1. Introduction
Several metal complexes have shown promising antineoplastic activity against cancer cells and tumors both in vitro and in vivo [
1]. A group of such complexes is casiopeinas® which was a result of the search for new anticancer drugs based on endogenous (essential) metals which could present less toxicity [
2,
3,
4]; they have proven cytotoxic to cancer cells sensitive or resistant to cisplatin, and to xenograph tumors in mice [
5].
Some of these Casiopeinas ® have exhibited greater antineoplastic potency than cisplatin
in vitro and
in vivo studies of a variety of tumor cell lines [
4,
6]; also have shown superoxide genomic instability through intrachromosomal recombination [
7], and a low potency to induce genomic instability through intrachromosomal recombination [
8]; these features suggest that these drugs have diminished undesirable side effects [
9]; stability constants and structural data have been reported [
10]. Casiopeina III-ia (CasIII-ia), (
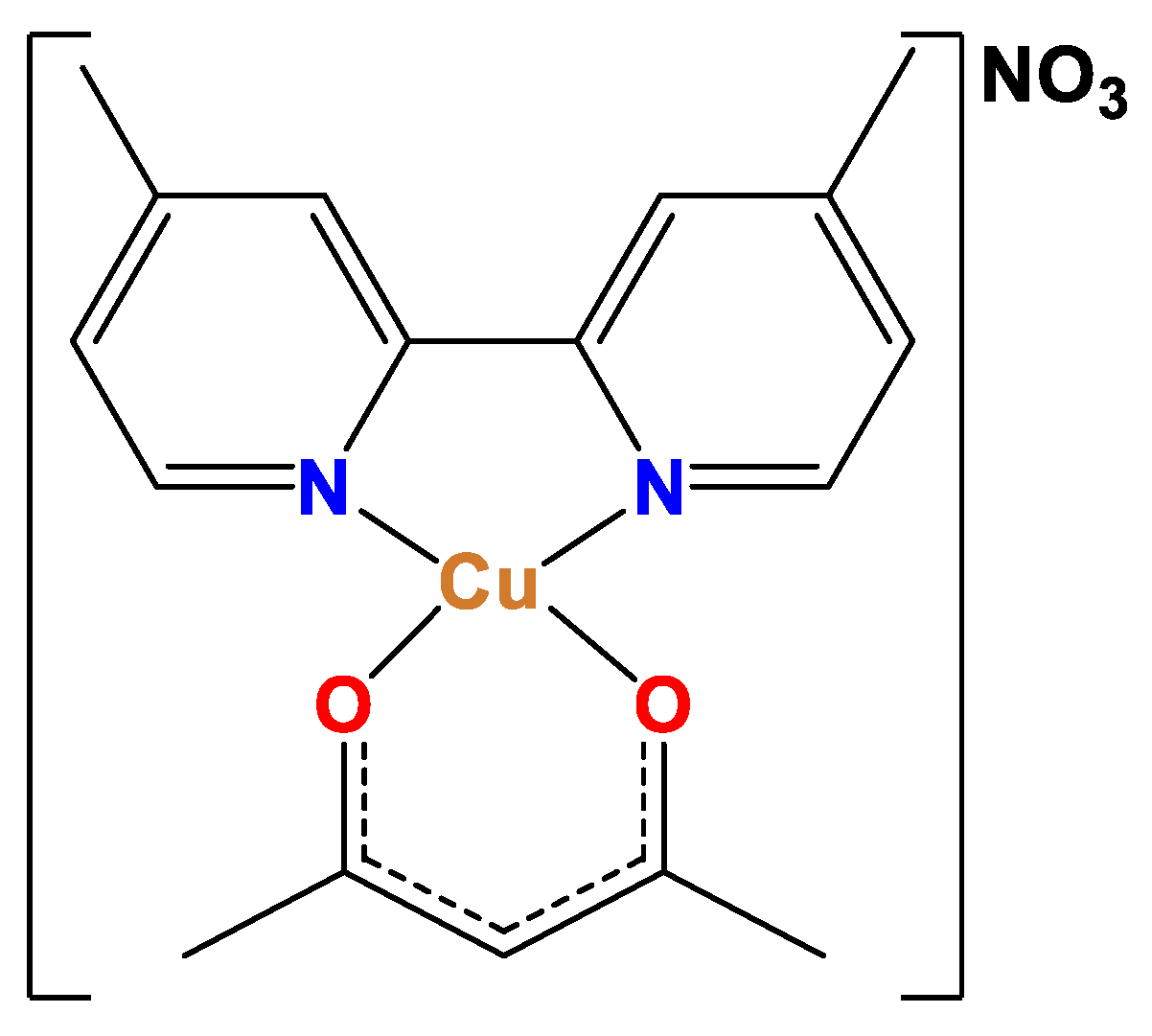
Figure 1) has shown in vitro a pharmacological effect and selectivity towards tumor lines (MCF-7, HCT-15, SK-N-SH neublastoma, HeLa and SiHa) and healthy cells, such as T lymphocytes and macrophages[
24,
25,
26]; in addition, in the case of CasIII-ia, its pharmacokinetic studies have been completed in different species such as rats [
27] and dogs [
20]. It should be noted that Cas III-ia is the first Mexican drug developed in a university with anticancer activity. that reaches phase 1 in clinical studies in Mexico.
Some of the mechanisms reported for casiopeinas® include DNA fragmentation and base oxidation, generating reactive oxygen species (ROS) and thereby causing copper reduction [
21]. ROS also affect the mitochondrial membrane by deporalizing it and causing mDNA damage by decreasing the levels of proteins involved in the respiratory chain, causing cell apoptosis via the caspase pathway. Casiopeinas have shown interaction with the cytochrome p450 isoform CYP1A1 enzyme, and an affinity for adenine [
22]. Additionally, it has been reported that casiopeinas interact with tubulins, integrins and proteins such as fibronectin, thereby producing changes in the cytoskeleton and finally cell death [
23]. Hemotoxicity in rat’s points to a more complex in vivo cytotoxicity of casiopeinas®, since the administration of a single dose of CII (5 mg/kg) did not generate serious damage and is within the functional range [
11,
12]. In terms of acute toxicological studies at a preclinical level in different species for casiopeinas®, the following results have been reported for casio III-Ea: NIH mice LD50 by intraperitoneal route = 12.47 mg/kg (females), 6.67 mg/kg (males); intravenously 7.12 mg/kg (females), 10.15 mg/kg (males); Wistar rat LD50 by intraperitoneal route 4.63 mg/kg (female), 5.26 (males); intravenously 8.48 mg/kg (females), 8.48 (males). The differences between species are visible, with Wistar rats being the most affected since their LD50 is smaller than in mice. The LD99 reported in dogs was 200 mg/m2 for casio III-ia and 160 mg/m2 for casio IIgly. These water toxicity data found in the different casiopeinas ® serve as a scientific basis to be able to extrapolate through allometric studies to select a dose that is safe to use in future clinical studies [
33,
34].
Studies have been reported on the pharmacokinetics of Cas III-ia in different animal species such as rats and dogs. The reported viability in terms of pharmacokinetic parameters is related to body weight and the physiological processes of each animal species [
20,
27,
28,
29].
Cas III-ia (
Figure 1) is a potentially useful antineoplastic agent [
13]. It is very active against L1210 leukemia cells and kills cells by induction of apoptosis [
11]; induces a weak recombinogenic action and can degrade DNA in vitro under a range of several cultures.
High-performed liquid chromatography (HPLC) methods for the quantification of Cas III-ia® and Cas IIgly® in rat plasma were reported. [
14,
15], however to determine the preclinical pharmacokinetic parameters in rabbits and
in vitro distribution, a sensitive and specific method of assay is needed in order to measure the drug in blood.Therefore, in this article we have developed and validated a simple and available gradient reversed-phase HPLC. The method was validated according to procedures and acceptance criteria based on national (NOM-177-SSA1-2013)[
31] ,international (FDA,2001) [
32] guidelines and recommendations of other authors [
16,
17,
18].
2. Materials and Methods
2.1. Reagents and Chemicals
Cas III-ia was obtained in our laboratory following the procedure reports in Patents [
2,
3]. Poolet rabbit total blood samples were used for the validation method. Acetaminophen (2.5 µg/ml) USP reference standard) was used as internal standard. It was added to academic solutions (calibration samples in methanol and control rabbit total blood samples). The relative peak area (drug peak/internal standard peak) was analyzed.
Methanol was HPLC grade. Water was produced by Milli-Q water system (Millipore, Bedford, MA, USA) Methanol was of HPLC grade, sodium phosphate sodium and other reagents are commercially available and were of analytical grade.
2.2. Animals
Male New Zealand rabbits, weighing between 2.0 and 3.0 kg were used in the study. The animals were kept under clean conventional conditions and had access to food and water ad libitum.
2.3. Chromatographic Conditions
The assay was performed using a high performance liquid chromatograph system with a Shimadzu pump Model LC10ADVP (Kyoto Japan), a Shimadzu variable-wavelength UV absorbance detector model SPD10ADVP, an automatic injector Shimadzu model SIL10ADVP fitted with a 50 μL sample loop (Cotati, CA, USA), a Shimadzu system controller model SCL10AVP (Kyoto Japan) and an integrator chromatography data station, (Shidmadzu Class VP Version 5.0, Shimadzu, 1999)., separations were achieved using a Symmetry ® C18 column of 250 x 4.6 mm I.D and particle size of 5 μm (Waters Associates, Millford, MA, USA) that was preceded by a C18, 5 μm guard column (Phenomenex®). The mobile phase was 0.01 M sodium phosphate buffer (pH 6.5)-Methanol (60:40) was kept at a flow-rate of 0.8 ml/min. The analyses were performed at room temperature. The absorbance at 262 nm was recorder at a sensitivity of 0.1 AUFS (absorbance units full scale) in the programmed parameters.
2.4. Sample Preparation
To 200 μL of blood was added to 0.6 μL of methanol and the mixture was shaken for 30 s in a vortex, then 50 μL of zinc sulfate (10% w/w) and 150 μL of Acetaminophen (concentration of 2.5 μg/ml) were added, followed by vigorous stirring for 30 s, and centrifugation for 5 min a 5000 g. The supernatant was transferred to vials and an aliquot of 50 μL was injected into the HPLC system.
2.4.1. Calibration Curves in Methanol
Stock solution of Cas III-ia was prepared dissolving 100 mg of Cas III-ia in methanol and then diluted to 10 ml with the same solvent. Concentrations of 10 –120 μg/ml were prepared in mobile phase.
2.4.2. Calibration Curves in Total Blood of Rabbit
4.0 mg of Cas III-ia was diluted to 10 ml in rabbit blood (400 μg/ml); the required concentrations (10-120 μg/ml) were prepared in rabbit blood at a dilution of 2 ml.
2.4.3. Intraday and Interday Variation Coefficient
We analyzed by quintuplicate, with three concentrations (high, medium and low quality control), 15, 35 and 75 ug/mL).
2.5. Stability Studies
For stability studies, control rabbit blood and methanol solutions were spiked with Cas III-ia (1mL) in Eppendorf tubes, at 6 °C (during 96 h) and at room temperature with or without protection of the light (during 96 h); each determination was performed in duplicate and the samples were treated in accordance to the sample preparation.
2.6. Preclinical Pharmacokinetics
Ten healthy male New Zealand rabbits, weighing between 2.0 and 3.0 kg were used in the study. A dose of 10.0 mg/kg of Cas III-ia was prepared in 30 ml of a mixture of saline solution and methanol (10:1), it was administered by slow infusion (0.5 mL/min) during 60 min. into the marginal ear vein. Blood samples were collected into small plastic centrifuge tubes through a cannula inserted into the marginal ear vein just before dosing and at 80, 90, 100, 110, 120, 140, 160, 180, 210, 240, 270 y 300 minutes after Cas IIIi-a administration. After each sample withdrawal, the cannula was flushed with an equal volume of heparinized solution. The blood samples were then immediately stored at 4°C until analysis.
3. Results and Discussion
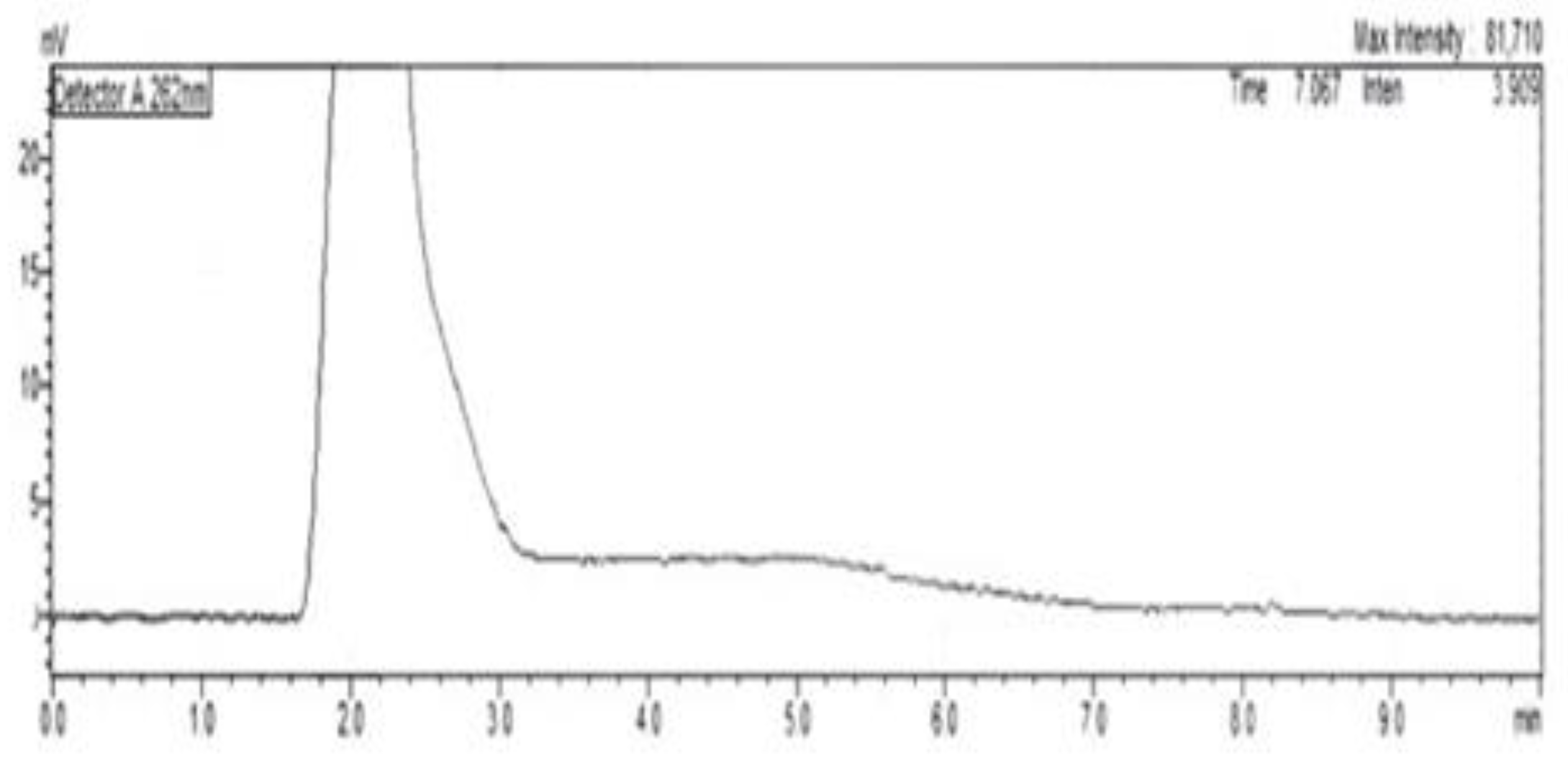
Representative chromatograms of rabbit blood are shown in (
Figure 2) blank total blood chromatogram, (
Figure 3) Retention time for Cas III-ia was 10.0 min. No interfering peaks from blood were detected at the retention time of Cas III-ia
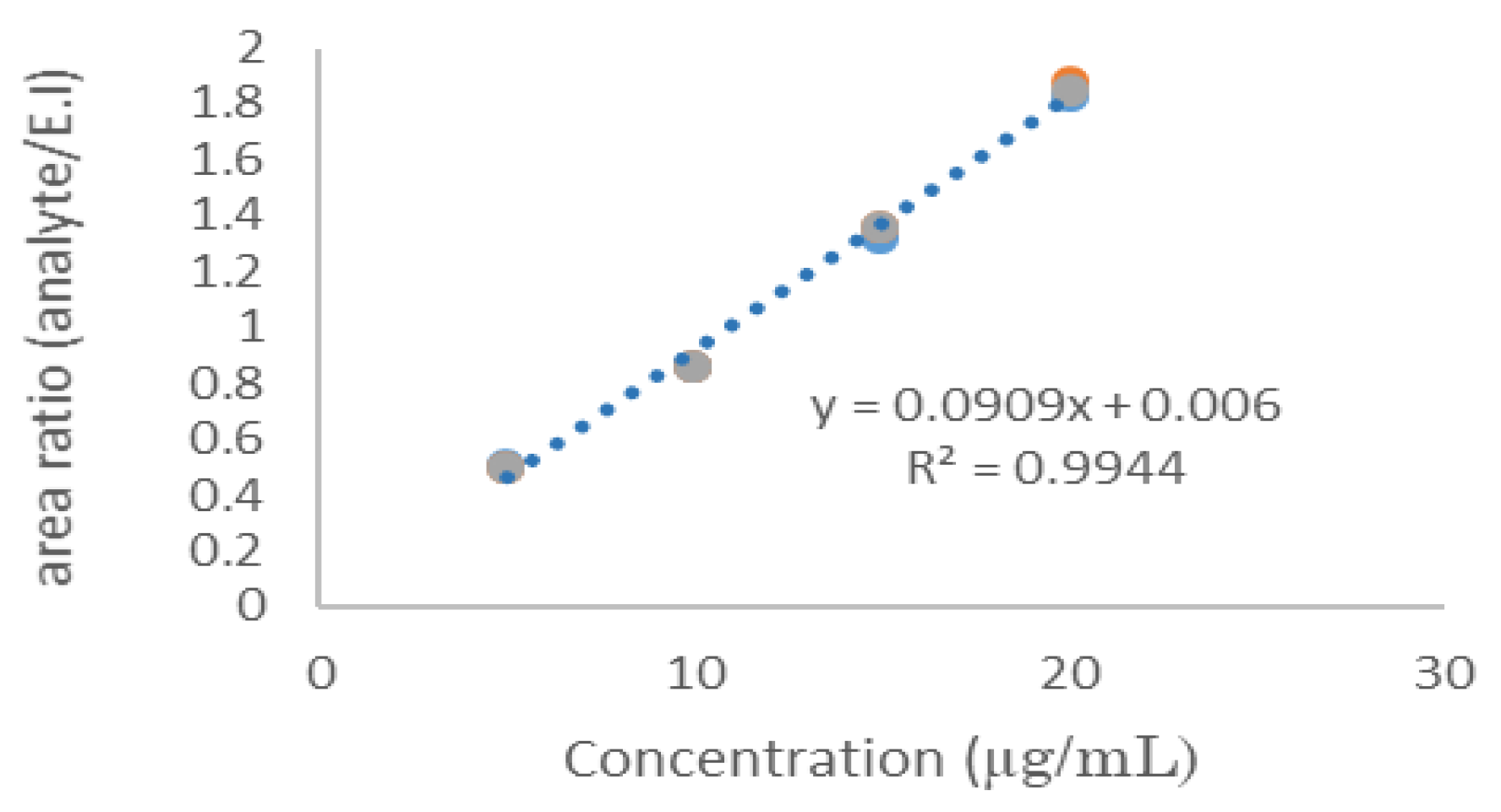
A relationship (r
2=0.9944) was found when the relative peak area of Cass-III-ia was plotted against various concentrations from 10 to 120 μg/mL, (10.0, 20.0, 40.0, 60.0, 80.0 and 120.0 μg/mL in 600 μL of rabbit blood, curves in triplicate assays (
Table 1 and
Figure 4).
Intra-day and inter-day precision of the method, assessed by analyzing samples, are shown in
Table 2. It was estimated from control curves samples prepared on the same day (n=15) and different days (n=30)., using different stock solutions. The corresponding coefficient of variation (C.V) was 0.89% to 5.10.
The recoveries of Cas III-ia was determined by comparing the relative peak area from total blood spiked with amounts of the compound (15, 35 and 70 μg/mL) using described extraction procedure vs. the relative peak area from the same series prepared in mobile phase and injected into HPLC. Each sample was determined in quintuplicate. The mean recovery of Cas III-ia in blood averaged 84.88% (n=15).There are previous reports where casiopeinas® in plasma protein binding assays present a significant accumulation in total blood compared to plasma because a significant binding to blood cells is reported in red blood cell/plasma ratios (Ke/p) above 2 for human blood and Beagle dogs at concentrations of 1 µg/mL.[
19]. Therefore, performing the extraction process in total blood was more efficient to determine pharmacokinetic parameters with respect to rabbit plasma.
El LOQ of 5.08 μg/mL was defined as the sample concentration from spiked blood resulting in a peak area of ten times the noise level.
The LOD was defined as the sample concentration resulting in a peak of three times noise level. A value of 3.5 μg/mL was determined.
The stability of Cas III-ia; before and after sample pre-treatment was determined. After 96 h at 40C 99.85% of Cas III-ia was still present in blood rabbit. There was 74.96% Cas III-ia at 370C protected of the light after 96 h and blood spiked with Cas III-ia at 370C without protection of the light was determined to be 97.5 % for 96 h.
Pharmacokinetic Results
No chromatographic interferences from any endogenous compounds were found.
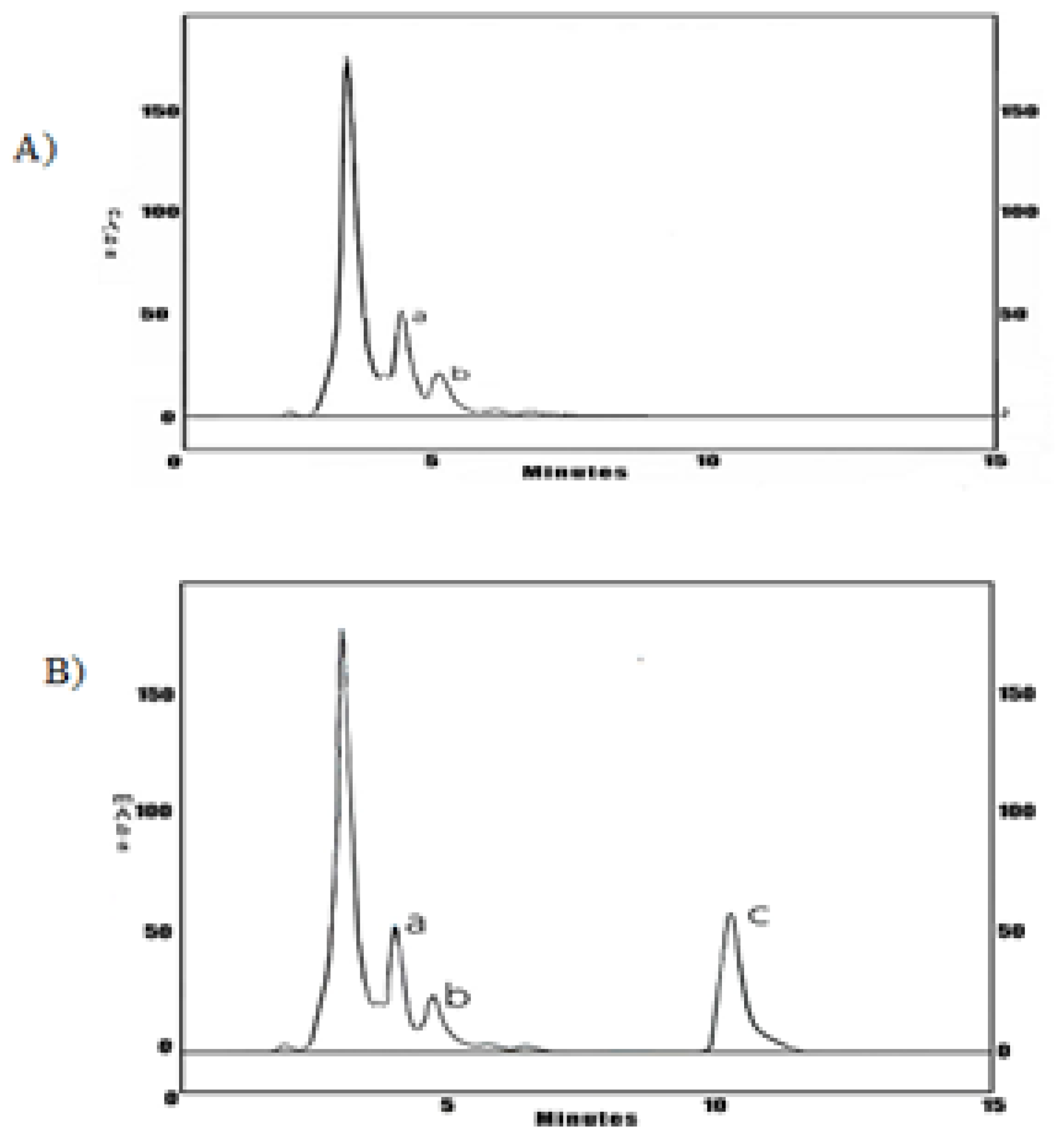
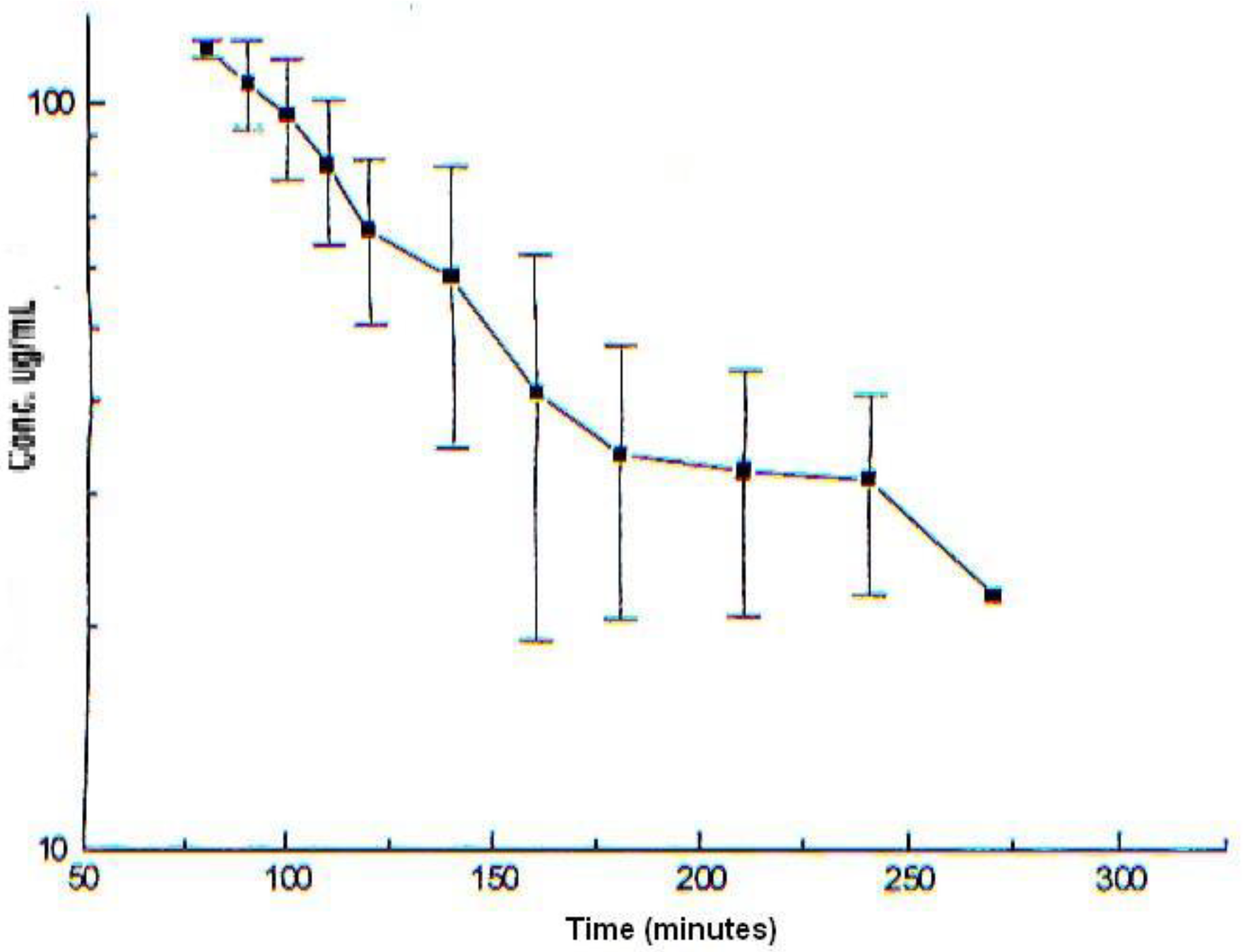
Shows (
Figure 5) the blood levels in the stationary state (Css) concentration-time profile of Cas III-ia were 117.42± 3.26 μg/mL in a one-compartment model, obtained by WINNOLIN software, and the half-life time was 53.92± 25.41 min.
The pharmacokinetic parameters obtained in rabbit were the following: (kel) = 0.0150 min-1, half-life time (T1/2) = 53.92 min, apparent volume of distribution (Vd) = 202.81 mL, clearance (Cl) = 2.08 mL /mi and area under the curve (AUC)=23163.85 µg/mL.min.
According to the results obtained, we can observe that the half-life time in the rabbit (0.88 h) is shorter compared to the rat (12.46 h) [
29]. When comparing the clearance in the rabbit, which was 2.08 mL/min, with respect to the clearance in the rat (0.45 mL/min), we can observe that the rabbit has a higher elimination rate. Comparing the volume of distribution in the different species rabbit (202.81 mL), rat (0.462 L)[
29] and dog TMR= approximately 2 weeks (data obtained from compartmental modeling program winnolin)[
29] we can observe that Cas III-ia presents a wide distribution in tissues; according to these results obtained, we can suggest that as reported for other casiopeinas® casiopeina III-ia has a wide distribution in tissues such as blood due to its high affinity to blood cells such as erythrocytes [
19]. However, although different doses were administered intravenously in each species, it is observed that it presents low bioavailability when comparing the 3 species dose 10 mg/kg, rabbit=23163.85 µg/mL.min, dose 10 mg/kg rat=22.27 mg/mL.min,[
29] dog dose 3.5 mg/kg=40472.75 µg/mL.min,[
29]. These data indicate that there is interspecies variability because of the body weight and physiological processes of each species. Pharmacokinetic scaling between species is necessary for the optimization of test doses in humans through allometric equations where biochemical, anatomical and physiological similarities between animals can be generalized and expressed in mathematical models [
30].
4. Conclusions
The method development proved to be useful and reliable for the determination of Cas III-ia in total blood rabbit. The pre-treatment procedure for the sample, involving direct precipitation with zinc sulphate, is fast and simple.
The method, validated for concentrations in the range of 10 to 120 μg/mL, had good repeatability and accuracy and low limits of quantification and detection. The recovery of Cas III-ia was good enough; it is reproducible and constant over the entire range of the calibration line. This method is sufficiently sensitive to perform pharmacokinetic studies and can be applied in future preclinical pharmacokinetic studies.
The pharmacokinetic data obtained in the present work and its comparison with the data in the different species reported [
29], contribute to the characterization of Cas III-ia, these data at a preclinical level will allow us to know the bases to be able to extrapolate an adequate dose through allometric studies to carry out studies in future clinical stages and also build on the adequate design of dosing intervals that are safe for humans.
Autor Contributions: All authors contributed to preparing and writing the text according to content, particularly as follows: Investigation, Nancy Gama, Julia Jarquin, Hector Ariel Morales † and Inés Noriega; Methodology, Kenneth Carrasco, Julia Jarquin and Hector Ariel Morales †; Project administration, Inés Noriega; Resources, Lena Azuara and Inés Noriega; Supervision, Kenneth Carrasco; Validation, Kenneth Carrasco; Writing – original draft, Nancy Gama.
Funding information: This work was funded by Project PAPIIT IN240214 biopharmaceutical research and preclinical kinetics of drugs and medications.
Ethical approval: The letter is in the process of delivery.
Acknowledgmets
Adrián Espinoza Guillen is dedicated to all the students and collaborators who have participated in the development of this article about casiopeinas®.
Conflicts of interest: Nancy Vara Gama declares that she has no conflict of interest. L. Ruiz Azuara declares that she has no conflict of interest. I. Fuentes Noriega declares that she has no conflict of interest. K. Rubio-Carrasco declares that she has no conflict of interest. J. Antonio-Jarquin declares that she has no conflict of interest and H. Rico- Morales declares that she has no conflict of interest.
Data Availability Statement
Not applicable.
References
- Guo, U.Z.; Sadler, P.I. Metals in Medicine. Angew Chem. Int. 1999, 38, 1512–1531. [Google Scholar] [CrossRef]
- Azuara, L.R. Process to obtain new mixed copper aminoacidate from methyl phenanthroline complexes to be used as anticancerigenic agents. U.S. Patent Pat. 1996, 19, A5576326. [Google Scholar]
- Azuara, L.R. Process to obtain new mixed copper aminoacidate complexes from phenylatephenanthroline to be used as anticancerigenic agent. US. Patent Re 1997, 35, 458. [Google Scholar]
- Ruiz-Ramírez, L.; Gracia, I.; Moreno, R.; Díaz, L.; Huerta, L.; Mayet, L.; Ortiz, V.; Lomeli, C. The antitumor activity of several transition metal complexes. J. Inorg. Biochem. 1991, 43, 615. [Google Scholar] [CrossRef]
- Rivero, M.; De Vizcaya, A.; Plant, N.; Ruiz, L.; Dobrota, M. Mixed chelate copper complex, Casiopeina II gly®, binds and degrades nucleic acids: A mechanism of cytotoxicity. Chemicol-Biol. 2007, 165, 189–199. [Google Scholar] [CrossRef]
- Hernández, L.; Marin, A.; Pavon, N.; Carvajal, K.; Moreno, R. Cardiotoxicity of Koper-based antineoplastic drugs casiopeinas is related to inhibition of energy metabolism. Toxicol Appl Pharmacol. 2006, 212, 79–88. [Google Scholar] [CrossRef]
- Ferrer, G.; Ruiz-Ramírez, L.; Radi, R. Ternary Koper complexes and manganese (III) tetrakis(4-benzoic acid) porphyrin catalyze peroxynitrile-dependent nitration of aromatics. Chem. Res. Toxicol. 1997, 10, 1338–1344. [Google Scholar] [CrossRef]
- Arnaudeau, C.; Tenorio, E.; Jenssen, D.; Helleday, T. Inhibition of DNA synthesis is a potent mechanism by which cytostatic drugs induce homologous recombination in mammalian cells. Mutant Res. 2000, 461, 221–228. [Google Scholar] [CrossRef]
- Marin, H.; Gracia, I.; Ruiz-Ramírez, L.; Moreno, R. Toxic effects of Koper-based antineoplastic drugs (Casiopeinas) on mitochondrial functions. Biochem Pharmacol. 2003, 65, 1979–1989. [Google Scholar] [CrossRef]
- Solans, X.; Ruiz-Ramírez, L.; Martínez, A.; Gasque, L.; Briansó, J.L. Structures of chloro(glycinato) (1,10-phenanthroline) Copper (II) monohydratade (I) and aqual(1,10-phenanthroline) (Lphenylalaninato)copper(II)nitrate monohydrate (II). Acta Crystallogr C. 1998, 44, 628–631. [Google Scholar] [CrossRef]
- De Vizcaya, A. Rivero, L. Ruiz-Ramírez, J.A. Howarth, M. Dobrota, Hematoxicity response in rats by the novel Copper-based anticancer agent: casiopeina II. Toxicol. 2003, 194, 103–113. [Google Scholar] [CrossRef]
- Lippard, S.J. Platinum complexes: probes of polynucleotide structure and antitumor drugs. Acc. Chem. Res. 1978, 11, 211–217. [Google Scholar] [CrossRef]
- Trejo, C.; Palencia, G.; Zúñiga, S.; Rodríguez, A.; Osorio, L.; Luvia, S.T.; Gracia, I.; Márquez, L.; Moreno, M.E.; Bravo, M.E.; Ruiz-Azuara, L.; Rodríguez, S.; Sotelo, J. Cas IIgly induces apoptosis in glioma C6 cells in vitro and in vivo through caspase-dependent and caspase-independent mechanisms. Neoplasia. 2005, 7, 563–574. [Google Scholar] [CrossRef]
- Fuentes, L. Ruiz-Ramírez, A. Tovar., H. Rico, I. Gracia, Development and validation of a liquid chromatographic method for Casiopeina IIIi in rat plasma. J Chromatograph B Analyt Technol Biomed Life Sci. 2002, 772, 115–121. [Google Scholar] [CrossRef]
- Reyes, L.; Fuentes, I.; Ruiz-Ramírez, L.; Macías, L. Development and validation of a liquid chromatographic method for Casiopeina IIgly in rat plasma. J Chromatograph B Analyt Technol Biomed Life Sci. 2003, 791, 111–116. [Google Scholar] [CrossRef]
- Braggio, S.; Barnab, R.J.; Grossi, P.; Cugola, M. A strategy for validation of bioanalytical methods. J. Pharm Biomed Anal. 1996, 14, 375–388. [Google Scholar] [CrossRef]
- Bresolle, F.; Bromet-Petit, M.; Audran, M. Validation of liquid chromatographic and gas chromatographic methods. Applications to pharmacokinetics. J. Chromatogr B Biomed Appl. 1996, 686, 3–10. [Google Scholar] [CrossRef]
- Shah, V.P.; Midha, K.K.; Dighe, S.; McGilveray, I.J.; Skelly, J.P.; Yacobi, A.; Layloff, T.; Viswanathan, C.T.; Cook, C.E.; McDowall, R.D. , et al. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J. Drug Metab Pharmaco 1991, 16, 249–255. [Google Scholar]
- Roberto Carlos Cañas-Alonso, Inés Fuentes-Noriega, and Lena Ruiz-Azuara, Blood to Plasma Ratio, Short-Term Stability and Plasma Protein Binding of Casiopeína IIgly, a Copper (II) Based Compound with Antineoplastic Activity, J. Mex. Chem. Soc. 2013, 57, 239–244.
- Cañas-Alonso, R.C. , Fuentes-Noriega I., Ruiz-Azuara L. Pharmacokinetics of Casiopeína IIgly in beagle dog: A copper based compound with antineoplastic activity. J. Bioanal. Biomed 2010, 2, 28–34. [Google Scholar]
- Rodrigo Galindo-Murillo, Juan Carlos García-Ramos, Lena Ruiz-Azuara, Thomas E. Cheatham III and FernandoCortés-Guzmán, Intercalation processes of copper complexes in DNA. Nucleic Acids Research 2015, 43, 5364–5376.
- Campero, P. C., Bravo, G. M. E., Hernández, O. S. L., Olguin, R. S. R., Espinosa, A. J. J., and Ruiz-Azuara, L. Effect of [Cu(4,7-dimethyl-1,10-phenanthroline) (acetylaceto- nato)]NO3, Casiopeína III-Ea, on the activity of cyto- chrome P450. Toxicol. In Vitro. 2016, 33, 16–22. [CrossRef]
- Becco, L., Rodríguez, A., Bravo, M. E., Prieto, M. J., Ruiz-Azuara, L., Garat, B., Moreno, V., and Gambino, D. New achievements on biological aspects of copper complexes Casiopeínas®: Interaction with DNA and proteins and anti-Trypanosoma cruzi activity. J. Inorg. Biochem 2012, 109, 49–56. [CrossRef]
- Juan Carlos García-Ramos, Yanis Toledano-Magaña, Anllely G Gutiérrez, Adriana Vázquez- Aguirre, Ana L Alonso-Sáenz, Virginia Gómez-Vidales, Marcos Flores-Álamo, Carmen Mejía, Lena Ruiz-Azuara, The mitochondrial apoptotic pathway is induced by Cu(II) antineoplastic compounds (Casiopeínas®) in SK-N-SH neuroblastoma cells after short exposure times. BioMetals. BIOM-D-16-00234 (2017) 30:43–58. [CrossRef]
- Francisco Carvallo-Chaigneau, Cristina Trejo-Solís,Celedonio Gómez-Ruiz, Ernesto Rodríguez-Aguilera, Lucía Macías-Rosales,Edith Cortés-Barberena,Carlos Cedillo-Peláez, Isabel Gracia-Mora, Lena Ruiz-Azuara, Vicente Madrid-Marina, Fernando Constantino-Casas, Casiopeina III-ia induces apoptosis in HCT-15 cells in vitro through caspase-dependent mechanisms and has antitumor effect in vivo, Biometals,2008,21(1): 17-28. [CrossRef]
- Ruiz-Azuara, Lena and Bravo Ma Elena, Copper Compounds in Cancer Chemotherapy, Curr. Med. Chem., 2010,17(31): 3606-3615. [CrossRef]
- Ines Fuentes-Noriega, Lena Ruiz-Ramírez, Araceli Tovar Tovar, Hector Rico-Morales, Isabel Gracia-Mora, Development and validation of a liquid chromatographic method for Casiopeina IIIi in rat plasma, J. Chromatogr. B, 2002, 772 : 115–121. [CrossRef]
- Romero, Estudio preliminar de farmacocinética de casiopeína III-ia (un nuevo anticancerígeno) en ratas, a partir del análisis de datos urinarios,2007, Universidad Nacional Autónoma de México, Facultad de Farmacia,México.
- Ines Fuentes Noriega, Farmacocinética preclínica de casiopeína IIIia y su unión a proteínas plasmáticas, 2005,Universidad Nacional Autónoma de México, Facultad de Farmacia,México.
- Paul R., V. Malik and Andrea N. Edginton,Physiologically-based pharmacokinetic modeling vs. allometric scaling for the prediction of infliximab pharmacokinetics in pediatric patients, cpt pharmacometrics syst pharmacol, 2019, 8(11): 835–844. [CrossRef]
- Mexican Official Standard NOM-177-SSA1-2013, Establishes the tests and procedures to demonstrate that a medicine is interchangeable and a biotechnological medicine is biocomparable.
- Guidance for Industry Statistical Approaches to Establishing Bioequivalence, U.S. Guidance for Industry Statistical Approaches to Establishing Bioequivalence, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), 2001,1-48.
- Rivera Huerta Marisol, Intravenous and intraperitoneal determination of lethal dose 50 (LD50) of casiopeina IIIE in rat and mouse, 1999, Universidad Nacional Autónoma de México. Facultad de Medicina Veterinaria, México.
- Marco Leal-García, Luis García-Ortuño, Lena Ruiz-Azuara, Isabel Gracia-Mora, Jorge Luna-delVillar and Héctor Sumano, Assessment of Acute Respiratory and Cardiovascular Toxicity of Casiopeinas in Anaesthetized Dogs, J. Comp. Nordic Pharmacol. Soc. Basic & Clin. Pharmacol. & Toxicol.,2007, 101:151–158. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).