Submitted:
20 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
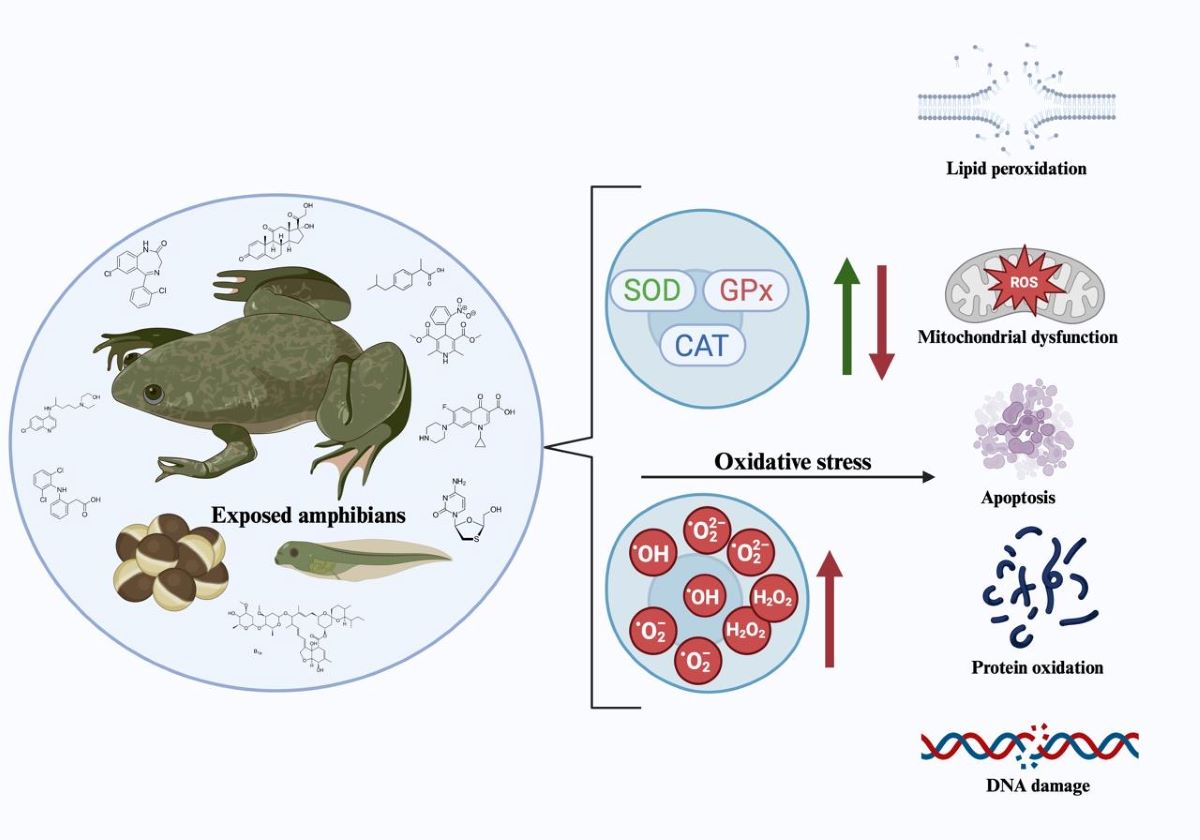
Keywords:
1. Introduction
1.1. Amphibians
1.2. Emerging Contaminants
1.3. Pharmaceuticals in the Environment
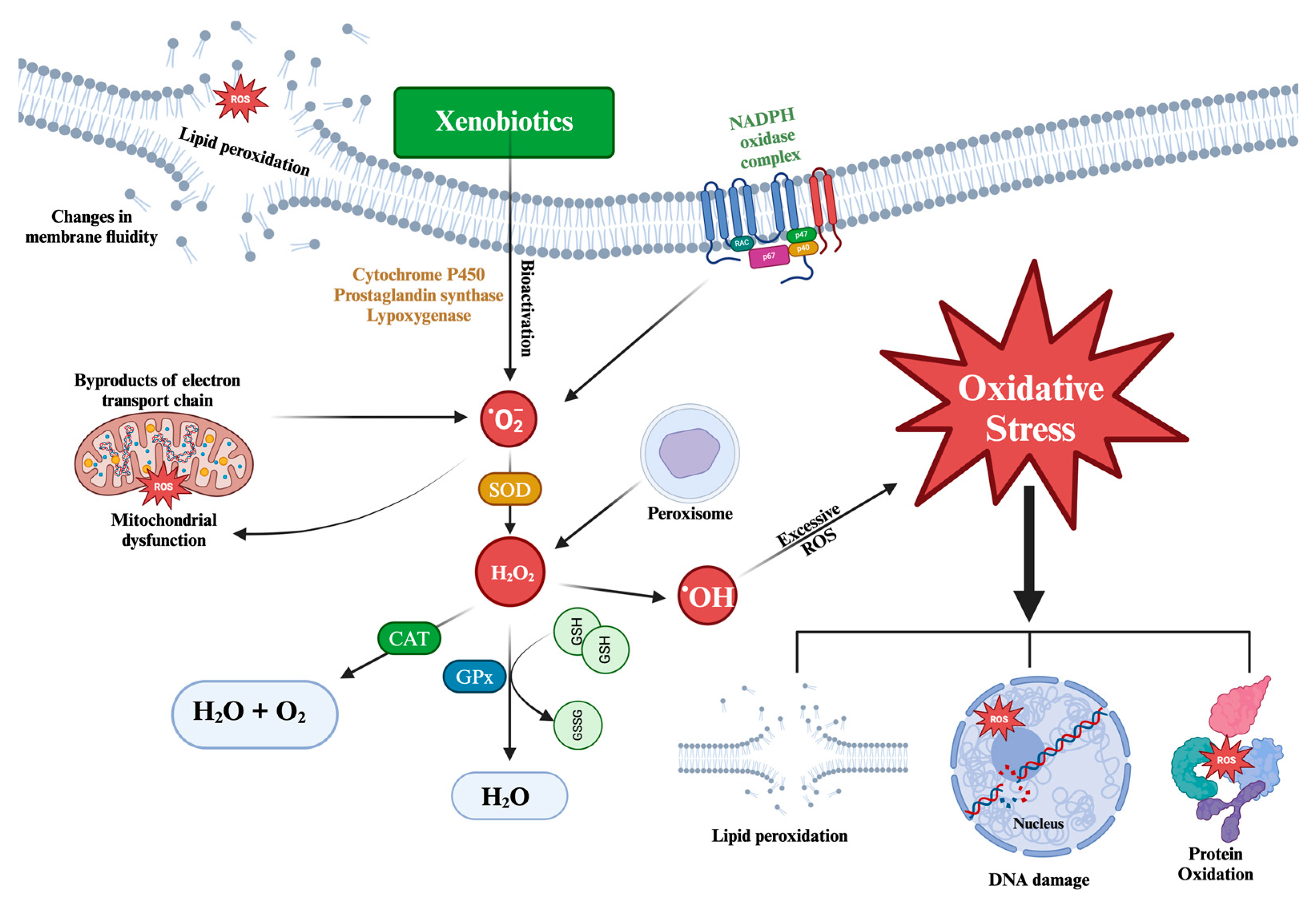
1.4. Oxidative Stress
1.4.1. Oxidative Stress Biomarkers
1.4.1.1. Oxidative Damage
1.4.1.2 Antioxidant Defenses
2. Methodology
- Experimental investigations analyzing the impact of drug exposure on oxidative stress biomarkers in amphibians.
- Articles published in peer-reviewed scientific journals in English.
- Studies providing data on at least one oxidative stress biomarker and drug-specific class.
- Research that clearly identifies the amphibian species used in this study.
- Studies using plant extracts or natural compounds instead of synthetic drugs.
- Studies that did not provide quantitative data on biomarkers of oxidative stress.
- Studies that focus exclusively on drug bioaccumulation without evaluating biomarkers of oxidative stress.
- Studies that do not provide sufficient information on experimental conditions.
3. Results
3.1. Antibiotics
3.2. Nonsteroidal Anti-Inflammatory Drugs
3.3. Antivirals
3.4. Antihypertensive
3.5. Glucocorticoids
3.6. Pharmaceutical Mixture
3.7. Anesthetic
3.8. Benzodiazepines
3.9. Antiparasitic
4. Conclusions and Future Research
Author Contributions
Conflicts of Interest
References
- Berkovitz, B.; Shellis, P. Amphibians. The Teeth of Non-Mammalian Vertebrates 2023, 203–257. [CrossRef]
- O’Rourke, D.P.; Rosenbaum, M.D. Biology and Diseases of Amphibians. Laboratory Animal Medicine: Third Edition 2015, 931–965. [CrossRef]
- Vitt, L.J.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles: Fourth Edition; 2013.
- Wells, K.D. The Ecology and Behavior of Amphibians; 2013.
- Gower, D.J.; Wilkinson, M. Conservation Biology of Caecilian Amphibians. Conservation Biology 2005, 19. [Google Scholar] [CrossRef]
- Rozenblit, F.; Gollisch, T. What the Salamander Eye Has Been Telling the Vision Scientist’s Brain. Semin Cell Dev Biol 2020, 106, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Elinson, R.P.; del Pino, E.M. Developmental Diversity of Amphibians. Wiley Interdiscip Rev Dev Biol 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.R. Amphibian Species of the World. Available at Http://Research.Amnh.Org/Herpetology/Amphibia/Index.Html. American Museum of Natural History, New York, USA. Accessed on 09 Sep 2023. 2023.
- Rivera-Correa, M.; Baldo, D.; Candioti, F.V.; Orrico, V.G.D.; Blackburn, D.C.; Castroviejo-Fisher, S.; Chan, K.O.N.N.; Gambale, P.; Gower, D.J.; Quah, E.S.H.; et al. Amphibians in Zootaxa: 20 Years Documenting the Global Diversity of Frogs, Salamanders, and Caecilians. Zootaxa 2021, 4979. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.C.; Wake, D.B. Class Amphibia Gray, 1825. In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-Level Classi fication and Survey of Taxonomic Richness. Zootaxa 2011, 3148. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive Review of Emerging Contaminants: Detection Technologies, Environmental Impact, and Management Strategies. Ecotoxicol Environ Saf 2024, 278, 116420. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, D. Global Performance and Trends of Research on Emerging Contaminants in Sewage Sludge: A Bibliometric Analysis from 1990 to 2023. Ecotoxicol Environ Saf 2024, 281, 116597. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E.B. Endocrine-Disrupting Chemicals and Disease Endpoints. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and Polyfluoroalkyl Substances in the Environment. Science (1979) 2022, 375. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in Water Systems: A Review of Their Impacts on the Environment and Their Potential Hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, J.; Yue, T.; Zhang, X.; Liu, J.; Bai, J. Distribution, Chemical Fractionation, and Potential Environmental Risks of Hg, Cr, Cd, Pb, and As in Wastes from Ultra-Low Emission Coal-Fired Industrial Boilers in China. J Hazard Mater 2023, 446, 130606. [Google Scholar] [CrossRef]
- Sorvari, J.; Wahlström, M. Industrial By-Products. Handbook of Recycling: State-of-the-art for Practitioners, Analysts, and Scientists 2024, 259–285. [CrossRef]
- Bean, T.G.; Chadwick, E.A.; Herrero-Villar, M.; Mateo, R.; Naidoo, V.; Rattner, B.A. Do Pharmaceuticals in the Environment Pose a Risk to Wildlife? Environ Toxicol Chem 2024, 43. [Google Scholar] [CrossRef]
- Toutain, P.L.; Ferran, A.; Bousquet-Mélou, A. Species Differences in Pharmacokinetics and Pharmacodynamics. Handb Exp Pharmacol 2010, 199. [Google Scholar]
- Fernández, C.; Beltrán, E.M.; Tarazona, J. V. Pharmaceuticals Effects in the Environment. Encyclopedia of Toxicology: Third Edition 2014, 844–848. [CrossRef]
- Xu, M.; Huang, H.; Li, N.; Li, F.; Wang, D.; Luo, Q. Occurrence and Ecological Risk of Pharmaceuticals and Personal Care Products (PPCPs) and Pesticides in Typical Surface Watersheds, China. Ecotoxicol Environ Saf 2019, 175, 289–298. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and Removal of Pharmaceuticals and Endocrine Disruptors in South Korean Surface, Drinking, and Waste Waters. Water Res 2007. [Google Scholar] [CrossRef]
- Ebele, A.J.; Oluseyi, T.; Drage, D.S.; Harrad, S.; Abou-Elwafa Abdallah, M. Occurrence, Seasonal Variation and Human Exposure to Pharmaceuticals and Personal Care Products in Surface Water, Groundwater and Drinking Water in Lagos State, Nigeria. Emerg Contam 2020. [Google Scholar] [CrossRef]
- Shen, X.; Chang, H.; Sun, Y.; Wan, Y. Determination and Occurrence of Natural and Synthetic Glucocorticoids in Surface Waters. Environ Int 2020, 134, 105278. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Li, Y.; Rehman, M.S.U.; Zubair, M.; Mustafa, G.; Nazar, M.F.; Yu, C.P.; Sun, Q. Occurrence, Spatial Variation and Risk Assessment of Pharmaceuticals and Personal Care Products in Urban Wastewater, Canal Surface Water, and Their Sediments: A Case Study of Lahore, Pakistan. Science of the Total Environment 2019, 688, 653–663. [Google Scholar] [CrossRef]
- Mainero Rocca, L.; Gentili, A.; Caretti, F.; Curini, R.; Pérez-Fernández, V. Occurrence of Non-Steroidal Anti-Inflammatory Drugs in Surface Waters of Central Italy by Liquid Chromatography–Tandem Mass Spectrometry. Int J Environ Anal Chem 2015, 95, 685–697. [Google Scholar] [CrossRef]
- Praveena, S.M.; Shaifuddin, S.N.M.; Sukiman, S.; Nasir, F.A.M.; Hanafi, Z.; Kamarudin, N.; Ismail, T.H.T.; Aris, A.Z. Pharmaceuticals Residues in Selected Tropical Surface Water Bodies from Selangor (Malaysia): Occurrence and Potential Risk Assessments. Science of The Total Environment 2018, 642, 230–240. [Google Scholar] [CrossRef]
- López-Serna, R.; Jurado, A.; Vázquez-Suñé, E.; Carrera, J.; Petrović, M.; Barceló, D. Occurrence of 95 Pharmaceuticals and Transformation Products in Urban Groundwaters Underlying the Metropolis of Barcelona, Spain. Environmental Pollution 2013. [Google Scholar] [CrossRef] [PubMed]
- Björlenius, B.; Ripszám, M.; Haglund, P.; Lindberg, R.H.; Tysklind, M.; Fick, J. Pharmaceutical Residues Are Widespread in Baltic Sea Coastal and Offshore Waters – Screening for Pharmaceuticals and Modelling of Environmental Concentrations of Carbamazepine. Science of the Total Environment 2018. [Google Scholar] [CrossRef] [PubMed]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and Spatial Distribution of 158 Pharmaceuticals, Drugs of Abuse and Related Metabolites in Offshore Seawater. Science of the Total Environment 2016. [Google Scholar] [CrossRef]
- Lolić, A.; Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Correia, M.; Delerue-Matos, C. Assessment of Non-Steroidal Anti-Inflammatory and Analgesic Pharmaceuticals in Seawaters of North of Portugal: Occurrence and Environmental Risk. Science of the Total Environment 2015, 508, 240–250. [Google Scholar] [CrossRef]
- Li, Y.; Sallach, J.B.; Zhang, W.; Boyd, S.A.; Li, H. Insight into the Distribution of Pharmaceuticals in Soil-Water-Plant Systems. Water Res 2019, 152. [Google Scholar] [CrossRef]
- Martínez-Piernas, A.B.; Plaza-Bolaños, P.; García-Gómez, E.; Fernández-Ibáñez, P.; Agüera, A. Determination of Organic Microcontaminants in Agricultural Soils Irrigated with Reclaimed Wastewater: Target and Suspect Approaches. Anal Chim Acta 2018. [Google Scholar] [CrossRef]
- Lees, K.; Fitzsimons, M.; Snape, J.; Tappin, A.; Comber, S. Pharmaceuticals in Soils of Lower Income Countries: Physico-Chemical Fate and Risks from Wastewater Irrigation. Environ Int 2016. [CrossRef] [PubMed]
- Fonseca, V.F.; Duarte, I.A.; Duarte, B.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Gillanders, B.M.; Reis-Santos, P. Environmental Risk Assessment and Bioaccumulation of Pharmaceuticals in a Large Urbanized Estuary. Science of the Total Environment 2021, 783. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, L.; Snape, J.R.; Verbruggen, B.; Owen, S.F.; Kristiansson, E.; Margiotta-Casaluci, L.; Österlund, T.; Hutchinson, K.; Leverett, D.; Marks, B.; et al. Pharmacology beyond the Patient – The Environmental Risks of Human Drugs. Environ Int 2019, 129, 320–332. [Google Scholar] [CrossRef]
- Jacob, R.S.; Araújo, C.V.M.; Santos, L.V. de S.; Moreira, V.R.; Lebron, Y.A.R.; Lange, L.C. The Environmental Risks of Pharmaceuticals beyond Traditional Toxic Effects: Chemical Differences That Can Repel or Entrap Aquatic Organisms. Environmental Pollution 2021, 268. [Google Scholar] [CrossRef]
- Gómez-Regalado, M. del C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E.; Zafra-Gómez, A. Bioaccumulation/Bioconcentration of Pharmaceutical Active Compounds in Aquatic Organisms: Assessment and Factors Database. Science of The Total Environment 2023, 861, 160638. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of Psychoactive Pharmaceuticals in Fish in an Effluent Dominated Stream. Water Res 2017. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lu, G.; Yan, Z.; Liu, J.; Wang, P.; Wang, Y. Bioaccumulation and Trophic Transfer of Pharmaceuticals in Food Webs from a Large Freshwater Lake. Environmental Pollution 2017. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.; Vijayanandan, A. Human Health and Ecological Risk Assessment of 98 Pharmaceuticals and Personal Care Products (PPCPs) Detected in Indian Surface and Wastewaters. Science of the Total Environment 2022, 807. [Google Scholar] [CrossRef] [PubMed]
- Teran-Velasquez, G.; Helm, B.; Krebs, P. High Spatiotemporal Model-Based Tracking and Environmental Risk-Exposure of Wastewater-Derived Pharmaceuticals across River Networks in Saxony, Germany. Water (Switzerland) 2023, 15. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Eustress and Distress in Redox Homeostasis. In Stress: Physiology, Biochemistry, and Pathology Handbook of Stress Series, Volume 3; 2019.
- Singh, A.K.; Rana, H.K.; Pandey, A.K. The Oxidative Stress: Causes, Free Radicals, Targets, Mechanisms, Affected Organs, Effects, Indicators. In Antioxidants Effects in Health: The Bright and the Dark Side; 2022.
- Freitas, J.S. Pollutants and Oxidative Stress in Tadpoles. In Toxicology of Amphibian Tadpoles; 2024.
- El-SiKaily, A.; Shabaka, S. Biomarkers in Aquatic Systems: Advancements, Applications and Future Directions. Egypt J Aquat Res 2024, 50, 169–182. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular Biomarkers of Oxidative Stress in Aquatic Organisms in Relation to Toxic Environmental Pollutants. Ecotoxicol Environ Saf 2006, 64. [Google Scholar] [CrossRef]
- Klran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative Stress and Antioxidants in Health and Disease. Journal of Laboratory Medicine 2023, 47. [Google Scholar]
- Kisty, E.A.; Falco, J.A.; Weerapana, E. Redox Proteomics Combined with Proximity Labeling Enables Monitoring of Localized Cysteine Oxidation in Cells. Cell Chem Biol 2023, 30. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M. 8-Oxoguanine and 8-Oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Castillo-González, C.; Barcenilla, B.B.; Young, P.G.; Hall, E.; Shippen, D.E. Quantification of 8-OxoG in Plant Telomeres. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Lee, T.H. Antioxidant Enzymes as Redox-Based Biomarkers: A Brief Review. BMB Rep 2015, 48. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singh, S.; Sharma, P. Role of Antioxidants in Neutralizing Oxidative Stress. Nutraceutical Fruits and Foods for Neurodegenerative Disorders 2024, 353–378. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alexandria Journal of Medicine 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An Insight on Superoxide Dismutase (SOD) from Plants for Mammalian Health Enhancement. J Funct Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Jing, M.; Han, G.; Wan, J.; Zhang, S.; Yang, J.; Zong, W.S.; Niu, Q.; Liu, R. Catalase and Superoxide Dismutase Response and the Underlying Molecular Mechanism for Naphthalene. Science of The Total Environment 2020, 736, 139567. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Orian, L. The Glutathione Peroxidase Family: Discoveries and Mechanism. Free Radic Biol Med 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Schueler, F.W.; Rollinson, N. A 91% Decline in a Common Anuran in an Otherwise Stable Amphibian Community Inferred from 17 Years of Rapid Road Surveys. Anim Conserv 2024, 27. [Google Scholar] [CrossRef]
- Campbell Grant, E.H.; Amburgey, S.M.; Gratwicke, B.; Acosta-Chaves, V.; Belasen, A.M.; Bickford, D.; Brühl, C.A.; Calatayud, N.E.; Clemann, N.; Clulow, S.; et al. Priority Research Needs to Inform Amphibian Conservation in the Anthropocene. Conserv Sci Pract 2023, 5. [Google Scholar] [CrossRef]
- Johnson, M.S.; Aubee, C.; Salice, C.J.; Leigh, K.B.; Liu, E.; Pott, U.; Pillard, D. A Review of Ecological Risk Assessment Methods for Amphibians: Comparative Assessment of Testing Methodologies and Available Data. Integr Environ Assess Manag 2017, 13. [Google Scholar] [CrossRef]
- Seneviratne, A.; Dissanayake, M.R.; Sumanasekara, V. Review on Use of Amphibian Taxa as a Bio-Indicator for Watershed Health and Stresses. Environmental Studies and Services Division, NBRO 2015.
- Melvin, S.D. Oxidative Stress, Energy Storage, and Swimming Performance of Limnodynastes Peronii Tadpoles Exposed to a Sub-Lethal Pharmaceutical Mixture throughout Development. Chemosphere 2016, 150, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Gnatyshyna, L.; Horyn, O.; Sokolova, I.; Stoliar, O. Endocrine and Cellular Stress Effects of Zinc Oxide Nanoparticles and Nifedipine in Marsh Frogs Pelophylax Ridibundus. Aquatic Toxicology 2017, 185. [Google Scholar] [CrossRef]
- Falfushynska, H.I.; Gnatyshyna, L.L.; Horyn, O.; Stoliar, O.B. Vulnerability of Marsh Frog Pelophylax Ridibundus to the Typical Wastewater Effluents Ibuprofen, Triclosan and Estrone, Detected by Multi-Biomarker Approach. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2017, 202, 26–38. [Google Scholar] [CrossRef]
- Peltzer, P.M.; Lajmanovich, R.C.; Attademo, A.M.; Junges, C.M.; Teglia, C.M.; Martinuzzi, C.; Curi, L.; Culzoni, M.J.; Goicoechea, H.C. Ecotoxicity of Veterinary Enrofloxacin and Ciprofloxacin Antibiotics on Anuran Amphibian Larvae. Environ Toxicol Pharmacol 2017, 51. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, P.M.; Lajmanovich, R.C.; Martinuzzi, C.; Attademo, A.M.; Curi, L.M.; Sandoval, M.T. Biotoxicity of Diclofenac on Two Larval Amphibians: Assessment of Development, Growth, Cardiac Function and Rhythm, Behavior and Antioxidant System. Science of the Total Environment 2019, 683. [Google Scholar] [CrossRef] [PubMed]
- Cuzziol Boccioni, A.P.; Peltzer, P.M.; Martinuzzi, C.S.; Attademo, A.M.; León, E.J.; Lajmanovich, R.C. Morphological and Histological Abnormalities of the Neotropical Toad, Rhinella Arenarum (Anura: Bufonidae) Larvae Exposed to Dexamethasone. J Environ Sci Health B 2020, 56. [Google Scholar] [CrossRef]
- Fernández, L.P.; Brasca, R.; Attademo, A.M.; Peltzer, P.M.; Lajmanovich, R.C.; Culzoni, M.J. Bioaccumulation and Glutathione S-Transferase Activity on Rhinella Arenarum Tadpoles after Short-Term Exposure to Antiretrovirals. Chemosphere 2020, 246. [Google Scholar] [CrossRef]
- da Luz, T.M.; Araújo, A.P. da C.; Estrela, F.N.; Braz, H.L.B.; Jorge, R.J.B.; Charlie-Silva, I.; Malafaia, G. Can Use of Hydroxychloroquine and Azithromycin as a Treatment of COVID-19 Affect Aquatic Wildlife? A Study Conducted with Neotropical Tadpole. Science of The Total Environment 2021, 780, 146553. [Google Scholar] [CrossRef]
- Fogliano, C.; Motta, C.M.; Venditti, P.; Fasciolo, G.; Napolitano, G.; Avallone, B.; Carotenuto, R. Environmental Concentrations of a Delorazepam-Based Drug Impact on Embryonic Development of Non-Target Xenopus Laevis. Aquatic Toxicology 2022, 250. [Google Scholar] [CrossRef]
- Lourido, M.; Peluso, J.; Aronzon, C.M. Lethal and Sublethal Effects of the Emerging Contaminant Oxytetracycline on the Embryo-Larval Development of Rhinella Arenarum. Environ Toxicol Pharmacol 2022, 89, 103783. [Google Scholar] [CrossRef]
- Rutkoski, C.F.; Grott, S.C.; Israel, N.G.; Carneiro, F.E.; de Campos Guerreiro, F.; Santos, S.; Horn, P.A.; Trentini, A.A.; Barbosa da Silva, E.; Coelho de Albuquerque, C.A.; et al. Hepatic and Blood Alterations in Lithobates Catesbeianus Tadpoles Exposed to Sulfamethoxazole and Oxytetracycline. Chemosphere 2022, 307. [Google Scholar] [CrossRef] [PubMed]
- Gavrilović, B.R.; Despotović, S.G.; Petrović, T.G.; Radovanović, T.B.; Gavrić, J.P.; Mirč, M.; Anđelković, M.; Vukov, T.; Tomašević Kolarov, N.; Prokić, M.D. Does the Anesthetic Tricaine Methanesulfonate (MS-222) Distort Oxidative Status Parameters in Tadpoles? Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2024, 278, 109859. [Google Scholar] [CrossRef]
- Laçin, C.; Turhan, D.O.; Güngördü, A. Assessing the Impact of Antiviral Drugs Commonly Utilized during the COVID-19 Pandemic on the Embryonic Development of Xenopus Laevis. J Hazard Mater 2024, 472, 134462. [Google Scholar] [CrossRef]
- Peluso, J.; Martínez Chehda, A.; Olivelli, M.S.; Aronzon, C.M. Ecotoxicological Effects of the Emerging Contaminant Ivermectin on Rhinella Arenarum: A Comparative Study of Active Ingredient and Commercial Formulation. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2024, 283, 109965. [Google Scholar] [CrossRef]
- Peluso, J.; Chehda, A.M.; Aronzon, C.M. A Multi-Approach Analysis of the Toxicity of a Commercial Formulation of Monensin on Rhinella Arenarum Embryos and Larvae. Environ Toxicol Pharmacol 2024, 108, 104454. [Google Scholar] [CrossRef]
- Rutkoski, C.F.; Grott, S.C.; Israel, N.G.; Guerreiro, F. de C.; Carneiro, F.E.; Bitschinski, D.; Warsneski, A.; Horn, P.A.; Lima, D.; Bastolla, C.L.V.; et al. Prednisone and Prednisolone Effects on Development, Blood, Biochemical and Histopathological Markers of Aquarana Catesbeianus Tadpoles. Aquatic Toxicology 2024, 268. [Google Scholar] [CrossRef]
- Alishiri, M.; Abdollahi, S.A.; Neysari, A.N.; Ranjbar, S.F.; Abdoli, N.; Afsharjahanshahi, M. Removal of Ciprofloxacin and Cephalexin Antibiotics in Water Environment by Magnetic Graphene Oxide Nanocomposites; Optimization Using Response Surface Methodology. Results in Engineering 2023, 20, 101507. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Chen, S.; Guan, X.; Zhong, Y.; Yang, Q. Occurrence, Risk Assessment, and in Vitro and in Vivo Toxicity of Antibiotics in Surface Water in China. Ecotoxicol Environ Saf 2023, 255, 114817. [Google Scholar] [CrossRef]
- Wat, C.C.Y.; Xin, X.; Lai, R.W.S.; Mao, X.; Leung, K.M.Y. Impact of Environmental Factors Changes Induced by Marine Heatwaves and Heavy Precipitation on Antibiotic Toxicity to Isochrysis Galbana: Implications for Climate Change Adaptation. Mar Pollut Bull 2024, 203, 116453. [Google Scholar] [CrossRef]
- Fu, L.; Huang, T.; Wang, S.; Wang, X.; Su, L.; Li, C.; Zhao, Y. Toxicity of 13 Different Antibiotics towards Freshwater Green Algae Pseudokirchneriella Subcapitata and Their Modes of Action. Chemosphere 2017, 168, 217–222. [Google Scholar] [CrossRef]
- Martins, A.; Guimarães, L.; Guilhermino, L. Chronic Toxicity of the Veterinary Antibiotic Florfenicol to Daphnia Magna Assessed at Two Temperatures. Environ Toxicol Pharmacol 2013, 36, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Yisa, A.G.; Chia, M.A.; Gadzama, I.M.K.; Oniye, S.J.; Sha’aba, R.I.; Gauje, B. Immobilization, Oxidative Stress and Antioxidant Response of Daphnia Magna to Amoxicillin and Ciprofloxacin. Environ Toxicol Pharmacol 2023, 98, 104078. [Google Scholar] [CrossRef]
- Bawa-Allah, K.A.; Ehimiyein, A.O. Ecotoxicological Effects of Human and Veterinary Antibiotics on Water Flea (Daphnia Magna). Environ Toxicol Pharmacol 2022, 94, 103932. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shen, Z.; Ding, J.; Qu, M.; Li, T.; Tong, M.; Di, Y. Sulfamethoxazole Induced Systematic and Tissue-Specific Antioxidant Defense in Marine Mussels (Mytilus Galloprovincialis): Implication of Antibiotic’s Ecotoxicity. Chemosphere 2021, 279, 130634. [Google Scholar] [CrossRef] [PubMed]
- Lacaze, E.; Pédelucq, J.; Fortier, M.; Brousseau, P.; Auffret, M.; Budzinski, H.; Fournier, M. Genotoxic and Immunotoxic Potential Effects of Selected Psychotropic Drugs and Antibiotics on Blue Mussel (Mytilus Edulis) Hemocytes. Environmental Pollution 2015, 202, 177–186. [Google Scholar] [CrossRef]
- Giannessi, J.; De Marchi, L.; Meucci, V.; Intorre, L.; Monni, G.; Baratti, M.; Pretti, C. Subcellular Tissue-Specific Responses of Mytilus Galloprovincialis to Fluoroquinolone Antibiotics. Environ Toxicol Pharmacol 2023, 104, 104306. [Google Scholar] [CrossRef]
- Liang, X.; Wang, F.; Li, K.; Nie, X.; Fang, H. Effects of Norfloxacin Nicotinate on the Early Life Stage of Zebrafish (Danio Rerio): Developmental Toxicity, Oxidative Stress and Immunotoxicity. Fish Shellfish Immunol 2020, 96, 262–269. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Gu, H.; Zhang, L.; Shang, Y.; Wang, T.; Wang, T.; Zeng, J.; Ma, L.; Huang, W.; et al. Short-Term Exposure to Norfloxacin Induces Oxidative Stress, Neurotoxicity and Microbiota Alteration in Juvenile Large Yellow Croaker Pseudosciaena Crocea. Environmental Pollution 2020, 267, 115397. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histological Alterations in Gills and Liver of Rainbow Trout (Oncorhynchus Mykiss) after Exposure to the Antibiotic Oxytetracycline. Environ Toxicol Pharmacol 2017, 53, 164–176. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Correia, A.T.; Golovko, O.; Žlábek, V.; Nunes, B. Assessment of Toxic Effects of the Antibiotic Erythromycin on the Marine Fish Gilthead Seabream (Sparus Aurata L.) by a Multi-Biomarker Approach. Chemosphere 2019, 216, 234–247. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, J. yuan; Bian, Y.; Ren, C. jie; Xiao-li, N.; Yang, C. yu; Xiao-xue, X.; Feng, X. song Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in the Environment: Updates on Pretreatment and Determination Methods. Ecotoxicol Environ Saf 2023, 267, 115624. [Google Scholar] [CrossRef]
- Russo, C.; Nugnes, R.; Orlo, E.; di Matteo, A.; De Felice, B.; Montanino, C.; Lavorgna, M.; Isidori, M. Diclofenac Eco-Geno-Toxicity in Freshwater Algae, Rotifers and Crustaceans. Environmental Pollution 2023, 335, 122251. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, M.; Mao, W.; Chen, C.; Fu, L.; Li, Z.; Du, S.; Liu, H. Photosynthetic Toxicity of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) on Green Algae Scenedesmus Obliquus. Science of The Total Environment 2020, 707, 136176. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, M.; Gorbi, S.; Da Ros, Z.; Fattorini, D.; d’Errico, G.; Milan, M.; Bargelloni, L.; Regoli, F. Ecotoxicological Potential of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Marine Organisms: Bioavailability, Biomarkers and Natural Occurrence in Mytilus Galloprovincialis. Mar Environ Res 2016, 121, 31–39. [Google Scholar] [CrossRef]
- Piedade, F.; Bio, S.; Nunes, B. Effects of Common Pharmaceutical Drugs (Paracetamol and Acetylsalicylic Acid) Short Term Exposure on Biomarkers of the Mussel Mytilus Spp. Environ Toxicol Pharmacol 2020, 73, 103276. [Google Scholar] [CrossRef] [PubMed]
- Chabchoubi, I. Ben; Bouchhima, R.A.; Louhichi, N.; Baanannou, A.; Masmoudi, S.; Hentati, O. Short-Term Effects of Various Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) on Danio Rerio Embryos. MethodsX 2023, 10, 102215. [Google Scholar] [CrossRef]
- Mikula, P.; Hollerova, A.; Hodkovicova, N.; Doubkova, V.; Marsalek, P.; Franc, A.; Sedlackova, L.; Hesova, R.; Modra, H.; Svobodova, Z.; et al. Long-Term Dietary Exposure to the Non-Steroidal Anti-Inflammatory Drugs Diclofenac and Ibuprofen Can Affect the Physiology of Common Carp (Cyprinus Carpio) on Multiple Levels, Even at “Environmentally Relevant” Concentrations. Science of The Total Environment 2024, 917, 170296. [Google Scholar] [CrossRef]
- Stancová, V.; Ziková, A.; Svobodová, Z.; Kloas, W. Effects of the Non-Steroidal Anti-Inflammatory Drug(NSAID) Naproxen on Gene Expression of Antioxidant Enzymes in Zebrafish (Danio Rerio). Environ Toxicol Pharmacol 2015, 40, 343–348. [Google Scholar] [CrossRef]
- Wang, R.; Luo, J.; Li, C.; Chen, J.; Zhu, N. Antiviral Drugs in Wastewater Are on the Rise as Emerging Contaminants: A Comprehensive Review of Spatiotemporal Characteristics, Removal Technologies and Environmental Risks. J Hazard Mater 2023, 457, 131694. [Google Scholar] [CrossRef]
- Silva, S.R.; Barbosa, F.A.R.; Mol, M.P.G.; Magalhães, S.M.S. Toxicity for Aquatic Organisms of Antiretroviral Tenofovir Disoproxil. J Environ Prot (Irvine, Calif) 2019, 10. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Occurrence, Ecotoxicological Effects and Risk Assessment of Antihypertensive Pharmaceutical Residues in the Aquatic Environment - A Review. Chemosphere 2015, 138, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Andrade de Sousa, J.; Hermes Pusceddu, F.; Dos Santos Barbosa Ortega, A.; Ueda de Carvalho, M.U. de C.; Amaral Gomes dos Santos, R.; Moledo de Souza Abessa, D.; Dias Seabra Pereira, C.; Alves Maranho, L. Biological Effects Caused by the Pharmaceuticals Losartan and Diclofenac, and Their Mixture on Marine Organisms. Ecotoxicology and Environmental Contamination 2022, 17. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Ecotoxicological Evaluation of Propranolol Hydrochloride and Losartan Potassium to Lemna Minor L. (1753) Individually and in Binary Mixtures. Ecotoxicology 2015, 24. [Google Scholar] [CrossRef]
- Reque, R.; Carneiro, R.D.; Yamamoto, F.Y.; Ramsdorf, W.A.; Martins, L.R.; Guiloski, I.C.; de Freitas, A.M. Ecotoxicity of Losartan Potassium in Aquatic Organisms of Different Trophic Levels. Environ Toxicol Pharmacol 2021, 87. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front Immunol 2019, 10. [Google Scholar] [CrossRef]
- Hardy, R.S.; Raza, K.; Cooper, M.S. Therapeutic Glucocorticoids: Mechanisms of Actions in Rheumatic Diseases. Nat Rev Rheumatol 2020, 16. [Google Scholar] [CrossRef]
- Hamilton, C.M.; Winter, M.J.; Margiotta-Casaluci, L.; Owen, S.F.; Tyler, C.R. Are Synthetic Glucocorticoids in the Aquatic Environment a Risk to Fish? Environ Int 2022, 162, 107163. [Google Scholar] [CrossRef]
- Mezzelani, M.; Peruzza, L.; d’Errico, G.; Milan, M.; Gorbi, S.; Regoli, F. Mixtures of Environmental Pharmaceuticals in Marine Organisms: Mechanistic Evidence of Carbamazepine and Valsartan Effects on Mytilus Galloprovincialis. Science of The Total Environment 2023, 860, 160465. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Soldado, N.; Mora-Medina, R.; Lora-Benítez, A.J.; Gonçalves Reis, L. de P.; Molina-López, A.M.; Moyano-Salvago, M. del R. Comparative Study of Tricaine Methanesulfonate (MS-222) and Eugenol as Euthanasia Agents in Zebrafish (Danio Rerio) as an Experimental Model. Lab Anim 2023, 57. [Google Scholar] [CrossRef]
- Balko, J.A.; Posner, L.P.; Chinnadurai, S.K. Immersion in Tricaine Methanesulfonate (MS-222) Is Not Sufficient for Euthanasia of Smokey Jungle Frogs (Leptodactylus Pentadactylus). Journal of Zoo and Wildlife Medicine 2019, 50. [Google Scholar] [CrossRef]
- Engin, E. GABAA Receptor Subtypes and Benzodiazepine Use, Misuse, and Abuse. Front Psychiatry 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.; Wild, I.; Caulet, B.; Leontiou, C.; Lugoboni, F.; Hajak, G. Long-Term Use of Benzodiazepines in Chronic Insomnia: A European Perspective. Front Psychiatry 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, C.; Carotenuto, R.; Panzuto, R.; Spennato, V.; De Bonis, S.; Simoniello, P.; Raggio, A.; Avallone, B.; Agnisola, C.; Motta, C.M. Behavioral Alterations and Gills Damage in Mytilus Galloprovincialis Exposed to an Environmental Concentration of Delorazepam. Environ Toxicol Pharmacol 2023, 97, 104030. [Google Scholar] [CrossRef]
- Lebreton, M.; Sire, S.; Carayon, J.L.; Malgouyres, J.M.; Vignet, C.; Géret, F.; Bonnafé, E. Low Concentrations of Oxazepam Induce Feeding and Molecular Changes in Radix Balthica Juveniles. Aquatic Toxicology 2021, 230, 105694. [Google Scholar] [CrossRef]
- Cerveny, D.; Brodin, T.; Cisar, P.; McCallum, E.S.; Fick, J. Bioconcentration and Behavioral Effects of Four Benzodiazepines and Their Environmentally Relevant Mixture in Wild Fish. Science of The Total Environment 2020, 702, 134780. [Google Scholar] [CrossRef]
- Pohl, J.; Ahrens, L.; Carlsson, G.; Golovko, O.; Norrgren, L.; Weiss, J.; Örn, S. Embryotoxicity of Ozonated Diclofenac, Carbamazepine, and Oxazepam in Zebrafish (Danio Rerio). Chemosphere 2019. [Google Scholar] [CrossRef] [PubMed]
- C. Campbell, W. History of Avermectin and Ivermectin, with Notes on the History of Other Macrocyclic Lactone Antiparasitic Agents. Curr Pharm Biotechnol 2012, 13. [CrossRef]
- Sulik, M.; Antoszczak, M.; Huczyński, A.; Steverding, D. Antiparasitic Activity of Ivermectin: Four Decades of Research into a “Wonder Drug. ” Eur J Med Chem 2023, 261, 115838. [Google Scholar] [CrossRef] [PubMed]
- Conforti, S.; Dietrich, J.; Kuhn, T.; Koppenhagen, N. van; Baur, J.; Rohner, P.T.; Blanckenhorn, W.U.; Schäfer, M.A. Comparative Effects of the Parasiticide Ivermectin on Survival and Reproduction of Adult Sepsid Flies. Ecotoxicol Environ Saf 2018, 163, 215–222. [Google Scholar] [CrossRef]
- Garric, J.; Vollat, B.; Duis, K.; Péry, A.; Junker, T.; Ramil, M.; Fink, G.; Ternes, T.A. Effects of the Parasiticide Ivermectin on the Cladoceran Daphnia Magna and the Green Alga Pseudokirchneriella Subcapitata. Chemosphere 2007, 69, 903–910. [Google Scholar] [CrossRef]
- González-Tokman, D.; Martínez M., I.; Villalobos-Ávalos, Y.; Munguía-Steyer, R.; Ortiz-Zayas, M. del R.; Cruz-Rosales, M.; Lumaret, J.P. Ivermectin Alters Reproductive Success, Body Condition and Sexual Trait Expression in Dung Beetles. Chemosphere 2017, 178, 129–135. [Google Scholar] [CrossRef]
- Powrie, Y.; Strydom, M.; Aucamp, M.; Schellack, N.; Steenkamp, V.; Smith, C. Zebrafish Behavioral Response to Ivermectin: Insights into Potential Neurological Risk. Med Drug Discov 2022, 16, 100141. [Google Scholar] [CrossRef]
- Lorente, C.J.; Mesa, L.; Montalto, L.; Gutiérrez, M.F.; Miró, M.V.; Lifschitz, A. Ivermectin Bioaccumulation and Transfer through Developmental Stages in Culex Pipiens (Diptera: Culicidae). Chemosphere 2023, 322, 138106. [Google Scholar] [CrossRef] [PubMed]
| Specie | Pharmaceutical | Concentration | Time of exposure | Main findings | References |
|---|---|---|---|---|---|
| Limnodynastes peronii | Diclofenac Naproxen Atenolol Gemfibrozil |
0.1, 1, 10 and 100 μgL-1 | 30 days | A significant increase in peroxidase activity was observed at the highest concentration of the drug mixture. | [62] |
| Pelophylax ridibundus | Nifedipine | 10 μM | 14 days | Increased ROS production, elevated SOD activity, and higher GSH and GSSG levels. | [63] |
| Pelophylax ridibundus | Ibuprofen Estrone |
250 100 ngL-1 |
14 days | Exposure can induce oxidative stress, although the magnitude of this effect varies depending on the compound. | [64] |
| Rhinella arenarum | Enrofloxacin Ciprofloxacin |
1, 10, 100 and 1000 μgL-1 | 96 h | An increase in LPO, decrease in CAT activity, and increase in GST activity was observed, particularly at the highest exposure concentrations. | [65] |
|
Trachycephalus typhonius Physalaemus albonotatus |
Diclofenac | 125 to 4000 μgL-1 125 to 2000 μgL-1 |
96 h 22 and 20 days |
An imbalance between ROS production and antioxidant systems was observed in both species, whereas GST activity exhibited interspecies variation. | [66] |
| Rhinella arenarum | Dexamethasone | 1-1000μgL-1 | 22 days | GST activity significantly increased in larvae exposed to the drug. | [67] |
| Rhinella arenarum | Lamivudine Stavudine Zidovudine Nevirapine |
0.5, 1, 2 and 4 μgmL-1 | 48 h | Biochemical imbalance between ROS production and induction of antioxidant systems. | [68] |
| Physalaemus cuvieri | Hydroxychloroquine Azithromycin |
12.5 μgL-1 | 72 h | Exposure to drugs did not elicit a significant oxidative stress response in tadpoles, potentially because of the activity of antioxidant enzymes. | [69] |
| Xenopus laevis | Delorazepam | 1, 5 and 10 μgL-1 | 96 h | Delorazepam alters redox equilibrium in embryos, potentially resulting in adverse effects on their development and viability. | [70] |
| Rhinella arenarum | Oxytetracycline | 10, 30 and 60 mgL-1 | 96 h | Exposure induced oxidative stress in both embryos and larvae, as evidenced by increased lipoperoxidation and altered antioxidant enzyme activities. | [71] |
| Lithobates catesbeianus | Sulfamethoxazole Oxytetracycline |
20, 90 and 460 ngL-1 | 16 days | Drug exposure induced OS in tadpoles as evidenced by the inhibition of antioxidant enzymes and increased oxidative damage to proteins. | [72] |
| Hyla arborea | Ethyl 3-aminobenzoate methanesulfonate (MS-222) | 0.1, 1 and 5 gL-1 | 15 min | MS-222 may potentially interfere with investigations of OS biomarkers, particularly those associated with GSH. | [73] |
| Xenopus laevis | Favipiravir Oseltamivir |
32.9 to 250 mgL-1 8.2 to 62.5 mgL-1 |
96 h | Biomarker responses indicate distinct detoxification and oxidative stress processes during organogenesis and the subsequent developmental stages. | [74] |
| Rhinella arenarum | Ivermectin | 1.25, 10 and 100 μgL-1 | 96 h | Induced OS, even at low concentrations, and the commercial formulation may exhibit higher toxicity than the active ingredient alone. | [75] |
| Rhinella arenarum | Monensin | 4, 12 and 120 μgL-1 | 96 h | A decrease in GST activity and GSH levels was observed, which was accompanied by an increase in TBARS levels. | [76] |
| Aquarana catesbeianus | Prednisone Prednisolone |
0.1, 1 and 10 μgL-1 | 16 days | Elevated SOD, CAT, GPx, and GST activities as well as increased MDA levels were observed in tadpoles exposed to prednisone. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





