar] [CrossRef]
- Mondal, M.F.; Asaduzzaman, M.; Ueno, M.; Kawaguchi, M.; Yano, S.; Ban, T.; Asao, T. Reduction of Potassium (K) Content in Strawberry Fruits Though KNO3 Management of Hydroponic. Hort. 2017, 86, 26–36. [Google Scholar] [CrossRef]
- Zhang, G.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. Plant Growth and Photosynthesis Response to Low Potassium Conditions in Three Lettuce (Lactuca Sativa) Types. Hortic. J. 2017, 86, 229–237. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Talukder, M.R.; Tanaka, H.; Ueno, M.; Kawaguchi, M.; Yano, S.; Ban, T.; Asao, T. Production of Low-Potassium Content Melon Through Hydroponic Nutrient Management Using Perlite Substrate. Front. Plant Sci. 2018, 9, 1382. [Google Scholar]
- Okada, H.; Abedin, T.; Yamamoto, A.; Hayashi, T.; Hosokawa, M. Production of Low-Potassium Onions Based on Mineral Absorption Patterns During Growth and Development. Sci. Hortic. 2020, 267, 109252. [Google Scholar] [CrossRef]
- Takahashi, S.; Che, J.; Horiuchi, N.; Cho, H.Y.; Onwona-Agyeman, S.; Kojima, K.; Yamada, M.; Ogiwara, I. Production of Low-Potassium Fruit of Potted and Fertigated Southern. Highbush Blueberry (Vaccinium Corymbosum L. Interspecific Hybrid). Hortic. J. 2021, 90, 161–171.
- Tsukagoshi, S.; Aoki, M.; Johkan, M.; Hohjo, M.; Maruo, T.A. Quantitative Management of Potassium Supply for Hydroponic Production of Low-Potassium Cherry-Type Tomato Fruit for Chronic Kidney Disease Patients. J. Horti. 2021, 7, 87. [Google Scholar]
- Son, Y.J.; Park, J.E.; Kim, J.; Yoo, G.; Lee, T.S.; Nho, C.W. Production of Low Potassium Kale with Increased Glucosinolate Content from Vertical Farming as A Novel Dietary Option for Renal Dysfunction Patients. Food Chem. 2021, 339, 128092. [Google Scholar] [CrossRef]
- Renna, M.; Castellino, M.; Leoni, B.; Paradiso, V.; Santamaria, P. Microgreens Production with Low Potassium Content for Patients with Impaired Kidney Function. Nutri. 2018, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Barrett, D.M. Standardization of A Rapid Spectrophotometric Method for Lycopene Analysis. Acta. Hortic. 2007, 758, 111–128. [Google Scholar]
- AOAC. Official Methods of Analysis. 16th ed., 5th Revision, Association of Official Analytical Chemists, Washington DC. 1999,.
- Allen, S.E. Chemical Analysis of Ecological Materials. 2nd ed., Blackwell Scientific Publications, Oxford and London. 1989,.
- Brand-Williams, W.; Cuvelier. M.E.; Berset, C. Use of A Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol. 1995, 28, 25 – 30.
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) As A Measure of “Antioxidant Power: The FRAP Assay”. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Setyorini, D.; Sugito, Y.; Aini, N.; Yudho Tyasmoro, S. Lycopene, Beta-Carotene and Productivity of Tomato Varieties at Different Shade Levels Under Medium Land of Indonesia. J. Appl. Hortic. 2018, 20, 92–96. [Google Scholar] [CrossRef]
- Mengel, K. Potassium. In: Barker, A.V.; Pilbeam, D.J. Eds., Handbook of Plant Nutrition, 1st Ed. Taylor & Francis, London, UK. 2007, pp. 91 – 120.
- Jákli, B.; Tavakol, E.; Tränkner, M.; Senbayram, M.; Dittert, K. Quantitative Limitations to Photosynthesis in K Deficient Sunflower and Their Implications on Water-Use Efficiency. J. Plant Physiol. 2017, 209, 20–30. [Google Scholar] [PubMed]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Nahar, K.; Shahadat Hossain, Md.; Al Mahmud, J.; Shahadat Hossen, Md.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agron. 8, 2018, 31.
- Szczerba, M.W.; Britto, D.T.; Kronzucker, H.J. K+ Transport in Plants: Physiology and Molecular Biology. J. Plant. Physiol. 2009, 166, 447- 466.
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants. 2021, 10, 419. [Google Scholar]
- Wang, X.; Ling, H.; Zhu, B.; Jiang, Z. Plant Calcium Signaling in Response to Potassium Deficiency. Int. J. Mol. Sci. 2018, 19, 3456. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ And K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z.; Damon, P.M.; Cakmak, I. Crops and Genotypes Differ in Efficiency of Potassium Uptake and Use. Physiol. Plant. 2008, 133, 624–636. [Google Scholar] [CrossRef]
- Ma, W.; Tang, S.; Dengzeng, Z.; Zhang, D.; Zhang, T; Ma, X. Root Exudates Contribute to Belowground Ecosystem Hotspots. A Review. Front. Microbiol. 2022, 13, 937940–937958.
- Wang, T.; Chen, X.; Ju, C.; Wang, C. Calcium Signaling in Plant Mineral Nutrient: From Uptake to Transport. Plant Comm. 2023, 4, 1000678. [Google Scholar]
- Barker, V.A.; Pilbeam, J.D. Handbook of Plant Nutrition. CRC Press, Taylor and Francis Group, Boca Raton, FL. 2007,.
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Plant. Signal. Behav. 2023, 18, e2030082-11.
- Bashir, A.; Khan, Q.U.; Alem, A.; Hendi, A.A.; Zaman, U.; Khan, S.U.; Rehman, K.U.; Khan, A.A.; Ullah, I.; Anwar, Y. Zinc and Potassium Fertilizer Synergizes Plant Nutrient Availability and Affects Growth, Yield, and Quality of Wheat Genotypes. Plants. 2023, 12, 2241. [Google Scholar] [CrossRef]
- Chaudhary, D.G.; Chaudhary, S.R.; Chaudhary, M.M.; Mor, V.B. Interaction Effect of Potassium and Zinc on Yield and Nutrient Uptake of Forage Maize (Zea Mays L.) Grown on Loamy Sand Soil. Int. J. Chem. Stud. 2017, 5, 1737–1739. [Google Scholar]
- Brosnan, J.T.; Brosnan, M.E. The Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef]
- Arif, M.; Tasneem, M.; Bashir, F.; Yaseen, G.; Anwar, A. Evaluation of Different Levels of Potassium and Zinc Fertilizer on The Growth and Yield of Wheat. Int. J. Biosen Bioelectron. 2017, 3, 242–246. [Google Scholar] [CrossRef]
- Oosterhuis, D.; Loka, D.; Kawakami, E.; Pettigrew, W. The Physiology of Potassium in Crop Production. Adv. Agron. 2014, 126, 203–234. [Google Scholar]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How Do Plants Respond to Nutrient Shortage by Biomass Allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar]
- Al-Moshileh, A.M.; Errebhi M.A.; Obiadalla-Ali. H.A. Effect of Potassium Fertilization on Tomato and Cucumber Plants Under Greenhouse Conditions. Biosci. Res. 2017, 14, 68–74.
- Wang, Y.; Wu, W.H. Potassium Transport and Signaling in Higher Plants. Annu. Rev. Plant. Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Cui, J.; Tcherkez, G. Potassium Dependency of Enzymes in Plant Primary Metabolism. Plant Physi. Biochem. 2021, 166, 522–530. [Google Scholar]
- Tränker, M.; Tavakol, A.; Jákli, B. Functioning of Potassium and Magnesium in Photosynthesis, Photosynthate Translocation and Photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol. Hortic. 2022, 2, 1–21. [Google Scholar]
- Bramley, P.M. Regulation of Carotenoid Formation During Tomato Fruit Ripening and Development. J. Exp. Bot. 2002, 53, 2107–2113. [Google Scholar] [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernicr, R.; Matteo, A.D.; Fogliano, V; Pellegrini. N. Antioxidant Nutrient Quality of Tomato. Mol. Nutr. Food Res. 2007, 51, 609–617.
- Gaaliche, B.; Ladhari, A.; Zarrelli, A.; Ben, M.M. Impact of Foliar Potassium Fertilization on Biochemical Composition and Antioxidant Activity of Fig (Ficus Carica L.). Sci. Hortic. (Amsterdam). 2019, 253, 111–119. [Google Scholar]
- Marlin, M.; Simarmata, M.; Salamah, U.; Nurcholis. W. Effect of Nitrogen and Potassium Application on Growth, Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Eleutherine Palmifolia. AIMS Agric. Food. 2022, 7, 580–593.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).