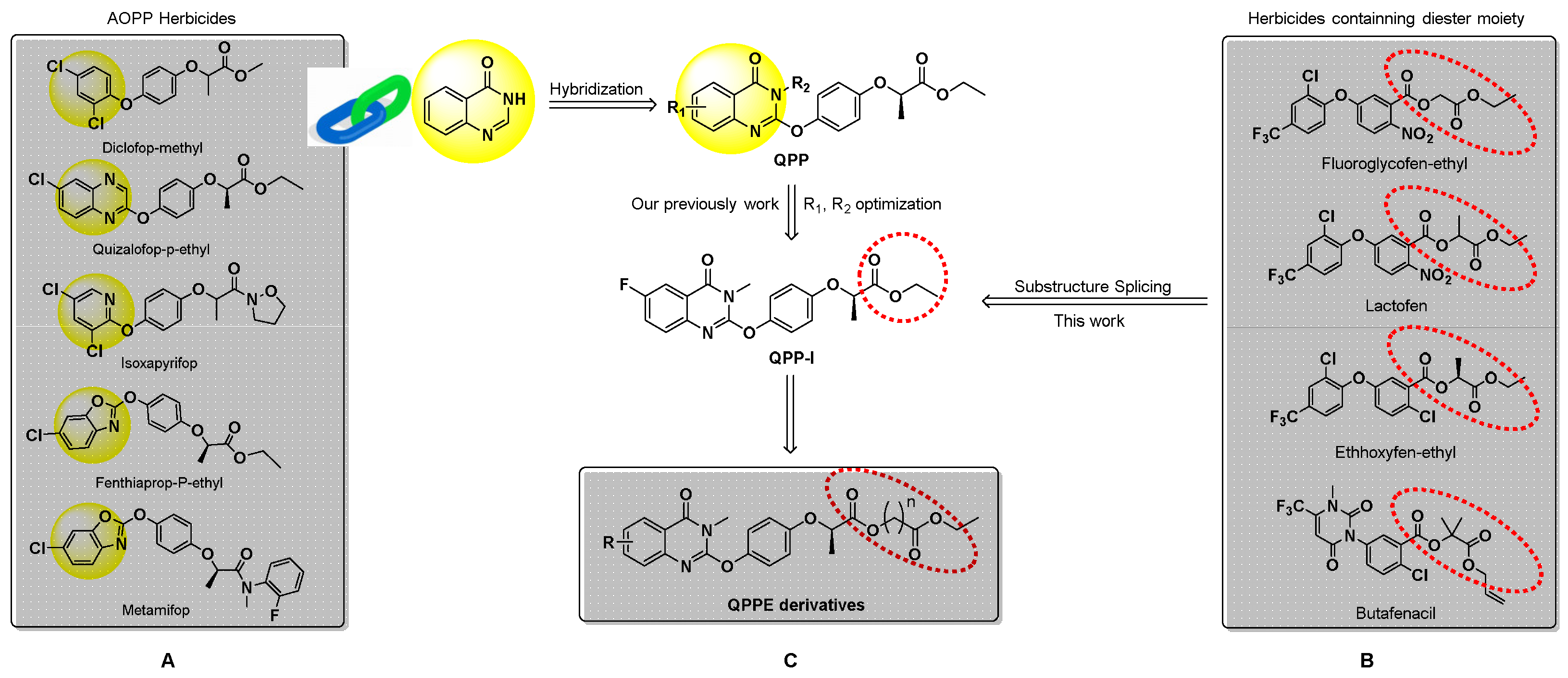
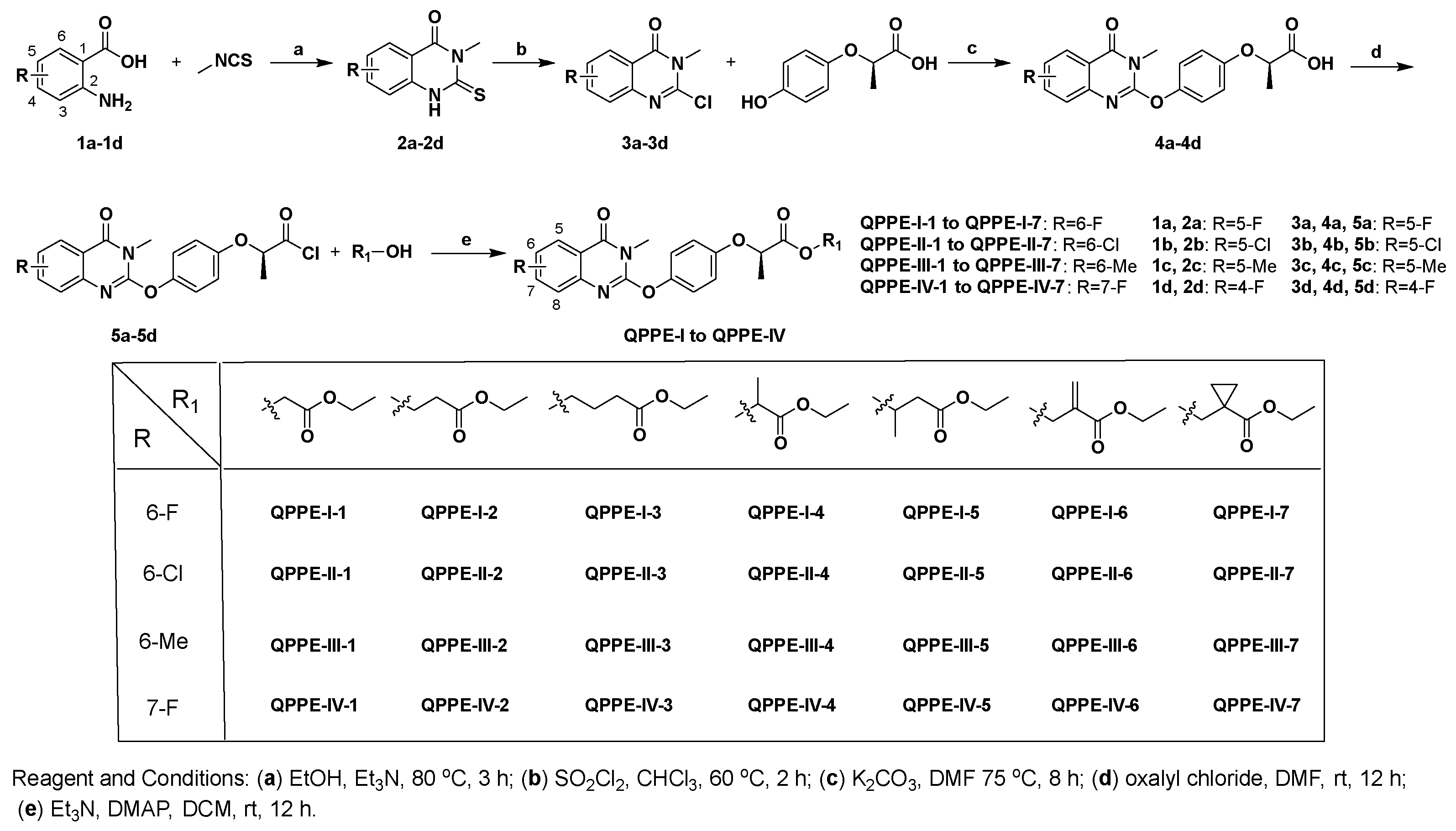
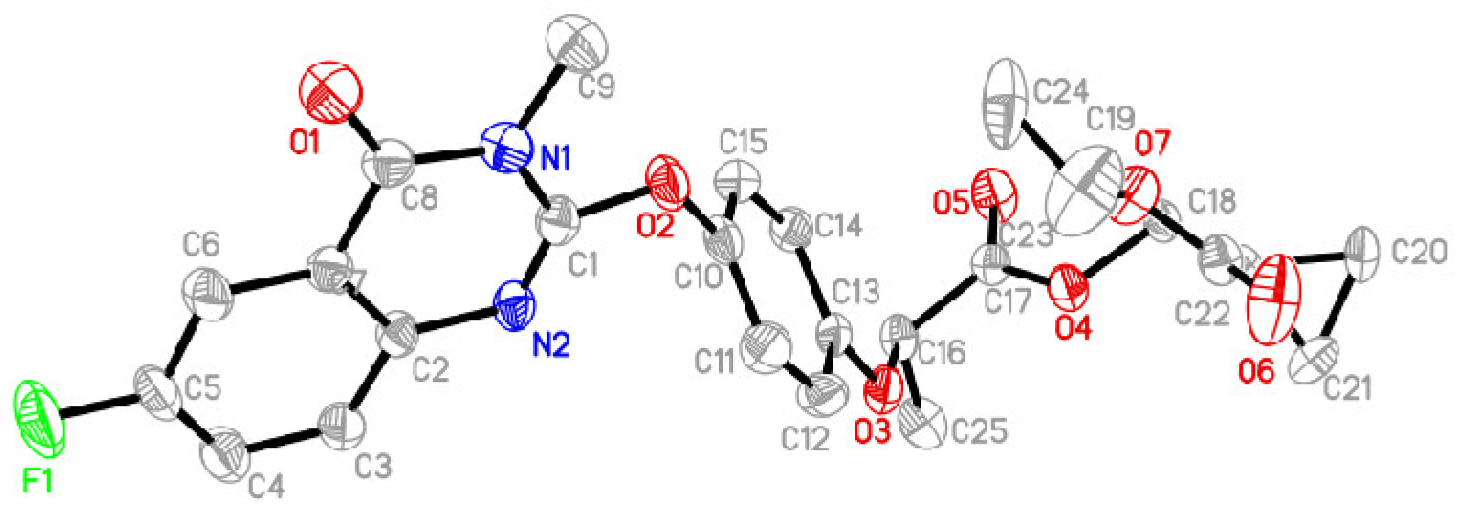
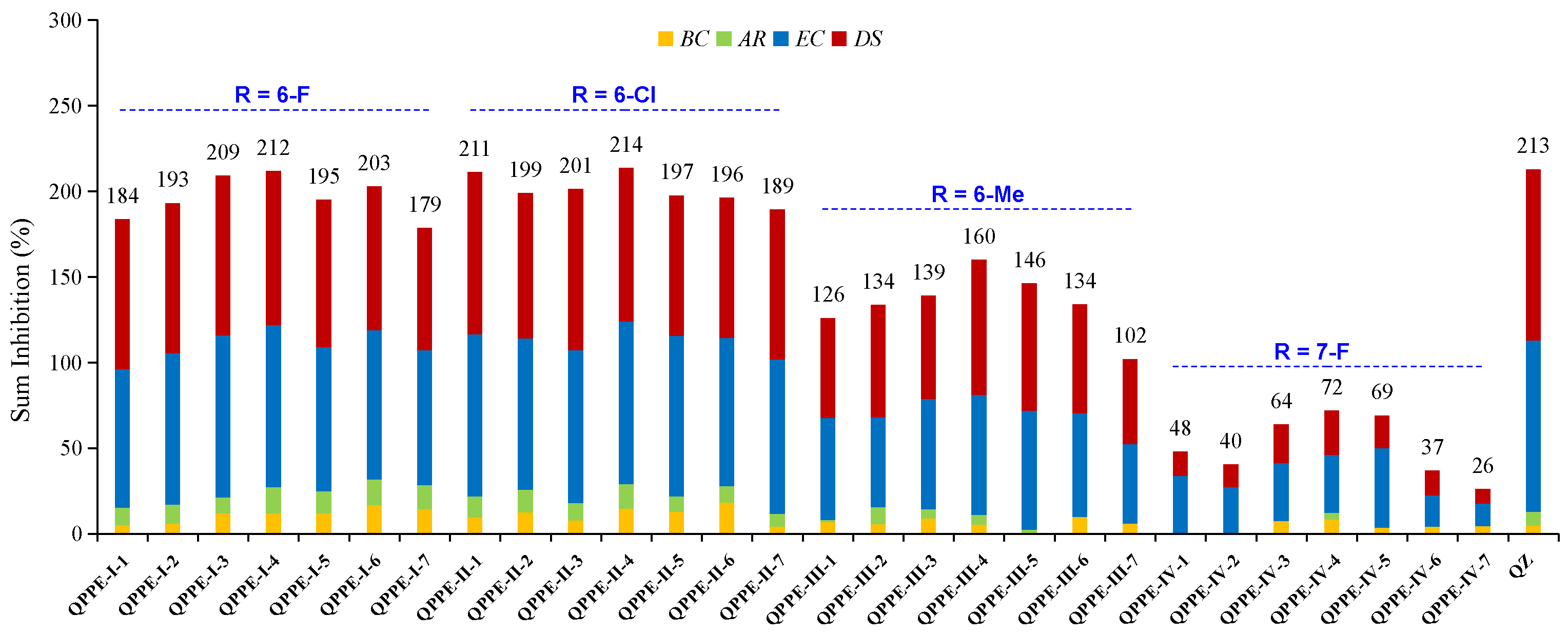
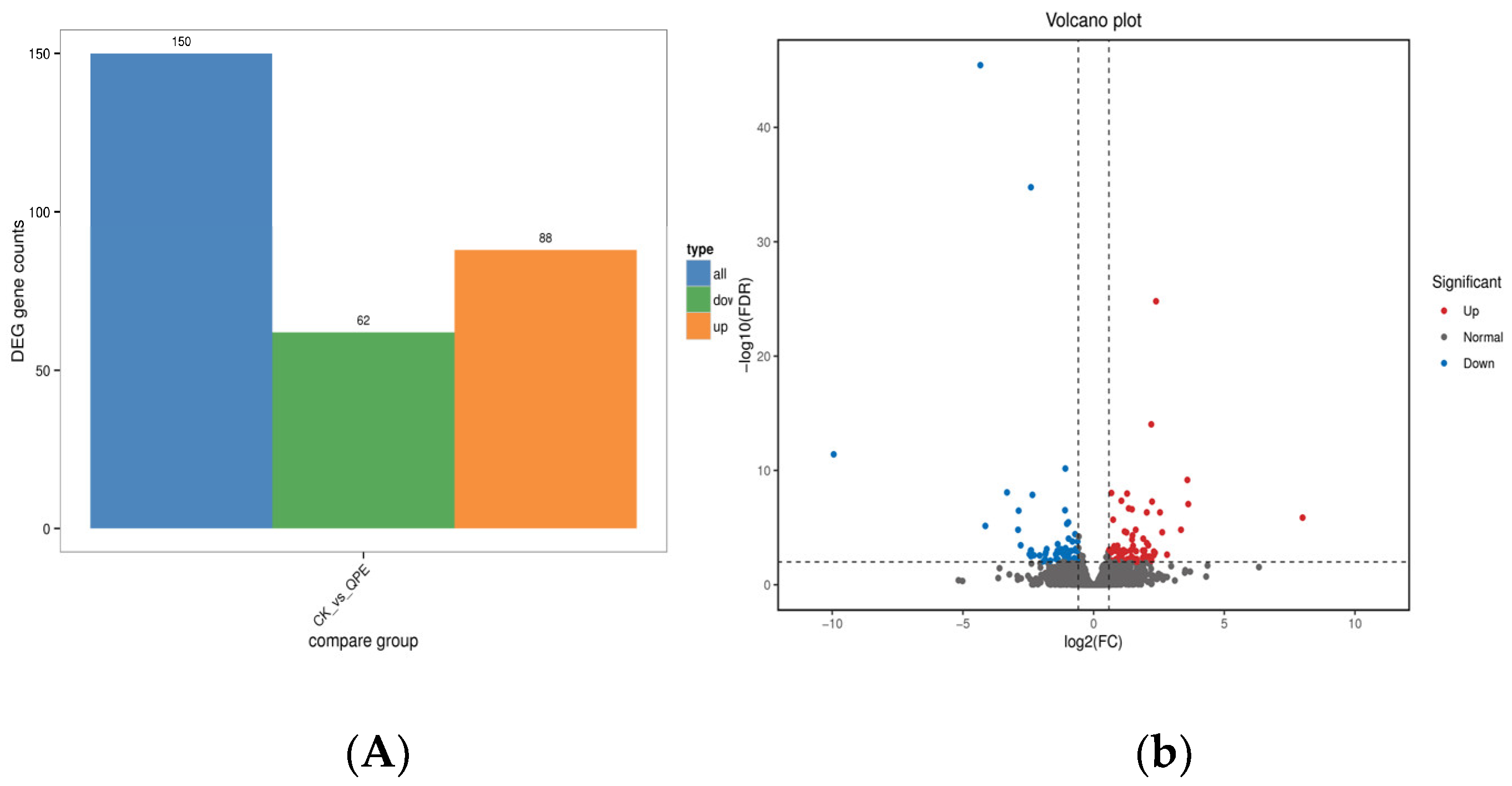
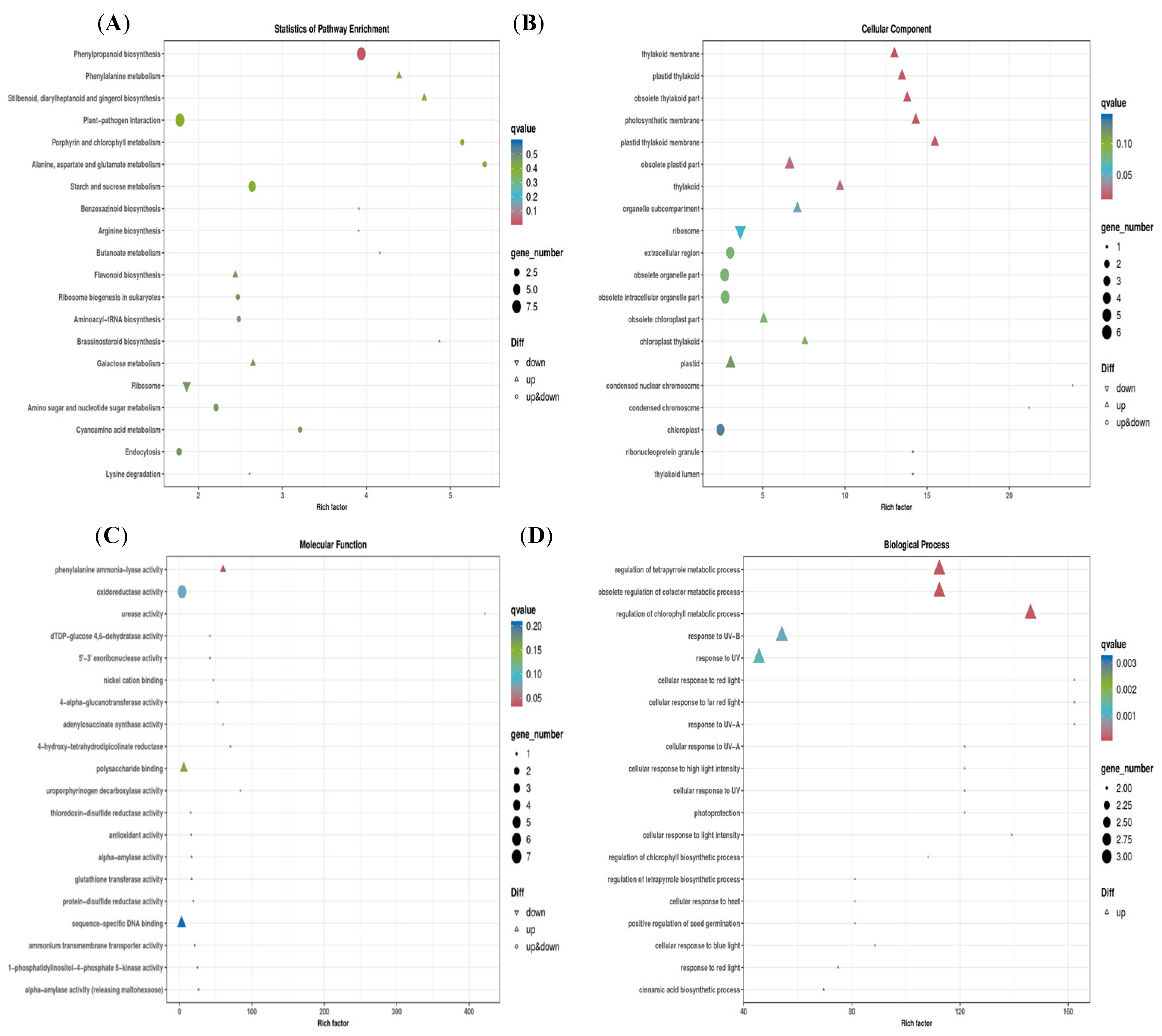
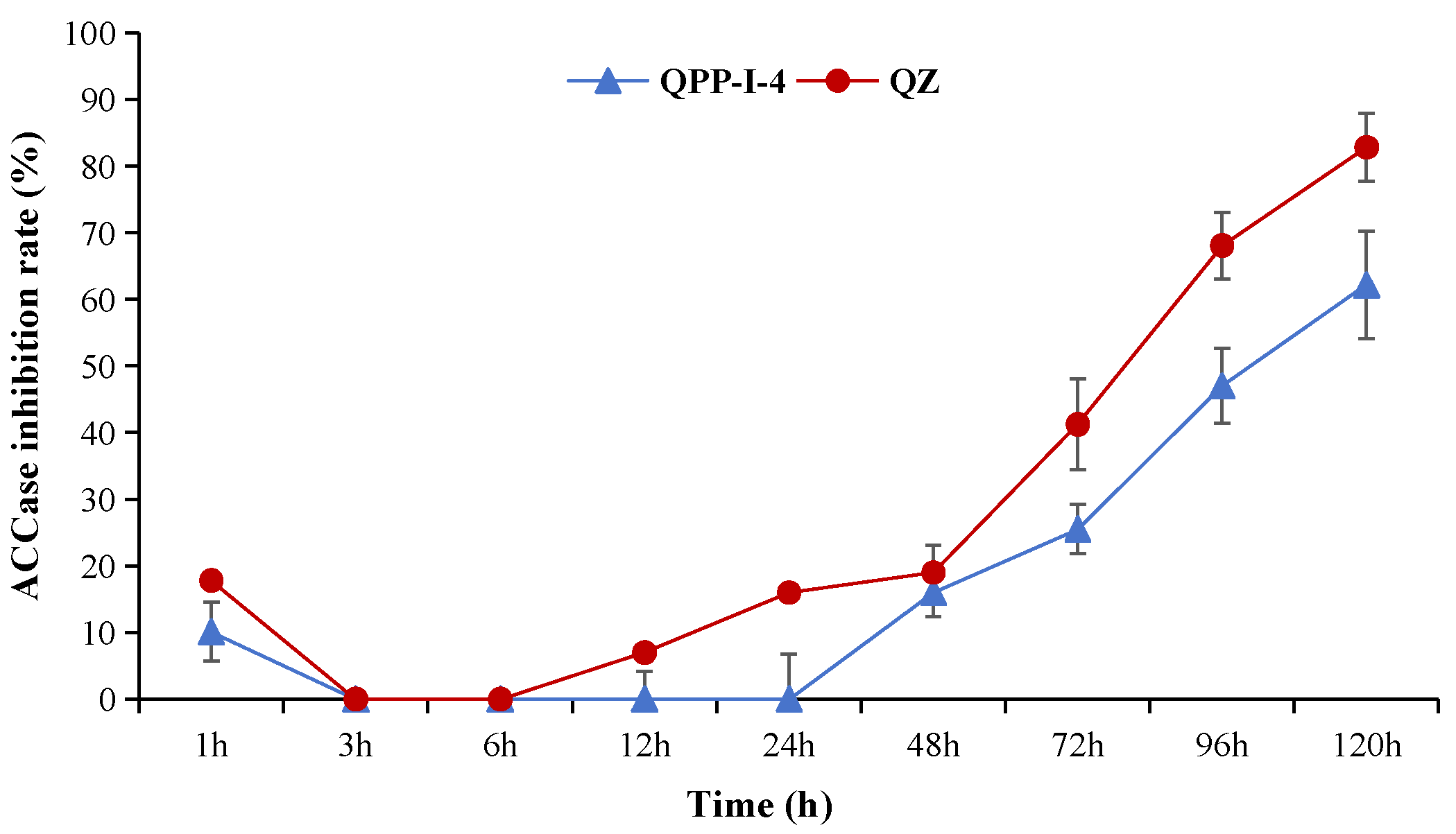
2.2.3. General Procedure for the Synthesis of Series Target Compounds QPPE−I to QPPE−IV
Representative example for the synthesis of QPPE-I-1: (R)-2-(4-Hydroxyphenoxy) propanoic acid (3.6 g, 20 mmol) was dissolved in 50 mL of DMF, and K2CO3 (5.5 g, 40 mmol) was then added in two batches. The reaction mixture was stirred for 1.0 h at 75 oC, then intermediate 3a (4.2 g, 20 mmol) was added. The reaction mixture was stirred for 7.0 h at 75 oC. After the reaction was complete (TLC monitoring), 150 mL of ice water was poured into the reaction system, and the pH was adjusted to 4-5 by 1 M HCl followed by extraction with ethyl acetate (3 x 50 mL). The organic layer was washed with saturated NaCl solution, dried with anhydrous Na2SO4. The solvent was removed using a rotary flash evaporator, and the crude product 4a was used without further purification.
The crude product 4a (358 mg, 1 mmol) was dissolved in 10 mL of DCM in a 25 mL flask, then oxalyl chloride (254 mg, 2 mmol) and DMF (one drop) was added. The mixture was stirred for 12 h at room temperature. After that, the solvent was removed by a rotary flash evaporator, and the crude product 5a was obtained. Subsequently, 5a was dissolved in 10 mL of DCM, and then, ethyl glycolate (156 mg, 1.5 mmol), 4-dimethylaminopyridine (DMAP, 12 mg, 0.1 mmol), and Et3N (202 mg, 2 mmol) were sequentially added to the solution, and the reaction mixture was stirred for 12 h at room temperature. After the reaction was complete (TLC monitoring), aqueous hydrochloric acid solution (1 M, 20 mL) was added to the reaction system. The organic layer was separated and washed with water, saturated NaCl solution, dried with anhydrous Na2SO4, concentrated by a rotary flash evaporator. The residue was purified through chromatograph on silica gel using petroleum ether/ethyl acetate (v/v, 20:1) as an elution to give target compound QPPE-I-1 as a white solid (193 mg, yield: 43.5%).
The target compounds QPPE-I-2 to QPPE-I-7 and series target compounds QPPE-II to QPPE-IV were synthesized by the similar procedure to compound QPPE-I-1.
2-Ethoxy-2-oxoethyl (R)-2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-I-1) white solid; yield 43.5%; m.p. 86–89 oC; 1H NMR (500 MHz, CDCl3) δ 7.84 (dd, J = 8.4, 2.3 Hz, 1H), 7.38 – 7.29 (m, 2H), 7.17 (d, J = 8.5 Hz, 2H), 6.99 (d, J = 8.3 Hz, 2H), 4.88 (q, J = 6.8 Hz, 1H), 4.72 (q, J = 15.8 Hz, 2H), 4.23 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 1.73 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 167.2, 162.3 (d, J = 3.5 Hz), 159.7 (d, J = 245.5 Hz), 155.3, 152.2, 145.9, 143.1, 128.2 (d, J = 8.0 Hz), 122.8 (d, J = 22.6 Hz), 122.7, 119.9 (d, J = 8.4 Hz), 116.0, 111.9 (d, J = 23.8 Hz), 72.8, 61.7, 61.1, 29.0, 18.7, 14.1; HRMS: calcd for C22H21FN2NaO7+ [M+Na]+ 467.1225, found 467.1234.
3-Ethoxy-3-oxopropyl (R)-2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-I-2) white solid; yield 42.0%; m.p. 67–70 oC; 1H NMR (500 MHz, CDCl3) δ 7.84 (dd, J = 8.5, 2.1 Hz, 1H), 7.38 – 7.29 (m, 2H), 7.15 (d, J = 8.9 Hz, 2H), 6.93 (d, J = 8.9 Hz, 2H), 4.76 (q, J = 6.7 Hz, 1H), 4.47 (t, J = 6.3 Hz, 2H), 4.15 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 2.67 (t, J = 6.3 Hz, 2H), 1.64 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 170.2, 162.3 (d, J = 3.5 Hz), 159.7 (d, J = 245.4 Hz), 155.4, 152.2, 145.9, 143.1, 128.2 (d, J = 7.8 Hz), 122.8 (d, J = 22.9 Hz), 122.6, 119.9 (d, J = 9.0 Hz), 116.0, 111.86 (d, J = 23.9 Hz), 72.8, 69.3, 61.6, 29.0, 18.6, 16.9, 14.1; HRMS: calcd for C23H23FN2NaO7+ [M+Na]+ 481.1382, found 481.1391.
Ethyl (R)-4-((2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)butanoate (QPPE-I-3) white solid; yield 50.0%; m.p. 51–53 oC; 1H NMR (500 MHz, CDCl3) δ 7.84 (dd, J = 8.3, 2.3 Hz, 1H), 7.38 – 7.29 (m, 2H), 7.18 – 7.13 (m, 2H), 6.97 – 6.91 (m, 2H), 4.78 (q, J = 6.8 Hz, 1H), 4.28 – 4.19 (m, 2H), 4.13 (q, J = 7.1 Hz, 2H), 3.70 (s, 3H), 2.34 (t, J = 7.4 Hz, 2H), 2.00 – 1.96 (m, 2H), 1.66 (d, J = 6.8 Hz, 3H), 1.30 – 1.14 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 172.6, 172.0, 162.3 (d, J = 3.1 Hz), 159.7 (d, J = 245.5 Hz), 155.4, 152.2, 145.8, 143.1, 128.2 (d, J = 8.1 Hz), 122.9, 122.8 (d, J = 24.0 Hz), 122.7, 119.8 (d, J = 8.5 Hz), 115.9, 111.9 (d, J = 23.6 Hz), 73.1, 64.3, 60.6, 30.5, 29.0, 24.0, 18.6, 14.2; HRMS: calcd for C24H25FN2NaO7+ [M+Na]+ 495.1538, found 495.1541.
1-Ethoxy-1-oxopropan-2-yl (2R)-2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-I-4) colorless oil; yield 43.1%; 1H NMR (500 MHz, CDCl3) δ 7.85 – 7.83 (m, 1H), 7.37 – 7.31 (m, 2H), 7.18 – 7.11 (m, 2H), 7.03 – 6.95 (m, 2H), 5.21 – 5.15 (m, 1H), 4.87 – 4.82 (m, 1H), 4.24 – 4.16 (m, 2H), 3.69 (s, 3H), 1.73 – 1.70 (m, 3H), 1.56 – 1.51 (m, 3H), 1.29 – 1.25 (m, 3H); 13C NMR (126 MHz, CDCl3) δ 171.2, 170.0 (d, J = 10.8 Hz), 161.6, 155.3, 152.7 (d, J = 3.4 Hz), 145.7 (d, J = 10.0 Hz), 145.0, 134.4, 130.0, 127.5, 126.0, 122.6, 122.6, 119.7, 116.0, 115.7, 72.7, 72.6, 69.2, 69.1, 61.5, 18.5, 16.8, 14.1; HRMS: calcd for C23H23FN2NaO7+ [M+Na]+ 481.1382, found 481.1393.
Ethyl 3-(((R)-2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)butanoate (QPPE-I-5) white solid; yield 36.6%; m.p. 51–54 oC; 1H NMR (500 MHz, CDCl3) δ 7.89 – 7.71 (m, 1H), 7.36 – 7.30 (m, 2H), 7.18 – 7.12 (m, 2H), 6.96 – 6.90 (m, 2H), 5.45 – 5.28 (m, 1H), 4.73 (q, J = 6.8 Hz, 1H), 4.18 – 4.06 (m, 2H), 3.69 (d, J = 1.3 Hz, 3H), 2.71 – 2.47 (m, 2H), 1.63 (dd, J = 6.8, 3.5 Hz, 3H), 1.30 – 1.21 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 171.2, 169.9, 169.8, 162.33, 162.3, 159.7 (d, J = 245.5 Hz), 155.4, 152.2 (d, J = 6.5 Hz), 145.8, 143.1, 128.2 (d, J = 8.1 Hz), 122.8 (d, J = 18.0 Hz), 122.7, 119.8 (d, J = 8.5 Hz), 115.9, 111.8 (d, J = 23.6 Hz), 73.1, 68.6, 68.4, 60.8, 40.7, 29.0, 19.9, 19.7, 18.5, 14.2; HRMS: calcd for C24H25FN2NaO7+ [M+Na]+ 495.1538, found 495.1548.
Ethyl (R)-2-(((2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)methyl)acrylate (QPPE-I-6) white solid; yield 38.7%; m.p. 112–115 oC; 1H NMR (500 MHz, CDCl3) δ 7.84 (dd, J = 8.5, 1.7 Hz, 1H), 7.37 – 7.30 (m, 2H), 7.15 (d, J = 8.9 Hz, 2H), 6.95 (d, J = 8.9 Hz, 2H), 6.37 (s, 1H), 5.77 (s, 1H), 4.97 – 4.88 (m, 2H), 4.82 (q, J = 6.8 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 3.70 (s, 3H), 1.68 (d, J = 6.8 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 165.0, 162.4, 159.8 (d, J = 245.3 Hz), 158.8, 155.4, 152.2, 145.9, 143.1, 135.0, 128.2 (d, J = 7.9 Hz), 128.0, 122.8 (d, J = 24.3 Hz), 122.7, 115.9, 111.9 (d, J = 23.5 Hz), 73.1, 63.2, 61.1, 29.0, 18.6, 14.2; HRMS: calcd for C24H23FN2NaO7+ [M+Na]+ 493.1382, found 493.1391.
Ethyl (R)-1-(((2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)methyl)cyclopropane-1-carboxylate (QPPE-I-7) white solid; yield 45.3%; m.p. 124–127 oC; 1H NMR (500 MHz, CDCl3) δ 7.83 (dd, J = 8.3, 1.8 Hz, 1H), 7.37 – 7.30 (m, 2H), 7.15 (d, J = 8.9 Hz, 2H), 6.94 (d, J = 8.9 Hz, 2H), 4.80 (q, J = 6.7 Hz, 1H), 4.37 – 4.31 (m, 2H), 4.17 – 4.10 (m, 2H), 3.69 (s, 3H), 1.66 (d, J = 6.7 Hz, 3H), 1.33 (d, J = 2.4 Hz, 2H), 1.23 (t, J = 7.1 Hz, 3H), 0.92 (d, J = 1.8 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 172.9, 172.0, 162.3 (d, J = 3.4 Hz), 159.7 (d, J = 245.4 Hz), 155.4, 152.2, 145.8, 143.1, 128.1 (d, J = 8.1 Hz), 122.8 (d, J = 25.1 Hz), 122.7, 119.9 (d, J = 8.6 Hz), 115.9, 111.9 (d, J = 23.7 Hz), 73.0, 67.3, 61.0, 29.0, 23.1, 18.6, 14.4, 14.3, 14.2; HRMS: calcd for C25H25FN2NaO7+ [M+Na]+ 507.1538, found 507.1547.
2-Ethoxy-2-oxoethyl (R)-2-(4-((6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-II-1) white solid; yield 45.7%; m.p. 85–88 oC; 1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 2.2 Hz, 1H), 7.53 (dd, J = 8.7, 2.2 Hz, 1H), 7.29 (d, J = 8.7 Hz, 1H), 7.16 (d, J = 8.9 Hz, 2H), 6.99 (d, J = 8.9 Hz, 2H), 4.88 (q, J = 6.8 Hz, 1H), 4.76 – 4.67 (m, 2H), 4.23 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 1.73 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 167.2, 162.0, 155.3, 152.8, 145.8, 145.1, 134.7, 130.5, 127.7, 126.4, 122.7, 119.9, 116.0, 72.8, 61.7, 61.1, 29.0, 18.7, 14.1; HRMS: calcd for C22H21ClN2NaO7+ [M+Na]+ 483.0929, found 483.0939.
3-Ethoxy-3-oxopropyl (R)-2-(4-((6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-II-2) brown solid; yield 38.1%; m.p. 72–75 oC; 1H NMR (500 MHz, CDCl3) δ 8.15 (d, J = 2.3 Hz, 1H), 7.52 (dd, J = 8.7, 2.3 Hz, 1H), 7.29 (d, J = 8.8 Hz, 1H), 7.15 (d, J = 9.0 Hz, 2H), 6.93 (d, J = 9.0 Hz, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.47 (t, J = 6.3 Hz, 2H), 4.15 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 2.67 (t, J = 6.2 Hz, 2H), 1.64 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.8, 170.3, 162.0, 155.4, 152.8, 145.7, 145.1, 134.6, 130.5, 127.7, 126.3, 122.7, 119.9, 115.9, 72.9, 60.9, 60.7, 33.8, 29.0, 18.6, 14.2; HRMS: calcd for C23H23ClN2NaO7+ [M+Na]+ 497.1086, found 497.1093.
Ethyl (R)-4-((2-(4-((6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)butanoate (QPPE-II-3) white solid; yield 44.9%; m.p. 62–65 oC; 1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 2.5 Hz, 1H), 7.52 (dd, J = 8.7, 2.5 Hz, 1H), 7.28 (d, J = 8.7 Hz, 1H), 7.17 – 7.12 (m, 2H), 6.97 – 6.92 (m, 2H), 4.78 (q, J = 6.8 Hz, 1H), 4.27 – 4.17 (m, 2H), 4.13 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 2.34 (t, J = 7.4 Hz, 2H), 2.01 – 1.95 (m, 2H), 1.66 (d, J = 6.8 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 172.6, 172.0, 162.0, 155.4, 152.8, 145.7, 145.1, 134.7, 130.5, 127.7, 126.3, 122.7, 119.9, 115.8, 73.0, 64.4, 60.6, 30.5, 29.0, 24.0, 18.6, 14.2; HRMS: calcd for C24H25ClN2NaO7+ [M+Na]+ 511.1242, found 511.1252.
1-Ethoxy-1-oxopropan-2-yl (2R)-2-(4-((6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-II-4) colorless oil; yield 40.1%; 1H NMR (500 MHz, CDCl3) δ 8.03 – 7.88 (m, 1H), 7.49 – 7.33 (m, 1H), 7.23 – 7.08 (m, 2H), 6.99 – 6.94 (m, 2H), 5.23 – 5.03 (m, 1H), 4.90 – 4.74 (m, 1H), 4.25 – 4.09 (m, 2H), 3.64 – 3.53 (m, 3H), 1.71 – 1.66 (m, 3H), 1.58 – 1.39 (m, 3H), 1.35 – 1.14 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 171.2, 170.0, 161.6, 155.3, 152.7, 145.7, 145.0, 134.4, 130.0, 127.5, 126.0, 122.6, 119.7, 116.0, 115.7, 72.6, 69.2, 61.5, 28.8, 18.5, 16.8, 14.1; HRMS: calcd for C23H23ClN2NaO7+ [M+Na]+ 497.1086, found 497.1095.
Ethyl 3-(((R)-2-(4-((6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)butanoate (QPPE-II-5) yellow oil; yield 41.7%; 1H NMR (500 MHz, CDCl3) δ 8.11 (d, J = 1.2 Hz, 1H), 7.49 (d, J = 7.5 Hz, 1H), 7.25 (t, J = 8.8 Hz, 1H), 7.16 (d, J = 8.4 Hz, 2H), 6.93 (t, J = 7.9 Hz, 2H), 5.38 (q, J = 6.2 Hz, 1H), 4.73 (q, J = 6.4 Hz, 1H), 4.16 – 4.06 (m, 2H), 3.67 (s, 3H), 2.74 – 2.46 (m, 2H), 1.63 (d, J = 6.4 Hz, 3H), 1.40 – 1.20 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 171.2, 169.9, 169.8, 161.9, 154.1, 152.8, 145.7, 145.1, 145.1, 134.5, 130.3, 127.6, 126.2, 119.8, 115.8, 73.0, 68.5, 68.4, 60.7, 40.6, 28.9, 19.8, 19.7, 18.5, 14.2; HRMS: calcd for C24H25ClN2NaO7+ [M+Na]+ 511.1242, found 511.1252.
Ethyl (R)-2-(((2-(4-((6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)methyl)acrylate (QPPE-II-6) white solid; yield 52.3%; m.p. 100–102 oC; 1H NMR (500 MHz, CDCl3) δ 8.17 (d, J = 2.4 Hz, 1H), 7.53 (dd, J = 8.7, 2.4 Hz, 1H), 7.29 (d, J = 8.7 Hz, 1H), 7.15 (d, J = 8.9 Hz, 2H), 6.95 (d, J = 8.8 Hz, 2H), 6.37 (s, 1H), 5.77 (s, 1H), 4.97 – 4.88 (m, 2H), 4.83 (q, J = 6.8 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 1.68 (d, J = 6.8 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 165.0, 162.0, 155.4, 152.8, 145.8, 145.1, 135.0, 134.7, 130.5, 128.0, 126.4, 122.8, 119.9, 115.9, 73.0, 63.3, 61.1, 29.0, 18.6, 14.2; HRMS: calcd for C24H23ClN2NaO7+ [M+Na]+ 509.1086, found 509.1095.
Ethyl (R)-1-(((2-(4-((6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)methyl)cyclopropane-1-carboxylate (QPPE-II-7) light yellow oil; yield 46.4%; 1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 2.4 Hz, 1H), 7.53 (dd, J = 8.7, 2.5 Hz, 1H), 7.29 (d, J = 8.7 Hz, 1H), 7.17 – 7.11 (m, 2H), 6.97 – 6.91 (m, 2H), 4.79 (q, J = 6.8 Hz, 1H), 4.34 (q, J = 11.7 Hz, 2H), 4.13 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 1.66 (d, J = 6.8 Hz, 3H), 1.33 (q, J = 4.8 Hz, 2H), 1.23 (t, J = 7.1 Hz, 3H), 0.94 – 0.88 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 172.9, 172.0, 162.0, 155.5, 152.8, 145.7, 145.2, 134.7, 130.5, 127.6, 126.4, 122.7, 119.9, 115.9, 73.0, 67.3, 61.0, 29.0, 23.1, 18.6, 14.3, 14.2; HRMS: calcd for C25H25ClN2NaO7+ [M+Na]+ 523.1242, found 523.1251.
2-Ethoxy-2-oxoethyl (R)-2-(4-((3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-III-1) white solid; yield 61.4%; m.p. 100–103 oC; 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.42 (d, J = 6.9 Hz, 1H), 7.26 (d, J = 7.9 Hz, 1H), 7.18 (d, J = 8.7 Hz, 2H), 6.99 (d, J = 8.5 Hz, 2H), 4.87 (q, J = 6.8 Hz, 1H), 4.71 (q, J = 15.8 Hz, 2H), 4.23 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 2.43 (s, 3H), 1.73 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 167.2, 163.1, 155.2, 152.1, 146.1, 144.4, 135.8, 134.9, 126.5, 125.8, 122.7, 118.6, 116.0, 72.8, 61.7, 61.1, 28.9, 21.1, 18.7, 14.1; HRMS: calcd for C23H24N2NaO7+ [M+Na]+ 463.1476, found 463.1481.
3-Ethoxy-3-oxopropyl (R)-2-(4-((3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-III-2) white solid; yield 43.6%; m.p. 97–98 oC; 1H NMR (500 MHz, CDCl3) δ 7.99 (s, 1H), 7.42 (dd, J = 8.3, 1.7 Hz, 2H), 7.26 (d, J = 9.3 Hz, 2H), 6.93 (d, J = 9.0 Hz, 1H), 4.76 (q, J = 6.8 Hz, 1H), 4.46 (t, J = 6.3 Hz, 2H), 4.15 (q, J = 7.1 Hz, 2H), 3.68 (s, 3H), 2.66 (t, J = 6.3 Hz, 2H), 2.43 (s, 3H), 1.64 (d, J = 6.8 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.8, 170.3, 163.1, 155.2, 152.1, 146.0, 144.5, 135.7, 134.9, 126.5, 125.8, 122.7, 118.6, 115.9, 73.0, 60.9, 60.7, 33.8, 28.8, 21.1, 18.6, 14.2; HRMS: calcd for C24H26N2NaO7+ [M+Na]+ 477.1632, found 477.1641.
Ethyl (R)-4-((2-(4-((3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)butanoate (QPPE-III-3) white solid; yield 48.2%; m.p. 83–85 oC; 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.42 (d, J = 7.3 Hz, 1H), 7.26 (d, J = 8.9 Hz, 1H), 7.17 (d, J = 8.7 Hz, 2H), 6.94 (d, J = 8.5 Hz, 2H), 4.78 (q, J = 6.7 Hz, 1H), 4.27 – 4.18 (m, 2H), 4.13 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 2.43 (s, 3H), 2.34 (t, J = 7.3 Hz, 2H), 2.02 – 1.94 (m, 2H), 1.65 (d, J = 6.8 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 172.7, 172.0, 163.1, 155.2, 152.1, 146.0, 144.4, 135.8, 134.9, 126.5, 125.9, 122.7, 118.6, 115.8, 73.1, 64.3, 60.6, 30.5, 28.8, 24.0, 21.1, 18.6, 14.2; HRMS: calcd for C25H28N2NaO7+ [M+Na]+ 491.1789, found 491.1798.
1-Ethoxy-1-oxopropan-2-yl (2R)-2-(4-((3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-III-4) colorless oil; yield 42.6%; 1H NMR (500 MHz, CDCl3) δ 7.99 (s, 1H), 7.41 (d, J = 8.1 Hz, 1H), 7.24 (dd, J = 8.2, 3.7 Hz, 1H), 7.17 (d, J = 8.8 Hz, 2H), 6.98 (t, J = 9.3 Hz, 2H), 5.18 (dq, J = 14.1, 7.1 Hz, 1H), 4.89 – 4.78 (m, 1H), 4.20 (p, J = 6.9 Hz, 2H), 3.68 (s, 3H), 2.42 (s, 3H), 1.73 – 1.69 (m, 3H), 1.55 – 1.51 (m, 3H), 1.26 (td, J = 7.0, 2.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 170.2, 163.0, 155.2, 152.1, 146.0, 144.5, 135.7, 134.8, 126.5, 125.8, 122.6, 118.6, 116.2, 115.9, 72.9, 69.2, 61.6, 28.8, 21.1, 18.6, 16.8, 14.1; HRMS: calcd for C24H26N2NaO7+ [M+Na]+ 477.1632, found 477.1642.
Ethyl 3-(((R)-2-(4-((3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)butanoate (QPPE-III-5) colorless oil; yield 41.7%; 1H NMR (500 MHz, CDCl3) δ 7.97 (s, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.22 (t, J = 8.3 Hz, 1H), 7.16 (d, J = 8.0 Hz, 2H), 6.92 (dd, J = 8.8, 7.0 Hz, 2H), 5.39 (dd, J = 12.8, 6.3 Hz, 1H), 4.72 (q, J = 6.7 Hz, 1H), 4.17 – 4.11 (m, 1H), 4.08 (q, J = 7.1 Hz, 1H), 3.66 (d, J = 1.2 Hz, 3H), 2.75 – 2.48 (m, 2H), 2.40 (s, 3H), 1.62 (dd, J = 6.7, 3.1 Hz, 3H), 1.38 – 1.20 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 171.2, 169.9, 162.9, 155.3, 152.1, 145.9, 144.4, 135.7, 134.7, 126.4, 125.8, 122.7, 118.6, 115.8, 73.0, 68.4, 60.7, 40.6, 28.8, 21.1, 19.7, 18.5, 14.2; HRMS: calcd for C25H28N2NaO7+ [M+Na]+ 491.1789, found 491.1797.
Ethyl (R)-2-(((2-(4-((3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)methyl)acrylate (QPPE-III-6) white solid; yield 36.7%; m.p. 99–101 oC; 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.42 (dd, J = 8.3, 1.6 Hz, 1H), 7.25 (d, J = 8.3 Hz, 1H), 7.16 (d, J = 9.0 Hz, 2H), 6.94 (d, J = 9.0 Hz, 2H), 6.37 (s, 1H), 5.77 (s, 1H), 4.97 – 4.88 (m, 2H), 4.82 (q, J = 6.8 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 2.43 (s, 3H), 1.67 (d, J = 6.8 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 165.0, 163.1, 155.2, 152.1, 146.0, 144.4, 135.8, 134.9, 128.0, 126.5, 125.8, 122.8, 118.6, 115.9, 73.1, 63.2, 61.1, 28.8, 21.1, 18.6, 14.2; HRMS: calcd for C25H26N2NaO7+ [M+Na]+ 489.1632, found 489.1642.
Ethyl (R)-1-(((2-(4-((3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)methyl)cyclopropane-1-carboxylate (QPPE-III-7) light yellow solid; yield 38.9%; m.p. 64–67 oC; 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.42 (dd, J = 8.3, 1.6 Hz, 1H), 7.25 (d, J = 9.9 Hz, 1H), 7.15 (d, J = 8.9 Hz, 2H), 6.94 (d, J = 9.0 Hz, 2H), 4.79 (q, J = 6.7 Hz, 1H), 4.37 – 4.30 (m, 2H), 4.13 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 2.43 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.36 – 1.30 (m, 2H), 1.23 (t, J = 7.1 Hz, 3H), 0.98 – 0.82 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 173.0, 172.1, 163.1, 155.3, 152.1, 145.9, 144.5, 135.8, 134.9, 126.5, 125.8, 122.7, 118.6, 115.9, 73.0, 67.4, 61.0, 28.9, 23.1, 21.1, 18.7, 14.4, 14.3, 14.2; HRMS: calcd for C26H28N2NaO7+ [M+Na]+ 503.1789, found 503.1798.
2-Ethoxy-2-oxoethyl (R)-2-(4-((7-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-IV-1) white solid; yield 42.2%; m.p. 72–74 oC; 1H NMR (500 MHz, CDCl3) δ 8.20 (dd, J = 8.5, 6.4 Hz, 1H), 7.16 (d, J = 8.9 Hz, 2H), 7.05 – 6.93 (m, 4H), 4.88 (q, J = 6.8 Hz, 1H), 4.72 (q, J = 15.8 Hz, 2H), 4.24 (q, J = 7.1 Hz, 2H), 3.68 (s, 3H), 1.73 (d, J = 6.8 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 167.2, 166.6 (d, J = 253.3 Hz), 162.2, 155.4, 153.5, 148.9 (d, J = 13.7 Hz), 145.8, 129.7 (d, J = 10.7 Hz), 122.7, 116.0, 115.6, 113.6 (d, J = 23.6 Hz), 111.4 (d, J = 22.3 Hz), 72.8, 61.7, 61.1, 28.8, 18.6, 14.1; HRMS: calcd for C22H21FN2NaO7+ [M+Na]+ 467.1225, found 467.1235.
3-Ethoxy-3-oxopropyl (R)-2-(4-((7-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-IV-2) light yellow oil; yield 47.7%; 1H NMR (500 MHz, CDCl3) δ 8.20 (dd, J = 8.7, 6.2 Hz, 1H), 7.17 – 7.12 (m, 2H), 7.07 – 6.99 (m, 2H), 6.97 – 6.90 (m, 2H), 4.77 (q, J = 6.8 Hz, 1H), 4.47 (t, J = 6.3 Hz, 2H), 4.16 (q, J = 7.1 Hz, 2H), 3.68 (s, 3H), 2.67 (t, J = 6.3 Hz, 2H), 1.64 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.8, 170.3, 166.6 (d, J = 253.6 Hz), 162.3, 155.4, 153.5, 148.8 (d, J = 13.8 Hz), 145.7, 129.7 (d, J = 10.9 Hz), 122.7, 115.9, 115.6 (d, J = 1.5 Hz), 113.7 (d, J = 23.6 Hz), 111.5 (d, J = 22.2 Hz), 73.0, 60.9, 60.7, 33.8, 28.8, 18.6, 14.2; HRMS: calcd for C23H23FN2NaO7+ [M+Na]+ 481.1382, found 481.1390.
Ethyl (R)-4-((2-(4-((7-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)butanoate (QPPE-IV-3) light yellow oil; yield 42.9%; 1H NMR (500 MHz, CDCl3) δ 8.20 (dd, J = 8.8, 6.2 Hz, 1H), 7.15 (d, J = 9.0 Hz, 2H), 7.06 – 6.98 (m, 2H), 6.94 (d, J = 9.0 Hz, 2H), 4.78 (q, J = 6.8 Hz, 1H), 4.27 – 4.19 (m, 2H), 4.13 (q, J = 7.2 Hz, 2H), 3.68 (s, 3H), 2.34 (t, J = 7.4 Hz, 2H), 2.02 – 1.96 (m, 2H), 1.66 (d, J = 6.8 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 172.6, 171.9, 166.6 (d, J = 253.5 Hz), 162.2, 155.5, 153.5, 148.8 (d, J = 13.7 Hz), 145.8, 129.6 (d, J = 10.9 Hz), 122.7, 115.9, 115.6, 113.6 (d, J = 23.6 Hz), 111.4 (d, J = 22.5 Hz), 73.1, 64.3, 60.5, 30.53, 28.8, 24.0, 18.6, 14.2; HRMS: calcd for C24H25FN2NaO7+ [M+Na]+ 495.1538, found 495.1546.
1-Ethoxy-1-oxopropan-2-yl (2R)-2-(4-((7-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPPE-IV-4) colorless oil; yield 31.6%; 1H NMR (500 MHz, CDCl3) δ 8.20 (dd, J = 8.8, 6.2 Hz, 1H), 7.15 (d, J = 9.0 Hz, 2H), 7.05 – 6.96 (m, 4H), 5.18 (dq, J = 9.9, 7.1 Hz, 1H), 4.87 – 4.82 (m, 1H), 4.29 – 4.13 (m, 2H), 3.68 (s, 3H), 1.73 – 1.70 (m, 3H), 1.56 – 1.51 (m, 3H), 1.27 (td, J = 7.1, 2.9 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 170.1, 166.6 (d, J = 253.5 Hz), 162.2, 155.4, 153.5, 148.9, 145.8 (d, J = 10.5 Hz), 129.7 (d, J = 10.8 Hz), 122.6, 116.2, 115.9, 115.6, 113.6 (d, J = 23.7 Hz), 111.4 (d, J = 23.0 Hz), 72.9, 69.3, 63.7, 61.6, 28.8, 18.6, 16.8, 14.1; HRMS: calcd for C23H23FN2NaO7+ [M+Na]+ 481.1382, found 481.1391.
Ethyl 3-(((R)-2-(4-((7-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)butanoate (QPPE-IV-5) colorless oil; yield 31.4%; 1H NMR (500 MHz, CDCl3) δ 8.22 – 8.19 (m, 1H), 7.14 (d, J = 8.3 Hz, 2H), 7.05 – 6.97 (m, 2H), 6.95 – 6.92 (m, 2H), 5.42 – 5.36 (m, 1H), 4.73 (q, J = 6.7 Hz, 1H), 4.25 – 3.96 (m, 2H), 3.68 (s, 3H), 2.73 – 2.44 (m, 2H), 1.63 (dd, J = 6.8, 3.1 Hz, 3H), 1.41 – 1.18 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 171.2, 169.9, 166.6 (d, J = 253.6 Hz), 162.3, 155.5, 153.5, 148.9 (d, J = 13.8), 145.7, 129.7 (d, J = 10.9 Hz), 122.6, 115.9, 115.6, 113.7 (d, J = 24.0), 73.1, 68.5, 60.8, 40.7, 28.8, 19.8, 18.5, 14.2; HRMS: calcd for C24H25FN2NaO7+ [M+Na]+ 495.1538, found 495.1548.
Ethyl (R)-2-(((2-(4-((7-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)methyl)acrylate (QPPE-IV-6) colorless oil; yield 37.2%; 1H NMR (500 MHz, CDCl3) δ 8.22 – 8.19 (m, 1H), 7.15 (d, J = 8.9 Hz, 2H), 7.07 – 6.98 (m, 2H), 6.95 (d, J = 8.9 Hz, 2H), 6.37 (s, 1H), 5.76 (s, 1H), 4.97 – 4.88 (m, 2H), 4.83 (q, J = 6.7 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 3.68 (s, 3H), 1.68 (d, J = 6.8 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 166.6 (d, J = 253.1 Hz), 164.9, 162.2, 155.4, 153.5, 148.8 (d, J = 13.7 Hz), 145.8, 134.9, 129.7 (d, J = 10.8 Hz), 127.9, 122.8, 115.9, 115.6, 113.6 (d, J = 23.6 Hz), 111.4 (d, J = 22.2 Hz), 73.0, 63.2, 61.1, 28.8, 18.6, 14.2; HRMS: calcd for C24H23FN2NaO7+ [M+Na]+ 493.1382, found 493.1392.
Ethyl (R)-1-(((2-(4-((7-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoyl)oxy)methyl)cyclopropane-1-carboxylate (QPPE-IV-7) white solid; yield 40.4%; m.p. 73–75 oC; 1H NMR (500 MHz, CDCl3) δ 8.22 – 8.19 (m, 1H), 7.17 – 7.09 (m, 2H), 7.05 – 7.01 (m, 2H), 6.95 (t, J = 6.2 Hz, 2H), 4.80 (q, J = 6.8 Hz, 1H), 4.34 (q, J = 11.7 Hz, 2H), 4.14 (q, J = 7.1 Hz, 2H), 3.68 (s, 3H), 1.66 (d, J = 6.8 Hz, 3H), 1.38 – 1.30 (m, 2H), 1.23 (t, J = 7.1 Hz, 3H), 0.96 – 0.85 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 172.9, 172.0, 166.6 (d, J = 253.1 Hz), 162.3, 155.5, 153.5, 148.9 (d, J = 13.9 Hz), 145.7, 129.7 (d, J = 10.7 Hz), 122.7, 115.9, 115.6, 113.7 (d, J = 23.3 Hz), 111.4 (d, J = 22.5 Hz), 73.0, 67.4, 61.0, 28.8, 23.1, 18.6, 14.4, 14.2, 14.2; HRMS: calcd for C25H25FN2NaO7+ [M+Na]+ 507.1538, found 507.1547.