1. Introduction
Recently, in the Republic of Korea, there has been a notable increase in cases of antifreeze poisoning among companion animals, particularly felines. Ethylene glycol (EG), a key component in antifreeze formulations, lowers the freezing points of automotive and industrial coolants [
1,
2]. Despite its utility and wide use, EG is colorless, odorless, and has a sweet taste that masks its highly toxic nature, leading to accidental or intentional ingestion and potentially fatal outcomes. In particular, the palatability of antifreeze poses a significant risk to animals [
3]. Transformation of EG into glycolic acid (GA), a toxic metabolite, through metabolic processes contributes to various adverse effects, such as metabolic acidosis and acute renal failure [
4]. In our process, we have primarily relied on necropsy finding
s, including hematoxylin and eosin (H&E
) staining of collected tissues and genetic testing, but there have been cases where the results were inconclusive, highlighting the need for a more precise diagnostic method. This diagnostic uncertainty poses immediate health implications, encompassing broader concerns regarding animal welfare and presenting substantial legal and ethical challenges. Therefore, there is an urgent need for reliable and rapid diagnostic methods capable of identifying EG and its metabolites in biological samples.
Traditional analytical methods, including gas chromatography-tandem mass spectrometry (GC-MS/MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS), have been instrumental in toxicological studies [
4]. However, these techniques often involve complex sample preparation and derivatization steps, such as the use of bis(trimethylsilyl)trifluoroacetamide (BSTFA) to enhance compound volatility and detection sensitivity [
5]. Although effective, these processes are time-consuming and prone to non-specific reactions. Additionally, the amount of sample that can be obtained from companion animals is typically small, which presents a challenge to achieving accurate and reliable results. Moreover, the limited sample volume makes it difficult to test for other potential compounds that could be involved. Therefore, a streamlined analytical process that enables rapid and precise detection of EG and GA, while allowing assessment of other possible toxicants, is critically needed to facilitate prompt and appropriate medical intervention.
This study introduces a novel analytical methodology that obviates the need for derivatization, simplifies the detection process, and significantly reduces the likelihood of analytical errors [
6]. This methodological innovation provides a dependable tool for rapid diagnosis of EG poisoning in companion animals, aiding in timely administration of treatment and supporting legal action in cases of suspected animal abuse [
7,
8]. Broadly, our study addresses a pressing public health concern with wide implications for animal welfare, forensic science, and toxicology.
2. Results
2.1. Analysis of ethylene glycol using GC-MS
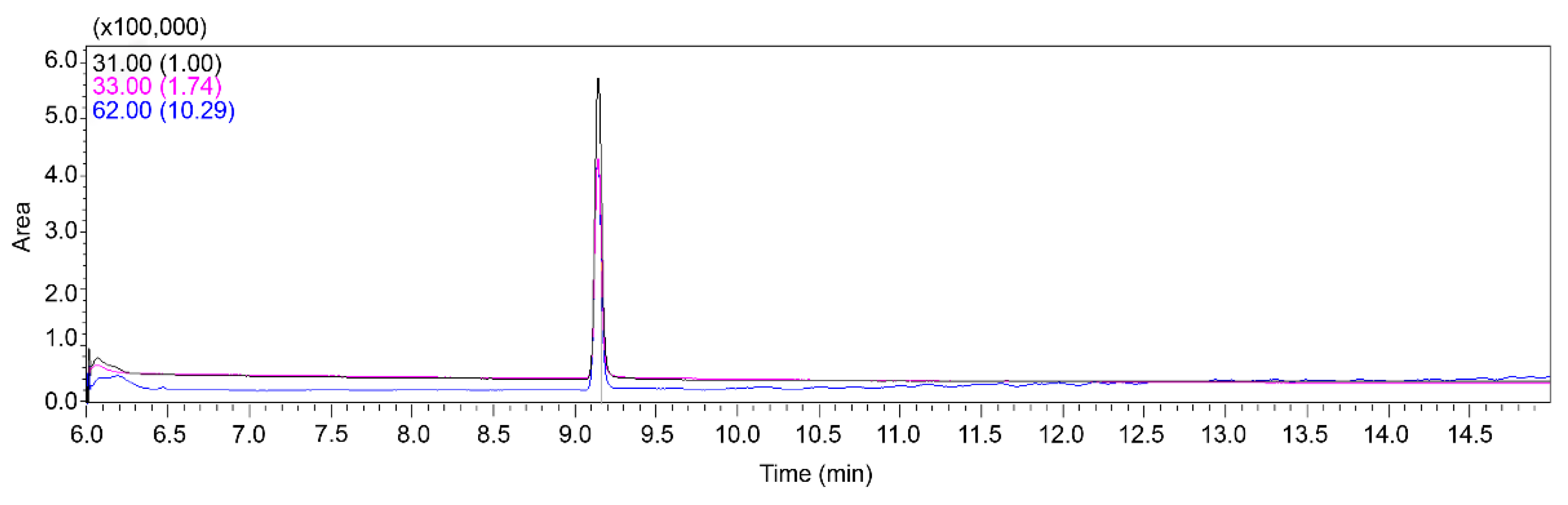
A new method using GC-MS/MS was developed for the accurate quantification of EG in the stomach tissues of felines. This approach involved the measurement of EG, with intense mass spectral peaks at m/z 62, 33, and 31. Based on these observations, the quantification ion was designated as 31 m/z, whereas the qualitative ions were set as m/z 33 and 62. The ion ratio (qualitative/quantitative) was 0.33. According to the standards, the range of quantifier:qualifier ratios allowed is usually less than ±30%. Before sample analyses, the procedure was validated using stomach tissue samples spiked with EG. The chromatograms produced (
Figure 1) under the specific chromatographic conditions used in this study demonstrated no significant interference from the tissue matrix, thereby ensuring reliability of the results. EG had a retention time of approximately 9.15 min. The method exhibited a linear response over the concentration range required for the objectives of the research, with a correlation coefficient (
R2) of 0.99 at concentrations ranging from 5 to 200 μg/mL in the tissue. The limits of detection (LOD) and quantification (LOQ) were 25 and 100 ng/mL, respectively. Recovery and precision of EG using this method were in the range of 83.05–91.73% (
Table 1). These results validated the repeatability and accuracy of the method for EG analysis.
2.2. Analysis of glycolic acid using LC-MS/MS
An analytical approach utilizing LC-MS/MS was used to quantify GA, a key metabolite of EG, in feline gastric tissues [
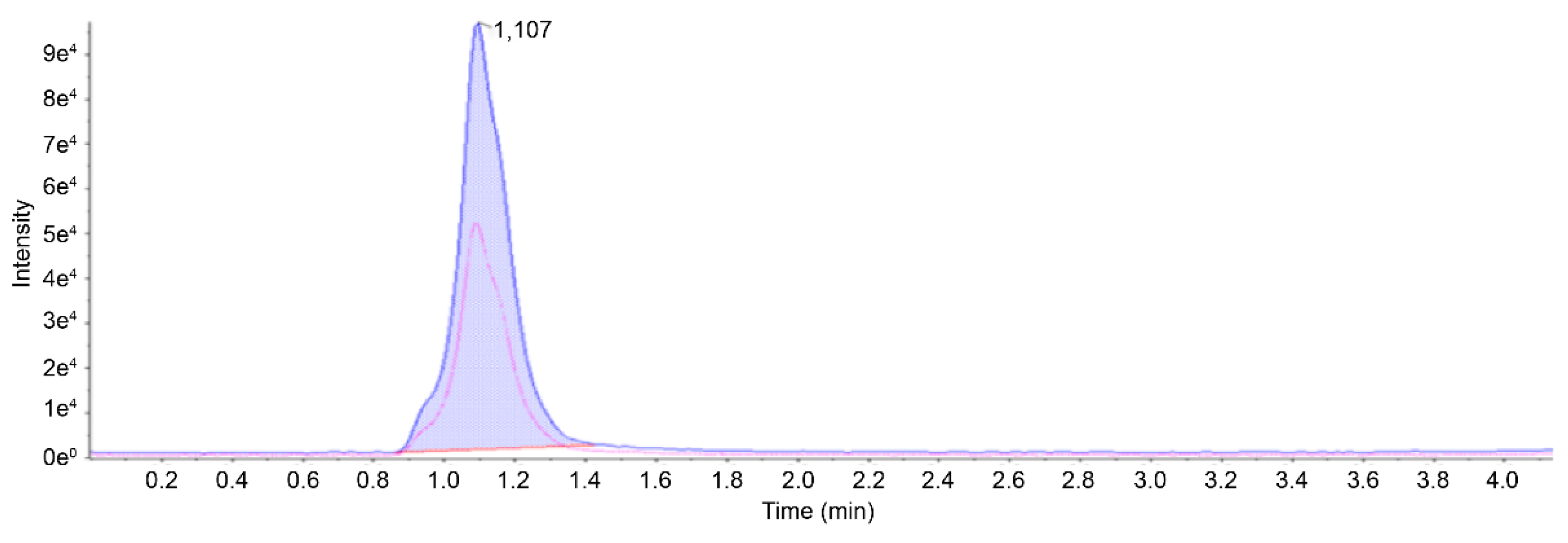
9]. Mass spectral peaks were identified at 74.9 m/z as the precursor ion, accompanied by product ions at 45.1 and 35.0 m/z. Based on these observations, the quantification ion was designated as 45.1 m/z, whereas the ion chosen for qualitative analysis was set as 35.0 m/z. The ratio of qualitative to quantitative ions was set to 0.5. To ensure the validity of the method, stomach tissue samples spiked with GA were analyzed before the technique was used for actual sample evaluation. The resulting chromatograms (
Figure 2) from this methodology highlight the absence of significant matrix-related interference, confirming the capacity to yield dependable outcomes for the measurement of biological GA levels. Under the chromatographic conditions specified above, GA exhibited a retention time of 1.10 min. This approach demonstrated a linear relationship across the range of concentrations deemed necessary to achieve the goals of this study. The correlation coefficient (
R2), LOD, and LOQ were 0.99, 5, and 20 ng/mL, respectively. The recovery rate using this method fell within 80.92–95.30%, and the precision estimates for GA concentrations of 1, 2, 5, 25, and 50 μg/mL in the tissues were consistently 0.81–1.34% and 0.41–0.96% for GA and EG, respectively (
Table 1). These findings confirmed the reliability and accuracy of the method within a single analytical session.
2.3. Analysis of samples suspected to be exposed to antifreeze
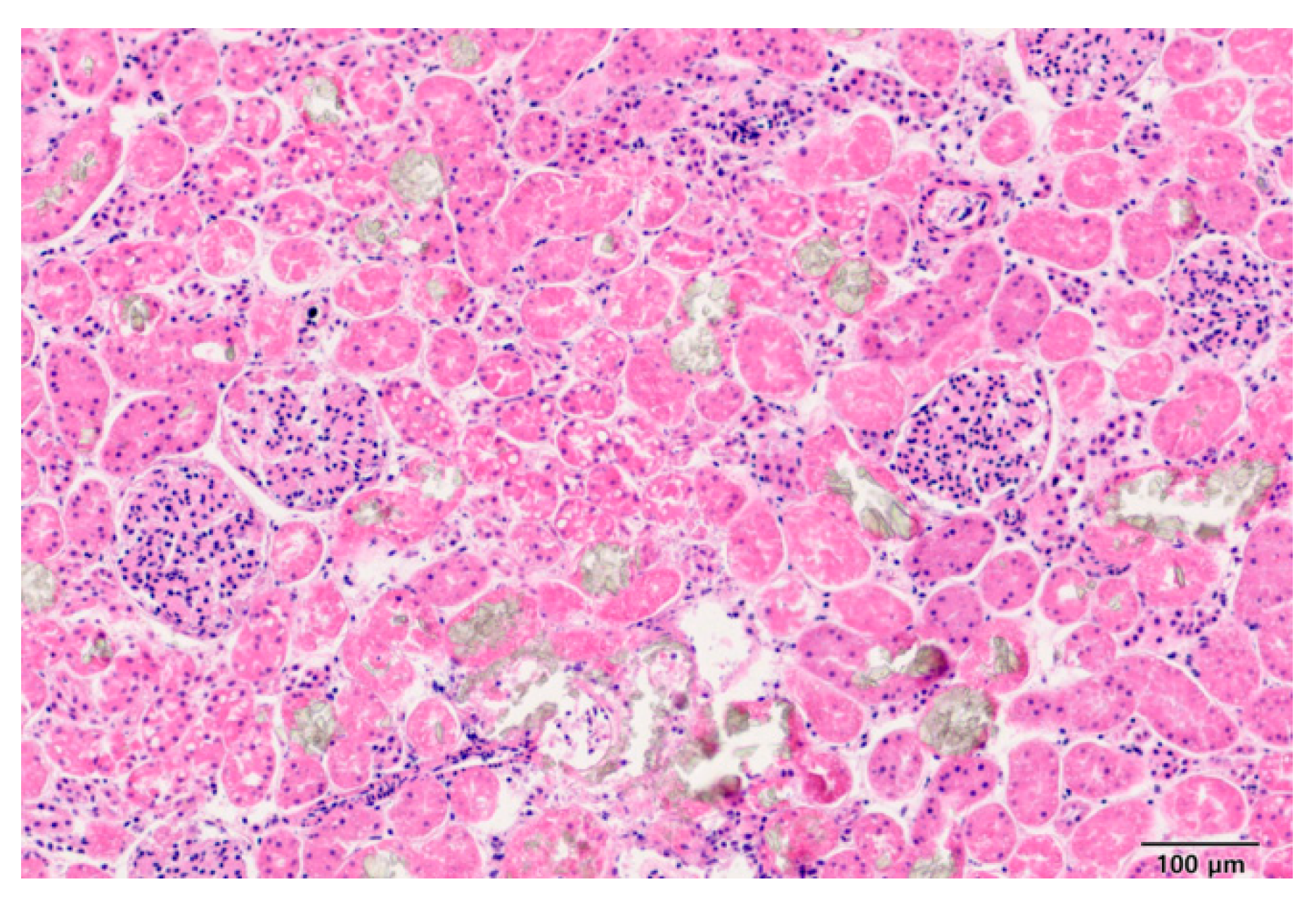
A stray cat (Case 1) was admitted to a veterinary hospital exhibiting symptoms of vomiting, respiratory distress, and decreased consciousness. Despite emergency interventions, the cat did not survive. A toxicology analysis was requested from the Animal and Plant Quarantine Agency to determine the cause of death. Upon necropsy, kidney lesions were observed, and histopathological examination using H&E staining confirmed the presence of crystalline deposits of brown-colored material in the renal tissue (
Figure 3). However, the specific nature of the crystals could not be identified. Genetic testing using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for markers typically associated with EG exposure was conducted, but no definitive results were obtained (data not shown).
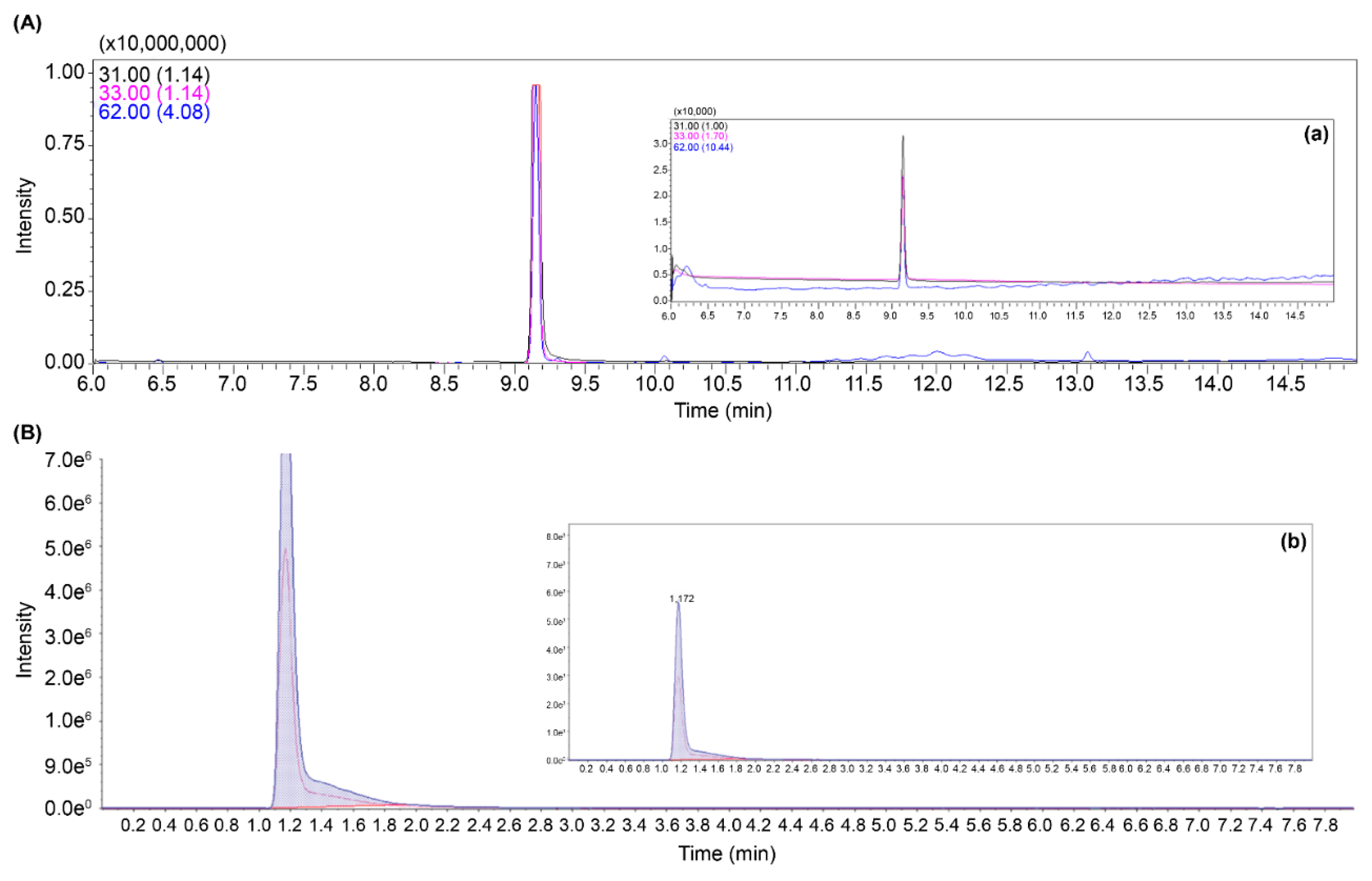
The presence of EG and GA in the stomach sample was confirmed using the mass spectrometry method developed in this study; 82 µg/mL EG and 27 µg/mL GA were detected in Case 1. Subsequently, four additional cases (Case 2–5) of suspected EG exposure were identified, and calcium oxalate crystals were observed in the kidney tissue upon necropsy. In total, five cases of EG poisoning were documented, with significant amounts of EG and GA detected. The concentrations of EG and GA in the samples exceeded the measurement capabilities of GC-MS/MS and LC-MS/MS. To accurately quantify these high concentrations and establish the ratios of the detected EG and GA ions, the samples were diluted 1,000-fold for re-analysis (
Figure 4). The detected concentrations were 82–590 μg/mL for EG and 27–98 μg/mL for GA in the stomach tissues.
As EG is metabolized in the liver, and the primary lesions appeared in the kidneys, additional liver and kidney samples were collected from the cases for further analysis. The results are presented in
Table 2. In the liver, EG and GA concentrations were 69–473 μg/mL and 29–62 μg/mL, respectively, whereas in the kidneys, EG and GA concentrations were 67–410 μg/mL and 28–55 μg/mL, respectively.
3. Discussion
EG and its toxic metabolite GA are of significant concern in veterinary forensics owing to their high toxicity and the frequent occurrence of antifreeze poisoning in pets [
10]. The need for precise diagnostic methods is underscored by the palatability of antifreeze compounds, which pose severe toxicological risks to companion animals [
11]. Upon ingestion, EG is metabolized to glycolaldehyde by alcohol dehydrogenase, which is subsequently oxidized to GA. This metabolite is further converted to oxalic acid, which can form calcium oxalate crystals in the kidneys and cause renal damage [
12,
13]. Recent incidents in Korea have highlighted the fatal consequences of antifreeze ingestion, with necropsies revealing gastric contents indicative of acute poisoning. Moreover, visual observations of renal tubular epithelial necrosis accompanied by calcium oxalate crystals underscore the severe and rapid progression of toxicity associated with EG exposure [
14].
In one notable case (case 1), the initial necropsy revealed no evidence of antifreeze exposure, and the presence of bleeding led to the suspicion of rodenticide poisoning. However, subsequent tests did not support this hypothesis. Further investigation of kidney tissues using H&E staining identified mild crystalline deposits, raising the possibility of antifreeze poisoning. To confirm this, molecular testing was performed, targeting genes typically responsive to EG exposure. However, the genetic tests showed no significant changes, likely owing to the low sensitivity of the assay. To accurately determine the cause of death, a mass spectrometry method was developed. This novel approach enabled the precise detection and quantification of EG and its metabolites, ultimately confirming antifreeze poisoning as the cause. Traditionally, methods for detecting EG and GA in cases of antifreeze poisoning rely on GC-MS/MS with BSTFA derivatization [
15]. However, these methods are limited by the low molecular weights of EG and GA and small amount of tissue available from feline necropsy. To overcome these challenges, we developed a novel method using GC-MS for EG and LC-MS/MS for GA without the need for BSTFA derivatization. This method was designed to rapidly detect and accurately quantify EG and GA in the same sample, even when other toxic substances, such as rodenticides or pesticides, were present. The method met all validation parameters, with proven reproducibility and stability [
16,
17].
Our analysis of EG and GA concentrations in five feline cases of antifreeze poisoning revealed a correlation between the presence of these compounds in various tissues and pathological outcomes. For example, in Case 1, EG was found in gastric tissue at a concentration of 82 μg/mL and in renal tissue at 67 μg/mL, with corresponding GA concentrations of 27 μg/mL in gastric and 28 μg/mL in renal tissues. These concentrations suggest that exposure at this level leads to organ bleeding and formation of mild calcium oxalate crystals in the kidneys, indicating renal damage. Similarly, in Case 2, higher levels of EG and GA were detected, with EG at 572 μg/mL in gastric tissue and 367 μg/mL in renal tissue, with GA at 59 μg/mL in gastric and 46 μg/mL in renal tissues. The presence of calcium oxalate crystals in the kidneys supported the diagnosis of antifreeze poisoning. This pattern of EG metabolism to GA and its subsequent conversion to oxalic acid, which forms calcium oxalate crystals, was observed in all cases. For instance, in Case 3, the levels of EG were 590 μg/mL in gastric tissue and 359 μg/mL in renal tissue, whereas GA levels reached 98 μg/mL in gastric and 53 μg/mL in renal tissues, again correlating with the presence of calcium oxalate crystals in the kidneys. In the cases 4 and 5, similar patterns were observed, as seen in the previous cases.
This variability in the metabolic response, combined with the consistent presence of calcium oxalate crystals across cases, underscores the deadly nature of EG poisoning and critical need for rapid diagnosis and treatment. These findings support forensic analyses of metabolites in cases of suspected antifreeze ingestion and demonstrate that even low concentrations of EG and GA can lead to fatal outcomes. The potential lethality of EG and its metabolites highlights the urgent need for rapid diagnostic methods and effective treatment strategies to mitigate the risks associated with antifreeze poisoning in pets. The presence of calcium oxalate crystals serves as a definitive indicator of toxicity progression, aiding in the forensic reconstruction of events leading to poisoning, which is vital for both therapeutic and legal outcomes.
4. Materials and Methods
4.1. Reagents and chemicals
High-purity EG and GA were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as standards. LC-MS-grade acetonitrile and methanol were purchased from J.T. Baker (Phillipsburg, NJ, USA), and formic acid solution was obtained from Merck (Darmstadt, Germany). Distilled water (EMD Millipore, Billerica, MA, USA) was used with a Milli-Q Integral Milli-Q membrane point-of-use cartridge (0.22 μm). All other reagents and solvents were of analytical grade.
4.2. Sample preparation for mass spectrometry analysis
Upon receiving a report of a deceased feline, necropsy was conducted to remove the maximum of stomach content or tissue. The samples were placed in a container designed for freezing for preservation. For the analysis, 2 g of the sample and 4 mL of cold acetonitrile with 0.1% formic acid were added to a conical tube. The supernatant obtained by centrifugation for 10 min at 3000 × g and 4 °C was put into an ultracentrifuge filter (cutoff membrane at 10 kDa, 4 mL) and purified by centrifugal separation (Beckman Coulter Avanti J-E; 3000 × g, 4 °C, 2 h). The purified solution was placed in a nitrogen evaporator, concentrated to 1 mL, and filtered using a 0.2-μm PVDF syringeless filter prior to analyses. The samples were divided equally into two groups. One half (0.5 mL) was used to measure EG by GC-MS. The remaining 0.5 mL was utilized for the quantification of GA using high-performance LC-MS/MS.
4.3. GC-MS method
EG was analyzed by GC-MS (Shimadzu GCMS-TQ8050 NX). The instrument was equipped with SH-PolarWax column (0.32 mm ID, 1 µm, 30 m) to achieve high-resolution separation. The analytical conditions were optimized as follows: injection volume was set at 1 μL, and injection temperature was maintained at 250 °C. The column flow was regulated at 2.0 mL/min in column flow mode, with a split injection mode of 5:1 to ensure optimal sample introduction. The column oven temperature program started at 60 °C, was held for 2 min, and was ramped up at a rate of 15 °C/min to 240 °C, where it was held for an additional 3 min. The ion source and interface temperatures were set at 200 °C and 230 °C, respectively. EG was quantified in selected ion monitoring (SIM) mode, targeting ions with m/z ratios of 31, 33, and 62 for high specificity and sensitivity.
4.4. LC-MS/MS method
GA in the solution was quantified using SCIEX Triple Quad 5500+ system (AB Sciex, Framingham, MA, USA) equipped with an electrospray ionization (ESI) interface for ion generation. Chromatographic separation was achieved on Phenomenex LC column (2 × 50 mm, 4 μm particle size maintained at a temperature of 40 °C). The flow rate was set to 0.2 mL/min, and the injection volume was 2 μL. The mobile phase consisted of two components: 5 mM ammonium acetate in distilled water (mobile phase A) and 0.1% formic acid in methanol (mobile phase B). A gradient program was applied to optimize the separation with a total run time of 8 min. The program was initiated with 50% B for 2.0 min, increased to 95% B from 2.5 to 5.0 min, and returned to 50% B from 5.1 to 8.0 min. The analytes were ionized in negative mode using an ESI source. For the quantification of GA, multiple reaction monitoring (MRM) was employed, focusing on the transitions 74.9, 47.0, 45.1, and 35.0, to ensure precise and accurate measurements.
4.5. Validation of GC-MS and LC-MS/MS methods
To assess the specificity of the quantification methods for detecting EG and GA in tissue samples, standard solutions of these compounds were prepared and used to spike untreated tissue samples with concentrations of 10, 50, 100, 500, and 1,000 ng/mL. This procedure ensured that matrix effects did not interfere with detection. The standard solutions were prepared in acetonitrile with 0.1% formic acid, and the pH was adjusted to maintain stability and ensure a linear response. Dilutions were performed to create calibration standards. The samples were analyzed using GC-MS and LC-MS/MS to determine the concentrations of EG and GA. The collected data were used to evaluate accuracy, linearity, recovery percentage, calibration curves, and regression coefficients. The consistency and reliability of the method were verified by injecting samples at three different concentrations, six times each. The limits of detection (LOD) and quantification (LOQ) were calculated from the calibration curve using the equations LOD = (3.3 × SD)/slope and LOQ = (10 × SD)/slope, where SD denotes standard deviation of signal responses [
18].
4.6. Forensic examination [necropsy and H&E staining]
Upon receiving samples from deceased animals suspected of EG exposure, a detailed necropsy was performed. Sample selection was based on the observation of specific pathological signs indicative of EG toxicity, such as renal tubular epithelial necrosis accompanied by calcium oxalate crystals, pulmonary edema, and hemorrhagic gastroenteritis [
19,
20]. H&E staining of kidney tissues was performed after fixation, processing, embedding, and sectioning using
Ventana HE 600 (Roche Diagnostics, Tucson, AZ, USA), following an in-house method. Five cases exhibiting these characteristics were identified. Stomach tissue samples were collected to test for EG and GA.
4.7. Genetic assay using PCR
The expression of differentially expressed microRNAs (miRNAs) after antifreeze poisoning was verified using RT-qPCR. Nucleic acid was extracted from the kidney tissue using the Maxwell RSC Viral TNA Kit (Promega, Madison, WI, USA), according to the manufacturer's instructions. RT-qPCR was conducted using specific primer sets based on a previous study [
21].
Author Contributions
Conceptualization, J.W.K.; methodology, J.W.K., H.Y.C.; formal analysis, J.W.K., H.Y.C., K.H.L., A.Y.K., G.E.S.; investigation, J.W.K., H.Y.C.; resources, J.W.K; data curation, J.W.K., H.Y.C.; writing—original draft preparation, J.W.K.; writing—review and editing, J.W.K. M.A.H., J.W.B., T.W.K.; project administration, J.W.K.; funding acquisition, B.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by veterinary science research project grants from the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea (B-1543069-2023-25-02).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest. The manuscript was suggested by the review committee, despite the lead author being an editor for the special issue.
References
- Patočka, J.; Hon, Z. Ethylene glycol, hazardous substance in the household. Acta Med. (Hradec Kralove) 2010, 53, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Baselt, R.C.; Cravey, R.H. Disposition of Toxic Drugs and Chemicals in Man; Biomedical Publications: Davis, CA, 1982; Volume 33. [Google Scholar]
- Amoroso, L.; Cocumelli, C.; Bruni, G.; Brozzi, A.; Tancredi, F.; Grifoni, G.; Mastromattei, A.; Meoli, R.; Di Guardo, G.; Eleni, C. Ethylene glycol toxicity: a retrospective pathological study in cats. Vet. Ital. 2017, 53, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Schweighauser, A.; Francey, T. Ethylene glycol poisoning in three dogs: importance of early diagnosis and role of hemodialysis as a treatment option. Schweiz. Arch. Tierheilkd. 2016, 158, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.H.; Rutter, P.W.; Yao, H.H. Simultaneous determination of ethylene glycol and glycolic acid in serum by gas chromatography-mass spectrometry. J. Anal. Toxicol. 1999, 23, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Edinboro, L.E.; Nanco, C.R.; Soghioan, D.M.; Poklis, A. Determination of ethylene glycol in serum utilizing direct injection on a wide-bore capillary column. Ther. Drug Monit. 1993, 15, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Tuero, G.; González, J.; Sahuquillo, L.; Freixa, A.; Gomila, I.; Elorza, M.Á.; Barceló, B. Value of glycolic acid analysis in ethylene glycol poisoning: A clinical case report and systematic review of the literature. Forensic Sci. Int. 2018, 290, e9–e14. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.; Hewlett, T.P.; Webb, R.; Brown, S.T.; Ordinario, A.T.; McMartin, K.E. Ethylene glycol intoxication: evaluation of kinetics and crystalluria. Am. J. Med. 1988, 84, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Eder, A.F.; McGrath, C.M.; Dowdy, Y.G.; Tomaszewski, J.E.; Rosenberg, F.M.; Wilson, R.B.; Wolf, B.A.; Shaw, L.M. Ethylene glycol poisoning: toxicokinetic and analytical factors affecting laboratory diagnosis. Clin. Chem. 1998, 44, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.R.; Brubacher, J. Methanol and ethylene glycol poisoning: a case study and review of current literature. Can. J. Emerg. Med. 2002, 4, 34–40. [Google Scholar] [CrossRef] [PubMed]
- David, D.P.; Bramwell, K.J.; Hamilton, R.S.; Williams, S.R. Ethylene glycol poisoning: case report of a record-high level and a review. J. Emerg. Med. 1997, 15, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.; Hewlett, T.P.; Webb, R.; Brown, S.T.; Ordinario, A.T.; McMartin, K.E. Ethylene glycol intoxication: evaluation of kinetics and crystalluria. Am. J. Med. 1988, 84, 145–152. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.J.; Dargan, P.I.; Wood, D.M. Challenges in the diagnosis of ethylene glycol poisoning. Ann. Clin. Biochem. 2014, 51, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Kurtz, I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin. J. Am. Soc. Nephrol. 2008, 3, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, B. Analysis of antifreeze poisoning trends in companion animals in Korea. J. Vet. Med. Toxicol. 2023, 29, 145–153. [Google Scholar] [PubMed]
- Hong, H.; Li, W.; Li, X.; Huang, B.; Shi, D. Determination of glycolic acid in natural seawater by liquid chromatography coupled with triple quadrupole mass spectrometry. Limnol. Oceanogr. Methods 2017, 15, 631–641. [Google Scholar] [CrossRef]
- Dziadosz, M. Direct analysis of ethylene glycol in human serum on the basis of analyte adduct formation and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1072, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Hossain. M.A.; Pervin, R.; Park, N.H.; Kang, J.W.; Lee, K.J.; Suh, J.W.; Park, S.C. Development of a simple and sensitive HPLC method for the determination of rifaximin in serum and its application. Indian J. Pharm. Sci. 2018, 80, 1108–1114. [Google Scholar] [CrossRef]
- Smith, M.; Lee, H. Innovative approaches to the rapid diagnosis of ethylene glycol poisoning in small animals. Vet. Sci. Today 2021, 17, 560–569. [Google Scholar] [PubMed]
- Merck Veterinary Manual. Ethylene glycol toxicosis in animals. Available online: https://www.msdvetmanual.com/toxicology/ethylene-glycol-toxicosis/ethylene-glycol-toxicosis-in-animals (accessed on 5 February 2024).
- Liu, Z.; Jiang, H.; Yang, J.; Wang, T.; Ding, Y.; Liu, J.; Wang, S.; Ye, Z. Analysis of altered microRNA expression profiles in the kidney tissues of ethylene glycol-induced hyperoxaluric rats. Mol. Med. Rep. 2016, 14, 4650–4658. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).