Submitted:
29 November 2024
Posted:
29 November 2024
You are already at the latest version
Abstract
The term “photocatalysis” has recently gained high popularity, and various products using photocatalytic functions have been commercialized. Of all the materials that may be used as photocatalysts, titanium dioxide (TiO2) is virtually the only one that is now and most likely will remain appropriate for industrial application. Water and air purification systems, sterilization, hydrogen evolution, self-cleaning surfaces, and photoelectrochemical conversion are just a few of the products and applications in the environmental and energy domains that make extensive use of TiO2 photocatalysis. This is due to the fact that TiO2 has the lowest cost, most stability, and most effective photoactivity. Furthermore, history attests to its safety for both people and the environment because it has been used as a white pigment since antiquity. This review discusses some important aspects and issues concerning different synthesis methods and their influence on the structure and properties of TiO2, as well as the concept of photocatalysis based on it as a promising biocompatible functional material that has been widely used in recent years. The advantages of TiO2 applications in various fields of science and technology are discussed, including environmental protection, photocatalysis including self-cleaning surfaces, water and air purification systems, hydrogen liberation, photovoltaic energy, cancer diagnosis and therapy, coatings and dental products, etc. Information on the structure and properties of TiO2 phases is presented, as well as modern methods of synthesizing functional materials based on it. A detailed review of the basic principles of TiO2 photocatalysis is then given, with a brief introduction to the modern concept of TiO2 photocatalysis. Recent advances in the fundamental understanding of TiO2 photocatalysis at the atomic-molecular level are highlighted, and advances in TiO2 photocatalysis from the perspective of design and engineering of new materials are discussed. The challenges and prospects of TiO2 photocatalysis are briefly discussed.
Keywords:
1. Introduction
2. TiO2, Synthesis, Characterization and Properties
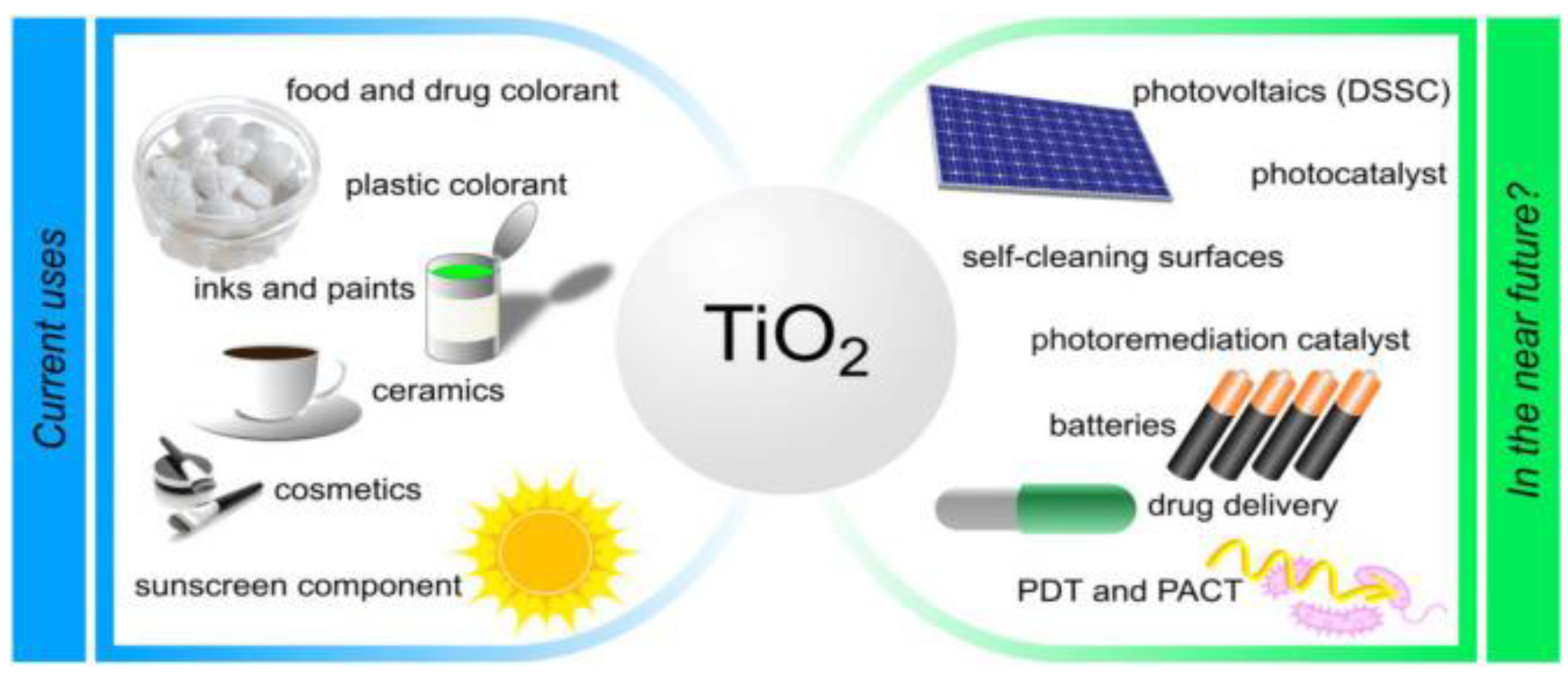
2.1. Brief Introduction and Applications of TiO2
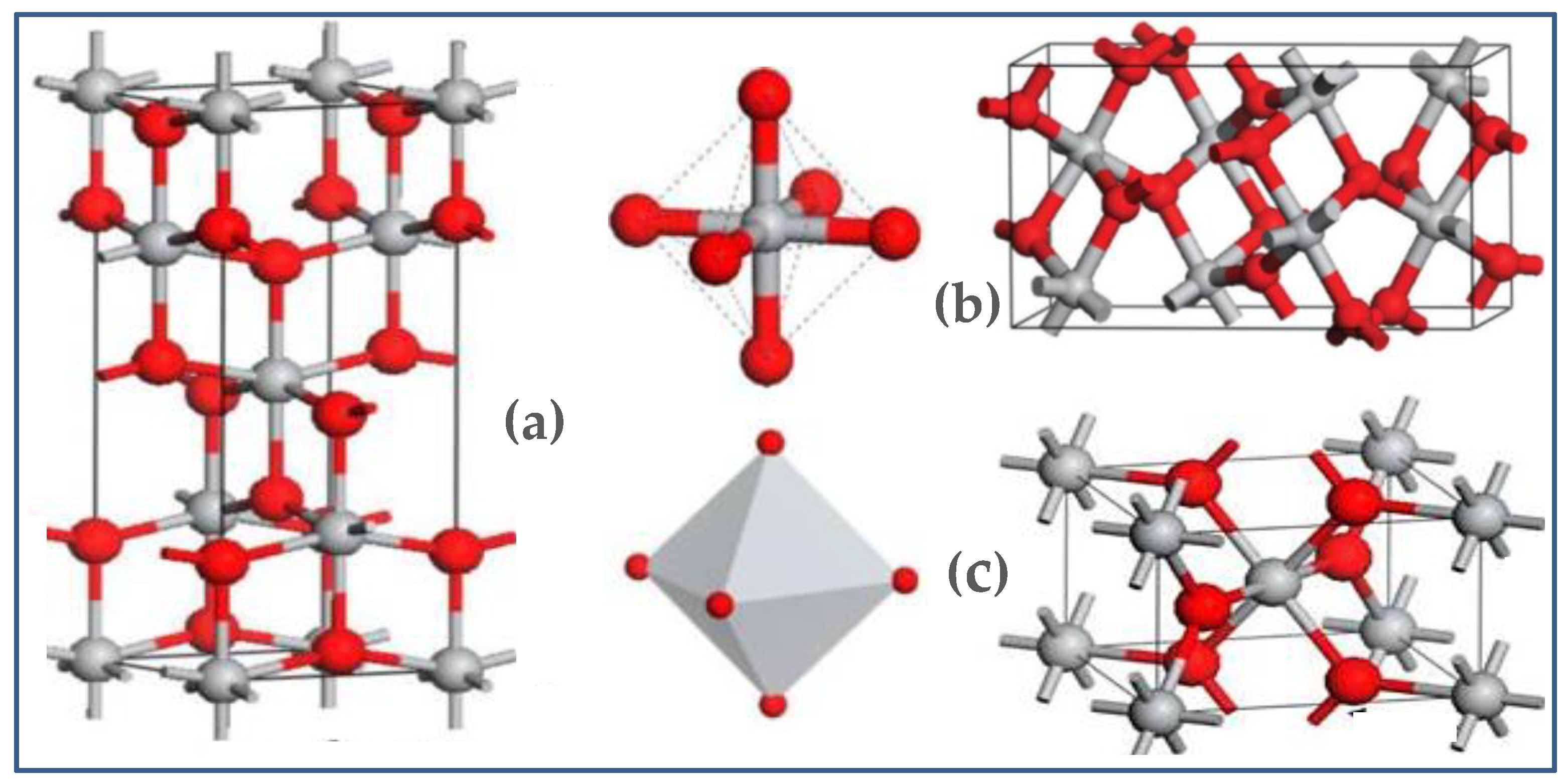
2.2. Crystal Structures of TiO2
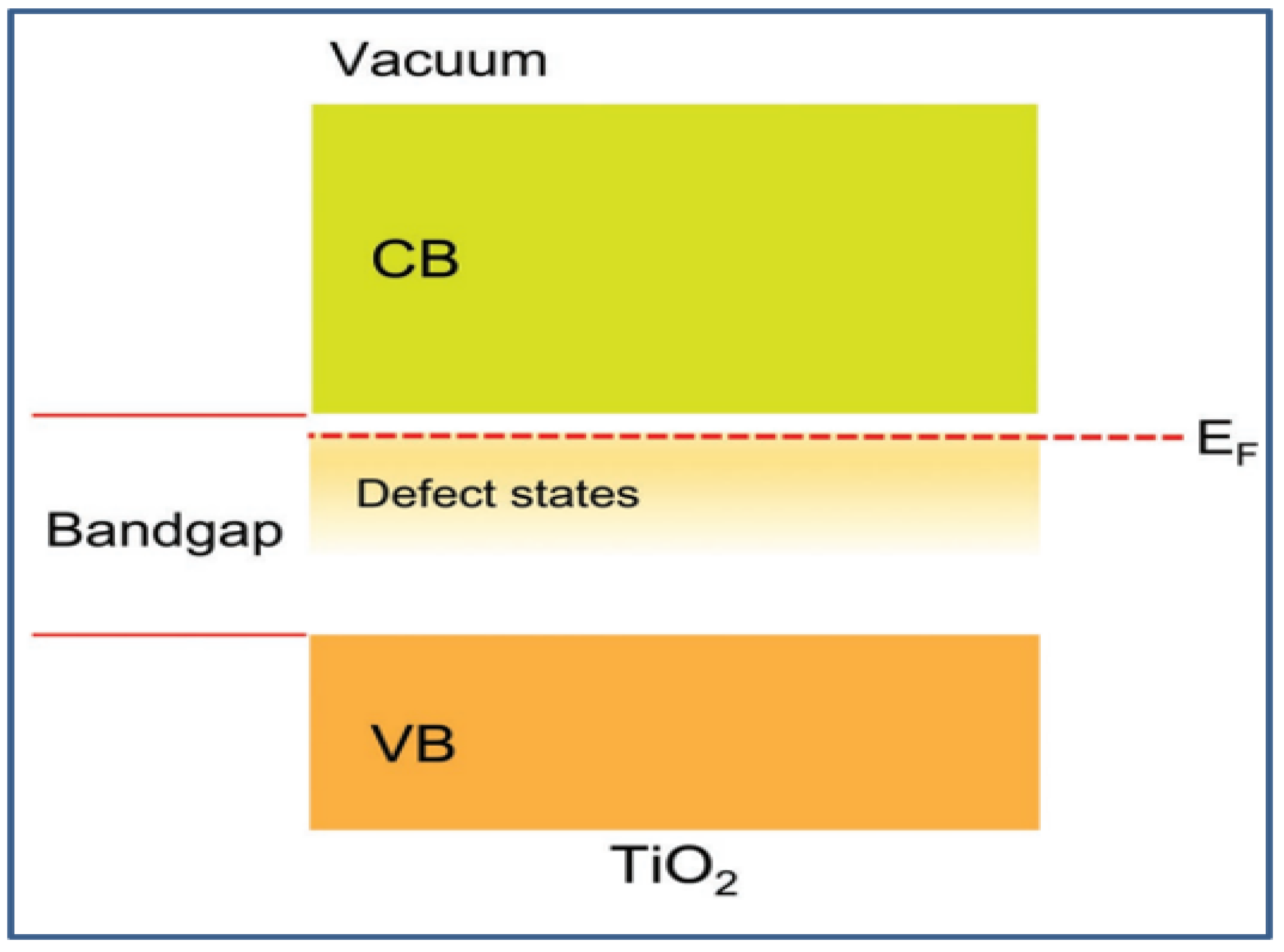
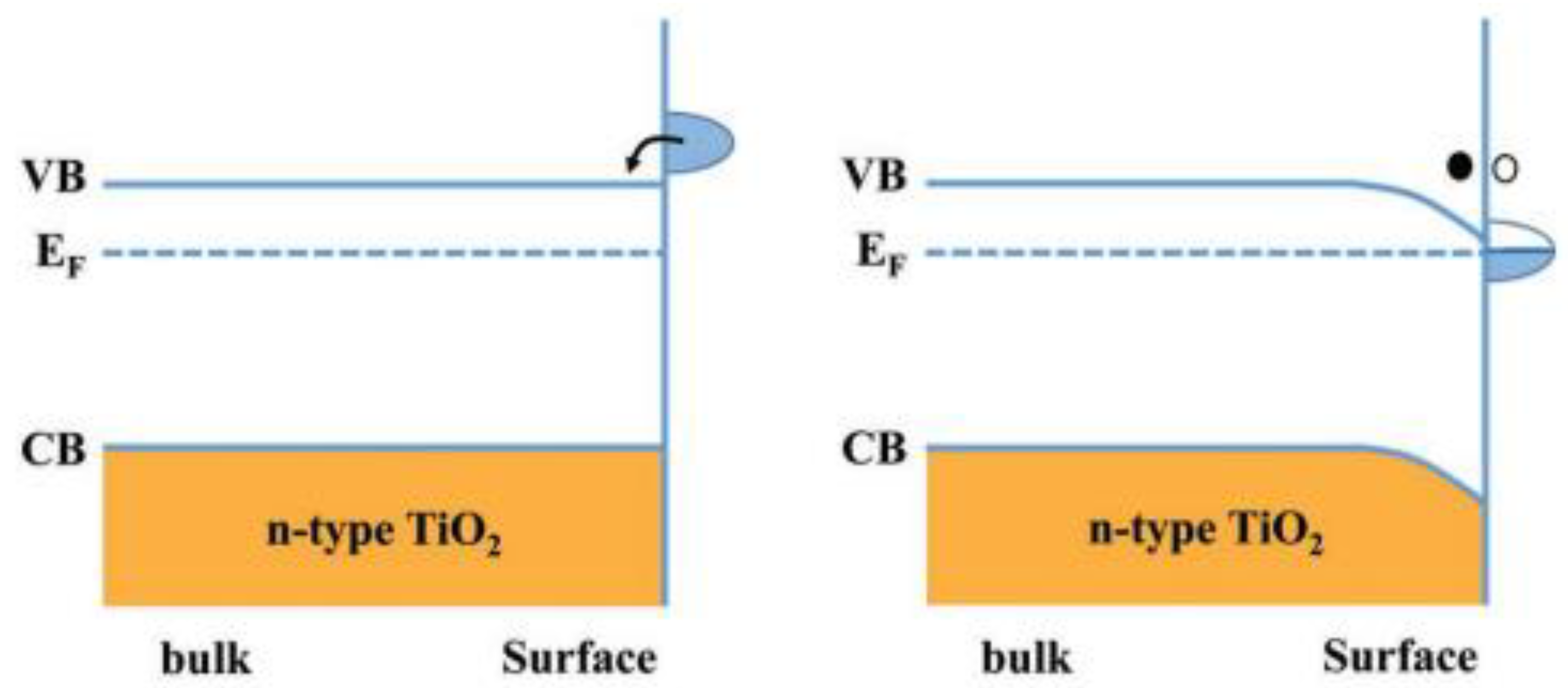
2.3. Electronic Properties and Band Structure
2.4. Optical Properties of TiO2
3. Efficient Methods OF TiO2 Synthesis
3.1. Hydrothermal Method of TiO2 Preparation
3.2. Solvothermal Synthesis of TiO2
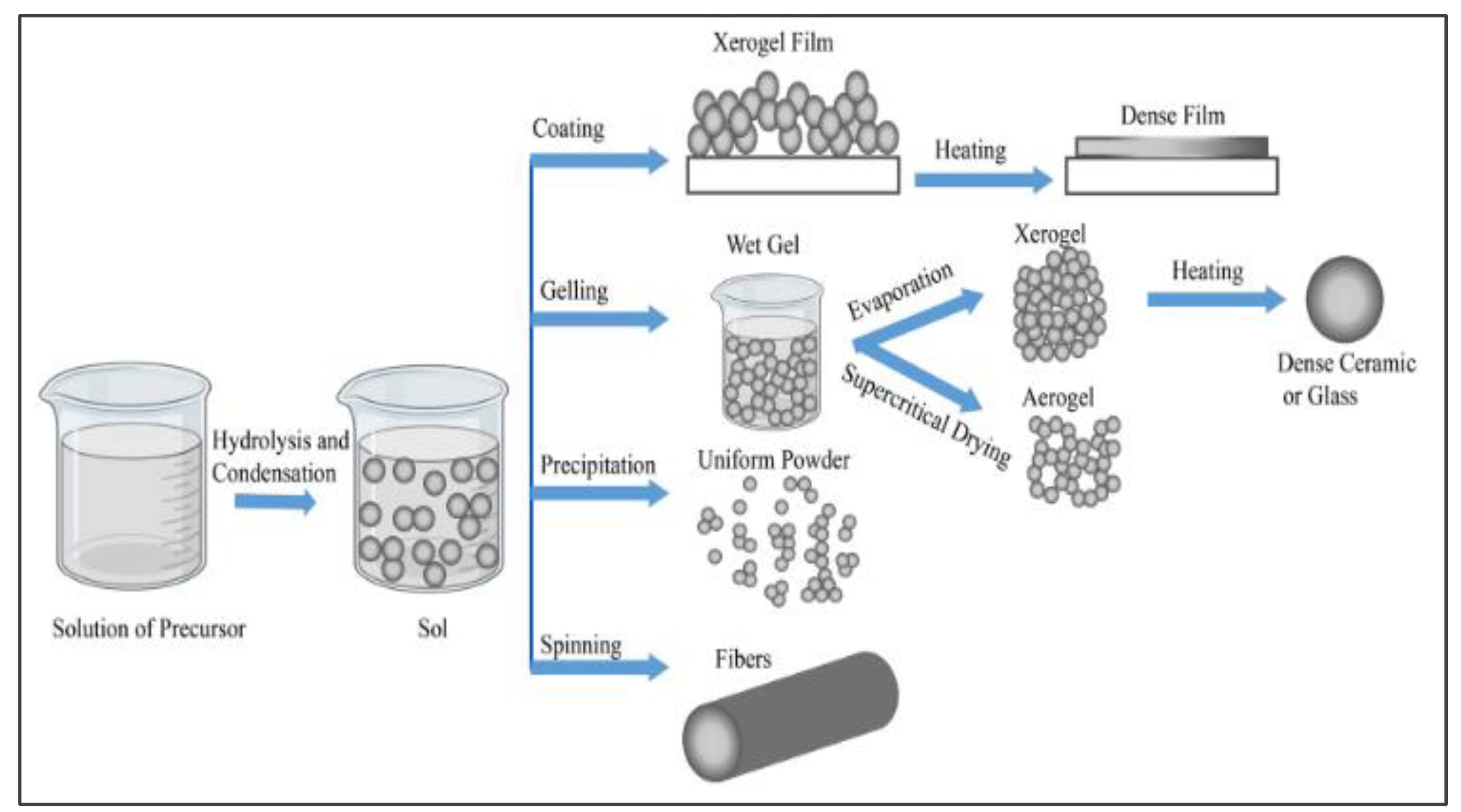
3.3. Sol-gel Method of TiO2 Production
3.4. Sonochemical and Microwave-assisted Methods of TiO2 Synthesis
3.5. Synthesis of TiO2 by Oxidation Method
3.6. Synthesis of TiO2 by Chemical Vapor Deposition (CVD)
3.7. Green Synthesis of TiO2
3.8. Electrodeposition and Ionic Liquid-assisted Methods
3.9. Synthesis of Nanoscale and Thin Film Structures of TiO2
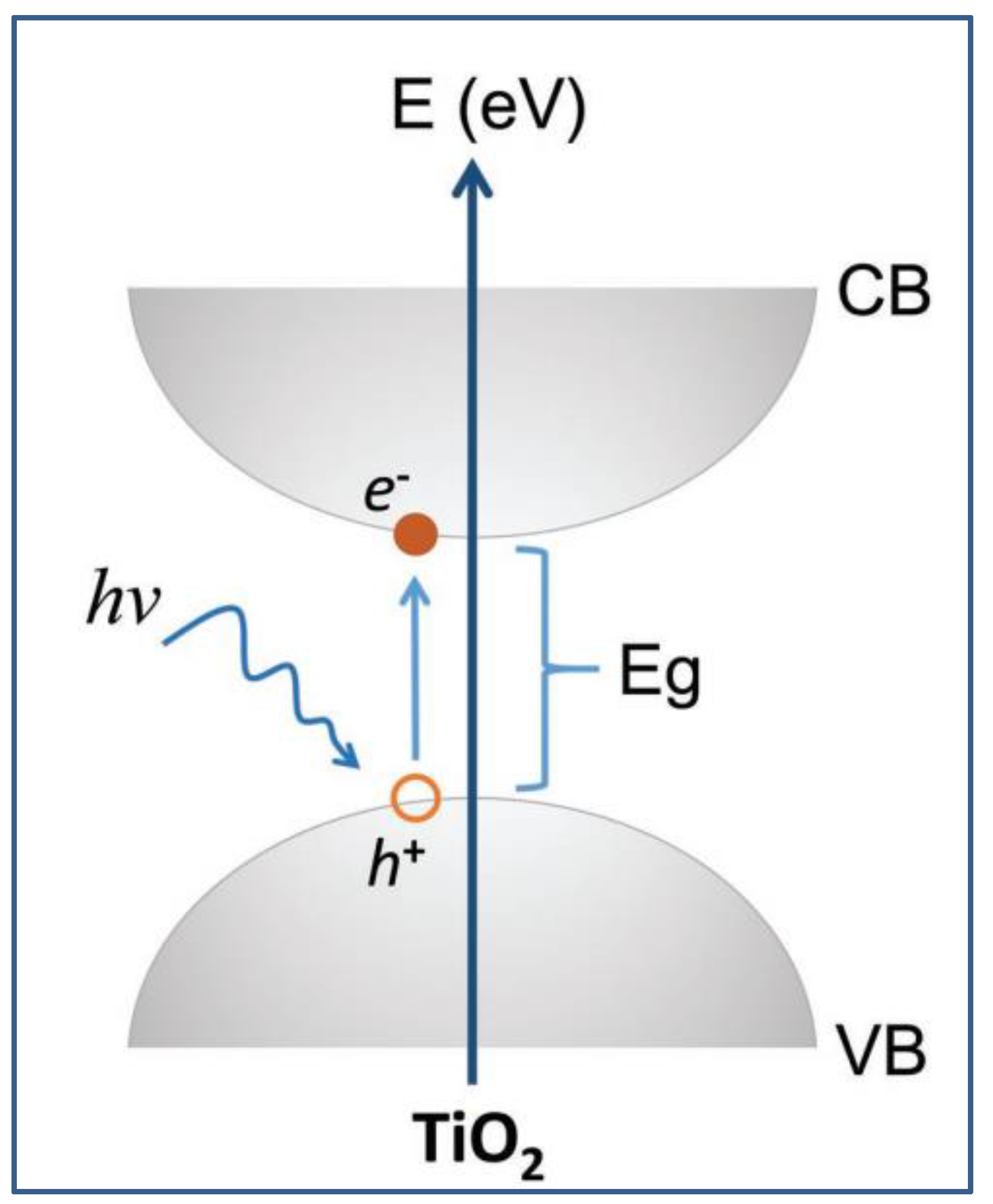
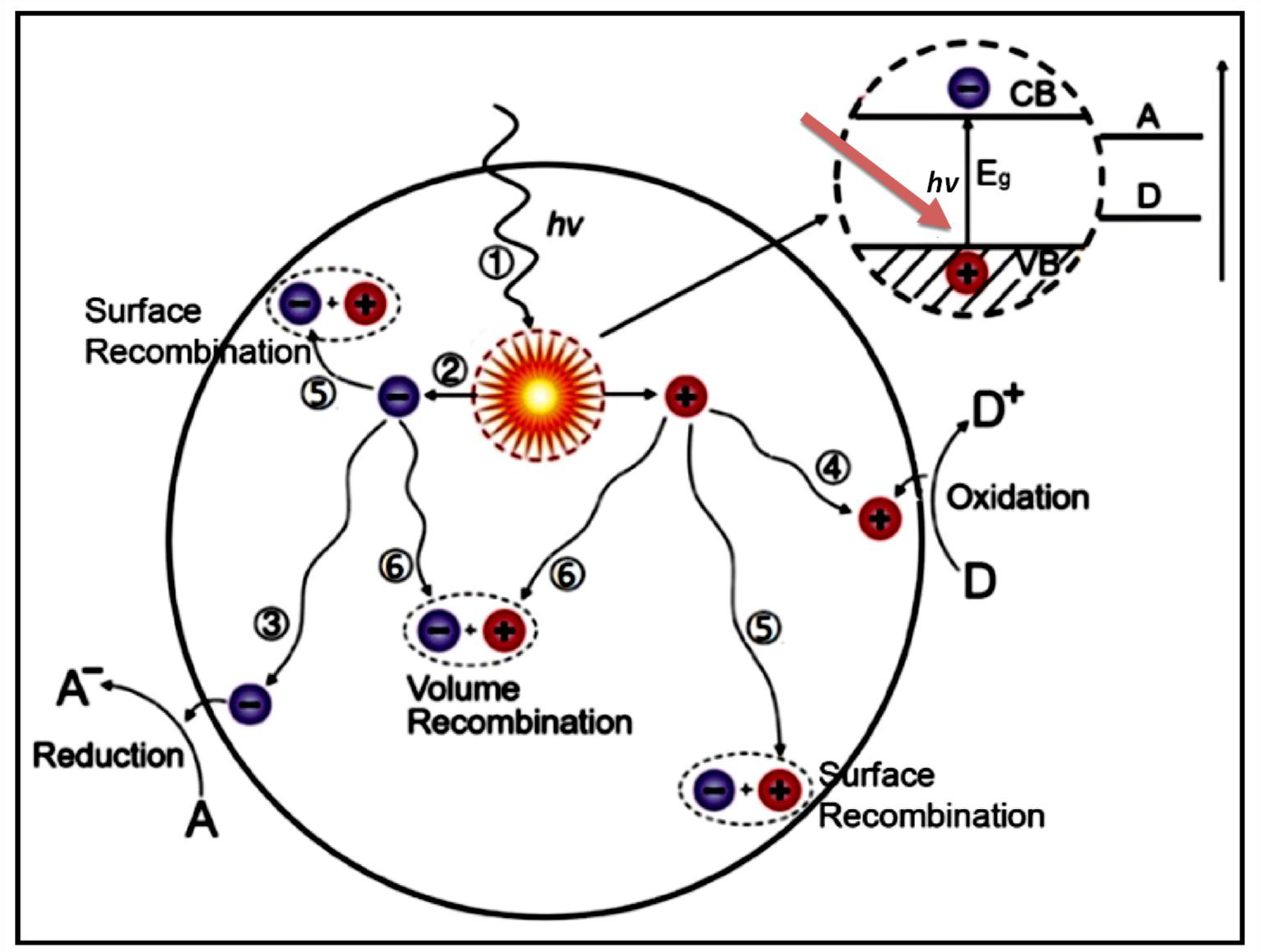
4. Concept of Photocatalysis Using TiO2
5. Growing Interest in the Application Of TiO2 Photocatalysis
5.1. Purification of Water and Air from Organic Pollutants
5.2. TiO2 and Water Photolysis
5.3. Treatment of Water from Inorganic Compounds
5.4. Medical Applications of TiO2
5.5. Photocatalytic Reduction of CO2
6. Current Challenges
7. Opportunities
8. Summary and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviation List
References
- Wang J, Azam W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geosci Front 2024, 15, 101757. [Google Scholar] [CrossRef]
- Schneider J, Matsuoka M, Takeuchi M et al. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem Rev 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Xuan VN, Hoa PX, Thu NTP et al. Factors affecting environmental pollution for green economy: The case of ASEAN countries. Environ Chall 2024, 14, 100827. [Google Scholar] [CrossRef]
- Kabir M, Habiba UE, Khan W et al. Climate change due to increasing concentration of carbon dioxide and its impacts on environment in 21st century; a mini review. J King Saud Univ Sci 2023, 35, 102693. [Google Scholar] [CrossRef]
- Bordet A, Leitner W. Adaptive Catalytic Systems for Chemical Energy Conversion. Angew Chem Int Edit 2023, 62, e202301956. [Google Scholar] [CrossRef]
- Hassan Q, Tabar V, Sameen AZ et al. A review of green hydrogen production based on solar energy; techniques and methods. Energ Harvesting Sys 2024, 11, 20220134. [Google Scholar] [CrossRef]
- Dang VH, Nguyen TA, Le MV et al. Photocatalytic hydrogen production from seawater splitting: Current status, challenges, strategies and prospective applications. Chem Eng J 2024, 484, 149213. [Google Scholar] [CrossRef]
- Zhang C, Li N, An G. Review of Concentrated Solar Power Technology Applications in Photocatalytic Water Purification and Energy Conversion: Overview, Challenges and Future Directions. Energies, 2024, 17, 463. [Google Scholar] [CrossRef]
- Mohtaram S, Mohtaram MS, Sabbaghi S et al. Enhancement strategies in CO2 conversion and management of biochar supported photocatalyst for effective generation of renewable and sustainable solar energy. Energ Convers Manage 2024, 300, 117987. [Google Scholar] [CrossRef]
- Worku AK, Ayele DW, Deepak DB et al. Recent Advances and Challenges of Hydrogen Production Technologies via Renewable Energy Sources. Adv Energy Sustain Res 2024, 5, 2300273. [Google Scholar] [CrossRef]
- Kumar A, Rana S, Dhiman P et al. Current progress in heterojunctions based on Nb2O5 for photocatalytic water treatment and energy applications. J Mol Liq 2024, 399, 124360. [Google Scholar] [CrossRef]
- Albini A, Fagnoni M. 1908, Giacomo Ciamician and the Concept of Green Chemistry. Chem Sus Chem 2008, 1, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu S, Wang D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv Energy Mater 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Bruner L, Kozak J. On the knowledge of photocatalysis. I. The light reaction in mixtures: uranium salt + oxalic acid [In German]. J Electroche Appl Phys Chem 1911, 17, 354–360. [Google Scholar]
- Eibner, A. Action of light on pigments I. Chem-Ztg 1911, 35, 753–755. [Google Scholar]
- Baur E, Perret A. On the effect of light on dissolved silver salts in the presence of zinc oxide [In German]. Helv Chim Acta 1924, 7, 910–915. [Google Scholar]
- Renz, C. On the effect of oxides on silver nitrate and gold chloride in light [In German]. Helv Chim Acta 1932, 15, 1077–1084. [Google Scholar] [CrossRef]
- Goodeve CF, Kitchener JA. The mechanism of photosensitisation by solids. Trans Faraday Soc 1938, 34, 902. [Google Scholar] [CrossRef]
- Ravelli D, Dondi D, Fagnoni M et al. Photocatalysis. A multi-faceted concept for green chemistry. Chem Soc Rev 2009, 38, 1999. [Google Scholar] [CrossRef]
- Lotfabadi, P. Analyzing passive solar strategies in the case of high-rise building. Renew Sust Energ Rev 2015, 52, 1340–1353. [Google Scholar] [CrossRef]
- Boddy, PJ. Oxygen Evolution on Semiconducting TiO2. J Electrochem Soc 1968, 115, 199. [Google Scholar] [CrossRef]
- Fujishima A, Honda K. Electroсhemicаl photolysisof water at a sеmiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tuntithavornwat S, Saisawang C, Ratvijitvech T et al. Recent development of black TiO2 nanoparticles for photocatalytic H2 production: An extensive review. Int J Hydrogen Energ 2024, 55, 1559–1593. [Google Scholar] [CrossRef]
- Schrauzer G, Guth T. Photolysis of water and photoreduction of nitrogen on titanium dioxide. J Am Chem Soc 2002, 99, 7189–7193. [Google Scholar]
- Aldosari OF, Hussain I. Unlocking the potential of TiO2-based photocatalysts for green hydrogen energy through water-splitting: Recent advances, future perspectives and techno feasibility assessment. Int J Hydrogen Energ 2024, 59, 958–981. [Google Scholar] [CrossRef]
- Dai W, Wang H, Xiao M et al. Visible Photocatalytic Hydrogen Production from CH3OH Over CuO/Wo3, The Effect of Electron Transfer Behavior of the Adsorbed CH3OH. Chem Eng J 2023, 459, 141616. [Google Scholar] [CrossRef]
- Arora I, Garg S, Sapi A et al. Insights into photocatalytic CO2 reduction reaction pathway: Catalytic modification for enhanced solar fuel production. J Ind Eng Chem 2024, 137, 1–28. [Google Scholar] [CrossRef]
- Alli YA, Oladoye PO, Onawole AT et al. Photocatalysts for CO2 reduction and computational insights. Fuel 2023, 344, 128101. [Google Scholar] [CrossRef]
- Chen Z, Zhang G, Cao S et al. Advanced semiconductor catalyst designs for the photocatalytic reduction of CO2. Mat R: Energy 2023, 3, 100193. [Google Scholar]
- Fang S, Rahaman M, Bharti J et al. Photocatalytic CO2 reduction. Nature Reviews Methods Primers 2023, 3, 61. [Google Scholar] [CrossRef]
- Wang Z, Seo J, Hisatomi T et al. Efficient visible-light-driven water oxidation by single-crystal Ta3N5 nanoparticles. Nano Res 2023, 16, 4562–4567. [Google Scholar] [CrossRef]
- Zhan X, Lei T, Wang L et al. Enhanced photocatalytic removal of tetracycline and methyl orange using Ta3N5@ZnIn2S4 nanocomposites. J Photoch Photobio A 2024, 451, 115538. [Google Scholar]
- Zhao T, Ye Z, Zeng M et al. Molten Salt Synthesis of Mg-Doped Ta3N5 Nanoparticles with Optimized Surface Properties for Enhanced Photocatalytic Hydrogen Evolution. Energy Fuels 2023, 37, 18194–18203. [Google Scholar] [CrossRef]
- Liu Z, Fan S, Li X et al. Synergistic effect of single-atom Cu and hierarchical polyhedron-like Ta3N5/CdIn2S4 S-scheme heterojunction for boosting photocatalytic NH3 synthesis. Appl Catal B-Environ 2023, 327, 122416. [Google Scholar] [CrossRef]
- Dong Y, Ai F, Sun-Waterhouse D et al. Optical and Photocatalytic Properties of Three-Dimensionally Ordered Macroporous Ta2O5 and Ta3N5 Inverse Opals. Chem Mater 2023, 35, 8281–8300. [Google Scholar] [CrossRef]
- Li S, Cai M, Wang C et al. Ta3N5/CdS Core–Shell S-scheme Heterojunction Nanofibers for Efficient Photocatalytic Removal of Antibiotic Tetracycline and Cr(VI): Performance and Mechanism Insights. Adv Fiber Mater 2023, 5, 994–1007. [Google Scholar] [CrossRef]
- Zhao T, Ye Z, Zeng M et al. Molten Salt Synthesis of Mg-Doped Ta3N5 Nanoparticles with Optimized Surface Properties for Enhanced Photocatalytic Hydrogen Evolution. Energy Fuels 2023, 37, 18194–18203. [Google Scholar] [CrossRef]
- Akter J, Hanif MdA, Islam MdA et al. Visible-light-active novel α-Fe2O3/Ta3N5 photocatalyst designed by band-edge tuning and interfacial charge transfer for effective treatment of hazardous pollutants. J Env Chem Eng 2021, 9, 106831. [Google Scholar] [CrossRef]
- Jia X, Wang C, Li Y et al. All-Solid-State Z-scheme Ta3N5/Bi/CaTaO2N photocatalyst transformed from perovskite CaBi2Ta2O9 for efficient overall water splitting. Chem Eng J 2022, 431, 134041. [Google Scholar] [CrossRef]
- Hussain E, Ishaq A, Abid MZ et al. Sunlight-Driven Hydrogen Generation: Acceleration of Synergism between Cu-Ag Cocatalysts on a CdS System. ACS Appl Energ Mater 2024, 7, 1914–1926. [Google Scholar] [CrossRef]
- Li M, Zhang J, Wang L et al. Direct Z-Scheme Oxygen-vacancy-rich TiO2/Ta3N5 heterojunction for degradation of ciprofloxacin under visible light: Degradation pathways and mechanism insight. Appl Surf Sci 2022, 583, 152516. [Google Scholar] [CrossRef]
- Lim KL, Sin JC, Lam SM et al. Controlled solvothermal synthesis of self-assembled SrTiO3 microstructures for expeditious solar-driven photocatalysis dye effluents degradation. Environ Res 2024, 251, 118647. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel S, Paramasivam S, Velusamy P et al. Experimental investigation of structural, morphological, and optical characteristics of SrTiO3 nanoparticles using a shock tube for photocatalytic applications. Z Phys Chem.
- Liu M, Xu X, Xiao W et al. Au nanoparticles decorated SrTiO3-x hollow structure for plasmatic enhanced hydrogen production in UV-visible and near-infrared region. J Alloy Compd 2024, 983, 173859. [Google Scholar] [CrossRef]
- Zhuang H, Chen X, Xia J et al. State-of-the-art progress in Ag3PO4-based photocatalysts: Rational design, regulation and perspective. Appl Mater Today 2023, 31, 101742. [Google Scholar] [CrossRef]
- Hamrouni A, Azzouzi H, Rayes A et al. Enhanced Solar Light Photocatalytic Activity of Ag Doped TiO2-Ag3PO4 Composites. Nanomaterials-Basel 2020, 10, 795. [Google Scholar] [CrossRef]
- Madanu TL, Mouchet SR, Deparis O et al. Tuning and transferring slow photons from TiO2 photonic crystals to BiVO4 nanoparticles for unprecedented visible light photocatalysis. J Colloid Interf Sci 2023, 634, 290–299. [Google Scholar] [CrossRef]
- Heckel S, Wittmann M, Reid M et al. An Account on BiVO4 as Photocatalytic Active Matter. Accounts Mater Res 2024, 5, 400–412. [Google Scholar] [CrossRef]
- Zhang X, Puttaswamy M, Bai H et al. CdS/ZnS core-shell nanorod heterostructures co-deposited with ultrathin MoS2 cocatalyst for competent hydrogen evolution under visible-light irradiation. J Colloid Interf Sci 2024, 665, 430–442. [Google Scholar] [CrossRef]
- Suresh M, Pravina R, Sivasamy A. Facile synthesis of MoS2 nanoparticles anchored graphene nanocomposite for enhanced photocatalytic activity under solar irradiation. J Mol Struct 2024, 1305, 137760. [Google Scholar] [CrossRef]
- Panchal D, Sharma A, Pal S. Engineered MoS2 nanostructures for improved photocatalytic applications in water treatment. Mater Today Sustain 2023, 21, 100264. [Google Scholar]
- Balan B, Xavier MM, Mathew S. MoS2 - Based Nanocomposites for Photocatalytic Hydrogen Evolution and Carbon Dioxide Reduction. ACS Omega 2023, 8, 25649–25673. [Google Scholar] [CrossRef] [PubMed]
- Xu Y, Liu K, Zhang J et al. NH4Cl-assisted synthesis of TaON nanoparticle applied to photocatalytic hydrogen and oxygen evolution from water. J Energy Chem 2024, 94, 541–550. [Google Scholar] [CrossRef]
- Jiang H, Shi Y, Zang S. Pd/PdO and hydrous RuO2 difunction-modified SiO2@TaON@Ta3N5 nano-photocatalyst for efficient solar overall water splitting. Int J Hydrogen Energ 2023, 48, 17827–17837. [Google Scholar] [CrossRef]
- Maekawa T, Huang Y, Tateishi N et al. Slow photon photocatalytic enhancement of H2 production in TaON inverse opal photonic crystals. J Solid State Chem 2024, 329, 124404. [Google Scholar] [CrossRef]
- Qu H, Yang S, Zhou X. Theoretical insights into the effect of surface structure and anion ordering on the properties of SrTaO2N for photocatalytic water splitting. Int J Quantum Chem 2024, 124, e27321. [Google Scholar] [CrossRef]
- Tao X, Tsugawa T, Hatakeyama K et al. Synthesis and photocatalytic activity of LaTiO2N using titanium oxide nanosheet/La3+ hybrids as a precursor. Sustain Energ Fuels 2024, 8, 1269–1279. [Google Scholar] [CrossRef]
- Xia Z, Wang X, Liu K et al. Preparation and photocatalytic properties of WSe2/BiVO4 p-n heterojunction photocatalytic materials. Catal Commun 2024, 187, 106857. [Google Scholar] [CrossRef]
- Rengifo-Herrera J, Pulgarin C. Why five decades of massive research on heterogeneous photocatalysis, especially on TiO2, has not yet driven to water disinfection and detoxification applications? Critical review of drawbacks and challenges. Chem Eng J 2023, 477, 146875. [Google Scholar] [CrossRef]
- Sohail M, Rauf S, Irfan M et al. Recent developments, advances and strategies in heterogeneous photocatalysts for water splitting. Nanoscale Adv 2024, 6, 1286–1330. [Google Scholar] [CrossRef]
- Qahtan TF, Owolabi TO, Olubi OE et al. State-of-the-art, challenges and prospects of heterogeneous tandem photocatalysis. Coordin Chem Rev 2023, 492, 215276. [Google Scholar] [CrossRef]
- Lotfi S, Ouardi ME, Ahsaine HA et al. Recent progress on the synthesis, morphology and photocatalytic dye degradation of BiVO4 photocatalysts: A review. Catal Rev 2024, 66, 214–258. [Google Scholar] [CrossRef]
- Low J, Zhang C, Ma J et al. Heterogeneous photocatalysis: what is being overlooked? Trends Chem 2023, 5, 121–132. [Google Scholar] [CrossRef]
- Okab AA, Jabbar ZH, Graimed BH et al. A comprehensive review highlights the photocatalytic heterojunctions and their superiority in the photo-destruction of organic pollutants in industrial wastewater. Inorg Chem Commun 2023, 158, 111503.
- Sohail M, Rauf S, Irfan M et al. Recent developments, advances and strategies in heterogeneous photocatalysts for water splitting. Nanoscale Adv 2024, 6, 1286–1330. [Google Scholar] [CrossRef] [PubMed]
- Masri M, Girisha KB, Hezam A et al. Metal halide perovskite-based photocatalysts for organic pollutants degradation: Advances, challenges, and future directions. Colloid Surface A 2024, 687, 133387. [Google Scholar] [CrossRef]
- Baaloudj O, Vu N, Assadi A et al. Recent advances in designing and developing efficient sillenite-based materials for photocatalytic applications. Adv Colloid Interface Sci 2024, 327, 103136. [Google Scholar] [CrossRef]
- Kumar A, Rana S, Dhiman P et al. Current progress in heterojunctions based on Nb2O5 for photocatalytic water treatment and energy applications. J Mol Liq 2024, 399, 124360. [Google Scholar] [CrossRef]
- Anucha CB, Altin I, Bacaksiz E et al. Titanium dioxide (TiO₂)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem Eng J Adv 2022, 10, 100262. [Google Scholar] [CrossRef]
- Dharma HNC, Jaafar J, Widiastuti N et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes-Basel 2022, 12, 345. [Google Scholar] [CrossRef]
- Gatou M, Syrrakou A, Lagopati N et al. Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review. Reactions-Basel 2024, 5, 135–194. [Google Scholar] [CrossRef]
- Sangeeta O, Sandhu N, Kumar C et al. Critical Review on Titania-Based Nanoparticles: Synthesis, Characterization, and Application as a Photocatalyst. Chem Afr 2024, 7, 1749–1768. [Google Scholar] [CrossRef]
- Rashid A, Hossain KT, Mondal S et al. Synthesis, characterization, and photocatalytic performance of methyl orange in aqueous TiO2 suspension under UV and solar light irradiation. S Afr J Chem Eng 2022, 40, 113–125. [Google Scholar]
- Ahmadpour N, Nowrouzi M, Madadi Avargani V et al. Design and optimization of TiO2-based photocatalysts for efficient removal of pharmaceutical pollutants in water: Recent developments and challenges. J Water Process Eng 2024, 57, 104597. [Google Scholar] [CrossRef]
- Yao Y, Han Y, Qi S et al. Metal-free thiophene-based covalent organic frameworks achieve efficient photocatalytic hydrogen evolution by accelerating interfacial charge transfer. Int J Hydrogen Energ 2024, 63, 184–192. [Google Scholar] [CrossRef]
- Liu X, Jing X, Liu R et al. Plasmon-Enhanced Perovskite Photocatalysts for CO2 Reduction: A Mini Review. Energ Fuel 2024, 38, 4966–4679. [Google Scholar] [CrossRef]
- Rawat J, Sharma H, Dwivedi C. Microwave-assisted synthesis of carbon quantum dots and their integration with TiO2 nanotubes for enhanced photocatalytic degradation. Diam Relat Mater 2024, 144, 111050. [Google Scholar] [CrossRef]
- Puri N, Gupta A. Water remediation using titanium and zinc oxide nanomaterials through disinfection and photo catalysis process: A review. Environ Res 2023, 227, 115786. [Google Scholar] [CrossRef]
- Chen X, Ren Y, Qu G et al. A review of environmental functional materials for cyanide removal by adsorption and catalysis. Inorg Chem Commun 2023, 157, 111298. [Google Scholar] [CrossRef]
- Ibhadon A, Fitzpatrick P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Hossain R, Uddin MA, Khan MA. Mechanistic Understanding in Manipulating Energetics of TiO2 for Photocatalysis. J Phys Chem C 2023, 127, 10897–10912. [Google Scholar] [CrossRef]
- Eder M, Tschurl M, Heiz U. Toward a Comprehensive Understanding of Photocatalysis: What Systematic Studies and Alcohol Surface Chemistry on TiO2 (110) Have to Offer for Future Developments. J Phys Chem Lett 2023, 14, 6193–6201. [Google Scholar] [CrossRef] [PubMed]
- Sumaria V, Rawal TB, Li Y et al. Machine Learning, Density Functional Theory, and Experiments to Understand the Photocatalytic Reduction of CO2 by CuPt/TiO2. Cond mat 2024, 1, 1–16. [Google Scholar]
- Henderson, MA. A surface science perspective on photocatalysis. Surf Sci Rep 2011, 66, 185–297. [Google Scholar]
- Hyunwoo Y, Eunsoo K, Sung H et al. Hole trap, charge transfer and photoelectrochemical water oxidation in thickness-controlled TiO2 anatase thin films. Appl Surf Sci 2020, 529, 147020. [Google Scholar] [CrossRef]
- Ali S, Ismail PM, Khan M et al. Charge transfer in TiO2 - based photocatalysis: fundamental mechanisms to material strategies. Nanoscale 2024, 16, 4352–4377. [Google Scholar] [CrossRef]
- Aldosari OF, Hussain I. Unlocking the potential of TiO2-based photocatalysts for green hydrogen energy through water-splitting: Recent advances, future perspectives and techno feasibility assessment. Int J Hydrogen Energ 2024, 59, 958–981. [Google Scholar] [CrossRef]
- Yin S, Liu L, Li J et al. Mesoporous TiO2 Single-Crystal Particles from Controlled Crystallization-Driven Mono-Micelle Assembly as an Efficient Photocatalyst. J Am Chem Soc 2024, 146, 1701–1709. [Google Scholar] [CrossRef]
- Alamoudi M, Katsiev K, Idriss H. Monitoring the Lifetime of Photoexcited Electrons in a Fresh and Bulk Reduced Rutile TiO2 Single Crystal Possible Anisotropic Propagation. J Phys Chem Lett 2023, 14, 9238–9244. [Google Scholar] [CrossRef]
- Li C, Shen S, Niu J et al. Mesoporous single-crystal-based TiO2 microspheres decorated by carbon nitride for obviously improved photocatalytic performance and recyclability. Inorg Chem Commun 2023, 150, 110524. [Google Scholar] [CrossRef]
- He T, Wang D, Xu Y et al. The Facile Construction of Anatase Titanium Dioxide Single Crystal Sheet-Connected Film with Observable Strong White Photoluminescence. Coat 2024, 14, 292. [Google Scholar] [CrossRef]
- Petrik NG, Baer MD, Mundy CJ et al. Mixed Molecular and Dissociative Water Adsorption on Hydroxylated TiO2 (110): An Infrared Spectroscopy and Ab Initio Molecular Dynamics Study. J Phys Chem C 2022, 126, 21616–21627. [Google Scholar] [CrossRef]
- Jiménez-Calvo P, Caps V, Keller V. Plasmonic Au-based junctions onto TiO2, gC3N4, and TiO2-gC3N4 systems for photocatalytic hydrogen production: Fundamentals and challenges. Renew Sust Energ Rev 2021, 149, 111095. [Google Scholar] [CrossRef]
- Qasim M, Arif MI, Naseer A et al. Biogenic Nanoparticles at the Forefront: Transforming Industrial Wastewater Treatment with TiO2 and Graphene. Sch J Agric Vet Sci 2024.
- Barakat NAM, Irfan OM, Mohamed OA. TiO2 NPs-immobilized silica granules: New insight for nano catalyst fixation for hydrogen generation and sustained wastewater treatment. PLoS One 2023, 18, e0287424. [Google Scholar]
- Azizi ZL, M Qasim, MI Arif, A Naseer,. Biogenic Nanoparticles at the Forefront: Transforming Industrial Wastewater Treatment with TiO2 and Graphene. Appl Biochem Biotech 2024.
- Suhan MdBK, Al-Mamun MdR, Farzana N et al. Sustainable pollutant removal and wastewater remediation using TiO2-based nanocomposites: A critical review. Nano-Struct Nano-Objects 2023, 36, 101050. [Google Scholar] [CrossRef]
- Ariaeinezhad F, Mohammadnezhad G, Zare M et al. Controllable and facile one-pot synthesis of high surface area amorphous, crystalline, and triphasic TiO2 : Catalytic and photocatalytic applications. J Mater Chem A 2024, 12, 6488–6506. [Google Scholar] [CrossRef]
- Chen Z, Chen H, Wang K et al. Enhanced TiO2 Photocatalytic 2 e– Oxygen Reduction Reaction via Interfacial Microenvironment Regulation and Mechanism Analysis. ACS Catal 2023, 13, 6497–6508. [Google Scholar] [CrossRef]
- Eder M, Tschurl M, Heiz U. Toward a Comprehensive Understanding of Photocatalysis: What Systematic Studies and Alcohol Surface Chemistry on TiO2 (110) Have to Offer for Future Developments. J Phys Chem Lett 2023, 14, 6193–6201. [Google Scholar] [CrossRef]
- Jabraoui H, Rouhani MD, Rossi C et al. Towards H2 production from water and ethanol interactions on hydrated TiO2(101): Insights from ReaxFF molecular dynamics. Appl Surf Sci 2024, 656, 159692. [Google Scholar] [CrossRef]
- Patidar A, Dugyala VR, Chakma S et al. Reactive oxygen species aided photocatalytic degradation of tetracycline using non-metal activated carbon doped TiO2 nanocomposite under UV-light irradiation. Res Chem Intermediat 2024, 50, 1035–1063. [Google Scholar] [CrossRef]
- Zhang R, Luo T, Zeng W et al. Methanol Adsorption and Reaction on TiO2 (110) at Near Ambient Pressure. J Phys Chem C 2023, 127, 1049–1056. [Google Scholar] [CrossRef]
- Lin Y, Bocharov D, Isakoviča I et al. Chlorine Adsorption on TiO2(110)/Water Interface: Nonadiabatic Molecular Dynamics Simulations for Photocatalytic Water Splitting. Electron Mater 2023, 4, 33–48. [Google Scholar] [CrossRef]
- Abbas, M. Factors influencing the adsorption and photocatalysis of direct red 80 in the presence of a TiO2, Equilibrium and kinetics modeling. J Chem Res 2021, 45, 694–701. [Google Scholar] [CrossRef]
- Lang X, Liang Y, Zhang J et al. Structure and reactivity of a water-covered anatase TiO2 (001) surface. Phys Chem Chem Phys 2020, 22, 1371–1380. [Google Scholar] [CrossRef]
- Guo Q, Zhou C, Ma Z et al. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv Mater 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Ali S, Ismail PM, Khan M et al. Charge transfer in TiO2 -based photocatalysis: fundamental mechanisms to material strategies. Nanoscale 2024, 16, 4352–4377. [Google Scholar] [CrossRef]
- Lettieri S, Pavone M, Fioravanti A et al. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Mater 2021, 14, 1645. [Google Scholar] [CrossRef]
- Rosa D, Abbasova N, Di Palma L. Titanium Dioxide Nanoparticles Doped with Iron for Water Treatment via Photocatalysis: A Review. Nanomaterials 2024, 14, 293. [Google Scholar] [CrossRef]
- Vorontsov AV, Valdés H, Smirniotis PG et al. Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis. Surface 2020, 3, 72–92. [Google Scholar] [CrossRef]
- Jeon JP, Kweon DH, Jang BJ et al. Enhancing the photocatalytic activity of TiO2 catalysts. Adv Sustain Syst 2020, 4, 2000197. [Google Scholar] [CrossRef]
- Mohsin M, Bhatti IA, Zeshan M et al. Prospects, challenges, and opportunities of the metals-modified TiO2 based photocatalysts for hydrogen generation under solar light irradiation: A review. Flatchem 2023, 42, 100547. [Google Scholar] [CrossRef]
- Kirk CH, Wang P, Chong CYD et al. TiO2 photocatalytic ceramic membranes for water and wastewater treatment: Technical readiness and pathway ahead. J Mater Sci Technol 2024, 183, 152–164. [Google Scholar] [CrossRef]
- Linsebigler AL, Lu G, Yates JT. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem Rev 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Wang C, Liu H, Qu Y. TiO2 - Based Photocatalytic Process for Purification of Polluted Water: Bridging Fundamentals to Applications. J Nanomater 2013, 2013, 319637. [Google Scholar] [CrossRef]
- Henderson MA, Lyubinetsky I. Molecular-Level Insights into Photocatalysis from Scanning Probe Microscopy Studies on TiO2 (110). Chem Rev 2013, 113, 4428–4455. [Google Scholar] [CrossRef]
- Guo Q, Zhou C, Ma Z et al. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv Mater 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Henderson MA, Shen M, Wang ZT. Characterization of the Active Surface Species Responsible for UV-Induced Desorption of O2 from the Rutile TiO2(110) Surface. The J of Phyl Che C 2013, 17, 5774–5784. [Google Scholar]
- Guo Q, Zhou C, Ma Z et al. Elementary Chemical Reactions in Surface Photocatalysis. Annu Rev Phys Chem 2018, 69, 451–472. [Google Scholar] [CrossRef]
- Waghmode MS, Gunjal AB, Mulla JA et al. Studies on the titanium dioxide nanoparticles: biosynthesis, applications and remediation. SN Appl Sci 2019, 1, 310. [Google Scholar] [CrossRef]
- Kang X, Liu S, Dai Z et al. Titanium dioxide: from engineering to applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Bai J, Zhou B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem Rev 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Ghosh S, Das AP. Modified titanium oxide (TiO2) nanocomposites and its array of applications: a review. Toxicol Environ Chem 2015, 97, 491–514. [Google Scholar] [CrossRef]
- Hsu CY, Mahmoud ZH, Abdullaev S et al. Nano titanium oxide (nano-TiO2): A review of synthesis methods, properties, and applications. Case Stud Chem Environ Eng 2024, 9, 100626. [Google Scholar] [CrossRef]
- Qamar OA, Jamil F, Hussain M et al. Advances in synthesis of TiO2 nanoparticles and their application to biodiesel production: A review. Chem Eng J 2023, 460, 141734. [Google Scholar] [CrossRef]
- Anisonian, KG. Physico-chemical bases of magnetizing roasting of leucoxene ores and concentrates for separation of leucoxene and quartz by magnetic separation. Novosibirsk, IA: Baikov Institute of Metallurgy and Materials Science, Russian Academy of Sciences, 2015.
- Zhang W, Zhu Z, Cheng CY. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Umar M, Aziz HA. Photocatalytic degradation of organic pollutants in water. Intech 2013, 8, 196–197. [Google Scholar]
- Wu J, Zhong L. Predictive modeling for VOC adsorption on TiO2 filters: Implications for enhanced photocatalytic oxidation in indoor air quality management. Chem Eng J 2024, 480, 148222. [Google Scholar] [CrossRef]
- Zhou W, Chen F, Li M et al. Facet-Dependent Photocatalytic Behavior of Rutile TiO2 for the Degradation of Volatile Organic Compounds: In Situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy and Density Functional Theory Investigations. Langmuir 2024, 40, 2120–2129. [Google Scholar] [CrossRef]
- Lin Z, Jiang X, Xu W et al. The effects of water, substrate, and intermediate adsorption on the photocatalytic decomposition of air pollutants over nano-TiO2 photocatalysts. Phys Chem Chem Phys 2024, 26, 662–678. [Google Scholar] [CrossRef]
- Kharouf M, Zyoud AH, Zyoud SH et al. Enhanced photocatalytic degradation of phenazopyridine using rutile TiO2/clay composite: catalyst recovery and environmental implications. Int J Environ Sci Technol 2024, 21, 7491–7508. [Google Scholar] [CrossRef]
- Pavel M, Anastasescu C, State RN et al. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Kanan S, Moyet MA, Arthur RB et al. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal Rev 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Badr P, Sajjadnejad M, Haghshenas SM. Influence of incorporating B4C nanoparticles and pulse electrodeposition parameters on the surface morphology and wear behavior of nickel based nanocomposite coatings. Prog Chem Biochem Res 2023, 6, 292–313. [Google Scholar]
- Belhachem A, Amara N, Belmekki H et al. Synthesis, characterization and anti-inflammatory activity of an alginate-zinc oxide nanocomposite. Asian J Nanosci Mater 2023, 3, 173–185. [Google Scholar]
- Puri J, Dhuldhaj U, Abdulbasit S. Nano-biochar production and its characteristics: overview. Int J Adv Biol Biom Res 2023, 11, 206–220. [Google Scholar]
- Eddy DR, Nur Sheha GA, Permana MD et al. Study on triphase of polymorphs TiO2 (anatase/rutile/brookite) for boosting photocatalytic activity of metformin degradation. Chemosphere 2024, 351, 141206. [Google Scholar] [CrossRef]
- Zahra Z, Habib Z, Chung S et al. Exposure Route of TiO2 NPs from Industrial Applications to Wastewater Treatment and Their Impacts on the Agro-Environment. Nanomaterials 2020, 10, 1469. [Google Scholar] [CrossRef]
- Berardinelli A, Parisi F. Introduction: TiO2 in the food industry and cosmetics. In Titanium Dioxide (TiO₂) and Its Applications. Publishing: Elsevier, Netherlands 2021, 353-371.
- Janczarek M, Klapiszewska I, Jesionowski T et al. Progress of functionalized TiO2-based nanomaterials in the construction industry: A comprehensive review. Chem Eng J 2022, 430, 132062. [Google Scholar] [CrossRef]
- Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT et al. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Samuel HS, Maraizu UN, Emmanuel EE. Supercritical fluids: Properties, formation and applications. J Eng Ind Res 2023, 4, 176–188. [Google Scholar]
- Taheri A, Rezayati-Zad Z. Characterization of tribological and electrochemical corrosion behavior of GO coatings formed on 6061 Al alloy processed by plasma electrolytic oxidation. Prog Chem Biochem Res 2023, 6, 261–276. [Google Scholar]
- Asaad Mahdi M, Farhan MA, Mahmoud ZH et al. Direct sunlight photodegradation of congo red in aqueous solution by TiO2/rGO binary system: Experimental and DFT study. Arab J Chem 2023, 16, 104992. [Google Scholar] [CrossRef]
- Mahmoud ZH, Hamrouni A, Kareem AB et al. Synthesis and characterization of chitosan sheet modified by varied weight ratio of anatase (TiO2) nano mixture with Cr(VI) adsorbing. Kuwait J Sci 2023, 50, 290–299. [Google Scholar] [CrossRef]
- Eddy DR, Nur Sheha GA, Permana MD et al. Study on triphase of polymorphs TiO2 (anatase/rutile/brookite) for boosting photocatalytic activity of metformin degradation. Chemosphere 2024, 351, 141206. [Google Scholar] [CrossRef]
- Simons PY, Dachille F. The structure of TiO2 II, a high-pressure phase of TiO2. Acta Cryst 1967, 23, 334–336. [Google Scholar] [CrossRef]
- Latroche M, Brohan L, Marchand R et al. New hollandite oxides: TiO2(H) and K0.06TiO2. J Solid State Chem 1989, 81, 78–82. [Google Scholar] [CrossRef]
- Cromer DT, Herrington K. The Structures of Anatase and Rutile. J Am Chem Soc 1955, 77, 4708–4709. [Google Scholar] [CrossRef]
- Mo SD, Ching WY. Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Phys Rev B 1995, 51, 13023–13032. [Google Scholar] [CrossRef]
- Kandiel TA, Robben L, Alkaima A et al. Brookite versus anatase TiO2 photocatalysts: phase transformations and photocatalytic activities. Photoch Photobio Sci 2013, 12, 602–609. [Google Scholar] [CrossRef]
- Samat M, Ali A, Taib M et al. Hubbard U calculations on optical properties of 3d transition metal oxide TiO2. Results in Physics 2016, 6, 891–896. [Google Scholar] [CrossRef]
- Landmann M, Rauls E, Schmidt WG. The electronic structure and optical response of rutile, anatase and brookite TiO2. J Phys-Condens Mat 2012, 24, 195503. [Google Scholar] [CrossRef] [PubMed]
- Thompson TL, Yates JT. Surface Science Studies of the Photoactivation of TiO2 - New Photochemical Processes. Chem Rev 2023, 106, 4428–4453. [Google Scholar]
- Tang H, Prasad K, Sanjines R et al. Electrical and optical properties of TiO2 anatase thin films. J Appl Phys 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Yang HG, Sun CH, Qiao SZ et al. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Guo Q, Zhou C, Ma Z et al. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv Mater 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Wang Z, Wen B, Hao Q et al. Localized Excitation of Ti3+ Ions in the Photoabsorption and Photocatalytic Activity of Reduced Rutile TiO2. J Am Chem Soc 2015, 137, 9146–9152. [Google Scholar] [CrossRef]
- Yin WJ, Wen B, Zhou C et al. Excess electrons in reduced rutile and anatase TiO2. Surf Sci Rep 2018, 73, 58–82. [Google Scholar] [CrossRef]
- Hardman PJ, Raikar GN, Muryn CA et al. Valence-band structure of TiO2 along the Γ-Δ-X and Γ-Σ-M directions. Phys Rev B 1994, 49, 7170. [Google Scholar] [CrossRef]
- Lindan PJD, Harrison NM, Gillan MJ et al. First-principles spin-polarized calculations on the reduced and reconstructed TiO2 (110) surface. Phys Rev B 1997, 55, 15919–15927. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf Sci Rep 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Li G, Chen L, Graham ME et al. A comparison of mixed phase titania photocatalysts prepared by physical and chemical methods: The importance of the solid–solid interface. J Mol Catal A-Chem 2007, 275, 30–35. [Google Scholar] [CrossRef]
- Morrison, SR. Electrochemistry at Semiconductor and Oxidized Metal Electrodes. Plenum Press: New York, USA, 1980.
- Zhao Y, Li C, Liu X et al. Synthesis and optical properties of TiO2 nanoparticles. Mater Lett 2007, 61, 79–83. [Google Scholar] [CrossRef]
- Ma J, Li W, Le N, et al. Red-shifted absorptions of cation-defective and surface-functionalized anatase with enhanced photoelectrochemical properties. ACS omega, 1092.
- Shon H, Phuntsho S, Okour Y et al. Visible light responsive titanium dioxide (TiO2). J Korean Ind Eng Chem 2008, 19, 1–16. [Google Scholar]
- Mathew RM, Jose J, Zachariah ES et al. Defect Induced Ultrafast Organic Dye Adsorption by Amorphous Titanium Dioxide/Phosphorus-Doped Carbon Nanodot Hybrid. J Clust Sci 2024, 35, 1045–1062. [Google Scholar] [CrossRef]
- Ahad A, Podder J, Saha T et al. Effect of chromium doping on the band gap tuning of titanium dioxide thin films for solar cell applications. Heliyon 2024, 10, e23096. [Google Scholar] [CrossRef]
- Kuranov D, Grebenkina A, Bogdanova A et al. Effect of Donor Nb(V) Doping on the Surface Reactivity, Electrical, Optical and Photocatalytic Properties of Nanocrystalline TiO2. Materials 2024, 17, 375. [Google Scholar] [CrossRef]
- Devi LG, Kumar SG. Influence of physicochemical - electronic properties of transition metal ion doped polycrystalline titania on the photocatalytic degradation of Indigo Carmine and 4-nitrophenol under UV/solar light. Appl Surf Sci 2011, 257, 2779–2790. [Google Scholar] [CrossRef]
- Basavarajappa PS, Patil SB, Ganganagappa N et al. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int J Hydrogen Energ 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Kuriechen SK, Murugesan S, Paul RS. Mineralization of Azo Dye Using Combined Photo-Fenton and Photocatalytic Processes under Visible Light. J Catalysts 2013, 1–6. [Google Scholar] [CrossRef]
- Chakraborty AK, Ganguli S, Sabur MA. Nitrogen doped titanium dioxide (N-TiO2): Electronic band structure, visible light harvesting and photocatalytic applications. J Water Process Eng 2023, 55, 104183. [Google Scholar] [CrossRef]
- Ishikawa T, Sahara R, Ohno K et al. Electronic structure analysis of light-element-doped anatase TiO2 using all-electron GW approach. Comp Mater Sci 2023, 220, 112059. [Google Scholar] [CrossRef]
- Gao C, Guo M, Liu Y et al. Surface modification methods and mechanisms in carbon nanotubes dispersion. Carbon 2023, 212, 118133. [Google Scholar] [CrossRef]
- Yang J, Mei S, Ferreira JMF. Hydrothermal synthesis of TiO2 nanopowders from tetraalkylammonium hydroxide peptized sols. Mat Sci Eng C 2001, 15, 183–185. [Google Scholar] [CrossRef]
- Wang D, Yu B, Zhou F et al. Synthesis and characterization of anatase TiO2 nanotubes and their use in dye-sensitized solar cells. Mater Chem Phys 2009, 113, 602–606. [Google Scholar] [CrossRef]
- Wahi RK, Liu Y, Falkner JC et al. Solvothermal synthesis and characterization of anatase TiO2 nanocrystals with ultrahigh surface area. J Colloid Interf Sci 2006, 302, 530–536. [Google Scholar] [CrossRef]
- Kobayashi M, Kato H, Miyazaki T et al. Hydrothermal Synthesis of Pseudocubic Rutile-Type Titania Particles. Ceramics 2019, 2, 56–63. [Google Scholar] [CrossRef]
- Govindaraj R, Santhosh N, Senthil Pandian M et al. Synthesis of nanocrystalline TiO2 nanorods via hydrothermal method: An efficient photoanode material for dye sensitized solar cells. J Cryst Growth 2017, 468, 125–128. [Google Scholar] [CrossRef]
- Rajamanickam G, Narendhiran S, Muthu SP et al. Hydrothermally derived nanoporous titanium dioxide nanorods / nanoparticles and their influence in dye-sensitized solar cell as a photoanode. Chem Phys Lett 2017, 689, 19–25. [Google Scholar] [CrossRef]
- Wang F, Shi Z, Gong F, Jiu J, Adachi M. Morphology Control of Anatase TiO2 by Surfactant-assisted Hydrothermal Method. Chinese J Chem Eng 2007, 15, 754–759. [Google Scholar] [CrossRef]
- Lei L, Yuming C, Bo L et al. Study on the surface erosion route to the fabrication of TiO2 hollow spheres. Appl Surf Sci 2010, 256, 2596–2601. [Google Scholar] [CrossRef]
- Liu Z, Sun DD, Guo P et al. One-Step Fabrication and High Photocatalytic Activity of Porous TiO2 Hollow Aggregates by Using a Low-Temperature Hydrothermal Method Without Templates. Chem A Eur J 2007, 13, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Xue B, Sun T, Mao F et al. Facile synthesis of mesoporous core-shell TiO2 nanostructures from TiCl3. Materials Research Bulletin 2011, 46, 1524–1529. [Google Scholar] [CrossRef]
- Yan XM, Kang J, Gao L et al. Solvothermal synthesis of carbon coated N-doped TiO2 nanostructures with enhanced visible light catalytic activity. Appl Surf Sci 2013, 265, 778–783. [Google Scholar] [CrossRef]
- Kang M, Lee SY, Chung CH et al. Characterization of a TiO2 photocatalyst synthesized by the solvothermal method and its catalytic performance for CHCl3 decomposition. J Photoch Photobio A 2001, 144, 185–191. [Google Scholar] [CrossRef]
- Nam WS, Han GY. Characterization and photocatalytic performance of nanosize TiO2 powders prepared by the solvothermal method. Korean J Chem Eng 2003, 20, 1149–1153. [Google Scholar] [CrossRef]
- Yang HG, Liu G, Qiao SZ et al. Solvothermal Synthesis and Photoreactivity of Anatase TiO2 Nanosheets with Dominant {001} Facets. J Am Chem Soc 2009, 131, 4078–4083. [Google Scholar] [CrossRef]
- Sencer S, Annabella S. Surface Structure and Reactivity of Anatase TiO2 Crystals with Dominant {001} Facets. Phys. Chem. C 2013, 117, 12–6358. [Google Scholar]
- Li X, Peng Q, Yi J et al. Near Monodisperse TiO2 Nanoparticles and Nanorods. Chem A Eur J 2006, 12, 2383–2391. [Google Scholar] [CrossRef]
- Lü X, Ding S, Xie Y et al. Non-Aqueous Preparation of High-Crystallinity Hierarchical TiO2 Hollow Spheres with Excellent Photocatalytic Efficiency. Eur J Inorg Chem 2011, 2879–2883. [Google Scholar] [CrossRef]
- Liu Z, Sun DD, Guo P et al. One-Step Fabrication and High Photocatalytic Activity of Porous TiO2 Hollow Aggregates by Using a Low-Temperature Hydrothermal Method Without Templates. Chem A Eur J 2007, 13, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Liu C, Sun H, Yang S. From Nanorods to Atomically Thin Wires of Anatase TiO2 : Nonhydrolytic Synthesis and Characterization. Chem A Eur J 2010, 16, 4381–4393. [Google Scholar] [CrossRef]
- Supphasrirongjaroen P, Praserthdam P, Panpranot J et al. Effect of quenching medium on photocatalytic activity of nano-TiO2 prepared by solvothermal method. Chem Eng J 2008, 138, 622–627. [Google Scholar] [CrossRef]
- Dinh C, Nguyen T, Kleitz F et al. A solvothermal single-step route towards shape-controlled titanium dioxide nanocrystals. Can J Chem Eng 2012, 90, 8–17. [Google Scholar] [CrossRef]
- Kim CS, Moon BK, Park JH et al. Solvothermal synthesis of nanocrystalline TiO2 in toluene with surfactant. J Cryst Growth 2003, 257, 309–315. [Google Scholar] [CrossRef]
- Gong D, Grimes CA, Varghese OK et al. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Zhang P, Yin S, Sato T. Synthesis of high-activity TiO2 photocatalyst via environmentally friendly and novel microwave assisted hydrothermal process. Appl Catal B-Environ 2009, 89, 118–122. [Google Scholar] [CrossRef]
- Zhang QH, Han WD, Hong YJ et al. Photocatalytic reduction of CO2 with H2O on Pt-loaded TiO2 catalyst. Catal Today 2009, 148, 335–340. [Google Scholar] [CrossRef]
- Zhang Y, Zheng H, Liu G et al. Synthesis and electrochemical studies of a layered spheric TiO2 through low temperature solvothermal method. Electrochim Acta 2009, 54, 4079–4083. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing D, Zaleska A, Bein T. Three-Dimensional Titanium Dioxide Nanomaterials. Chem Rev 2014, 114, 9487–9558. [Google Scholar] [CrossRef]
- Attar AS, Ghamsari MS, Hajiesmaeilbaigi F et al. J Am Chem Soc 1981, 103, 6116.
- Oskam G, Nellore A, Penn RL et al. The Growth Kinetics of TiO2 Nanoparticles from Titanium(IV) Alkoxide at High Water/Titanium Ratio. J Phys Chem B 2003, 107, 1734–1738. [Google Scholar] [CrossRef]
- Mehrotra RC, Singh A. ChemInform Abstract: Recent Trends in Metal Alkoxide Chemistry. J Cheminformatics 1997, 28, 12869.
- Livage J, Henry M, Sanchez C. Sol-gel chemistry of transition metal oxides. Prog Solid State Ch 1988, 18, 259–341. [Google Scholar] [CrossRef]
- Rahman I, Mostafa M, Kazem M et al. An inclusive review on inorganic gels: classifications, synthesis methods and applications. J Ir Cheml Soc 2023, 20, 1757–1779. [Google Scholar] [CrossRef]
- Yu JC, Yu J, Ho W et al. Preparation of highly photocatalytic active nano-sized TiO2 particles via ultrasonic irradiation. Chem Commun 2001, 19, 1942–1943. [Google Scholar]
- Chen, X. Titanium Dioxide Nanomaterials and Their Energy Applications. Chinese J Catal 2009, 30, 839–851. [Google Scholar] [CrossRef]
- Zhu YJ, Chen F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem Rev 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Collins, MJ. Future Trends in Microwave Synthesis. Future Med Chem 2010, 2, 151–155. [Google Scholar] [CrossRef]
- Jacob J, Chia LHL, Boey FYC. Thermal and non-thermal interaction of microwave radiation with materials. J Mater Sci 1995, 30, 5321–5327. [Google Scholar] [CrossRef]
- De La Hoz A, Díaz-Ortiz Á, Moreno A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem Soc Rev 2005, 34, 164–178. [Google Scholar]
- Gressel-Michel E, Chaumont D, Stuerga D. From a microwave flash-synthesized TiO2 colloidal suspension to TiO2 thin films. J Colloid Interf Sci 2005, 285, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Corradi AB, Bondioli F, Focher B et al. Conventional and Microwave-Hydrothermal Synthesis of TiO2 Nanopowders. J Am Ceram Soc 2005, 88, 2639–2641. [Google Scholar] [CrossRef]
- Shang J, Zhou Y, Yan H, et al. Atom layer deposited TiO2 electron transport layer for silicon heterojunction solar cells to achieve high performance. Langmuir 2011, 27, 10191–10196. [Google Scholar]
- Baldassari S, Komarneni S, Mariani E et al. Rapid Microwave - Hydrothermal Synthesis of Anatase Form of Titanium Dioxide. J Am Ceram Soc 2005, 88, 3238–3240. [Google Scholar] [CrossRef]
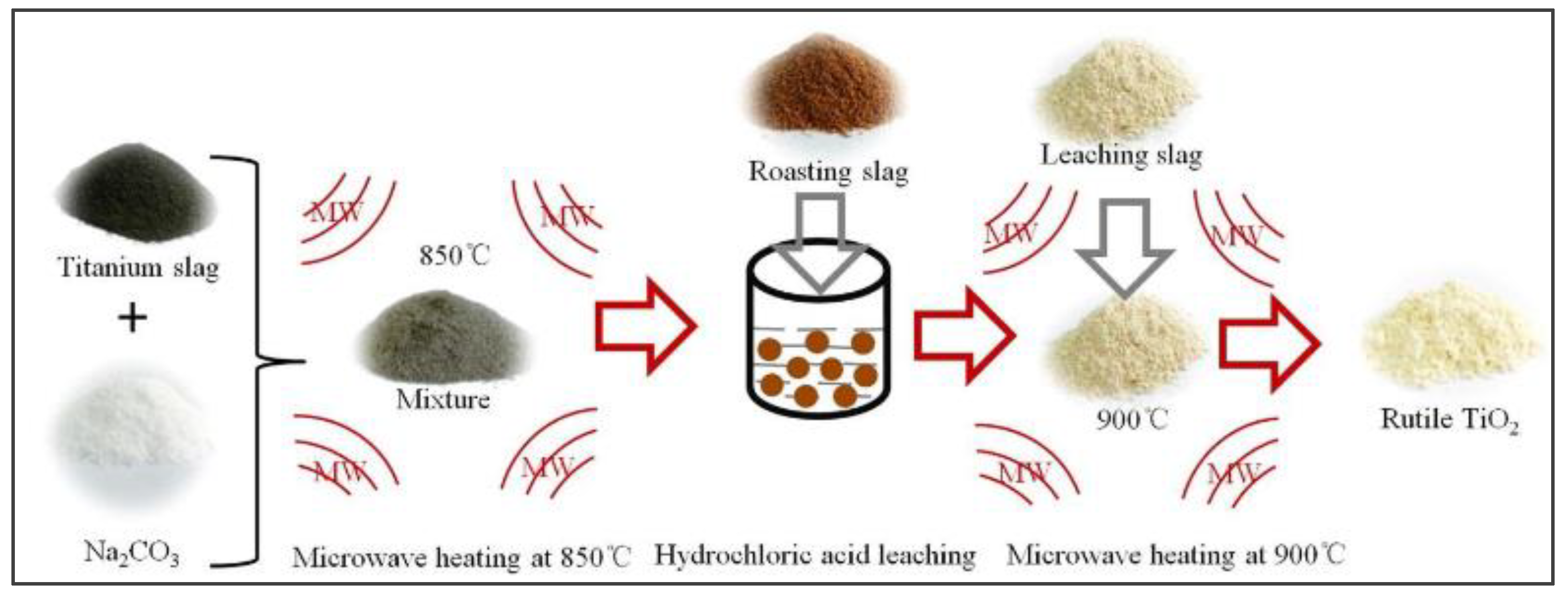
- Kang J, Gao L, Zhang M et al. Synthesis of rutile TiO2 powder by microwave-enhanced roasting followed by hydrochloric acid leaching. Adv Powder Technol 2020, 31, 1140–1147. [Google Scholar] [CrossRef]
- Kim H, Noh K, Choi C et al. Extreme Superomniphobicity of Multiwalled 8 nm TiO2 Nanotubes. Langmuir 2011, 27, 10191–10196. [Google Scholar] [CrossRef]
- Malekshahi BM, Nemati KA, Fatholahi L et al. A review on synthesis of nano-TiO2 via different methods. J Nanostructures 2013, 3, 1–9. [Google Scholar]
- Chen X, Mao SS. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem Rev 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Seifried S, Winterer M, Hahn H. Nanocrystalline Titania Films and Particles by Chemical Vapor Synthesis. Chem Vap Depos 2000, 6, 239–244. [Google Scholar] [CrossRef]
- Oh SM, Ishigaki T. Preparation of pure rutile and anatase TiO2 nanopowders using RF thermal plasma. Thin Solid Films 2004, 457, 186–191. [Google Scholar] [CrossRef]
- Gablenz S, Voltzke D, Abicht HP et al. Preparation of fine TiO2 powders via spray hydrolysis of titanium tetraisopropoxide. J Mater Sci Lett 1998, 17, 537–539. [Google Scholar] [CrossRef]
- Yu J, Zhao X. Effect of substrates on the photocatalytic activity of nanometer TiO2 thin films. Mater Res Bull 2000, 35, 1293–1301. [Google Scholar] [CrossRef]
- Zhang Q, Li C. High Temperature Stable Anatase Phase Titanium Dioxide Films Synthesized by Mist Chemical Vapor Deposition. Nanomaterials 2020, 10, 911. [Google Scholar] [CrossRef]
- Sedky NK, Mahdy NK, Abdel-kader NM et al. Facile sonochemically-assisted bioengineering of titanium dioxide nanoparticles and deciphering their potential in treating breast and lung cancers: Biological, molecular, and computational-based investigations. RSC Adv 2024, 14, 8583–8601. [Google Scholar] [CrossRef]
- Aswini R, Murugesan S, Kannan K. Bio-engineered TiO2 nanoparticles using Ledebouria revoluta extract: Larvicidal, histopathological, antibacterial and anticancer activity. Int J Environ An Ch 2021, 101, 2926–2936. [Google Scholar] [CrossRef]
- Danyliuk NV, Tatarchuk TR, Shyichuk AV. Batch microreactor for photocatalytic reactions monitoring. PCSS 2020, 21, 338–346. [Google Scholar] [CrossRef]
- Mbonyiryivuze A, Zongo S, Diallo A et al. Titanium dioxide nanoparticles biosynthesis for dye sensitized solar cells application: Review. Phys Mater Chem 2015, 3, 12–17. [Google Scholar]
- Saikumari N, Preethi T, Abarna B et al. Ecofriendly, green tea extract directed sol - gel synthesis of nano titania for photocatalytic application. J Mater Sci: Mater Electron 2019, 30, 6820–6831. [Google Scholar]
- Sundrarajan M, Bama K, Bhavani M et al. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J Photoch Photobio B 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Hariharan D, Jegatha CA, Mayandi J et al. Visible light active photocatalyst: Hydrothermal green synthesized TiO2 NPs for degradation of picric acid. Mater Lett 2018, 222, 45–49. [Google Scholar] [CrossRef]
- Thandapani K, Kathiravan M, Namasivayam E et al. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ Sci Pollut Res 2018, 25, 10328–10339. [Google Scholar] [CrossRef] [PubMed]
- Gurrappa I, Binder L. Electrodeposition of nanostructured coatings and their characterization - A review. Sci Technol Adv Mat 2008, 9, 043001. [Google Scholar] [CrossRef] [PubMed]
- Hanaor D, Michelazzi M, Leonelli C et al. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J Eur Ceram Soc 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Jiang LC, Zhang WD. Electrodeposition of TiO2 nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanal 2019, 21, 988–993. [Google Scholar]
- Wasserscheid P, Keim W. Ionic Liquids - New “Solutions” for Transition Metal Catalysis. Angew Chem Int Ed 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Wasserscheid P, Welton T. Ionic liquids in synthesis. Wiley Vch Press: New Jersey, USA, 2008.
- Ma Z, Yu J, Dai S. Preparation of Inorganic Materials Using Ionic Liquids. Adv Mater 2010, 22, 261–285. [Google Scholar] [CrossRef]
- Hong K, Yoo KS. Synthesis of TiO2 hollow spheres using binary ionic liquids as an electrocatalyst. Res Chem Intermed 2011, 37, 1325–1331. [Google Scholar] [CrossRef]
- Mali SS, Betty CA, Bhosale PN et al. Hydrothermal synthesis of rutile TiO2 nanoflowers using Brønsted Acidic Ionic Liquid [BAIL]: Synthesis, characterization and growth mechanism. Cryst Eng Comm 2012, 14, 1920. [Google Scholar] [CrossRef]
- He Z, Yue G, Gao Y et al. Efficient flexible dye-sensitized solar cells from rear illumination based on different morphologies of titanium dioxide photoanode. J Semicond 2024, 45, 022801. [Google Scholar] [CrossRef]
- Kakihana M, Kobayashi M, Tomita K et al. Application of Water-Soluble Titanium Complexes as Precursors for Synthesis of Titanium-Containing Oxides via Aqueous Solution Processes. B Chem Soc Jpn 2010, 83, 1285–1308. [Google Scholar] [CrossRef]
- Younus LA, Mahmoud ZH, Hamza AA et al. Photodynamic therapy in cancer treatment: properties and applications in nanoparticles. Braz J Biol 2024, 84, e268892. [Google Scholar]
- Abdul-Reda HU, Mahmoud ZH, Alaziz KMA et al. Antimicrobial finishing of textiles using nanomaterials. Braz J Biol 2024, 84, e264947. [Google Scholar]
- Mohammadkhani A, Mohammadkhani F, Farhadyar N et al. Novel nanocomposite zinc phosphate / polyvinyl alcohol / carboxymethyl cellulose: Synthesis, characterization and investigation of antibacterial and anticorrosive properties. Case Stud Chem Environ Eng 2024, 9, 100591. [Google Scholar] [CrossRef]
- Kianfar, E. A review of recent advances in carbon dioxide absorption–stripping by employing a gas - liquid hollow fiber polymeric membrane contactor. Polym Bull 2023, 80, 11469–11505. [Google Scholar] [CrossRef]
- Yi Hsu C, Mahmoud ZH, Abdullaev S et al. Nanocomposites based on Resole / graphene / carbon fibers: A review study. Case Stud Chem Environ Eng 2023, 8, 100535. [Google Scholar] [CrossRef]
- AL-Salman HNK, Hsu CY, Nizar JZ et al. Graphene oxide-based biosensors for detection of lung cancer: A review. Results Chem 2024, 7, 101300. [Google Scholar] [CrossRef]
- Gong D, Grimes CA, Varghese OK et al. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Paulose M, Shankar K, Yoriya S et al. Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134μm in Length. J Phys Chem B 2006, 110, 16179–16184. [Google Scholar] [CrossRef]
- Ali G, Chen C, Yoo SH et al. Fabrication of complete titania nanoporous structures via electrochemical anodization of Ti. Nanoscale Res Lett 2011, 6, 332. [Google Scholar] [CrossRef]
- Parmon, VN. Development of physico-chemical foundations of transformation solar energy by decomposing water into molecular photocatalytic systems [doctor’s thesis]. Novosibirsk, 1984. [Google Scholar]
- Shijubo N, Honda Y, Fujishima T et al. Lung surfactant protein-A and carcinoembryonic antigen in pleural effusions due to lung adenocarcinoma and malignant mesothelioma. Eur Respir J 1995, 8, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Jasim SA, Ali MH, Mahmood ZH et al. Role of Alloying Composition on Mechanical Properties of CuZr Metallic Glasses During the Nanoindentation Process. Met Mater Int 2022, 28, 2075–2082. [Google Scholar] [CrossRef]
- Bokov DO, Mustafa YF, Mahmoud ZH et al. Cr-SiNT, Mn-SiNT, Ti-C70 and Sc-CNT as Effective Catalysts for CO2 Reduction to CH3OH. Silicon 2022, 14, 8493–8503. [Google Scholar] [CrossRef]
- Krakowiak R, Musial J, Bakun P et al. Titanium Dioxide-Based Photocatalysts for Degradation of Emerging Contaminants including Pharmaceutical Pollutants. Appl Sci 2021, 11, 8674. [Google Scholar] [CrossRef]
- Magaña-López R, Zaragoza-Sánchez PI, Jiménez-Cisneros BE et al. The Use of TiO2 as a Disinfectant in Water Sanitation Applications. Water 2021, 13, 1641. [Google Scholar] [CrossRef]
- Yang X, Zhao R, Zhan H et al. Modified Titanium dioxide-based photocatalysts for water treatment: Mini review. Environ Funct Mater 2024, Epub ahead of print.
- Khan, M. Principles and mechanisms of photocatalysis. Photocatalytic Syst Des 2021, 1, 1–22. [Google Scholar]
- Yadav HM, Kim JS, Pawar SH. Developments in photocatalytic antibacterial activity of nano TiO2, A review. Korean J Chem Eng 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Hassan SA, Ghadam P. Introduction: Photocatalytic Properties of Metal-Based Nanoparticles. In: Handbook of Green and Sustainable Nanotechnology. Springer Publishing: New York 2022, 1-25.
- Sedita SR, Yasunori B, Naohiro S. Introduction: Pasteur scientists meet the market: An empirical illustration of the innovative performance of university–industry relationships. In: Innovation, Alliances, and Networks in High-Tech Environments. Routledge: London, 2015; 263–281.
- Mills A, Le HS. An overview of semiconductor photocatalysis. J Photoch Photobio A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Munuera G, Gonzalez-Elipe AR, Rives-Arnau V et al. Photo-adsorption of oxygen on acid and basic TiO2 surfaces. Sud Surf Sci Catal 1985, 21, 113–126. [Google Scholar]
- Linsebigler AL, Lu G, Yates JT. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem Rev 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Watson SS, Beydoun D, Scott JA et al. The effect of preparation method on the photoactivity of crystalline titanium dioxide particles. Chem Eng J 2003, 95, 213–220. [Google Scholar] [CrossRef]
- Ohno T, Sarukawa K, Tokieda K et al. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J Catal 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Gerischer H, Heller A. Photocatalytic Oxidation of Organic Molecules at TiO2 Particles by Sunlight in Aerated Water. J Electro chem Soc 1992, 139, 113–118. [Google Scholar] [CrossRef]
- Gao L, Zhang Q. Effects of amorphous contents and particle size on the photocatalytic properties of TiO2 nanoparticles. Scripta Mater 2001, 44, 1195–1198. [Google Scholar] [CrossRef]
- Mohd Raub AA, Bahru R, Mohamed MA et al. Photocatalytic activity enhancement of nanostructured metal-oxides photocatalyst: A review. Nanotechnol 2024, 35, 242004. [Google Scholar] [CrossRef]
- Ahmed SN, Haide W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: a review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Tanos F, Razzouk A, Lesage G et al. A Comprehensive Review on Modification of Titanium Dioxide-Based Catalysts in Advanced Oxidation Processes for Water Treatment. Chem Sus Chem 2024, 17, e202301139. [Google Scholar] [CrossRef]
- Hamad TG, Salman TA. Green synthesis of titanium oxide nanoparticles using pomegranate leaf extract: Characterization and antibacterial activity evaluation: Proceedings of the AIP Conference. Baghdad, Iraq, 26-27 August 2023.
- Kubota Y, Shuin T, Kawasaki C et al. Photokilling of T-24 human bladder cancer cells with titanium dioxide. Clin Oncol 1994, 70, 1107–1111. [Google Scholar]
- Sun S, Vikrant K, Kim KH et al. Titanium dioxide–supported mercury photocatalysts for oxidative removal of hydrogen sulfide from the air using a portable air purification unit. J Hazard Mater 2024, 470, 134089. [Google Scholar] [CrossRef]
- Shang H, Jia H, Zhang W et al. Surface Hydrogen Bond-Induced Oxygen Vacancies of TiO2 for Two-Electron Molecular Oxygen Activation and Efficient NO Oxidation. Environ Sci Technol 2023, 57, 20400–20409. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N. Heterogeneous Photocatalysis and Prospects of TiO2-Based Photocatalytic DeNOxing the Atmospheric Environment. Catal 2018, 8, 553. [Google Scholar] [CrossRef]
- Safni S, Patricia EO, Ollinovela T et al. Degradation of Phenol Using Sonolysis and Photolysis by TiO2 / RHAC Catalyst and Analysis with Spectrophotometer UV-VIS and HPLC. Al Kimiya 2023, 10, 50–56. [Google Scholar] [CrossRef]
- Yamaguti K, Sato S. Photolysis of water over metallized powdered titanium dioxide. J Chem Soc 1985, 81, 1237. [Google Scholar]
- Stevens CG, Wessman NJ, Bowman JE et al. Photolysis of water vapor on titanium / TiO2 surfaces. Chem Phys Lett 1982, 91, 335–338. [Google Scholar] [CrossRef]
- Fujishima A, Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Nosaka, Y. Water Photo-Oxidation over TiO2 - History and Reaction Mechanism. Catal 2022, 12, 1557. [Google Scholar] [CrossRef]
- Bentouba S, Vajeeston P, Yohi S et al. TiO2 as a Photocatalyst for Water Splitting - An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Ni M, Leung MKH, Leung DYC et al. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sust Energ Rev 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Al-Rasheed, RA. Water Treatment by Heterogeneous Photocatalysis: An overview. 2005; 14, 1-15.
- Jafari S, Mahyad B, Hashemzadeh H et al. Biomedical applications of TiO2 nanostructures: recent advances. Int J Nanomed 2020, 3447–3470.
- Lee SY, Park SJ. TiO2 photocatalyst for water treatment applications. J Ind Eng Chem 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Dharma HNC, Jaafar J, Widiastuti N et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Raya I, Mansoor AS, Widjaja G et al. ZnMoO4 nanoparticles: Novel and facile synthesis, characterization, and photocatalytic performance. J Nanostruct 2022, 12, 446–454. [Google Scholar]
- Mahmood ZH, Jarosova M, Kzar HH et al. Synthesis and characterization of Co3O4 nanoparticles: Application as performing anode in Li-ion batteries. J Chinese Chem Soc 2022, 69, 657–662. [Google Scholar] [CrossRef]
- Bahadoran A, Jabarabadi M, Mahmood Z et al. Quick and sensitive colorimetric detection of amino acid with functionalized-silver / copper nanoparticles in the presence of cross linker, and bacteria detection by using DNA-template nanoparticles as peroxidase activity. Spectrochim Acta A 2022, 268, 120636. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud ZH, AL-Bayati RA, Khadom AA. Electron transport in dye-sanitized solar cell with tin-doped titanium dioxide as photoanode materials. J Mater Sci: Mater Electron 2022, 33, 5009–5023. [Google Scholar]
- Raya I, Widjaja G, Mahmood ZH et al. Kinetic, isotherm, and thermodynamic studies on Cr (VI) adsorption using cellulose acetate / graphene oxide composite nanofibers. Appl Phys A 2022, 128, 167. [Google Scholar] [CrossRef]
- Çeşmeli S, Biray Avci C. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J Drug Target 2019, 27, 762–766. [Google Scholar] [CrossRef]
- Li Q, Wang X, Lu X et al. The incorporation of daunorubicin in cancer cells through the use of titanium dioxide whiskers. Biomaterials 2009, 30, 4708–4715. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J Can Res Ther 2014, 10, 853. [Google Scholar] [CrossRef]
- Wang Q, Huang JY, Li HQ et al. TiO2 nanotube platforms for smart drug delivery: a review. Int J Nanomed 2016, 11, 4819–4834. [Google Scholar] [CrossRef]
- Raja G, Cao S, Kim DH et al. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mat Sci Eng C 2020, 107, 110303. [Google Scholar] [CrossRef]
- Akram MW, Raziq F, Fakhar-e-Alam M et al. Tailoring of Au-TiO2 nanoparticles conjugated with doxorubicin for their synergistic response and photodynamic therapy applications. J Photoch Photobio A 2019, 384, 112040. [Google Scholar]
- Inoue T, Fujishima A, Konishi S et al. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Kurzmann C, Verheyen J, Coto M et al. In vitro evaluation of experimental light activated gels for tooth bleaching. Photoch Photobio Sci 2019, 18, 1009–1019. [Google Scholar] [CrossRef]
- Woolerton TW, Sheard S, Reisner E et al. Efficient and Clean Photoreduction of CO2 to CO by Enzyme-Modified TiO2 Nanoparticles Using Visible Light. J Am Chem Soc 2010, 132, 2132–2133. [Google Scholar] [CrossRef]
- Roy SC, Varghese OK, Paulose M et al. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Thampi KR, Kiwi J, Grätzel M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature 1987, 327, 506–508. [Google Scholar] [CrossRef]
- Kaya S, Krupin O, LaRue J et al. Real-time observation of surface bond breaking with an x-ray laser. Science 2013, 339, 1302–1305. [Google Scholar] [CrossRef]
- Farouk HU, Raman AAA, Daud WMAW. Surface transformations of TiO2 anatase deactivated in methylene blue solution with Cl− ions in the colloid. J of the Taiwan Inst of Chem Engin 2017, 80, 203–214. [Google Scholar] [CrossRef]
- Zhang H, Banfield JF. Structural Characteristics and Mechanical and Thermodynamic Properties of Nanocrystalline TiO2. Chem Rev 2014, 114, 9613–9644. [Google Scholar] [CrossRef] [PubMed]
- Penn RL, Banfield JF. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from titania. Geochim Cosmochim Ac 1999, 63, 1549–1557. [Google Scholar] [CrossRef]
- Rahimi N, Pax RA, Gray EMacA. Review of functional titanium oxides. I: TiO2 and its modifications. Prog Solid State Ch 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Farouk HU, Raman AAA, Daud WMAW. TiO2 catalyst deactivation in textile wastewater treatment: Current challenges and future advances. J Ind Eng Chem 2016, 33, 11–21. [Google Scholar] [CrossRef]
- Kääriäinen ML, Kääriäinen TO, Cameron DC. Titanium dioxide thin films, their structure and its effect on their photoactivity and photocatalytic properties. Thin Solid Films 2009, 517, 6666–6670. [Google Scholar] [CrossRef]
- Ta N, Liu J, Shen W. Tuning the shape of ceria nanomaterials for catalytic applications. Chinese J Catal 2013, 34, 838–850. [Google Scholar] [CrossRef]
- Lee K, Yoon H, Ahn C et al. Strategies to improve the photocatalytic activity of TiO2 : 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef]
- Nakata K, Fujishima A. TiO2 photocatalysis: Design and applications. J. Photochem Photobiol C Photochem Rev 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Yang S, Xiong F, Chen K et al. Impact of Titanium Dioxide and Fullerenol Nanoparticles on Caco-2 Gut Epithelial Cells. J nanosci nanotechnol 2018, 18, 2387–2393. [Google Scholar] [CrossRef]
- Ayorinde T, Sayes CM. An updated review of industrially relevant titanium dioxide and its environmental health effects. J Hazard Mater Lett 2023, 4, 100085. [Google Scholar] [CrossRef]
- Bauer M, Lei C, Read K et al. Direct Observation of Surface Chemistry Using Ultrafast Soft-X-Ray Pulses. Phys Rev Lett 2001, 87, 025501. [Google Scholar] [CrossRef]
- Nematov, D. Investigation optical properties of the orthorhombic system CsSnBr3-xIx: application for solar cells and optoelectronic devices. Journal of Human, Earth, and Future 2021, 2, 404–411. [Google Scholar]
- Nematov, D. D., Burhonzoda, A. S., Khusenov, M. A., Kholmurodov, K. T., & Ibrahim, M. A. (2019). The quantum-chemistry calculations of electronic structure of boron nitride nanocrystals with density Functional theory realization. Egyptian Journal of Chemistry, 62(The First International Conference on Molecular Modeling and Spectroscopy 19-22 February, 2019), 21-27.
- Dilshod, N. , Kholmirzo, K., Aliona, S., Kahramon, F., Viktoriya, G., & Tamerlan, K. (2022). A DFT Study of Structure, Electronic and Optical Properties of Se-Doped Kesterite Cu2ZnSnS4 (CZTSSe).
- Nematov, D. Analysis of the optical properties and electronic structure of semiconductors of the Cu2NiXS4 (X= Si, Ge, Sn) family as new promising materials for optoelectronic devices. Journal of Optics and Photonics Research 2024, 1, 91–97. [Google Scholar] [CrossRef]
- Nematov, D. D. , Burhonzoda, A. S., Kholmurodov, K. T., Lyubchyk, A. I., & Lyubchyk, S. I.. A detailed comparative analysis of the structural stability and Electron-Phonon properties of ZRO2, mechanisms of water adsorption on T-ZRO2 (101) and T-YSZ (101) surfaces. Nanomaterials 2023, 13, 2657. [Google Scholar] [PubMed]
- Nematov, D. Bandgap tuning and analysis of the electronic structure of the Cu2NiXS4 (X= Sn, Ge, Si) system: mBJ accuracy with DFT expense. Chemistry of Inorganic Materials 2023, 1, 100001. [Google Scholar] [CrossRef]
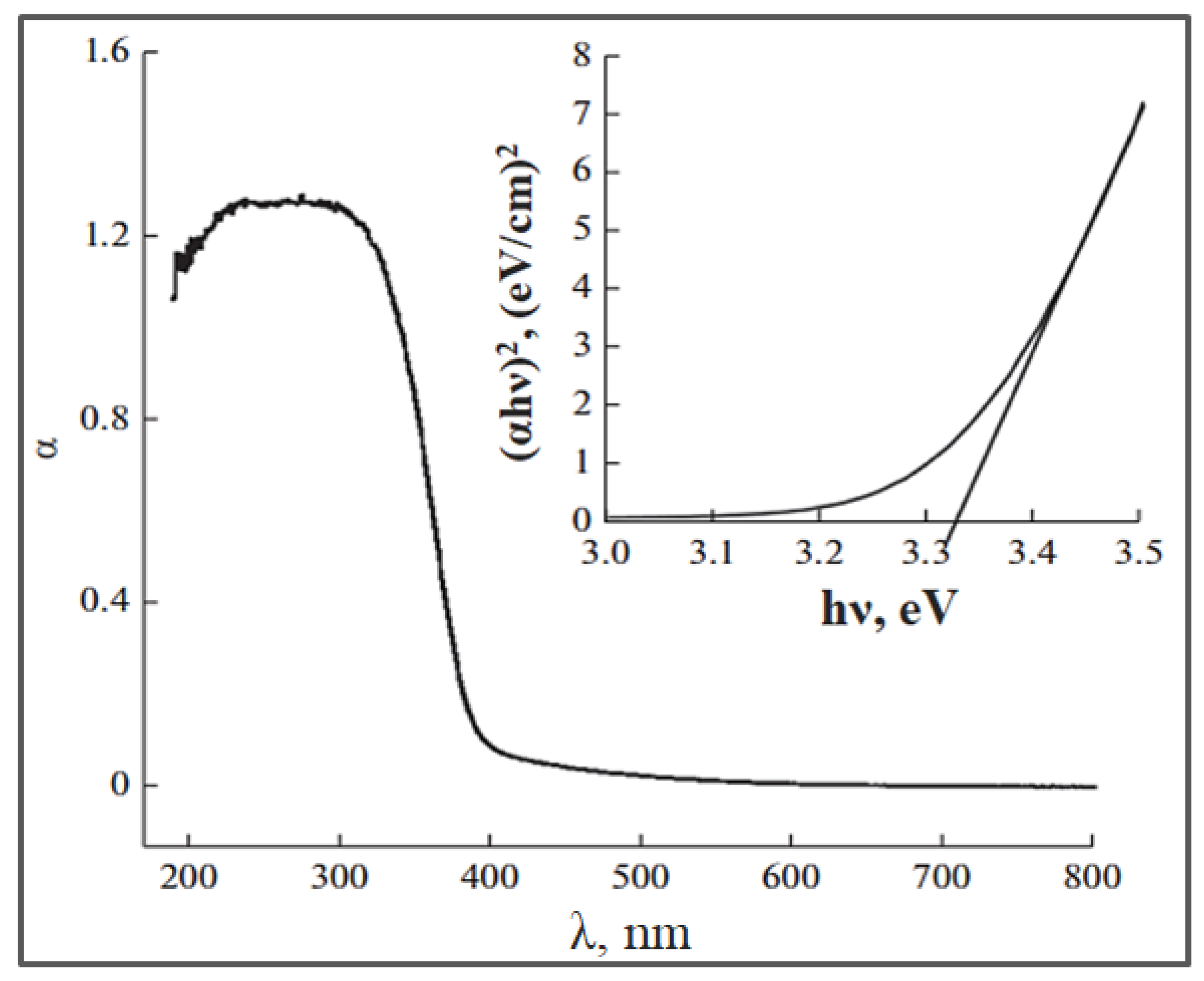
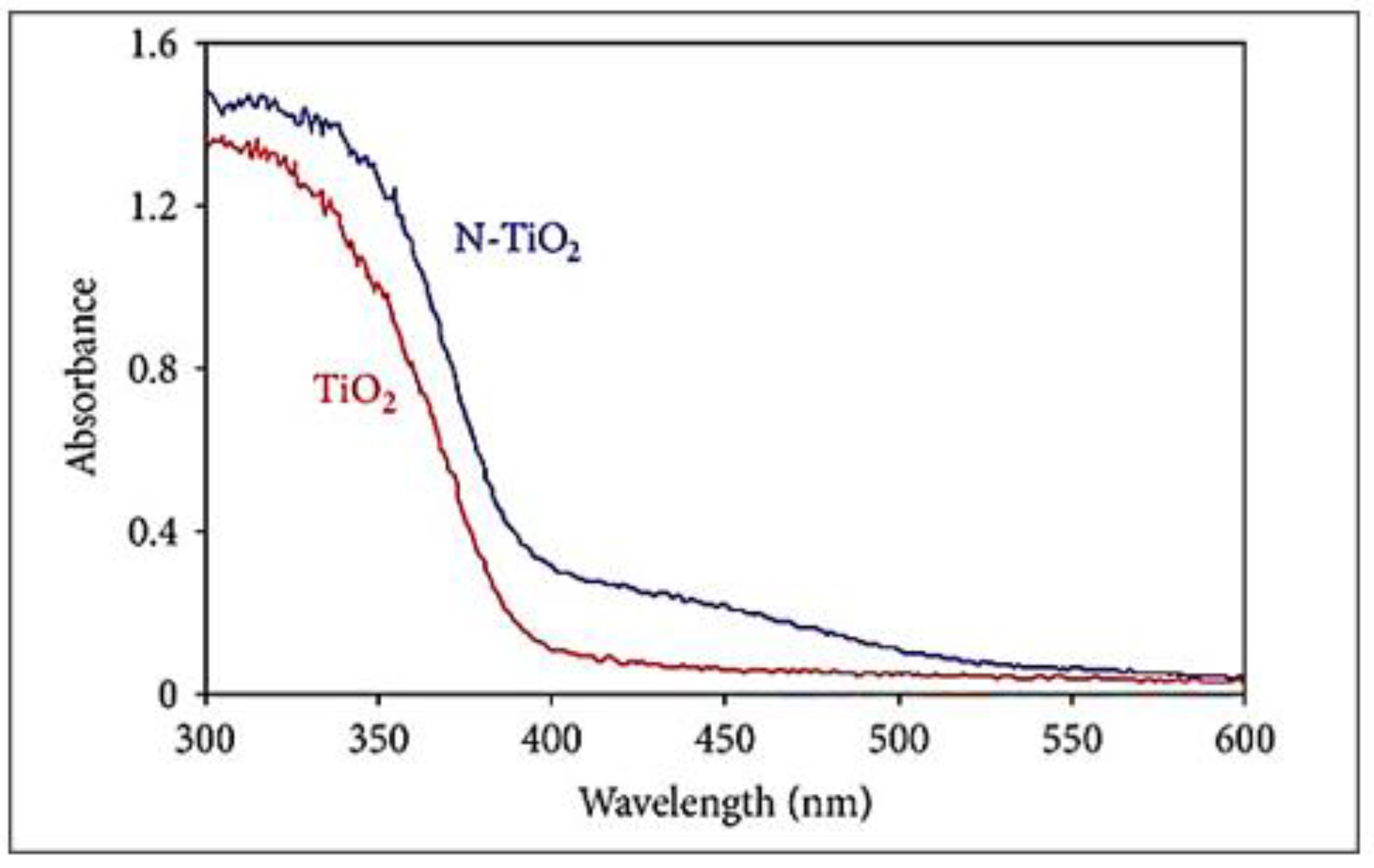
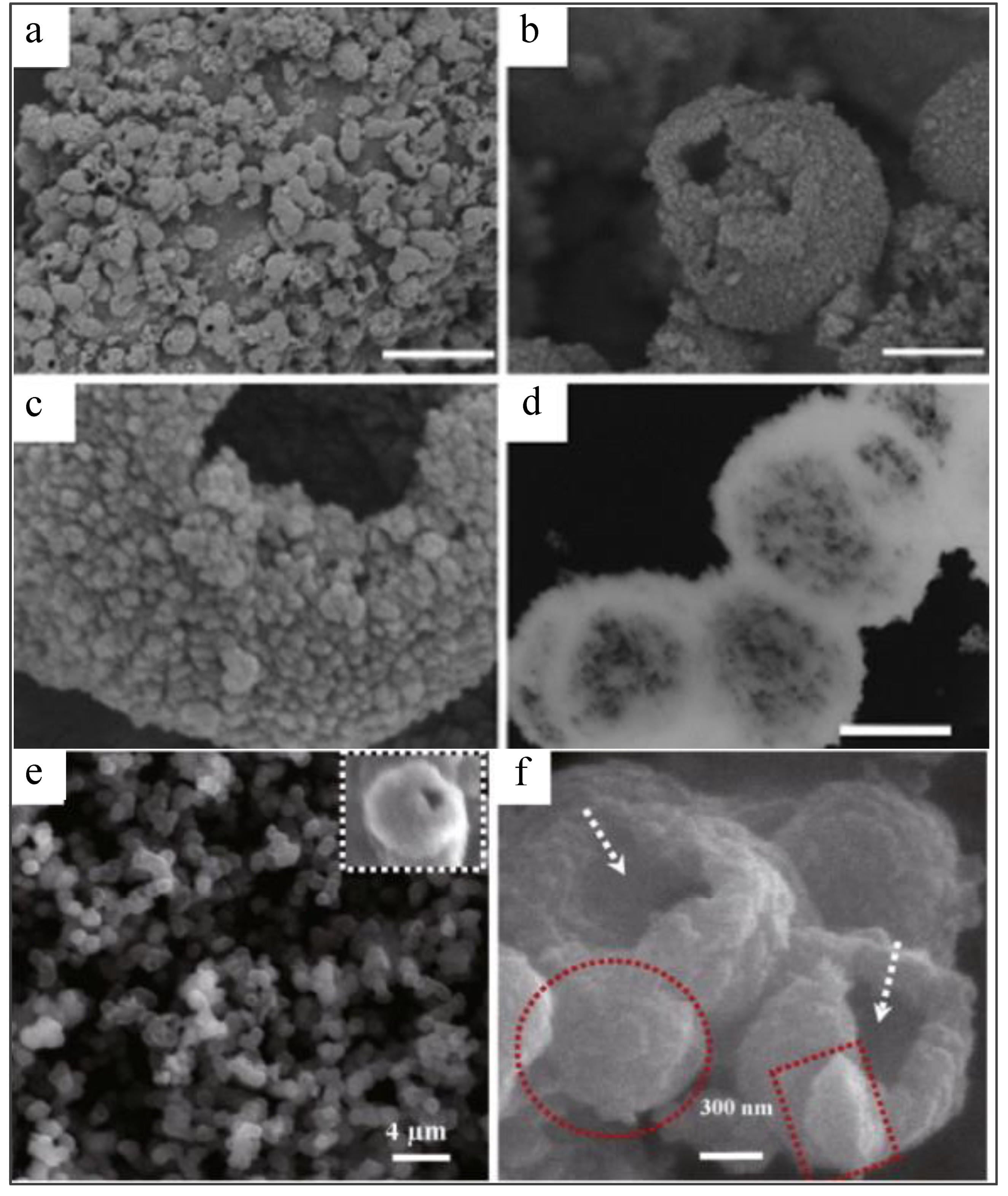
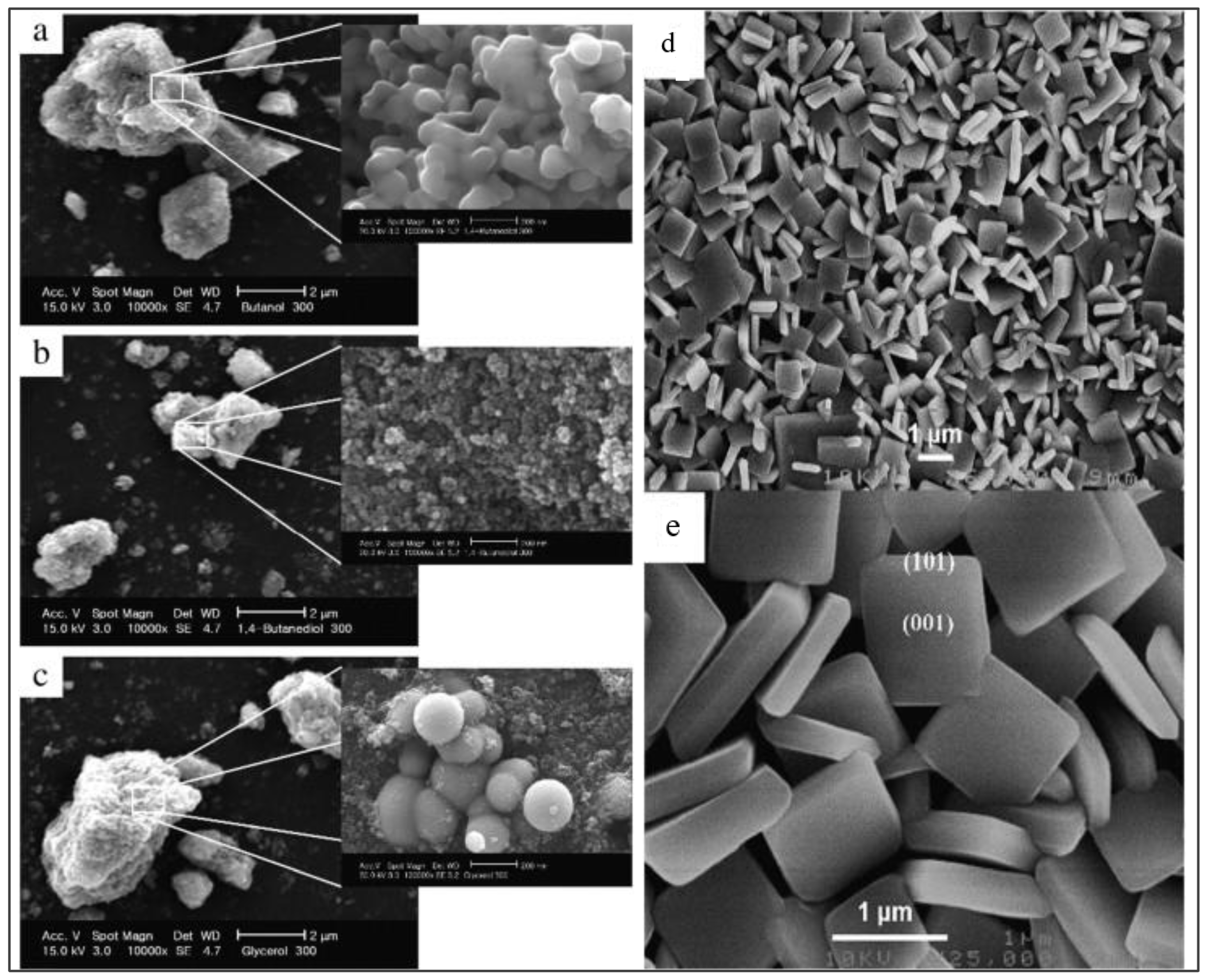
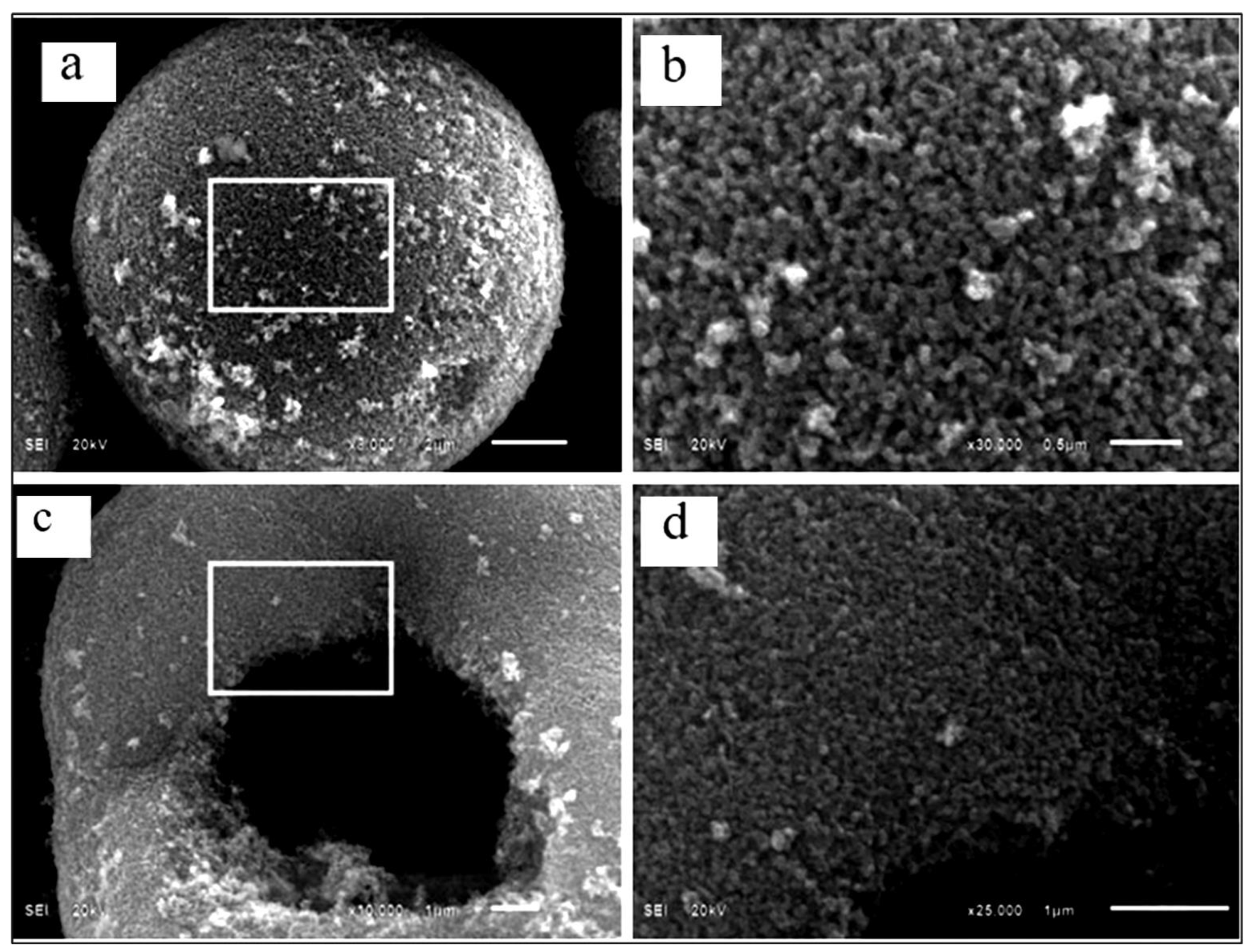
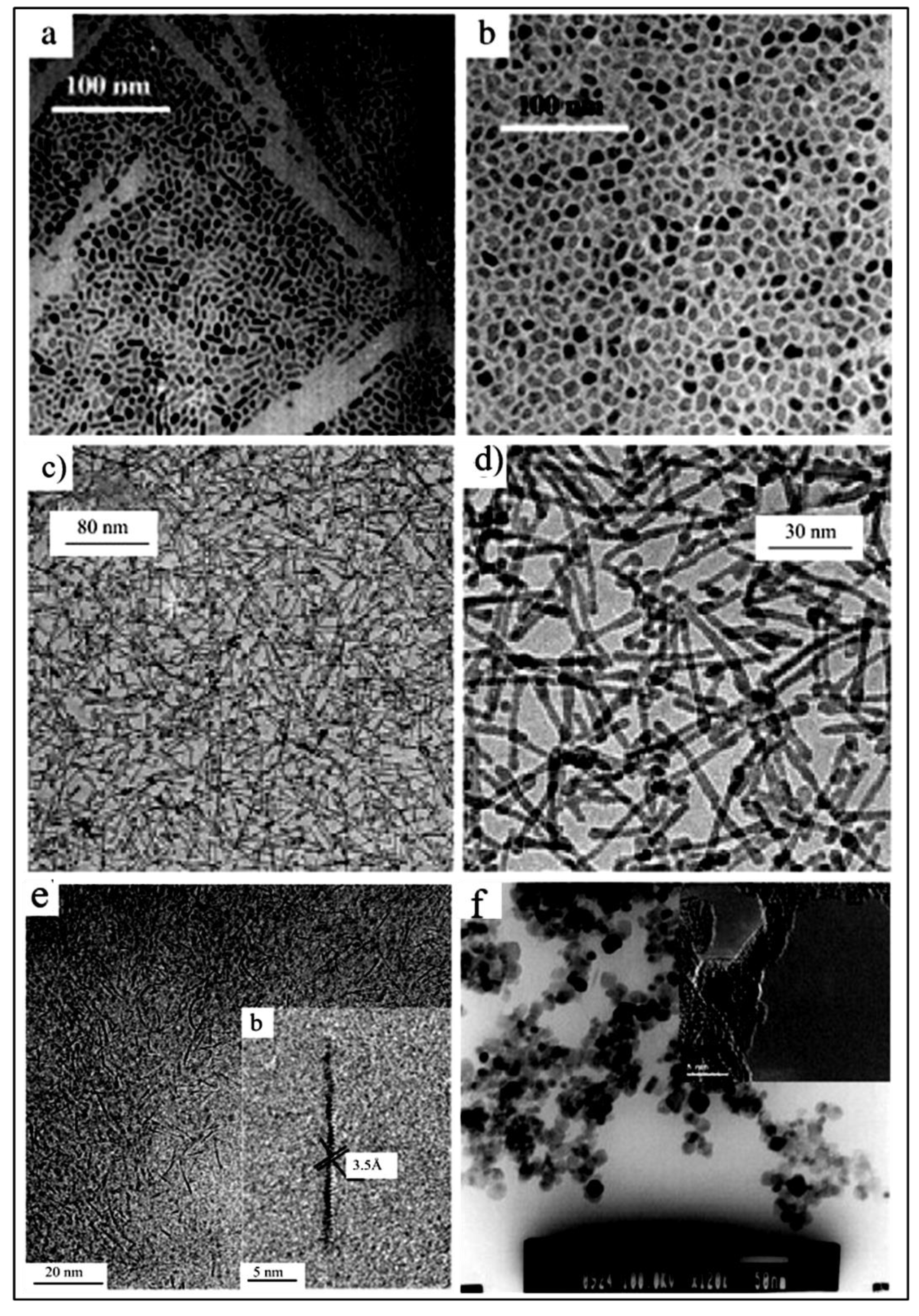
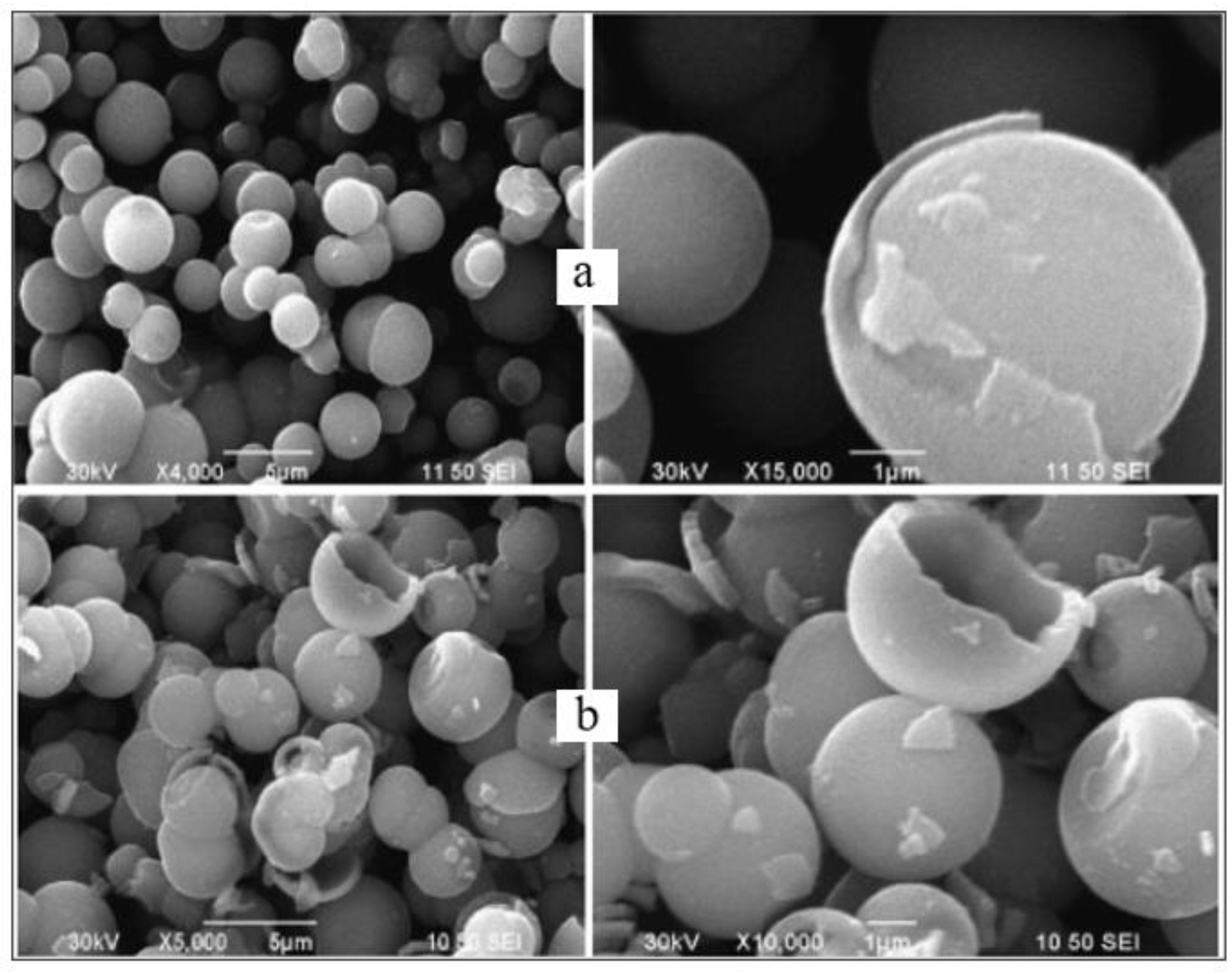

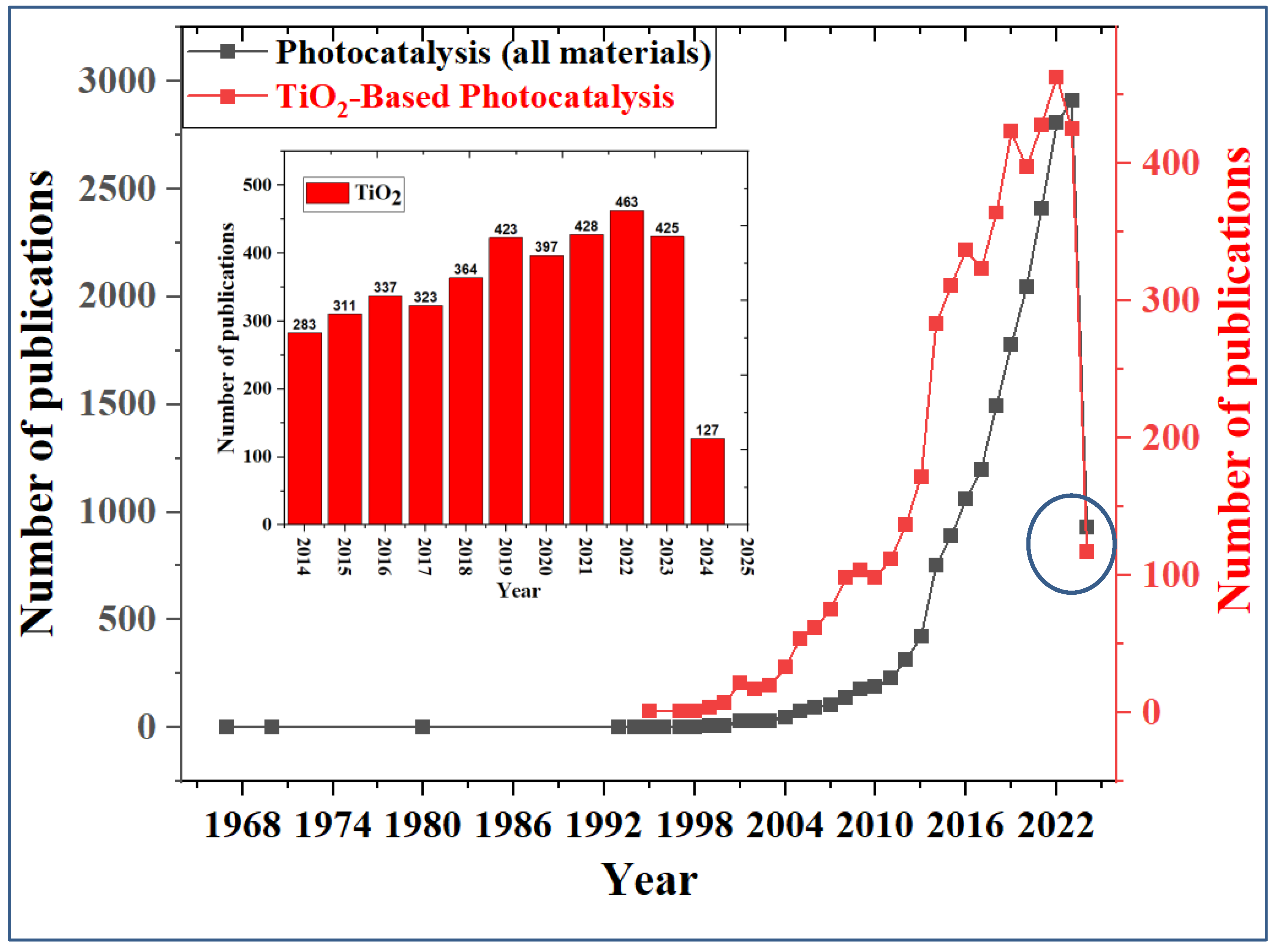
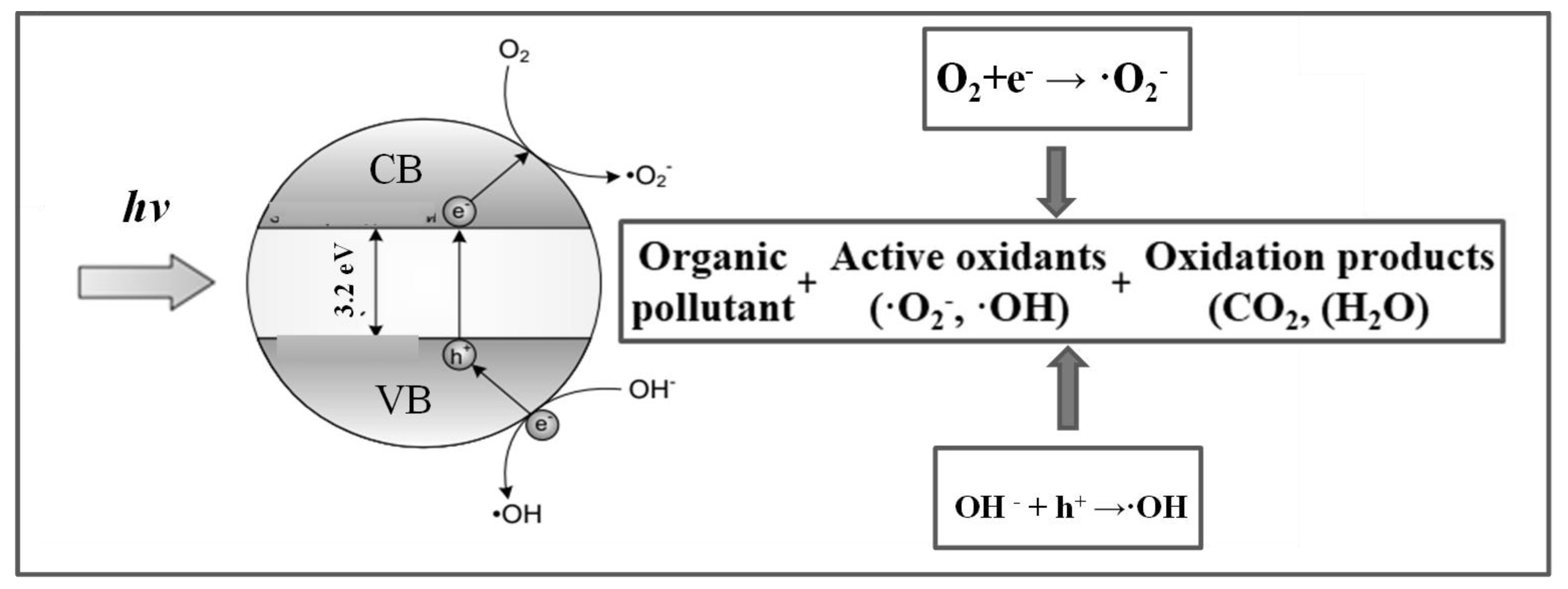
| Parameter | Anatase | Rutile | Brookite |
|---|---|---|---|
| Crystal structure | Tetragonal | Tetragonal | Rhombic |
| Lattice parameters (nm) | а=0.3784 с=0.9515 |
а=0.45936 с=0.29587 |
а=0.9184 b=0.5447 c=0.5154 |
| Density (g/cm3) | 3.79 | 4.13 | 3.99 |
| Space group | L4/amd | P4/mnm | Pbca |
| Number of units in cell | 2 | 2 | 4 |
| O-Ti-O bond angle | 77.7º, 92.6º | 81.2º, 90.0º | 77.0º-105º |
| Ti-O bond length (nm) | 0.1937(4) 0.1965(2) |
0.1949(4) 0.1980(2) |
0.187-0.204 |
| Processing Time (h) | Crystal Size (nm) | ||
|---|---|---|---|
| Anatase | Brookite | Rutile | |
| 2 | 4.9 | 7.7 | 29.1 |
| 3 | 5.7 | 9.7 | 34.8 |
| 12 | 8.6 | 14.0 | 51.7 |
| 24 | 10.0 | 15.6 | 57.4 |
| 72 | 12.9 | 19.8 | 63.7 |
| 168 | 16.6 | 23.5 | 67.2 |
| Oxidizing Agent | Oxidizing Potential (V) |
|---|---|
| Hydroxyl radical, HO• | +2.80 |
| Electron hole in VB, h+ | +2.70 |
| Ozone, O3 | +2.07 |
| Hydrogen peroxide, H2O2 | +1.78 |
| Permanganate ion, (MnO4)2- | +1.70 |
| Chlorine dioxide, ClO2 | +1.15 |
| Chlorine, Cl2 | +1.40 |
| Oxygen, O2 | +1.20 |
| Superoxide radical, O2- | -0.33 |
| Electron in the CB, e– | -0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





