Submitted:
30 September 2024
Posted:
02 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Lithraea Molleoides Fruit Gum Flour (LMFG)
2.2.1. Proximate Analysis of Biomass
2.2.4. Antioxidant Activity Assays
2.2.5. Reducing Power
2.2.6. Hydroxyl Radical Scavenging
2.2.7. DPPH Scavenging Activity
2.2.8. Total Polyphenol Content
2.2.9. Fourier Transform Infrared Spectroscopy
2.2.10. X-ray Diffraction (XRD)
2.2.11. Differential Scanning Calorimetric Analysis (DSC)
2.2.12. Thermogravimetric Analysis—Differential Thermogravimetric Analysis
2.3. Lithraea Molleoides Fruit Gum Films (LMFGf)
2.3.1. Scanning Electron Microscopy–Electron Dispersive X-ray Spectroscopy (SEM-EDX)
2.3.2. Mechanical Tests
2.3.4. Water Vapor Permeability
2.3.5. Biodegradability
3. Results
3.1. Lithraea Molleoides Fruit Gum Flour (LMFG Flour)
3.1.1. Proximate Analysis of Biomass and Antioxidant Capacity
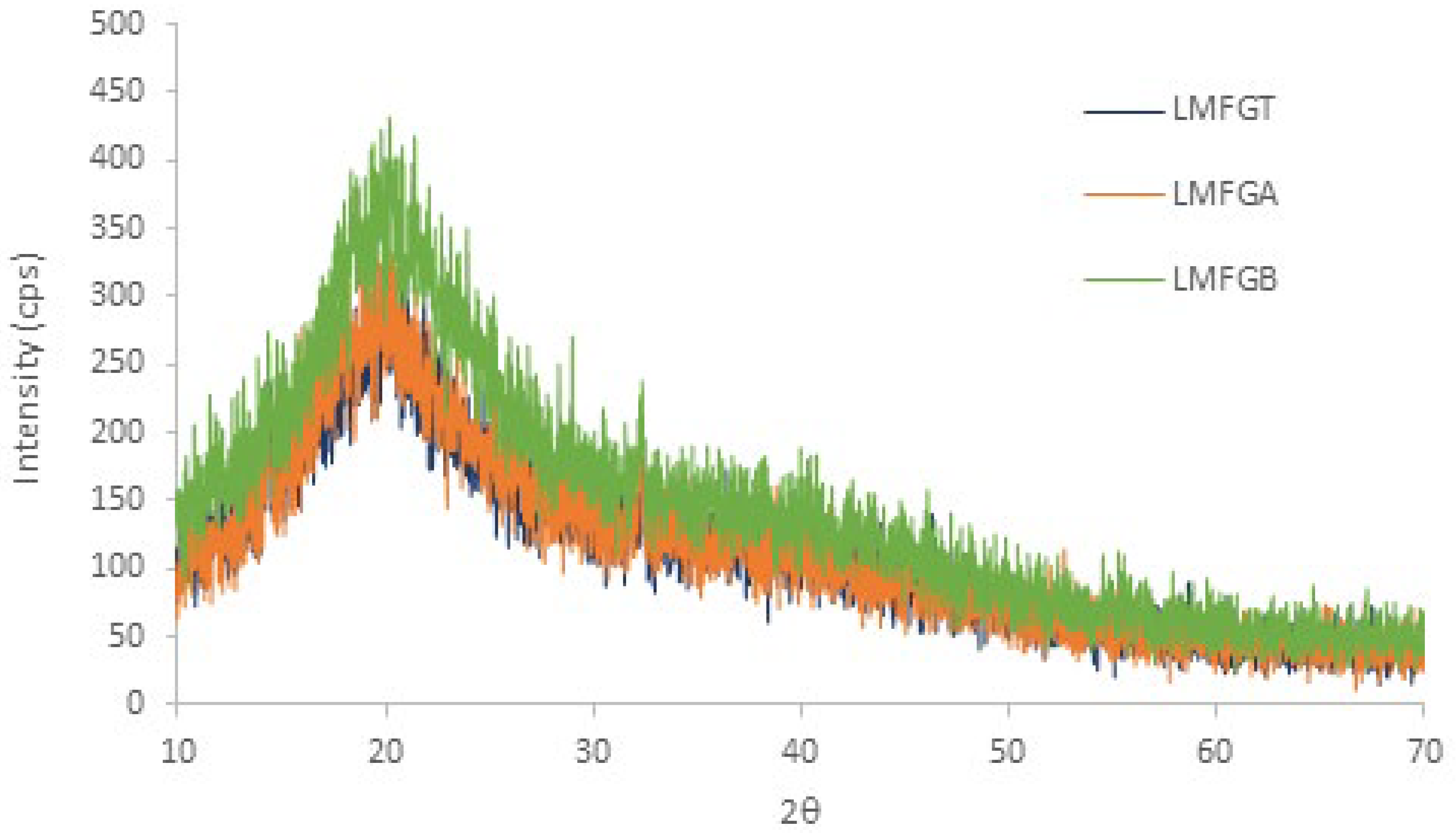
3.1.3. DRX
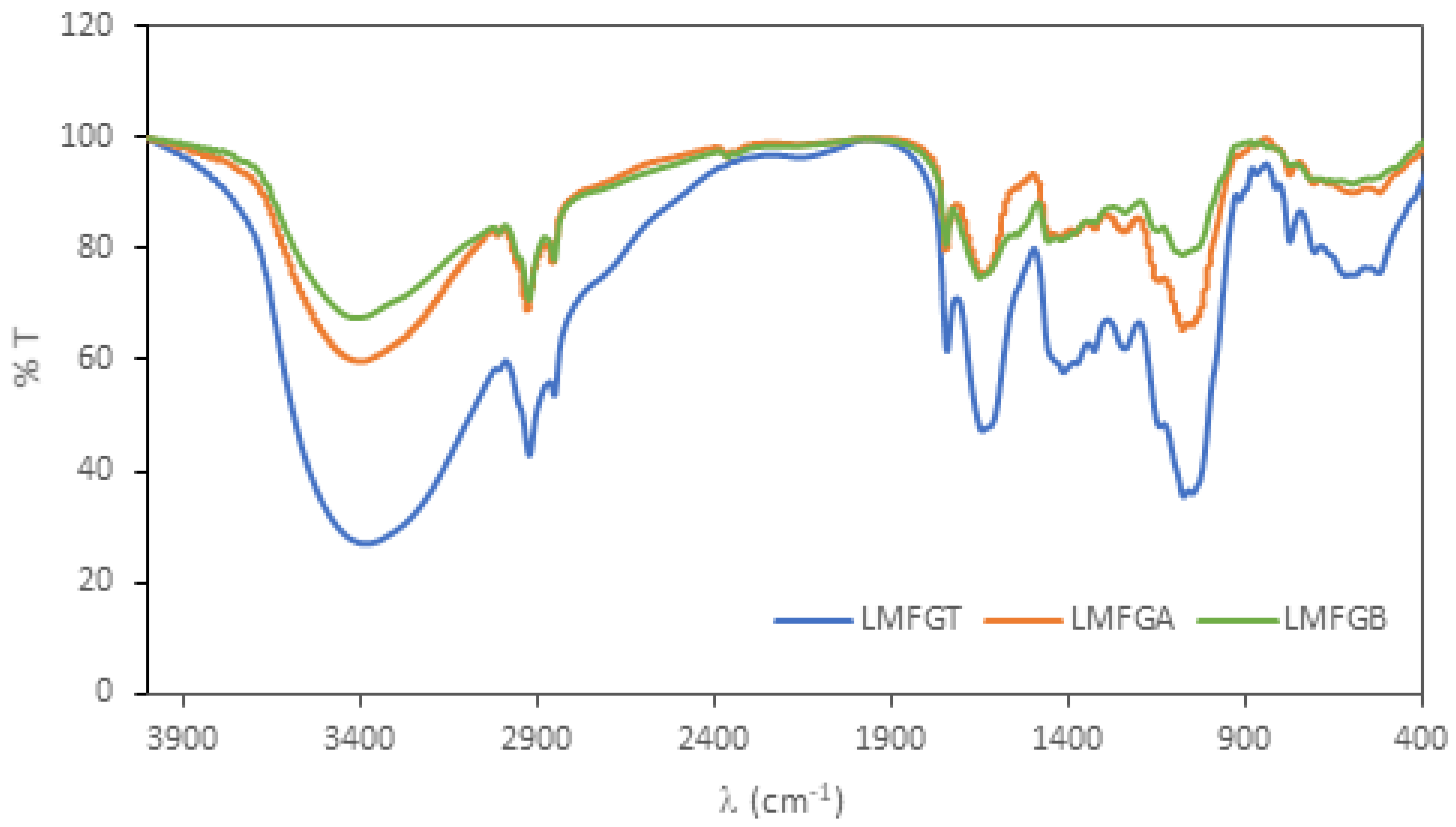
3.1.4. FTIR
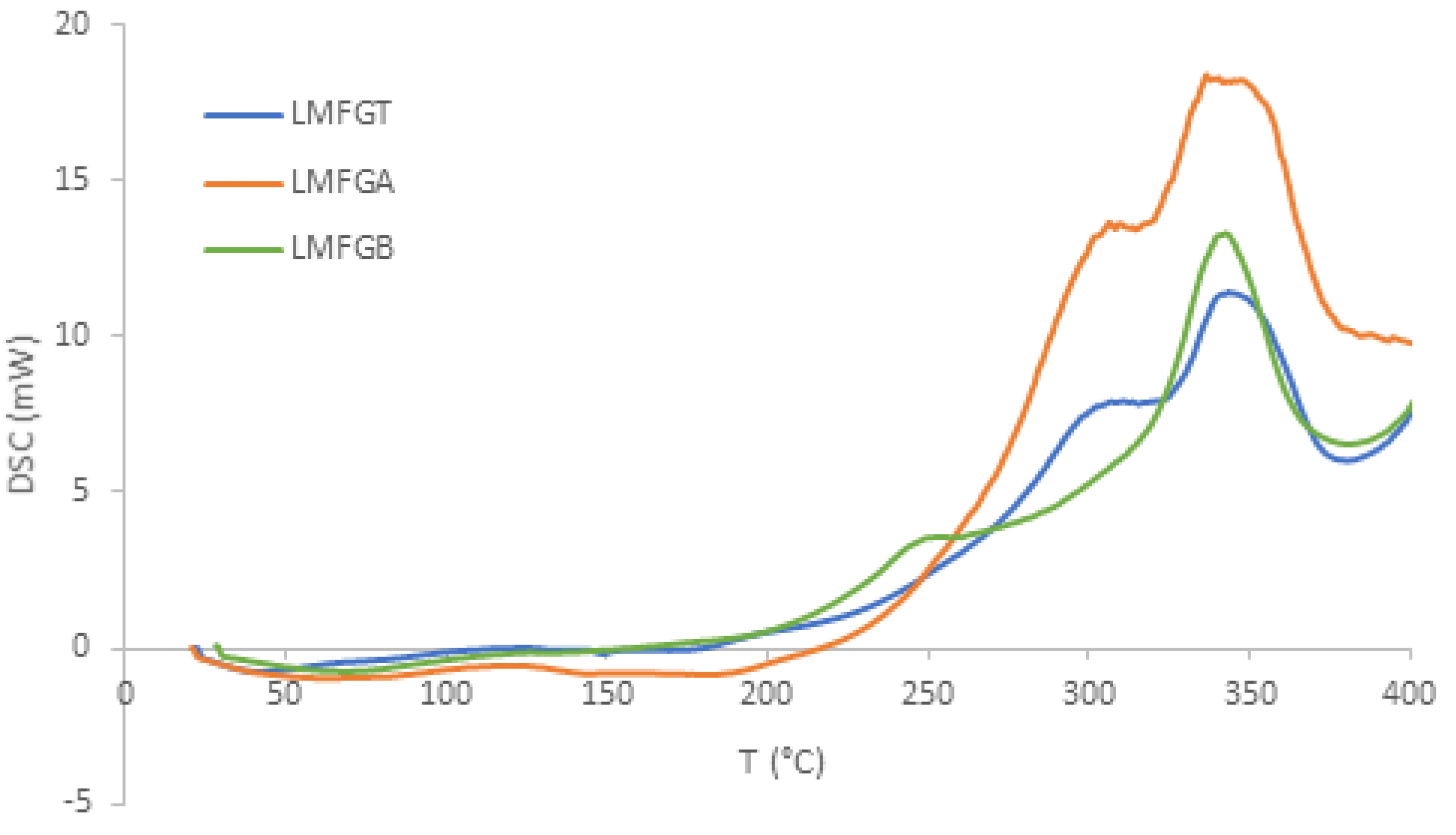
3.1.5. DSC
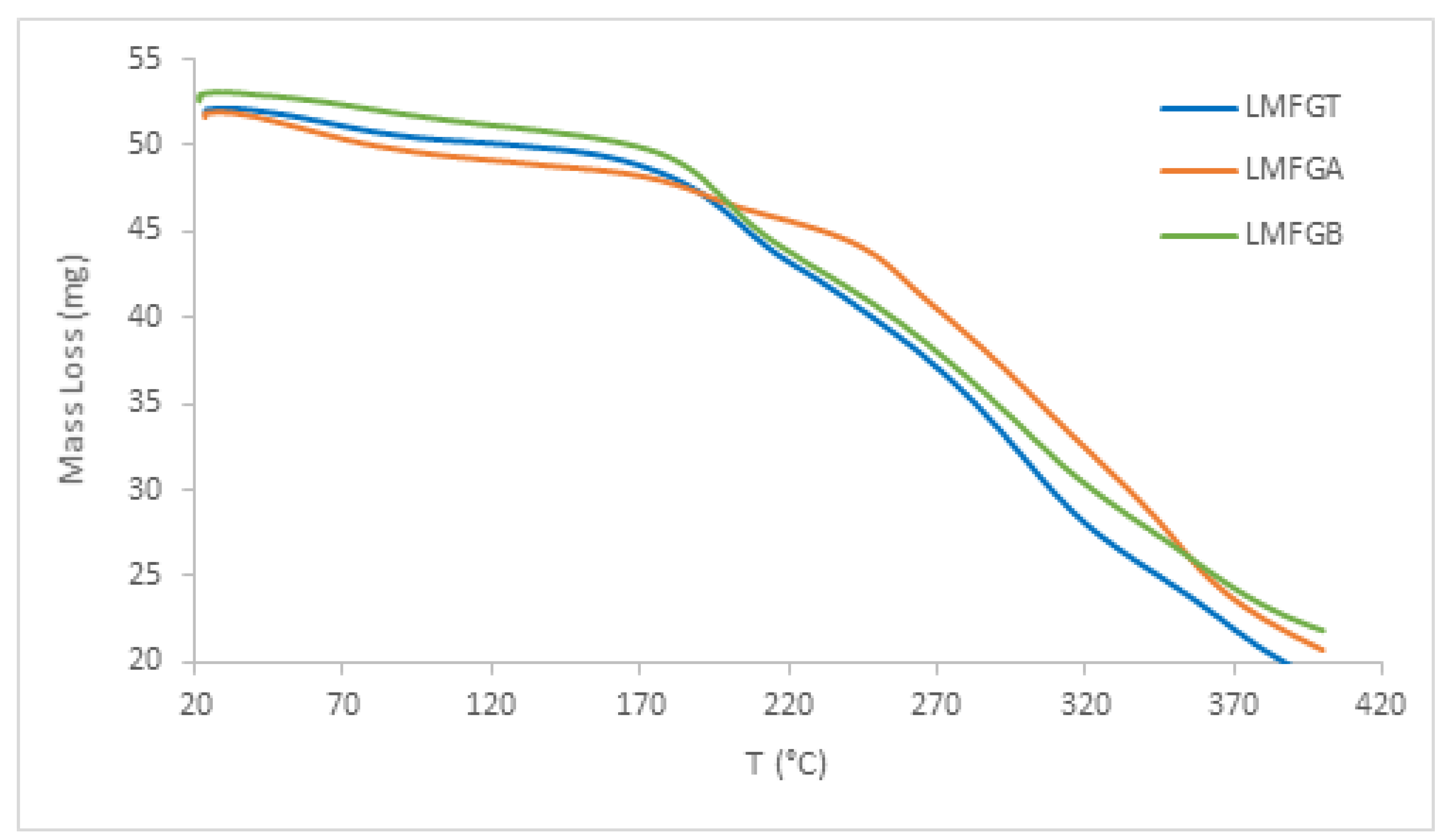
3.1.6. TGA
3.2. Lithraea Molleoides Fruit Gum Films (LMFG Films)
3.2.1. SEM-EDX
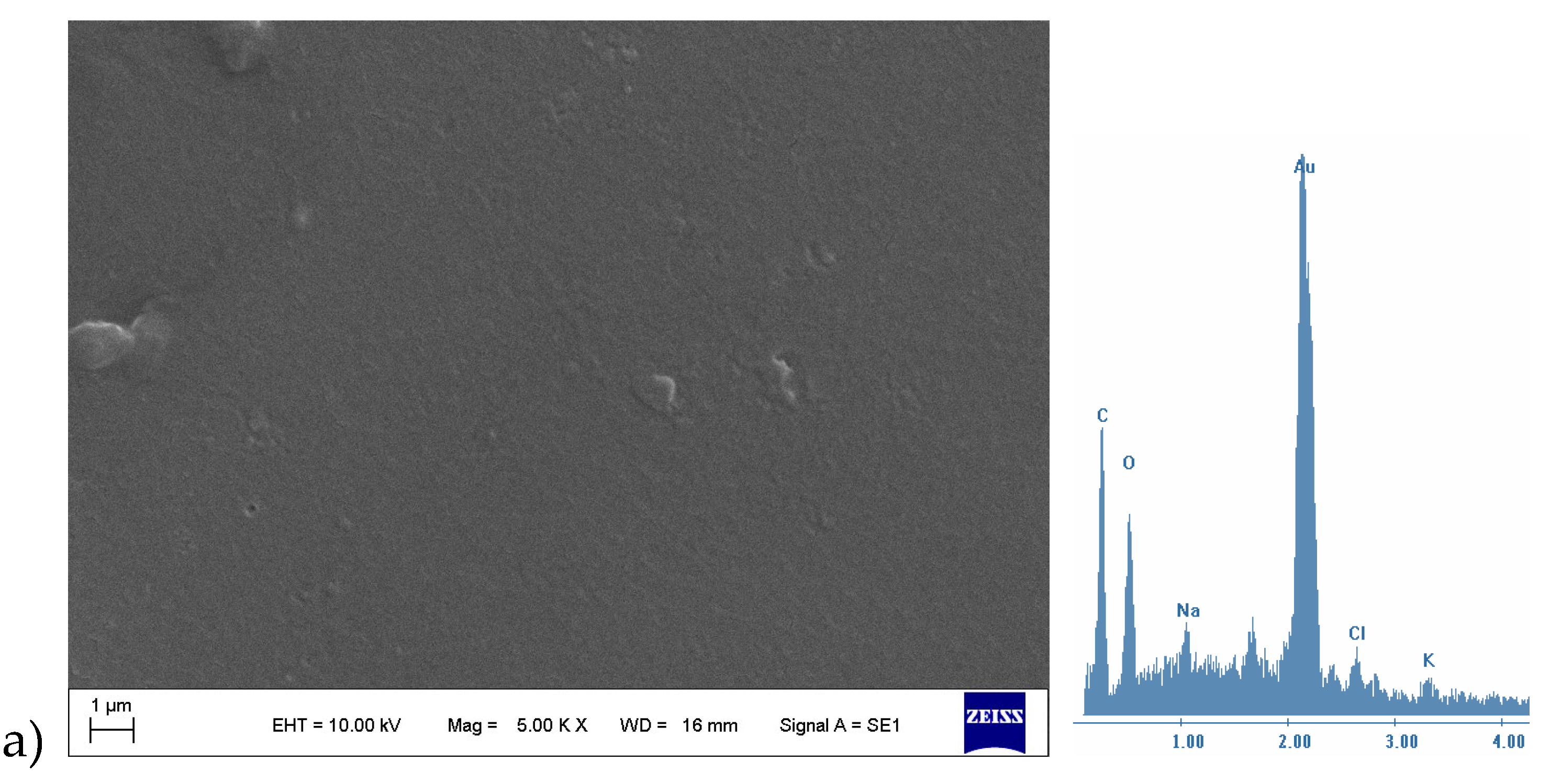
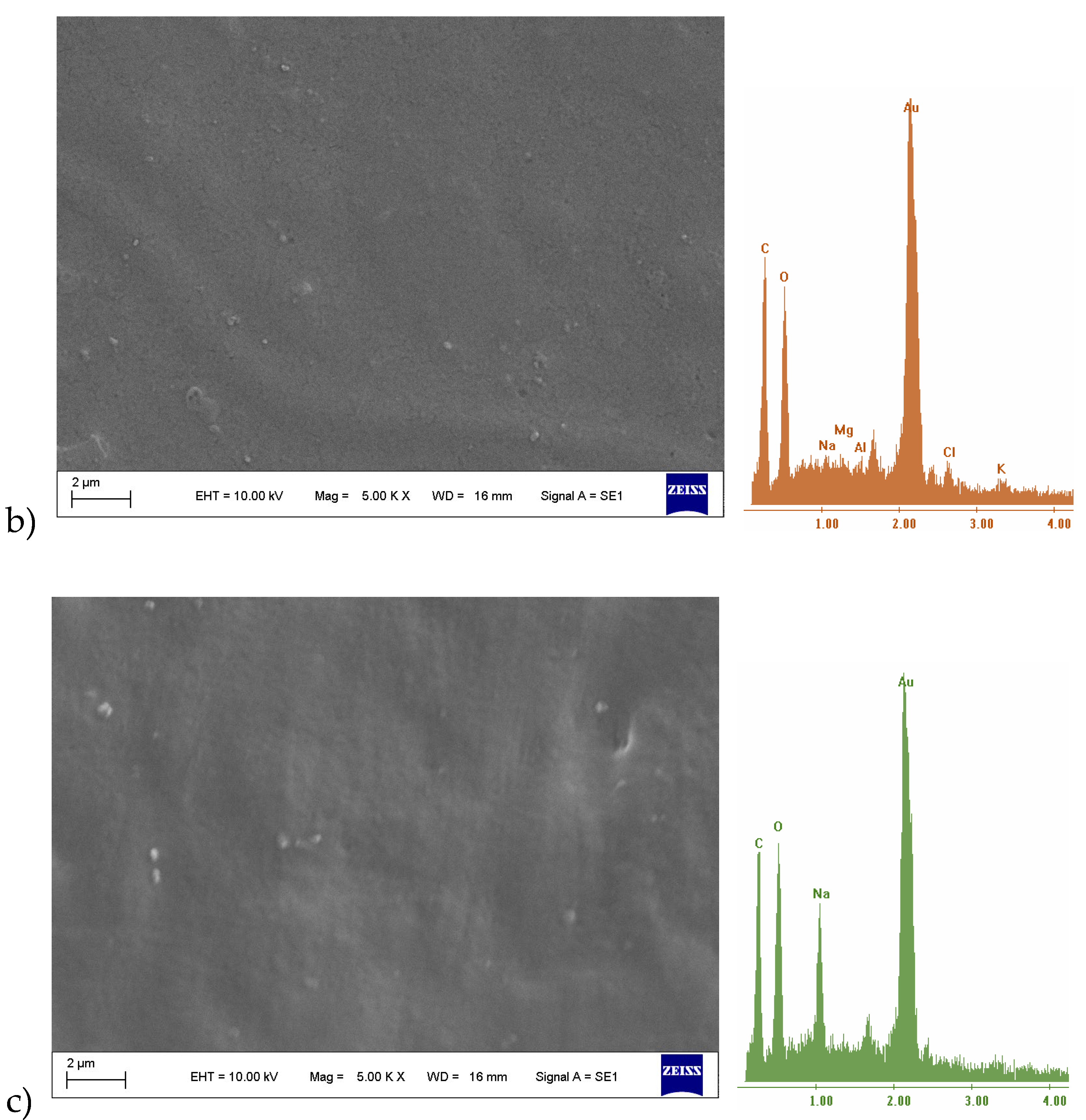
3.2.2. Mechanical Test, WVP-Water Vapor Sorption (WVS)
| Films | e (µm) | εmax% | σmax (MPa) | E (MPa) | WVP P (ng.m/ m2 s Pa) |
|---|---|---|---|---|---|
| Pec2.5 | 207.8 ± 9.2 | 20.08 ± 0.90 | 23.79 ± 0.97 | 32.85 ± 1.1 | 0.55 |
| LMFGTf | 224.4 ± 8.1 | 24.47 ± 0.78 | 8.44 ± 0.34 | 19.65 ± 0.91 | 0.97 |
| LMFGAf | 264.6 ± 6.5 | 25.58 ± 0.06 | 21.87 ± 0.87 | 47.23 ± 0.82 | 1.52 |
| LMFGBf | 221.9 ± 5.8 | 19.40 ± 0.88 | 13.37 ± 0.56 | 26.05 ± 0.89 | 2.06 |
3.2.3. Biodegradability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics derived from food packaging waste—Their origin and health risks. Materials 2023, 16(2), 674. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Linhares, R.; Martelli, M. Films and Coatings from Agro-Industrial Residues. Chapter 11, In Edible films and Coatings; CRC Press: London, UK, 2016; pp. 211–232. [Google Scholar]
- Nussinovitch, A. Biopolymer films and composite coatings. In Modern Biopolymer Science; Academic Press: London, UK, 2009; pp. 295–326. [Google Scholar]
- da Silva, M.A.; Bierhalz, A.C.K. Biopolymer Films and Composite Coatings: Applications. In Handbook of Biopolymers; Springer Nature: Singapore, 2023; pp. 1229–1261. [Google Scholar]
- Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Novel sources of edible films and coatings. Stewart Postharvest Review 2010, 3, 2, 1–8. [Google Scholar]
- Beyza, H.; Fatma, K.; Hecer, C. Edible films coatings: A good idea from past to future technology. J. Food Technol. 2018, 5, 28–33. [Google Scholar]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food and Bioprocess Technology 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Dyshlyuk, L.; Babich, O.; Belovа, D.; Prosekov, A. Comparative analysis of physical and chemical properties of biodegradable edible films of various compositions. Journal of Food Process Engineering 2017, 40(1), e12331. [Google Scholar] [CrossRef]
- Subroto, E.; Indiarto, R.; Pangawikan, A.D.; Prakoso, F. Production and Characteristics of Composite Edible Films Based on Polysaccharides and Proteins. International Journal of Emerging Trends in Engineering Research 2021, 9(2), 42–48. [Google Scholar]
- Han, J.H. Edible films and coatings: A review. Chapter 9. In Innovations in food packaging; Academic Press: San Diego, CA, USA, 2014; pp. 213–255. [Google Scholar]
- Šuput, D.Z.; Lazić, V.L.; Popović, S.Z.; Hromiš, N.M. Edible films and coatings: Sources, properties and application. Food and Feed Research 2015, 42(1), 11–22. [Google Scholar] [CrossRef]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Merah, O. Recent developments in edible films and coatings for fruits and vegetables. Coatings 2023, 13(7), 1177. [Google Scholar]
- Drevet, R.; Benhayoune, H. Advanced Biomaterials and Coatings. Coatings 2022, 12(7), 965. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Coltro, L.; Dantas, F.B.H.; Vieira, R.P. Research on food packaging and storage. Coatings 2022, 12(11), 1714. [Google Scholar] [CrossRef]
- Dhanapal, A.; Sasikala, P.; Rajamani, L.; Kavitha, V.; Yazhini, G.; Banu, S. Edible films from polysaccharides. Food Science and Quality Management 2012, 3, 9–17. [Google Scholar]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A review. Agronomy 2021, 11(5), 813. [Google Scholar] [CrossRef]
- Nieto, M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. Chapter 2. In Edible Films and Coatings for food Applications; Springer-Nature: Berlin, Germany, 2009; pp. 57–112. [Google Scholar]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10(7), 674. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and functionalized films/coatings—Performances and perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends in Food Science Technology 2011, 22(6), 292–303. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Delattre, C. Modification of chitosan for the generation of functional derivatives. Applied Sciences 2019, 9(7), 1321. [Google Scholar]
- Roman, M.; Nechita, P.; Vasile, M.A.; Cantaragiu Ceoromila, A.M. Barrier and Antimicrobial Properties of Coatings Based on Xylan Derivatives and Chitosan for Food Packaging Papers. Coatings 2023, 13(10), 1761. [Google Scholar] [CrossRef]
- Pinto, J.P.; D’souza, O.J.; Hiremani, V.D.; Dalbanjan, N.P.; Kumar, S.P.; Narasagoudr, S.S.; Chougale, R.B. Functional properties of taro starch reinforced polysaccharide based films for active packaging. Food Bioscience 2023, 56, 103340. [Google Scholar] [CrossRef]
- Tarnowiecka-Kuca, A.; Peeters, R.; Bamps, B.; Stobińska, M.; Kamola, P.; Wierzchowski, A.; Mizielińska, M. Paper Coatings Based on Polyvinyl Alcohol and Cellulose Nanocrystals Using Various Coating Techniques and Determination of Their Barrier Properties. Coatings 2023, 13(11), 1975. [Google Scholar]
- Thiviya, P.; Gamage, A.; Liyanapathiranage, A.; Makehelwala, M.; Dassanayake, R.S.; Manamperi, A.; Madhujith, T. Algal polysaccharides: Structure, preparation and applications in food packaging. Food Chemistry 2023, 405, 134903. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, I.L.; Lin, C.; Hang, Y.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Intelligent films of marine polysaccharides and purple cauliflower extract for food packaging and spoilage monitoring. Carbohydrate Polymers 2023, 299, 120133. [Google Scholar] [CrossRef] [PubMed]
- Kokkuvayil Ramadas, B.; Rhim, J.W.; Roy, S. Recent Progress of Carrageenan-Based Composite Films in Active and Intelligent Food Packaging Applications. Polymers 2024, 16(7), 1001. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Zou, X.; Zhu, B.; You, L.; Zhao, Z.; Hileuskaya, K. The characteristics of polysaccharide from Gracilaria chouae and its application in food packaging with carboxymethyl cellulose and lysozyme. Food Hydrocolloids 2023, 135, 108109. [Google Scholar] [CrossRef]
- Long, J.; Zhang, W.; Zhao, M.; Ruan, C.Q. The reduce of water vapor permeability of polysaccharide-based films in food packaging: A comprehensive review. Carbohydrate Polymers 2023, 121267. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.C.M.; Dos Santos, P.N.; Santos, F.H.; Molina, G.; Pelissari, F.M. Sustainability approaches for agrowaste solution: Biodegradable packaging and microbial polysaccharides bio-production. Science of The Total Environment 2023, 886, 163922. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, X.; Xu, S.; Shao, P.; Li, J.; Chen, Z. ;... Renard, C.M. Advances in green solvents for production of polysaccharide-based packaging films: Insights of ionic liquids and deep eutectic solvents. Comprehensive Reviews in Food Science and Food Safety 2023, 22(2), 1030–1057. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Z.; Yao, L.; Feng, T.; Song, S.; Sun, M. Insights into the Edible and Biodegradable Ulvan-Based Films and Coatings for Food Packaging. Foods 2023, 12(8), 1622. [Google Scholar] [CrossRef]
- da Silva Bruni, A.R.; Friedrichsen, J.D.S.A.; de Jesus, G.A.M.; da Silva Alves, E.; da Costa, J.C.M.; Souza, P.R.; Bonafe, E.G. Characterization and application of active films based on commercial polysaccharides incorporating ZnONPs. International Journal of Biological Macromolecules 2023, 224, 1322–1336. [Google Scholar] [CrossRef]
- Tang, H.; Han, Z.; Zhao, C.; Jiang, Q.; Tang, Y.; Li, Y.; Cheng, Z. Preparation and characterization of Aloe vera polysaccharide-based packaging film and its application in blueberry preservation. Progress in Organic Coatings 2023, 177, 107445. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.; Souza, M.P.; Teixeira, J.A.; Vicente, A.A. Physical properties of edible coatings films made with a polysaccharide from Anacardium occidentale, L. Journal of Food Engineering 2009, 95(3), 379–385. [Google Scholar] [CrossRef]
- Irimia, A.; Popescu, C.M. Bioactive Paper Packaging for Extended Food Shelf Life. Coatings 2023, 13(9), 1658. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M. Polysaccharides/propolis composite as promising materials with biomedical and packaging applications: A review. Biomass Conversion and Biorefinery 2024, 14(4), 4555–4565. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Ahmadigol, A.; Khubber, S.; Altintas, Z. Bionanocomposite films with plasticized WPI-jujube polysaccharide/starch nanocrystal blends for packaging fresh-cut carrots. Food Packaging and Shelf Life 2023, 36, 101042. [Google Scholar] [CrossRef]
- Huang, J.; Wu, W.; Niu, B.; Fang, X.; Chen, H.; Wang, Y.; Gao, H. Characterization of Zizania latifolia polysaccharide-corn starch composite films and their application in the postharvest preservation of strawberries. LWT 2023, 173, 114332. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Zhang, H.; Zhang, D.; Li, Z.; Yu, J. Characterization of polysaccharide-based antibacterial films properties of loaded with Nisin and preservation of fresh-cut watermelon. Food Science and Technology 2023, 43, e127522. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, Y.; Zhuang, Y.; Tan, K.B.; Chen, J. Effects of grape seed extract on the properties of pullulan polysaccharide/xanthan gum active films for apple preservation. International Journal of Biological Macromolecules 2023, 241, 124617. [Google Scholar] [CrossRef]
- Jafarian, M.; Taghinia, P.; Sedaghati, S. Development and characterization of a new active and intelligent packaging system based on soluble soybean polysaccharide-Malva sylvestris extract. Journal of Food Science and Technology 2023, 60(7), 1944–1951. [Google Scholar] [CrossRef]
- Şahin, S.; Eyüboğlu, S.; Karkar, B.; Ata, G.D. Development of bioactive films loaded with extract and polysaccharide of Pinus brutia bark. Journal of Food Science 2024. [CrossRef]
- Yu, T.; Wu, D.; Liang, B.; Wang, J.; Shang, X.; Wu, Q. Preparation, characterization of Auricularia auricula polysaccharide-based films and application in meat preservation. International Journal of Biological Macromolecules 2023, 244, 125242. [Google Scholar] [CrossRef]
- Janik, W.; Jakubski, Ł.; Kudła, S.; Dudek, G. Modified polysaccharides for food packaging applications: A review. International Journal of Biological Macromolecules 2023, 128916. [Google Scholar] [CrossRef]
- Yang, X.; Niu, Y.; Fan, Y.; Zheng, T.; Fan, J. Green synthesis of Poria cocos polysaccharides-silver nanoparticles and their applications in food packaging. International Journal of Biological Macromolecules 2024, 269, 131928. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, H.M.S.; Ahmed, S.; Olewnik-Kruszkowska, E.; Gierszewska, M.; Brzezinska, M.S.; Dembińska, K.; Kalwasińska, A. Carboxymethyl cellulose based films enriched with polysaccharides from mulberry leaves (Morus alba L.) as new biodegradable packaging material. International Journal of Biological Macromolecules 2023, 253, 127633. [Google Scholar] [CrossRef] [PubMed]
- Kafashan, A.; Joze-Majidi, H.; Kazemi-Pasarvi, S.; Babaei, A.; Jafari, S.M. Nanocomposites of soluble soybean polysaccharides with grape skin anthocyanins and graphene oxide as an efficient halochromic smart packaging. Sustainable Materials and Technologies 2023, 38, e00755. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, S.; Das, S.; De, S.; Paul, P.; Dua, T.K.; Nandi, G. Current Status of Marine Animal Derived Polysaccharides in Sustainable Food Packaging. ACS Food Science Technology 2023, 3(11), 1877–1889. [Google Scholar]
- Liu, J.; Dong, Y.; Zheng, X.; Pei, Y.; Tang, K. Citric acid crosslinked soluble soybean polysaccharide films for active food packaging applications. Food Chemistry 2024, 438, 138009. [Google Scholar] [CrossRef]
- Chi, K.; He, J.; Lin, W.S.; Bokhari, S.M.; Catchmark, J.M. Electrostatically complexed natural polysaccharides as aqueous barrier coatings for sustainable and recyclable fiber-based packaging. ACS Applied Materials Interfaces 2023, 15(9), 12248–12260. [Google Scholar] [CrossRef]
- Kaya, E.C.; Yucel, U. Advances in cellulose-based packaging films for food products. In Cellulose-Fundamentals and Conversion into Biofuel and Useful Chemicals; Intech Open: London, UK, 2023; pp. 1–21. [Google Scholar]
- Dong, Y.; Li, Y.; Ma, Z.; Rao, Z.; Zheng, X.; Tang, K.; Liu, J. Effect of polyol plasticizers on properties and microstructure of soluble soybean polysaccharide edible films. Food Packaging and Shelf Life 2023, 35, 101023. [Google Scholar] [CrossRef]
- Garro, M.F.; Salinas Ibáñez, A.G.; Vega, A.E.; Arismendi Sosa, A.C.; Pelzer, L.; Saad, J.R.; Maria, A.O. Gastroprotective effects and antimicrobial activity of Lithraea molleoides and isolated compounds against Helicobacter pylori. Journal of Ethnopharmacology 2015, 176, 469–474. [Google Scholar] [CrossRef]
- Becerra, F.; Garro, M.F.; Masuelli, M. Influence of the Type of Hydrolysis on the Intrinsic Viscosity of Lithraea Molleoides Fruit Gum, J. Mate. Poly. Sci. 2023, 3(2), 1–6. [Google Scholar]
- Rulli, M.M.; Villegas, L.B.; Barcia, C.S.; Colin, V.L. Bioconversion of sugarcane vinasse into fungal biomass protein and its potential use in fish farming. Journal of Environmental Chemical Engineering 2021, 9(5), 106136. [Google Scholar] [CrossRef]
- Deng, C.; Hu, Z.; Fu, H.; Hu, M.; Xu, X.; Chen, J. Chemical analysis and antioxidant activity in vitro of β-d-glucan isolated from Dictyophora indusiate. Int. J. Biol. Macromol. 2012, 51, 70–75. [Google Scholar] [CrossRef]
- Du, X.; Mu, H.; Zhou, S.; Zhang, Y.; Zhu, X. Chemical analysis and antioxidant activity of polysaccharides extracted from Inonotus obliquus sclerotia. Int. J. Biol. Macromol. 2013, 62, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Arora, S.; Singh, B. Antioxidant activity of the phenol rich fractions of leaves of Chukrasia tabularis A. Juss. Bioresour. Technol. 2008, 99, 7692–7698. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M.J.N.P. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Zanon, M.; Masuelli, M. Purification and characterization of alcayota gum. Biopolym. Res. 2018, 2, 105. [Google Scholar]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Barbosa, H.F.; Francisco, D.S.; Ferreira, A.P.; Cavalheiro, É.T. A new look towards the thermal decomposition of chitins and chitosans with different degrees of deacetylation by coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef]
- Zanon, M.; Masuelli, M.A. Alcayota gum films: Experimental reviews. J. Mater. Sci. Chem. Eng. 2018, 6, 11–58. [Google Scholar] [CrossRef]
- Lazo, L.; Melo, G.M.; Auad, M.L.; Filippa, M.; Masuelli, M.A. Synthesis and characterization of Chañar gum films. Colloids and Interfaces 2022, 6(1), 10. [Google Scholar] [CrossRef]
- Illanes, C.O.; Takara, E.A.; Masuelli, M.A.; Ochoa, N.A. pH-responsive gum tragacanth hydrogels for high methylene blue adsorption. Journal of Chemical Technology Biotechnology 2024, 99(1), 31–39. [Google Scholar] [CrossRef]
- Masuelli, M.A.; Lazo, L.; Becerra, F.; Torres, F.; Illanes, C.O.; Takara, A. ;... Bercea, M. Physical and Chemical Properties of Pachycymbiola brasiliana Eggshells—From Application to Separative Processes. Processes 2024, 12(4), 814. [Google Scholar] [CrossRef]
- Zapata-Luna, R.L.; Davidov-Pardo, G.; Pacheco, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; García-Márquez, E.; Cuevas-Bernardino, J.C. Structural and physicochemical properties of bio-chemical chitosan and its performing in an active film with quercetin and Phaseolus polyanthus starch. Revista Mexicana de Ingeniería Química 2023, 22(2), 1–11. [Google Scholar] [CrossRef]
- Acosta-Ferreira, S.; Castillo, O.S.; Madera-Santana, J.T.; Mendoza-García, D.A.; Núñez-Colín, C.A.; Grijalva-Verdugo, C.; Villa-Lerma, A.G.; Morales-Vargas, A.T.; Rodríguez-Núñez, J.R. Production and physicochemical characterization of chitosan for the harvesting of wild microalgae consortia. Biotechnology Reports 2020, 28, e00554. [Google Scholar] [CrossRef] [PubMed]
- Chel-Guerrero, L.; Betancur-Ancona, D.; Aguilar-Vega, M.; Rodríguez-Canto, W. Films properties of QPM corn starch with Delonix regia seed galactomannan as an edible coating material. International Journal of Biological Macromolecules 2024, 255, 128408. [Google Scholar] [CrossRef]
- Leite, K.; Nascimento, J.; Neto JH, L.; Gomes, K. (2024) Influence of citric acid on the properties of galactomanan bioplastics from Prosopis juliflora (Sw) DC: Influência do ácido cítrico nas propriedades dos bioplásticos de galactomanana de Prosopis juliflora (sw), D.C. Concilium, 24.
- Meng, F.; Zhang, Y.; Xiong, Z.; Wang, G.; Li, F.; Zhang, L. Mechanical, hydrophobic and thermal properties of an organic-inorganic hybrid carrageenan-polyvinyl alcohol composite film. Composites Part B: Engineering 2018, 143, 1–8. [Google Scholar] [CrossRef]
- Ruano, P.; Lazo Delgado, L.; Picco, S.; Villegas, L.; Tonelli, F.; Aguilera Merlo, M.E.; Rigau, J.; Diaz, D.; Masuelli, M. Extraction and Characterization of Pectins from Peels of Criolla Oranges (Citrus sinensis). Experimental Reviews. Chapter 1. In Pectins - Extraction, Purification, Characterization and Applications; Masuelli, M., Ed.; Intech Publishers: London, UK, 2020. [Google Scholar]
- Lin, X.; Chen, S.; Wang, R.; Li, C.; Wang, L. Fabrication, characterization and biological properties of pectin and/or chitosan-based films incorporated with noni (Morinda citrifolia) fruit extract. Food Hydrocolloids 2023, 134, 108025. [Google Scholar] [CrossRef]
- Ciaramitaro, V.; Piacenza, E.; Paliaga, S.; Cavallaro, G.; Badalucco, L.; Laudicina, V.A.; Chillura Martino, D.F. Exploring the Feasibility of Polysaccharide-Based Mulch Films with Controlled Ammonium and Phosphate Ions Release for Sustainable Agriculture. Polymers 2024, 16(16), 2298. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Wang, P.; Liu, Z.; An, L.; Zhang, X.; Gao, W. Evalua-tion of citrus pectin extraction methods: Synergistic enhancement of pectin’s antioxi-dant capacity and gel properties through combined use of organic acids, ultrasoni-cation, and microwaves. International Journal of Biological Macromolecules 2024, 266, 131164. [Google Scholar] [CrossRef]
| Moisture (%wt.) | Ash (%wt.) | Total Grass (g/100g) | Proteins (%wt.) | Fibers (%wt.) | |
|---|---|---|---|---|---|
| HLM | 10.60 ± 0.25 | 1.84 ± 0.02 | 9.92 ± 0.33 | 0.0454 | 15.22 ± 0.53 |
| LMFGT | 2.40 ± 0.74 | 1.45 ± 0.03 | 7.30 ± 0.31 | 0.0851 | 28.49 ± 1.24 |
| LMFGA | 3.12 ± 0.18 | 0.82 ± 0.01 | 3.40 ± 0.14 | 0.0189 | 27.78 ± 1.55 |
| LMFGB | 3.25 ± 0.17 | 0.84 ± 0.01 | 2.84 ± 0.13 | 0.0330 | 42.20 ± 1.89 |
| Reducing Power (µeq gal/mL) | Antioxidant Activity (µeq AA/ml) | Total Polyphenols (µeq garl/mL) | |
|---|---|---|---|
| HLM | 4.04 ± 0.22 | 14.78 ± 0.55 | 13.13 ± 0.39 |
| LMFGT | 1.91 ± 0.17 | 3.48 ± 0.22 | 28.58 ± 0.62 |
| LMFGA | 3.49 ± 0.33 | 2.71 ± 0.37 | 11.96 ± 0.48 |
| LMFGB | 9.91 ± 0.42 | 9.46 ± 0.45 | 41.82 ± 1.06 |
| Flour | 2θ | dspacing (nm) | ICr % |
| LMFGT | 20.18 ± 0.81 | 4.39 ± 1.03 | 47.75 ± 1.23 |
| LMFGA | 20.62 ± 0.79 | 4.30 ± 0.98 | 48.10 ± 1.01 |
| LMFGB | 20.34 ± 0.84 | 4.36 ± 0.95 | 48.83 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





