1. Introduction
Coccinellidae is a globally distributed family with a high diversity in ecology, morphology, behavior, and diet [
1]. Three main types of feeding habits have been reported for Coccinellidae: phytophagous, predatory, and mycetophagous [
2]. Phytophagous ladybeetles that feed on leaves are considered as agricultural pests, while mycophagous ladybeetles that feed on fungi [
3,
4], as well as predatory ladybeetles that prey on aphids [
5], whiteflies [
6], and mites [
7], are excellent biological control agents that play an important role in protecting crops and maintaining ecological balance [
8]. Diversity in feeding habits has been reported to be related to the evolution of mouthparts [
9], and studying the structure of the mouthparts is the key to understanding their feeding mechanisms.
Mouthparts are the main feeding organs of insects, which play vital roles in feeding process [
10,
11]. The morphology of insect mouthparts have greatly evolved according to different foods and feeding habits [
12]. For example, the nectar-feeding insects of Meloidae have developed proboscis-like mouthparts specifically adapted for the purpose of nectar consumption [
13]. By examining the mouthpart morphology and sensillas types of insects, we can not only understand their feeding mechanism, but also establish a fundation for subsequent biocontrol strategies [
14]. So far, a large number of Coleoptera insects have been extensively characterized in terms of their morphology and ultrastructure of mouthparts, including Coccinellidae [
15], Bruchinae [
16], Curculionoidea [
11], Meloidae [
13], Scarabaeidae [
17], Nitidulidae [
18] and Cerambycidae [
19]. Most of these insects are phytophagous and predatory species [
16,
20]. In contrast, limited research has been conducted on mycetophagous beetles, particularly regarding the mouthparts of mycetophagous ladybeetles [
21].
Vibidia duodecimguttata is a mycetophagous ladybird beetle species that exhibits a wide distribution in the Palearctic region [
22,
23]. It serves as an obligate consumer of various powdery mildew fungi throughout its life stages [
24]. Besides, experiment has provided evidence for the overwintering advantage of
V. duodecimguttata, characterized by relatively low winter mortality [
21]. In conclusion, this species exhibits significant potential as a biological control agent [
25,
26]. This study aims to elucidate the fine morphology of the mouthparts of
V. duodecimguttata as well as to describe the morphological types, number, and distribution of sensilla on these mouthparts using scanning electron microscopy (SEM). The results hold significant implications for inferring feeding mechanism.
2. Materials and Methods
2.1. Insect Collection
Adults of Vibidia duodecimguttata were collected from Lanzhou, Gansu Province, China, on 7 July 2022. After collection, specimens were preserved in 75% ethanol solution and stored at 4°C in a refrigerator until use.
2.2. Scanning Electron Microscopy
Ten female and ten male ladybeetles were selected and placed in centrifuge tubes with 75% ethanol, and then they were washed twice with an ultrasonic cleaner (SB-5200DTD, Scientz, Ningbo, China) for twenty seconds each time. After that, there heads were dissected from the bodies under a stereomicroscope (Stemi 305, Zeiss, Suzhou, China), and then were cleaned; and dehydrated with ethanol at concentrations of 80%, 85%, 90%, and 95% respectively for 20 minutes each, and followed by two twenty-minute periods of dehydration in 99.9% ethanol. After dehydration, the mouthparts of females and males were placed individually in a clean petri dish in an electrically heated thermostatic drying oven (GZX-GF101-2-BS-II/H, Hengzi, Shanghai, China) at 40℃ for 12 hours until all the specimens were completely dry. Each part of the dried mouthparts were dissected and mounted on aluminum stubs with double-sided copper sticky tape, and were then sprayed with gold using a high-resolution sputter coater (ACE600, Leica, Vienna, Austria), they were observed and photographed with a scanning electron microscope (S3400N, Hitachi, Tokyo, Japan) at 5 kv.
2.3. Image Processing and Data Analysis
Images were imported into Photoshop 2016 (Adobe Systems, San Jose, CA, USA) for image combination and measurement. At least ten sensilla of the same type from different position and different samples were measured to detemine the length and diameter of this sensillum. For the identification of various types of sensilla, the traditional morphological classification method was used according to the external morphology, length, and distribution [
27,
28].
3. Results
3.1. Gross Morphology of the Mouthparts
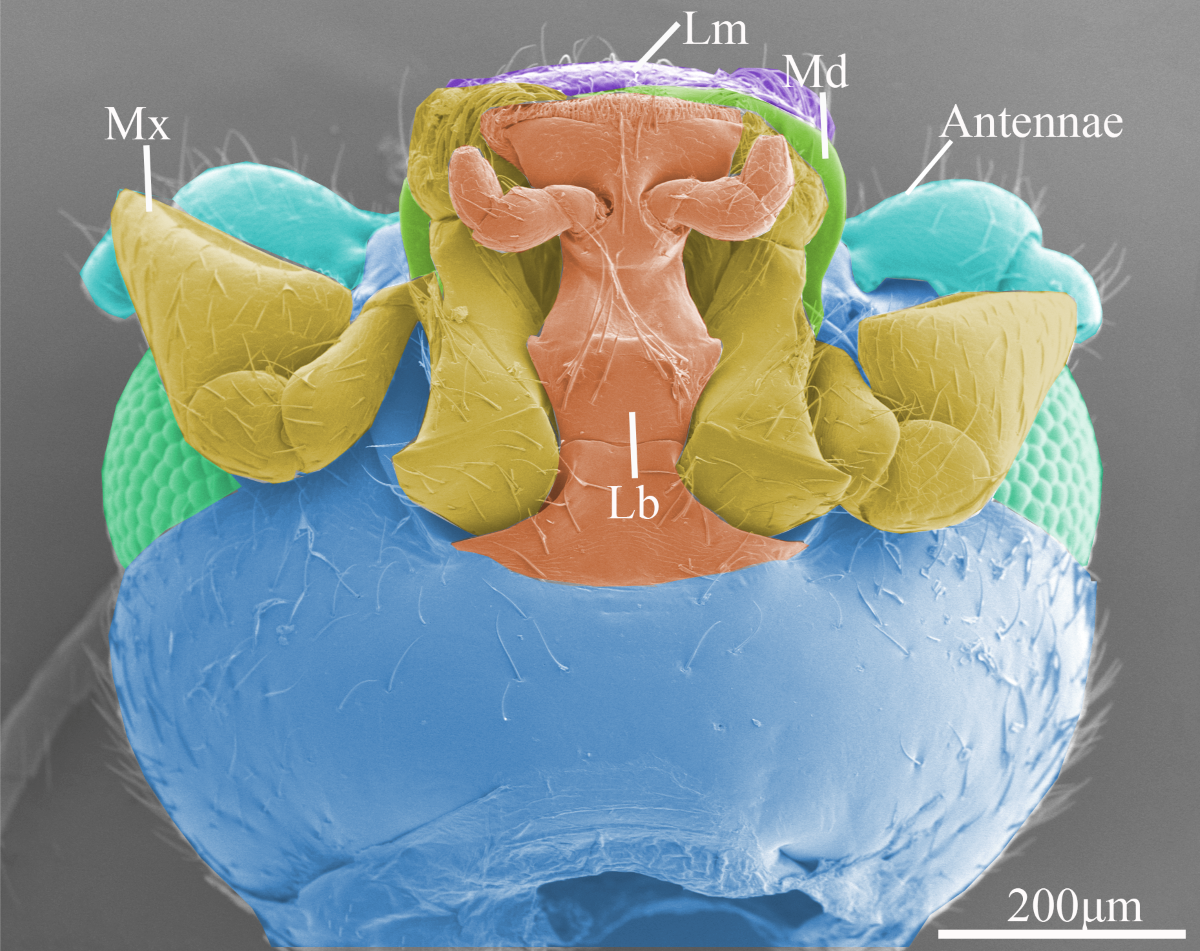
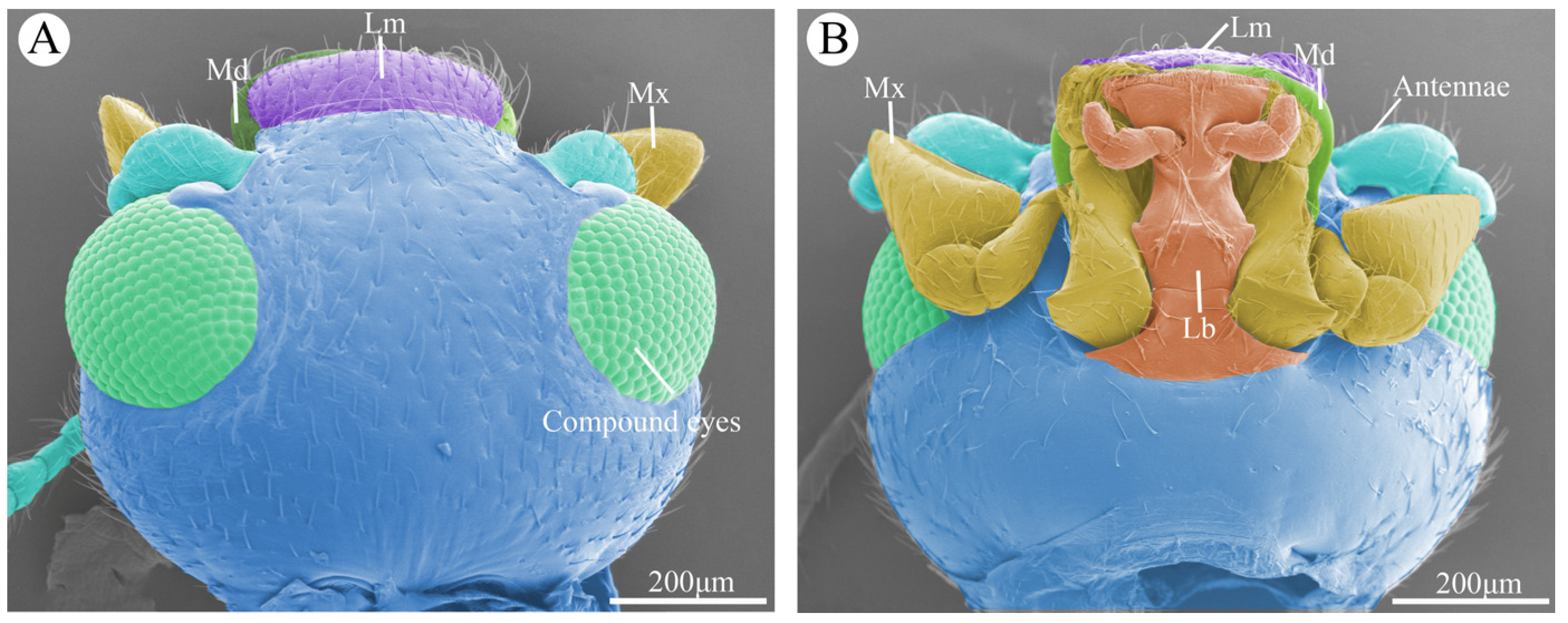
The mouthparts of
Vibidia duodecimguttata adults were typical chewing mouthparts, which were composed of a labrum, a pair of mandibles, a pair of maxillaes, a labium, and a soft hypopharynx. Hypopharynx was a none-sclerotized structure and not visible externally. This study focuses only on the morphology of the sclerotized structures. From the dorsal side, only labrum, part of the maxillary palp and part of the mandibles can be seen in front of the head (
Figure 1A). From the ventral side, the whole labium and maxillae, part of the labrum and part of the mandibles that sandwiched between the labrum and labium can be seen (
Figure 1B).
3.2. Types and Morphology of Sensilla on Mouthparts
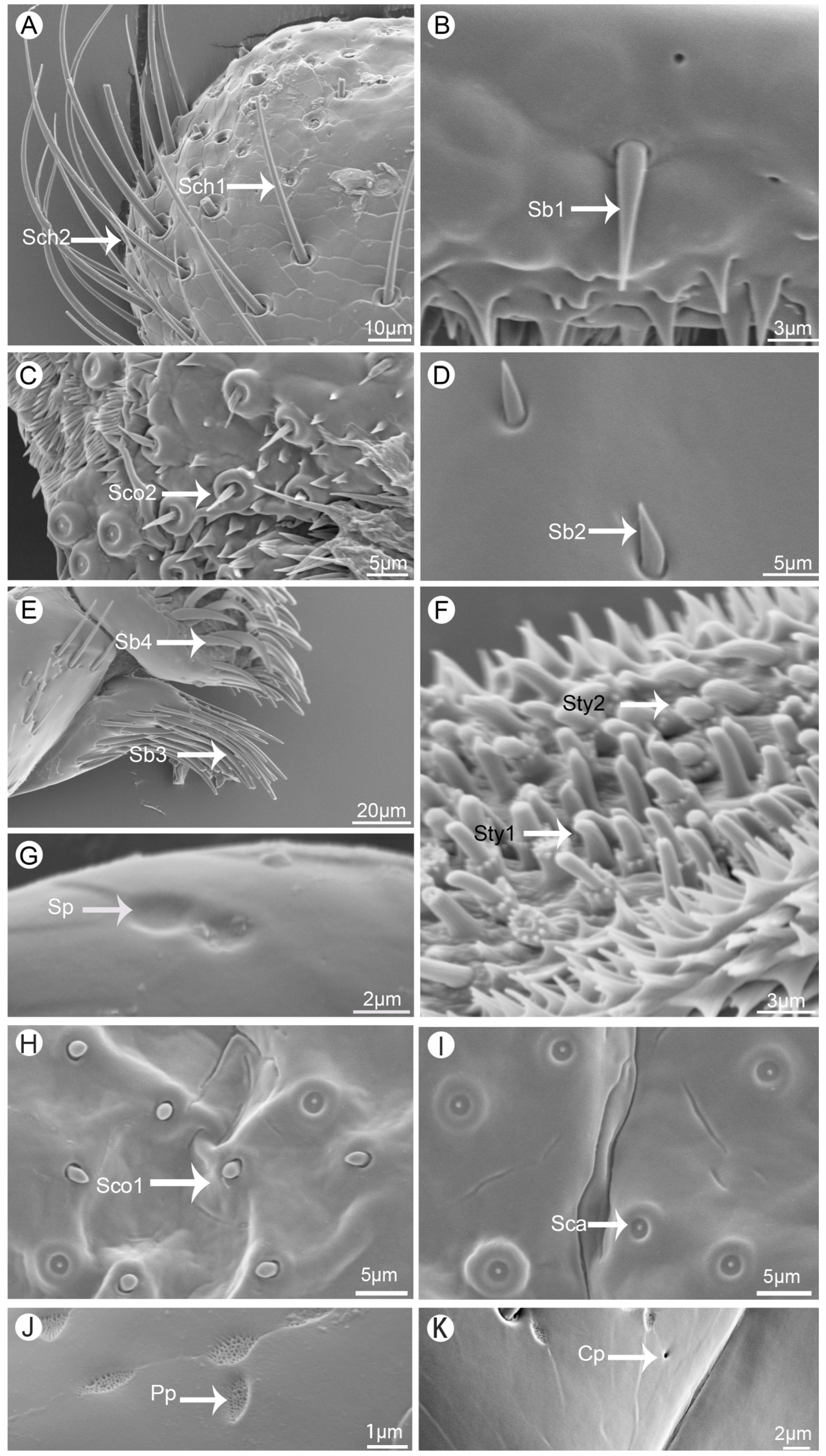
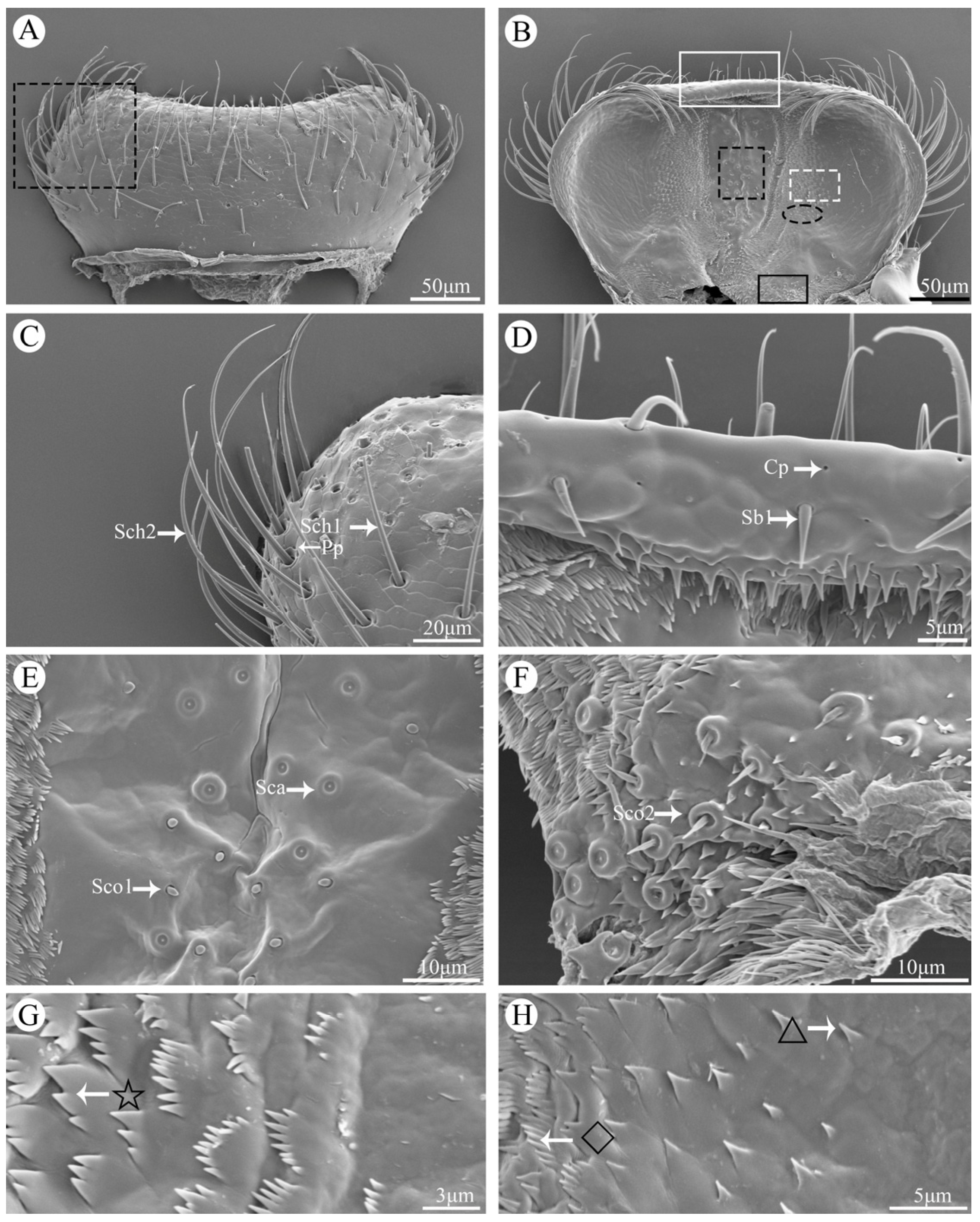
In total, six kinds of sensilla were identified on the mouthparts of
V. duodecimguttata by scanning electron micrographs, which including two types of sensilla chaetica (Sch), four types of sensilla basiconica (Sb), two types of sensilla styloconica (Sty), one types of sensilla campaniformia (Sca), two types of sensilla coeloconica (Sco), and one type of sensilla placodea (Sp), for a total of twelve types of sensilla. Besides, two kinds of glandular structures were identified, namely cuticular pores (Cp) and perforated plates (Pp) (
Table 1).
Sensilla chaetica (Sch) can be divided into two types. Sensilla chaetica I (Sch1) are spiniform and upright, inserted into a round socket, and with sharp tips. Their surfaces are longitudinally grooved with no pore (
Figure 2A). Their lengths range from 50.3 to 59.6 μm, and their diameters range from 2.2 to 3.2 μm. They are widely distributed on all mouthpart surfaces (
Table 1).
Sensilla chaetica II (Sch2) are much longer than Sch1 and their tips are slightly curved (
Figure 2A). Their surfaces are quite similar with Sch1. Their lengths range from 93.2 to 115.2 μm, and their diameters range from 2.1 to 3.9 μm. They are widely distributed on labrum, maxillae and labium (
Table 1).
Sensilla basiconica (Sb) can be divided into four types. Sensilla basiconica I (Sb1) are conical and straight, inserted into a round and concave socket, stout at the base and sharp at the top. They have smooth surface (
Figure 2B). Their lengths range from 8.1 to 12.9 μm, and their diameters range from 1.7 to 2.1 μm. They are distributed on labrum, maxillae and labium (
Table 1).
Sensilla basiconica II (Sb2) are similar to Sb1 but much shorter, with circular socket, smooth surface, and sharp tip (
Figure 2D). Their lengths range from 2.4 to 4.2 μm, and their diameters range from 0.7 to 1.7 μm. They are often distributed on mandibles and labium (
Table 1).
Sensilla basiconica III (Sb3) are the longest among four types of this kind of sensilla. They have smooth surfaces, slightly curved and thin tips (
Figure 2E). Their lengths range from 39.7 to 50.8 μm, and their diameters range from 1.9 to 4.1 μm. They are only found on lacinia of maxillae and often gather together (
Table 1).
Sensilla basiconica IV (Sb4) are different from the other three in that it is sickle-shaped and has a smooth, non-porous surface (
Figure 2E). Their lengths range from 32.4 to 40.3 μm, and their diameters range from 3.1 to 5.4 μm. They are only found on galea of maxillae and often gather together (
Table 1).
Sensilla styloconica (Sty) can be divided into two types. Sensilla styloconica I (Sty1) are conical with convex socket, and covered with longitudinalis grooves on the surface, with the micro-digitations at the top and have an obvious terminal pore (
Figure 2F). They are densely distributed on the maxillary palpi and labial palpi. Their lengths range from 1.6 to 2.9 μm, and their socket diameters range from 0.6 to 1.1 μm (
Table 1).
Sensilla styloconica II (Sty2) are cylindrical, with longitudinalis stria on the surface and a obvious convex cylindrical socket at the base, which is surrounded by a ring of globular processes (
Figure 2F). They are slightly longer (2.8-3.7 μm) than Sty1 while their socket diameters (0.6 to 1.3 μm) are similar to Sty1. They are only distributed on maxillary palpi (
Table 1).
Sensilla campaniformia (Sca) are bell-shaped, convex on the outside, concave in the center, their surfaces are smooth with a small round protuberance in the middle (
Figure 2I). Their diameters range from 3.4 to 5.1 μm. They are distributed on epipharynx (
Table 1).
Sensilla coeloconica (Sco) can be divided into two types. Sensilla coeloconica I (Sco1) are conical and short, inserted into an slightly round socket (
Figure 2H). Their diameters range from 1.4 to 1.8 μm. They are only distributed on epipharynx (
Table 1).
Sensilla coeloconica II (Sco2) are longer than Sco1, with a sharp tip, with surface wrinkled and surrounded by round smooth protrusions (
Figure 2C). Their lengths range from 1.6 to 2.9 μm, and their socket diameters range from 3.2 to 5.0 μm. They are just distributed on epipharynx (
Table 1).
Sensilla placodea (Sp) are circular and slightly concave, and their surfaces are smooth with no pore (
Figure 2G). Their diameters range from 1.6 to 2.8 μm. They are distributed on the first segment of labial palpi (
Table 1).
3.3. Glandular Structures on Mouthparts
Perforated plates (Pp) are concave structure with many small holes on the smooth surface (
Figure 2J), with a variety of irregular shapes, such as round, oval, diamond shaped and so on. Their diameters range from 1.7 to 4.6 μm. They are widely distributed on the surface of all structures of the mouthpart (
Table 1).
Cuticular pores (Cp) are generally round and concave hole on the surface (
Figure 2K). They are quite small (0.4 to 0.8 μm) and widely distributed on all structures of the mouthpart (
Table 1).
3.4. Labrum
The labrum is a nearly oblong bilaminar structure that attached to the front margin of the anteclypeus (
Figure 3A,B). The outer surface of the labrum is sculptured and bears a number of sensilla and glandular structures, such as Sch1, Sch2, Sb1, Pp and Cp (
Figure 3C,D). The inner surface of the labrum, called epipharynx, is smooth and bears fewer sensilla than the outer surface, such as Sco1, Sco2 and Sca (
Figure 3E,F). These sensilla are surrounded by various types of cuticle protrusions (e.g., spiny processes, palmate processes, and scaly processes) (
Figure 3G,H).
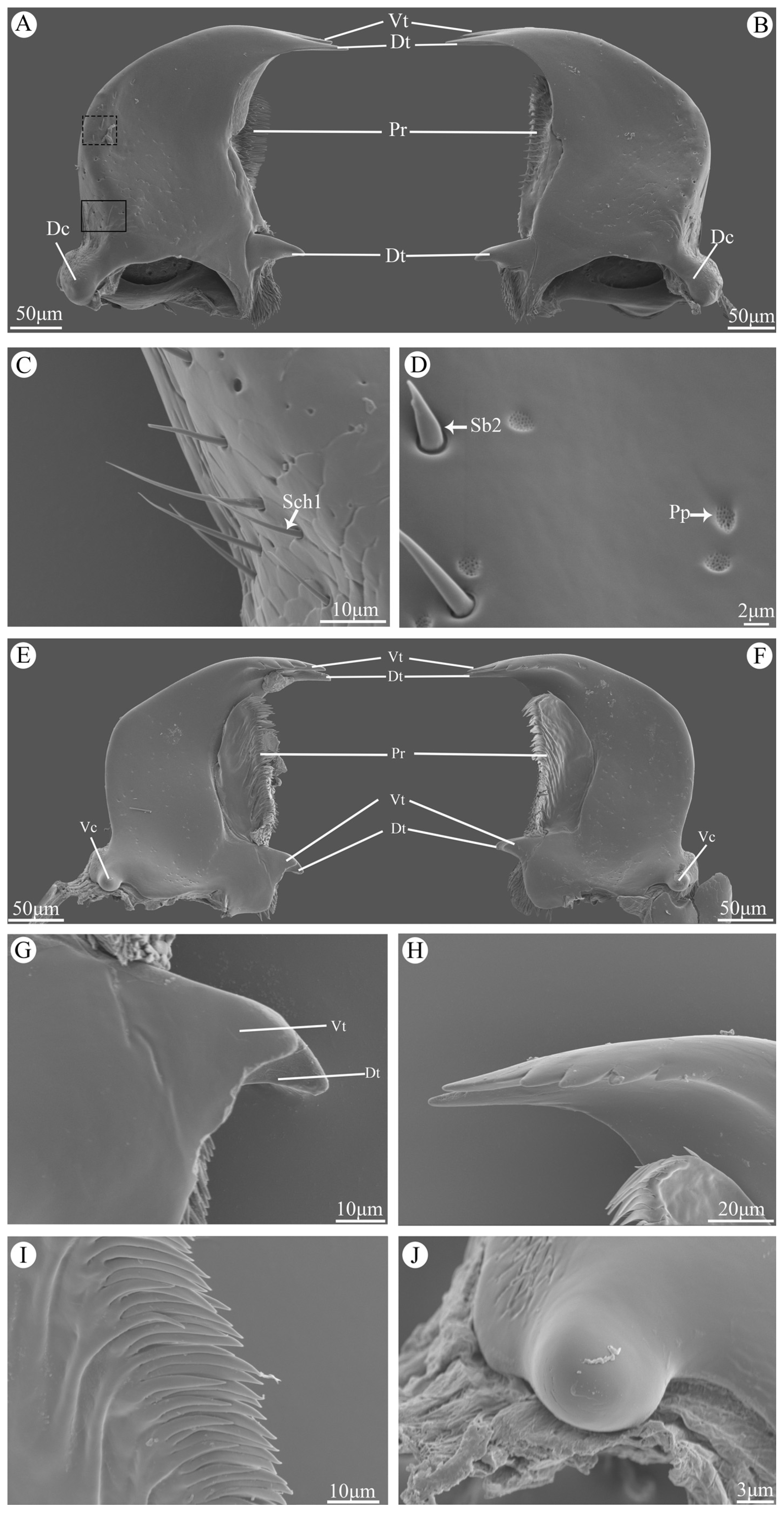
3.5. Mandible
The mandibles are highly sclerotised and symmetrically distributed under the labrum. Their surface are quite smooth with some Cp on the central part and Sch1, Sb2 and Pp on the marginal region (
Figure 4C,D). The apical incisor region consists of two teeth, namely dorsal teeth and ventral teeth, among which the ventral teeth are smooth on the dorsal side while serrated on the ventral side with four little accessory teeth (
Figure 4H). The prostheca lies on the central part of the ventral side of mandibles, and bears numerous multi-layered slender bristles on the margin (
Figure 4I). The molar region consists of two teeth, whereas the morphology of these two teeth are different between left and right mandible. On the right mandible, the ventral tooth is slightly smaller and blunt than the dorsal tooth (
Figure 4G), while on the left mandible, these two teeth are similar in size and shape (
Figure 4F). The dorsal condyle is ellipsoidal and covered with scaly processes (
Figure 4A,B), while the ventral condyle is globular with smooth surface (
Figure 4J).
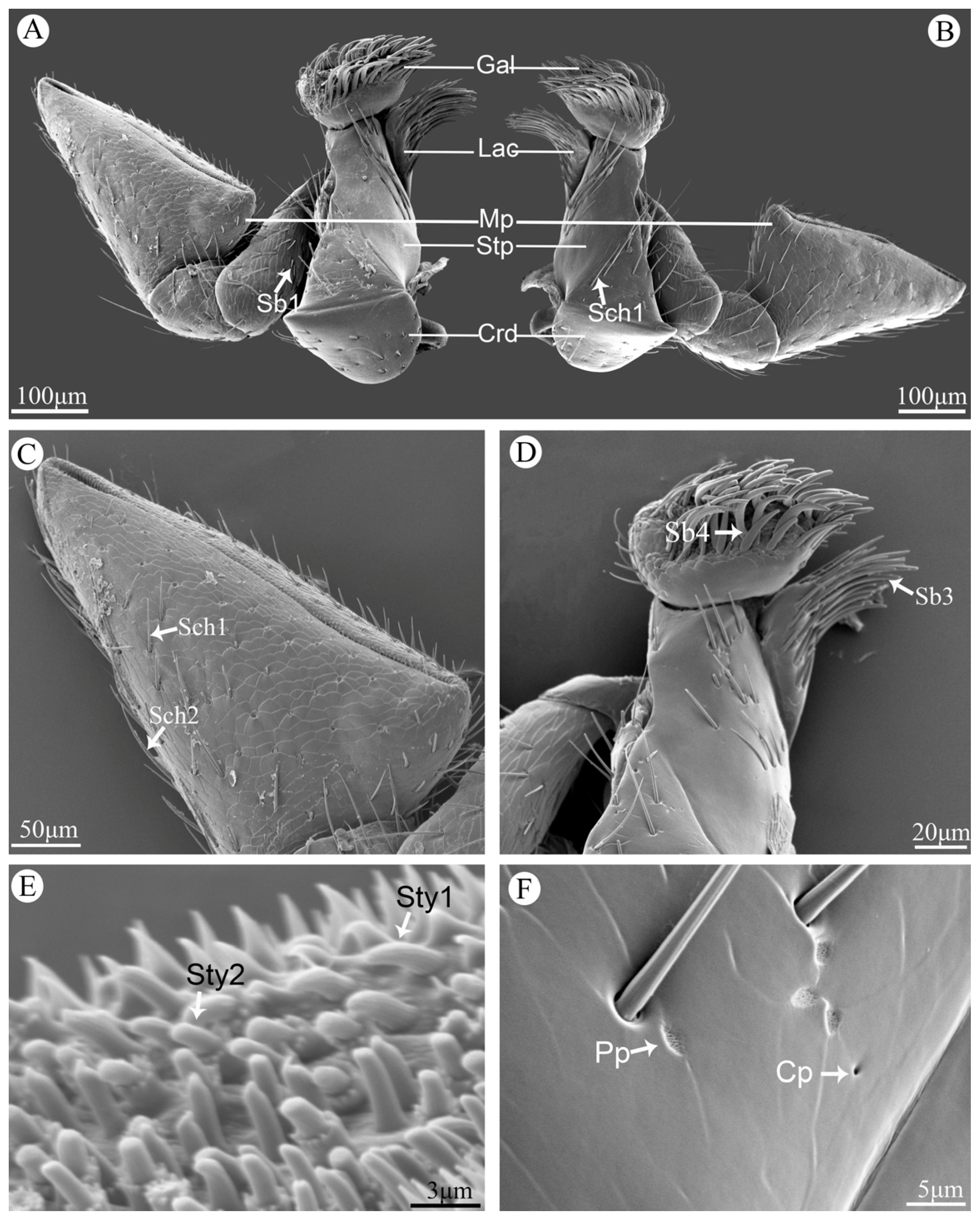
3.6. Maxillae
The maxillae are paired and symmetrical structures, and each maxilla consists of cardo, stipe, lacinia, galea, and a maxillary palp (
Figure 5A,B). Cardo is hemispherical and attached to the head by an articulation. Stipe is trapezoidal and bears Sch1 and Cp. Galea is a spoon-like structure situated at the distal part of the maxillae, with plenty of Sch1 and Sb4 on the top. Lacinia is a brush-like structure with many Sb3 on the margin (
Figure 5D).
The maxillary palp is divided into four segments. The first segment is quite small and attaches to the stipe. The second segment is rectangular and covered with a small amount of Sb1. The third segment is fan-shaped, and the last segment is more dilated in the form of an isosceles triangle (
Figure 5C). The surface of all segments of maxillary palpi are scaly and covered with Sch1, Sch2, Sb1, Pp and Cp (
Figure 5C,F), and the apical region of the last segment of maxillary palp is a sensory region that densely covered with sensilla and cuticular processes. The main types of sensilla are Sty1 and Sty2 (
Figure 5E).
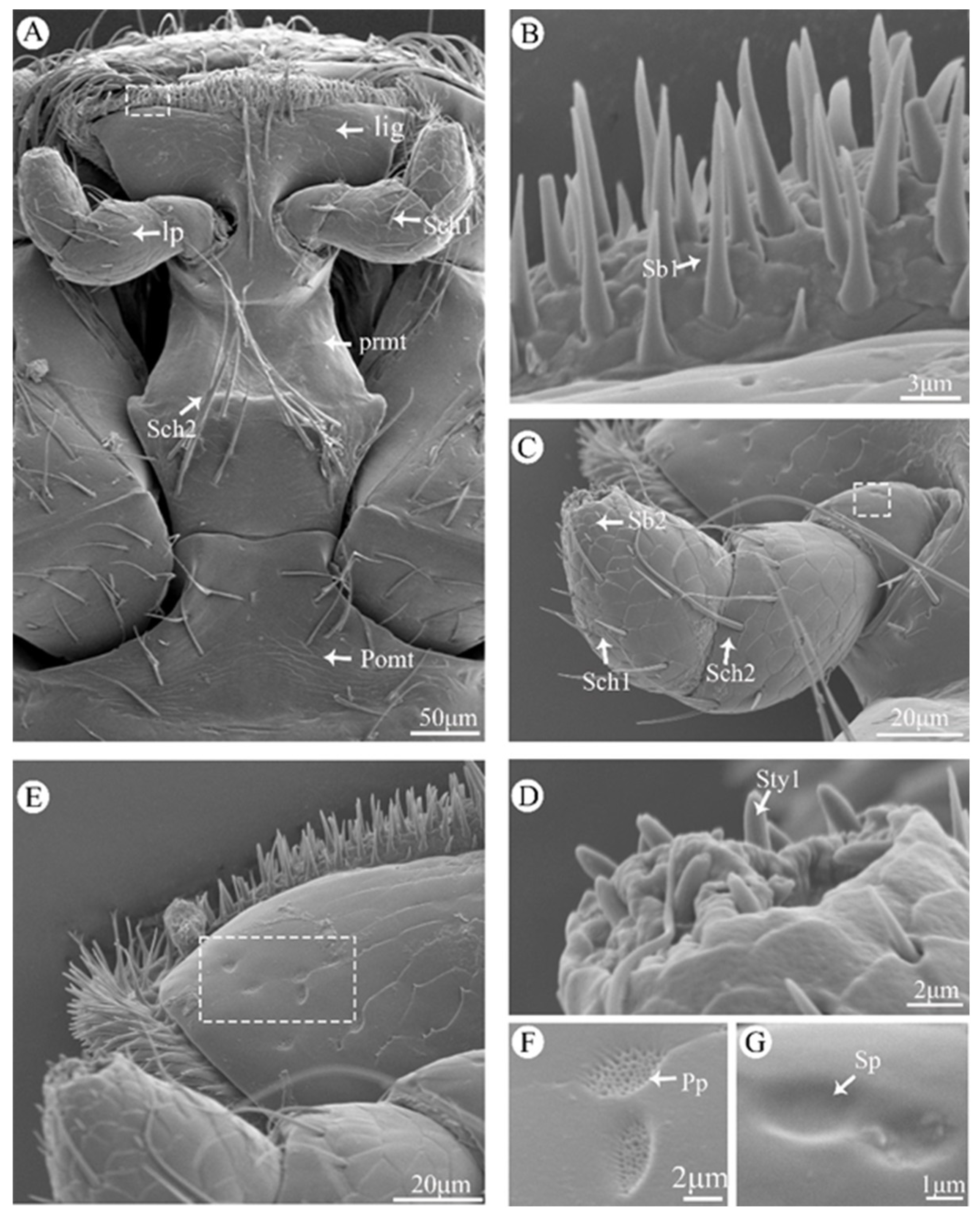
3.7. Labium
The labium consists of a postmentum, prementum, ligula, and a pair of labial palpi (
Figure 6A). The postmentum is hatchet-shaped, have a smooth surface and covered with several Sch1 on the marginal region (
Figure 6A). The prementum is wide in the middle and narrow at both ends, the surface are covered with Sch1, Sch2 and Cp (
Figure 6A). The labial palp consists of three segments, the first segment of the surface is smooth and only with Sp (
Figure 6C,G); the second segment is conical, with scaly processes on the surface and covered with Sch2 (
Figure 6C). The last segment is bullet-shaped, covered with Sch1 on the surface and the extremity around part is covered with Sb2 (
Figure 6C), and a large number of Sty1 are distributed on the top (
Figure 6D). The ligula is trapezoidal, and the top region is covered with Sb1 (
Figure 6B), Sch1, Pp (
Figure 6E,F) and Cp.
4. Discussion
4.1. The Morphology of Mouthparts
In the present study, the mouthparts of Vibidia duodecimguttata are typical chewing mouthparts like other ladybeetles. However, the specific structure of the mouthparts have evolved different morphology with different diets [
29]. Although in the same diet, their food resource maybe different. For example, predatory ladybeetles may feed on aphids, scale insects, whiteflies and mites. Therefore, their mouthpart structure will evolve into different forms according to different diets and food resources [
12]. Besides, the mouthparts structure in the same genus also be different discrepancy [
30]. For example, a detailed comparative study of the mouthparts of Hippodamia variegata and Coccinella transversoguttata showed many differences in the types of sensilla and the specific shapes of the mouthparts [
20].
The morphology of the mandible was reported to be determined by the feeding method [
29], and as a consequence, the mandible is the key to distinguishing between different feeding habits of ladybeetles [
31]. Predatory ladybeetles have either bicuspids or an unidentate apex of mandibles used for piercing the prey and sucks out the juice [
31]. For phytophagous species, their mandibles have multiple denticles that are used to scrape the surface of the leaves and ingest plant sap. While for mycetophagous species, mandibles have several accessory teeth on the ventral tooth that are used for the collection of fungal spores. The mandibles of V. duodecimguttata in this study are similar to the other mycetophagous ladybeetles, the incisors of mandible bear two teeth, the ventral tooth is smaller than the dorsal one, and the ventral tooth bears several accessory teeth. As reported in Illeis chinensis, there is one accessory tooth found on the dorsal tooth of incisor, while 12-16 accessory tooth can be found on the ventral tooth [
21]. For Psyllobora vigintiduopunctata, they have only three accessory teeth [
31]. While for V. duodecimguttata in this study, they have four accessory teeth. This difference in the number of accessory teeth may be related to their feeding habitat.
In phytophagous and predatory species, the comb-like prostheca are used to transport plant sap and empty ‘skin’ [
32], while in mycetophagous species, the shorter but denser comb-like prostheca are used to collect spores from the incisor area to the molar area
[31–33].
In addition to the mandibles, the morphology of the other parts of the mouthparts is also related to feeding habits. Compared to ladybeetles with other diets, the labrum, maxillae and labium of the mycetophagous ladybeetles V. duodecimguttata are in agreement with the morphology of the mouthpart of I. chinensis. Their labrum are enlarged on two sides of the middle part, which may better protect the other structures of the mouthparts. The ligula of the labium are broader, which may better enclose the mandibles, allowing more fungal spores taken in, and preventing them from flowing out better [
21]. Secondly, compared with the phytophagous and predatory species, the mycetophagous ladybeetles have distinct dilated sensory field on the top of last segment of maxillary palpi, which may contribute to search and access food faster and more accurate. Additionally, the curved setae of the maxillary galea and lacinia are well-suited for effcient release and collection of spores and conidia [
34].
4.2. Difference between V. duodecimguttata and I. chinensis
Compared with
I. chinensis, the only mycetophagous ladybeetles reported in previous studies, the first and most significant difference lies in the type of sensilla: both sensilla digitiformia and böhm bristles are notably absent in
V. duodecimguttata. Sensilla digitiformia serve as receptors for heat, water or carbon dioxide stimuli and have been shown to play a role as tactile mechanoreceptors capable of detecting contact and vibration stimuli [
35]. This suggests that
V. duodecimguttata may rely more heavily on antennal sensilla for heat, water or CO2 reception. On the other hand, böhm bristles respond to external stimuli associated with gravity perception. Schneider’s study on insect antennae revealed that böhm bristles on the antennae responded to gravity stimulation when all other body joints were mechanically fixed [
36]. Therefore, we hypothesize that
V. duodecimguttata utilizes its antennal böhm bristles for responding to gravity stimulation. Further research and discussion regarding the antennal sensilla of
V. duodecimguttata is necessary to substantiate these claims.
The other significant difference between these two ladybeetles was found on the maxillary palpi, especially the topical part. In I. chinensis, the end of the maxillary palpi expanded in a fan-shaped manner, whereas in V. duodecimguttata it assumed a triangular shape, from which we speculated that the fan shape may allow for greater range of motion to allow for better search and perception. Futhermore, the sensilla on the surface of mouthpart of I. chinensis is more than that of V. duodecimguttata, especially on the maxillary palpi and labial palpi surface. The surface of the maxillary palpi were found to be covered with an increased amount of Sch1 while only a few Sch3 were present on the prementum of the labium; additionally, there were 28 Sty1 at the end of the labial palpi in I. chinensis as opposed to only about 14 Sty1 in V. duodecimguttata.
The dorsal and ventral teeth of the mandible in
I. chinensis are much sharper [
20]. Additionally, the ligula in
I. chinensis is broader, and the labial palpi are longer compared to other species [
21]. These morphological variations may be attributed to specific ecological adaptions and evolutionary processes. Notably,
V. duodecimguttata primarily consumes fungal spores found on broad-leaved trees and shrubs infected with powdery fungi [
22], while
I. chinensis mainly feeds on hyphae and spores of powdery mildew affecting crops and fruit trees.
4.3. The Function of Sensilla
The numerous sensilla distributed on the mouthparts serve various functions in feeding, host and mate detection, etc. [
37]. During feeding, maxillary palpi and labial palpi play a crucial role in gustatory and olfactory perception [
38], with a significant number of sensilla located in the sensory field on the distal region of these organs [
20]. The predominant types of sensilla found on both maxillary palpi and labial palpi of
V. duodecimguttata are consistent with those observed in other ladybeetles: Sty1 and Sty2 at the tip of the maxillary palpi, as well as Sty1 at the end of labial palpi. Sty2 has been considered an olfactory sensillum [
39], whereas Sty1 has been associated with gustatory and mechanical functions [
38]. Several potential roles for olfaction can be hypothesized including habitat selection, spawning site localization, prey detection or intersexual communication. Olfactory sensilla present on mouthparts primarily contribute to prey search activities [
40]. In addition, same as
I. chinensis, mycophagous ladybeetles have only one Sp on the labial palpi, It was reported that its function is to register the cuticular stress exerted on the palpal tips during prey capture and feeding [
41].
Two kinds of glandular structures were identified on the mouthparts of
V. duodecimguttata namely Cp and Pp. Cp represents a common type of stomata in ladybirds, widely distributed on the surface of the mouthparts, serving as terminal apparatus for secretory cells [
42]. On the other hand, Pp is also extensively distributed across all surfaces of the mouthparts, characterized by numerous perforated plates on the dorsal side of the maxillae that facilitate drainage of glandular cells onto the cuticular surface [
43]. Despite their resemblance to sensory organs [
30], our study observed abundant secretions emanating from these pores, confirming their nature as glandular structures rather than sensory organs.
The other sensilla identified in this study exhibit functional similarities to those commonly observed in other insects, serving as mechano-, temperature- or humidity-reception [
44]. Sensilla chaetica, as a prominent mechanoreceptor on the mouthparts, detects external stimuli [
38,
45]. Sensilla basiconica is the second most abundant sensilla and is typically involved in taste perception and food detection [
38,
46,
47], However, sensilla coeloconic and sensilla campaniformia is exclusively located on the surface of epipharynx in
V. duodecimguttata. Sensilla coeloconica play a role in chemoreception while also sensing changes in temperature and humidity [15,38;43], whereas sensilla campaniformia function as cuticular strain detectors that respond to tension and strain within the cuticle when it comes into contact with food [
48,
49,
50,
51].
This study provides a detailed examination of the fine morphology of each component of the mouthpart of V. duodecimguttata, while also investigating the type, distribution, and morphological structure of the sensilla present on these mouthparts. As one of the few reports on mouthpart morphology in mycetophagous ladybird beetle species, we summarize both similarities and differences observed in comparison to published species, thereby establishing a solid foundation for further research into inter-diet comparisons. Future studies should focus on exploring sensory functions to elucidate variations in sensilla among different species and diets, ultimately shedding light on the feeding mechanisms employed by mycetophagous ladybird beetles.
Author Contributions
Conceptualization, Y.H., Y.S. and L.C.; methodology, Y.H., L.C., K.W. and Y.S. software, L.C. and Y.H.; data curation, Y.H. and L.C.; writing—original draft preparation, L.C., Y.S. and Y.H.; writing—review and editing, Y.H.; supervision, Y.H.; funding acquisition, Y.H., Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (Grant No. 32160120); the Funds for Fuxi Young Scientific Talents of Gansu Agricultural University (Gaufx-03Y05).
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
We sincerely acknowledge the invaluable assistance provided by Xingmin Wang’s team from South China Agricultural University, Guangzhou, China, in accurately identifying ladybird species. Furthermore, we would like to express our appreciation for the help of all staff and students in the Biocontrol Engineering Laboratory of Crop Diseases and Pests of Gansu Province, College of Plant Protection, Gansu Agricultural University, Lanzhou, China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, M.L.; Zhang, L.J.; Zhang, Q.L.; Zhang, L.; Li, M.; Wang, X.T.; Feng, R.Q.; Tang, P.A. Mitogenome evolution in ladybirds: Potential association with dietary adaptation. Ecol. Evol. 2020, 10, 1042–1053. [Google Scholar] [CrossRef]
- Minelli, A.; Pasqual, C. The Mouthparts of Ladybirds: Structure and Function. Ital. J. Zool. 1977, 44, 183–187. [Google Scholar] [CrossRef]
- Sutherland, A.M; Gubler, W.D.; Parrella, M.P. Effects of fungicides on a mycophagous coccinellid may represent integration failure in disease management. Biol. Control. 2010, 54, 292–299. [Google Scholar] [CrossRef]
- Guo, X.L.; Wu, X.B. Utility value and breeding of the fungivorous ladybugs. J. Gansu. Agric. Univ. 1990, 4, 394–400. [Google Scholar]
- Jiang, Y.; Xiu, C.L.; Pan, H.S.; Liu, X.N. Recruitment of Hippodamia variegata by active volatiles from Glycyrrhiza uralensis and Alhagi sparsifolia plants infested with Aphis atrata. Pest. Manag. Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.M.; Legaspi, J.C.; Legaspi, B.C. Adult Survival of Delphastus catalinae (Coleoptera: Coccinellidae), a Predator of Whiteflies (Hemiptera: Aleyrodidae), on Diets of Whiteflies, Honeydew, and Honey. Environ. Entomol. 2012, 41, 669–675. [Google Scholar] [CrossRef]
- Biddinger, D.J.; Weber, D.C.; Hull, L.A. Coccinellidae as predators of mites: Stethorini in biological control. Biol. Control. 2009, 51, 268–283. [Google Scholar] [CrossRef]
- Giorgi, J.A.; Vandenberg, N.J.; McHugh, J.V.; Forrester, J.A.; Ślipiński, S.A.; Miller, K. B.; Shapiro, L.R.; Whiting, M.F. The evolution of food preferences in Coccinellidae. Biol. Control. 2009, 51, 215–231. [Google Scholar] [CrossRef]
- Pervez, A.; Yadav, M.; Bozdoğan, H. Functional morphology of mouthparts and antennal sensillae of two co-generic aphidophagous ladybirds. Int. J. Trop. Insect. Sc. 2022, 42, 2531–2546. [Google Scholar] [CrossRef]
- Rani, P.U.; Madhavendra, S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stall) and Nezara viridula L. (Hemiptera). Int. J. Insect Morphol. Embryol. 1995, 24, 119–132. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, C.N.; Dai, W. Fine Structure and Sensory Apparatus of the Mouthparts of the Maize Weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionoidea: Dryophthoridae). Ann. Entomol. Soc. Am. 2016, 109, 881–889. [Google Scholar] [CrossRef]
- Betz, O.; Thayer, M.K.; Newton, A.F. Comparative morphology and evolutionary pathways of the mouthparts in spore-feeding Staphylinoidea (Coleoptera). Acta Zool-Stockholm 2003, 84, 179–238. [Google Scholar] [CrossRef]
- Wilhelmi, A.; Krenn, H.W. Elongated mouthparts of nectar-feeding Meloidae (Coleoptera). Zoomorphology 2012, 131, 325–337. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.Y.; Fang, H. Morphological study on the mouthparts of four adult Aphodiinae beetles (Coleoptera: Aphodiinae). J. Asia-Pac. Entomo. 2023, 26, 102143. [Google Scholar] [CrossRef]
- Hao, Y.N.; Sun, Y.X.; Liu, C.Z. Functional morphology of the mouthparts of lady beetle Coccinella transversoguttata (Coccinellidae, Coleoptera), with reference to their feeding mechanism. J. Morphol. 2019, 280, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Li, Y.; Xu, F.L.; Yang, M.F.; Wang, X.R.; Wu, C.X. Ultrastructure of the Sensilla on the Antennae and Mouthparts of Bean Weevils, Megabruchidius dorsalis (Coleoptera: Bruchinae). Insects 2021, 12, 1112. [Google Scholar] [CrossRef] [PubMed]
- Eilers, E.J.; Talarico, G.; Hansson, B.S.; Hilker, M.; Reinecke, A. Sensing the Underground—Ultrastructure and Function of Sensory Organs in Root-Feeding Melolontha melolontha (Coleoptera: Scarabaeinae) Larvae. Plos One 2012, 7, e41357. [Google Scholar] [CrossRef]
- Li, Q.H.; Chen, L.Y.; Liu, M.K.; Wang, W.K.; Sabatelli, S.; Giulio, A.D.; Audisio, P. Scanning Electron Microscope Study of Antennae and Mouthparts in the Pollen-Beetle Meligethes (Odonthogethes) chinensis (Coleoptera: Nitidulidae: Meligethinae). Insects 2021, 12, 659. [Google Scholar] [CrossRef]
- Liu, C.T.; Tong, X. Functional morphology of the mouthparts of longhorn beetle adult Psacothea hilaris (Coleoptera: Cerambycidae) and sensilla comparisons between the sexes. Arthropod. Struct. Dev. 2023, 77, 101312. [Google Scholar] [CrossRef]
- Hao, Y.N.; Sun, Y.X.; Liu, C.Z. Functional morphology of the mouthparts of lady beetle Hippodamia variegata (Coleoptera: Coccinellidae), with reference to their feeding mechanism. Zoomorphology 2019, 139, 199–212. [Google Scholar] [CrossRef]
- Wang, K.; Lu, Y.Y.; Bai, M.; Sun, Y.X.; Hao, Y.N. The Microscopic Morphology of Mouthparts and Their Sensilla in the Mycophagous Ladybeetle Illeis chinensis (Coleoptera: Coccinellidae). Insects 2024, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Ceryngier, P.; Godeau, J.F. Predominance of Vibidia duodecimguttata (Poda, 1761) in the assemblages of ladybird beetles (Coleoptera:Coccinellidae) overwintering in floodplain forests. Baltic. J. Coleopterol. 2013, 13, 41–50. [Google Scholar]
- Yan, J.Y.; Song, P.F.; Li, Y.; Tong, X.; Wang, J.L.; Liu, D.X. Characterization of the complete mitochondrial genome of Vibidia duodecimguttata (Coleoptera: Coccinellidae). Mitochondr. Dna. 2020, 5, 1565–1566. [Google Scholar] [CrossRef]
- Sutherland, A.; Parrella, M.P. Biology and Co-Occurrence of Psyllobora vigintimaculata taedata (Coleoptera: Coccinellidae) and Powdery Mildews in an Urban Landscape of California. Ann. Entomol. Soc. Am. 2009, 102, 484–491. [Google Scholar] [CrossRef]
- Tabata, J.; Moraes, C.M.D.; Mescher, M.C. Olfactory Cues from Plants Infected by Powdery Mildew Guide Foraging by a Mycophagous Ladybird Beetle. Plos One 2011, 6, e23799. [Google Scholar] [CrossRef]
- Sutherland, A.M.; Parrella, M.P. Mycophagy in Coccinellidae: a review and synthesis. Biol. Control. 2009, 51, 284–293. [Google Scholar] [CrossRef]
- Altner, H.; Prillinger, L. Ultrastructure of Invertebrate Chemo-, Thermo-, and Hygroreceptors and Its Functional Significance. Int. Rev. Cytol. 1980, 0, 69–139. [Google Scholar]
- Brożek, J.; Bourgoin, T. Morphology and distribution of the external labial sensilla in Fulgoromorpha (Insecta: Hemiptera). Zoomorphology 2012, 132, 33–65. [Google Scholar] [CrossRef] [PubMed]
- Krenn, H.W.; A.H., *!!! REPLACE !!!*. Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod. Struct. Dev. 2012, 41, 101–118. [Google Scholar] [CrossRef]
- Chi, D.F.; Wang, G.L.; Liu, J.W.; Wu, Q.Y; Zhu, Y.P. Antennal Morphology and Sensilla of Asian Multicolored Ladybird Beetles, Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Entomol. News. 2009, 120, 137–152. [Google Scholar] [CrossRef]
- Samways, M.J.; Osborn, R.; Saunders, T.L. Mandible Form Relative to the Main Food Type in Ladybirds (Coleoptera: Coccinellidae). Biocontrol Sci. Techn. 1997, 7, 275–286. [Google Scholar] [CrossRef]
- Karolyi, F.; Hansal, T.; Krenn, H.W.; Colville, J.F. Comparative morphology of the mouthparts of the megadiverse South African monkey beetles (Scarabaeidae: Hopliini): Feeding adaptations and guild structure. PeerJ 2016, 4, e1597. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Huag, J.T.; Maas, A.; Waloszek, D. Functional aspects of the gammaridean mandibles with special reference to the lacinia mobilis (Crustacea, Amphipoda). Zool. Anz. 2013, 252, 536–547. [Google Scholar] [CrossRef]
- Cai, C.Y.; Newton, A.F.; Thayer, M.K.; Leschen, R.A.B.; Huang, D.Y. Specialized proteinine rove beetles shed light on insect—Fungal associations in the Cretaceous. Proc. R. Soc. 2016, 283, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zacharuk, R.Y.; Albert, P.J.; Bellamy, F.W. Ultrastructure and function of digitiform sensilla on the labial palp of a larval elaterid (Coleoptera). Can. J. Zool. 1977, 55, 569–578. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Giglio, A.; Ferrero, E.A.; Perrotta, E.; Talarico, F.F.; Brandmayr, T.Z. Sensory structures involved in prey detection on the labial palp of the ant-hunting beetle Siagona europaea dejean 1826 (coleoptera, carabidae). Acta Zool-Stockholm 2010, 91, 328–334. [Google Scholar] [CrossRef]
- Cao, Y.K.; Huang, M. A SEM study of the antenna and mouthparts of Omosita colon (Linnaeus) (Coleoptera: Nitidulidae). Mirosc. Res. Techniq. 2016, 79, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Vosshall, L. B.; Stocker, R.F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007, 30, 505–533. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, H.; Barbier, R.; Bernard, J.; Ferran, A. Antennal sensilla and sexual dimorphism of the adult ladybird beetle Semiadalia undecimnotata Schn. (Coleoptera: Coccinellidae). Int. J. Ins. Morph. Embryol. 1995, 24, 307–322. [Google Scholar] [CrossRef]
- Baker, G.T.; Monroe, W.A. Sensory receptors on the adult labial and maxillary palpi and galea of Cicindela sexguttata (Coleoptera: Cicindelidae). J. Morphol. 1995, 226, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sevarika, M.; Rondoni, G.; Conti, E.; Romani, R. Antennal sensory organs and glands of the harlequin ladybird, Harmonia axyridis. Entomol. Exp. Appl. 2021, 169, 111–124. [Google Scholar] [CrossRef]
- Skilbeck, C.A.; Anderson, M. The fine structure of glandular units on the antennae of two species of the parasitoid, Aleochara (Coleoptera: Staphylinidae). Int. J. Ins. Morph. Embryol. 1994, 23, 319–328. [Google Scholar] [CrossRef]
- Ruchty, M.; Romani, R.; Kuebler, L.S.; Ruschioni, S.; Roces, F.; Isidoro, N.; Kleineidam, C.J. The thermo-sensitive sensilla coeloconica of leaf-cutting ants (Atta vollenweideri). Arthropod. Struct. Dev. 2009, 38, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Broeckling, C.D.; Salom, S.M. Antennal morphology of two specialist predators of Hemlock woolly adelgid, Adelges tsugae Annand (Homoptera: Adelgidae). Ann. Entomol. Soc. Am. 2003, 96, 153–160. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, L.L.; Luo, Y.Q.; Zong, S.X. Scanning electron microscopy analysis of the cephalic sensilla of Chrysolina aeruginosa Fald. (Coleoptera, Chrysomelidae). Microsc. Res. Techniq. 2013, 76, 423–431. [Google Scholar] [CrossRef]
- Hallberg, E. Sensory Organs in Ips typographus (Insecta: Coleoptera) Fine structure of the sensilla of the maxillary and labial palps. Acta Zool-Stockholm 1982, 63, 191–198. [Google Scholar] [CrossRef]
- Meng, Y.F.; Qin, D.Z. Structure and sensilla of the antennae and mouthparts of Loxocephala perpunctata Jacobi (Hemiptera: Fulgomorpha: Eurybrachidae). Acta Zool-Stockholm 2017, 100, 135–152. [Google Scholar] [CrossRef]
- Ochieng, S.A.; Park, K.C.; Jun, Z.W; Baker, T.C. Functional morphology of antennal chemoreceptors of the parasitoid Microplitis croceipes (Hymenoptera: Braconidae). Arthropod. Struct. Dev. 2000, 29, 231–240. [Google Scholar] [CrossRef]
- Gnatzy, W.; Grünert, U.; Bender, M. Campaniform sensilla of Calliphora vicina (Insecta, Diptera). Zoomorphology 1987, 106, 312–319. [Google Scholar] [CrossRef]
- Keil, TA. Functional morphology of insect mechanoreceptors. Microsc. Res. Techniq. 1997, 39, 506–531. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).