Submitted:
26 September 2024
Posted:
27 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. HFpEF
2.1. The Worldwide Prevalence, Morbidity and Mortality
2.2. The Pathophysiology of HFpEF
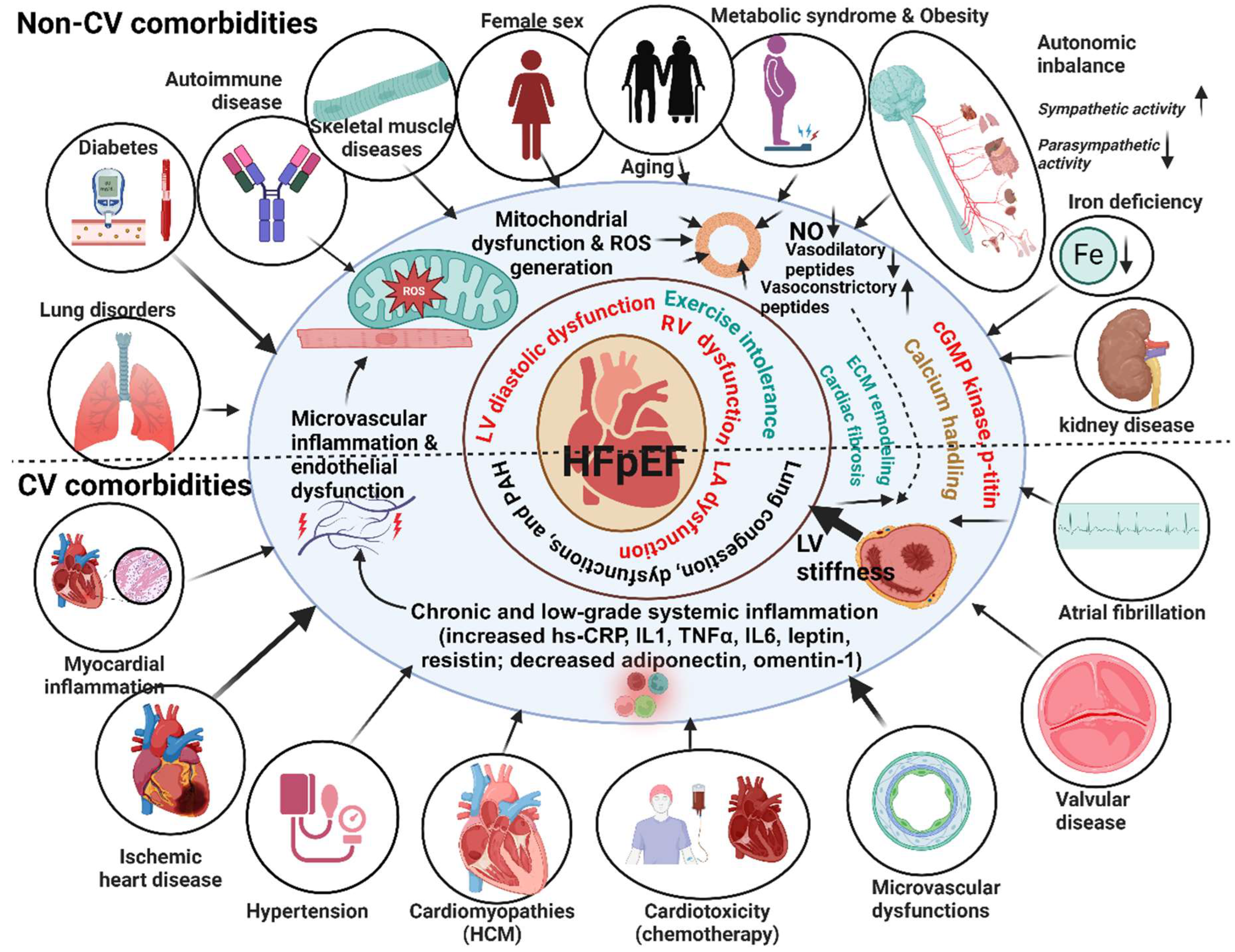
3. Animal Models of HFpEF
4. Mitochondrial Metabolism in Cardiac Haemostasis and Injury-Induced Remodelling
4.1. Mitochondrial-the Powerhouse of Heart
4.2. Mitochondrial Metabolism and Metabolites in Cardiac Haemostasis
4.3. Mitochondrial Metabolism and Metabolites in Injury-Induced Cardiac Remodeling, with a Focus on HFpEF
5. Mitochondrial Quality Control (MQC): The Protective “Mito Orchestra” in the Context of HFpEF
5.1. Mitochondrial Biogenesis
5.2. Mitochondrial Dynamics
5.3. Mitophagy
6. Mitochondrial Dysfunction and ROS Generation: Implications in HFpEF
6.1. Mitochondrial ROS Generation and Damage
6.2. Mitochondrial ROS as Coordinators of Cellular Function and Mitohormesis
6.3. Mitochondrial ROS and Heart Failure
6.4. Mitochondrial ROS and HFpEF: A Fraction of the Whole?
7. Therapeutics for HFpEF: Targeting mtROS and Associated Signal Molecules?
7.1. Conventional Pharmacological Therapy
7.1.1. Diuretics
7.1.2. Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors
7.1.3. Mineralocorticoid Receptor Antagonists (MARs)
7.1.4. Angiotensin Receptor–Neprilysin Inhibitors
7.1.5. Angiotensin Receptor Blockers
7.1.6. β-Blockers
7.2. Mitochondria Oxidative Stress-Targeted Therapy
7.2.1. Elamipretide
7.2.2. CoQ10
7.2.3. MitoQ
7.3. Non-Pharmacological Management
7.3.1. Dietary Interventions-Low Carbohydrate Diet
7.3.2. Exercise Training
8. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. , et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N. , et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143. [Google Scholar] [CrossRef] [PubMed]
- Walter, J. Paulus, M., PHD,* Carsten Tschöpe, MD, PHDy. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. Journal of the American College of Cardiology 2013. [Google Scholar] [CrossRef]
- Mishra, S.; Kass, D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nature Reviews Cardiology 2021, 18, 400–423. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V. , et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. , et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Teramoto, K.; Teng, T.-H.K.; Chandramouli, C.; Tromp, J.; Sakata, Y.; Lam, C.S. Epidemiology and Clinical Features of Heart Failure with Preserved Ejection Fraction. Cardiac Failure Review 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Robert, J. Mentz, M., Jacob P. Kelly, MD*, Thomas G. von Lueder, MD, PhD†, Adriaan A. Voors, MD‡, Carolyn S. P. Lam, MBBS§, Martin R. Cowie, MD, MSc||, Keld Kjeldsen, MD, DSc¶, Ewa A. Jankowska, MD, PhD#, Dan Atar, MD, PhD†, Javed Butler, MD, MPH**, Mona Fiuzat, PharmD*, Faiez Zannad, MD††, Bertram Pitt, MD‡‡, and Christopher M. O’Connor, MD*. Noncardiac Comorbidities in Heart Failure With Reduced Versus Preserved Ejection Fraction. J Am Coll Cardiol, 2014. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Lund, G.S.a.L.H. Global Public Health Burden of Heart Failure. Card Fail Rev 2017. [Google Scholar] [CrossRef]
- Bhatia, R.S.; Tu, J.V.; Lee, D.S.; Austin, P.C.; Fang, J.; Haouzi, A.; Gong, Y.; Liu, P.P. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. New England Journal of Medicine 2006, 355, 260–269. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Internal Medicine 2015, 175, 996. [Google Scholar] [CrossRef] [PubMed]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C. , et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. New England Journal of Medicine 2008, 359, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L. , et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Muthiah Vaduganathan, M. , MPH,a Ravi B. Patel, MD,b Alexander Michel, MD, MSC,c Sanjiv J. Shah, MD,b Michele Senni, MD,d Mihai Gheorghiade, MD,e Javed Butler, MD, MPH, MBAf. Mode of Death in Heart Failure With Preserved Ejection Fraction. JOU RNA L O F T H E AMER I CAN C O LL EG E O F C AR DI OLOG Y 2017, 69. [Google Scholar] [CrossRef]
- Rahul, S. Loungani, M., a John R. Teerlink, MD,b Marco Metra, MD, PHD,c Larry A. Allen, MD, MHS,d Javed Butler, MD, MBA, MPH,e Peter E. Carson, MD,f Chien-Wei Chen, PHD,g Gad Cotter, MD,h Beth A. Davison, PHD,h Zubin J. Eapen, MD, MHS,i Gerasimos S. Filippatos, MD,j,k Claudio Gimpelewicz, MD,l Barry Greenberg, MD,m Thomas Holbro, PHD,l James L. Januzzi, JR, MD,n David E. Lanfear, MD, MS,o Peter S. Pang, MD,p Ileana L. Piña, MD, MPH,q Piotr Ponikowski, MD, PHD,r Alan B. Miller, MD,s Adriaan A. Voors, MD, PHD,t G. Michael Felker, MD, MHSa. Cause of Death in Patients With Acute Heart Failure: Insights From RELAX-AHF-2. JA C C: HEAR T F AI L U RE 2020, 8. [Google Scholar] [CrossRef]
- Lee, D.S.; Gona, P.; Albano, I.; Larson, M.G.; Benjamin, E.J.; Levy, D.; Kannel, W.B.; Vasan, R.S. A Systematic Assessment of Causes of Death After Heart Failure Onset in the Community. Circulation: Heart Failure 2011, 4, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Shahim, A.; Hourqueig, M.; Lund, L.H.; Savarese, G.; Oger, E.; Venkateshvaran, A.; Benson, L.; Daubert, J.C.; Linde, C.; Donal, E. , et al. Long-term outcomes in heart failure with preserved ejection fraction: Predictors of cardiac and non-cardiac mortality. ESC Heart Failure 2023, 10, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Köseoğlu, F.D.; Özlek, B. Anemia and Iron Deficiency Predict All-Cause Mortality in Patients with Heart Failure and Preserved Ejection Fraction: 6-Year Follow-Up Study. Diagnostics 2024, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Omote, K.; Verbrugge, F.H.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction: Mechanisms and Treatment Strategies. Annual Review of Medicine 2022, 73, 321–337. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Alcaide, P.; Condorelli, G.; Gillette, T.G.; Heymans, S.; Jones, E.A.V.; Kallikourdis, M.; Lichtman, A.; Marelli-Berg, F.; Shah, S. , et al. Immunometabolic Mechanisms of Heart Failure with Preserved Ejection Fraction. Nat Cardiovasc Res 2022, 1, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Shinya; Jessica; Vangala, P. ; Tencerova, M.; Sarah; Joseph; Shen, Y.; Michael; Aouadi, M. Local Proliferation of Macrophages Contributes to Obesity-Associated Adipose Tissue Inflammation. Cell Metabolism 2014, 19, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nature Reviews Immunology 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Dubrock, H.M.; Abouezzeddine, O.F.; Redfield, M.M. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLOS ONE 2018, 13, e0201836. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart Failure With Preserved Ejection Fraction In Perspective. Circulation Research 2019, 124, 1598–1617. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Baicu, C.F.; Gaasch, W.H. Diastolic Heart Failure — Abnormalities in Active Relaxation and Passive Stiffness of the Left Ventricle. New England Journal of Medicine 2004, 350, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Jaber, W.A.; Ommen, S.R.; Lam, C.S.P.; Redfield, M.M.; Nishimura, R.A. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart 2011, 97, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Methawasin, M.; Strom, J.G.; Slater, R.E.; Fernandez, V.; Saripalli, C.; Granzier, H. Experimentally Increasing the Compliance of Titin Through RNA Binding Motif-20 (RBM20) Inhibition Improves Diastolic Function In a Mouse Model of Heart Failure With Preserved Ejection Fraction. Circulation 2016, 134, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.V.; Bronzwaer, J.G.F.; Van Der Velden, J.; Stienen, G.J.M.; Laarman, G.J.; Somsen, A. , et al. Low Myocardial Protein Kinase G Activity in Heart Failure With Preserved Ejection Fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef]
- Zile, M.R.; Baicu, C.F.; S. Ikonomidis, J.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M., et al. Myocardial Stiffness in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef]
- Thanh, T. Phan, M., CHB (HONS),* Khalid Abozguia, MBBCH,* Ganesh Nallur Shivu, MBBS,*; Gnanadevan Mahadevan, M., * Ibrar Ahmed, MB, CHB,* Lynne Williams, MB, CHB,*; Girish Dwivedi, M., * Kiran Patel, PHD,* Paul Steendijk, PHD,† Houman Ashrafian, MA,‡; Anke Henning, P., § Michael Frenneaux, MD*. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. Journal of the American College of Cardiology. [CrossRef]
- Sorimachi, H.; Burkhoff, D.; Verbrugge, F.H.; Omote, K.; Obokata, M.; Reddy, Y.N.V.; Takahashi, N.; Sunagawa, K.; Borlaug, B.A. Obesity, venous capacitance, and venous compliance in heart failure with preserved ejection fraction. European Journal of Heart Failure 2021, 23, 1648–1658. [Google Scholar] [CrossRef]
- Barry, A. Borlaug, M., Carolyn S. P. Lam, MBBS, Véronique L. Roger, MD, MPH, Richard J. Rodeheffer, MD, Margaret M. Redfield, MD. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. 2009. [CrossRef]
- Yu Ting Tan, M. , * Frauke Wenzelburger, MD,*† Eveline Lee, MBCHB,† Grant Heatlie, MBBS, PHD,† Francisco Leyva, MD,* Kiran Patel, MBBCHIR,PHD,* Michael Frenneaux, MD,* John E. Sanderson, MD*. The Pathophysiology of Heart Failure With Normal Ejection Fraction. Journal of the American College of Cardiology. [CrossRef]
- Backhaus, S.J.; Lange, T.; George, E.F.; Hellenkamp, K.; Gertz, R.J.; Billing, M.; Wachter, R.; Steinmetz, M.; Kutty, S.; Raaz, U. , et al. Exercise Stress Real-Time Cardiac Magnetic Resonance Imaging for Noninvasive Characterization of Heart Failure With Preserved Ejection Fraction. Circulation 2021, 143, 1484–1498. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. European Journal of Heart Failure 2019, 21, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Obokata, M.; Verbrugge, F.H.; Lin, G.; Borlaug, B.A. Atrial Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation. Journal of the American College of Cardiology 2020, 76, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Barry, A. Borlaug, M., Yogesh N.V. Reddy, MBBS, MSC. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JA CC: HEART F AIL U RE 2019. [CrossRef]
- Freed, B.H.; Daruwalla, V.; Cheng, J.Y.; Aguilar, F.G.; Beussink, L.; Choi, A.; Klein, D.A.; Dixon, D.; Baldridge, A.; Rasmussen-Torvik, L.J. , et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction. Circulation: Cardiovascular Imaging 2016, 9. [Google Scholar] [CrossRef]
- Telles, F.; Nanayakkara, S.; Evans, S.; Patel, H.C.; Mariani, J.A.; Vizi, D.; William, J.; Marwick, T.H.; Kaye, D.M. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. European Journal of Heart Failure 2019, 21, 495–505. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Pislaru, S.; Borlaug, B.A. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. European Heart Journal 2019, 40, 689–697. [Google Scholar] [CrossRef]
- Zakeri, R.; Chamberlain, A.M.; Roger, V.L.; Redfield, M.M. Temporal Relationship and Prognostic Significance of Atrial Fibrillation in Heart Failure Patients With Preserved Ejection Fraction. Circulation 2013, 128, 1085–1093. [Google Scholar] [CrossRef]
- Marco Guazzi, M. , PHD,a Robert Naeije, MD, PHDb. Pulmonary Hypertension in Heart Failure. JOU RNA L O F T H E AMER I CAN C O LL EG E O F C AR DI OLOG Y, /: ttp, 2017; 1. [Google Scholar] [CrossRef]
- Gorter, T.M.; Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Borlaug, B.A. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. European Heart Journal 2018, 39, 2825–2835. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Guazzi, M.; Testani, J.M.; Borlaug, B.A. Altered Hemodynamics and End-Organ Damage in Heart Failure. Circulation 2020, 142, 998–1012. [Google Scholar] [CrossRef]
- Gorter, T.M.; Hoendermis, E.S.; Van Veldhuisen, D.J.; Voors, A.A.; Lam, C.S.P.; Geelhoed, B.; Willems, T.P.; Van Melle, J.P. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. European Journal of Heart Failure 2016, 18, 1472–1487. [Google Scholar] [CrossRef]
- Gorter, T.M.; Van Veldhuisen, D.J.; Bauersachs, J.; Borlaug, B.A.; Celutkiene, J.; Coats, A.J.S.; Crespo-Leiro, M.G.; Guazzi, M.; Harjola, V.P.; Heymans, S. , et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure 2018, 20, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Marius, M. Hoeper, M., Katrin Meyer, MD, Jessica Rademacher, MD, Jan Fuge, MPH, Tobias Welte, MD, Karen M. Olsson, MD. Diffusion Capacity and Mortality in Patients With Pulmonary Hypertension Due to Heart Failure With Preserved Ejection Fraction. JA C C: HEAR T F AI L U RE, 2016. [Google Scholar] [CrossRef]
- Fermoyle, C.C.; Stewart, G.M.; Borlaug, B.A.; Johnson, B.D. Simultaneous Measurement of Lung Diffusing Capacity and Pulmonary Hemodynamics Reveals Exertional Alveolar-Capillary Dysfunction in Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Bhattacharya, P.; Kumar, A.; Proto, E.; Konda, P.; Segers, P.; Akers, S.R.; Townsend, R.R.; Zamani, P. Impact of Diabetes Mellitus on Ventricular Structure, Arterial Stiffness, and Pulsatile Hemodynamics in Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Andersen, M.J.; Obokata, M.; Koepp, K.E.; Kane, G.C.; Melenovsky, V.; Olson, T.P.; Borlaug, B.A. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. Journal of the American College of Cardiology 2017, 70, 136–148. [Google Scholar] [CrossRef]
- Shmuel Schwartzenberg, M. , Margaret M. Redfield, MD, Aaron M. From, MD, Paul Sorajja, MD, Rick A. Nishimura, MD, Barry A. Borlaug, MD. Effects of vasodilation in heart failure with preserved or reduced ejection fraction: implications ofdistinct pathophysiologies on response to therapy. Journal of the American College of Cardiology 2012. [CrossRef]
- Eiichi Akiyama, M. , *† Seigo Sugiyama, MD, PHD,* Yasushi Matsuzawa, MD,*† Masaaki Konishi, MD, PHD,*† Hiroyuki Suzuki, MD,† Toshimitsu Nozaki, MD, PHD,* Keisuke Ohba, MD,* Junichi Matsubara, MD, PHD,* Hirofumi Maeda, MD,* Yoko Horibata, MD,* Kenji Sakamoto, MD, PHD,* Koichi Sugamura, MD, PHD,* Megumi Yamamuro, MD, PHD,* Hitoshi Sumida, MD, PHD,‡ Koichi Kaikita, MD, PHD,* Satomi Iwashita, MT,* Kunihiko Matsui, MD, MPH,§ Kazuo Kimura, MD, PHD,† Satoshi Umemura, MD, PHD,储 Hisao Ogawa, MD, PHD*. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. Journal of the American College of Cardiology 2012. [Google Scholar] [CrossRef]
- Sanders-Van Wijk, S.; Tromp, J.; Beussink-Nelson, L.; Hage, C.; Svedlund, S.; Saraste, A.; Swat, S.A.; Sanchez, C.; Njoroge, J.; Tan, R.-S. , et al. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction. Circulation 2020, 142, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Walter, J. Paulus, M., PHD,* Carsten Tschöpe, MD, PHDy. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology 2013. [CrossRef]
- Ahmad, A.; Corban, M.T.; Toya, T.; Verbrugge, F.H.; Sara, J.D.; Lerman, L.O.; Borlaug, B.A.; Lerman, A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. European Journal of Heart Failure 2021, 23, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Kane, G.C.; Olson, T.P.; Jarolim, P.; Borlaug, B.A. Myocardial Injury and Cardiac Reserve in Patients With Heart Failure and Preserved Ejection Fraction. Journal of the American College of Cardiology 2018, 72, 29–40. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.-S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.A. , et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. European Heart Journal 2018, 39, 3439–3450. [Google Scholar] [CrossRef]
- Jeong Hoon Yang, M.O. , Yogesh N.V. Reddy, Margaret M. Redfield, Amir Lerman,and BarryA.Borlaug. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. European Journal of Heart Failure 2020. [CrossRef]
- Abudiab, M.M.; Redfield, M.M.; Melenovsky, V.; Olson, T.P.; Kass, D.A.; Johnson, B.D.; Borlaug, B.A. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. European Journal of Heart Failure 2013, 15, 776–785. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Melenovsky, V.; Russell, S.D.; Kessler, K.; Pacak, K.; Becker, L.C.; Kass, D.A. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation 2006, 114, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Stoller, D.; Hendrix, J.; Howden, E.; Lawley, J.; Livingston, S.; Adams-Huet, B.; Holmes, C.; Goldstein, D.S.; Levine, B.D. Mechanisms of Chronotropic Incompetence in Heart Failure With Preserved Ejection Fraction. Circulation: Heart Failure 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Howden, E.; Lawley, J.; Samels, M.; Levine, B.D. Central Command and the Regulation of Exercise Heart Rate Response in Heart Failure With Preserved Ejection Fraction. Circulation 2021, 143, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Kouta Funakoshi, M.; Kazuya Hosokawa, P.; Takuya Kishi, P.; Tomomi Ide, P.; Kenji Sunagawa, P. Striking volume intolerance is induced by mimicking arterial baroreflex failure in normal left ventricular function. Basic Science and Experimental Study 2013. [Google Scholar] [CrossRef]
- Bhella, P.S.; Prasad, A.; Heinicke, K.; Hastings, J.L.; Arbab-Zadeh, A.; Adams-Huet, B.; Pacini, E.L.; Shibata, S.; Palmer, M.D.; Newcomer, B.R. , et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. European Journal of Heart Failure 2011, 13, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.E.; Eisman, A.S.; Pappagianopoulos, P.P.; Wooster, L.; Bailey, C.S.; Wagner, P.D.; Lewis, G.D. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. Circulation 2018, 137, 148–161. [Google Scholar] [CrossRef]
- Kitzman DW, N.B. , Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 2014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Kelly, D.P.; Chirinos, J.A. Mitochondrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circulation 2019, 139, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Okayama, H.; Hamada, M.; Kawakami, H.; Ikeda, S.; Hashida, H.; Shigematsu, Y.; Hiwada, K. Alterations in expression of sarcoplasmic reticulum gene in Dahl rats during the transition from compensatory myocardial hypertrophy to heart failure. Journal of Hypertension 1997, 15, 1767–1774. [Google Scholar] [CrossRef]
- Qu, P.; Hamada, M.; Ikeda, S.; Hiasa, G.; Shigematsu, Y.; Hiwada, K. Time-Course Changes in Left Ventricular Geometry and Function during the Development of Hypertension in Dahl Salt-Sensitive Rats. Hypertension Research 2000, 23, 613–623. [Google Scholar] [CrossRef]
- Chen-Izu, Y.; Chen, L.; Bányász, T.; McCulle, S.L.; Norton, B.; Scharf, S.M.; Agarwal, A.; Patwardhan, A.; Izu, L.T.; Balke, C.W. Hypertension-induced remodeling of cardiac excitation-contraction coupling in ventricular myocytes occurs prior to hypertrophy development. American Journal of Physiology-Heart and Circulatory Physiology 2007, 293, H3301–H3310. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Janicki, J.S.; Pick, R.; Capasso, J.; Anversa, P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol 1990, 65, 1g–7g. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Sakata, Y.; Mano, T.; Yoshida, J.; Ohtani, T.; Takeda, Y.; Miwa, T.; Masuyama, T.; Yamamoto, K.; Hori, M. Therapeutic effects of angiotensin II type 1 receptor blocker at an advanced stage of hypertensive diastolic heart failure. J Hypertens 2007, 25, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Masuyama, T.; Sakata, Y.; Mano, T.; Nishikawa, N.; Kondo, H.; Akehi, N.; Kuzuya, T.; Miwa, T.; Hori, M. Roles of renin-angiotensin and endothelin systems in development of diastolic heart failure in hypertensive hearts. Cardiovasc Res 2000, 47, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Lupón, J.; Gavidia-Bovadilla, G.; Ferrer, E.; de Antonio, M.; Perera-Lluna, A.; López-Ayerbe, J.; Domingo, M.; Núñez, J.; Zamora, E.; Moliner, P. , et al. Heart Failure With Preserved Ejection Fraction Infrequently Evolves Toward a Reduced Phenotype in Long-Term Survivors. Circ Heart Fail 2019, 12, e005652. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Liao, R.; Eberli, F.R.; Satoh, N.; Chevalier, B.; Apstein, C.S.; Suter, T.M. Early changes in excitation-contraction coupling: transition from compensated hypertrophy to failure in Dahl salt-sensitive rat myocytes. Cardiovasc Res 1998, 37, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Capasso, J.M.; Palackal, T.; Olivetti, G.; Anversa, P. Left ventricular failure induced by long-term hypertension in rats. Circ Res 1990, 66, 1400–1412. [Google Scholar] [CrossRef]
- Wallner, M.; Eaton, D.M.; Berretta, R.M.; Liesinger, L.; Schittmayer, M.; Gindlhuber, J.; Wu, J.; Jeong, M.Y.; Lin, Y.H.; Borghetti, G. , et al. HDAC inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction. Sci Transl Med 2020, 12. [Google Scholar] [CrossRef]
- Gallet, R.; de Couto, G.; Simsolo, E.; Valle, J.; Sun, B.; Liu, W.; Tseliou, E.; Zile, M.R.; Marbán, E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci 2016, 1, 14–28. [Google Scholar] [CrossRef]
- Tofovic, S.P.; Kusaka, H.; Kost, C.K., Jr.; Bastacky, S. Renal function and structure in diabetic, hypertensive, obese ZDFxSHHF-hybrid rats. Ren Fail 2000, 22, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.; Cerqueira, R.J.; Ibarrola, J.; Fontoura, D.; Fernández-Celis, A.; Zannad, F.; Falcão-Pires, I.; Paulus, W.J.; Leite-Moreira, A.F.; Rossignol, P. , et al. Arterial Remodeling and Dysfunction in the ZSF1 Rat Model of Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2019, 12, e005596. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Tabima, D.M.; Dube, J.J.; Hughan, K.S.; Vanderpool, R.R.; Goncharov, D.A.; St Croix, C.M.; Garcia-Ocaña, A.; Goncharova, E.A.; Tofovic, S.P. , et al. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation 2016, 133, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Hopf, A.E.; Andresen, C.; Kötter, S.; Isić, M.; Ulrich, K.; Sahin, S.; Bongardt, S.; Röll, W.; Drove, F.; Scheerer, N. , et al. Diabetes-Induced Cardiomyocyte Passive Stiffening Is Caused by Impaired Insulin-Dependent Titin Modification and Can Be Modulated by Neuregulin-1. Circ Res 2018, 123, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Salah, E.M.; Bastacky, S.I.; Jackson, E.K.; Tofovic, S.P. Captopril Attenuates Cardiovascular and Renal Disease in a Rat Model of Heart Failure With Preserved Ejection Fraction. J Cardiovasc Pharmacol 2018, 71, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Schiattarella, G.G.; Jiang, N.; May, H.I.; Lavandero, S.; Gillette, T.G.; Hill, J.A. Female Sex Is Protective in a Preclinical Model of Heart Failure With Preserved Ejection Fraction. Circulation 2019, 140, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Munagala, V.K.; Hart, C.Y.; Burnett, J.C., Jr.; Meyer, D.M.; Redfield, M.M. Ventricular structure and function in aged dogs with renal hypertension: a model of experimental diastolic heart failure. Circulation 2005, 111, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Hay, I.; Fetics, B.; Kass, D.A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 2003, 107, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Reiter, U.; Reiter, G.; Manninger, M.; Adelsmayr, G.; Schipke, J.; Alogna, A.; Rajces, A.; Stalder, A.F.; Greiser, A.; Mühlfeld, C. , et al. Early-stage heart failure with preserved ejection fraction in the pig: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2016, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Sorop, O.; Heinonen, I.; van Kranenburg, M.; van de Wouw, J.; de Beer, V.J.; Nguyen, I.T.N.; Octavia, Y.; van Duin, R.W.B.; Stam, K.; van Geuns, R.J. , et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res 2018, 114, 954–964. [Google Scholar] [CrossRef]
- Schwarzl, M.; Hamdani, N.; Seiler, S.; Alogna, A.; Manninger, M.; Reilly, S.; Zirngast, B.; Kirsch, A.; Steendijk, P.; Verderber, J. , et al. A porcine model of hypertensive cardiomyopathy: implications for heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2015, 309, H1407–1418. [Google Scholar] [CrossRef]
- Ferrari, R.; Censi, S.; Mastrorilli, F.; Boraso, A. Prognostic benefits of heart rate reduction in cardiovascular disease. European Heart Journal Supplements 2003, 5, G10–G14. [Google Scholar] [CrossRef]
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial function in the heart: the insight into mechanisms and therapeutic potentials. Br J Pharmacol 2019, 176, 4302–4318. [Google Scholar] [CrossRef] [PubMed]
- Barth, E.; Stämmler, G.; Speiser, B.; Schaper, J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 1992, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Schaper, J.; Meiser, E.; Stämmler, G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res 1985, 56, 377–391. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ Res 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Chaanine, A.H.; Joyce, L.D.; Stulak, J.M.; Maltais, S.; Joyce, D.L.; Dearani, J.A.; Klaus, K.; Nair, K.S.; Hajjar, R.J.; Redfield, M.M. Mitochondrial Morphology, Dynamics, and Function in Human Pressure Overload or Ischemic Heart Disease With Preserved or Reduced Ejection Fraction. Circ Heart Fail 2019, 12, e005131. [Google Scholar] [CrossRef] [PubMed]
- Stoldt, S.; Wenzel, D.; Kehrein, K.; Riedel, D.; Ott, M.; Jakobs, S. Spatial orchestration of mitochondrial translation and OXPHOS complex assembly. Nat Cell Biol 2018, 20, 528–534. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat Rev Mol Cell Biol 2022, 23, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Redout, E.M.; Wagner, M.J.; Zuidwijk, M.J.; Boer, C.; Musters, R.J.; van Hardeveld, C.; Paulus, W.J.; Simonides, W.S. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res 2007, 75, 770–781. [Google Scholar] [CrossRef]
- Karamanlidis, G.; Lee, C.F.; Garcia-Menendez, L.; Kolwicz, S.C., Jr.; Suthammarak, W.; Gong, G.; Sedensky, M.M.; Morgan, P.G.; Wang, W.; Tian, R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 2013, 18, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Lesnefsky, E.J.; Chen, Q.; Hoppel, C.L. Mitochondrial Metabolism in Aging Heart. Circ Res 2016, 118, 1593–1611. [Google Scholar] [CrossRef] [PubMed]
- Rosca, M.; Minkler, P.; Hoppel, C.L. Cardiac mitochondria in heart failure: normal cardiolipin profile and increased threonine phosphorylation of complex IV. Biochim Biophys Acta 2011, 1807, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Wu, J.M.F.; Schulze, P.C. Mitochondrial Homeostasis Mediates Lipotoxicity in the Failing Myocardium. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J Biol Chem 2023, 299, 102838. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 2020, 11, 102. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Wisneski, J.A.; Stanley, W.C.; Neese, R.A.; Gertz, E.W. Effects of acute hyperglycemia on myocardial glycolytic activity in humans. J Clin Invest 1990, 85, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Vega, R.B.; Kelly, D.P. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochimica et biophysica acta 2013, 1833, 840–847. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 2013, 113, 603–616. [Google Scholar] [CrossRef]
- Da Dalt, L.; Cabodevilla, A.G.; Goldberg, I.J.; Norata, G.D. Cardiac lipid metabolism, mitochondrial function, and heart failure. Cardiovascular Research 2023, 119, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf) 2010, 199, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Mahieu, N.G.; Huang, X.; Singh, M.; Crawford, P.A.; Johnson, S.L.; Gross, R.W.; Schaefer, J.; Patti, G.J. Lactate metabolism is associated with mammalian mitochondria. Nat Chem Biol 2016, 12, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ Res 2023, 132, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol 2017, 595, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.L.; Karwi, Q.G.; Wagg, C.; Zhang, L.; Vo, K.; Altamimi, T.; Uddin, G.M.; Ussher, J.R.; Lopaschuk, G.D. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc Res 2021, 117, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat Rev Cardiol 2023, 20, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Sivanand, S.; Viney, I.; Wellen, K.E. Spatiotemporal Control of Acetyl-CoA Metabolism in Chromatin Regulation. Trends Biochem Sci 2018, 43, 61–74. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J. , et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A. , et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Vujic, A.; Koo, A.N.M.; Prag, H.A.; Krieg, T. Mitochondrial redox and TCA cycle metabolite signaling in the heart. Free Radic Biol Med 2021, 166, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wei, W.; Yang, M.; Du, Y.; Wan, Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab 2014, 20, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S. The failing heart--an engine out of fuel. N Engl J Med 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Leone, T.C.; Keller, M.P.; Martin, O.J.; Broman, A.T.; Nigro, J.; Kapoor, K.; Koves, T.R.; Stevens, R.; Ilkayeva, O.R. , et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail 2014, 7, 1022–1031. [Google Scholar] [CrossRef]
- Allard, M.F.; Schönekess, B.O.; Henning, S.L.; English, D.R.; Lopaschuk, G.D. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol 1994, 267, H742–750. [Google Scholar] [CrossRef] [PubMed]
- Pound, K.M.; Sorokina, N.; Ballal, K.; Berkich, D.A.; Fasano, M.; Lanoue, K.F.; Taegtmeyer, H.; O’Donnell, J.M.; Lewandowski, E.D. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res 2009, 104, 805–812. [Google Scholar] [CrossRef]
- Hahn, V.S.; Petucci, C.; Kim, M.S.; Bedi, K.C., Jr.; Wang, H.; Mishra, S.; Koleini, N.; Yoo, E.J.; Margulies, K.B.; Arany, Z. , et al. Myocardial Metabolomics of Human Heart Failure With Preserved Ejection Fraction. Circulation 2023, 147, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Schiattarella, G.G.; Jiang, N.; Altamirano, F.; Szweda, P.A.; Elnwasany, A.; Lee, D.I.; Yoo, H.; Kass, D.A.; Szweda, L.I. , et al. NAD(+) Repletion Reverses Heart Failure With Preserved Ejection Fraction. Circ Res 2021, 128, 1629–1641. [Google Scholar] [CrossRef]
- O’Sullivan, J.F.; Li, M.; Koay, Y.C.; Wang, X.S.; Guglielmi, G.; Marques, F.Z.; Nanayakkara, S.; Mariani, J.; Slaughter, E.; Kaye, D.M. Cardiac Substrate Utilization and Relationship to Invasive Exercise Hemodynamic Parameters in HFpEF. JACC Basic Transl Sci 2024, 9, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Güven, B.; Wagg, C.S.; Almeida de Oliveira, A.; Silver, H.; Zhang, L.; Chen, B.; Wei, K.; Ketema, E.B.; Karwi, Q.G. , et al. Mitochondrial fatty acid oxidation is the major source of cardiac adenosine triphosphate production in heart failure with preserved ejection fraction. Cardiovascular Research 2024, 120, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Sung, M.M.; Ezekowitz, J.; Mandal, R.; Han, B.; Bjorndahl, T.C.; Bouatra, S.; Anderson, T.; Oudit, G.Y.; Wishart, D.S. , et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One 2015, 10, e0124844. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E. , et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G. , et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med 2021, 27, 1954–1960. [Google Scholar] [CrossRef]
- Koleini, N.; Meddeb, M.; Zhao, L.; Keykhaei, M.; Kwon, S.; Farshidfar, F.; Hahn, V.S.; Pearce, E.L.; Sharma, K.; Kass, D.A. Landscape of glycolytic metabolites and their regulating proteins in myocardium from human heart failure with preserved ejection fraction. Eur J Heart Fail, 2024. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Deng, Y.; Yang, L.; Ou, W.; Xie, M.; Ding, L.; Jiang, C.; Yu, H.; Li, Q. , et al. Mitochondrial protein hyperacetylation underpins heart failure with preserved ejection fraction in mice. J Mol Cell Cardiol 2022, 165, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Fernández, A.; Fernández-Mora, Á.; Bernal-Ramírez, J.; Alves-Figueiredo, H.; Nieblas, B.; Salazar-Ramírez, F.; Maldonado-Ruiz, R.; Zazueta, C.; García, N.; Lozano, O. , et al. Distinguishing pathophysiological features of heart failure with reduced and preserved ejection fraction: A comparative analysis of two mouse models. J Physiol, 2024. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R. , et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y. , et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ Res 2021, 128, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Levitas, A.; Muhammad, E.; Harel, G.; Saada, A.; Caspi, V.C.; Manor, E.; Beck, J.C.; Sheffield, V.; Parvari, R. Familial neonatal isolated cardiomyopathy caused by a mutation in the flavoprotein subunit of succinate dehydrogenase. Eur J Hum Genet 2010, 18, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Van Vranken, J.G.; Bricker, D.K.; Dephoure, N.; Gygi, S.P.; Cox, J.E.; Thummel, C.S.; Rutter, J. SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration. Cell Metab 2014, 20, 241–252. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Cao, K.; Zeng, M.; Fu, X.; Zheng, A.; Zhang, F.; Gao, F.; Zou, X.; Li, H. , et al. Cardiac disruption of SDHAF4-mediated mitochondrial complex II assembly promotes dilated cardiomyopathy. Nat Commun 2022, 13, 3947. [Google Scholar] [CrossRef]
- Gibb, A.A.; Murray, E.K.; Eaton, D.M.; Huynh, A.T.; Tomar, D.; Garbincius, J.F.; Kolmetzky, D.W.; Berretta, R.M.; Wallner, M.; Houser, S.R. , et al. Molecular Signature of HFpEF: Systems Biology in a Cardiac-Centric Large Animal Model. JACC Basic Transl Sci 2021, 6, 650–672. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Silva, D.; Wüst, R.C.I.; Conceição, G.; Gonçalves-Rodrigues, P.; Gonçalves, N.; Gonçalves, A.; Kuster, D.W.D.; Leite-Moreira, A.F.; van der Velden, J.; de Sousa Beleza, J.M. , et al. Disturbed cardiac mitochondrial and cytosolic calcium handling in a metabolic risk-related rat model of heart failure with preserved ejection fraction. Acta Physiol (Oxf) 2020, 228, e13378. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Gjini, P.; Gopal, D.M.; Fetterman, J.L. Inefficient Batteries in Heart Failure: Metabolic Bottlenecks Disrupting the Mitochondrial Ecosystem. JACC Basic Transl Sci 2022, 7, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.S.; Knutsdottir, H.; Luo, X.; Bedi, K.; Margulies, K.B.; Haldar, S.M.; Stolina, M.; Yin, J.; Khakoo, A.Y.; Vaishnav, J. , et al. Myocardial Gene Expression Signatures in Human Heart Failure With Preserved Ejection Fraction. Circulation 2021, 143, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Tavori, H.; Pirillo, A.; Fazio, S.; Catapano, A.L. Biology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol lowering. Cardiovasc Res 2016, 112, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Da Dalt, L.; Castiglioni, L.; Baragetti, A.; Audano, M.; Svecla, M.; Bonacina, F.; Pedretti, S.; Uboldi, P.; Benzoni, P.; Giannetti, F. , et al. PCSK9 deficiency rewires heart metabolism and drives heart failure with preserved ejection fraction. Eur Heart J 2021, 42, 3078–3090. [Google Scholar] [CrossRef]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol 2015, 4, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat Rev Nephrol 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Suomalainen, A.; Battersby, B.J. Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol 2018, 19, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and Mitophagy in Cardiovascular Disease. Circ Res 2017, 120, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality control of the mitochondrion. Dev Cell 2021, 56, 881–905. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.; Katuri, J.; Liang, T.; Bai, Y. Mitochondrial chaperones in human health and disease. Free Radic Biol Med 2022, 179, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Craig, E.A. Heat-shock proteins as molecular chaperones. Eur J Biochem 1994, 219, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.T.; Ciechanover, A. The Ubiquitin Code in the Ubiquitin-Proteasome System and Autophagy. Trends Biochem Sci 2017, 42, 873–886. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem 2010, 47, 69–84. [Google Scholar] [CrossRef]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol Rev 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Gupta, S.; Kass, G.E.; Szegezdi, E.; Joseph, B. The mitochondrial death pathway: a promising therapeutic target in diseases. J Cell Mol Med 2009, 13, 1004–1033. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C. , et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Zong, H.; Ren, J.M.; Young, L.H.; Pypaert, M.; Mu, J.; Birnbaum, M.J.; Shulman, G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A 2002, 99, 15983–15987. [Google Scholar] [CrossRef] [PubMed]
- Virbasius, J.V.; Scarpulla, R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A 1994, 91, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Bedja, D.; Campbell, N.; Dunkerly, B.; Chenna, V.; Maitra, A.; Steenbergen, C. miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS One 2014, 9, e96820. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ferlito, M.; Kent, O.A.; Fox-Talbot, K.; Wang, R.; Liu, D.; Raghavachari, N.; Yang, Y.; Wheelan, S.J.; Murphy, E. , et al. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 2012, 110, 1596–1603. [Google Scholar] [CrossRef]
- Dogan, A.E.; Hamid, S.M.; Yildirim, A.D.; Yildirim, Z.; Sen, G.; Riera, C.E.; Gottlieb, R.A.; Erbay, E. PACT establishes a posttranscriptional brake on mitochondrial biogenesis by promoting the maturation of miR-181c. J Biol Chem 2022, 298, 102050. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.J.; Wang, C.J.; He, Y.; Zhou, Y.L.; Peng, X.D.; Liu, S.K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol Sin 2018, 39, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Büttner, S.; Schmidt, A.; van de Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K. , et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med 2011, 17, 1076–1085. [Google Scholar] [CrossRef]
- Martin, O.J.; Lai, L.; Soundarapandian, M.M.; Leone, T.C.; Zorzano, A.; Keller, M.P.; Attie, A.D.; Muoio, D.M.; Kelly, D.P. A role for peroxisome proliferator-activated receptor γ coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res 2014, 114, 626–636. [Google Scholar] [CrossRef]
- Lai, L.; Leone, T.C.; Zechner, C.; Schaeffer, P.J.; Kelly, S.M.; Flanagan, D.P.; Medeiros, D.M.; Kovacs, A.; Kelly, D.P. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev 2008, 22, 1948–1961. [Google Scholar] [CrossRef]
- Sihag, S.; Cresci, S.; Li, A.Y.; Sucharov, C.C.; Lehman, J.J. PGC-1α and ERRα target gene downregulation is a signature of the failing human heart. Journal of Molecular and Cellular Cardiology 2009, 46, 201–212. [Google Scholar] [CrossRef]
- Sun, C.-K.; Chang, L.-T.; Sheu, J.-J.; Wang, C.-Y.; Youssef, A.A.; Wu, C.-J.; Chua, S.; Yip, H.-K. Losartan Preserves Integrity of Cardiac Gap Junctions and PGC-1α Gene Expression and Prevents Cellular Apoptosis in Remote Area of Left Ventricular Myocardium Following Acute Myocardial Infarction. International Heart Journal 2007, 48, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Reusch, J.E.B.; McCune, S.A.; Leinwand, L.A.; Luckey, S.W.; Konhilas, J.P.; Brown, D.A.; Chicco, A.J.; Sparagna, G.C.; Long, C.S. , et al. Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. American Journal of Physiology-Heart and Circulatory Physiology 2007, 293, H246–H259. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.O.; Wende, A.R.; Crum, A.; Hunter, D.; Olsen, C.D.; Rawlings, T.; Riehle, C.; Ward, W.F.; Abel, E.D. Maintaining PGC-1α expression following pressure overload-induced cardiac hypertrophy preserves angiogenesis but not contractile or mitochondrial function. The FASEB Journal 2014, 28, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.K.; Mansfield, C.M.; Lehman, J.J.; Kovacs, A.; Courtois, M.; Saffitz, J.E.; Medeiros, D.M.; Valencik, M.L.; McDonald, J.A.; Kelly, D.P. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 2004, 94, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, X.; Lu, Z.; Zhang, P.; Fassett, J.; Zhang, Y.; Xin, Y.; Hall, J.L.; Viollet, B.; Bache, R.J. , et al. AMP Activated Protein Kinase-α2 Regulates Expression of Estrogen-Related Receptor-α, a Metabolic Transcription Factor Related to Heart Failure Development. Hypertension 2011, 58, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Chaanine, A.H.; Joyce, L.D.; Stulak, J.M.; Maltais, S.; Joyce, D.L.; Dearani, J.A.; Klaus, K.; Nair, K.S.; Hajjar, R.J.; Redfield, M.M. Mitochondrial Morphology, Dynamics, and Function in Human Pressure Overload or Ischemic Heart Disease With Preserved or Reduced Ejection Fraction. Circulation: Heart Failure 2019, 12, e005131. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu Rev Pathol 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Parra, V.; Verdejo, H.; del Campo, A.; Pennanen, C.; Kuzmicic, J.; Iglewski, M.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. The complex interplay between mitochondrial dynamics and cardiac metabolism. J Bioenerg Biomembr 2011, 43, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Atici, A.E.; Crother, T.R.; Noval Rivas, M. Mitochondrial quality control in health and cardiovascular diseases. Frontiers in Cell and Developmental Biology 2023, 11. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Olichon, A.; ElAchouri, G.; Baricault, L.; Delettre, C.; Belenguer, P.; Lenaers, G. OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death & Differentiation 2007, 14, 682–692. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. Journal of Cell Biology 2003, 160, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, H.; Fiket, M.; Alexander, C.; Chan, D.C. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. Journal of Cell Biology 2007, 178, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Elgass, K.; Pakay, J.; Ryan, M.T.; Palmer, C.S. Recent advances into the understanding of mitochondrial fission. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2013, 1833, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-B.; Hausenloy, D.J. Mitochondrial morphology and cardiovascular disease. Cardiovascular Research 2010, 88, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.; Dasgupta, A.; Chen, K.-H.; Mewburn, J.; Archer, Stephen L. The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease. Clinical Science 2016, 130, 1861–1874. [Google Scholar] [CrossRef]
- Otera, H.; Ishihara, N.; Mihara, K. New insights into the function and regulation of mitochondrial fission. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2013, 1833, 1256–1268. [Google Scholar] [CrossRef]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBenedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER Tubules Mark Sites of Mitochondrial Division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Gustafsson, Å.B. The role of mitochondrial fission in cardiovascular health and disease. Nature Reviews Cardiology 2022, 19, 723–736. [Google Scholar] [CrossRef]
- Zaffagnini, G.; Martens, S. Mechanisms of Selective Autophagy. Journal of Molecular Biology 2016, 428, 1714–1724. [Google Scholar] [CrossRef]
- Song, M.; Mihara, K.; Chen, Y.; Scorrano, L.; Dorn, Gerald W. , II. Mitochondrial Fission and Fusion Factors Reciprocally Orchestrate Mitophagic Culling in Mouse Hearts and Cultured Fibroblasts. Cell Metabolism 2015, 21, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Moore, X.-L.; Gao, X.-M.; Dart, A.M.; Lim, Y.L.; Du, X.-J. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sciences 2007, 80, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guo, Y.; Mi, L.; Wang, X.; Li, L.; Gao, W. Mitofusin 2 Inhibits Angiotensin II-Induced Myocardial Hypertrophy. Journal of Cardiovascular Pharmacology and Therapeutics 2011, 16, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, C.; Liu, J.; Wang, Z.; Liu, Y.; Luo, C.; Chen, Y.; Wen, S. Expression Profile of microRNAs in Hypertrophic Cardiomyopathy and Effects of microRNA-20 in Inducing Cardiomyocyte Hypertrophy Through Regulating Gene MFN2. DNA and Cell Biology 2019, 38, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Qin, X.; Xu, J.; Hou, Z.; Yang, H.; Mao, X.; Xing, W.; Li, X.; Zhang, X. , et al. Enhancing fatty acid utilization ameliorates mitochondrial fragmentation and cardiac dysfunction via rebalancing optic atrophy 1 processing in the failing heart. Cardiovascular Research 2018, 114, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Franco, A.; Fleischer, J.A.; Zhang, L.; Dorn, G.W., II. Abrogating Mitochondrial Dynamics in Mouse Hearts Accelerates Mitochondrial Senescence. Cell Metabolism 2017, 26, 872–883.e875. [Google Scholar] [CrossRef]
- Chaanine, A.H.; Joyce, L.D.; Stulak, J.M.; Maltais, S.; Joyce, D.L.; Dearani, J.A.; Klaus, K.; Nair, K.S.; Hajjar, R.J.; Redfield, M.M. Mitochondrial Morphology, Dynamics, and Function in Human Pressure Overload or Ischemic Heart Disease With Preserved or Reduced Ejection Fraction. Circulation: Heart Failure 2019, 12. [Google Scholar] [CrossRef]
- Molina, A.J.A.; Bharadwaj, M.S.; Van Horn, C.; Nicklas, B.J.; Lyles, M.F.; Eggebeen, J.; Haykowsky, M.J.; Brubaker, P.H.; Kitzman, D.W. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC: Heart Failure 2016, 4, 636–645. [Google Scholar] [CrossRef]
- Bode, D.; Wen, Y.; Hegemann, N.; Primessnig, U.; Parwani, A.; Boldt, L.-H.; M. Pieske, B.; R. Heinzel, F.; Hohendanner, F. Oxidative Stress and Inflammatory Modulation of Ca2+ Handling in Metabolic HFpEF-Related Left Atrial Cardiomyopathy. Antioxidants 2020, 9, 860. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Yao, W.; Li, L.; Niu, P.; Huo, Y.; Tan, W. Morphometric, Hemodynamic, and Multi-Omics Analyses in Heart Failure Rats with Preserved Ejection Fraction. International Journal of Molecular Sciences 2020, 21, 3362.e435. [Google Scholar] [CrossRef]
- McWilliams, T.G.; Prescott, A.R.; Montava-Garriga, L.; Ball, G.; Singh, F.; Barini, E.; Muqit, M.M.K.; Brooks, S.P.; Ganley, I.G. Basal Mitophagy Occurs Independently of PINK1 in Mouse Tissues of High Metabolic Demand. Cell Metabolism 2018, 27, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, M.; Wu, G.; Lou, J.; Feng, M.; Xu, J.; Zhou, J.; Zhang, P.; Yang, H.; Dong, L. , et al. Short-term but not long-term high fat diet feeding protects against pressure overload-induced heart failure through activation of mitophagy. Life Sciences 2021, 272, 119242. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Saito, T.; Zhai, P.; Oka, S.-i.; Mizushima, W.; Nakamura, M.; Ikeda, S.; Shirakabe, A.; Sadoshima, J. Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy. Circulation Research 2019, 124, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Bi, Y.; Zhao, Z.; Wang, S.; Lin, S.; Yang, Z.; Wang, X.; Mao, J. Emerging role of mitophagy in heart failure: from molecular mechanism to targeted therapy. Cell Cycle 2023, 22, 906–918. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Chiong, M.; Lavandero, S.; Klionsky, D.J.; Ren, J. Mitophagy in cardiovascular diseases: molecular mechanisms, pathogenesis, and treatment. Trends Mol Med 2022, 28, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza-Olivares, F.; Troncoso, M.F.; Pino-De La Fuente, F.; Martinez-Bilbao, J.; Riquelme, J.A.; Norambuena-Soto, I.; Villa, M.; Lavandero, S.; Castro, P.F.; Chiong, M. A potential role of autophagy-mediated vascular senescence in the pathophysiology of HFpEF. Frontiers in Endocrinology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Titus, A.S.; Sung, E.A.; Zablocki, D.; Sadoshima, J. Mitophagy for cardioprotection. Basic Res Cardiol 2023, 118, 42. [Google Scholar] [CrossRef]
- Rüb, C.; Wilkening, A.; Voos, W. Mitochondrial quality control by the Pink1/Parkin system. Cell and Tissue Research 2017, 367, 111–123. [Google Scholar] [CrossRef]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metabolism 2014, 19, 757–766. [Google Scholar] [CrossRef]
- Twig, G.; Shirihai, O.S. The Interplay Between Mitochondrial Dynamics and Mitophagy. Antioxidants & Redox Signaling 2010, 14, 1939–1951. [Google Scholar] [CrossRef]
- Kubli, D.A.; Gustafsson, Å.B. Mitochondria and Mitophagy. Circulation Research 2012, 111, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.E.; Arias-Durán, C.; Ávalos-Guajardo, Y.; Aedo, G.; Verdejo, H.E.; Parra, V.; Lavandero, S. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Aspects Med 2020, 71, 100822. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J. , et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–360. [Google Scholar] [CrossRef] [PubMed]
- Kassiotis, C.; Ballal, K.; Wellnitz, K.; Vela, D.; Gong, M.; Salazar, R.; Frazier, O.H.; Taegtmeyer, H. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation 2009, 120, S191–197. [Google Scholar] [CrossRef]
- Billia, F.; Hauck, L.; Konecny, F.; Rao, V.; Shen, J.; Mak, T.W. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A 2011, 108, 9572–9577. [Google Scholar] [CrossRef]
- Wang, B.; Nie, J.; Wu, L.; Hu, Y.; Wen, Z.; Dong, L.; Zou, M.H.; Chen, C.; Wang, D.W. AMPKα2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ Res 2018, 122, 712–729. [Google Scholar] [CrossRef] [PubMed]
- Summer, G.; Kuhn, A.R.; Munts, C.; Miranda-Silva, D.; Leite-Moreira, A.F.; Lourenço, A.P.; Heymans, S.; Falcão-Pires, I.; van Bilsen, M. A directed network analysis of the cardiome identifies molecular pathways contributing to the development of HFpEF. Journal of Molecular and Cellular Cardiology 2020, 144, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.D.; Houstis, N.; Yu, A.; Chang, B.; Yeri, A.; Li, H.; Hobson, R.; Lerchenmüller, C.; Vujic, A.; Chaudhari, V. , et al. Exercise training reverses cardiac aging phenotypes associated with heart failure with preserved ejection fraction in male mice. Aging Cell 2020, 19, e13159. [Google Scholar] [CrossRef]
- Shinmura, K.; Tamaki, K.; Sano, M.; Murata, M.; Yamakawa, H.; Ishida, H.; Fukuda, K. Impact of long-term caloric restriction on cardiac senescence: Caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. Journal of Molecular and Cellular Cardiology 2011, 50, 117–127. [Google Scholar] [CrossRef]
- Hoshino, A.; Mita, Y.; Okawa, Y.; Ariyoshi, M.; Iwai-Kanai, E.; Ueyama, T.; Ikeda, K.; Ogata, T.; Matoba, S. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nature Communications 2013, 4, 2308. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A. , et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nature Medicine 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Bou-Teen, D.; Kaludercic, N.; Weissman, D.; Turan, B.; Maack, C.; Di Lisa, F.; Ruiz-Meana, M. Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic Biol Med 2021, 167, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Gioscia-Ryan, R.; Yang, D.; Sutton, N.R.; Tyrrell, D.J. Cardiovascular aging: spotlight on mitochondria. Am J Physiol Heart Circ Physiol 2024, 326, H317–h333. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Redox-optimized ROS balance: A unifying hypothesis. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2010, 1797, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Donhauser, E.; Maske, T.; Bischof, C.; Dumitrescu, D.; Rudolph, V.; Klinke, A. Mitochondrial Integrity Is Critical in Right Heart Failure Development. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radical Biology and Medicine 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.B.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a Source of Reactive Oxygen and Nitrogen Species: From Molecular Mechanisms to Human Health. Antioxidants & Redox Signaling 2012, 18, 2029–2074. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiological Reviews 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bharathi, S.S.; Beck, M.E.; Goetzman, E.S. The fatty acid oxidation enzyme long-chain acyl-CoA dehydrogenase can be a source of mitochondrial hydrogen peroxide. Redox Biology 2019, 26, 101253. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Kakimoto, P.A.H.B.; Kowaltowski, A.J. Diet-Sensitive Sources of Reactive Oxygen Species in Liver Mitochondria: Role of Very Long Chain Acyl-CoA Dehydrogenases. PLOS ONE 2013, 8, e77088. [Google Scholar] [CrossRef]
- Kaludercic, N.; Carpi, A.; Menabò, R.; Di Lisa, F.; Paolocci, N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2011, 1813, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jin, Z.X.; Cai, J.; Li, R.; Deng, K.Q.; Ji, Y.X.; Lei, F.; Li, H.P.; Lu, Z.; Li, H. Energy substrate metabolism and oxidative stress in metabolic cardiomyopathy. J Mol Med (Berl) 2022, 100, 1721–1739. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nature Reviews Gastroenterology & Hepatology 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Boudina, S.; Sena, S.; O’Neill, B.T.; Tathireddy, P.; Young, M.E.; Abel, E.D. Reduced Mitochondrial Oxidative Capacity and Increased Mitochondrial Uncoupling Impair Myocardial Energetics in Obesity. Circulation 2005, 112, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Bugger, H.; Sena, S.; O’Neill, B.T.; Zaha, V.G.; Ilkun, O.; Wright, J.J.; Mazumder, P.K.; Palfreyman, E.; Tidwell, T.J. , et al. Contribution of Impaired Myocardial Insulin Signaling to Mitochondrial Dysfunction and Oxidative Stress in the Heart. Circulation 2009, 119, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Simões, I.C.M.; Fontes, A.; Pinton, P.; Zischka, H.; Wieckowski, M.R. Mitochondria in non-alcoholic fatty liver disease. The International Journal of Biochemistry & Cell Biology 2018, 95, 93–99. [Google Scholar] [CrossRef]
- Muftuoglu, M.; Mori, M.P.; Souza-Pinto, N.C.d. Formation and repair of oxidative damage in the mitochondrial DNA. Mitochondrion 2014, 17, 164–181. [Google Scholar] [CrossRef]
- Kawahara, H.; Fukura, M.; Tsuchishima, M.; Takase, S. Mutation of Mitochondrial DNA in Livers From Patients With Alcoholic Hepatitis and Nonalcoholic Steatohepatitis. Alcoholism: Clinical and Experimental Research 2007, 31, S54–S60. [Google Scholar] [CrossRef]
- Pohjoismäki, J.L.O.; Goffart, S.; Tyynismaa, H.; Willcox, S.; Ide, T.; Kang, D.; Suomalainen, A.; Karhunen, P.J.; Griffith, J.D.; Holt, I.J. , et al. Human Heart Mitochondrial DNA Is Organized in Complex Catenated Networks Containing Abundant Four-way Junctions and Replication Forks *<sup> </sup>. Journal of Biological Chemistry 2009, 284, 21446–21457. [Google Scholar] [CrossRef]
- Frahm, T.; Mohamed, S.A.; Bruse, P.; Gemünd, C.; Oehmichen, M.; Meissner, C. Lack of age-related increase of mitochondrial DNA amount in brain, skeletal muscle and human heart. Mechanisms of Ageing and Development 2005, 126, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wilhelmsson, H.; Graff, C.; Li, H.; Oldfors, A.; Rustin, P.; Brüning, J.C.; Kahn, C.R.; Clayton, D.A.; Barsh, G.S. , et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nature Genetics 1999, 21, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Wilhelmsson, H.; Hansson, A.; Thorén, P.; Duffy, J.; Rustin, P.; Larsson, N.-G. Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proceedings of the National Academy of Sciences 2000, 97, 3467–3472. [Google Scholar] [CrossRef] [PubMed]
- Marin-Garcia, J.; Goldenthal, M.J.; Moe, G.W. Mitochondrial pathology in cardiac failure. Cardiovascular Research 2001, 49, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Elorza, A.A.; Soffia, J.P. mtDNA Heteroplasmy at the Core of Aging-Associated Heart Failure. An Integrative View of OXPHOS and Mitochondrial Life Cycle in Cardiac Mitochondrial Physiology. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef]
- Lakshmanan, L.N.; Yee, Z.; Ng, L.F.; Gunawan, R.; Halliwell, B.; Gruber, J. Clonal expansion of mitochondrial DNA deletions is a private mechanism of aging in long-lived animals. Aging Cell 2018, 17, e12814. [Google Scholar] [CrossRef]
- Tuppen, H.A.L.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA mutations and human disease. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2010, 1797, 113–128. [Google Scholar] [CrossRef]
- Li, H.; Slone, J.; Fei, L.; Huang, T. Mitochondrial DNA Variants and Common Diseases: A Mathematical Model for the Diversity of Age-Related mtDNA Mutations. Cells 2019, 8, 608. [Google Scholar] [CrossRef]
- Li, M.; Schönberg, A.; Schaefer, M.; Schroeder, R.; Nasidze, I.; Stoneking, M. Detecting Heteroplasmy from High-Throughput Sequencing of Complete Human Mitochondrial DNA Genomes. The American Journal of Human Genetics 2010, 87, 237–249. [Google Scholar] [CrossRef]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Roy, P.; Cifani, C.; Amenta, F.; Tayebati, S.K. Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats. International Journal of Molecular Sciences 2020, 21, 2035. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radical Biology and Medicine 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Romestaing, C.; Han, X.; Li, Y.; Hao, X.; Wu, Y.; Sun, C.; Liu, X.; Jefferson, L.S.; Xiong, J. , et al. Cardiolipin Remodeling by ALCAT1 Links Oxidative Stress and Mitochondrial Dysfunction to Obesity. Cell Metabolism 2010, 12, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Shah, A.K.; Tappia, P.S. Role of Oxidative Stress in Metabolic and Subcellular Abnormalities in Diabetic Cardiomyopathy. International Journal of Molecular Sciences 2020, 21, 2413. [Google Scholar] [CrossRef] [PubMed]
- Tappia, S.P.; Adameova, A.; Dhalla, S.N. Attenuation of Diabetes-induced Cardiac and Subcellular Defects by Sulphur-containing Amino Acids. Current Medicinal Chemistry 2018, 25, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Gill, E.K.; Abudalo, R.A.; Edgar, K.S.; Watson, C.J.; Grieve, D.J. Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart 2018, 104, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 2007, 6, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hekimi, S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 2010, 8, e1000556. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.; Song, W.; Perrimon, N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 2013, 155, 699–712. [Google Scholar] [CrossRef]
- Pan, Y.; Schroeder, E.A.; Ocampo, A.; Barrientos, A.; Shadel, G.S. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab 2011, 13, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Zarse, K.; Schmeisser, S.; Groth, M.; Priebe, S.; Beuster, G.; Kuhlow, D.; Guthke, R.; Platzer, M.; Kahn, C.R.; Ristow, M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab 2012, 15, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Hwang, A.B.; Kenyon, C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol 2010, 20, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Roy, R. Increased levels of hydrogen peroxide induce a HIF-1-dependent modification of lipid metabolism in AMPK compromised C. elegans dauer larvae. Cell Metab 2012, 16, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Otani, H. Reactive Oxygen Species as Mediators of Signal Transduction in Ischemic Preconditioning. Antioxidants & Redox Signaling 2004, 6, 449–469. [Google Scholar] [CrossRef]
- Antonucci, S.; Mulvey, J.F.; Burger, N.; Di Sante, M.; Hall, A.R.; Hinchy, E.C.; Caldwell, S.T.; Gruszczyk, A.V.; Deshwal, S.; Hartley, R.C. , et al. Selective mitochondrial superoxide generation in vivo is cardioprotective through hormesis. Free Radical Biology and Medicine 2019, 134, 678–687. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by mitochondrial oxidants. J Biol Chem 2012, 287, 4434–4440. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Embo j 2019, 38. [Google Scholar] [CrossRef]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 1998, 95, 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Honda, S.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Paek, J.; Lo, J.Y.; Narasimhan, S.D.; Nguyen, T.N.; Glover-Cutter, K.; Robida-Stubbs, S.; Suzuki, T.; Yamamoto, M.; Blackwell, T.K.; Curran, S.P. Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metab 2012, 16, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama 2007, 297, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Rubens, M.; Saxena, A.; Shehadeh, N. Selenium and vitamin E for prostate cancer--justifications for the SELECT study. Asian Pac J Cancer Prev 2015, 16, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Lonn, E.; Bosch, J.; Yusuf, S.; Sheridan, P.; Pogue, J.; Arnold, J.M.; Ross, C.; Arnold, A.; Sleight, P.; Probstfield, J. , et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama 2005, 293, 1338–1347. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Rass, U.; Ahel, I.; West, S.C. Defective DNA repair and neurodegenerative disease. Cell 2007, 130, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.; Krogvold, L.; Zhitomirsky, S.; Swisa, A.; Fischman, M.; Lax, T.; Dahan, T.; Hurvitz, N.; Weinberg-Corem, N.; Klochendler, A. , et al. β-Cell DNA Damage Response Promotes Islet Inflammation in Type 1 Diabetes. Diabetes 2018, 67, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Schroyer, A.L.; Stimes, N.W.; Abi Saab, W.F.; Chadee, D.N. MLK3 phosphorylation by ERK1/2 is required for oxidative stress-induced invasion of colorectal cancer cells. Oncogene 2018, 37, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Simpson, C.M.; Berner, J.; Chong, H.B.; Fang, J.; Ordulu, Z.; Weiss-Sadan, T.; Possemato, A.P.; Harry, S.; Takahashi, M. , et al. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell 2023, 186, 2361–2379. [Google Scholar] [CrossRef]
- van Soest, D.M.K.; Polderman, P.E.; den Toom, W.T.F.; Keijer, J.P.; van Roosmalen, M.J.; Leyten, T.M.F.; Lehmann, J.; Zwakenberg, S.; De Henau, S.; van Boxtel, R. , et al. Mitochondrial H(2)O(2) release does not directly cause damage to chromosomal DNA. Nat Commun 2024, 15, 2725. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxidative Medicine and Cellular Longevity 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Deng, J.; Liu, X.; Li, Q.; Zhang, J.; Bai, H.; Zhang, J. Echinacoside reverses myocardial remodeling and improves heart function via regulating SIRT1/FOXO3a/MnSOD axis in HF rats induced by isoproterenol. J Cell Mol Med 2021, 25, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W.; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N. , et al. Mitochondrial Function, Biology, and Role in Disease. Circulation Research 2016, 118, 1960–1991. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A 2015, 112, 11389–11394. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Liu, X.; Wang, W. Regulation of metabolism in individual mitochondria during excitation-contraction coupling. J Mol Cell Cardiol 2014, 76, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Pytel, G.; Schrepper, A.; Amorim, P.; Färber, G.; Shingu, Y.; Mohr, F.W.; Schwarzer, M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovascular Research 2009, 86, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.M.; Thapa, D.; Shepherd, D.L. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. American Journal of Physiology-Heart and Circulatory Physiology 2014, 307, H1–H14. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. The Journal of Clinical Investigation 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S.; Aragonés, J.; Landázuri, M.O. Mitochondrial reprogramming through cardiac oxygen sensors in ischaemic heart disease. Cardiovascular Research 2010, 88, 219–228. [Google Scholar] [CrossRef]
- Cesselli, D.; Jakoniuk, I.; Barlucchi, L.; Beltrami, A.P.; Hintze, T.H.; Nadal-Ginard, B.; Kajstura, J.; Leri, A.; Anversa, P. Oxidative Stress–Mediated Cardiac Cell Death Is a Major Determinant of Ventricular Dysfunction and Failure in Dog Dilated Cardiomyopathy. Circulation Research 2001, 89, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Barlaka, E.; Görbe, A.; Gáspár, R.; Pálóczi, J.; Ferdinandy, P.; Lazou, A. Activation of PPARβ/δ protects cardiac myocytes from oxidative stress-induced apoptosis by suppressing generation of reactive oxygen/nitrogen species and expression of matrix metalloproteinases. Pharmacological Research 2015, 95-96, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Laviola, L.; Leonardini, A.; Melchiorre, M.; Orlando, M.R.; Peschechera, A.; Bortone, A.; Paparella, D.; Natalicchio, A.; Perrini, S.; Giorgino, F. Glucagon-Like Peptide-1 Counteracts Oxidative Stress-Dependent Apoptosis of Human Cardiac Progenitor Cells by Inhibiting the Activation of the c-Jun N-terminal Protein Kinase Signaling Pathway. Endocrinology 2012, 153, 5770–5781. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; D’Oria, R.; Incalza, M.A.; Caccioppoli, C.; Andrulli Buccheri, V.; Cignarelli, A.; Paparella, D.; Margari, V.; Natalicchio, A.; Perrini, S. , et al. GLP-1 Receptor Activation Inhibits Palmitate-Induced Apoptosis via Ceramide in Human Cardiac Progenitor Cells. The Journal of Clinical Endocrinology & Metabolism 2017, 102, 4136–4147. [Google Scholar] [CrossRef]
- Sawyer, D.B. Oxidative Stress in Heart Failure: What Are We Missing? The American Journal of the Medical Sciences 2011, 342, 120–124. [Google Scholar] [CrossRef]
- Sam, F.; Kerstetter, D.L.; Pimental, D.R.; Mulukutla, S.; Tabaee, A.; Bristow, M.R.; Colucci, W.S.; Sawyer, D.B. Increased Reactive Oxygen Species Production and Functional Alterations in Antioxidant Enzymes in Human Failing Myocardium. Journal of Cardiac Failure 2005, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Coskun, P.; Patel, M.; Tuinstra, R.; Cottrell, B.; Jun, A.S.; Zastawny, T.H.; Dizdaroglu, M.; Goodman, S.I.; Huang, T.-T. , et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proceedings of the National Academy of Sciences 1999, 96, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Doctrow, S.R.; Schneider, J.A.; Haberson, J.; Patel, M.; Coskun, P.E.; Huffman, K.; Wallace, D.C.; Malfroy, B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci 2001, 21, 8348–8353. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; DeMazumder, D.; Sidor, A.; Foster, D.B.; O’Rourke, B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circulation Research 2018, 123, 356–371. [Google Scholar] [CrossRef]
- Ago, T.; Liu, T.; Zhai, P.; Chen, W.; Li, H.; Molkentin, J.D.; Vatner, S.F.; Sadoshima, J. A Redox-Dependent Pathway for Regulating Class II HDACs and Cardiac Hypertrophy. Cell 2008, 133, 978–993. [Google Scholar] [CrossRef]
- Wei, J.; Joshi, S.; Speransky, S.; Crowley, C.; Jayathilaka, N.; Lei, X.; Wu, Y.; Gai, D.; Jain, S.; Hoosien, M. , et al. Reversal of pathological cardiac hypertrophy via the MEF2-coregulator interface. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Oudot, A.; Vergely, C.; Ecarnot-Laubriet, A.; Rochette, L. Angiotensin II activates NADPH oxidase in isolated rat hearts subjected to ischaemia–reperfusion. European Journal of Pharmacology 2003, 462, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lyamzaev, K.G.; Panteleeva, A.A.; Simonyan, R.A.; Avetisyan, A.V.; Chernyak, B.V. Mitochondrial Lipid Peroxidation Is Responsible for Ferroptosis. Cells 2023, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Dixon, Scott J. ; Lemberg, Kathryn M.; Lamprecht, Michael R.; Skouta, R.; Zaitsev, Eleina M.; Gleason, Caroline E.; Patel, Darpan N.; Bauer, Andras J.; Cantley, Alexandra M.; Yang, Wan S., et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Jang, S.; Chapa-Dubocq, X.R.; Tyurina, Y.Y.; St Croix, C.M.; Kapralov, A.A.; Tyurin, V.A.; Bayır, H.; Kagan, V.E.; Javadov, S. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biology 2021, 45, 102021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nature Reviews Molecular Cell Biology 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, K.; Gao, C.; Ma, W.; Liu, M.; Guo, X.; Bao, G.; Han, B.; Hu, H.; Zhao, Z. Activation of FMS-like tyrosine kinase 3 protects against isoprenaline-induced cardiac hypertrophy by improving autophagy and mitochondrial dynamics. FASEB Journal 2022, 36. [Google Scholar] [CrossRef]
- Mercer, J.R.; Cheng, K.K.; Figg, N.; Gorenne, I.; Mahmoudi, M.; Griffin, J.; Vidal-Puig, A.; Logan, A.; Murphy, M.P.; Bennett, M. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome. Circulation Research 2010, 107, 1021–1031. [Google Scholar] [CrossRef]
- Xu, H.-x.; Cui, S.-m.; Zhang, Y.-m.; Ren, J. Mitochondrial Ca2+ regulation in the etiology of heart failure: physiological and pathophysiological implications. Acta Pharmacologica Sinica 2020, 41, 1301–1309. [Google Scholar] [CrossRef]
- Luan, Y.; Ren, K.D.; Luan, Y.; Chen, X.; Yang, Y. Mitochondrial Dynamics: Pathogenesis and Therapeutic Targets of Vascular Diseases. Frontiers in Cardiovascular Medicine 2021, 8. [Google Scholar] [CrossRef]
- Luan, Y.; Luan, Y.; Feng, Q.; Chen, X.; Ren, K.D.; Yang, Y. Emerging Role of Mitophagy in the Heart: Therapeutic Potentials to Modulate Mitophagy in Cardiac Diseases. Oxidative Medicine and Cellular Longevity 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q. , et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circulation Research 2020, 127, 486–501. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, B.; Han, X. The role of autophagy in angiotensin II-induced pathological cardiac hypertrophy. Journal of Molecular Endocrinology 2016, 57, R143–R152. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Chen, M.; Chen, M.; Cui, C.; Zhu, Y.; Luo, X.; Wu, B. Nr2f2 Overexpression Aggravates Ferroptosis and Mitochondrial Dysfunction by Regulating the PGC-1 α Signaling in Diabetes-Induced Heart Failure Mice. Mediators of Inflammation 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Fan, X.; Xu, D.; Zhao, L.; Zhang, B.; Li, X. Deciphering the mitochondria-inflammation axis: Insights and therapeutic strategies for heart failure. International Immunopharmacology 2024, 139, 112697. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.; Ahuja, S.; Rani, V. Transcription Factors in Heart: Promising Therapeutic Targets in Cardiac Hypertrophy. Current Cardiology Reviews 2011, 7, 262–271. [Google Scholar] [CrossRef]
- Pu, W.T.; Ma, Q.; Izumo, S. NFAT Transcription Factors Are Critical Survival Factors That Inhibit Cardiomyocyte Apoptosis During Phenylephrine Stimulation In Vitro. Circulation Research 2003, 92, 725–731. [Google Scholar] [CrossRef]
- Qiu, Z.; Fan, Y.; Wang, Z.; Huang, F.; Li, Z.; Sun, Z.; Hua, S.; Jin, W.; Chen, Y. Catestatin Protects Against Diastolic Dysfunction by Attenuating Mitochondrial Reactive Oxygen Species Generation. J Am Heart Assoc 2023, 12, e029470. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Kim, S.Y.; Tong, D.; Ferdous, A.; Piristine, H.; Dasgupta, S.; Wang, X.; French, K.M.; Villalobos, E. , et al. Xbp1s-FoxO1 axis governs lipid accumulation and contractile performance in heart failure with preserved ejection fraction. Nat Commun 2021, 12, 1684. [Google Scholar] [CrossRef]
- Shirakawa, R.; Yokota, T.; Nakajima, T.; Takada, S.; Yamane, M.; Furihata, T.; Maekawa, S.; Nambu, H.; Katayama, T.; Fukushima, A. , et al. Mitochondrial reactive oxygen species generation in blood cells is associated with disease severity and exercise intolerance in heart failure patients. Scientific Reports 2019, 9. [Google Scholar] [CrossRef]
- Negi, S.I.; Jeong, E.-M.; Shukrullah, I.; Veleder, E.; Jones, D.P.; Fan, T.-H.M.; Varadarajan, S.; Danilov, S.M.; Fukai, T.; Dudley Jr., S. C. Renin-Angiotensin Activation and Oxidative Stress in Early Heart Failure with Preserved Ejection Fraction. BioMed Research International 2015, 2015, 825027. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. Nitric oxide and mitochondrial respiration in the heart. Cardiovascular Research 2007, 75, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Mital, S.; Loke, K.E.; Addonizio, L.J.; Oz, M.C.; Hintze, T.H. Left ventricular assist device implantation augments nitric oxide dependent control of mitochondrial respiration in failing human hearts. J Am Coll Cardiol 2000, 36, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Xu, H.; DeVore, A.D.; Schulte, P.J.; Butler, J.; Yancy, C.W.; Bhatt, D.L.; Hernandez, A.F.; Heidenreich, P.A.; Fonarow, G.C. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: Findings from Get With The Guidelines-Heart Failure registry. Am Heart J 2016, 182, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.; Tay, W.T.; Teng, T.K.; Anand, I.; Richards, A.M.; Ling, L.H.; MacDonald, M.R.; Chandramouli, C.; Tromp, J.; Siswanto, B.B. , et al. Association of Diabetes Mellitus on Cardiac Remodeling, Quality of Life, and Clinical Outcomes in Heart Failure With Reduced and Preserved Ejection Fraction. J Am Heart Assoc 2019, 8, e013114. [Google Scholar] [CrossRef]
- Schleicher, E.; Friess, U. Oxidative stress, AGE, and atherosclerosis. Kidney International 2007, 72, S17–S26. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metabolism 2018, 28, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kho, A.L.; Anilkumar, N.; Chibber, R.; Pagano, P.J.; Shah, A.M.; Cave, A.C. Glycated Proteins Stimulate Reactive Oxygen Species Production in Cardiac Myocytes. Circulation 2006, 113, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Maack, C.; Lehrke, M.; Backs, J.; Heinzel, F.R.; Hulot, J.-S.; Marx, N.; Paulus, W.J.; Rossignol, P.; Taegtmeyer, H.; Bauersachs, J. , et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the Translational Research Committee of the Heart Failure Association–European Society of Cardiology. European Heart Journal 2018, 39, 4243–4254. [Google Scholar] [CrossRef]
- Kasner, M.; Aleksandrov, A.S.; Westermann, D.; Lassner, D.; Gross, M.; von Haehling, S.; Anker, S.D.; Schultheiss, H.-P.; Tschöpe, C. Functional iron deficiency and diastolic function in heart failure with preserved ejection fraction. International Journal of Cardiology 2013, 168, 4652–4657. [Google Scholar] [CrossRef]
- Bates, A.; Shen, Q.; Hiebert, J.B.; Thimmesch, A.; Pierce, J.D. Myocardial energetics and ubiquinol in diastolic heart failure. Nursing & Health Sciences 2014, 16, 428–433. [Google Scholar] [CrossRef]
- Luptak, I.; Qin, F.; Sverdlov, A.L.; Pimentel, D.R.; Panagia, M.; Croteau, D.; Siwik, D.A.; Bachschmid, M.M.; He, H.; Balschi, J.A. , et al. Energetic Dysfunction Is Mediated by Mitochondrial Reactive Oxygen Species and Precedes Structural Remodeling in Metabolic Heart Disease. Antioxidants & Redox Signaling 2019, 31, 539–549. [Google Scholar] [CrossRef]
- Hu, Q.; Wood, C.R.; Cimen, S.; Venkatachalam, A.B.; Alwayn, I.P.J. Mitochondrial Damage-Associated Molecular Patterns (MTDs) Are Released during Hepatic Ischemia Reperfusion and Induce Inflammatory Responses. PLOS ONE 2015, 10, e0140105. [Google Scholar] [CrossRef]
- Zhao, C.; Itagaki, K.; Gupta, A.; Odom, S.; Sandler, N.; Hauser, C.J. Mitochondrial damage-associated molecular patterns released by abdominal trauma suppress pulmonary immune responses. J Trauma Acute Care Surg 2014, 76, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Pollara, J.; Edwards, R.W.; Lin, L.; Bendersky, V.A.; Brennan, T.V. Circulating mitochondria in deceased organ donors are associated with immune activation and early allograft dysfunction. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nature Reviews Immunology 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Unterreiner, A.; Rubert, J.; Kauffmann, M.; Fruhauf, A.; Heiser, D.; Erbel, P.; Schlapbach, A.; Eder, J.; Bodendorf, U.; Boettcher, A. , et al. Pharmacological inhibition of IKKβ dampens NLRP3 inflammasome activation after priming in the human myeloid cell line THP-1. Biochemical and Biophysical Research Communications 2021, 545, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hulina, A.; Grdić Rajković, M.; Jakšić Despot, D.; Jelić, D.; Dojder, A.; Čepelak, I.; Rumora, L. Extracellular Hsp70 induces inflammation and modulates LPS/LTA-stimulated inflammatory response in THP-1 cells. Cell Stress and Chaperones 2018, 23, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Leung, J.C.K.; Yiu, W.H.; Chan, L.Y.Y.; Li, B.; Lok, S.W.Y.; Xue, R.; Zou, Y.; Lai, K.N.; Tang, S.C.W. Tubular β-catenin alleviates mitochondrial dysfunction and cell death in acute kidney injury. Cell Death & Disease 2022, 13, 1061. [Google Scholar] [CrossRef]
- Ummarino, D. Mitochondrial calcium efflux essential for heart function. Nature Reviews Cardiology 2017, 14, 317–317. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Luan, Y.; Jiao, Y.; Liu, H.; Huang, Z.; Feng, Q.; Pei, J.; Yang, Y.; Ren, K. Broadening Horizons: Exploring mtDAMPs as a Mechanism and Potential Intervention Target in Cardiovascular Diseases. Aging Dis, 2023. [Google Scholar] [CrossRef]
- Silvis, M.J.M.; Kaffka Genaamd Dengler, S.E.; Odille, C.A.; Mishra, M.; van der Kaaij, N.P.; Doevendans, P.A.; Sluijter, J.P.G.; de Kleijn, D.P.V.; de Jager, S.C.A.; Bosch, L. , et al. Damage-Associated Molecular Patterns in Myocardial Infarction and Heart Transplantation: The Road to Translational Success. Front Immunol 2020, 11, 599511. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Shah, S.J.; Butler, J.; Kellogg, D.L., Jr.; Lewis, G.D.; Forman, D.E.; Mentz, R.J.; Borlaug, B.A.; Simon, M.A.; Chirinos, J.A. , et al. Exercise Intolerance in Older Adults With Heart Failure With Preserved Ejection Fraction: JACC State-of-the-Art Review. J Am Coll Cardiol 2021, 78, 1166–1187. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R. , et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Obokata, M.; Testani, J.M.; Felker, G.M.; Tang, W.H.W.; Abou-Ezzeddine, O.F.; Sun, J.L.; Chakrabothy, H.; McNulty, S.; Shah, S.J. , et al. Adverse Renal Response to Decongestion in the Obese Phenotype of Heart Failure With Preserved Ejection Fraction. J Card Fail 2020, 26, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F. , et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Rocca, H.-P.B.L.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E. , et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. New England Journal of Medicine 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F. , et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M. , et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J. , et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Obesity-Associated Heart Failure as a Theoretical Target for Treatment With Mineralocorticoid Receptor Antagonists. JAMA Cardiol 2018, 3, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ricchiuti, V.; Lian, B.Q.; Yao, T.M.; Coutinho, P.; Romero, J.R.; Li, J.; Williams, G.H.; Adler, G.K. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 2008, 117, 2253–2261. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L. , et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I. , et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B. , et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Jackson, A.M.; Lam, C.S.P.; Redfield, M.M.; Anand, I.S.; Ge, J.; Lefkowitz, M.P.; Maggioni, A.P.; Martinez, F.; Packer, M. , et al. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation 2020, 141, 338–351. [Google Scholar] [CrossRef]
- Chyou, J.Y.; Qin, H.; Butler, J.; Voors, A.A.; Lam, C.S.P. Sex-related similarities and differences in responses to heart failure therapies. Nature Reviews Cardiology 2024, 21, 498–516. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Panjrath, G.S.; Amancherla, K.; Davis, L.L.; Deswal, A.; Dixon, D.L.; Januzzi, J.L., Jr.; Yancy, C.W. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023, 81, 1835–1878. [Google Scholar] [CrossRef]
- Yusuf, S.; Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; McMurray, J.J.; Michelson, E.L.; Olofsson, B.; Ostergren, J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003, 362, 777–781. [Google Scholar] [CrossRef]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C. , et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008, 359, 2456–2467. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Bunting, K.V.; Flather, M.D.; Altman, D.G.; Holmes, J.; Coats, A.J.S.; Manzano, L.; McMurray, J.J.V.; Ruschitzka, F.; van Veldhuisen, D.J. , et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018, 39, 26–35. [Google Scholar] [CrossRef]
- Palau, P.; de la Espriella, R.; Seller, J.; Santas, E.; Domínguez, E.; Bodí, V.; Sanchis, J.; Núñez, E.; Bayés-Genís, A.; Bertomeu-González, V. , et al. β-Blocker Withdrawal and Functional Capacity Improvement in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol 2024, 9, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Kaddoura, R.; Madurasinghe, V.; Chapra, A.; Abushanab, D.; Al-Badriyeh, D.; Patel, A. Beta-blocker therapy in heart failure with preserved ejection fraction (B-HFpEF): A systematic review and meta-analysis. Curr Probl Cardiol 2024, 49, 102376. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Jin, Y.; Zhang, P.; Li, H.; Yang, Y. Mitochondria-associated endoplasmic reticulum membranes and cardiac hypertrophy: Molecular mechanisms and therapeutic targets. Front Cardiovasc Med 2022, 9, 1015722. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Ren, K.D.; Luan, Y.; Chen, X.; Yang, Y. Mitochondrial Dynamics: Pathogenesis and Therapeutic Targets of Vascular Diseases. Front Cardiovasc Med 2021, 8, 770574. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Nikiforov, N.G.; Starodubova, A.V.; Popkova, T.V.; Orekhov, A.N. The Role of Mitochondria-Derived Peptides in Cardiovascular Diseases and Their Potential as Therapeutic Targets. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.H.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Birk, A.V.; Chao, W.M.; Bracken, C.; Warren, J.D.; Szeto, H.H. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. British Journal of Pharmacology 2014, 171, 2017–2028. [Google Scholar] [CrossRef]
- Eirin, A.; Ebrahimi, B.; Kwon, S.H.; Fiala, J.A.; Williams, B.J.; Woollard, J.R.; He, Q.; Gupta, R.C.; Sabbah, H.N.; Prakash, Y.S. , et al. Restoration of Mitochondrial Cardiolipin Attenuates Cardiac Damage in Swine Renovascular Hypertension. Journal of the American Heart Association 2016, 5, e003118. [Google Scholar] [CrossRef]
- Dai, D.-F.; Chen, T.; Szeto, H.; Nieves-Cintrón, M.; Kutyavin, V.; Santana, L.F.; Rabinovitch, P.S. Mitochondrial Targeted Antioxidant Peptide Ameliorates Hypertensive Cardiomyopathy. Journal of the American College of Cardiology 2011, 58, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, M.; Wang, X.; Zhang, H.; Ding, J.; Cheng, Z.; Qian, L. Peptide Szeto-Schiller 31 ameliorates doxorubicin-induced cardiotoxicity by inhibiting the activation of the p38 MAPK signaling pathway. Int J Mol Med 2021, 47, 63. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dai, W.; Hale, S.L.; Brown, D.A.; Wang, M.; Han, X.; Kloner, R.A. Bendavia restores mitochondrial energy metabolism gene expression and suppresses cardiac fibrosis in the border zone of the infarcted heart. Life Sciences 2015, 141, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Shi, J.; Gupta, R.C.; Sabbah, H.N.; Hale, S.L.; Kloner, R.A. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. Journal of Cardiovascular Pharmacology 2014, 64, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N.; Gupta, R.C.; Kohli, S.; Wang, M.; Hachem, S.; Zhang, K. Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circulation: Heart Failure 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, K.C.; Sparagna, G.C.; Chau, S.; Phillips, E.K.; Ambardekar, A.V.; Aftab, M.; Mitchell, M.B.; Sucharov, C.C.; Miyamoto, S.D.; Stauffer, B.L. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC: Basic to Translational Science 2019, 4, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Khan, M.S.; Anker, S.D.; Fonarow, G.C.; Kim, R.J.; Nodari, S.; O’Connor, C.M.; Pieske, B.; Pieske-Kraigher, E.; Sabbah, H.N. , et al. Effects of Elamipretide on Left Ventricular Function in Patients With Heart Failure With Reduced Ejection Fraction: The PROGRESS-HF Phase 2 Trial. Journal of Cardiac Failure 2020, 26, 429–437. [Google Scholar] [CrossRef]
- Gibson, C.M.; Giugliano, R.P.; Kloner, R.A.; Bode, C.; Tendera, M.; Jánosi, A.; Merkely, B.; Godlewski, J.; Halaby, R.; Korjian, S. , et al. EMBRACE STEMI study: A Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. European Heart Journal 2016, 37, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L.; Hatefi, Y.; Lester, R.L.; Widmer, C. Isolation of a quinone from beef heart mitochondria. Biochimica et Biophysica Acta 1957, 25, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Baggio, E.; Gandini, R.; Plancher, A.C.; Passeri, M.; Carmosino, G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. Molecular Aspects of Medicine 1994, 15, s287–s294. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The Effect of Coenzyme Q10 on Morbidity and Mortality in Chronic Heart Failure: Results From Q-SYMBIO: A Randomized Double-Blind Trial. JACC: Heart Failure 2014, 2, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. International Journal of Cardiology 2013, 167, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Johansson, P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLOS ONE 2018, 13, e0193120. [Google Scholar] [CrossRef] [PubMed]
- Botting, K.J.; Skeffington, K.L.; Niu, Y.; Allison, B.J.; Brain, K.L.; Itani, N.; Beck, C.; Logan, A.; Murray, A.J.; Murphy, M.P. , et al. Translatable mitochondria-targeted protection against programmed cardiovascular dysfunction. Science Advances 2020, 6, eabb1929. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C. , et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biology 2017, 11, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Pak, O.; Scheibe, S.; Esfandiary, A.; Gierhardt, M.; Sydykov, A.; Logan, A.; Fysikopoulos, A.; Veit, F.; Hecker, M.; Kroschel, F. , et al. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. European Respiratory Journal 2018, 51, 1701024. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Sun, Q.; Zhong, W.; Zhang, W.; Sun, X.; Zhou, Z. Mitochondria-targeted ubiquinone (MitoQ) enhances acetaldehyde clearance by reversing alcohol-induced posttranslational modification of aldehyde dehydrogenase 2: A molecular mechanism of protection against alcoholic liver disease. Redox Biology 2018, 14, 626–636. [Google Scholar] [CrossRef]
- Goh, K.Y.; He, L.; Song, J.; Jinno, M.; Rogers, A.J.; Sethu, P.; Halade, G.V.; Rajasekaran, N.S.; Liu, X.; Prabhu, S.D. , et al. Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice. Redox Biology 2019, 21, 101100. [Google Scholar] [CrossRef]
- Kim, S.; Song, J.; Ernst, P.; Latimer, M.N.; Ha, C.-M.; Goh, K.Y.; Ma, W.; Rajasekaran, N.-S.; Zhang, J.; Liu, X. , et al. MitoQ regulates redox-related noncoding RNAs to preserve mitochondrial network integrity in pressure-overload heart failure. American Journal of Physiology-Heart and Circulatory Physiology 2020, 318, H682–H695. [Google Scholar] [CrossRef]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P. , et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Mikhalkova, D.; Holman, S.R.; Jiang, H.; Saghir, M.; Novak, E.; Coggan, A.R.; O’Connor, R.; Bashir, A.; Jamal, A.; Ory, D.S. , et al. Bariatric Surgery-Induced Cardiac and Lipidomic Changes in Obesity-Related Heart Failure with Preserved Ejection Fraction. Obesity (Silver Spring) 2018, 26, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Mauro, A.G.; Mezzaroma, E.; Kraskauskas, D.; Marchetti, C.; Buzzetti, R.; Van Tassell, B.W.; Abbate, A.; Toldo, S. A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. International Journal of Cardiology 2015, 198, 66–69. [Google Scholar] [CrossRef] [PubMed]
- von Bibra, H.; Ströhle, A.; St. John Sutton, M.; Worm, N. Dietary therapy in heart failure with preserved ejection fraction and/or left ventricular diastolic dysfunction in patients with metabolic syndrome. International Journal of Cardiology 2017, 234, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Canada, J.M.; Buckley, L.F.; Trankle, C.R.; Billingsley, H.E.; Dixon, D.L.; Mauro, A.G.; Dessie, S.; Kadariya, D.; Mezzaroma, E. , et al. Dietary Fat, Sugar Consumption, and Cardiorespiratory Fitness in Patients With Heart Failure With Preserved Ejection Fraction. JACC: Basic to Translational Science 2017, 2, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats With Total and Cause-Specific Mortality. JAMA Internal Medicine 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Tucker, W.J.; Lijauco, C.C.; Hearon, C.M., Jr.; Angadi, S.S.; Nelson, M.D.; Sarma, S.; Nanayakkara, S.; La Gerche, A.; Haykowsky, M.J. Mechanisms of the Improvement in Peak VO(2) With Exercise Training in Heart Failure With Reduced or Preserved Ejection Fraction. Heart Lung Circ 2018, 27, 9–21. [Google Scholar] [CrossRef]
- Pandey, A.; Parashar, A.; Kumbhani, D.; Agarwal, S.; Garg, J.; Kitzman, D.; Levine, B.; Drazner, M.; Berry, J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 2015, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.; Gelbrich, G.; Düngen, H.D.; Fröhling, S.; Wachter, R.; Stahrenberg, R.; Binder, L.; Töpper, A.; Lashki, D.J.; Schwarz, S. , et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 2011, 58, 1780–1791. [Google Scholar] [CrossRef]
- Mueller, S.; Winzer, E.B.; Duvinage, A.; Gevaert, A.B.; Edelmann, F.; Haller, B.; Pieske-Kraigher, E.; Beckers, P.; Bobenko, A.; Hommel, J. , et al. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. Jama 2021, 325, 542–551. [Google Scholar] [CrossRef] [PubMed]
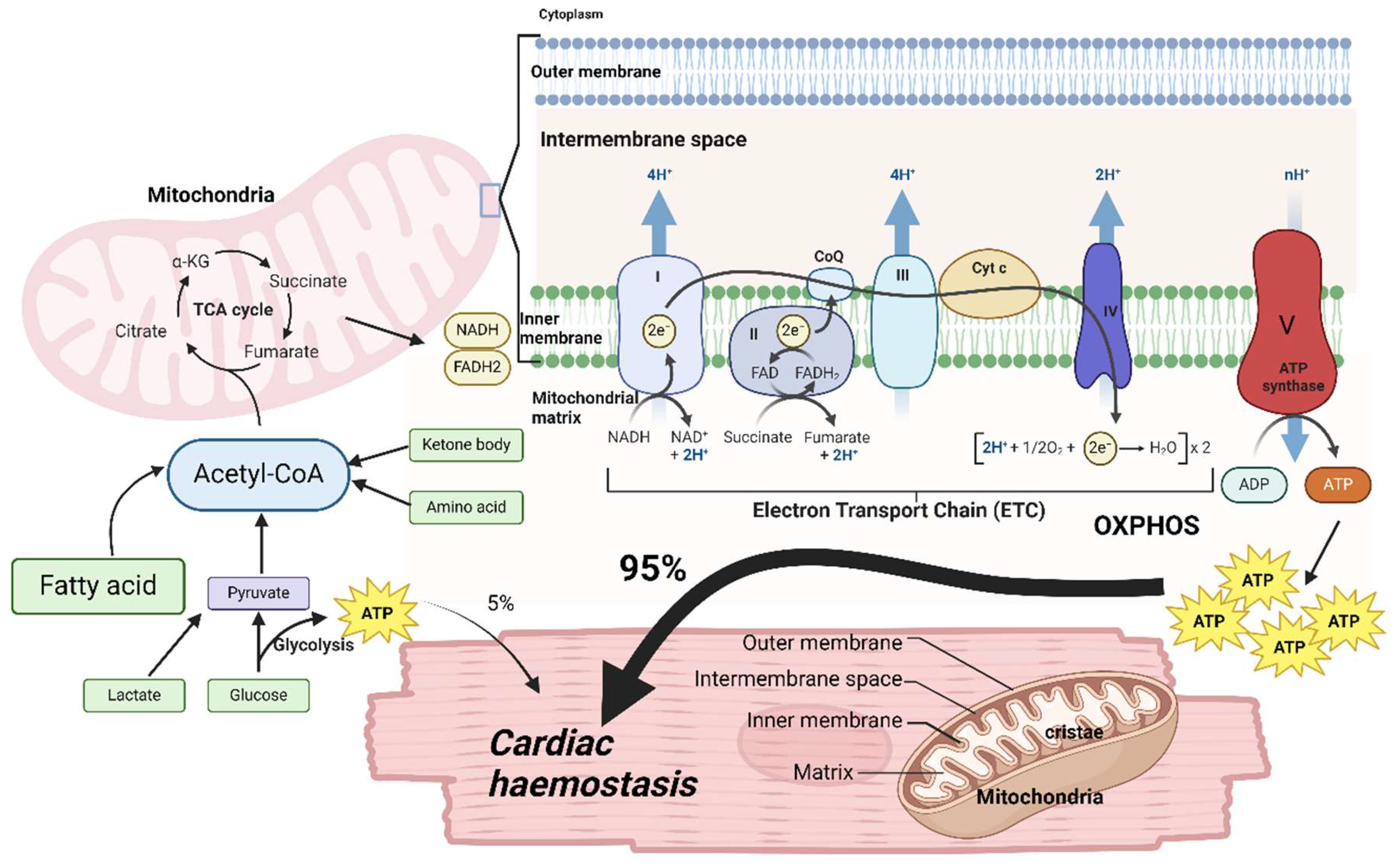
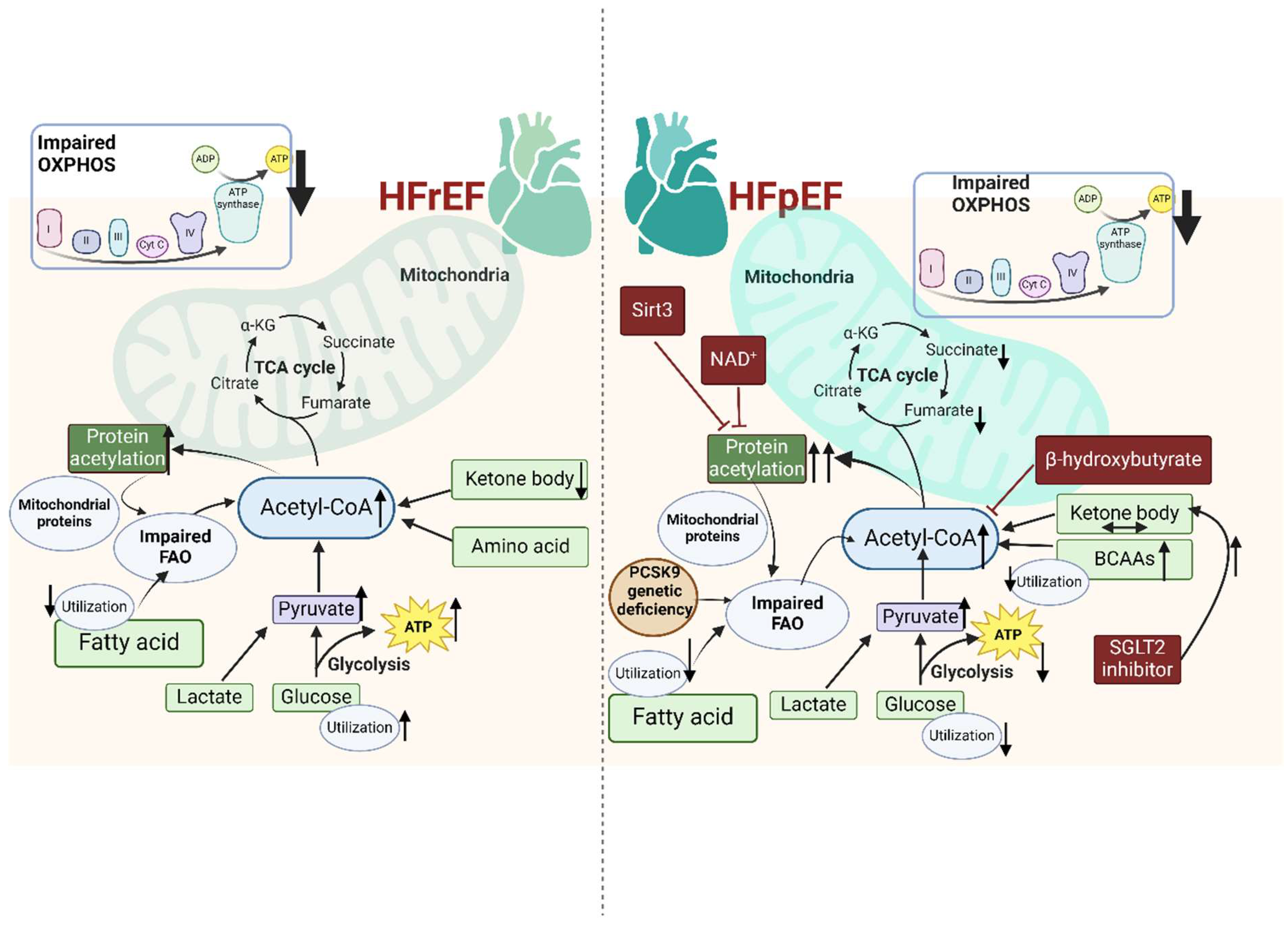
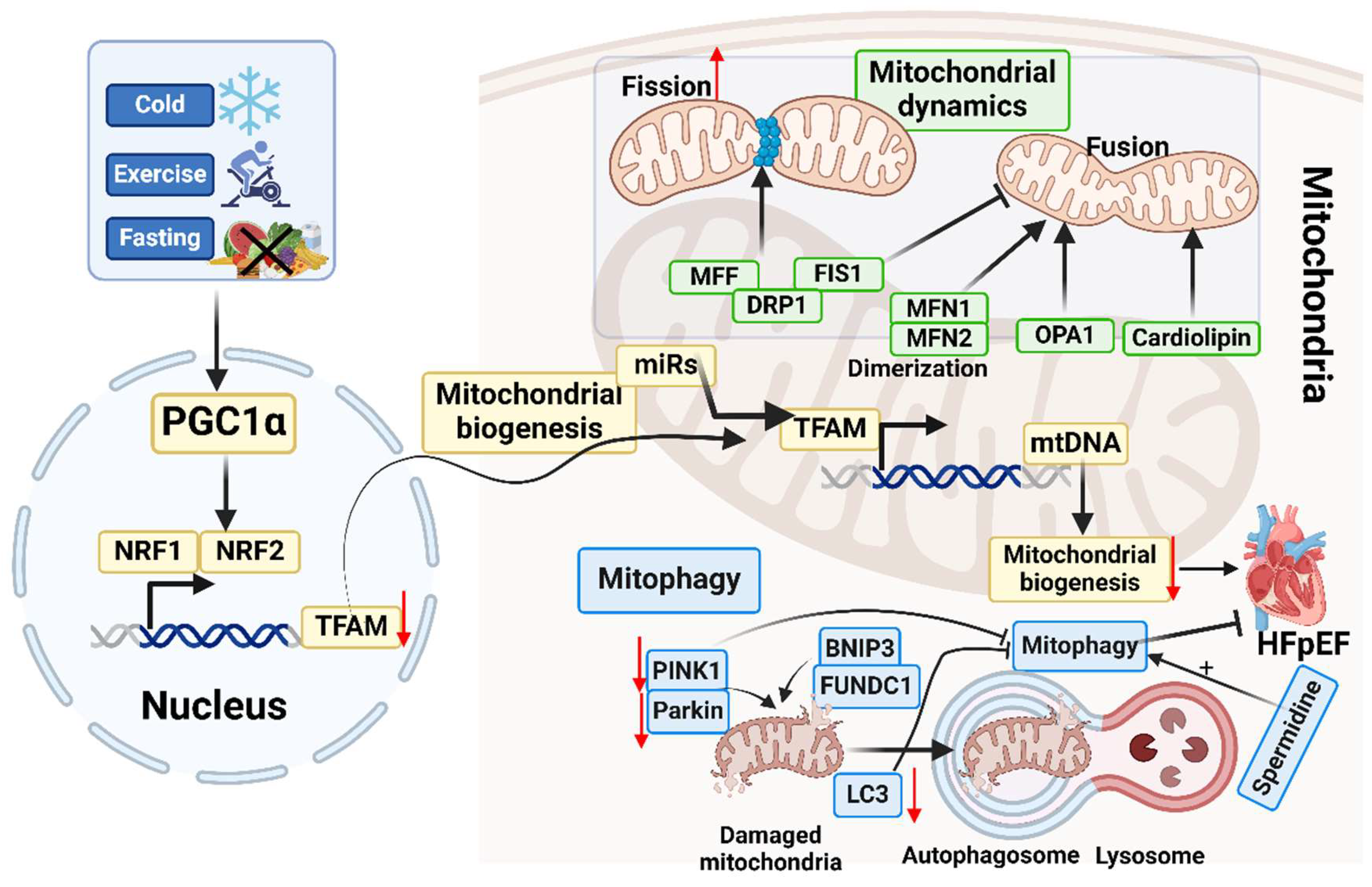
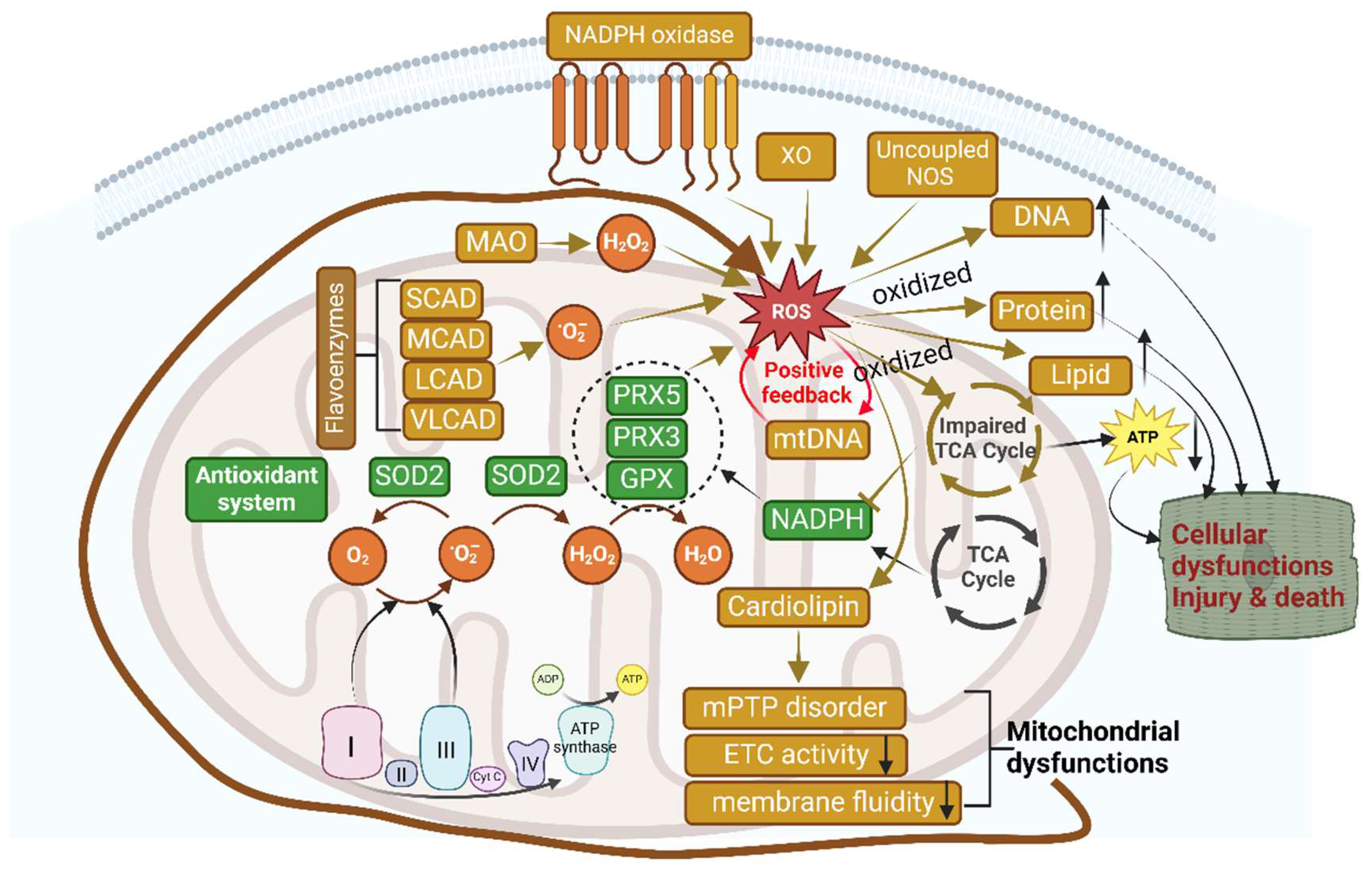
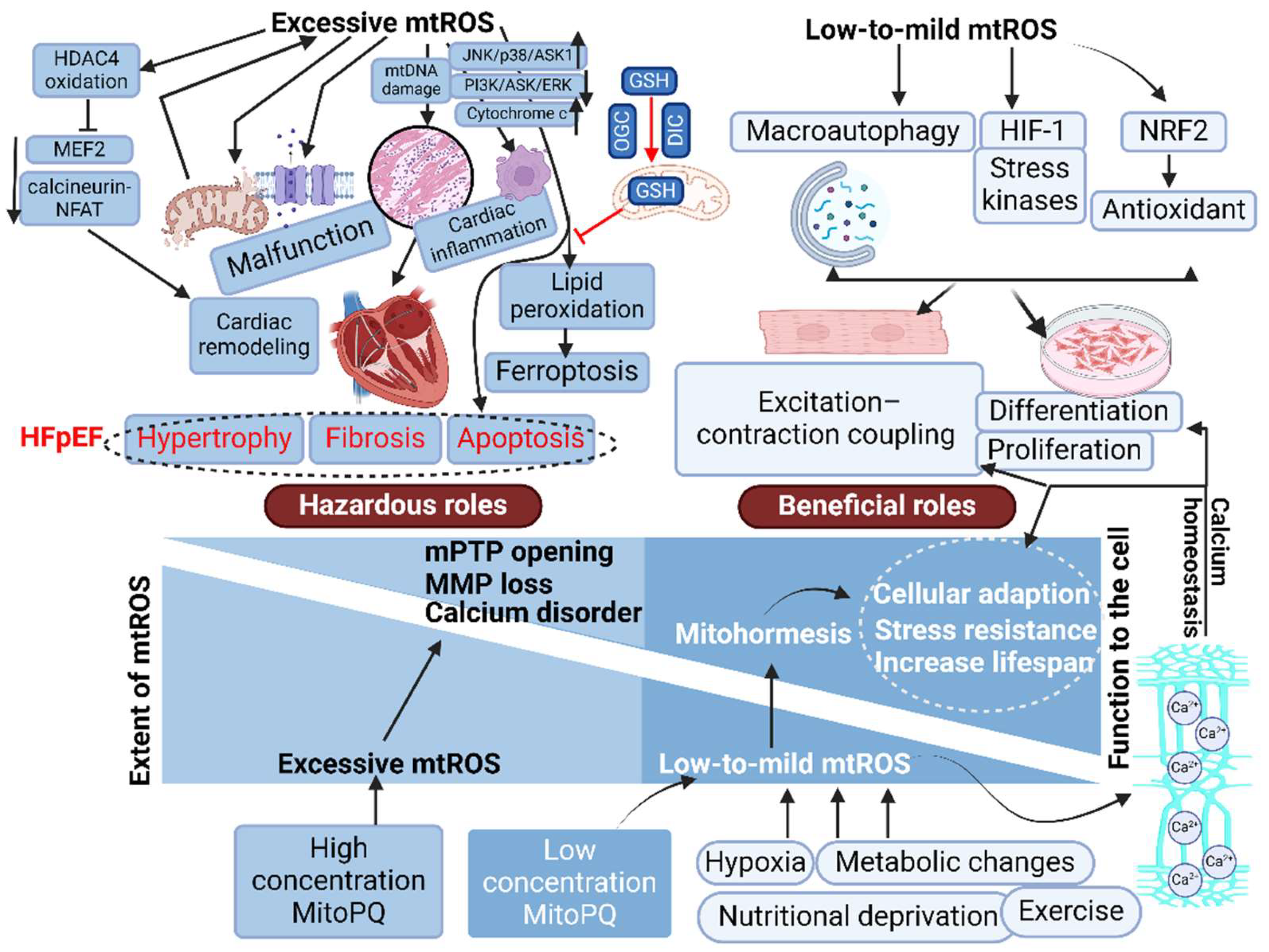
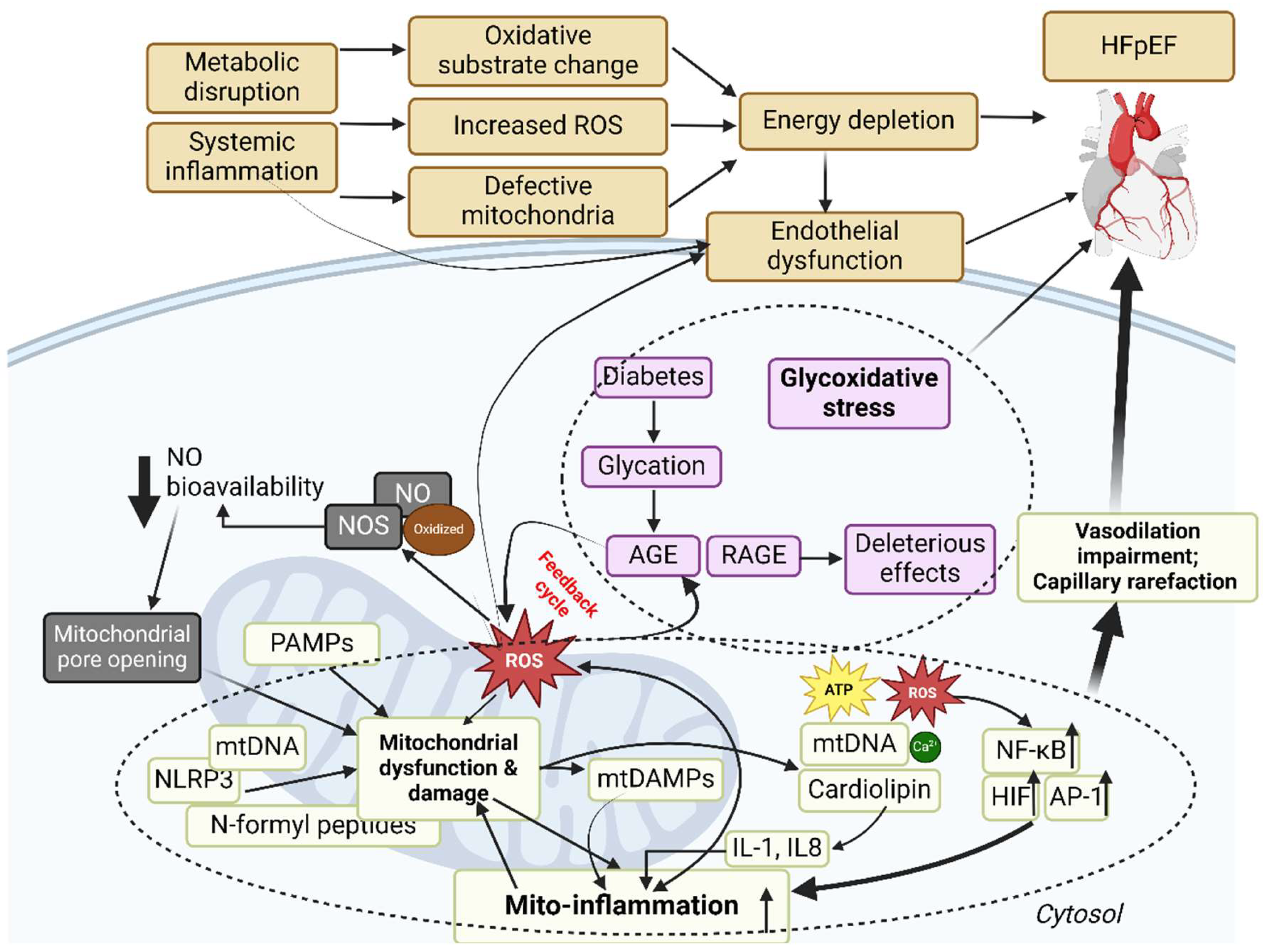
| Animal models | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hypertensive phenotype | Cardio-metabolic phenotype | |||||||||
| Features | Rodent | Large animal | Rodent | Large animal | ||||||
| Model | DSSRs | SHRs | Aortic constriction | Aldosterone infusion | Aged dogs + perinephritis | Aortic-banded cats | ZSF1 | Ageing | l-NAME + HFD | DOCA + salt-loaded pigs +HFD |
| Hypertension | Y | Y | N | Y | Y | Y | Y | N | Y | Y |
| Pulmonary congestion | N | N | Mild | Mild | - | Y | Y | - | Y | - |
| Diastolic Dysfunction | Y | Y | Mild | Y | Y | Y | Y | Y | Y | Y |
| LVH | Y | Y | Y | Y | Y | Y | Y | Mild | Y | Y |
| Exercise Intolerance | Y | Y | - | - | - | - | Y | Y | Y | Y |
| Obesity | N | N | N | N | N | N | Y | N | Y | Y |
| Preserved LVEF | Y* | Y* | Y* | Y* | Y | Y* | Y | Y | Y | Y |
| Therapeutics | Target(s) | Rationale | Major findings/outcome | Main adverse effects |
|---|---|---|---|---|
| Non-pharmacological management | ||||
| Dietary interventions- Low carbohydrate diet | Adipose tissue (especially visceral fat), liver and pancreas | Improve insulin sensitivity, reduce inflammation and glycoxidative stress |
Improve MetS manifestations and cardiovascular risk | Risk of hypoglycaemia in individuals on medication |
| Exercise training | Cardiac and skeletal muscles |
Improve LV diastolic function and cardiac output | Improvement in VO2peak peak and physical capacity |
Muscle soreness, fatigue, and injury from over-exertion |
| Conventional/repurposing drugs | ||||
| Diuretics | Kidney Cardiovascular system |
Alleviate symptoms of fluid overload | Reduce congestion Control of blood pressure and pulmonary congestion |
Electrolyte imbalance Hypotension |
| SGLT2 inhibitors | Kidney | Reduce glucose and sodium reabsorption, leading to natriuresis and osmotic diuresis | Reduce fluid overload, inflammation and oxidative stress Improve cardiac energy efficiency Reduce hospitalization and cardiovascular death |
Volume depletion Electrolyte imbalance |
| Mineralocorticoid receptor antagonists | Mineralocorticoid receptors in the kidneys, heart, and blood vessels. | Reduce sodium retention, lower blood pressure, and alleviate fluid overload. Reduce myocardial fibrosis |
Reduce hospitalizations Reduce myocardial remodelling |
Hyperkalaemia Gynecomastia Renal impairment |
| Angiotensin receptor–neprilysin inhibitors | Angiotensin II type 1 receptor and neprilysin | Reduce vascular stiffness, blood pressure, and increase natriuresis | Reduce hospitalizations Reverse cardiac remodelling |
Hypotension Hyperkalaemia Angioedema |
| Angiotensin receptor blockers | Angiotensin II type 1 receptor / Renin-angiotensin-aldosterone system | Reduce the heart’s workload by lowering blood pressure | Reduce hospitalizations |
Hypotension Hyperkalaemia Renal dysfunction |
| β-Blockers | β-adrenergic receptors (heart and blood vessels) | Reduce heart rate and improve diastolic filling time | Slight reduction in hospitalizations Can improve exercise tolerance |
Bradycardia Hypotension |
| Mitochondria oxidative stress-targeted therapy | ||||
| Elamipretide- Bendavia | Mitochondria | Improve mitochondrial bioenergetics Improve heart’s energy capacity |
Improve mitochondrial function and cardiac remodelling | Injection site reactions |
| Coenzyme Q10 | Mitochondria | Improve energy production Reduce oxidative stress |
Improve LVEF and exercise intolerance May reduce hospitalization |
Gastrointestinal issues |
| MitoQ-mitoquinone | Mitochondria | Neutralize oxidative stress Restore mitochondrial function |
Enhance mitochondrial respiration May improve exercise tolerance and reduce fatigue |
Gastrointestinal issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





