Submitted:
01 October 2025
Posted:
02 October 2025
You are already at the latest version
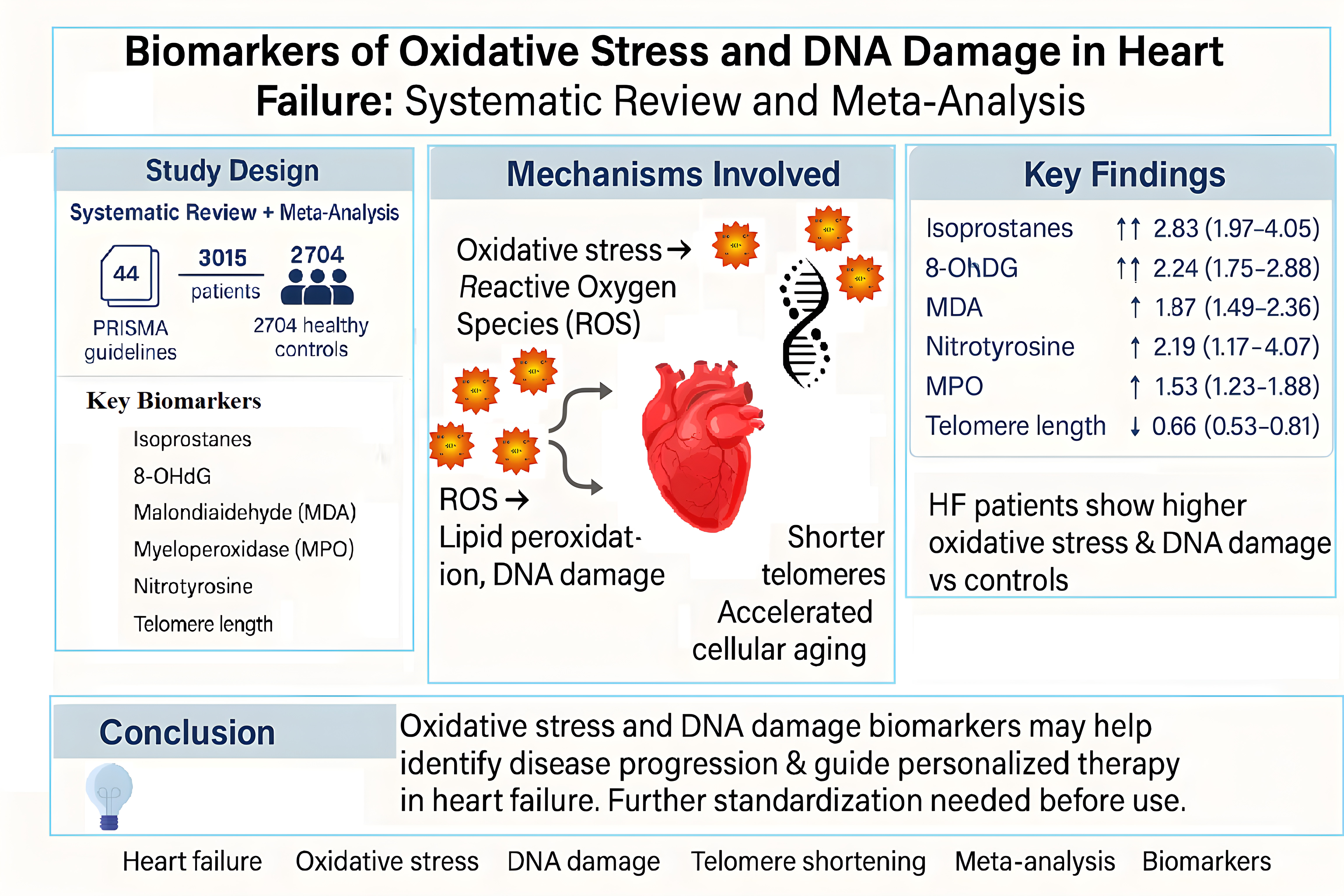
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Outcome
2.3. Statistical Analysis
3. Results
3.1. Data Collection
3.2. Biomarkers of Oxidative Stress and DNA Damage in HF Patients
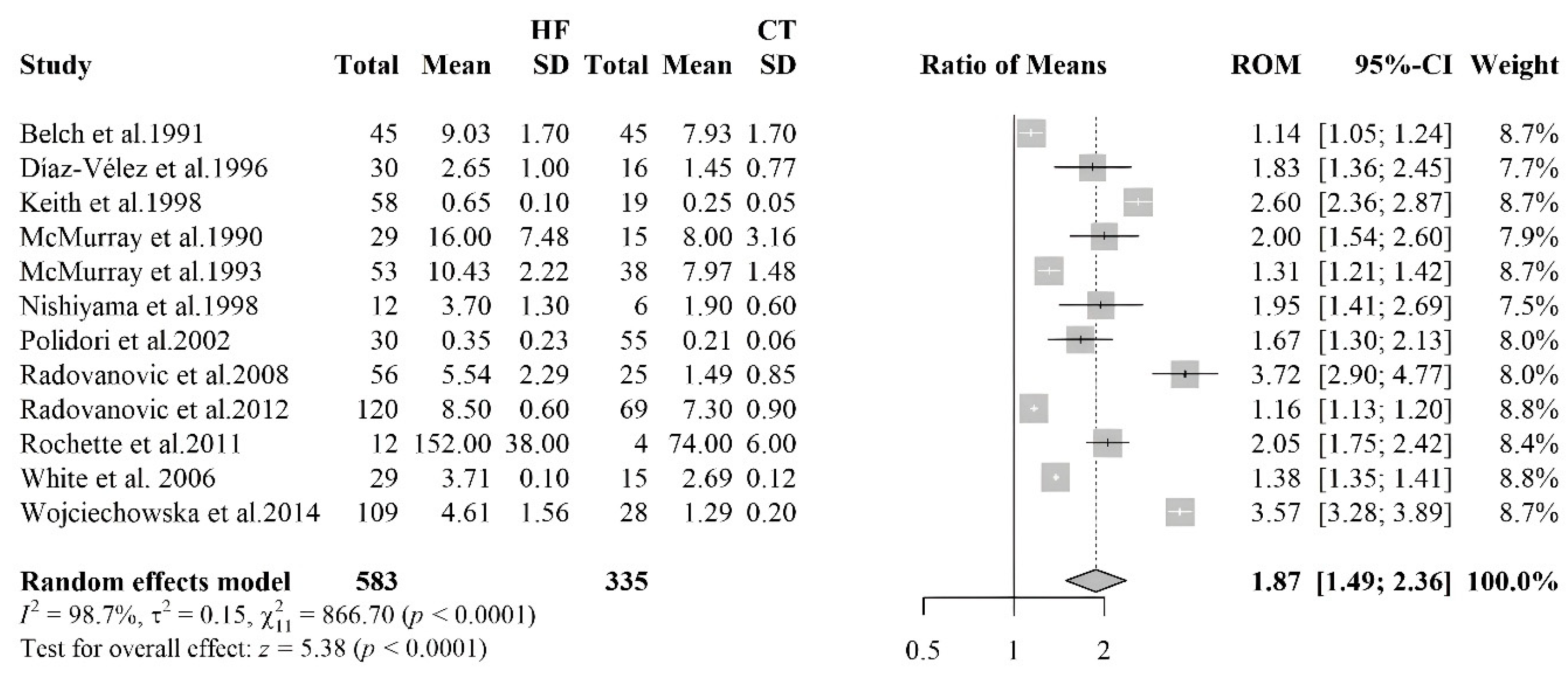
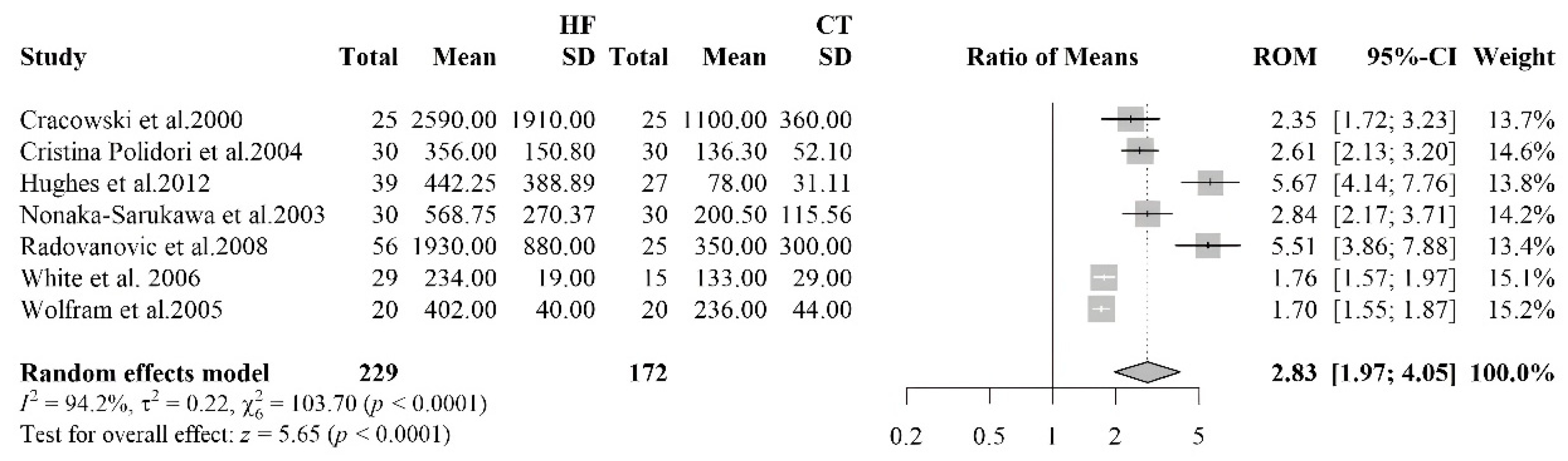
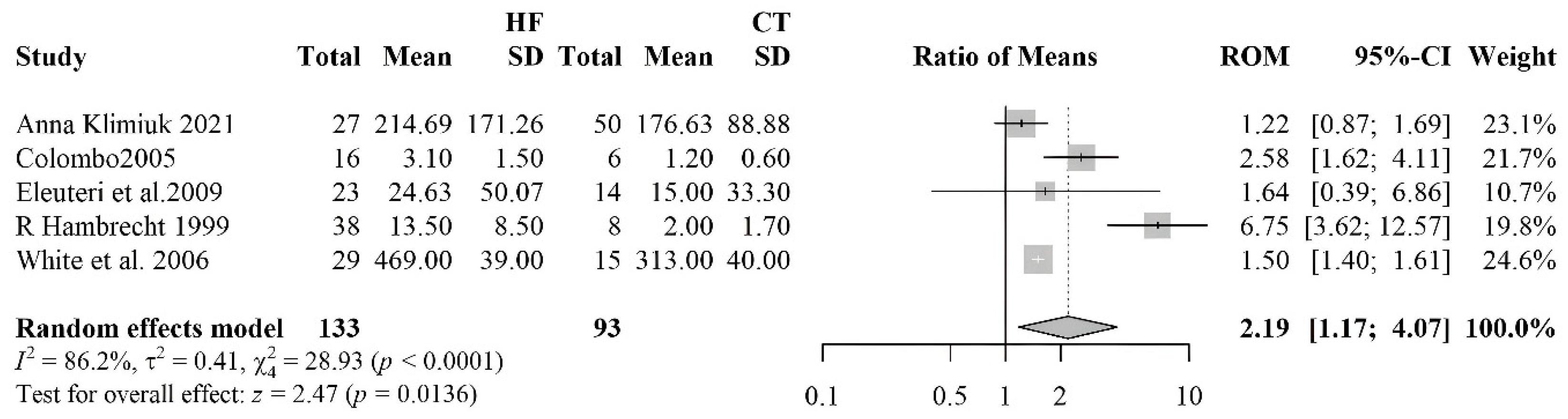
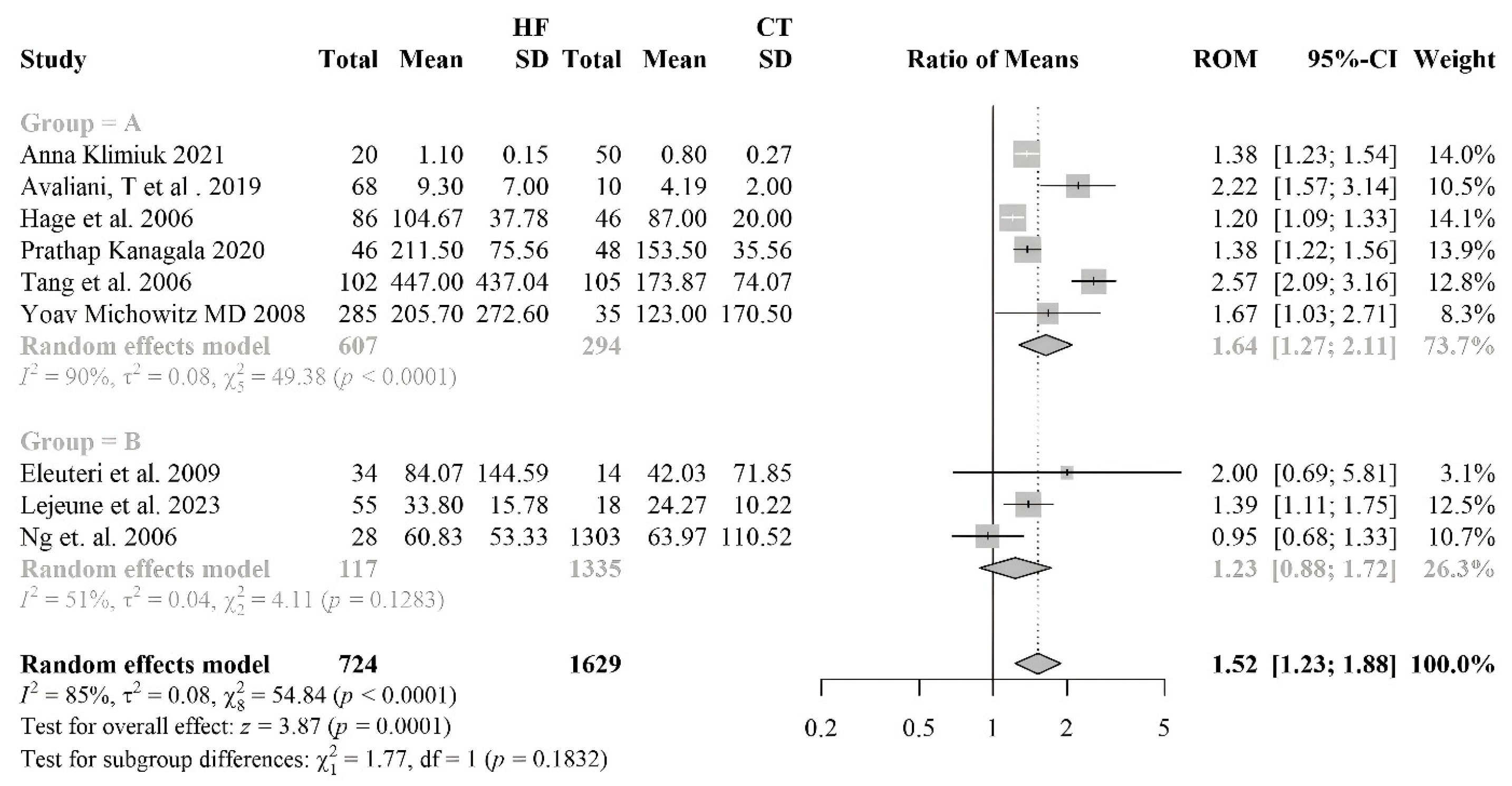
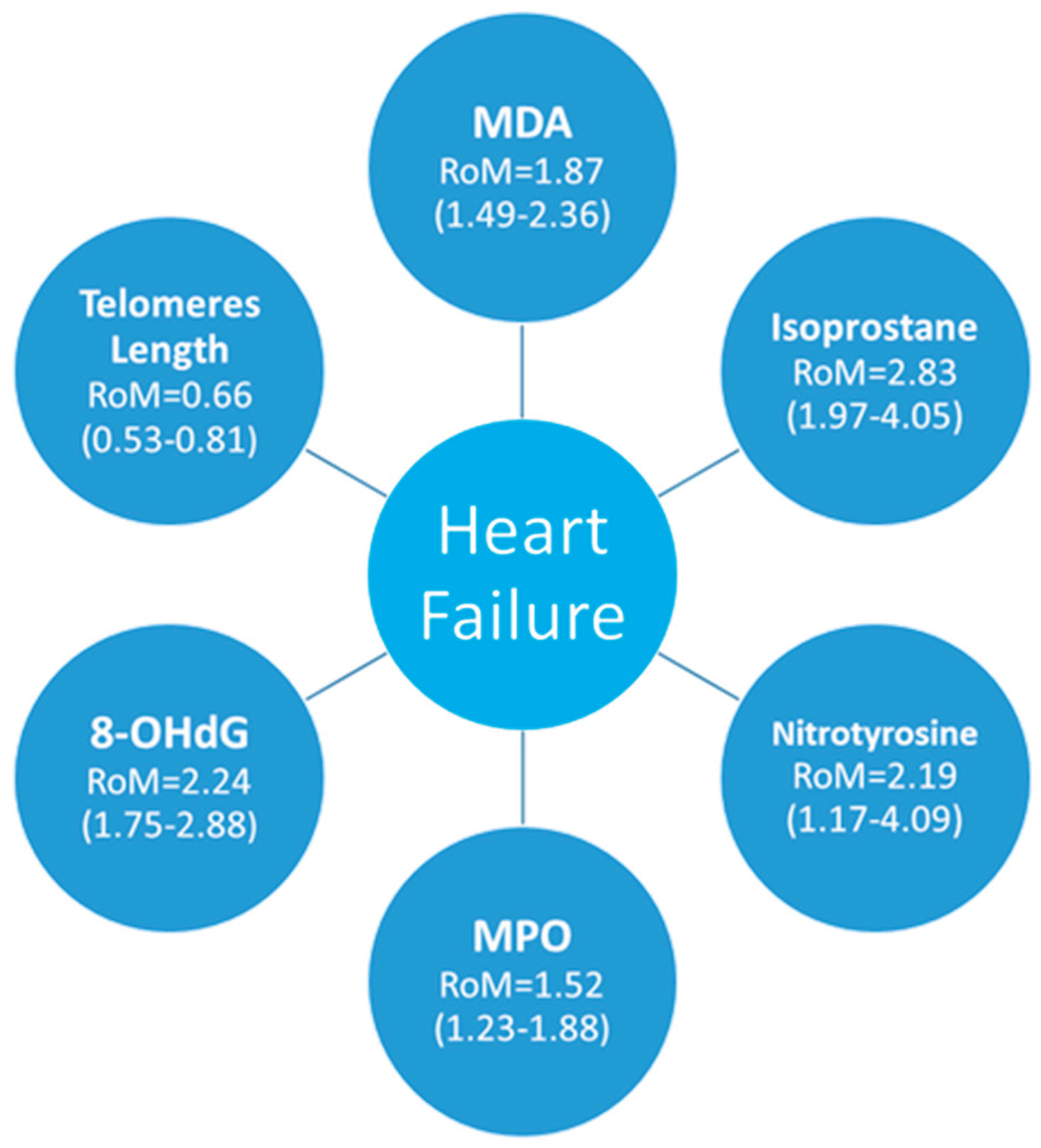
3.2.1. Meta-Analyses of Biomarkers of Oxidative Stress in HF Patients
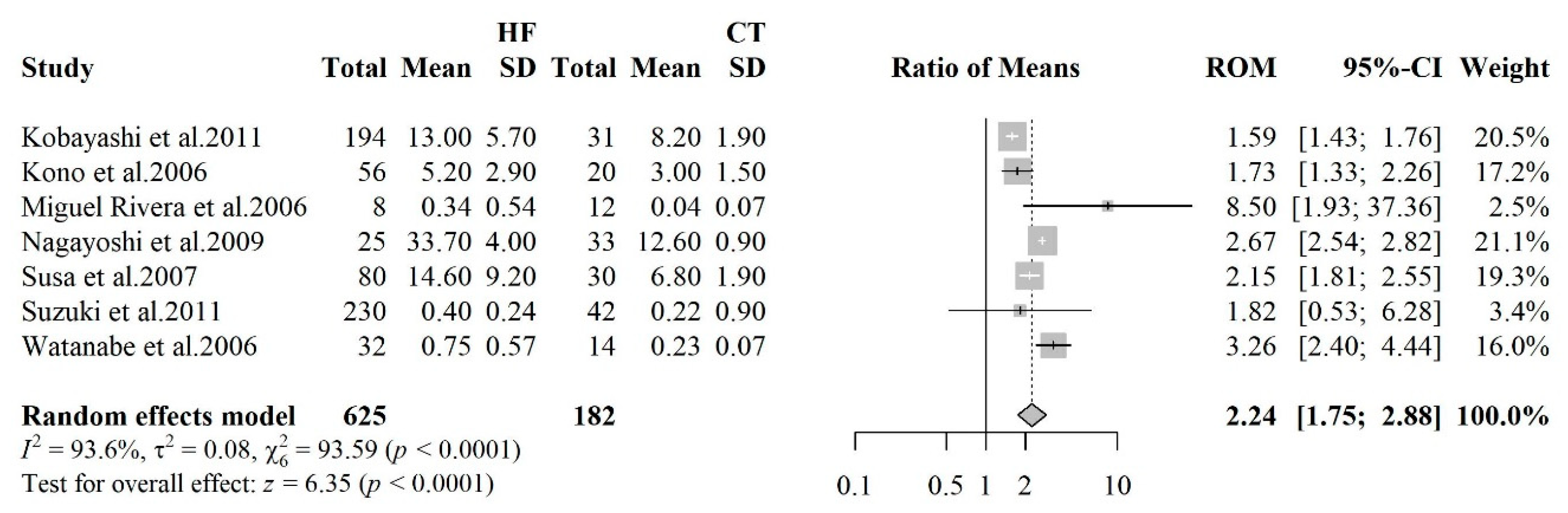
3.2.2. 8-Hydroxy-2'-Deoxyguanosine
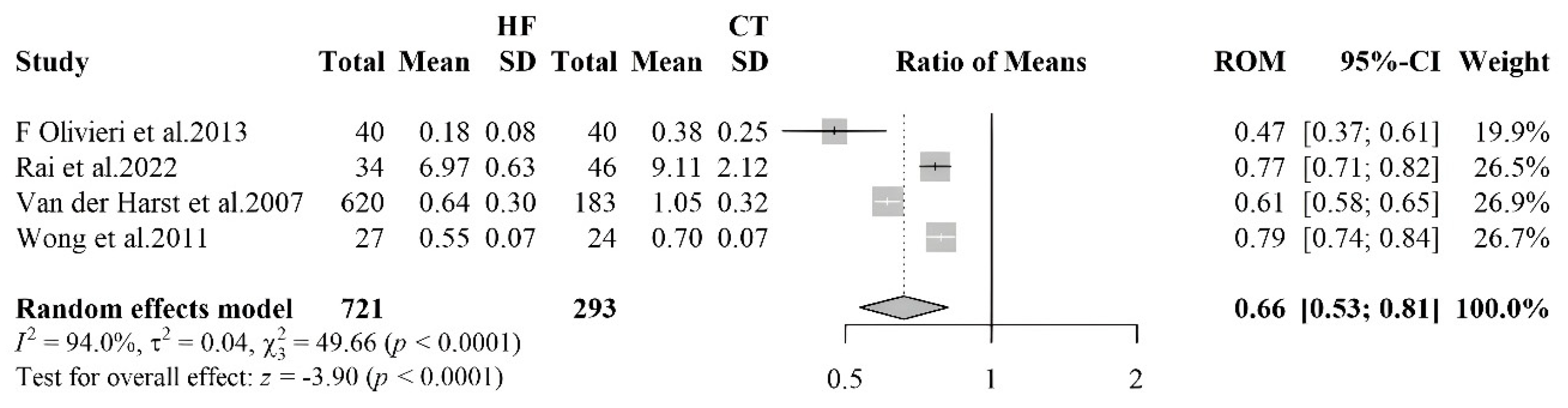
3.2.3. Telomere Length
3.2.4. Malondialdehyde
3.2.5. Isoprostane
3.2.6. Nitrotyrosine
3.2.7. Myeloperoxydase
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A8-OHdG | 8-hydroxy-2′-deoxyguanosine; |
| Aas | aldosterone antagonists; |
| ACEi | angiotensin-converting enzyme inhibitors; |
| AP | antiplatelets; |
| ARBs | angiotensin receptor blockers; |
| ASA | acetylsalicylic acid (aspirin); |
| BB | beta-blockers; |
| BNP | B-type natriuretic peptide; |
| CAT | catalase; |
| CCBs | calcium channel blockers; |
| CHD | congenital heart disease; |
| CHF | congestive heart failure; |
| CVD | cardiovascular disease; |
| DCM | dilated cardiomyopathy; |
| EF | ejection fraction; |
| GPx | glutathione peroxidase; |
| GSH | glutathione; |
| HF | heart failure; |
| HFmrEF | mid-range or mildly reduced ejection fraction; |
| HFpEF | preserved ejection fraction; |
| HFrEF | reduced ejection fraction; |
| HHD | hypertensive heart disease; |
| HVD | heart valve disease; |
| ICHF | ischaemic congestive heart failure; |
| ICM | ischaemic cardiomyopathy; |
| IDCM | idiopathic dilated cardiomyopathy; |
| MDA | malondialdehyde; |
| MI | myocardial infarction; |
| MPO | myeloperoxidase; |
| MRAs | mineralocorticoid receptor antagonists; |
| NAD+ | nicotinamide adenine dinucleotide; |
| NICHF | non-ischaemic congestive heart failure; |
| NOS | nitric oxide synthase; |
| NSAIDs | non-steroidal anti-inflammatory drugs. |
| NT-proBNP | N-terminal proBNP; |
| NYHA | New York Heart Association; |
| OACs | oral anticoagulants; |
| RNS | reactive nitrogen species; |
| ROM | ratio of means; |
| ROS | reactive oxygen species; |
| SOD | superoxide dismutase |
References
- Bozkurt B, Fonarow GC, Goldberg LR, et al. Cardiac Rehabilitation for Patients With Heart Failure: JACC Expert Panel. J Am Coll Cardiol 2021;77:1454–69. [CrossRef]
- Simmonds SJ, Cuijpers I, Heymans S, et al. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020;9:242. [CrossRef]
- Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 2023;118:3272–87. [CrossRef]
- Schwinger RHG. Pathophysiology of heart failure. Cardiovasc Diagn Ther 2021;11:263–76. [CrossRef]
- Ng ML, Ang X, Yap KY, et al. Novel Oxidative Stress Biomarkers with Risk Prognosis Values in Heart Failure. Biomedicines 2023;11:917. [CrossRef]
- Pizzino G, Irrera N, Cucinotta M, et al. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev 2017;2017:8416763. [CrossRef]
- Sawyer DB, Colucci WS. Mitochondrial Oxidative Stress in Heart Failure: “Oxygen Wastage” Revisited. Circ Res 2000;86:119–20. [CrossRef]
- van der Pol A, van Gilst WH, Voors AA, et al. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 2019;21:425–35. [CrossRef]
- Afzal S, Abdul Manap AS, Attiq A, et al. From imbalance to impairment: the central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front Pharmacol 2023;14:1269581. [CrossRef]
- Research C for DE and. Treatment for Heart Failure: Endpoints for Drug Development Guidance for Industry 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/treatment-heart-failure-endpoints-drug-development-guidance-industry (accessed , 2025). 1 April.
- González A, Richards AM, de Boer RA, et al. Cardiac remodelling - Part 1: From cells and tissues to circulating biomarkers. A review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2022;24:927–43. [CrossRef]
- Januzzi JL, Canty JM, Das S, et al. Gaining Efficiency in Clinical Trials With Cardiac Biomarkers. J Am Coll Cardiol 2021;77:1922–33. [CrossRef]
- Wei X, Zhang X, Cui M, et al. An Exploration of the Relationship Between Gut Virome and Cardiovascular Disease: A Comprehensive Review. Rev Cardiovasc Med 2025;26:36386. [CrossRef]
- Triantafyllis AS, Sfantou D, Karapedi E, et al. Coronary Implications of COVID-19. Med Princ Pract 2024:1–12. [CrossRef]
- Giacconi R, Piacenza F, Maggi F, et al. Association Between TTV Viremia, Chronic Inflammation, and Ischemic Heart Disease Risk: Insights From MARK-AGE and Report-Age Projects. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 2024;79:glae228. [CrossRef]
- Russo P, Milani F, Limongi D, et al. The effect of torque teno virus (TTV) infection on clinical outcomes, genomic integrity, and mortality in COPD patients. Mechanisms of Ageing and Development 2025;224:112024. [CrossRef]
- Russo P, Milani F, Limongi D, et al. The effect of torque teno virus (TTV) infection on clinical outcomes, genomic integrity, and mortality in COPD patients. Mechanisms of Ageing and Development 2025;224:112024. [CrossRef]
- Ayala A, Muñoz MF, Argüelles S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev 2014;2014:1–31. [CrossRef]
- Omari Shekaftik S, Nasirzadeh N. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker of oxidative DNA damage induced by occupational exposure to nanomaterials: a systematic review. Nanotoxicology 2021;15:850–64. [CrossRef]
- Ndrepepa, G. Myeloperoxidase – A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta 2019;493:36–51. [CrossRef]
- Rouhi L, Cheedipudi SM, Cathcart B, et al. Cytosolic DNA sensing protein pathway is activated in human hearts with dilated cardiomyopathy. J Cardiovasc Aging 2023;3:32. [CrossRef]
- Tubić Vukajlović J, Simić I, Smiljanić Z, et al. Genome instability in peripheral blood lymphocytes of patients with heart failure and reduced ejection fraction. Mutagenesis 2023;38:84–92. [CrossRef]
- Mondal NK, Sorensen E, Hiivala N, et al. Oxidative stress, DNA damage and repair in heart failure patients after implantation of continuous flow left ventricular assist devices. Int J Med Sci 2013;10:883–93. [CrossRef]
- Rivera M, Roselló-Lletí E, García de Burgos F, et al. [8-hydroxy-2’-deoxyguanosine and lipid peroxidation in patients with heart failure]. Rev Esp Cardiol 2006;59:1140–5.
- Kobayashi S, Susa T, Tanaka T, et al. Urinary 8-hydroxy-2’-deoxyguanosine reflects symptomatic status and severity of systolic dysfunction in patients with chronic heart failure. Eur J Heart Fail 2011;13:29–36. [CrossRef]
- Suzuki S, Shishido T, Ishino M, et al. 8-Hydroxy-2’-deoxyguanosine is a prognostic mediator for cardiac event. Eur J Clin Invest 2011;41:759–66. [CrossRef]
- Kono Y, Nakamura K, Kimura H, et al. Elevated Levels of Oxidative DNA Damage in Serum and Myocardium of Patients With Heart Failure. Circ J 2006;70:1001–5. [CrossRef]
- Susa T, Kobayashi S, Kawamura S, et al. Abstract 1703: Urinary 8-Hydroxy-2′-Deoxyguanosine Level reflects symptomatic status and severity of systolic dysfunction in Patients with Chronic Heart Failure. Circulation 2007;116:II_358-II_358. [CrossRef]
- Nagayoshi Y, Kawano H, Hokamaki J, et al. Differences in oxidative stress markers based on the aetiology of heart failure: Comparison of oxidative stress in patients with and without coronary artery disease. Free Radic Res 2009;43:1159–66. [CrossRef]
- Watanabe E, Matsuda N, Shiga T, et al. Significance of 8-hydroxy-2’-deoxyguanosine levels in patients with idiopathic dilated cardiomyopathy. J Card Fail 2006;12:527–32. [CrossRef]
- van der Harst P, van der Steege G, de Boer RA, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol 2007;49:1459–64. [CrossRef]
- Rai S, Badarinath ARS, George A, et al. Association of telomere length with diabetes mellitus and idiopathic dilated cardiomyopathy in a South Indian population: A pilot study. Mutat Res Toxicol Environ Mutagen 2022;874–875:503439. [CrossRef]
- Wong LSM, Huzen J, de Boer RA, et al. Telomere Length of Circulating Leukocyte Subpopulations and Buccal Cells in Patients with Ischemic Heart Failure and Their Offspring. PLoS ONE 2011;6:e23118. [CrossRef]
- Olivieri F, Antonicelli R, Recchioni R, et al. Telomere/telomerase system impairment in circulating angiogenic cells of geriatric patients with heart failure. Int J Cardiol 2013;164:99–105. [CrossRef]
- Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol 1998;31:1352–6. [CrossRef]
- Nishiyama Y, Ikeda H, Haramaki N, et al. Oxidative stress is related to exercise intolerance in patients with heart failure. Am Heart J 1998;135:115–20. [CrossRef]
- Rochette L, Tatou E, Maupoil V, et al. Atrial and Vascular Oxidative Stress in Patients with Heart Failure. Cell Physiol Biochem 2011;27:497–502. [CrossRef]
- Wojciechowska C, Romuk E, Tomasik A, et al. Oxidative Stress Markers and C-Reactive Protein Are Related to Severity of Heart Failure in Patients with Dilated Cardiomyopathy. Mediators Inflamm 2014;2014:147040. [CrossRef]
- Radovanovic S, Savic-Radojevic A, Pljesa-Ercegovac M, et al. Markers of Oxidative Damage and Antioxidant Enzyme Activities as Predictors of Morbidity and Mortality in Patients With Chronic Heart Failure. J Card Fail 2012;18:493–501. [CrossRef]
- Díaz-Vélez CR, García-Castiñeiras S, Mendoza-Ramos E, et al. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am Heart J 1996;131:146–52. [CrossRef]
- Belch JJ, Bridges AB, Scott N, et al. Oxygen free radicals and congestive heart failure. Heart 1991;65:245–8. [CrossRef]
- McMURRAY J, Chopra M, Abdullah I, et al. Evidence of oxidative stress in chronic heart failure in humans. Eur Heart J 1993;14:1493–8. [CrossRef]
- McMurray J, McLay J, Chopra M, et al. Evidence for enhanced free radical activity in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol 1990;65:1261–2. [CrossRef]
- Polidori MC, Savino K, Alunni G, et al. Plasma lipophilic antioxidants and malondialdehyde in congestive heart failure patients: relationship to disease severity. Free Radic Biol Med 2002;32:148–52. [CrossRef]
- White M, Ducharme A, Ibrahim R, et al. Increased systemic inflammation and oxidative stress in patients with worsening congestive heart failure: improvement after short-term inotropic support. Clin Sci 2006;110:483–9. [CrossRef]
- Cracowski, JL. Increased formation of F2-isoprostanes in patients with severe heart failure. Heart 2000;84:439–40. [CrossRef]
- Hughes CM, Woodside JV, McGartland C, et al. Nutritional intake and oxidative stress in chronic heart failure. Nutr Metab Cardiovasc Dis 2012;22:376–82. [CrossRef]
- Cristina Polidori M, Praticó D, Savino K, et al. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J Card Fail 2004;10:334–8. [CrossRef]
- Wolfram R, Oguogho A, Palumbo B, et al. Enhanced oxidative stress in coronary heart disease and chronic heart failure as indicated by an increased 8-epi-PGF2α. Eur J Heart Fail 2005;7:167–72. [CrossRef]
- Nonaka-Sarukawa M, Yamamoto K, Aoki H, et al. Increased urinary 15-F2t-isoprostane concentrations in patients with non-ischaemic congestive heart failure: a marker of oxidative stress. Heart 2003;89:871–4.
- Tang WHW, Brennan M-L, Philip K, et al. Plasma Myeloperoxidase Levels in Patients With Chronic Heart Failure. Am J Cardiol 2006;98:796–9. [CrossRef]
- Kanagala P, Arnold JR, Singh A, et al. Characterizing heart failure with preserved and reduced ejection fraction: An imaging and plasma biomarker approach. PLOS ONE 2020;15:e0232280. [CrossRef]
- Hage C, Michaëlsson E, Kull B, et al. Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Fail 2020;7:1534–46. [CrossRef]
- Lejeune S, Ginion A, Menghoum N, et al. Association of Plasma Myeloperoxidase with Inflammation and Diabetic status in HFpEF. Rev Cardiovasc Med 2023;24:56. [CrossRef]
- Ng LL, Pathik B, Loke IW, et al. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am Heart J 2006;152:94–101. [CrossRef]
- Eleuteri E, Di Stefano A, Ricciardolo FL, et al. Increased nitrotyrosine plasma levels in relation to systemic markers of inflammation and myeloperoxidase in chronic heart failure. Int J Cardiol 2009;135:386–90. [CrossRef]
- Michowitz Y, Kisil S, Guzner-Gur H, et al. Usefulness of serum myeloperoxidase in prediction of mortality in patients with severe heart failure. Isr Med Assoc J IMAJ 2008;10:884–8.
- Avaliani T, Talakvadze T, Tabagari S. INFLUENCE OF NUTRITIONAL STATE ON OUTCOME IN PATIENTS WITH CHRONIC HEART FAILURE. Georgian Med News 2019:61–6.
- Klimiuk A, Zalewska A, Knapp M, et al. Salivary Gland Dysfunction in Patients with Chronic Heart Failure Is Aggravated by Nitrosative Stress, as Well as Oxidation and Glycation of Proteins. Biomolecules 2021;11:119. [CrossRef]
- Hambrecht R, Adams V, Gielen S, et al. Exercise intolerance in patients with chronic heart failure and increased expression of inducible nitric oxide synthase in the skeletal muscle. J Am Coll Cardiol 1999;33:174–9. [CrossRef]
- Meijers WC, Bayes-Genis A, Mebazaa A, et al. Circulating heart failure biomarkers beyond natriuretic peptides: review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur J Heart Fail 2021;23:1610–32. [CrossRef]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [CrossRef]
- Fenech M, Knasmueller S, Knudsen LE, et al. “Micronuclei and Disease” special issue: Aims, scope, and synthesis of outcomes. Mutat Res.
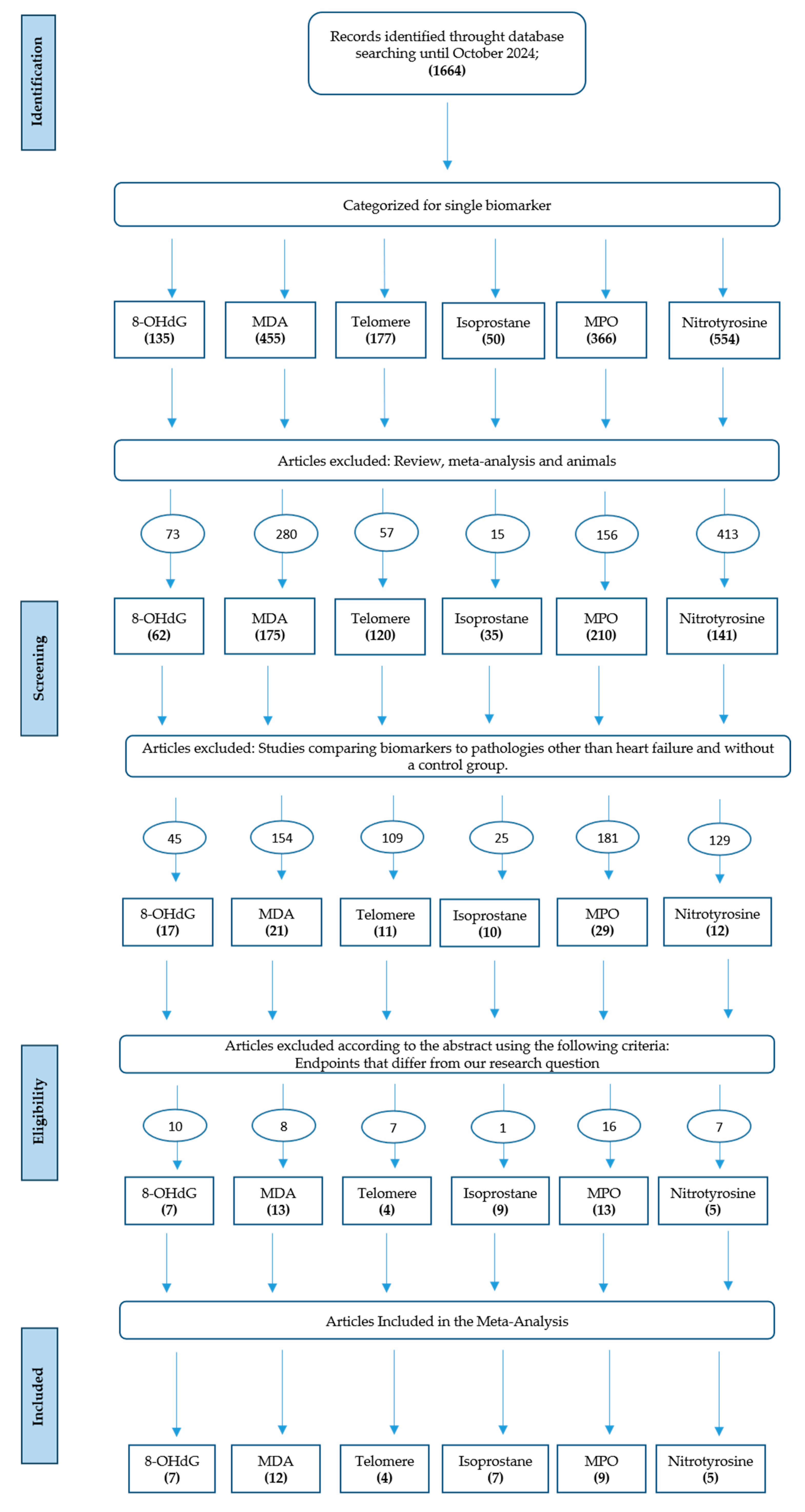
| Biomarker | Studies included (n) | HF patients (n) | Controls (n) |
| 8-OHdG | 7 | 625 | 182 |
| MDA | 12 | 583 | 335 |
| Telomere | 4 | 721 | 293 |
| Isoprostanes | 7 | 229 | 172 |
| MPO | 9 | 724 | 1629 |
| Nitrotyrosine | 5 | 133 | 93 |
| Total | 44 | 3015 | 2704 |
| Study, Year | Population, Sex (M/F) | Mean, age, years (SD/IC) | HF classification | HF Aethiology | Pharmacological treatment | Sample | Biomarker | Results | P value |
| 8-OHdG | |||||||||
| [24] | HF 78 (57M/21F) Controls 12 | HF 64±14 | HFrEF | IHD,DCM, HCM | Diuretics 74%; ACEi 73%; BB 51%; anti-aldosterone agents 42%; digoxin 26%; CAs 10% and ARBs 16% | 8-OHdG | Blood sample | HF: 0.34±0.54 Controls: 0.04±0.07 | <0,05 |
| [25] | HF 194 (108M/86F) Controls 31 (20M/11F) | HF 57.1±14.4 Controls 52.5+13.2 | HFrEF | DCM, MI | BB 80%, ACE-I 70%, 23% ARB, LDs 78%, 57% aldosterone antagonist, e 21% statin. | 8-OHdG | Urine sample | HF: 13.0±5.7 Controls: 8.2±1.9 | <0,0001 |
| [26] | HF 230 (140M/90F) Controls 42 (27M/15F) | HF 70.3±12,8 | HFrEF and HFpEF | DCM, IHD, HVD, HHD, HVD | 73% ACEi; 51% BB; CCBs 19%; 67% Diuretics; Statins 16% | 8-OHdG | Blood sample | HF: 0,40 ± 0,24 Controls: 0,22 ± 0,09 ng/ml | <0,001 |
| [27] | HF 56 (42M/14F) Controls 20 | HF 53±12 | HFrEF and HFpEF | DCM | 71% ACEi; ARBs 22%; CCBs 23%; Diuretics 55% | 8-OHdG | Blood sample | HF: 5,2±2,9; Controls: 3,0±1,5 | 0,0018 |
| [28] | HF 111 (M52%/F48%) Controls 30 | HF 57±16 | HFrEF and HFpEF | 8-OHdG | Urine sample | HF: 14.6±9.2, Controls: 6.8±1.9 | < 0.01 | ||
| [29] | 25 HF and 33 Controls | HF Mean 69 | HFrEF | CHD | 8-OHdG | Urine sample | HF: 33.7±4.0 Controls: 12.6±0.9 | < 0.01 | |
| [30] | HF (24M/8F) Controls (8M/6F) | HF 46.6±18.2 Controls 34.6±6.9 | HFrEF | DCM | 84% Diuretics; ACEi/ARBs 94%; BB 78%; Digoxin 38% | 8-OHdG | Blood sample | HF : 0,75 ±0,57 Controls: 0.23±0.07 | 0,003 |
| Telomere | |||||||||
| [31] | HF 620 (493M e 127F) 183 Controls (145M e 38F) | HF 66.2±8.9 Controls 66.2±8.7 | HFrEF | ICHF and NICHF | 51% BB; 88% ACEi ; 10% ARBs ; 93% Diuretics | Telomere | Blood samples | HF: 0,64±0,30 Controls: 1,05±0,32 | < 0.001 |
| [32] | HF 34 (24M/10F) Controls 46 (31M/15F) | HF 56±9 Controls 46±16 | HFrEF | IDC | Telomere | Blood samples | IDCM: 6.97±0.63 Controls: 9.11±2.12 | < 0.001 | |
| [33] | CHF 27 (23M/4F) Controls 24 (20M/4F) | HF 66±6,6 Controls 69±6.9 | HFrEF | 100% ACEI/ARB; 96% BB; 81% Diuretics; 88% Statins; 96% anticoagulants ; 25% aldosterone antagonists | Telomere | Blood samples | HF: 0,55±0,074 Controls: 0,70±0,074 | 0,002 | |
| [34] | 40 CHF (17M e 23F) Controls 40 (18M e 22F) | HF 82 (77–89) Controls 80 (76–85) | HFrEF | ICHF | Telomere | leukocytes | HF: 0.18±0.08 Controls: 0.38 ±0.25 | < 0.01 | |
| Malondialdehyde | |||||||||
| [35] | HF 58 (43M/15F) Controls 17 (12M/7F) | HFrEF | IDCM, IHD | 76% ACEI; 24% BB; 79% Diuretics; 58% Digoxin; 41% ASA; 15% Amiodarone | MDA | Blood sample | HF: 0.65±0,10 Controls: 0,25±0,05 | < 0,005 | |
| [36] | HF 12 (10M/2F) Controls 6 (6M) | HF (mean 52) Controls (mean 23) | HFrEF and HFpEF | HVD, HHD, DCM | 100% diuretics; 100% digitalis | MDA | Blood sample | HF: 3.7±1.3 Controls: 1.9±0.6 | < 0.01 |
| [37] | HF 12 (M10/F2) Controls 4 (4M) | DCM (54 ± 2) Controls (18, 25-27, 37) | HFrEF | DCM | MDA | Heart tissue | HF: 152±38 Controls: 74 ± 6 | ||
| [38] | HF 109 (93M/16F) Controls 28 | HF (45.97±10.8) | HFrEF | DCM | 94.5% BB, 92.66% ACEIs, 35.78% ARBs, 90.83% MRAs, 13.76% Amiodarone, LDs 60.55%, TDs 18.35%, OACs 44.95%, 63.30% digoxin | MDA | Blood sample | HF: 4.37 (3.68–5.78) Controls: 1.31 (1.14–1.41 | < 0.001 |
| [39] | HF 10 (7M/3F) Controls 69 (40M/29F) | 61.6±5.5 | HFrEF | 81.8% BB, 36.4% CCBs, 81.8% aspirin, 63.9% nitrates, 90.9% ACEi, 9.1% ARBs, 54.5% statins, 90.9% spironolactone, furosemide | MDA | Blood sample | HF: 8.5±0.6 Controls: 7.3±0.9 | < 0.05 | |
| [40] | HF 30 (30M) Controls 16 (14M/2F) | HF 63±8.2 Controls 53.8±15.7 | HFrEF | 76.7% ACEI, 87.7% nitrates, 63.3% diuretics, 73.3% digitalis, 3% hydralazine | MDA | Blood sample | HF: 2.65±1.3 Controls: 1.45±0.77 | < 0,05 | |
| [41] | CHF 45 (37M/8F) Controls 45 (29M/16F) | HF 58 range (27-68) Controls 62 range (40-74) | Atherosclerosis | MDA | Blood sample | HF: 9 (IQR 7,9-10,2) Controls: 7,7 (IQR 6,9-9,2) | < 0,01 | ||
| [42] | CHF 53 Controls 38 | HFrEF. HFpEF | IHD, HVD, IDC, CCHD | Diuretics, digoxin and vasodilators. | MDA | Blood sample | HF: 10,3 (IQR 9-12) Controls: 7,9 (IQR 7-9) | <0,001 | |
| [43] | HF 29 (19M/10F) Controls 15 (8M/7F) | HFrEF | IHD | MDA | Blood sample | HF: 16±7.48 Controls: 8±3.16 | <0,001 | ||
| [44] | CHF 30 (13M/17F) Controls 55 (30M/25F) | CHF 73.1±7.4, Controls 80±17.4 | HFrEF | Diuretics, BB, CAs, ACEi. | MDA | Blood sample | HF: 0.32 (0.21–0.52) Controls: 0.21 (0.17–0.25) | < 0.001 | |
| [39] | HF 12 (9M/3F) Controls 25 (17M/8F) | HF 60.8 ± 4.6 Controls 56.0 ± 4.6 | HFrEF | IHD | MDA | Blood sample | HF: 5.54±2.29 Controls: 1.49±0.85 | < 0.001 | |
| [44] | HF 28 (24M/4F) Controls 15 (10M/5F) | HF 61.9±2.6 | HFrEF | IHD, DCM | ACEi 38%, ARBs 38%, Diuretics 86%, Spironolactone 38%, Digoxin 66%, BB 24% | MDA | Blood sample | HF: 3.71±0.10; Controls: 2.69±0.12 | < 0.001 |
| Isoprostanes | |||||||||
| [39] | HF 12 (9M/3F) Controls 25 (17M/8F) | HF 60.8 ± 4.6 Controls 56.0 ± 4.6 | HFrEF | IHD | Isoprostanes | Urine sample | HF: 1930±880 Controls: 350±300 | < 0.001 | |
| [46] | HF 25 (22M/3F) Controls 25 (22M/3F) | HF 57 (27–75) | HFrEF | ICM, IDCM | ACEi 92%, LDs 88%, Digoxin 72%, Spironolactone 48%, BB 40%, ASA 40%, OAC 28%, Nitrates 4% | Isoprostaglandin F2α type III | Urine sample | HF: 2590±1910 Controls: 1100±360 |
< 0,0001 |
| [47] | CHF 39 (33M/ 6F) Controls 27 (21M/6F) | HF 66±10 Controls 60±10 | IHD, IDCM, VHD | ACEi/ARBs 22%, BB 79%, ASA 38%, Diuretics 77%, Digoxin 46%, Spironolactone 54%, Warfarin 41%, Statins 67% | Isoprostanes | Blood sample | HF: 449 (IQR: 173-698) Controls: 82 (IQR: 53-95) | <0.001 | |
| [48] | CHF 30 (14M/16F) Controls 30 (18M/12F) | HFrEF, HFpEF | Ischaemic origin | Diuretics, BB, CCBs, ACEi | Isoprostanes 8,12-iso-iPF2α-VI | Blood sample | CHF: 356.1±150.8 Controls: 136.3±52.1 | < 0.0001 | |
| [49] | HF 20 (16M/4F) controls 20 (16M/4F) | HF 46±7 controls 47±6 | HFrEF | DCM | Antiplatelets, ACE inhibitors, Nitrates, α-receptor blockers, CCBs, NSAIDs, Diuretics | Isoprostanes 8-epi-PGF2a | Urine sample | HF: 402 ± 40 Controls 236 ± 44 | < 0.001 |
| [50] | HF 15 (7M/8F) Controls 15 (8M/7F) | HFrEF | HVD, HHD, IDCM, CHD | Nitrates n=6, Ca antagonists n=6, BB n=3, ACEi n=15, Digoxin n=13, Diuretics n=23 | Isoprostane 15-F2t | urine sample | HF: 600 (IQR 355–720) Controls: 198 (IQR 125–281) | < 0,001 | |
| [45] | HF 28 (24M/4F) controls 15 (10M/5F) | HF 61.9±2.6 | HFrEF | IHD, IDCM | ACEi 38%, ARBs 38%, Diuretics 86%, Spironolactone 38%, Digoxin 66%, BB 24% | isoprostanes 8-epi-PGF2a | Blood sample | HF: 234±19 Controls: 133 ± 29 | <0,01 |
| Myeloperoxidase | |||||||||
| [51] | HF 102 (58%M/42%F) controls 105 | HF 65±14 Controls 44±11 |
HFrEF | IHD | MPO | Blood sample | HF: 212 (160-262) Controls: 153 (138-178) | < 0,0001 | |
| [52] | HF 46 (23M/23F) control 48 (24M/24F) | HF 72±8 controls 73±5 | HFrEF | MPO | Blood sample | HF: 212 (160-262) Controls: 153 (138-178) | < 0,05 | ||
| [53] | HF 86 (42M/44F) controls 46 | HF 73 (66-79) | HFrEF | ARBs 33%, ACEi 49%, Thiazide 16%, Potassium-sparing 21%, LDs 73%, CCBs 31%, BB 80%, Anticoags 55%, AP 34%, Statins 44%, Nitro 14%, Glucose-lowering 20% | MPO | Blood sample | HF: 101 (81-132) Controls: 86 (74-101) | < 0,015 | |
| [54] | HF 55 (19M/36F) controls 18 | HF 80±8.7 | HFrEF | ICM | LDs 76%, Mineralocorticoid receptor antagonists 33%, BB 62%, ACEi/ARBs 78%, Statins 64% | MPO | Blood sample | HF: 34.7 (22.7-44.0) Controls: 22.6 (18.2-32.0) | < 0,026 |
| [55] | HF 28 (22M/6F) Controls 1303 (730 M /573F) |
HF 68 (51-80) Controls 63 (45-80) | HFpEF | IHD | ACEi 88%, LDs 70%, Other diuretics 12%, BB 67%, Aldosterone antagonists 33%, CCBs 19% | MPO | Blood sample | HF: 49.5 (30.5-102.5) Controls: 27.3 (7.7-156.9) | < 0.0001 |
| [56] | HF 23 (20M/3F) Controls 14 (14M) | HF 68 (51-80) Controls 63 (45-80) | HFrEF | ICM, IHD | MPO | Blood sample | HF: 33.6 (11.7–206.9) Controls: 18.3 (5.4–102.4) | <0,02 | |
| [57] | HF 285 (215M/70F) controls 35 | HF 71.2 ± 11.3 | HFrEF, HFpEF | IHD | ACEi/ARBs 82.8%, BB 69.8%, Digoxin 23.8%, ASA 67.7%, Spironolactone 54%, Diuretics 82.1%, Statins 62.4% | MPO | Blood sample | HF: 205.7±272.6 Controls: 123± 170.5 | =0.01 |
| [58] | HF 68 (45M/23F) controls 10 | HF 64.3±13.4 | HFrEF | IHD | MPO | Blood sample | HF: 9,3±7 Controls: 4,19±2 | < 0,007 | |
| [56] | HF 27 (14M/13F) Control 40 (29M/11F) | HF 64 (49–85) Control 66 (42–87) | HFrEF | Diuretics 51,85%, ACE 48,15, Cardiac glycosides 12,5%, Organic nitrate 4,17%, Statins 48,15% | MPO | Blood sample | HF: 1.1 (1.0–1.2) Controls: 0.80 (0.62–0.98) | p ˂ 0.05 | |
| Nitrotirosine | |||||||||
| [56] | HF 23 (20M/3F) Controls 14 (14M) | HFrEF | ICM, IHD | Nitrotirosine | Blood sample | HF: 6.3 (0.0–67.6) Controls: 0.0 (0.0–45.0) | <0,02 | ||
| [45] | HF 28 (24M/4F) 15 Controls (10M/5F) | HF 61.9±2.6 | HFrEF | IHD, IDCM | ACEi 38%, ARBS 38%,Diuretics 86%,Spironolactone 38%,Digoxin 66%,BB 24% | Nitrotirosine | Blood sample | HF: 469±39 Controls: 313±40 | <0.05 |
| [59] | HF 27 (14M/13F) Controls 40 (29M/11F) | HF 64 (49–85) Controls 66 (42–87) | HFrEF | Diuretics 51,85%, ACE 48,15, Cardiac glycosides 12,5%, Organic nitrate 4,17%, Statins 48,15% | Nitrotirosine | Blood sample | HF: 206.6 (154.6–307.2) Controls: 181.1 (114.4–234.4) | =0.0005 | |
| [60] | 38 HF and 8 Controls | HF 55.5±10.0 Controls 47.6±13.0 | HFrEF | IHD, DCM | ACEi 89.5%, digitalis 84.2%, diuretic 89.5%, and nitrates 23.6% | Nitrotirosine | Muscle tissue | HF: 13.5±8.5 Controls: 2.0±1.7 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





