Submitted:
09 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Procedure
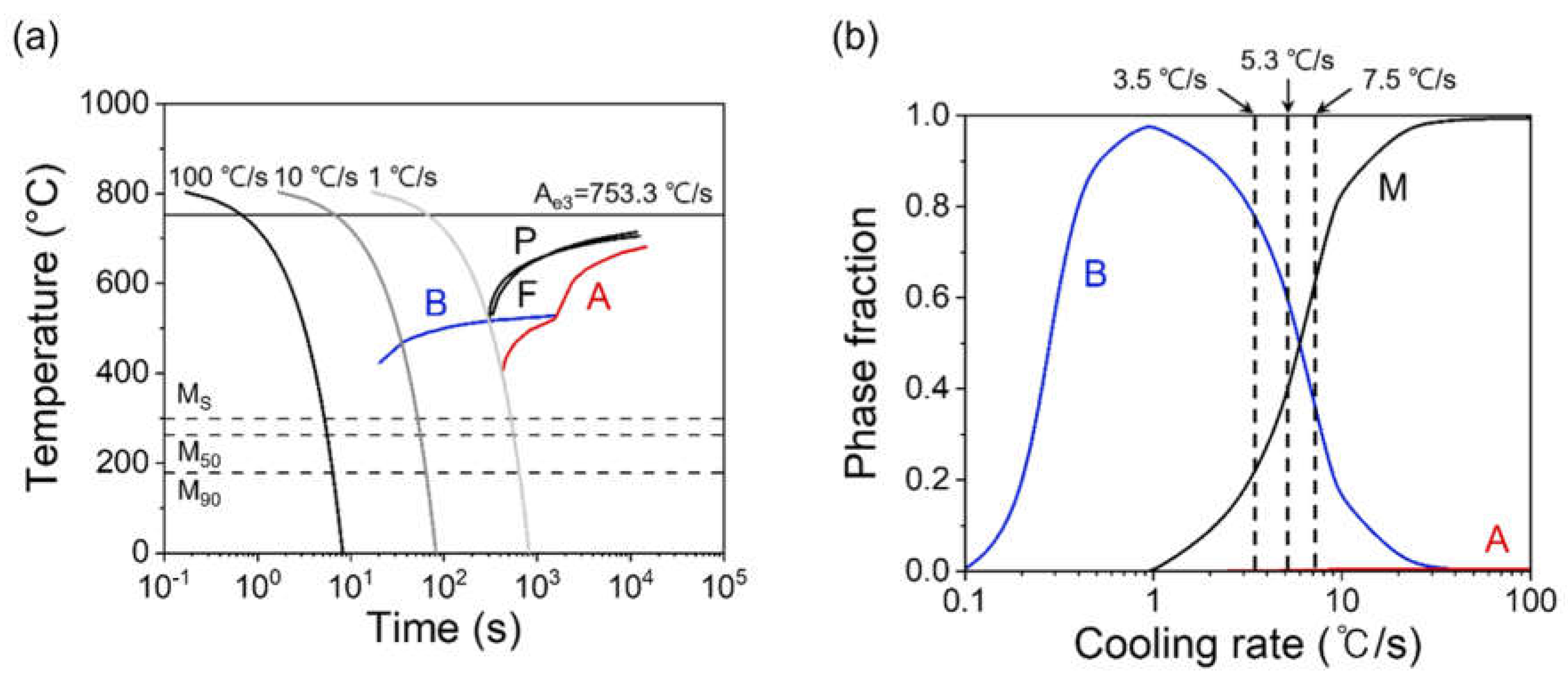
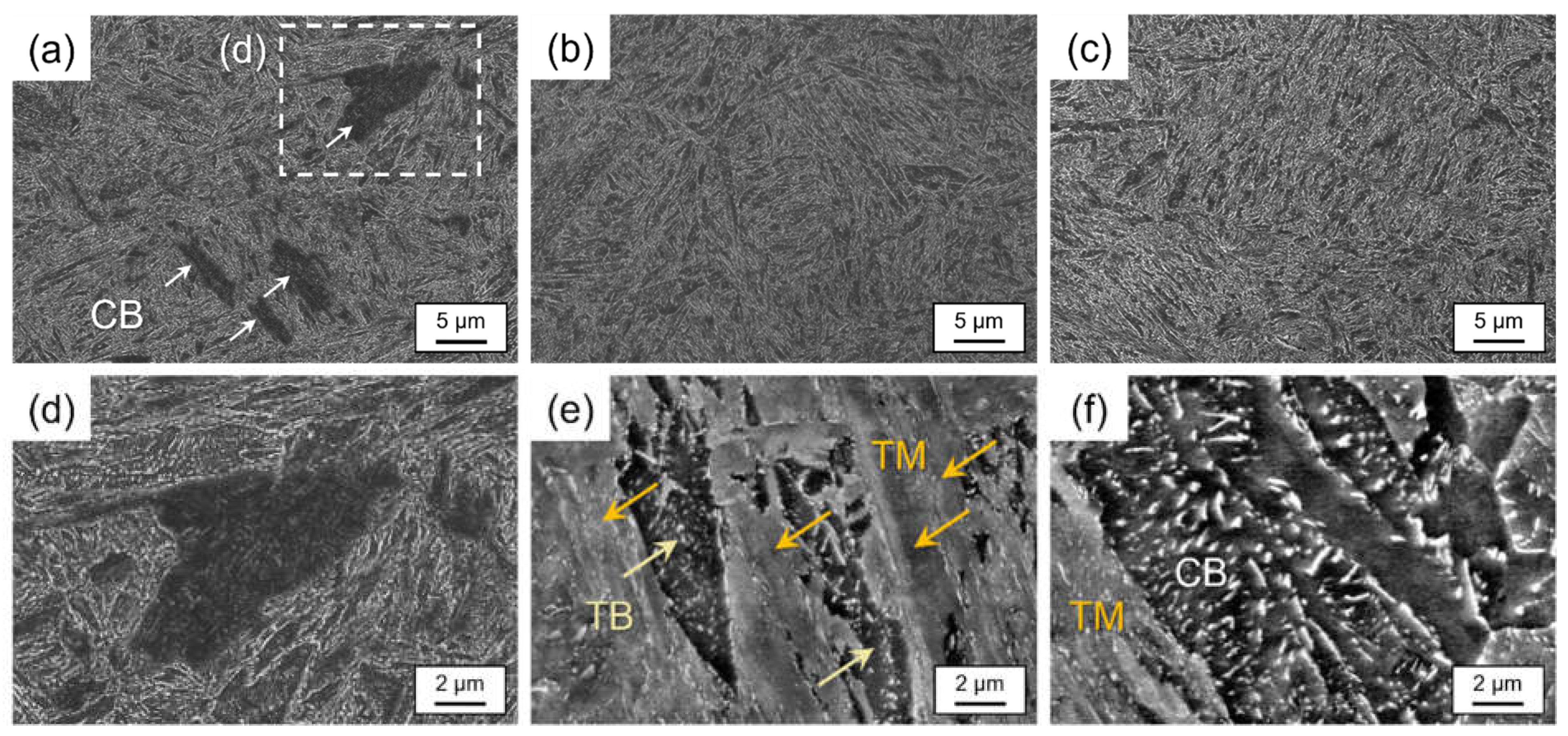
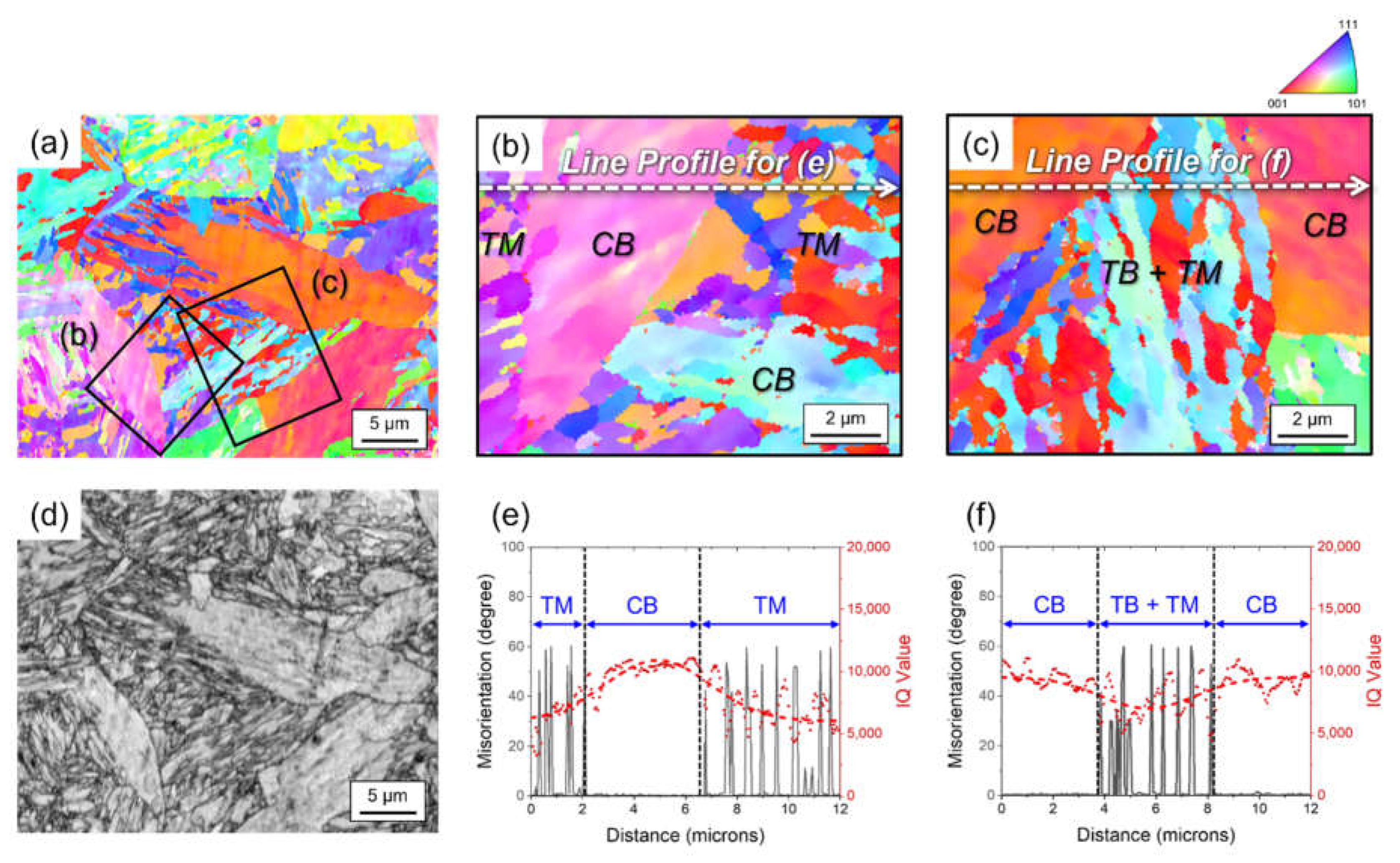
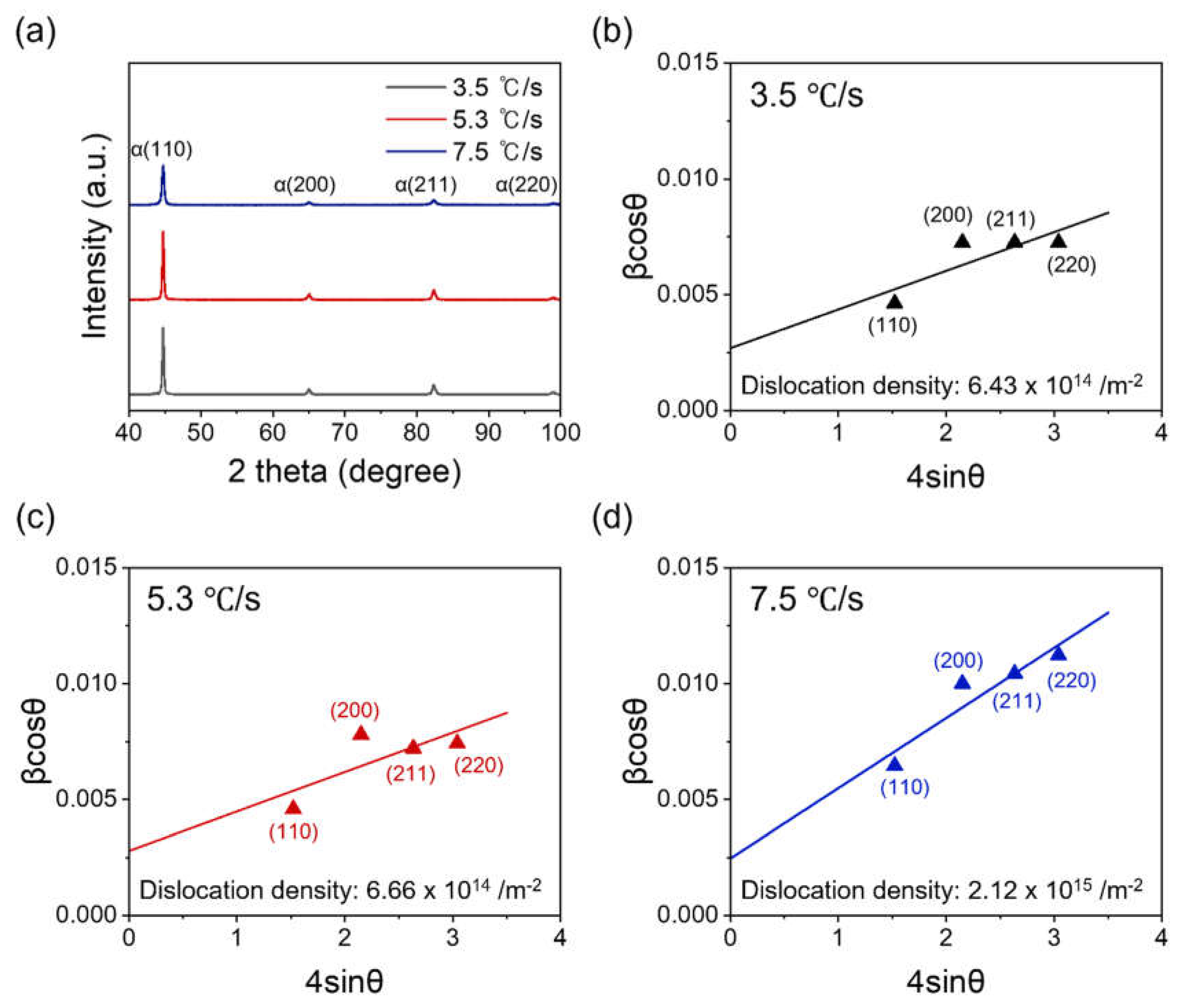
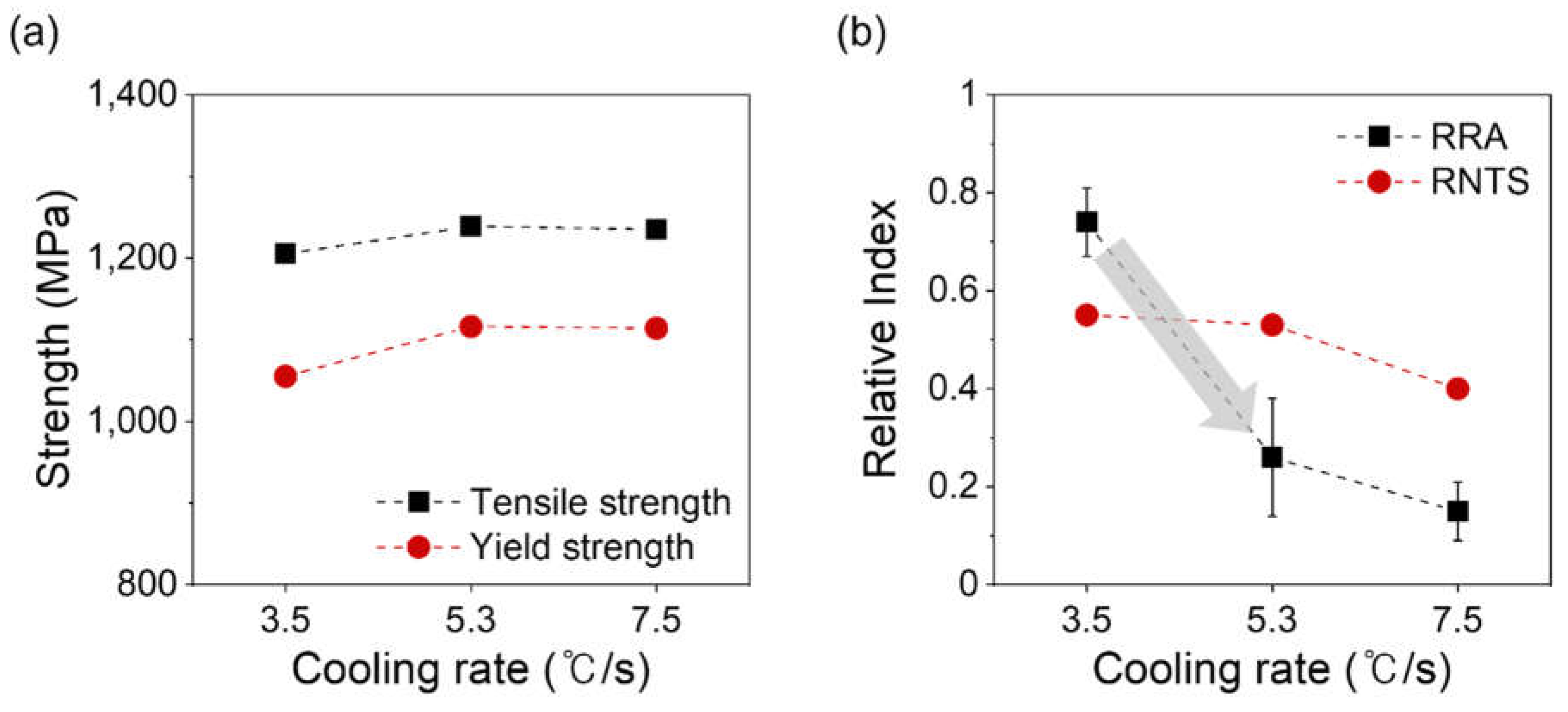
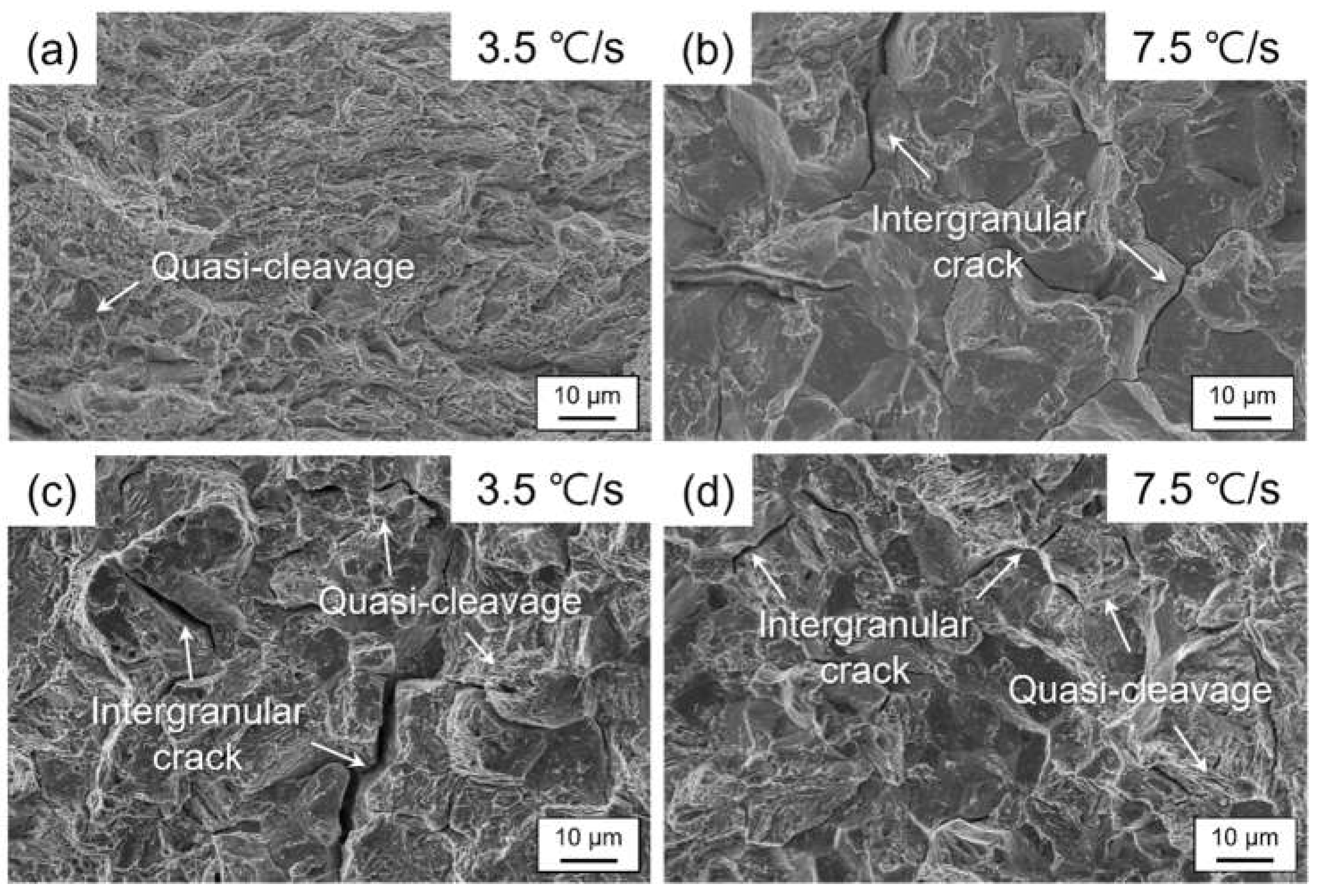
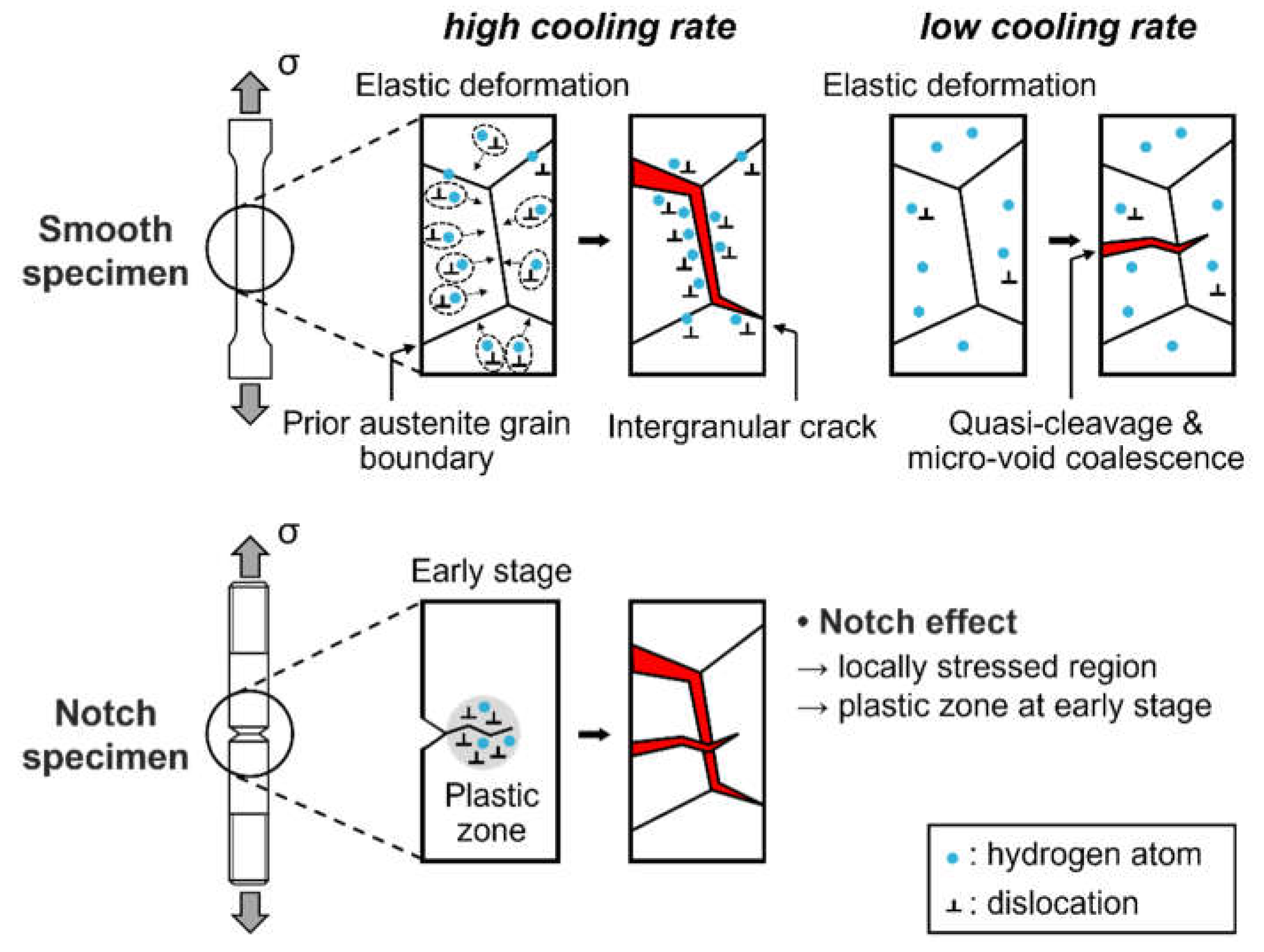
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Zielinski, A.; Domzalicki, P. Hydrogen degradation of high-strength low-alloyed steels. J. Mech. Work. Technol. 2003, 133, 230–235. [Google Scholar] [CrossRef]
- Liu, Q.; Atrens, A. A critical review of the influence of hydrogen on the mechanical properties of medium-strength steels. Corros. Rev. 2013, 31, 85–103. [Google Scholar] [CrossRef]
- Cotterill, P. The hydrogen embrittlement of metals. Prog. Mater. Sci. 1961, 9, 205–250. [Google Scholar] [CrossRef]
- Bernstein, I. The role of hydrogen in the embrittlement of iron and steel. Mater. Sci. Eng. 1970, 6, 1–19. [Google Scholar] [CrossRef]
- A Oriani, R. Hydrogen Embrittlement of Steels. Annu. Rev. Mater. Sci. 1978, 8, 327–357. [Google Scholar] [CrossRef]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen and stress concentration on the notch tensile strength of AISI 4135 steel. Mater. Sci. Eng. A 2005, 398, 37–46. [Google Scholar] [CrossRef]
- Kim, S.-G.; Shin, S.-H.; Hwang, B. Machine learning approach for prediction of hydrogen environment embrittlement in austenitic steels. J. Mater. Res. Technol. 2022, 19, 2794–2798. [Google Scholar] [CrossRef]
- Yoo, I.; Lee, J.-M.; Lim, H.-S.; Suh, J.-Y.; Lee, J.; Hwang, B. Comparative Study of Hydrogen Embrittlement of Three Heat-resistant Cr-Mo Steels Subjected to Electrochemical and Gaseous Hydrogen Charging. Met. Mater. Trans. A 2020, 51, 2118–2125. [Google Scholar] [CrossRef]
- Nagumo, M. Hydrogen related failure of steels – a new aspect. Mater. Sci. Technol. 2004, 20, 940–950. [Google Scholar] [CrossRef]
- Lee, S.-I.; Lee, J.-M.; Lee, S.-Y.; Kim, H.-J.; Suh, J.-Y.; Shim, J.-H.; Baek, U.-B.; Nahm, S.-H.; Lee, J.; Hwang, B. Tensile and fracture behaviors of austenitic high-manganese steels subject to different hydrogen embrittlement test methods. Mater. Sci. Eng. A 2019, 766, 138367. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Lee, Y.-K.; Raabe, D.; Tsuzaki, K. Overview of hydrogen embrittlement in high-Mn steels. Int. J. Hydrogen Energy 2017, 42, 12706–12723. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Met. Sin. 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Huang, S.; Hui, H.; Peng, J. Prediction of hydrogen-assisted fracture under coexistence of hydrogen-enhanced plasticity and decohesion. Int. J. Hydrogen Energy 2023, 48, 36987–37000. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Troiano, A.R. The Role of Hydrogen and Other Interstitials in the Mechanical Behavior of Metals. Met. Microstruct. Anal. 2016, 5, 557–569. [Google Scholar] [CrossRef]
- Nagao, A.; Dadfarnia, M.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. Hydrogen-enhanced-plasticity mediated decohesion for hydrogen-induced intergranular and “quasi-cleavage” fracture of lath martensitic steels. J. Mech. Phys. Solids 2018, 112, 403–430. [Google Scholar] [CrossRef]
- Dong, X.; Wang, D.; Thoudden-Sukumar, P.; Tehranchi, A.; Ponge, D.; Sun, B.; Raabe, D. Hydrogen-associated decohesion and localized plasticity in a high-Mn and high-Al two-phase lightweight steel. Acta Mater. 2022, 239, 118296. [Google Scholar] [CrossRef]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Met. Trans. 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Birnbaum, H.; Sofronis, P. Hydrogen-enhanced localized plasticity—a mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The detrimental effect of hydrogen at dislocations on the hydrogen embrittlement susceptibility of Fe-C-X alloys: An experimental proof of the HELP mechanism. Int. J. Hydrogen Energy 2018, 43, 3050–3061. [Google Scholar] [CrossRef]
- Martin, M.L.; Dadfarnia, M.; Nagao, A.; Wang, S.; Sofronis, P. Enumeration of the hydrogen-enhanced localized plasticity mechanism for hydrogen embrittlement in structural materials. Acta Mater. 2018, 165, 734–750. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Yonemura, T.; Bai, Y.; Tsuji, N. Effect of initial dislocation density on hydrogen accumulation behavior in martensitic steel. Scr. Mater. 2020, 178, 318–323. [Google Scholar] [CrossRef]
- Windle, A.H.; Smith, G.C. The Effect of Hydrogen on the Plastic Deformation of Nickel Single Crystals. Met. Sci. J. 1968, 2, 187–191. [Google Scholar] [CrossRef]
- Tien, J.K.; Richards, R.J.; Buck, O.; Marcus, H.L. Model of dislocation sweep-in of hydrogen during fatigue crack growth. Scr. Met. 1975, 9, 1097–1101. [Google Scholar] [CrossRef]
- Kurkela, M.; Latanision, R. The effect of plastic deformation on the transport of hydrogen in nickel. Scr. Met. 1979, 13, 927–932. [Google Scholar] [CrossRef]
- Hwang, C.; Bernstein, I. Dislocation transport of hydrogen in iron single crystals. Acta Met. 1986, 34, 1001–1010. [Google Scholar] [CrossRef]
- Itoh, G.; Jinkoji, T.; Kanno, M.; Koyama, K. Effect of impurity hydrogen on the deformation and fracture in an Al-5 mass Pct Mg alloy. Met. Mater. Trans. A 1997, 28, 2291–2295. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Martin, M.L.; Nagao, A.; Sofronis, P.; Robertson, I.M. Modeling hydrogen transport by dislocations. J. Mech. Phys. Solids 2015, 78, 511–525. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- ASTM E8/E8M-2013; Standard Test Methods for Tension Testing of Metallic Materials. ASTM, 2001.
- ASTM G142 Standard Test Methods for Determination of Susceptibility of Metals to Embrittlement in Hydrogen Containing Environments at High Pressure, High Temperature, or Both, 2016.
- Crimp, M.A. Scanning electron microscopy imaging of dislocations in bulk materials, using electron channeling contrast. Microsc. Res. Tech. 2006, 69, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Urrutia, I.; Zaefferer, S.; Raabe, D. Electron channeling contrast imaging of twins and dislocations in twinning-induced plasticity steels under controlled diffraction conditions in a scanning electron microscope. Scr. Mater. 2009, 61, 737–740. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Raabe, D. Dislocation and twin substructure evolution during strain hardening of an Fe–22wt.% Mn–0.6wt.% C TWIP steel observed by electron channeling contrast imaging. Acta Mater. 2011, 59, 6449–6462. [Google Scholar] [CrossRef]
- Yao, M.; Pradeep, K.; Tasan, C.; Raabe, D. A novel, single phase, non-equiatomic FeMnNiCoCr high-entropy alloy with exceptional phase stability and tensile ductility. Scr. Mater. 2013, 72, 5–8. [Google Scholar] [CrossRef]
- He, Y.; Jung, J.; Xu, L.; Liu, S.; Shin, K. A highly efficient and flexible route to study multi-scale microstructures in steels via BSE observation. Mater. Lett. 2022, 306, 130871. [Google Scholar] [CrossRef]
- Gao, J.; Xu, Z.; Fang, X.; He, J.; Li, W.; Du, X.; He, Y.; Jia, X.; Zhou, S. Enhancing creep resistance of aged Fe–Cr–Ni medium-entropy alloy via nano-sized Cu-rich and NbC precipitates investigated by nanoindentation. J. Mater. Res. Technol. 2022, 20, 1860–1872. [Google Scholar] [CrossRef]
- H. K. D. H. Bhadeshia, E. Keehan, L. Karlsson, H. O. Andren. Trans. Indian Inst. Met. 2006, 59, 689–694.
- Keehan, E.; Karlsson, L.; Bhadeshia, H.; Thuvander, M. Three-dimensional analysis of coalesced bainite using focused ion beam tomography. Mater. Charact. 2008, 59, 877–882. [Google Scholar] [CrossRef]
- Keehan, E.; Karlsson, L.; Bhadeshia, H.K.D.H.; Thuvander, M. Electron backscattering diffraction study of coalesced bainite in high strength steel weld metals. Mater. Sci. Technol. 2008, 24, 1183–1188. [Google Scholar] [CrossRef]
- Pak, J.H.; Bhadeshia, H.K.D.H.; Karlsson, L.; Keehan, E. Coalesced bainite by isothermal transformation of reheated weld metal. Sci. Technol. Weld. Join. 2008, 13, 593–597. [Google Scholar] [CrossRef]
- He, S.; He, B.; Zhu, K.; Huang, M. On the correlation among dislocation density, lath thickness and yield stress of bainite. Acta Mater. 2017, 135, 382–389. [Google Scholar] [CrossRef]
- Su, C.-H.; Li, Q.-G.; Huang, X.-F.; Huang, W.-G. Effect of bainite microstructure during two-step quenching and partitioning process on strength and toughness properties of a 0.3%C bainitic steel. J. Iron Steel Res. Int. 2018, 25, 235–242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




