1. Introduction
Fossil fuels have been the main source of energy since the industrial revolution and continue to serve an essential function in the worldwide energy grids(Wang et al., 2024). However, petroleum and natural gas are non-renewable diminishing energy sources with increased global warming potential (Yolcular et al., 2022). Engineers and scientists have investigated and proposed alternative energy solutions for solving increasing energy demand and problems with environmental pollution.
In this scene of technological hurry, hydrogen enters the picture. Hydrogen is a valuable fuel and source of energy. In comparison to traditional fuels, hydrogen combustion produces water vapour, whilst other fuels emit carbon dioxide and carbon monoxide. Hydrogen has a gross calorific value of almost 39.4 kWh kg-1, higher than gasoline's 12.89 kWh kg-1. The value of hydrogen rises quickly since conventional fuels have limited resources and are becoming more expensive by the day. Aluminium is the most abundant metals to utilized for restore low carbon aluminium, billets, wire rods, slabs, and other useful products. There is the misconceptions overcome wise revenge to reduce by product during smelting process through molten aluminium i.e., aluminium dross.
1.1. Background on Aluminium Dross
Aluminium dross is a by-product formed during the smelting and refining of aluminium. It consists of a mixture of aluminium oxide, aluminium nitride, various metal impurities and the removal of anode and cathode materials (Yang et al., 2021). Traditionally, the disposal of aluminium dross has been problematic due to its hazardous nature, leading to environmental contamination and health risks. Paper review finds modus operandi to implement for hydrogen production.
It is commonly known that when metal comes into contact with water, metal oxide and hydroxide are formed(Wibner et al., 2021). This is accompanied by the emission of hydrogen gas. The rate of gas evolution depends on the metal and the metal-water reaction rate. When highly reactive metals are used for hydrogen evolution, the possibilities of an explosion increase, making it extremely dangerous. Researchers have always been interested in using aluminium for metal-water reactions.
Researchers from all over the world have researched hydrogen generation utilizing aluminium dross as a raw material. Electrolytic aluminium, known for its high energy consumption and emissions, poses a significant environmental risk(Yang et al., 2021). The fine powder of aluminium dross was treated to high energy ball milling to reduce particle size to a few micrometres. Because of the significant reduction in particle size, the particle's specific surface area rapidly increases. This results in more surface exposure to water and a greater likelihood for hydrogen evolution. It should be emphasized that the reaction was carried out with tap water rather than an alkaline solution. The evolution of hydrogen is caused by the combination of tiny particle size and large specific surface area. The implementation of high-energy ball milling is the process's main downside. This is an extremely energy-intensive operation, and the amount of sample prepared for the experiment is minimal. Commercial uses of this method are not feasible due to financial restrictions.
There hasn't been much research done on using alkaline solutions for aluminium-water reactions. Using white aluminium dross as the raw material, the production of hydrogen offers a fantastic chance to investigate and develop an additional, more effective technique of producing hydrogen. The current study focuses on recycling white aluminium dross and producing hydrogen from alkaline solutions.
1.2. Aluminium Dross Recycling
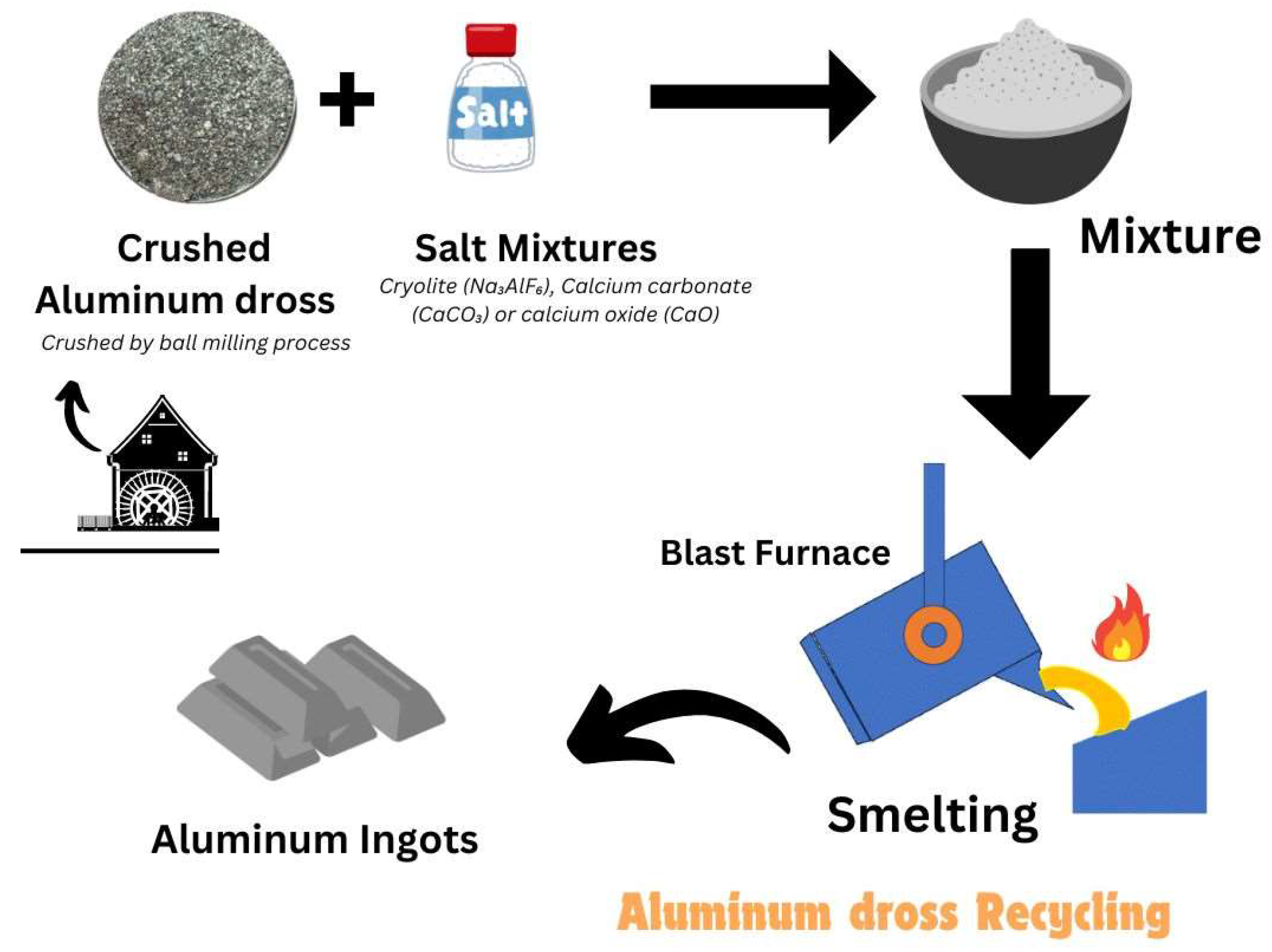
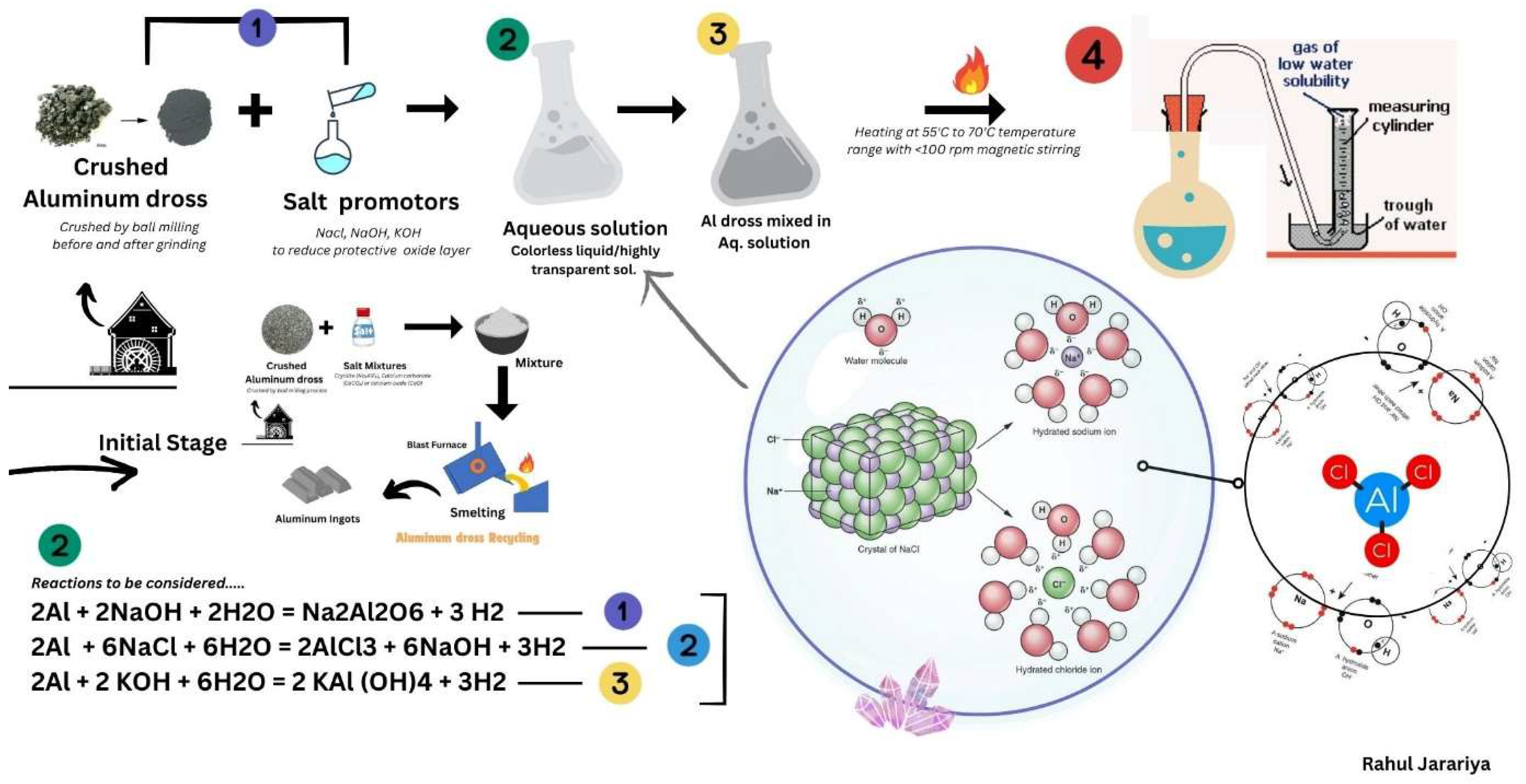
As we discussed in previous about the Al Dross, the sector comes under the Al dross possible reaction by two major methods namely pyrometallurgical and hydrometallurgical routes, (Meshram et al., 2020) this research avail the structure of potash alum production by innovative technique for Al dross recycling. It was estimated that potash alums also extracted from white aluminium dross with good quality and also utilizing waste materials into useful products in water treatment plants. Investigation focused on aluminium dross indispensable to reduce or recycle and processing it to zero waste concept(Wibner et al., 2021). Pyrometallurgical and hydrometallurgical concept have overcome to conversion into pure sustainable products by addition of salt promotors. According to this
Figure 1, here is the conventional process with the addition of salt in blast furnace then go to separate salt slag and remaining metallic aluminium utilize for production and rest dues not subsequently useful. If salt slag contacted with water will produce some amount of hydrogen, methane, ammonia, hydrogen sulphide etc. Instead blast furnace high temperature treatment process, another one innovative research overcome to minimize salt slag residue problem, Alcan plasma dross treatment, this may reduce the salt slag residue and also ensures environmental benefits, highly metal recovery(Meshram & Singh, 2018). Hydro-Quebec DROSCAR graphite arc process, ALUREC pro- cess, ECOCENT process and PyroGenesis DROSRITE process are there to deep review.
There are two primary reasons for this:
The aluminium-water reaction is vigorous enough to produce a significant amount of hydrogen. In theory, 1g of Al can produce roughly 1.2 Liters of H2. In addition, the reactivity with aluminium can be controlled. Aluminium reactivity is far lower than that of sodium and other reactive metals, which reduces the possibility of an explosion.
Aluminium is relatively abundant compared to other metals.
1.3. The Emergence of White Hydrogen
White hydrogen, produced from the reaction of aluminium with water, offers a clean and efficient energy source. The transformation of aluminium dross into white hydrogen not only provides a sustainable way to manage industrial waste but also contributes to the growing demand for clean energy. Researchers utilized the H2 productions promotors to reduce oxidation during Aluminium water chemical reaction like NaOH, KCl, NaCl, KOH etc (Bolt et al., 2020). The following chemical Al+H2O reaction products:
2Al + 2NaOH + 6H2O → 2NaAl (OH)4 + 3H2 - (1)
At, this stage the production of H2 gas at 70°C (Bolt et al., 2020), with highest yield qualified with NaOH chemical reaction within the reaction system, still other temperatures awaited in this study.
1.4. Chemical Processes for White Hydrogen Production
2. Basic Chemistry
The production of white hydrogen from aluminium involves the reaction of aluminium with water to produce hydrogen gas and aluminium hydroxide:
2Al + 6H
2O → 2Al (OH)
3 + 3H
2 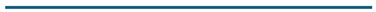 (1)
(1)
(Aluminium Oxide)
2Al + 2NaOH + 2H
2O = Na
2Al
2O
6 + 3H
2 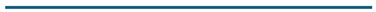 (2)
(2)
(Sodium Aluminate)
2Al + 6NaCl + 6H
2O = 2AlCl
3 + 6NaOH + 3H
2 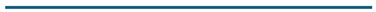 (3)
(3)
(Aluminium Chloride)
2Al + 2 KOH + 6H
2O = 2 K Al (OH)
4 + 3H
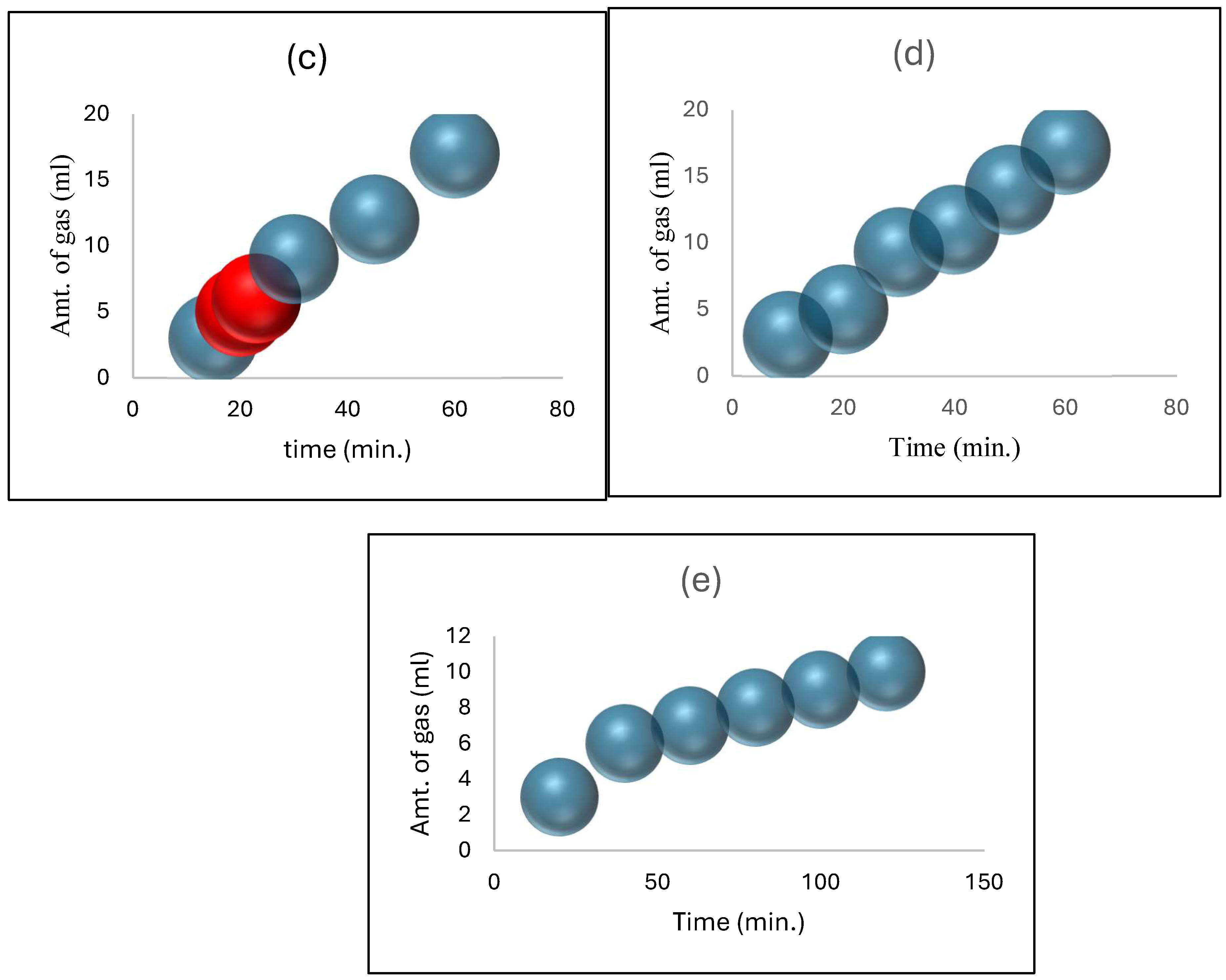
2 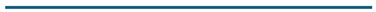 (4)
(4)
(Potassium aluminate)
3. Experimental Models
The are numerous of experimental procedure for H
2 production and technologies upgrading vast during upcoming years. Aluminium dross after recycling process with hot-spring utilized at various temperature ranges (Alviani et al., 2021) and one of the prominent feature using inverted cylinder process utilized for a laboratory procedure after downsized Al dross powder achieved(Singh et al., 2019). As earlier discussion, we reviewed two methods to recycle aluminium dross with or without salt. The reactor based method, using 45μm sized Aluminium dross(David & Kopac, 2012) pressed by Ball milling, therefore powder formed materials achieved, the reason of sizes still awaited for industrial usage, connections with Gas Chromatography (GC) not successfully determined by this laboratory scale, also gas controlling activity should be mentioned. SEM, XRD, TGA, EDS are important characterization to determine impurities, crystalline composition, decomposition rate, elemental studies(Knoks et al., 2024). Precursors amount respect with volume reduce H
2 gas production in vessel, pressure should be idealized because when hydrogen gas evolved from aluminium and water chemical reaction at a constant rate, it is sustainable to procure to target condition can receive thermodynamically(Godart et al., 2020). On the other hand, our review also focused on another electrochemical reaction which is based on catalytic activity, called Ni
3S
2-based electrocatalysts(Wang et al., 2024) which is utilized by industrialized alkaline water electrolysis. In
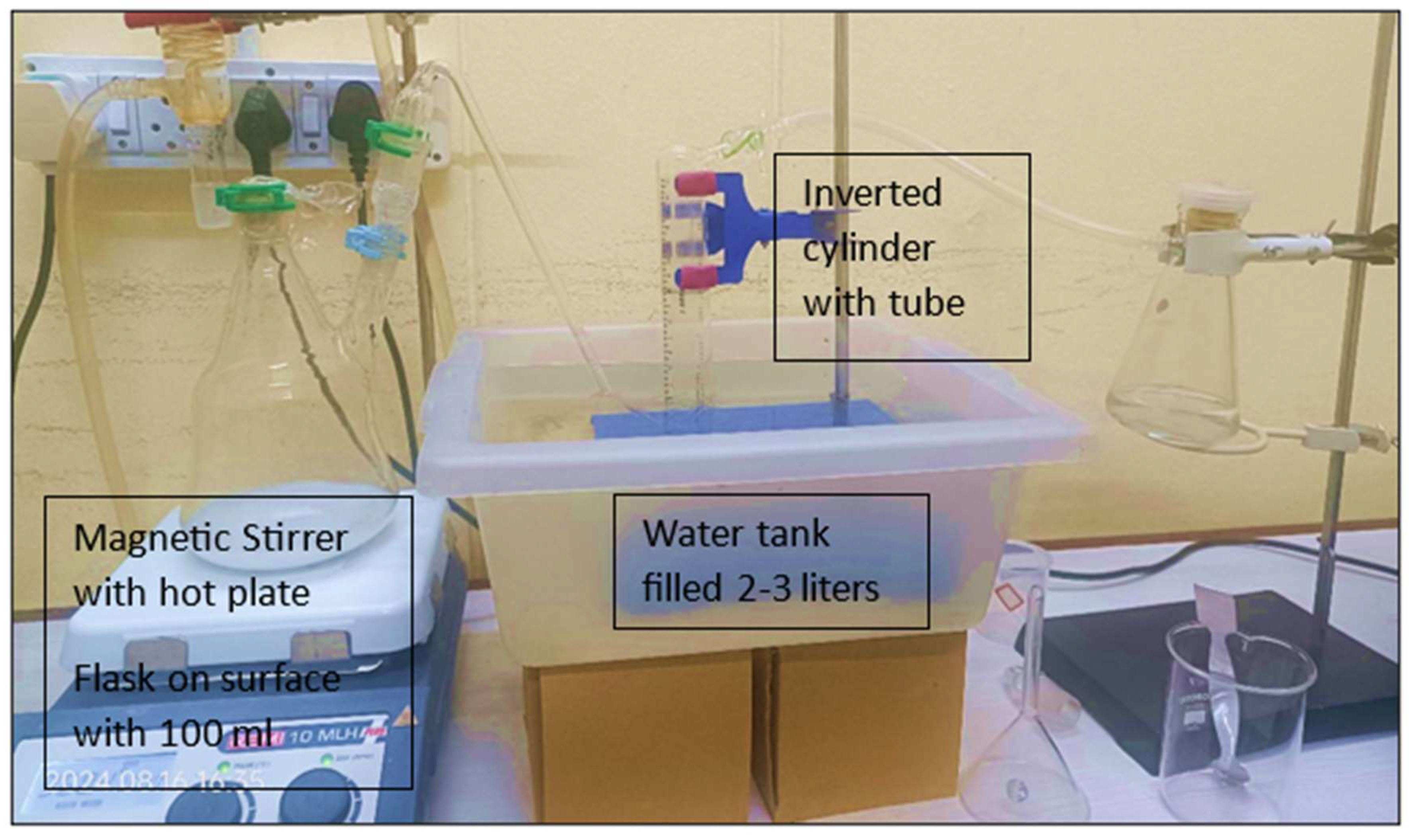
Figure 2, Aluminium powder is used in the aluminium-water reaction. The water is heated on a hot plate with a magnetic stirrer. Separately, aluminium fine powder is combined with sodium hydroxide pellets to form a homogenous mixture using a mortar and pestle. The weight of aluminium in the mixture is increased while maintaining the amount of alkali constant. The goal is to investigate the variation in the amount of gas created as the amount of Al given into the reaction and the temperature change.
In this
Figure 3, aluminium-water reaction takes conducted in a 100 mL bottom flask (with two necks). When a mixture of aluminium dross and alkali is applied to tap water at the reaction temperature, a sudden burst of gas occurs. It is important to ensure that the combination does not evolve into gas before being put to water. Because of alkalis' hygroscopic nature, hydrogen can be produced even before that. It is critical that, as soon as the combination comes into contact with water, the flask is sealed with a glass vessel tube attached with a pipe or rubber bung. This configuration facilitates the transfer of gas created in the flask. This gas is collected and measured in an inverted measuring cylinder filled with water and immersed in a water bath. The amount of gas collected is measured in a fixed period of time (100 sec.) and simultaneously the rate of gas evolution is determined. When the rate of gas evolution drops down to mL sec
-1, the reaction is stopped and the final reading is measured. The final liquor is filtered, and the solution is separated from the remaining solid. In all of the experiments, the weight of the powder has been varied from 2 g and 4 g, while the weight of the alkali (1M NaOH) was kept constant. This resulted in variations in the weight ratios used in the studies.
Figure 3.
Experimentation setup at ICT-IOC Bhubaneshwar laboratory followed with
Figure 2.
Figure 3.
Experimentation setup at ICT-IOC Bhubaneshwar laboratory followed with
Figure 2.
One of the greatest approaches in laboratory, with sodium chloride (98%) purity, reaction with extracted Aluminium from Al dross. The super hydrogen produced in continuous manner.
2Al + 6NaCl + 6H2O = 2AlCl3 + 6NaOH + 3H2
According to stoichiometry, Hydrogen production rates are higher as comparison to NaOH. Sodium Hydroxide can easily reduce oxide layer on Al
3+ without any external temperature support, it releases heat with hydrogen gas. Aluminium loosing electrons to form Al
3+ ions. This reaction instant product bubbles while (NaCl) does not produces significant amount of gas and it requires specific temperature that we observed in
Figure 5. Al
3+ oxidises reducing electrons to form Aluminium ions while sodium is reduced. This reaction is simple displacement reaction where Al
3+ displaces a less reactive metal Sodium metal form NaCl solution. The above experiment demonstrates how temperature varies with chemical reaction and the various temperature possibilities.
Table 1.
Hydrogen Production methods.
Table 1.
Hydrogen Production methods.
| Technology |
Steam reforming |
Gasification |
Super Critical water gasification |
Partial Oxidation |
Plasma reforming |
Proton exchange membrane electrolysis |
Alkaline electrolysis |
Solid Oxide Electrolysis |
Photo fermentation |
Dark Fermentation |
References |
| Operating Conditions |
700-800°C, Pressure 3-25 bar., Steam to carbon ratio 3.5. |
Operating temperature 800-900°C. |
Temperature 350-600°C., Pressure 22.12 MPa |
Operating Temperature 1150°C to 1500°C |
Operating temperature >2000°C. High degree of dissociation and ionisation |
Temperature 50-90°C. Pressure 15-30 bar |
Temperature 60-90°C |
Temperature 500-1000°C |
Ambient conditions, |
Ambient Conditions |
(Alviani et al., 2021)(Wang et al., 2024)(Xu et al., 2024) |
| Technical aspects |
High Purity in H2 Production., Deactivation of catalyst during coke deposition. |
Highly Impurities tar and H2. Efficient process., Corrosion due to slag formation., Deactivation of catalyst., Further optimisation is required for high yield. |
Further optimization required for high yield. |
Deactivation of catalyst due to coke deposition |
High electrode erosion, High conversion efficiency |
Simplicity in design, High current density |
Low current density, |
High energy efficiency |
Slow process |
Simple reactor design, low yield and slow process |
(Alviani et al., 2021)(Wang et al., 2024)(Xu et al., 2024) |
| Economic aspects |
High cost of precious metal catalyst |
High capital cost and high operating cost |
High cost of feedstock harvesting. High capital cost due to high temperature and pressure operation. |
Economically attractive due no heat requirement |
No costly catalyst required |
High cost of membrane and catalyst material |
Metal Based requirement of a catalyst |
Ni (Cathod) and Perovskite (Anode) requirement of a catalyst |
Low cost due to operating conditions |
Low cost due to the low operating temperature and pressure |
(Alviani et al., 2021)(Wang et al., 2024)(Xu et al., 2024) |
| Environmental aspects |
CO2 emission due to fuel combustion. |
CO2 emission |
CO2 emission |
CO2 emission |
CO2 emission |
Acidic environment |
Corrosive electrolyte environment |
Environment friendly technology |
CO2 emission |
CO2 emission |
(Alviani et al., 2021)(Wang et al., 2024)(Xu et al., 2024) |
| Energy requirement |
High energy consumption |
High energy requirement due to high temperature operations |
High energy requirement |
No heat requirement |
Extremely high energy requirement for plasma generation |
High electricity requirement for electrolysis due to low operating temperature |
High efficiency requirement for electrolysis due to low operating temperature |
High hear energy requirement |
High energy requirement for enzymes |
Low energy consumption and pilot scale |
(Alviani et al., 2021)(Wang et al., 2024)(Xu et al., 2024) |
| Process Development |
Mature technology and full commercial phase. |
Large scale investigation |
Lab-scale investigation |
Mature technology and full commercial phase |
Lab-scale investigation |
Early commercial phase |
Full commercial phase |
Research and development |
Pilot-scale |
Pilot scale |
(Alviani et al., 2021)(Wang et al., 2024)(Xu et al., 2024) |
Production of Hydrogen with their advancements:
Table 2.
Short Discussion on Hydrogen Production Technologies for future (Zhang et al., 2024), (Fu et al., 2021),(Akhlaghi & Najafpour-Darzi, 2020).
Table 2.
Short Discussion on Hydrogen Production Technologies for future (Zhang et al., 2024), (Fu et al., 2021),(Akhlaghi & Najafpour-Darzi, 2020).
| S.No. |
Methods |
Benefits |
Limitations |
Future Advancements |
| 1 |
Thermochemical Process |
High Conversion efficiencies, high production rate, |
Depend on fossils fuels, Required significant amount of energy, |
Solar Driven-thermochemical process using biomass as feedstock, awaiting advances with solar and wind energy |
| 2 |
Electrochemical process |
Low cost, high purity hydrogen |
Energy intensive process required high costing with expensive catalysis, if required. |
Anion Exchange membrane, Bipolar membrane electrolysis, microbial electrolysis cell. |
| 3 |
Biological process |
More sustainable and environment friendly, good efficiency, cost-effectiveness, low green house gas emission |
Low hydrogen yields, slow rate due to its inherent limitations of biological systems. |
Cynobacterial biohydrogen production, genetic engineering and biology and improve efficiencies further. |
| 4. |
Photocatalytic process |
Good in hydrogen yields, low green house gas emission, |
This process required sunlight or light source which may not require on time basis. |
Novel materials, catalyst and hybrid systems to improve hydrogen efficiency, Tandem or Z-scheme photo catalytic systems. |
4. Results and Discussions
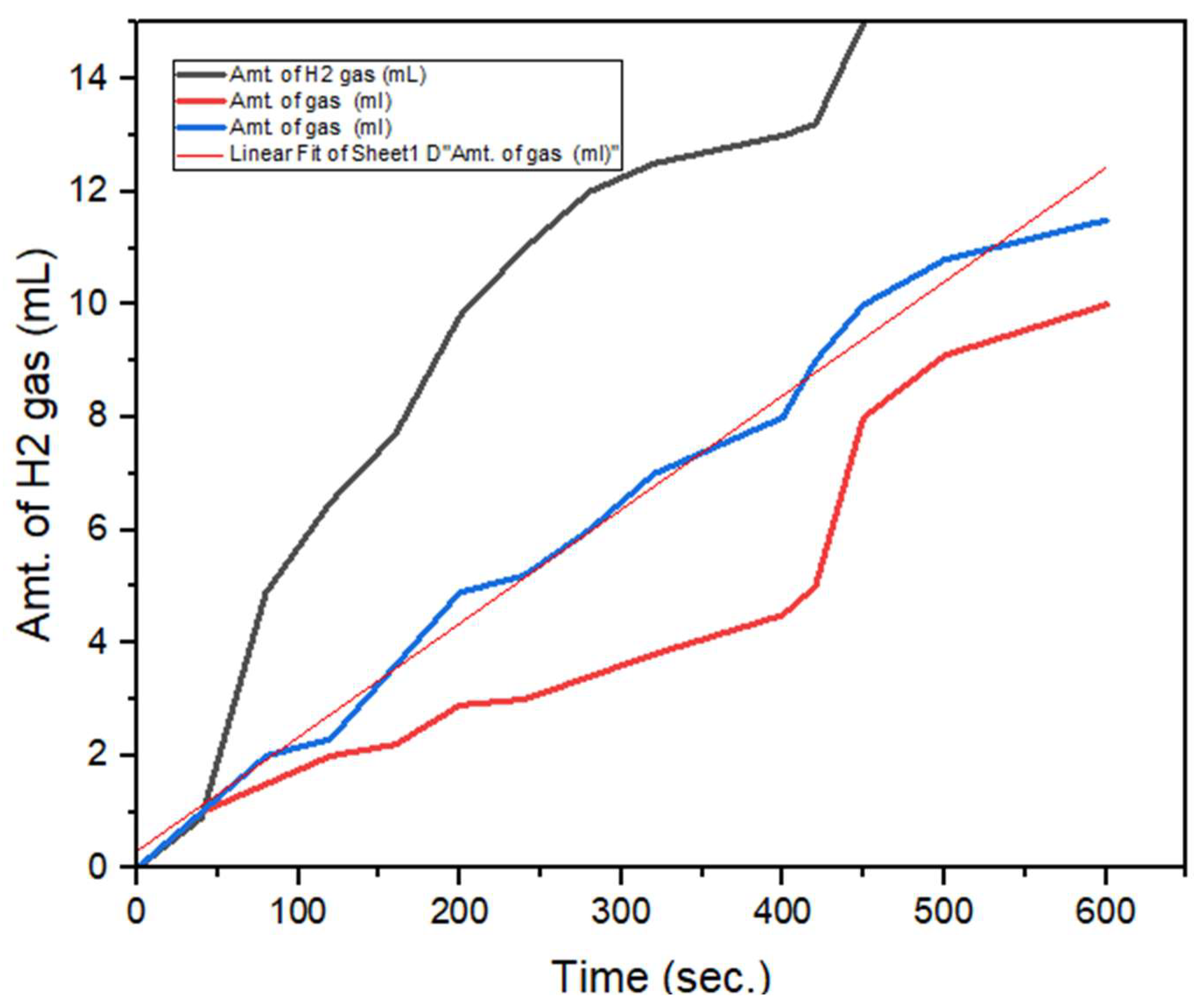
In
Figure 3: Hydrogen gas variation can see with time, black line show that amount of aluminium dross calculated 4g of wt. in aq. NaOH solution. Hydrogen gas evolution in bottom flask rapidly rises initially then withdrawn till 180 sec. then suddenly attack in inverted cylinder which is calculated by flow rate of gas 0.15 L/min. Similarly, with 2 g of Aluminium dross extracted aluminium rapidly rises the rate initially after 400 sec. hydrogen gas busted due to the surface area of glass vessel and then reached up to 600 sec with flow rate 0.10 L/min of H
2 gas. In blue line shows, that gas evolution faster than beginning at 120ºC. So, it can be estimated that when you rise the temperature, pressure will rise as per Gas law is application. This is executed due to promote the higher amount of Aluminium-water chemical reaction and the result can see with black line. After the experiment done, with 4g of Al dross will be enough for separation as residue and rest of homogenous mixture transfer to distillation unit for further separation for products.
Figure 3.
Amount of hydrogen calculated w.r.t. of time in (sec.).
Figure 3.
Amount of hydrogen calculated w.r.t. of time in (sec.).
In
Table 3: Shows our experimentation results based on the time (sec.) of hydrogen gas release in terms of L.min
-1. Hydrogen gas became stuck due to the large area of the container and required external compression. After compression by magnetism between 120 and 200 seconds due to the surface area of the gas pipe with loaded water filled inverted glass tube in water tank, the H
2 gas that emerged showed insolubility and provided pressure on the surface of the inverted gas tube. This table depicts how the flow rate of gas varies over time at constant temperature. At the period of hydrogen gas, a perfect hydrogen sensor was necessary to obtain an accurate hydrogen concentration (ppm). As a result, it became a point of analysis for hydrogen gas at all time intervals.
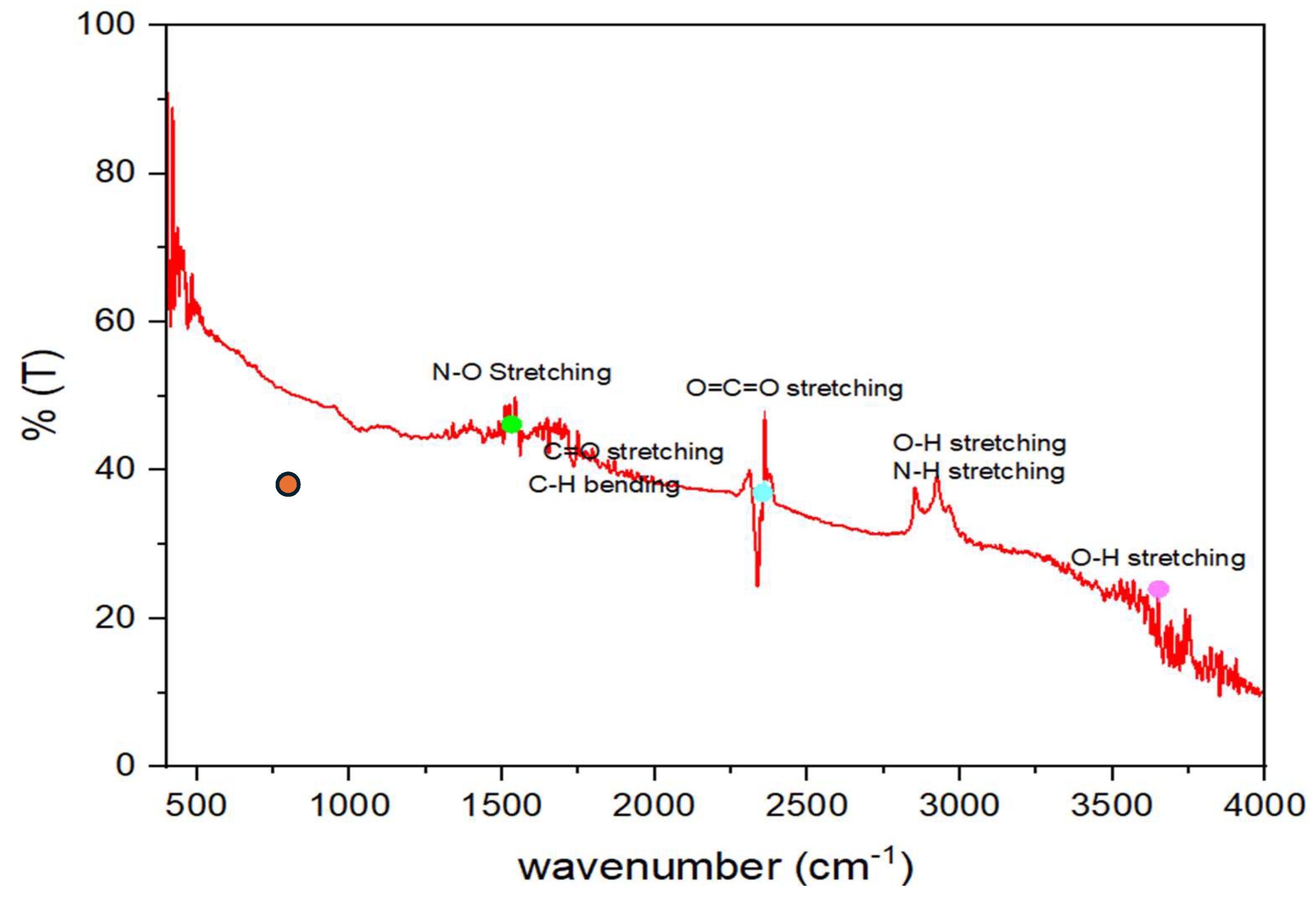
Figure 4.
FITR analysis for Residue material.
Figure 4.
FITR analysis for Residue material.
5. FTIR Analysis
FTIR confirms that sodium aluminate white shiny powdered material has prepared with taken reactions. The Broad spectrum of O-H stretching peaks around 3449 cm-1 which is shows the water molecules present in structure even during experimentation 100 ml H2O utilized for reactions with NaOH crystals. That orange spot shows that few amount of Al attached with lattice. 780-800cm-1 because NaOH reduce the formation of oxidation and protective layer of Al2O3. During reaction, Aluminium may reduce at High temperature from 70ºC to 120ºC. This FTIR spectrum also show the impurities of N-H stretching with O-H stretching and N-O between 1500 to 2000 cm-1. This shown that FTIR operation crystals unpolished finely. Before proceeding for characterization must filtered 2 times with acetone or distilled water.
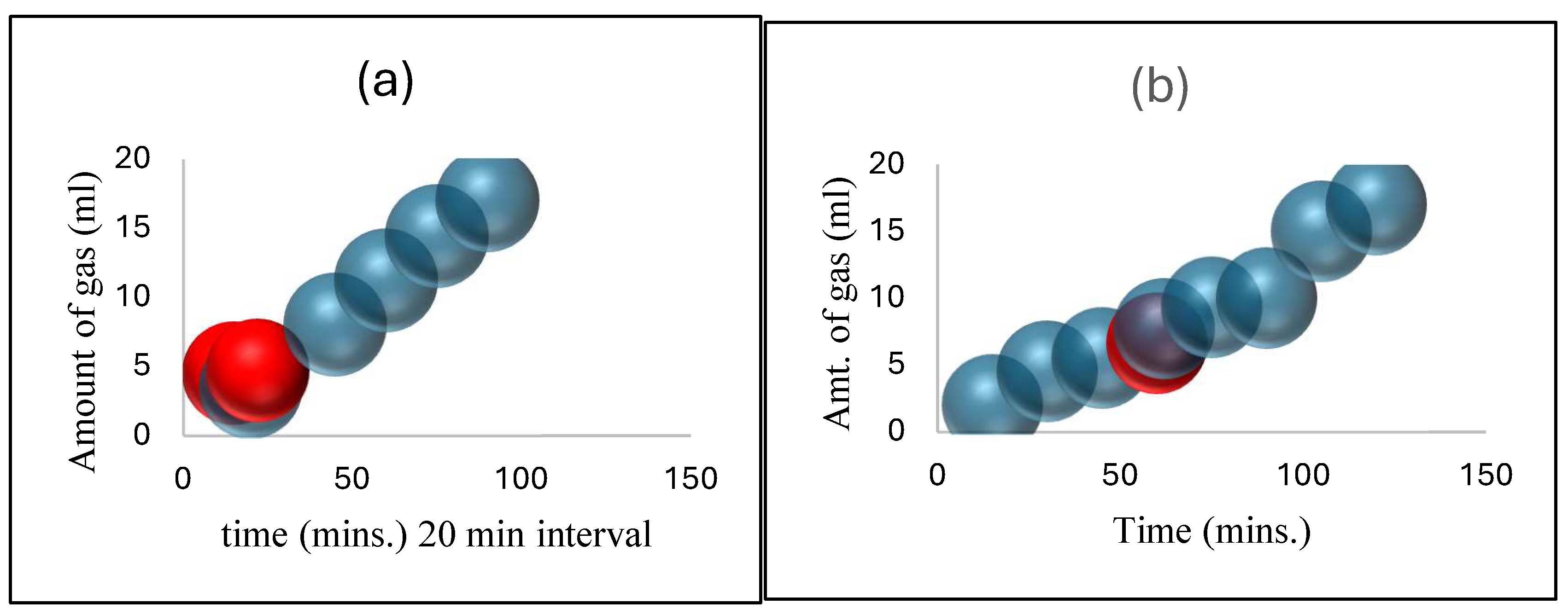
Figure 5.
(a) Initially, high pressure at 120ºC caused a violent gas attack. These two points highlight the issue with temperature measuring. Readings were taken at every 20-minute interval. (b) Initially, high pressure at 120ºC caused a violent gas attack. That point illustrates the issue with temperature measuring. Readings were taken at 20-minute intervals. (c) At 100ºC, high pressure caused a quick attack of gas. These two points highlight the issue with temperature measuring. According to our observations, these two demonstrate that the amount of gas can rapidly increase at certain values. Readings are taken at 10-minute intervals. (d) At 80ºC, gas levels ranged from moderate to high pressure. Bubbles rise linearly with time (t), and pressure increases inside the flask. This temperature is performing well in the parameter ranges. Readings are taken at 10-minute intervals. (e) The gas amount was originally low due to the huge area in the bottom flask at a low-pressure range of 60ºC. After 40 minutes, the inverted cylinder rises gently and linearly. Not excellent for gas estimation because certain spots were poorly built. Readings are taken at 20-minute intervals. This temperature measurement is not recommended for an experimentation due to errors.
Figure 5.
(a) Initially, high pressure at 120ºC caused a violent gas attack. These two points highlight the issue with temperature measuring. Readings were taken at every 20-minute interval. (b) Initially, high pressure at 120ºC caused a violent gas attack. That point illustrates the issue with temperature measuring. Readings were taken at 20-minute intervals. (c) At 100ºC, high pressure caused a quick attack of gas. These two points highlight the issue with temperature measuring. According to our observations, these two demonstrate that the amount of gas can rapidly increase at certain values. Readings are taken at 10-minute intervals. (d) At 80ºC, gas levels ranged from moderate to high pressure. Bubbles rise linearly with time (t), and pressure increases inside the flask. This temperature is performing well in the parameter ranges. Readings are taken at 10-minute intervals. (e) The gas amount was originally low due to the huge area in the bottom flask at a low-pressure range of 60ºC. After 40 minutes, the inverted cylinder rises gently and linearly. Not excellent for gas estimation because certain spots were poorly built. Readings are taken at 20-minute intervals. This temperature measurement is not recommended for an experimentation due to errors.
6. Role of Aluminium Dross
Aluminium dross, containing residual aluminium, can be treated to enhance its reactivity with water. Processes such as mechanical milling, chemical activation, and thermal treatment are employed to optimize the hydrogen yield refer
Table 1 for more methods to utilize the process and its applications.
Aluminium dross recycling process,
Separating aluminium from aluminium dross involves several steps to recover the valuable metal. Here are some common methods as mentioned on
Figure 1:
Mechanical Separation: This is the primary step where dross is crushed and ground to separate aluminium particles from non-metallic components. Techniques like ball milling and pressing are often used1.
Screening and Sizing: After mechanical separation, the particles are screened and sized. Larger aluminium -rich particles are separated from the finer non-metallic fractions.
Remelting: The aluminium -rich particles are then remelted at high temperatures (around 900°C) to recover pure aluminium. This process helps in obtaining a high percentage of metallic aluminium .
Advanced Separation Techniques: Some advanced methods include using Eddy Current Separators, Drum Magnets, and Electro Static Separators to enhance the recovery of aluminium from dross.
6.1. Smelting Process Mixtures for Aluminium Product from Aluminium Dross
Smelting salt mixtures
Figure 1. are used in the process of extracting metals from their ores. These mixtures often include various ingredients that help in the smelting process by lowering the melting point of the ore and aiding in the separation of metal from impurities. A common smelting salt mixture might include as Un-iodized table salt, Soda ash (sodium carbonate), Borax (sodium borate), Silicon sand, Wheat flour, Lard or oil. These ingredients are mixed with the crushed ore and heated in a furnace. The salt and other components help to form a slag, which is a byproduct that can be easily separated from the molten metal. Temperature is to maintain around 1600°C. One of best laboratory job done, with Al-dross and results considered from H2 production with Al
3+ had consisted measurement from Al
3+ dissolution without using any equipment of H2 gas sensors utilized. This would be effected only in theoretical significance(Alviani et al., 2021) and this review can excess for vast experimental theories with actual calculation could recognize for valuable H
2 production methods but the time intervals are still questionable in laboratory status.
6.2. Catalytic and Non-Catalytic Processes
Research has explored both catalytic and non-catalytic processes to maximize hydrogen production. Catalysts such as alkali metals and transition metal oxides can significantly increase the reaction rate and hydrogen yield.
7. Environmental and Economic Benefits
7.1. Waste Reduction
Transforming aluminium dross into hydrogen effectively reduces the volume of hazardous waste, mitigating environmental pollution and landfill use.
7.2. Energy Efficiency
The production of white hydrogen from aluminium dross is an energy-efficient process, as it utilizes existing industrial waste rather than requiring new raw materials.
7.3. Economic Viability
The economic benefits of this transformation include reduced waste disposal costs, potential revenue from hydrogen sales, and the development of new industrial processes and technologies.
8. Challenges and Limitations
8.1. Technical Challenges
Impurities in Aluminium Dross: The presence of impurities can affect the efficiency of the hydrogen production process.
Reaction Control: Controlling the reaction to prevent excessive heat and ensure consistent hydrogen production is critical.
8.2. Environmental Concerns
By-products Management: Proper management of by-products such as aluminium hydroxide is necessary to prevent secondary pollution.
Energy Consumption: While the process is energy-efficient, the initial treatment of aluminium dross may require significant energy input.
8.3. Economic Barriers
Initial Investment: High initial investment costs for setting up the necessary infrastructure can be a barrier for widespread adoption.
Market Acceptance: The market for white hydrogen is still developing, and establishing a stable demand is essential for economic viability.
9. Future Perspectives
Continued research into more efficient catalysts, improved reaction control methods, and better waste management practices will enhance the feasibility of this technology. Supportive policies and regulations can incentivize the adoption of white hydrogen production from aluminium dross, promoting investment and innovation. Integrating this technology with renewable energy sources can further enhance its sustainability and contribute to a circular economy.
10. Conclusions
The transformation of aluminium dross into white hydrogen presents a promising solution to the dual challenges of waste management and clean energy production. Many Industrial technologies are with or without salt for aluminium dross recycling. Therefore, advance changes overcome in future. While significant technical, environmental, and economic challenges remain, ongoing research and innovation hold the potential to overcome these barriers. Aluminium dross is used as raw materials for the production of hydrogen and Most of the Hydrogen Gas production by Industrial waste produced with NaOH aq. Sol & NaCl solution because byproduct utility will produce another advancement in wastewater or in cement industries. Utilized but different salt promotors still awaited to do research. Current review work found that hydrogen gas produced basis on materials as per stoichiometry, sufficient amount of volume should increase the rate of H2 gas and bubbling formed. The process is called as Aluminolysis process, it can be further utilizing as specific clean energy sources which can be used as a clean energy source for power generation, industrial processes, and transportation. The hydrogen gas produced through aluminosis can be used as a reactant for various chemical synthesis processes, such as the production of ammonia, methanol, and other chemicals, fuel cells, which can power electric vehicles, backup power systems, and other applications, it is also generate electricity in gas turbines, internal combustion engines, or fuel cells, reducing agent in steel production, replacing fossil fuels and reducing greenhouse gas emissions, renewable sources, such as solar or wind power, by converting it into hydrogen gas. However, for industrial-scale applications, further research and development are needed to Scale up the process, Increase the reaction rate and efficiency to produce large quantities of hydrogen gas. Improve safety in develop safe handling and storage procedures for the reactants and products. Optimize the process to reduce costs and make it economically viable. Establish infrastructure for the transportation and storage of hydrogen gas. By leveraging industrial waste as a resource, we can move towards a more sustainable and efficient energy future.
Author Contributions
Dr. Pyarimohan Dehury provides administrative and technical support on waste materials from industries. Mr. Rahul Jarariya is working under his supervision. .
Data Availability Statement
The data supporting the findings of this study publicly available. Data Not Available: The data supporting the findings of this study are not publicly available due to lack of vast awareness and Indian prime minster already issued budget for hydrogen production (white or green).
Conflicts of Interest
I declare that I have no competing interests that could influence the impartiality of this review. Specifically, I have: No Financial Interests: I do not have any financial interests or relationships with organizations or entities that might gain or lose financially from the publication of this work. No Personal Relationships: I have no personal relationships with the authors or organizations involved in this manuscript that could influence my review. No Professional Conflicts: I do not have any professional conflicts, such as collaborations, employment, or advisory roles with the authors or organizations involved. No Intellectual Bias: I do not have any intellectual biases or beliefs that could potentially affect my impartiality in reviewing this work.
References
- Akhlaghi, N., & Najafpour-Darzi, G. (2020). A comprehensive review on biological hydrogen production. International Journal of Hydrogen Energy, 45(43), 22492–22512. [CrossRef]
- Alviani, V. N., Hirano, N., Watanabe, N., Oba, M., Uno, M., & Tsuchiya, N. (2021). Local initiative hydrogen production by utilization of aluminum waste materials and natural acidic hot-spring water. Applied Energy, 293(April), 116909. [CrossRef]
- Bolt, A., Dincer, I., & Agelin-Chaab, M. (2020). Experimental study of hydrogen production process with aluminum and water. International Journal of Hydrogen Energy, 45(28), 14232–14244. [CrossRef]
- David, E., & Kopac, J. (2012). Hydrolysis of aluminum dross material to achieve zero hazardous waste. Journal of Hazardous Materials, 209–210, 501–509. [CrossRef]
- Fu, Q., Wang, D., Li, X., Yang, Q., Xu, Q., Ni, B. J., Wang, Q., & Liu, X. (2021). Towards hydrogen production from waste activated sludge: Principles, challenges and perspectives. Renewable and Sustainable Energy Reviews, 135(March 2020), 110283. [CrossRef]
- Godart, P., Fischman, J., Seto, K., & Hart, D. (2020). Corrigendum to “Hydrogen production from aluminum-water reactions subject to varied pressures and temperatures” (International Journal of Hydrogen Energy (2019) 44(23) (11448–11458), (S0360319919311486), (10.1016/j.ijhydene.2019.03.140)). International Journal of Hydrogen Energy, 45(1), 1195–1197. [CrossRef]
- Knoks, A., Mezulis, A., Richter, C., Varnagiris, S., Urbonavicius, M., Milcius, D., Meirbekova, R., Gunnarsson, G., Kleperis, J., Knoks, A., Mezulis, A., Richter, C., & Varnagiris, S. (2024). Investigation of Aluminium White Dross for Hydrogen Generation via Hydrolysis Investigation of Aluminium White Dross for Hydrogen Generation via Hydrolysis. [CrossRef]
- Meshram, A., Gautam, D., & Singh, K. K. (2020). Recycling of White Aluminium Dross: Production of Potash Alum. Transactions of the Indian Institute of Metals, 73(5), 1239–1248. [CrossRef]
- Meshram, A., & Singh, K. K. (2018). Resources , Conservation & Recycling Recovery of valuable products from hazardous aluminum dross: A review. Resources, Conservation & Recycling, 130(September 2017), 95–108. [CrossRef]
- Singh, K. K., Meshram, A., Gautam, D., & Jain, A. (2019). Hydrogen production using waste aluminium dross: From industrial waste to next-generation fuel. Agronomy Research, 17, 1199–1206. [CrossRef]
- Wang, S., Geng, Z., Bi, S., Wang, Y., Gao, Z., Jin, L., & Zhang, C. (2024). Recent advances and future prospects on Ni3S2-Based electrocatalysts for efficient alkaline water electrolysis. Green Energy and Environment, 9(4), 659–683. [CrossRef]
- Wibner, S., Antrekowitsch, H., & Meisel, T. C. (2021). Studies on the formation and processing of aluminium dross with particular focus on special metals. Metals, 11(7), 12. [CrossRef]
- Xu, R., Liu, S., Yang, M., Yang, G., Luo, Z., Ran, R., Zhou, W., & Shao, Z. (2024). Advancements and prospects of perovskite-based fuel electrodes in solid oxide cells for CO2 electrolysis to CO. Chemical Science, 11166–11187. [CrossRef]
- Yang, F., Yu, Q., Zuo, Z., & Hou, L. (2021). Thermodynamic analysis of waste heat recovery of aluminum dross in electrolytic aluminum industry. Energy Sources, Part A: Recovery, Utilization and Environmental Effects, 43(9), 1047–1059. [CrossRef]
- Yolcular, S., Karaoglu, S., & Karasoglu, M. (2022). Hydrogen generation performance of waste aluminum alloy chips and powders. Energy Sources, Part A: Recovery, Utilization and Environmental Effects, 44(1), 1529–1540. [CrossRef]
- Zhang, L., Jia, C., Bai, F., Wang, W., An, S., Zhao, K., Li, Z., Li, J., & Sun, H. (2024). A comprehensive review of the promising clean energy carrier: Hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel, 355(April 2023). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 (1)
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)