Submitted:
29 August 2024
Posted:
30 August 2024
You are already at the latest version
Abstract
Keywords:
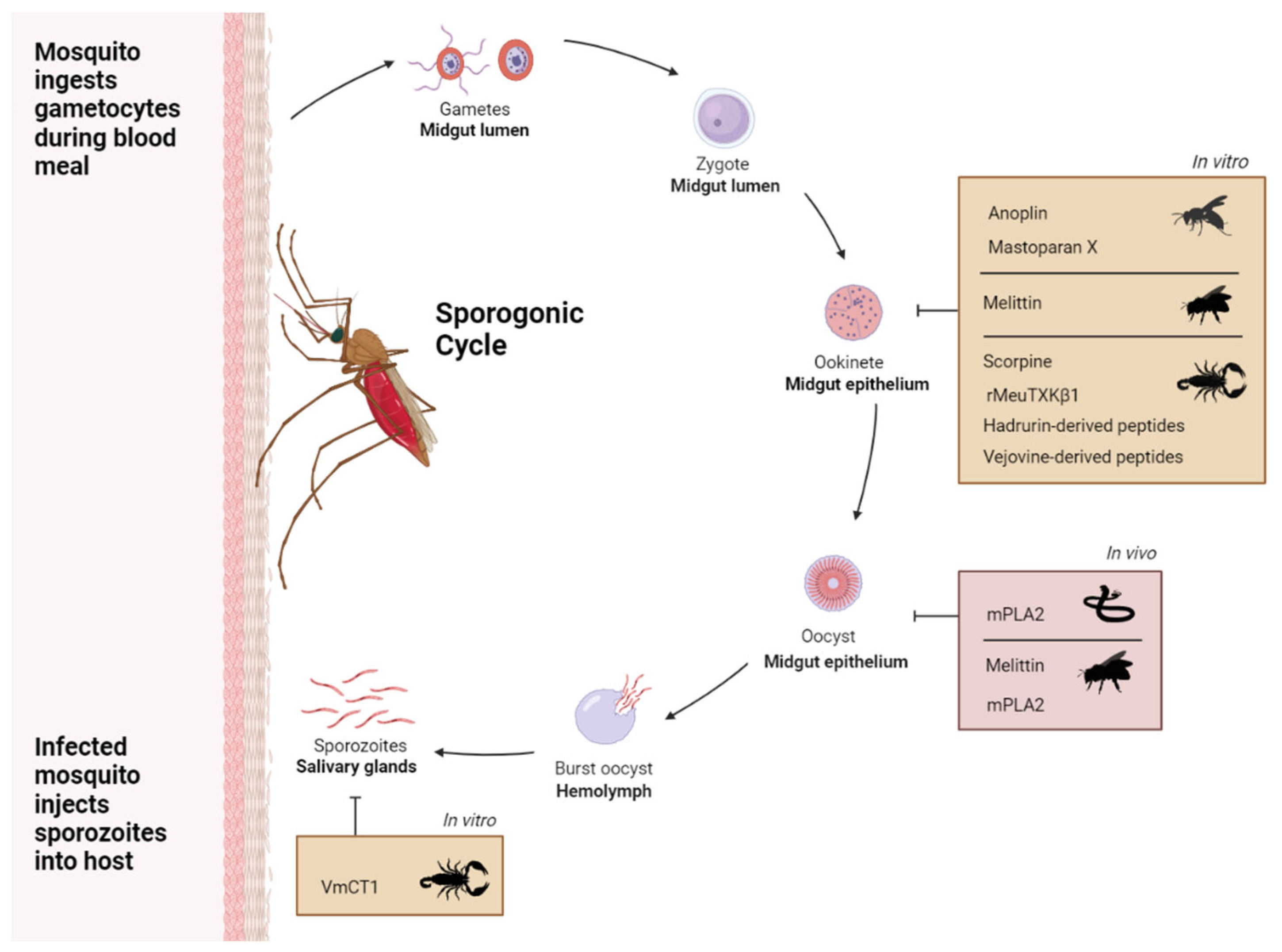
Overall Introduction and Aims
Antiparasitic Venom Toxins as Effectors to Target Plasmodium
Could Paratransgenesis Be Used to Target Arboviruses?
Conclusions
Supplementary Materials
List of Abbreviations
References
- Lee H, Halverson S, Ezinwa N. Mosquito-Borne Diseases. Primary Care - Clinics in Office Practice. 2018, 45, 393–407. [Google Scholar] [CrossRef] [PubMed]
- World malaria report 2023. World Health Organisation. Geneva: Licence: CC BY-NC-SA 3.0 IGO.; 2023. p. 1–356.
- Global Arbovirus Initiative: preparing for the next pandemic tackling mosquito-borne viruses with epidemic and pandemic potential. World Health Organization. Geneva: Licence: CC BY-NCSA 3.0 IGO; 2024. p. 1–24.
- Wu X, Lu Y, Zhou S, Chen L, Xu B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ Int. 2016, 86, 14–23. [Google Scholar] [CrossRef]
- Semenza JC, Rocklöv J, Ebi KL. Climate change and cascading risks from infectious disease. Infect Dis Ther. 2022, 11, 1371–90. [Google Scholar] [CrossRef] [PubMed]
- Fouque F, Reeder JC. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infect Dis Poverty. 2019, 8, 1–9. [Google Scholar]
- Baitharu I, Shroff S, Naik PP, Sahu JK. Environmental Management and Sustainable Control of Mosquito Vector: Challenges and Opportunities. Molecular Identification of Mosquito Vectors and Their Management. Singapore: Springer Singapore; 2020. p. 129–47.
- Durvasula R, V.; , Gumbs A, Panackal A, Kruglov O, Aksoy S, Merrifield RB, et al. Prevention of insect-borne disease: An approach using transgenic symbiotic bacteria. Proc Natl Acad Sci U S A. 1997, 94, 3274–8. [Google Scholar] [CrossRef]
- Ratcliffe NA, Furtado Pacheco JP, Dyson P, Castro HC, Gonzalez MS, Azambuja P, et al. Overview of paratransgenesis as a strategy to control pathogen transmission by insect vectors. Parasit Vectors. 2022, 15, 1–31. [Google Scholar]
- Wilke ABB, Marrelli MT. Paratransgenesis: A promising new strategy for mosquito vector control. Parasit Vectors. 2015, 8, 1–9. [Google Scholar]
- Casewell NR, Wüster W, Vonk FJ, Harrison RA, Fry BG. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol Evol. 2013, 28, 219–29. [Google Scholar] [CrossRef]
- Mackessy, SP. Venom production and secretion in reptiles. Journal of Experimental Biology. 2022, 225, 227348. [Google Scholar] [CrossRef]
- Guido-Patiño JC, Plisson F. Profiling hymenopteran venom toxins: Protein families, structural landscape, biological activities, and pharmacological benefits. Toxicon X. 2022, 14, 100119. [Google Scholar]
- Escoubas P, Diochot S, Corzo G. Structure and pharmacology of spider venom neurotoxins. Biochimie. 2000, 82, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Xia Z, He D, Wu Y, Kwok HF, Cao Z. Scorpion venom peptides: Molecular diversity, structural characteristics, and therapeutic use from channelopathies to viral infections and cancers. Pharmacol Res. 2023, 197, 106978. [Google Scholar] [CrossRef]
- Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. 2017, 3, 1–21. [Google Scholar]
- King, GF. Tying pest insects in knots: the deployment of spider-venom-derived knottins as bioinsecticides. Pest Manag Sci. 2019, 75, 2437–45. [Google Scholar] [CrossRef]
- Almeida JR, Gomes A, Mendes B, Aguiar L, Ferreira M, Brioschi MBC, et al. Unlocking the potential of snake venom-based molecules against the malaria, Chagas disease, and leishmaniasis triad. Int J Biol Macromol. 2023, 242 Pt. 2, 124745. [Google Scholar]
- Salimo ZM, Barros AL, Adrião AAX, Rodrigues AM, Sartim MA, de Oliveira IS, et al. Toxins from animal venoms as a potential source of antimalarials: A comprehensive review. Toxins (Basel). 2023, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Samy RP, Foo SL, Franco OL, Stiles BG, Kumar AP, Sethi G, et al. Identification of natural peptides as a new class of antimalarial drugs by in silico approaches. Frontiers in Bioscience. 2017, 9, 88–110. [Google Scholar] [CrossRef]
- Utkin Y, Siniavin A, Kasheverov I, Tsetlin V. Antiviral effects of animal toxins: is there a way to drugs? Int J Mol Sci. 2022, 23, 3634. [Google Scholar] [CrossRef]
- da Mata ÉCG, Mourão CBF, Rangel M, Schwartz EF. Antiviral activity of animal venom peptides and related compounds. Journal of Venomous Animals and Toxins Including Tropical Diseases 2017, 23, 1–12. [Google Scholar]
- Lima WG, Maia CQ, de Carvalho TS, Leite GO, Brito JCM, Godói IPD, et al. Animal venoms as a source of antiviral peptides active against arboviruses: a systematic review. Arch Virol. 2022, 167, 1763–72. [Google Scholar] [CrossRef] [PubMed]
- Teixeira SC, Borges BC, Oliveira VQ, Carregosa LS, Bastos LA, Santos IA, et al. Insights into the antiviral activity of phospholipases A2 (PLA2s) from snake venoms. Int J Biol Macromol. 2020, 164, 616–25. [Google Scholar] [CrossRef] [PubMed]
- Whitten MMA, Shiao SH, Levashina EA. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. 2006, 28, 121–30. [Google Scholar] [CrossRef]
- Riehle MA, Moreira CK, Lampe D, Lauzon C, Jacobs-Lorena M. Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int J Parasitol. 2007, 37, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc Natl Acad Sci U S A. 2012, 109, 12734–9. [Google Scholar] [CrossRef]
- Wang S, Dos-Santos ALA, Huang W, Liu KC, Oshaghi MA, Wei G, et al. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science (1979). 2017, 357, 1399–402. [Google Scholar]
- Bongio NJ, Lampe DJ. Inhibition of Plasmodium berghei development in mosquitoes by effector proteins secreted from Asaia sp. bacteria using a novel native secretion signal. PLoS One. 2015, 10, e0143541. [Google Scholar]
- De Freece C, Damiani C, Valzano M, D’Amelio S, Cappelli A, Ricci I, et al. Detection and isolation of the α-proteobacterium Asaia in Culex mosquitoes. Med Vet Entomol. 2014, 28, 438–42. [Google Scholar] [CrossRef]
- Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi , an Asian malarial mosquito vector. Proc Natl Acad Sci U S A. 2007, 104, 9047–51. [Google Scholar] [CrossRef]
- Maffo CGT, Sandeu MM, Fadel AN, Tchouakui M, Nguete DN, Menze B, et al. Molecular detection and maternal transmission of a bacterial symbiont Asaia species in field-caught Anopheles mosquitoes from Cameroon. Parasit Vectors. 2021, 14, 1–11. [Google Scholar]
- Rami A, Raz A, Zakeri S, Dinparast Djadid N. Isolation and identification of Asaia sp. in Anopheles spp. mosquitoes collected from Iranian malaria settings: Steps toward applying paratransgenic tools against malaria. Parasit Vectors. 2018, 11, 1–8. [Google Scholar]
- Zouache K, Raharimalala FN, Raquin V, Tran-Van V, Raveloson LHR, Ravelonandro P, et al. Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti , from different geographic regions of Madagascar. FEMS Microbiol Ecol. 2011, 75, 377–89. [Google Scholar] [CrossRef] [PubMed]
- Chouaia B, Rossi P, Montagna M, Ricci I, Crotti E, Damiani C, et al. Molecular evidence for multiple infections as revealed by typing of Asaia bacterial symbionts of four mosquito species. Appl Environ Microbiol. 2010, 76, 7444–50. [Google Scholar] [CrossRef] [PubMed]
- Boissière A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, et al. Midgut microbiota of the Malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012, 8, e1002742. [Google Scholar]
- Shane JL, Grogan CL, Cwalina C, Lampe DJ. Blood meal-induced inhibition of vector-borne disease by transgenic microbiota. Nat Commun. 2018, 9, 1–10. [Google Scholar]
- Carter V, Underhill A, Baber I, Sylla L, Baby M, Larget-Thiery I, et al. Killer bee molecules: Antimicrobial peptides as effector molecules to target sporogonic stages of Plasmodium. PLoS Pathog. 2013, 9, e1003790. [Google Scholar]
- Zhu S, Gao B, Aumelas A, del Carmen Rodríguez M, Lanz-Mendoza H, Peigneur S, et al. MeuTXKβ1, a scorpion venom-derived two-domain potassium channel toxin-like peptide with cytolytic activity. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2010, 1804, 872–83. [Google Scholar] [CrossRef] [PubMed]
- Pedron C, Silva A, Torres M, de Oliveira C, Andrade G, Cerchiaro G, et al. Net charge tuning modulates the antiplasmodial and anticancer properties of peptides derived from scorpion venom. J Pept Sci. 2021, 27, e3296. [Google Scholar] [CrossRef]
- Sánchez-Vásquez L, Silva-Sanchez J, Jiménez-Vargas JM, Rodríguez-Romero A, Muñoz-Garay C, Rodríguez MC, et al. Enhanced antimicrobial activity of novel synthetic peptides derived from vejovine and hadrurin. Biochim Biophys Acta Gen Subj. 2013, 1830, 3427–36. [Google Scholar] [CrossRef]
- Carballar-Lejarazú R, Rodríguez MH, De La Cruz Hernández-Hernández F, Ramos-Castañeda J, Possani LD, Zurita-Ortega M, et al. Recombinant scorpine: A multifunctional antimicrobial peptide with activity against different pathogens. Cellular and Molecular Life Sciences. 2008, 65, 3081–92. [Google Scholar] [CrossRef]
- Conde R, Zamudio FZ, Rodríguez MH, Possani LD. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000, 471, 165–8. [Google Scholar] [CrossRef]
- Zieler H, Keister DB, Dvorak JA. A snake venom phospholipase A2 blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J Exp Biol. 2001, 204, 4157–67. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues FG, Santos MN, De Carvalho TXT, Rocha BC, Riehle MA, Pimenta PFP, et al. Expression of a mutated phospholipase A2 in transgenic Aedes fluviatilis mosquitoes impacts Plasmodium gallinaceum development. Insect Mol Biol. 2008, 17, 175–83. [Google Scholar] [CrossRef] [PubMed]
- Smith RC, Vega-Rodríguez J, Jacobs-Lorena M. The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Mem Inst Oswaldo Cruz. 2014, 109, 644–61. [Google Scholar] [CrossRef]
- Delves M, Lafuente-Monasterio MJ, Upton L, Ruecker A, Leroy D, Gamo FJ, et al. Fueling open innovation for malaria transmission-blocking drugs: hundreds of molecules targeting early parasite mosquito stages. Front Microbiol. 2019, 10, 1–10. [Google Scholar]
- Lee MF, Wu YS, Poh CL. Molecular mechanisms of antiviral agents against Dengue virus. Viruses. 2023, 15, 1–37. [Google Scholar]
- Beard CB, O’Neill SL, Tesh RB, Richards FF, Aksoy S. Modification of arthropod vector competence via symbiotic bacteria. Parasitology Today. 1993, 9, 179–83. [Google Scholar] [CrossRef]
- Dias EHV, de Sousa Simamoto BB, da Cunha Pereira DF, Ribeiro MSM, Santiago FM, de Oliveira F, et al. Effect of BaltPLA2, a phospholipase A2 from Bothrops alternatus snake venom, on the viability of cells infected with dengue virus. Toxicology in Vitro. 2023, 88, 1–6. [Google Scholar]
- Cecilio AB, Caldas S, De Oliveira RA, Santos ASB, Richardson M, Naumann GB, et al. Molecular characterization of Lys49 and Asp49 phospholipases A2 from snake venom and their antiviral activities against Dengue virus. Toxins (Basel). 2013, 5, 1780–98. [Google Scholar] [CrossRef]
- Brenes H, Loría GD, Lomonte B. Potent virucidal activity against Flaviviridae of a group IIA phospholipase A2 isolated from the venom of Bothrops asper. Biologicals. 2020, 63, 48–52. [Google Scholar] [CrossRef]
- Cassani NM, Santos IA, Grosche VR, Ferreira GM, Guevara-Vega M, Rosa RB, et al. Roles of Bothrops jararacussu toxins I and II: Antiviral findings against Zika virus. Int J Biol Macromol. 2023, 227, 630–40. [Google Scholar] [CrossRef]
- Ayusso GM, Lima MLD, da Silva Sanches PR, Santos IA, Martins DOS, da Conceição PJP, et al. The dimeric peptide (KKYRYHLKPF)2K shows broad-spectrum antiviral activity by inhibiting different steps of chikungunya and zika virus infection. Viruses. 2023, 15, 1–17. [Google Scholar]
- Muller VD, Soares RO, Santos-Junior NN Dos, Trabuco AC, Cintra AC, Figueiredo LT, et al. Phospholipase A2 isolated from the venom of Crotalus durissus terrificus inactivates dengue virus and other enveloped viruses by disrupting the viral envelope. PLoS One. 2014, 9, e112351. [Google Scholar]
- Muller VDM, Russo RR, Oliveira Cintra AC, Sartim MA, De Melo Alves-Paiva R, Figueiredo LTM, et al. Crotoxin and phospholipases A 2 from Crotalus durissus terrificus showed antiviral activity against dengue and yellow fever viruses. Toxicon. 2012, 59, 507–15. [Google Scholar] [CrossRef] [PubMed]
- Russo RR, dos Santos Júnior NN, Cintra ACO, Figueiredo LTM, Sampaio SV, Aquino VH. Expression, purification and virucidal activity of two recombinant isoforms of phospholipase A 2 from Crotalus durissus terrificus venom. Arch Virol. 2019, 164, 1159–71. [Google Scholar] [CrossRef] [PubMed]
- Santos IA, Shimizu JF, de Oliveira DM, Martins DOS, Cardoso-Sousa L, Cintra ACO, et al. Chikungunya virus entry is strongly inhibited by phospholipase A2 isolated from the venom of Crotalus durissus terrificus. Sci Rep. 2021, 11, 1–12. [Google Scholar]
- Chen M, Aoki-Utsubo C, Kameoka M, Deng L, Terada Y, Kamitani W, et al. Broad-spectrum antiviral agents: secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci Rep. 2017, 7, 1–8. [Google Scholar]
- Miyashita M, Mitani N, Kitanaka A, Yakio M, Chen M, Nishimoto S, et al. Identification of an antiviral component from the venom of the scorpion Liocheles australasiae using transcriptomic and mass spectrometric analyses. Toxicon. 2021, 191, 25–37. [Google Scholar] [CrossRef]
- Xing M, Ji M, Hu J, Zhu T, Chen Y, Bai X, et al. Snake cathelicidin derived peptide inhibits zika virus infection. Front Microbiol. 2020, 11, 1–11. [Google Scholar]
- Ji Z, Li F, Xia Z, Guo X, Gao M, Sun F, et al. The scorpion venom peptide Smp76 inhibits viral infection by regulating Type-I interferon response. Virol Sin. 2018, 33, 545–56. [Google Scholar] [CrossRef]
- El-Bitar AMH, Sarhan M, Abdel-Rahman MA, Quintero-Hernandez V, Aoki-Utsubo C, Moustafa MA, et al. Smp76, a scorpine-Like peptide isolated from the venom of the scorpion Scorpio maurus palmatus , with a potent antiviral activity against Hepatitis C virus and Dengue virus. Int J Pept Res Ther. 2020, 26, 811–21. [Google Scholar] [CrossRef]
- Li F, Lang Y, Ji Z, Xia Z, Han Y, Cheng Y, et al. A scorpion venom peptide Ev37 restricts viral late entry by alkalizing acidic organelles. Journal of Biological Chemistry. 2019, 294, 182–94. [Google Scholar] [CrossRef] [PubMed]
- Flipse J, Wilschut J, Smit JM. Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic. 2013, 14, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Fox JM, Pierson TC. Chikungunya virus assembly and egress. Nat Microbiol. 2022, 7, 1112–3. [Google Scholar] [CrossRef] [PubMed]
- Ji M, Zhu T, Xing M, Luan N, Mwangi J, Yan X, et al. An antiviral peptide from Alopecosa nagpag spider targets NS2B-NS3 protease of flaviviruses. Toxins (Basel). 2019, 11, 584. [Google Scholar] [CrossRef]

| Venom Effector | Mosquito Species | Vector Symbiont | Effect | Ref |
|---|---|---|---|---|
| Scorpine | An. stephensi | Asaia bogorensis | 63% reduction in oocyst number of Plasmodium berghei | [29] |
| Scorpine | An. stephensi | Asaia bogorensis | 90% reduction in oocyst number of Plasmodium berghei | [37] |
| mPLA2 | An. stephensi | Escherichia coli | 23% reduction in oocyst number of Plasmodium berghei | [26] |
| Scorpine & mPLA2 | An.gambiea An. stephensi | Pantoea agglomerans | 97.8% reduction (scorpine) and 85.3% reduction (mPLA2) in oocyst number of Plasmodium falciparum | [27] |
| Scorpine & mPLA2 | An. gambiae | Serratia marcescens | 93% reduction (scorpine) and 86% reduction (mPLA2) in oocyst number of Plasmodium falciparum | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





