Submitted:
09 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Current Status of CTC Research
2.1. Old and New Blood-Based Cancer Biomarkers for Screening and Progression
2.2. Biology and the Role of CTCs in the Metastatic Process
2.3. Molecular Characteristics of CTCs
2.4. Clinical Significance of CTC Enumeration and Profiling
2.5. Shortcomings of a Single, Non-Recurrent CTC Analysis
2.5.1. The Methodology of CTC Detection Requires Standardization
2.5.2. Fluctuations in CTC Numbers Depend on Circadian Rhythm, Clinico-Pathological Features and Therapeutic Interventions
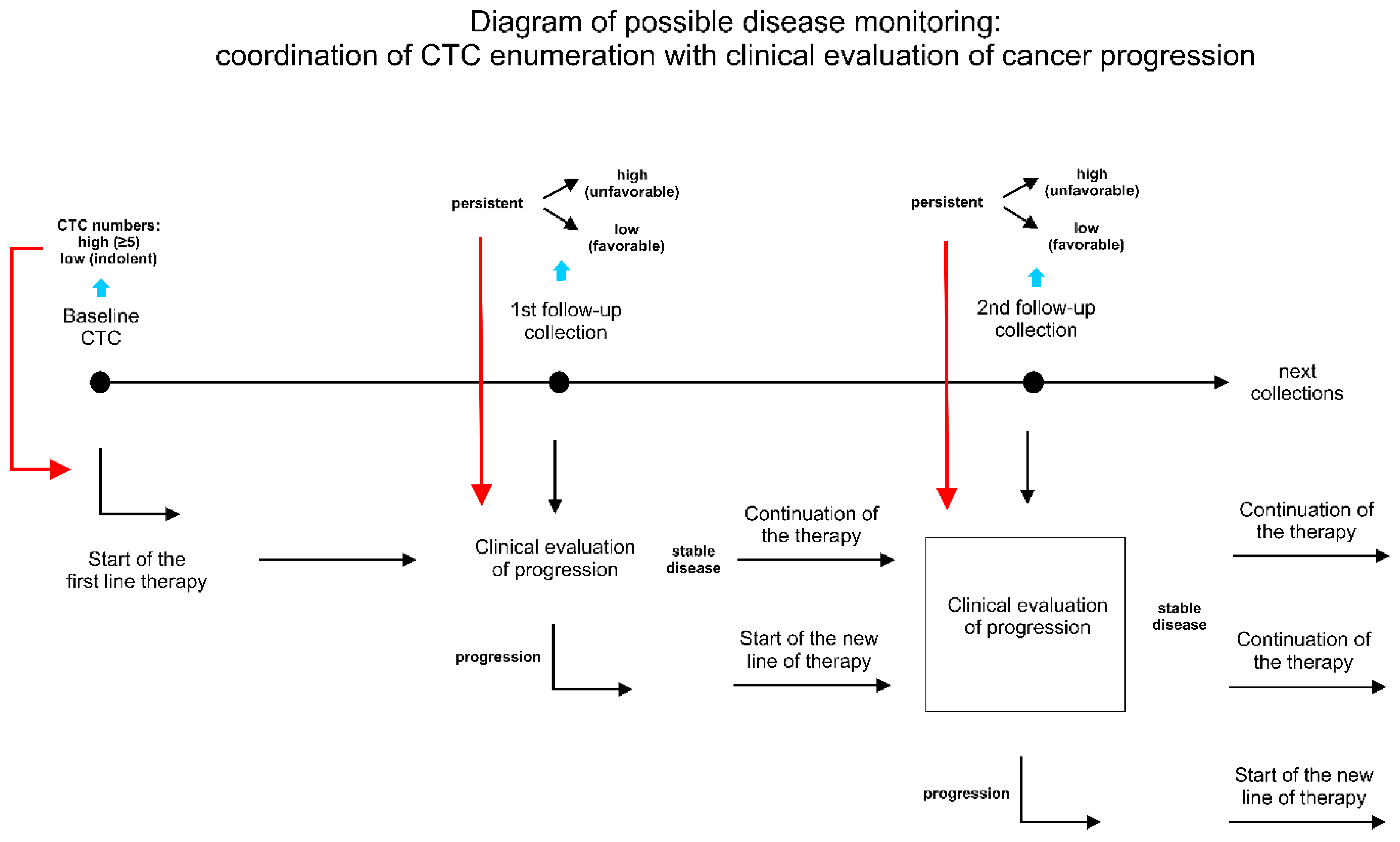
3. Principles and Advantages of Longitudinal Analysis
3.1. Serial Monitoring of CTC Dynamic in a Single Patient is Prognostically Superior to Single or Baseline/Follow-Up Blood Collection
3.2. Clinical Relevance of Long Monitoring in Relation to Treatment
4. Conclusions
Funding
Conflicts of Interest
References
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct Target Ther 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors (Basel) 2023, 24. [Google Scholar] [CrossRef]
- Uygur, M.M.; Gumus, M. The utility of serum tumor markers CEA and CA 15-3 for breast cancer prognosis and their association with clinicopathological parameters. Cancer Treat Res Commun 2021, 28, 100402. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Matsuda, T. Limited usefulness of serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for gastrointestinal and whole-body cancer screening. Sci Rep 2020, 10, 18202. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Evoy, D.; McDermott, E.W. CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta 2010, 411, 1869–1874. [Google Scholar] [CrossRef]
- Varzaru, V.B.; Eftenoiu, A.E.; Vlad, D.C.; Vlad, C.S.; Moatar, A.E.; Popescu, R.; Cobec, I.M. The Influence of Tumor-Specific Markers in Breast Cancer on Other Blood Parameters. Life (Basel) 2024, 14. [Google Scholar] [CrossRef]
- T.R., A. A Case of Cancer in Which Cells Similar to Those in the Tumours Were Seen in the Blood after Death. The Medical Journal of Australia 1869, 14, 146–147. [Google Scholar]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.J.; Padmanaban, V.; Silvestri, V.; Schipper, K.; Cohen, J.D.; Fairchild, A.N.; Gorin, M.A.; Verdone, J.E.; Pienta, K.J.; Bader, J.S.; et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A 2016, 113, E854–863. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Wei, R.R.; Sun, D.N.; Yang, H.; Yan, J.; Zhang, X.; Zheng, X.L.; Fu, X.H.; Geng, M.Y.; Huang, X.; Ding, J. CTC clusters induced by heparanase enhance breast cancer metastasis. Acta Pharmacol Sin 2018, 39, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.M.; Jansson, S.; Bendahl, P.O.; Levin Tykjaer Jorgensen, C.; Loman, N.; Graffman, C.; Lundgren, L.; Aaltonen, K.; Ryden, L. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat 2017, 161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Merino, D.; Weber, T.S.; Serrano, A.; Vaillant, F.; Liu, K.; Pal, B.; Di Stefano, L.; Schreuder, J.; Lin, D.; Chen, Y.; et al. Barcoding reveals complex clonal behavior in patient-derived xenografts of metastatic triple negative breast cancer. Nat Commun 2019, 10, 766. [Google Scholar] [CrossRef]
- Mego, M.; Karaba, M.; Sedlackova, T.; Benca, J.; Repiska, G.; Krasnicanova, L.; Macuch, J.; Sieberova, G.; Jurisova, S.; Pindak, D.; et al. Circulating tumor cells and breast cancer-specific mutations in primary breast cancer. Mol Clin Oncol 2020, 12, 565–573. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, L.; Han, L.; Tuo, X.; Ma, S.; Wang, Y.; Feng, X.; Liang, D.; Sun, C.; Wang, Q.; et al. The Discordance of Gene Mutations between Circulating Tumor Cells and Primary/Metastatic Tumor. Mol Ther Oncolytics 2019, 15, 21–29. [Google Scholar] [CrossRef]
- D'Oronzo, S.; Lovero, D.; Palmirotta, R.; Stucci, L.S.; Tucci, M.; Felici, C.; Cascardi, E.; Giardina, C.; Cafforio, P.; Silvestris, F. Dissection of major cancer gene variants in subsets of circulating tumor cells in advanced breast cancer. Sci Rep 2019, 9, 17276. [Google Scholar] [CrossRef]
- Genna, A.; Vanwynsberghe, A.M.; Villard, A.V.; Pottier, C.; Ancel, J.; Polette, M.; Gilles, C. EMT-Associated Heterogeneity in Circulating Tumor Cells: Sticky Friends on the Road to Metastasis. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Li, W.; Yang, R.; Zhang, X.; Ye, Y.; Yu, J.; Ye, L.; Tang, W. Circulating tumor cells undergoing EMT are poorly correlated with clinical stages or predictive of recurrence in hepatocellular carcinoma. Sci Rep 2019, 9, 7084. [Google Scholar] [CrossRef]
- Vardas, V.; Politaki, E.; Pantazaka, E.; Georgoulias, V.; Kallergi, G. Epithelial-to-mesenchymal transition of tumor cells: cancer progression and metastasis. Int J Dev Biol 2022, 66, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Gires, O.; Stoecklein, N.H. Dynamic EpCAM expression on circulating and disseminating tumor cells: causes and consequences. Cell Mol Life Sci 2014, 71, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Agnoletto, C.; Corra, F.; Minotti, L.; Baldassari, F.; Crudele, F.; Cook, W.J.J.; Di Leva, G.; d'Adamo, A.P.; Gasparini, P.; Volinia, S. Heterogeneity in Circulating Tumor Cells: The Relevance of the Stem-Cell Subset. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, X.; Zheng, J.; Dong, D. DNA methylome profiling of circulating tumor cells in lung cancer at single base-pair resolution. Oncogene 2021, 40, 1884–1895. [Google Scholar] [CrossRef]
- Markou, A.; Londra, D.; Tserpeli, V.; Kollias, I.; Tsaroucha, E.; Vamvakaris, I.; Potaris, K.; Pateras, I.; Kotsakis, A.; Georgoulias, V.; et al. DNA methylation analysis of tumor suppressor genes in liquid biopsy components of early stage NSCLC: a promising tool for early detection. Clin Epigenetics 2022, 14, 61. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Y.; Li, B.; Lu, X.; Wang, R.; Li, H.; Yan, B.; Gu, A.; Wang, W.; Huang, A.; et al. Whole-Exome Sequencing on Circulating Tumor Cells Explores Platinum-Drug Resistance Mutations in Advanced Non-small Cell Lung Cancer. Front Genet 2021, 12, 722078. [Google Scholar] [CrossRef]
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.C.; et al. Whole-exome sequencing of cell-free DNA and circulating tumor cells in multiple myeloma. Nat Commun 2018, 9, 1691. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Huang, B.; Lan, G.; Zhang, B.; Wei, J.; Deng, Z.; Li, Y.; Qin, Y.; Li, B.; Lu, Y.; et al. Comparison of whole exome sequencing in circulating tumor cells of primitive and metastatic nasopharyngeal carcinoma. Transl Cancer Res 2020, 9, 4080–4092. [Google Scholar] [CrossRef]
- Szostakowska, M.; Trebinska-Stryjewska, A.; Grzybowska, E.A.; Fabisiewicz, A. Resistance to endocrine therapy in breast cancer: molecular mechanisms and future goals. Breast Cancer Res Treat 2019, 173, 489–497. [Google Scholar] [CrossRef]
- Jeselsohn, R.; Buchwalter, G.; De Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015, 12, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Mack, P.C.; Banks, K.C.; Espenschied, C.R.; Burich, R.A.; Zill, O.A.; Lee, C.E.; Riess, J.W.; Mortimer, S.A.; Talasaz, A.; Lanman, R.B.; et al. Spectrum of driver mutations and clinical impact of circulating tumor DNA analysis in non-small cell lung cancer: Analysis of over 8000 cases. Cancer 2020, 126, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.K.; Lee, E.; Ng, C.C.; Yang, V.S.; Farid, M.; Teh, B.T.; Chan, J.Y.; Somasundaram, N. Circulating Tumor DNA Mutations in Progressive Gastrointestinal Stromal Tumors Identify Biomarkers of Treatment Resistance and Uncover Potential Therapeutic Strategies. Front Oncol 2022, 12, 840843. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.M.; van Marion, R.; Atmodimedjo, P.N.; de Jonge, E.; Mathijssen, R.H.J.; Paats, M.S.; de Bruijn, P.; Koolen, S.L.; von der Thusen, J.H.; Aerts, J.; et al. Clinical Utility of Circulating Tumor DNA in Patients With Advanced KRAS(G12C)-Mutated NSCLC Treated With Sotorasib. J Thorac Oncol 2024, 19, 995–1006. [Google Scholar] [CrossRef]
- Rothe, F.; Venet, D.; Peeters, D.; Rouas, G.; Rediti, M.; Smeets, D.; Dupont, F.; Campbell, P.; Lambrechts, D.; Dirix, L.; et al. Interrogating breast cancer heterogeneity using single and pooled circulating tumor cell analysis. NPJ Breast Cancer 2022, 8, 79. [Google Scholar] [CrossRef]
- Janni, W.J.; Rack, B.; Terstappen, L.W.; Pierga, J.Y.; Taran, F.A.; Fehm, T.; Hall, C.; de Groot, M.R.; Bidard, F.C.; Friedl, T.W.; et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res 2016, 22, 2583–2593. [Google Scholar] [CrossRef]
- Goodman, C.R.; Seagle, B.L.; Friedl, T.W.P.; Rack, B.; Lato, K.; Fink, V.; Cristofanilli, M.; Donnelly, E.D.; Janni, W.; Shahabi, S.; et al. Association of Circulating Tumor Cell Status With Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol 2018, 4, e180163. [Google Scholar] [CrossRef]
- Bidard, F.C.; Michiels, S.; Riethdorf, S.; Mueller, V.; Esserman, L.J.; Lucci, A.; Naume, B.; Horiguchi, J.; Gisbert-Criado, R.; Sleijfer, S.; et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J Natl Cancer Inst 2018, 110, 560–567. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Pierga, J.Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit Rev Oncol Hematol 2019, 134, 39–45. [Google Scholar] [CrossRef]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O'Rourke, M.A.; Lew, D.L.; et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 2014, 32, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Jacot, W.; Dureau, S.; Brain, E.; Bachelot, T.; Bourgeois, H.; Goncalves, A.; Ladoire, S.; Naman, H.; Dalenc, F.; et al. Abstract GS3-07: Clinical utility of circulating tumor cell count as a tool to chose between first line hormone therapy and chemotherapy for ER+ HER2- metastatic breast cancer: Results of the phase III STIC CTC trial. Cancer Research 2019, 79 (4_Supplement), GS3-07. [Google Scholar] [CrossRef]
- Jacot, W.; Cottu, P.; Berger, F.; Dubot, C.; Venat-Bouvet, L.; Lortholary, A.; Bourgeois, H.; Bollet, M.; Servent, V.; Luporsi, E.; et al. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: the CirCe T-DM1 trial. Breast Cancer Res 2019, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Arkadius Polasik, A.S. , Thomas W. P. Friedl, Brigitte Kathrin Rack, Elisabeth Katharina Trapp, Peter A. Fasching, Florin-Andrei Taran, Andreas D. Hartkopf, Andreas Schneeweiss, Volkmar Mueller, Bahriye Aktas, Klaus Pantel, Franziska Meier-Stiegen, Pauline Wimberger, Wolfgang Janni, Tanja N. Fehm. The DETECT study concept: Individualized therapy of metastatic breast cancer. Journal of Clinical Oncology 2016, 34. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther 2021, 6, 404. [Google Scholar] [CrossRef]
- Stoecklein, N.H.; Oles, J.; Franken, A.; Neubauer, H.; Terstappen, L.; Neves, R.P.L. Clinical application of circulating tumor cells. Med Genet 2023, 35, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Wang, P.; Peng, J.; Wang, X.; Zhu, Y.W.; Shen, N. Meta-analysis Reveals the Prognostic Value of Circulating Tumour Cells Detected in the Peripheral Blood in Patients with Non-Metastatic Colorectal Cancer. Sci Rep 2017, 7, 905. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Rahbari, N.N.; Buchler, M.W.; Koch, M.; Weitz, J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol 2013, 20, 2156–2165. [Google Scholar] [CrossRef]
- Huang, X.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Xu, H.; Wang, Z. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015, 15, 202. [Google Scholar] [CrossRef]
- Krebs, M.G.; Renehan, A.G.; Backen, A.; Gollins, S.; Chau, I.; Hasan, J.; Valle, J.W.; Morris, K.; Beech, J.; Ashcroft, L.; et al. Circulating Tumor Cell Enumeration in a Phase II Trial of a Four-Drug Regimen in Advanced Colorectal Cancer. Clin Colorectal Cancer 2015, 14, 115–122.e111-112. [Google Scholar] [CrossRef]
- Krebs, M.G.; Sloane, R.; Priest, L.; Lancashire, L.; Hou, J.M.; Greystoke, A.; Ward, T.H.; Ferraldeschi, R.; Hughes, A.; Clack, G.; et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011, 29, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Kapeleris, J.; Kulasinghe, A.; Warkiani, M.E.; Vela, I.; Kenny, L.; O'Byrne, K.; Punyadeera, C. The Prognostic Role of Circulating Tumor Cells (CTCs) in Lung Cancer. Front Oncol 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, E.A.; Atwal, S.; Liu, W.; Raja, R.; Fine, B.M.; Hughes, B.G.; Hicks, R.J.; Hampton, G.M.; Amler, L.C.; Pirzkall, A.; et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012, 18, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, Y.; Zhao, J.; Chen, M.; Xu, Y.; Zhong, W.; Xing, J.; Wang, M. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology 2016, 21, 519–525. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- De Souza, L.M.; Robertson, B.M.; Robertson, G.P. Future of circulating tumor cells in the melanoma clinical and research laboratory settings. Cancer Lett 2017, 392, 60–70. [Google Scholar] [CrossRef]
- Aktar, S.; Baghaie, H.; Islam, F.; Gopalan, V.; Lam, A.K. Current Status of Circulating Tumor Cells in Head and Neck Squamous Cell Carcinoma: A Review. Otolaryngol Head Neck Surg 2023, 168, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- Rack, B.; Schindlbeck, C.; Juckstock, J.; Andergassen, U.; Hepp, P.; Zwingers, T.; Friedl, T.W.; Lorenz, R.; Tesch, H.; Fasching, P.A.; et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014, 106. [Google Scholar] [CrossRef]
- Massard, C.; Borget, I.; Farace, F.; Aspeslagh, S.; Le Deley, M.C.; Le Tourneau, C.; Bidard, F.C.; Pierga, J.Y.; Dieras, V.; Hofman, P.; et al. RECIST response and variation of circulating tumour cells in phase 1 trials: A prospective multicentric study. Eur J Cancer 2017, 83, 185–193. [Google Scholar] [CrossRef]
- Muller, V.; Riethdorf, S.; Rack, B.; Janni, W.; Fasching, P.A.; Solomayer, E.; Aktas, B.; Kasimir-Bauer, S.; Pantel, K.; Fehm, T.; et al. Prognostic impact of circulating tumor cells assessed with the CellSearch System and AdnaTest Breast in metastatic breast cancer patients: the DETECT study. Breast Cancer Res 2012, 14, R118. [Google Scholar] [CrossRef]
- Templeman, A.; Miller, M.C.; Cooke, M.J.; O'Shannessy, D.J.; Gurung, Y.; Pereira, T.; Peters, S.G.; Piano, M.; Teo, M.; Khazan, N.; et al. Analytical performance of the FDA-cleared Parsortix((R)) PC1 system. J Circ Biomark 2023, 12, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wishart, G.; Templeman, A.; Hendry, F.; Miller, K.; Pailhes-Jimenez, A.S. Molecular Profiling of Circulating Tumour Cells and Circulating Tumour DNA: Complementary Insights from a Single Blood Sample Utilising the Parsortix((R)) System. Curr Issues Mol Biol 2024, 46, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Zavridou, M.; Mastoraki, S.; Strati, A.; Koutsodontis, G.; Klinakis, A.; Psyrri, A.; Lianidou, E. Direct comparison of size-dependent versus EpCAM-dependent CTC enrichment at the gene expression and DNA methylation level in head and neck squamous cell carcinoma. Sci Rep 2020, 10, 6551. [Google Scholar] [CrossRef] [PubMed]
- Braso-Maristany, F.; Griguolo, G.; Pascual, T.; Pare, L.; Nuciforo, P.; Llombart-Cussac, A.; Bermejo, B.; Oliveira, M.; Morales, S.; Martinez, N.; et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat Commun 2020, 11, 385. [Google Scholar] [CrossRef]
- Ju, S.; Chen, C.; Zhang, J.; Xu, L.; Zhang, X.; Li, Z.; Chen, Y.; Zhou, J.; Ji, F.; Wang, L. Detection of circulating tumor cells: opportunities and challenges. Biomark Res 2022, 10, 58. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBioMedicine 2022, 83, 104237. [Google Scholar] [CrossRef]
- Edd, J.F.; Mishra, A.; Smith, K.C.; Kapur, R.; Maheswaran, S.; Haber, D.A.; Toner, M. Isolation of circulating tumor cells. iScience 2022, 25, 104696. [Google Scholar] [CrossRef]
- Mathias, T.J.; Chang, K.T.; Martin, S.S.; Vitolo, M.I. Gauging the Impact of Cancer Treatment Modalities on Circulating Tumor Cells (CTCs). Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Donato, C.; Kunz, L.; Castro-Giner, F.; Paasinen-Sohns, A.; Strittmatter, K.; Szczerba, B.M.; Scherrer, R.; Di Maggio, N.; Heusermann, W.; Biehlmaier, O.; et al. Hypoxia Triggers the Intravasation of Clustered Circulating Tumor Cells. Cell Rep 2020, 32, 108105. [Google Scholar] [CrossRef]
- Zhu, X.; Suo, Y.; Fu, Y.; Zhang, F.; Ding, N.; Pang, K.; Xie, C.; Weng, X.; Tian, M.; He, H.; et al. In vivo flow cytometry reveals a circadian rhythm of circulating tumor cells. Light Sci Appl 2021, 10, 110. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The metastatic spread of breast cancer accelerates during sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N. Fluctuating numbers of circulating tumor cells in cancer patients and the meaning of zero counts. Oncotarget 2019, 10, 2658–2659. [Google Scholar] [CrossRef] [PubMed]
- Juratli, M.A.; Siegel, E.R.; Nedosekin, D.A.; Sarimollaoglu, M.; Jamshidi-Parsian, A.; Cai, C.; Menyaev, Y.A.; Suen, J.Y.; Galanzha, E.I.; Zharov, V.P. In Vivo Long-Term Monitoring of Circulating Tumor Cells Fluctuation during Medical Interventions. PLoS One 2015, 10, e0137613. [Google Scholar] [CrossRef] [PubMed]
- Gall, T.M.; Jacob, J.; Frampton, A.E.; Krell, J.; Kyriakides, C.; Castellano, L.; Stebbing, J.; Jiao, L.R. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg 2014, 149, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Xiao, W.; Dong, S.L.; Liang, H.F.; Zhang, Z.W.; Zhang, B.X.; Huang, Z.Y.; Chen, Y.F.; Zhang, W.G.; Luo, H.P.; et al. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma. BMC Cancer 2018, 18, 835. [Google Scholar] [CrossRef]
- Haga, N.; Onagi, A.; Koguchi, T.; Hoshi, S.; Ogawa, S.; Akaihata, H.; Hata, J.; Hiraki, H.; Honda, R.; Tanji, R.; et al. Perioperative Detection of Circulating Tumor Cells in Radical or Partial Nephrectomy for Renal Cell Carcinoma. Ann Surg Oncol 2020, 27, 1272–1281. [Google Scholar] [CrossRef]
- Camara, O.; Kavallaris, A.; Noschel, H.; Rengsberger, M.; Jorke, C.; Pachmann, K. Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol 2006, 4, 67. [Google Scholar] [CrossRef]
- Pang, S.; Li, H.; Xu, S.; Feng, L.; Ma, X.; Chu, Y.; Zou, B.; Wang, S.; Zhou, G. Circulating tumour cells at baseline and late phase of treatment provide prognostic value in breast cancer. Sci Rep 2021, 11, 13441. [Google Scholar] [CrossRef]
- Martin, O.A.; Anderson, R.L.; Russell, P.A.; Cox, R.A.; Ivashkevich, A.; Swierczak, A.; Doherty, J.P.; Jacobs, D.H.; Smith, J.; Siva, S.; et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys 2014, 88, 395–403. [Google Scholar] [CrossRef]
- Mathenge, E.G.; Dean, C.A.; Clements, D.; Vaghar-Kashani, A.; Photopoulos, S.; Coyle, K.M.; Giacomantonio, M.; Malueth, B.; Nunokawa, A.; Jordan, J.; et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia 2014, 16, 950–960. [Google Scholar] [CrossRef]
- Kusukawa, J.; Suefuji, Y.; Ryu, F.; Noguchi, R.; Iwamoto, O.; Kameyama, T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J Oral Pathol Med 2000, 29, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.A.; Anderson, R.L.; Narayan, K.; MacManus, M.P. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat Rev Clin Oncol 2017, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kobuchi, S.; Kawakita, A.; Tosaka, K.; Matsunaga, Y.; Yoshioka, S.; Jonan, S.; Amagase, K.; Hashimoto, K.; Kanda, M.; et al. Mobilization of Circulating Tumor Cells after Short- and Long-Term FOLFIRINOX and GEM/nab-PTX Chemotherapy in Xenograft Mouse Models of Human Pancreatic Cancer. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Ortiz-Otero, N.; Marshall, J.R.; Lash, B.; King, M.R. Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer 2020, 20, 873. [Google Scholar] [CrossRef]
- Vetter, M.; Landin, J.; Szczerba, B.M.; Castro-Giner, F.; Gkountela, S.; Donato, C.; Krol, I.; Scherrer, R.; Balmelli, C.; Malinovska, A.; et al. Denosumab treatment is associated with the absence of circulating tumor cells in patients with breast cancer. Breast Cancer Res 2018, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Bendahl, P.O.; Belting, M.; Gezelius, E. Longitudinal Assessment of Circulating Tumor Cells and Outcome in Small Cell Lung Cancer: A Sub-Study of RASTEN-A Randomized Trial with Low Molecular Weight Heparin. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Lozano, R.; Lorente, D.; Aragon, I.M.; Romero-Laorden, N.; Nombela, P.; Mateo, J.; Reid, A.H.M.; Cendon, Y.; Bianchini, D.; Llacer, C.; et al. Value of Early Circulating Tumor Cells Dynamics to Estimate Docetaxel Benefit in Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Bidard, F.C.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Costa, C.; Muinelo-Romay, L.; Cebey-Lopez, V.; Pereira-Veiga, T.; Martinez-Pena, I.; Abreu, M.; Abalo, A.; Lago-Leston, R.M.; Abuin, C.; Palacios, P.; et al. Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-clusters) as Predictors of Patient Outcomes. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Gerratana, L.; Davis, A.A.; Zhang, Q.; Basile, D.; Rossi, G.; Strickland, K.; Franzoni, A.; Allegri, L.; Mu, Z.; Zhang, Y.; et al. Longitudinal Dynamics of Circulating Tumor Cells and Circulating Tumor DNA for Treatment Monitoring in Metastatic Breast Cancer. JCO Precis Oncol 2021, 5, 943–952. [Google Scholar] [CrossRef]
- Szostakowska-Rodzos, M.; Fabisiewicz, A.; Wakula, M.; Tabor, S.; Szafron, L.; Jagiello-Gruszfeld, A.; Grzybowska, E.A. Longitudinal analysis of circulating tumor cell numbers improves tracking metastatic breast cancer progression. Sci Rep 2024, 14, 12924. [Google Scholar] [CrossRef] [PubMed]
- Forsare, C.; Bendahl, P.O.; Moberg, E.; Levin Tykjaer Jorgensen, C.; Jansson, S.; Larsson, A.M.; Aaltonen, K.; Ryden, L. Evolution of Estrogen Receptor Status from Primary Tumors to Metastasis and Serially Collected Circulating Tumor Cells. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.N.; Jayachandran, G.; Gao, H.; Peabody, P.; McBride, H.B.; Alvarez, F.D.; Kai, M.; Song, J.; Shen, Y.; Willey, J.S.; et al. Phenotypic Plasticity in Circulating Tumor Cells Is Associated with Poor Response to Therapy in Metastatic Breast Cancer Patients. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulou, D.; Markou, A.; Strati, A.; Zavridou, M.; Tzanikou, E.; Mastoraki, S.; Kallergi, G.; Georgoulias, V.; Lianidou, E. Comprehensive liquid biopsy analysis as a tool for the early detection of minimal residual disease in breast cancer. Sci Rep 2023, 13, 1258. [Google Scholar] [CrossRef]
- Hendricks, A.; Dall, K.; Brandt, B.; Geisen, R.; Roder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Longitudinal Analysis of Circulating Tumor Cells in Colorectal Cancer Patients by a Cytological and Molecular Approach: Feasibility and Clinical Application. Front Oncol 2021, 11, 646885. [Google Scholar] [CrossRef]
- Ko, J.M.; Vardhanabhuti, V.; Ng, W.T.; Lam, K.O.; Ngan, R.K.; Kwong, D.L.; Lee, V.H.; Lui, Y.H.; Yau, C.C.; Kwan, C.K.; et al. Clinical utility of serial analysis of circulating tumour cells for detection of minimal residual disease of metastatic nasopharyngeal carcinoma. Br J Cancer 2020, 123, 114–125. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Hendrix, L.H.; Hyslop, T.; Barry, W.T.; Winer, E.P.; Hudis, C.; Toppmeyer, D.; Carey, L.A.; Partridge, A.H.; Pierga, J.Y.; et al. Serial Analysis of Circulating Tumor Cells in Metastatic Breast Cancer Receiving First-Line Chemotherapy. J Natl Cancer Inst 2021, 113, 443–452. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Savenkov, O.; Asmus, E.J.; Ballman, K.V.; Scott, J.H.; Park, J.W.; Dickler, M.; Partridge, A.; Carey, L.A.; Winer, E.P.; et al. Clinical Significance of Circulating Tumor Cells in Hormone Receptor-positive Metastatic Breast Cancer Patients who Received Letrozole with or Without Bevacizumab. Clin Cancer Res 2020, 26, 4911–4920. [Google Scholar] [CrossRef]
- Ko, J.M.Y.; Lam, K.O.; Kwong, D.L.W.; Wong, I.Y.; Chan, F.S.; Wong, C.L.; Chan, K.K.; Law, T.T.; Chiu, K.W.H.; Lam, C.C.S.; et al. Circulating Tumor Cell Enumeration for Serial Monitoring of Treatment Outcomes for Locally Advanced Esophageal Squamous Cell Carcinoma. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
| Cancer Type | Authors | Year | No. of patients included to analysis | No. of collections | CTC detection method | Main findings | REF# |
|---|---|---|---|---|---|---|---|
| Breast Cancer | Wang C, Mu Z, Chervoneva I,….,Cristofanilli M, Yang, H. | 2017 | 128 | 3 | CellSearch | CTC-clusters added additional prognostic values to CTC enumeration alone, and a larger-size CTC-cluster conferred a higher risk of death in MBC patients | [13] |
| Breast Cancer | Larsson AM, Jansson S, Bendahl PO, …., Rydén L. | 2018 | 152 | 4 | CellSearch | Longitudinal evaluation of CTC and CTC clusters improves prognostication and monitoring in patients with MBC starting first-line systemic therapy. Changes in CTC count throughout treatment significantly correlated with survival and the prognostic value was more prominent at later time points. High CTC counts and presence of clusters were identified as prognostic factors for OS and PFS. | [12] |
| Breast Cancer | Forsare C, Bendahl PO, Moberg E, …., Rydén L. | 2020 | 147 | 3 | CellSearch | A shift in ER-status from PT to DM/CTCs was demonstrated. Retained ER positivity of CTCs after initiation of systemic therapy was associated with better prognosis for PFS. This effect was observed only for followu-up samples, highlighting the importance of CTCs phenotyping during the treatment. | [92] |
| Nasopharyngeal carcinoma (NPC) | Ko JMY, Vardhanabhuti VV, Ng WT,…... Lung ML. | 2020 | 21 | 4 | CTChip®FR1 | CTCs were characterized as a more sensitive biomarker for MRD, when compared with imaging. Longitudinal changes in CTCs and EBV DNA along CT treatment for mNPC was found predictive for disease relapse. | [96] |
| Breast Cancer | Magbanua MJM, Hendrix LH, Hyslop T, ……. Rugo HS. | 2021 | 469 | ≥3 | CellSearch | The authors conducted the CTC trajectory model, which divided the patients into groups predicting the consistent trend for negative CTCs, low CTCs, mid CTCs and high CTCs status. The mid and high tCTC groups were identified with higher risk of early prgression, shorter PFS and OS. | [97] |
| Breast Cancer | Magbanua MJM, Savenkov O, Asmus EJ, ...., Rugo HS. | 2021 | 294 | 4 | CellSearch | CTCs positive patients at the baseline were identified with worse PFS and OS than CTC negative patients. The CTC positivity during the treatment or baseline was identified as risk factor for PFS and OS. Patients that became CTC positive in 1st follow-up had poorer prognosis for OS than patients that stayed CTC negative or patients that remain CTC positive since baseline. Patients who stayed CTC positive had poorer PFS and OS that patients who stayed CTC negative since baseline. | [98] |
| Breast Cancer | Gerratana L, Davis AA, Zhang Q, …….., Cristofanilli M. | 2021 | 107 | 3 | CellSearch | The ctDNA analysis revelaed that mutant allele frequency (MAF) changes follow the response to treatment, while CTC numbers increased only at the time of clinical progression. Conclusion: MAF could be more suitable for real-time disease monitoring, while CTCs could be more likely linked to metastatic biology. | [90] |
| Breast Cancer | Pang S, Li H, Xu S, …..., Zhou G. | 2021 | 164 | 4 | IMNs (immunomagnetic nanospheres) | Surgery led to an increase in the number and prevalence of CTCs on the first day after surgery and did not return to the preoperative level until 14 days after surgery. The CTC prevalence at the baseline and end-point follow-up visits was related to PFS and OS, while the CTCs detected before chemotherapy were only related to PFS. | [78] |
| Colorectal Cancer | Hendricks A, Dall K, Brandt B,…..., Sebens S. | 2021 | 47 | 5 | NYONE, RT-PCR | Surgery did not have any statistically significant effect on the quantity of CTC detected by the cytological approach utilizing the cell imager NYONE. In one of the patients constant increase in CTCs detected via both methods (9 months after the surgery) occured before the local clinical reccurence (13 months after surgery). | [95] |
| Breast Cancer | Stergiopoulou D, Markou A, Strati A,…….., Lianidou E. | 2023 | 13 | ≥10 | CellSearch | The molecular characteristics of CTCs were highly different even for the same patient at different time points, and always increased before the clinical relapse. Rapid increases in CTC numbers at months 74 and 122, were associated with metastatic disease documented by biopsy 6 months earlier. | [94] |
| Breast Cancer | Cohen EN, Jayachandran G, Gao H, ……….., Reuben JM. | 2023 | 184 | 9 | MCA (microactivity array) | The study reaffirmed the cut-off of ≥5 CTCs for inferior prognosis of patients with MBC. It also highlighted that epithelial CTC counts were prognostic before initiation of therapy and early in therapy, whereas a shift towards mesenchymal CTC phenotypes as detected by gene expression was associated with disease progression. | [93] |
| Esophageal Squamous Cell Carcinoma | Ko JMY, Lam KO, Kwong DLW, ………..., Lung ML | 2023 | 88 | 12 | CTChip®FR1 | The changes in CTCs status pre-surgery and 1 or 3 month after surger and pT staging after resection are independent prognostic factors of poor prognosis for locally advanced ESCC patients receiving surgical treatment, as well as the presence of CTC clusters, unfavorable CTC status at baseline, 1-month and 3-month post-surgery. | [99] |
| Small Cell Lung Cancer | Bendahl PO, Belting M, Gezelius E. | 2023 | 42 | 2 | CellSearch | CTCs presence at the baseline was identified as the poor prognostic factor for the survival in patients. The persistent CTCs presence at 2-month follow-up and baseline was associated with significantly higher HR for OS. | [86] |
| Breast Cancer | Szostakowska-Rodzos M, Fabisiewicz A, Wakula M, …………….., Grzybowska EA. | 2024 | 135 | 3 | CytoTrack | High CTCs count was independent poor prognosis marker for PFS and OS, regardless of time of the enumeration. The consistnet low CTCs numbers during the treatment was revealed t be favorable prognostic marker for PFS and OS. The rising values of CTC count was identified as predictor for rapid progression. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





