1. Introduction
An electrochemical device such as a supercapacitor can store a large number of electrical charges by using electrodes with a large surface area. Supercapacitors, have attracted attention due to its high energy and power density [
1,
2]. Supercapacitors can be categorized into three types: electrochemical double layer supercapacitors, pseudocapacitors and hybrid supercapacitors based on their storage concept [
3]. Carbon based materials have been widely studied due to its high specific surface area, tunable pores, and outstanding chemical stability [
4]. Especially, graphene and its counterpart was investigated as an electrode for supercapacitor [
5] due to its tenability and conjugated network [
6]. Even this material can be added with bionanocomposite to improve the conductivity in all possible ways [
7]. Metal-organic frameworks (MOFs) are crystalline porous organic-inorganic hybrid materials that have a very high internal space due to their hollow nature [
8]. Several metal ions are used in the synthesis of MOFs. MOFs consist of two major components, metal ions and organic linkers. MOFs are typically prepared by combining metal ions and organic linkers under mild conditions using the solvothermal method to create a crystalline and porous network [
9,
10]. Similarly, polyaniline shows promise in energy storage due to its light weight, high conductivity, mechanical flexibility, and low cost, allowing it to work under pseudocapacitance [
11,
12,
13,
14,
15,
16,
17]. Even widening of the potential window can be accomplished by polymer based electrolyte [
18].
Yanhong et al. [
9] found that the manganese-based MOF maintained 105% capacitance even after 5000 cycles. MOF based materials used as an anode in a supercapacitor with rich porosity, and wide surface area. Zhang et al. [
19] proposed the use of Bimetallic Ni Mn-MOF nanosheets on NiCo
2O
4 Nanowire arrays to enhance electrochemical performance. Super capattery was developed using Co-MOF/polyaniline-based electrode material by Iqbal and co-authors [
20]. The composite electrode of MOF and PANI with an equal ration demonstrated a specific capacitance of 162.5 Cg
-1 at 0.4 Ag
-1 and a very low ESR in electrochemical impedance spectroscopy. Similarly, Co-MOF/PANI composite electrode was proposed by Srinivasan et al. [
21] using cobalt nitrate, benzene tricarboxylic acid, and aniline through chemically in situ oxidative polymerization. The constructed electrode exhibited a specific capacitance of 504 Fg
-1 at 1 Ag
-1, and retained 90 % Capacity Retention Ratio (CRR) after 5000 cycles at 2 Ag
-1.
A flexible and lightweight supercapacitor was designed by Zhao et al. [
22] using a chemical fluid method that employed bimetallic MOF (Co and Mn) on carbon cloth with specific capacitance of 2028 F g
-1 at 1 A g
-1. PANI/MIL-101 nanocomposites were created by growing PANI into the holes of MIL-101 with the nanocomposites achieved specific capacitance of 1197 F g
-1 at 1 A g
-1 [
23] and maintained a CRR of 81 % after 10,000 cycles. Co
3O
4 /Ni-based MOFs on carbon cloth using a two-step hydrothermal heating technology with the specific capacitance of 209 mAh g
-1 at 1 A g
-1 [
24]. Zhu et al. [
25] described the development of a ternary composite by means of MOF, metal oxide and conducting polymer through electro deposition and hydrothermal methods. The composite delivered specific capacitance of 340.7 F g
-1 at 1 A g
-1 and CRR of 82.5 % after 5000 cycles. Fe/Ni MOF was prepared by solvothermal method. When compared to pristine Ni MOF, Fe/Ni MOF exhibited higher conductivity [
26,
27]. Fe/Ni MOF prepared by solvothermally at temperature of 120
oC for OER [
28]. Fe/Ni MOF synthesized through solvothermal method directly used as OER electrocatalyst with high electrochemical stability in strong basic solution [
29]. Fe/Ni MOF exhibits superior electocatalytic properties [
30,
31]. Fe/Ni MOF nanosheets expose more active metal sites and enhance the intrinsic catalytic activity [
32]. Fe/Ni MOF exhibited an overpotential of 251 mV @100 mA.cm
-2 [
33]. Further, this bimetallic MOF exhibited Tafel slope of 45.4 mV dec
-1 which outperforms commercial ruthenium oxide [
34]. With controlled manner, placing Fe/Ni MOF composite vertically over carbon nanofibers led to reduction in the ion diffusion path. Thus, increase the redox reaction along with the enhancement of overall electrochemical performance [
35]. Similarly, by introducing the proper electrochemically active substance into the electrolyte causes the improvement in specific capacitance [
36]. Flexible electrodes contributed for several applications such as displays, wearable devices, laptops etc. [
37].
This work focuses on the development of bimetallic Ni-Fe MOF through a solvothermal method. Subsequently, polyaniline is embedded onto the bimetallic MOF using an in situ polymerization method. Finally, this composite was tested as electrode for supercapacitor. The bimetallic Fe/Ni MOF with PANI exhibited the specific capacitance of 33.26 F.g-1 at 1 A.g-1.
2. Experimental Section
Nickel nitrate hexahydrate, ferrous chloride tetrahydrate, terephthalic acid, polyaniline, ammonium persulphate, sulphuric acid, ethanol, and potassium hydroxide were purchased from Sisco Research Laboratories Pvt. Ltd and used to synthesize a novel hybrid nanocomposite of bimetallic MOF and polyaniline for supercapacitor application. All chemicals used in this study were analytical grade and were not purified further. The MOF was prepared using nickel nitrate hexahydrate and ferrous chloride tetrahydrate which act as node and terephthalic acid act as a linker. 5 g of 0.35 M concentration nickel nitrate hexahydrate dissolved in 48 ml of DD water and stirred for 15 minutes in magnetic stirrer. Similarly, 5 g of 0.35 M concentration ferrous chloride tetrahydrate dissolved in 71.4 ml of DD water and stirred for 15 minutes in magnetic stirrer. And then 5 g of 0.35 M terephthalic acid dissolved in 42 ml of DD water and stirred for 20 minutes in magnetic stirrer. The final mixture was transferred to an autoclave instrument and left for 2 hours. The solvothermal process was conducted using an autoclave instrument to precisely control the size, shape, and phase of the sample. Subsequently, the sample was transferred to a centrifuge machine and treated for 5 min at 2500 rpm. Finally, the obtained sample was kept in a furnace at 90oC for 3 hours.
To synthesize MOF/PANI, the following procedure was proposed. Firstly, 0.25g of bimetallic MOF was dispersed in 100 ml of distilled water and stirred for 30 minutes. Secondly, 2ml of aniline was added to the solution and stirred for an hour. 1g of ammonium persulphate was mixed with 40ml of 1 M sulphuric acid on a magnetic stirrer, and kept in a place for 5-7 minutes. The final solution was added to the prepared solution and stirred for 4 hours. The mixture was then washed with ethanol, filtered and heated with hot air.
First, a slurry of materials was produced by mixing the binder (40%) with 60 weight percent of active material. In order to use the obtained slurry as a working electrode in a three- electrode system, it was coated on graphite lead and dried for a period. In this study, 6 M KOH is used as an electrolyte for the analysis of CV, GCD, and EIS.
The specific capacitance values were calculated from the CV curves according the following equation: C = ∫Idt/ m Δ V where I is the oxidation or reduction current, dt is time differential, m indicates the mass of active material, and V represents the voltage range.
The specific capacitance (Cs, F g
−1 ) was calculated from the GCD curves according to the following equation: C = I × Δ t / m × Δ V where I (A) is the discharge current, Δt (s) is the discharge time, ΔV (V) represents the voltage window, and m (g) is the total mass of active materials in the working electrode [
38].
All the prepared samples were structurally characterized using FTIR and XRD measurements. The IR spectra (4500-400 cm-1) were performed on powdered samples on functional carbon surface of Model Perkin Elmer FT-IR spectrophotometer with the SOFTWARE-OPUS version 6.5. Scanning electron microscopy (FESEM) JEOL MODEL JSM 6360 was used to examine the morphology of the prepared functional carbon. The Diffraction patterns were analysed using XPERT PRO.For electrochemical analysis, the 3-electrode system was applied, in which the as-synthesized composite materials: bimetallic Fe/Ni MOF [BM]and bimetallic Fe/Ni MOF - polyaniline [BMP]were utilized as a working electrode, platinum as counter electrode, and silver/silver chloride as reference electrode, respectively. For the preparation of working electrode, 80% of active material (bimetallic Fe/Ni MOF and bimetallic Fe/Ni MOF – polyaniline) was mixed with 10% of Super P Carbon and 10% of Styrene-Butadine-Rubber (SBR) binder were mixed to make a homogenously dispersed slurry. Then 250 µg slurry is coated on 2 mm graphite tip. The OrigaLys electrochemical workstation (France) was used to conduct galvanostatic charge discharge (GCD), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) analyses under the 6M KOH electrolyte.
3. Results and Discussion
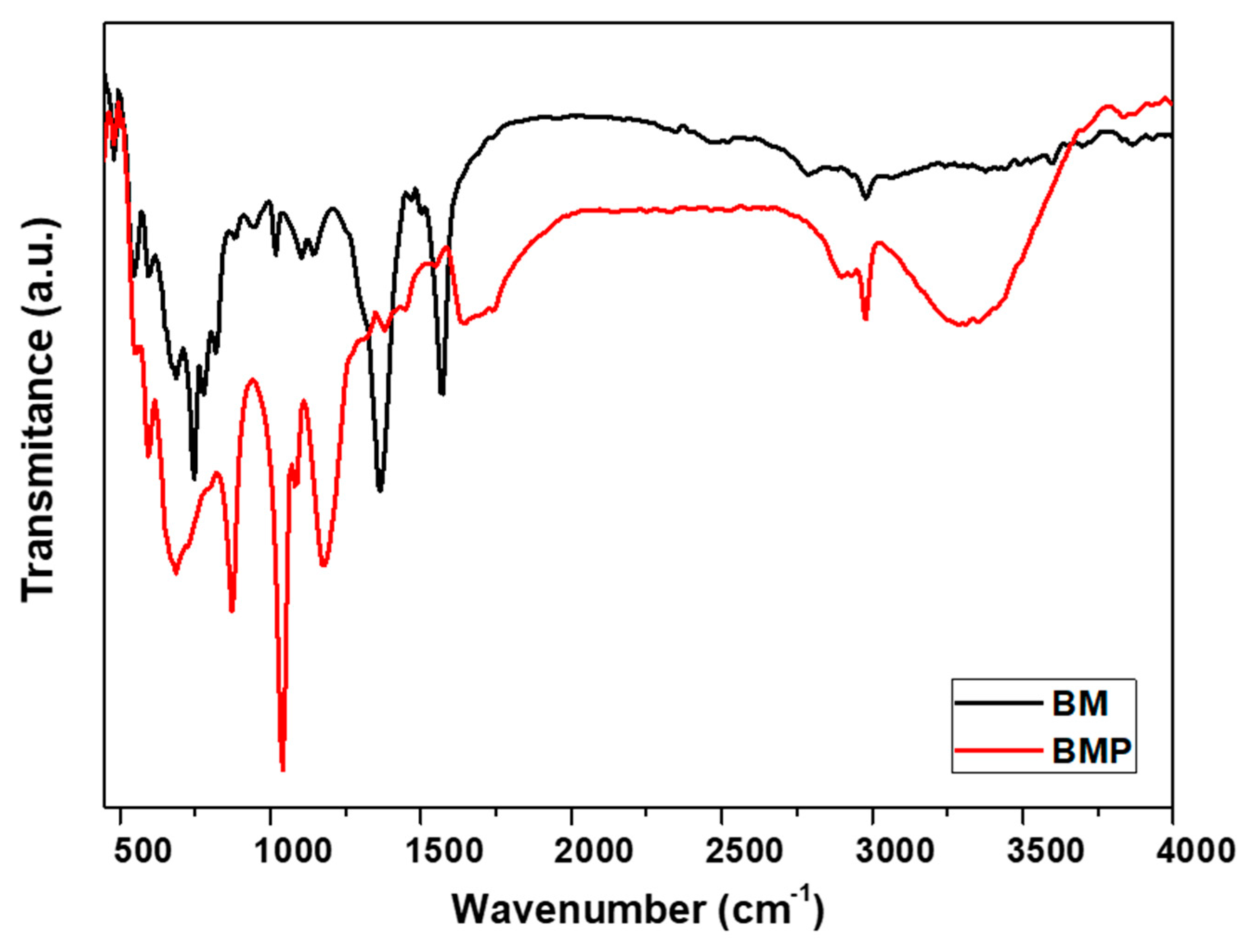
Figure 1 shows the FTIR analysis of the molecular structures and compositions of Fe/Ni MOF and Fe/Ni MOF-polyaniline composite. The sample Fe/Ni MOF exhibits C=O stretching and bending vibrations at 1504 cm
-1, COO stretching vibrations at 1365 cm
-1, and C-C vibrations at 856 cm
-1. The broad absorption peaks between 3300 – 3600 cm
-1 can be assigned to the OH bonds. The sample Fe/Ni-MOF with PANI also features C-H and –NH
+ = stretching and bending vibrations at 810cm
-1, and 1180cm
-1, respectively.
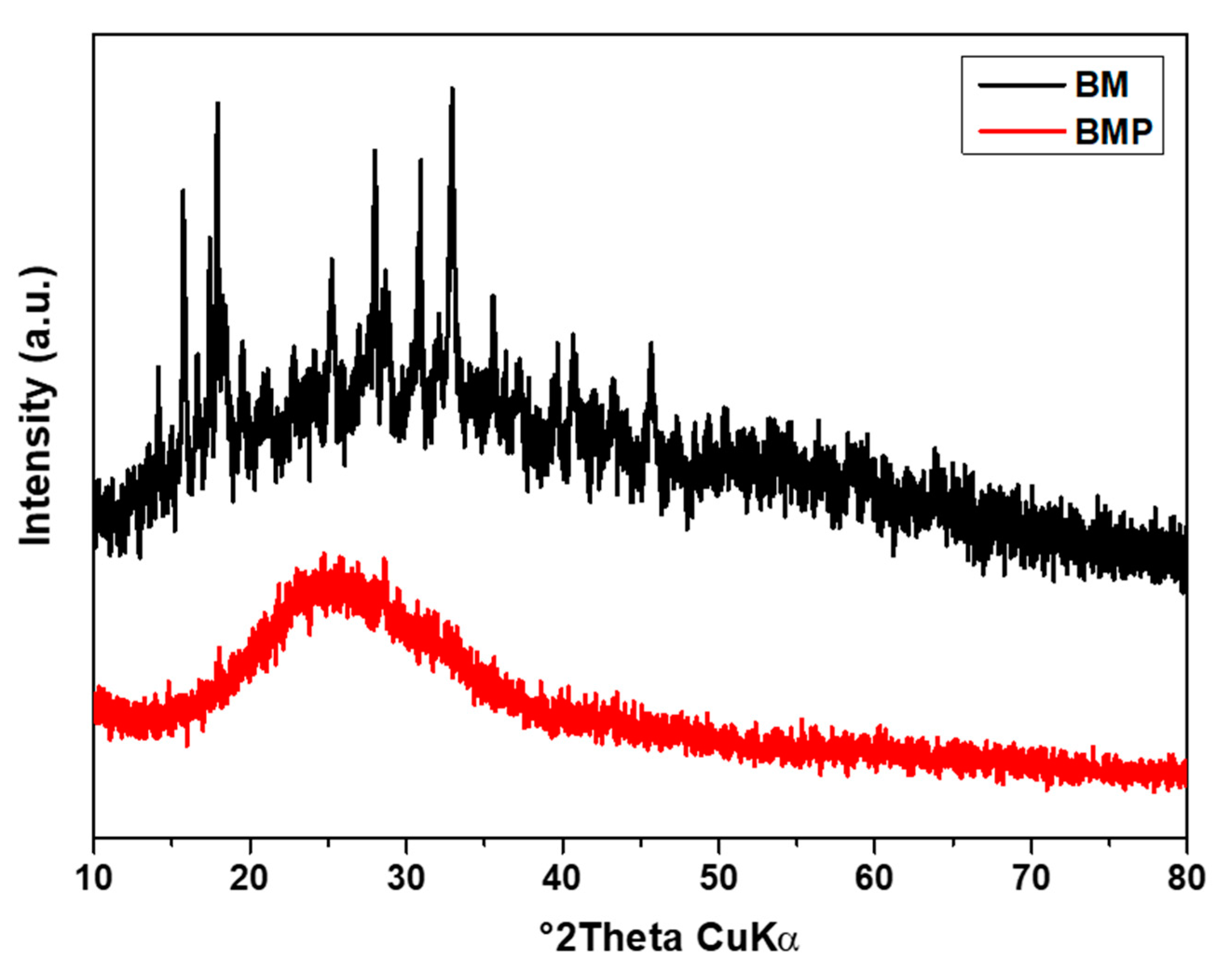
Figure 2 shows the XRD analysis of both samples, which revealed an amorphous structure with a few crystalline lines at 27°, 31°, 32°, and 35° which corresponds to the miller indices of (513), (060), (444) and (604). The amorphous samples had more defects on the surface, which is necessary for storing a higher amount of energy than the crystalline materials [
39].
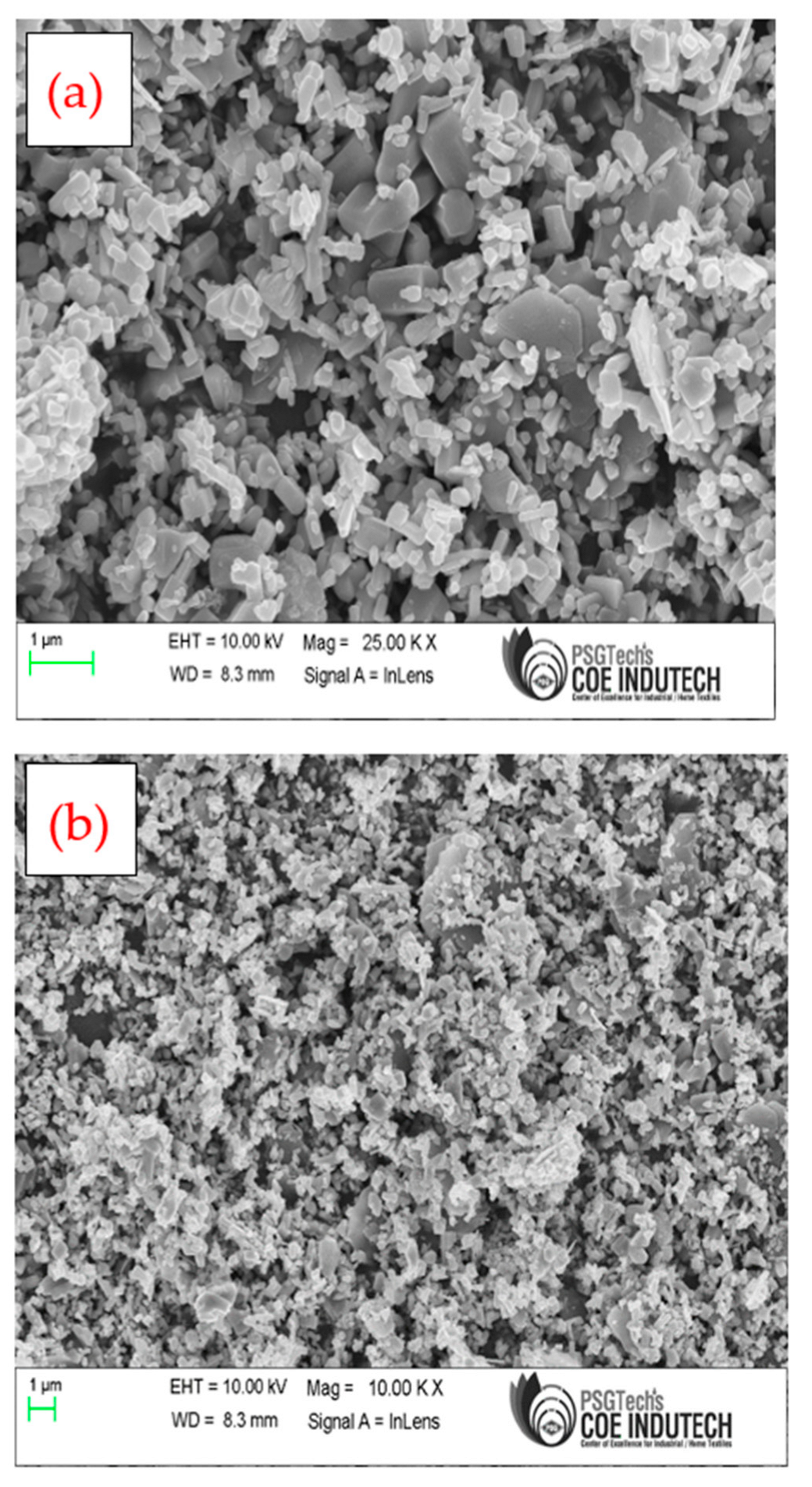
SEM was used to understand the morphology of the prepared samples.
Figure 3a depicts that the bimetallic Fe/Ni MOF displayed a flake-like morphology, agglomerated and irregular in shape.
Figure 3b indicates that polyaniline was grown on the surface of the bimetallic Fe/Ni MOF [
40].
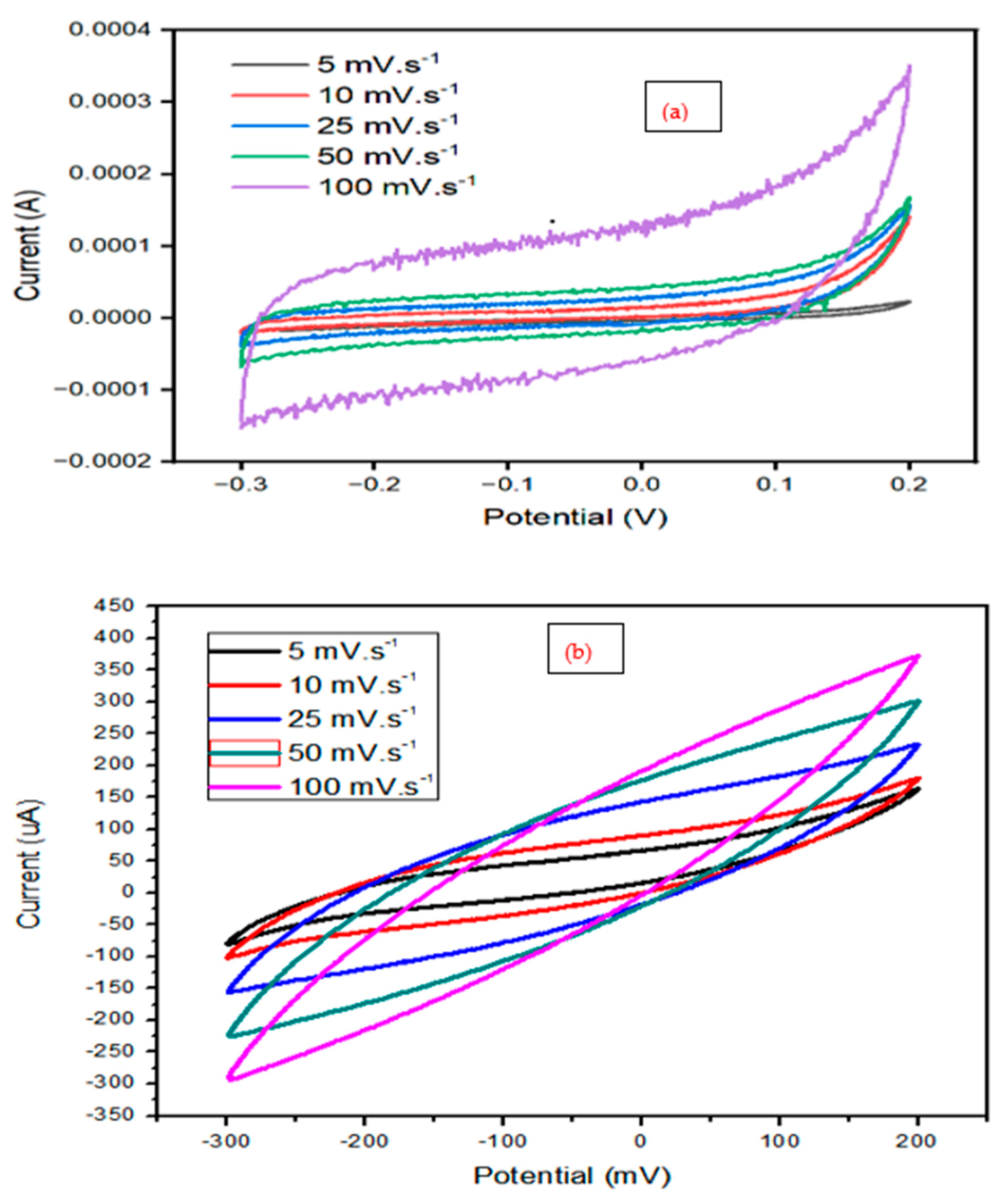
The CV curves were collected at different scan rates such as 10, 25, 50, and 100 mV.s
-1 in the potential window of -0.3 to 0.2 V. Based on the CV analysis presented in
Figure 4a& b, the specific capacitance of the samples was calculated. The CV curves of the bimetallic Fe/NI MOF, indicated the quick mobility of ions toward the electrodes’ surface as the scan rate increased, the shape of CV altered to a leaf-like shape. No redox peaks in the CV plot observed which indicates that the EDLC is not subjected oxidation or reduction. Due to this condition, the EDLC can be regarded to have a capacitive property since the electrodes are exposed to ion accumulation rather than ions undergoing intercalation/deintercalation [
41].
Table 1 shows that at 10 mVs
-1 scan rate, the specific capacitance of the bimetallic Fe/Ni MOF was higher (104 Fg
-1) than that of the composite of bimetallic Fe/Ni MOF and PANI (90 Fg
-1) due to the presence of the conducting polymer. The pores in the Fe/Ni metal organic framework may be hindered by PANI. Additionally, SEM images in
Figure 3b support this observation.
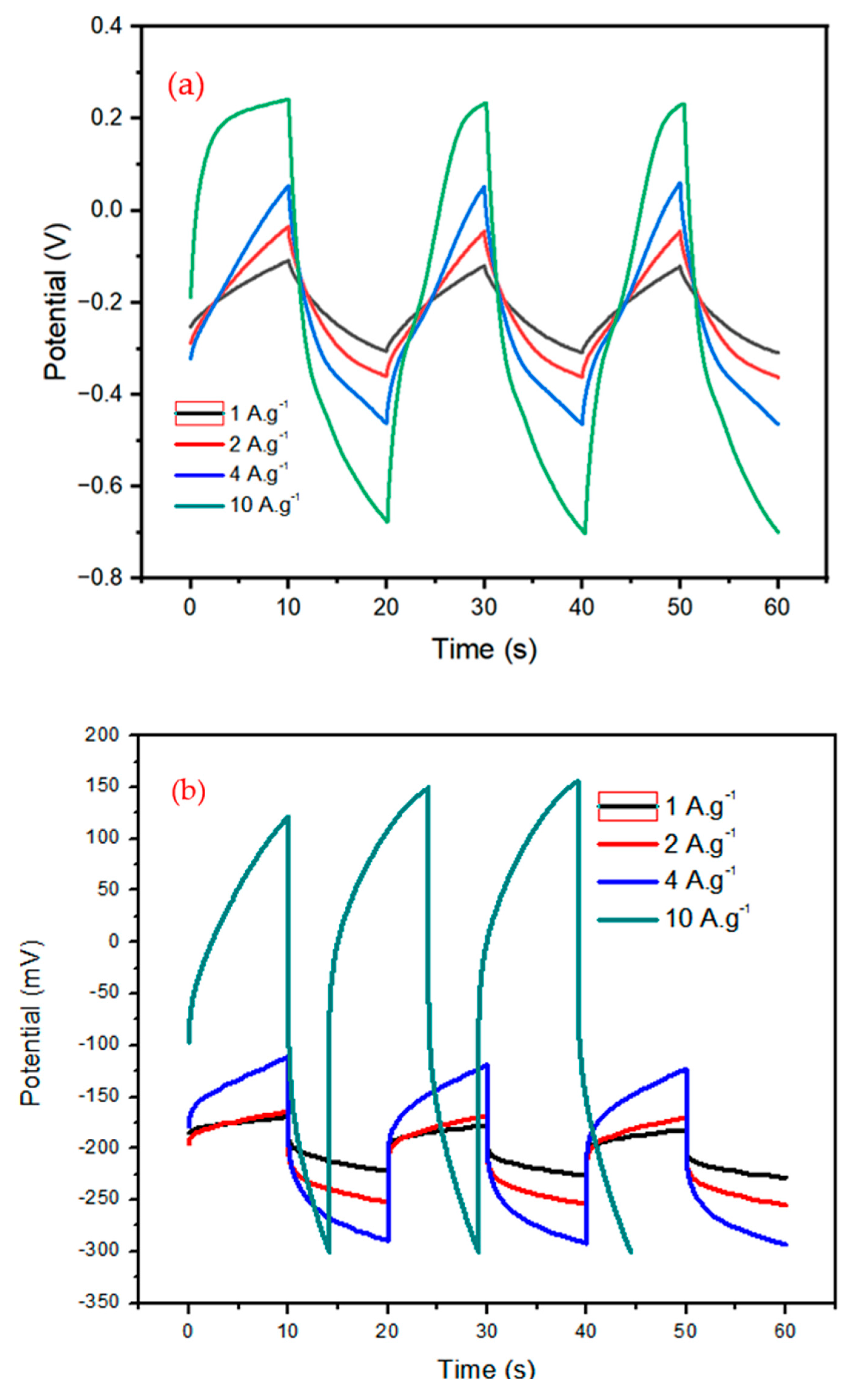
Figure 5a and b show the
Galvanostatic Charge-Discharge curves for the prepared samples at current densities of 1, 2, 4, and 10 Ag-1, respectively. At 1 Ag-1 current density, the bimetallic Fe/Ni MOF had an outstanding specific capacitance (228.88 Fg-1) than the bimetallic Fe/Ni MOF-PANI (33.26 Fg-1). Both the CV and GCD analyses led to the conclusion that the incorporation of a conducting polymer into an iron and nickel based metal organic framework reduces the specific capacitance and affects its stability.
From the overall results, the capacitance values from GCD results are much lower than that of CV results. One of the reasons is that the applied voltage or potential during the GCD was set to only 0.8 V and it was 0.5 V during the CV analysis. This applied to the different measurement techniques which mean the variation of voltage per time scan rate for CV, whilst varying the current for the charge/discharge technique.
Figure 5 plots the C
sp as a function of current density for electrodes during GCD analysis. It was found that Fe/Ni MOF showed the highest calculated capacitance value. Unfortunately, this is contra to that of CV analysis results. It can be suggested that this could be due to the inconsistency preparation of the active materials which may affected the quality of the fabricated electrode during the coating process [
42].
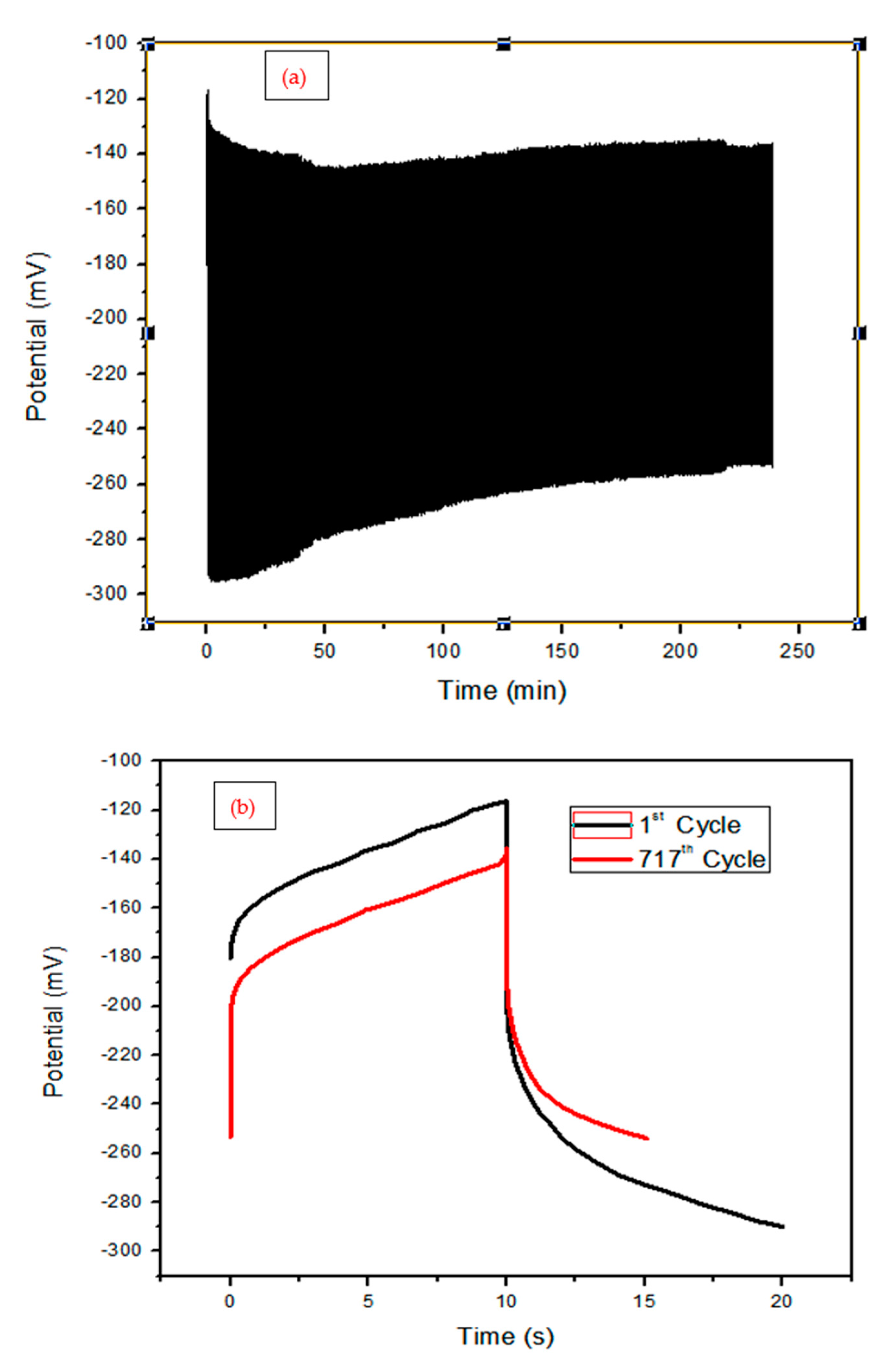
From the
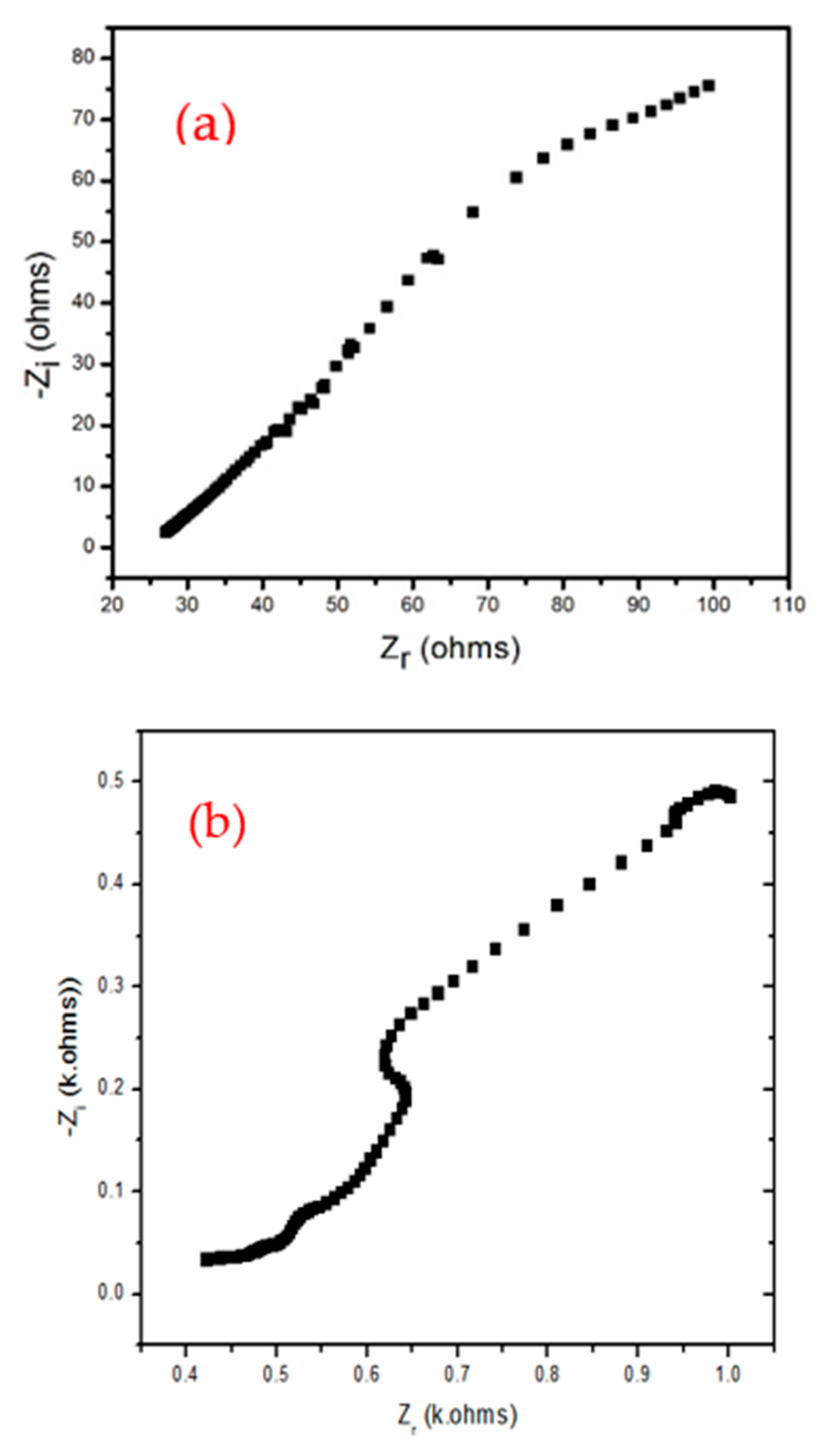
Figure 6 a and b, the sample was tested for 717 cycles and capacity retention ratio of 75 %. The degradation is due to the polymer. EIS spectra of the samples are shown in
Figure 7 a and b. The bimetallic Fe/Ni-MOF exhibited a lower charge transfer resistance (R
ct) but solution resistance (R
s) based on the EIS spectra (see,
Figure 7a). Conversely, the composite showed lower R
s but higher R
ct. Higher resistance is observed for BMP samples due to persistent problems with content of polymers.
4. Conclusions
In this study, a Fe/Ni-MOF PANI composite electrode material was developed for electrochemical energy storage. Fe/Ni-MOF and Fe/Ni-MOF PANI composite were synthesized using simple chemical methods followed by a solvothermal procedure. The material was synthesized using a MOF-template due to its porosity and well-defined topologies. The morphology and structure of the electrode material were evaluated using SEM, XRD, and FTIR. The electrochemical performances were evaluated in 6 M KOH using the OrigaLys electrochemical workstation. The bimetallic MOF exhibited a flake-like morphology. The CV analysis showed that, within the potential window of -0.3 to 0.2 V, the specific capacitance of the bimetallic Fe/Ni MOF was higher (104 F.g-1) than that of the composite (90 F.g-1) at 10 mV.s-1. The GCD also showed a higher capacitance for the bimetallic Fe/Ni MOF at all current densities. The electrochemical measurements indicate that the incorporation of conducting polymer in the metal organic framework resulted in a reduction in specific capacitance and stability.
References
- Wujie Dong, Miao Xie, Siwei Zhao, Qiuliang Qin, Fuqiang Huang, Materials design and preparation for high energy density and high power density electrochemical supercapacitors, Materials Science and Engineering: R: Reports, Volume 152, 2023, 100713, ISSN 0927-796X. [CrossRef]
- Tianqi He, Xiaoya Kang, Fujuan Wang, Junlei Zhang, Tianyun Zhang, Fen Ran, Capacitive contribution matters in facilitating high power battery materials toward fast-charging alkali metal ion batteries, Materials Science and Engineering: R: Reports, Volume 154, 2023, 100737, ISSN 0927-796X. [CrossRef]
- Kandasamy, S. K., & Kandasamy, K. (2018). Recent advances in electrochemical performances of graphene composite (graphene-polyaniline/polypyrrole/activated carbon/carbon nanotube) electrode materials for supercapacitor: a review. Journal of Inorganic and Organometallic Polymers and Materials, 28, 559-584.
- Xiaomin Yang, Huihui He, Ting Lv, Jieshan Qiu, Fabrication of biomass-based functional carbon materials for energy conversion and storage, Materials Science and Engineering: R: Reports, Volume 154, 2023, 100736, ISSN 0927-796X. [CrossRef]
- Yingkui Yang, Cuiping Han, Beibei Jiang, James Iocozzia, Chengen He, Dean Shi, Tao Jiang, Zhiqun Lin, Graphene-based materials with tailored nanostructures for energy conversion and storage, Materials Science and Engineering: R: Reports, Volume 102, 2016, Pages 1-72, ISSN 0927-796X. [CrossRef]
- Debin Kong, Zhichang Xiao, Yang Gao, Xinghao Zhang, Ruiying Guo, Xiaoxiong Huang, Xianglong Li, Linjie Zhi, Sp2-carbon dominant carbonaceous materials for energy conversion and storage, Materials Science and Engineering: R: Reports, Volume 137, 2019, Pages 1-37, ISSN 0927-796X. [CrossRef]
- Rui Xiong, Anise M. Grant, Ruilong Ma, Shuaidi Zhang, Vladimir V. Tsukruk, Naturally-derived biopolymer nanocomposites: Interfacial design, properties and emerging applications, Materials Science and Engineering: R: Reports, Volume 125, 2018, Pages 1-41, ISSN 0927-796X. [CrossRef]
- V. Pavlenko, S. Khosravi H, S. Żółtowska, A.B. Haruna, M. Zahid, Z. Mansurov, Z. Supiyeva, A. Galal, K.I. Ozoemena, Q. Abbas, T. Jesionowski, A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications, Materials Science and Engineering: R: Reports, Volume 149, 2022, 100682, ISSN 0927-796X. [CrossRef]
- Yanhong Liu, Jiahong Liu, Yijun Cao, Wei Shang, Ning Peng, Xianhao Long, Shengjun Zhou and Yuqing Wen (2021) 'Facile Synthesis and Characterization of the Mn-MOF Electrode Material for Flexible Supercapacitors', J. Electrochem. Energy Convers. Storage, Vol.19 (3) p. 031002. [CrossRef]
- Abdelkarim Chaouiki, Maryam Chafiq, Young Gun Ko, The art of controlled nanoscale lattices: A review on the self-assembly of colloidal metal–organic framework particles and their multifaceted architectures, Materials Science and Engineering: R: Reports, Volume 159, 2024, 100785, ISSN 0927-796X. [CrossRef]
- Kandasamy, S. K., & Kandasamy, K. (2019). Graphene–PolyanilineNanocomposite Treated with Microwave as a New Supercapacitor Electrode and its Structural, Electrochemical Properties Graphene–Polyaniline Nanocomposite Treated with Microwave as a New Supercapacitor Electrode and its Structural, Electrochemical Properties. J of New Materials for Electrochem Sys, 22(3), 125-131. [CrossRef]
- Sivalingam, D., Elangovan, H., Subramanian, M., Kandasamy, S. K., & Govindasamy, M. (2013). Synthesis and characterization of PANI/ferric chloride composite for fabrication of electrodes in supercapacitor. In Advanced Materials Research (Vol. 768, pp. 334-337). Trans Tech Publications Ltd.
- Kandasamy, S. K., Singaram, K. N., Krishnamoorthy, H., Arumugam, C., Palanisamy, S., Kandasamy, K., ...& D.-Cancar, H. (2021). Microwave-assisted graphene-based conducting polymer materials for supercapacitors. Handbook of Supercapacitor Materials: Synthesis, Characterization, and Applications, 299-326.
- Khan, A., & Asiri, A. M. (2022). Handbook of Supercapacitor Materials. R. Boddula (Ed.). Wiley-VCH.
- Arumugam, C., Kandasamy, S. K., Gunasekaran, K., Somasundaram, K., & Eswaramoorthi, K. P. (2021, November). Hierarchical structure of graphene oxide/polyaniline composite flexible supercapacitor. In AIP Conference Proceedings (Vol. 2387, No. 1, p. 090003). AIP Publishing LLC.
- Arumugam, C., Kandasamy, S. K., & Subramaniam, T. K. (2023). Structural and Electrochemical Analysis of Activated Carbon Derived from Seeds of Ziziphus jujuba and Shells of Prunus dulcis Decorated over Graphene Oxide/Polyaniline Binary Composite for High Energy Density Supercapacitor Applications. High Energy Chemistry, 57(3), 177-185. [CrossRef]
- Arumugam, C., Kandasamy, S. K., &Kumaravel Subramaniam, T. (2023). Enhancement of the Carbon Content and Electrochemical Performance by Decorating Zinc Oxide Over Graphene Oxide/Polyaniline Composite. Journal of Electrochemical Energy Conversion and Storage, 20(2), 020904. [CrossRef]
- Zifeng Wang, Minshen Zhu, Zengxia Pei, Qi Xue, Hongfei Li, Yan Huang, Chunyi Zhi, Polymers for supercapacitors: Boosting the development of the flexible and wearable energy storage, Materials Science and Engineering: R: Reports, Volume 139, 2020, 100520, ISSN 0927-796X. [CrossRef]
- Jing Zhang, Jiajie Huang, Liang Wang, Peiheng Sun, Peijia Wang, Zhujun Yao and Yefeng Yang (2021) 'Coupling Bimetallic NiMn-MOF Nanosheets on NiCo2O4 Nanowire Arrays with Boosted Electrochemical Performance for Hybrid Supercapacitor', Mater. Res. Bulletin, Vol.149, p 111707. [CrossRef]
- Muhammad Zahir Iqbal, Mian Muhammad Faisal, Syeda Ramsha Ali, Sidra Farid and Amir Muhammad Afzal (2020) 'Co-MOF/polyaniline-based electrode material for high performance supercapattery devices' Electochem. Acta, Vol. 346, p 136039. [CrossRef]
- Rajkumar Srinivasan, Elanthamilan Elaiyappillai, Evangeline Jafneel Nixon, I.Sharmila Lydia and Princy merlin Johnson (2020) 'Enhanced electrochemical behaviour of Co-MOF/PANI composite electrode for supercapacitors', Inorgan. Chem. Acta, Vol.502, p 119393. [CrossRef]
- Yunhe Zhao, Hongxing Dong, Xinyi He, Jing Yu, Rongrong Chen, Qi Liu, Jingyuan Liu, Hongsen Zhang and Jun Wang (2019) 'Carbon Cloth Modified with Metal-Organic Framework Derived CC@CoMoO4-Co(OH)2Nanosheets Array as a Flexible Energy-Storage Material', ChemElectroChem, Vol. 6 (13) p 3355-3366. [CrossRef]
- Qian Wang, Liang Shao, Zhonglei Ma, Juanjuan Xu, Ying Li, and Caiyun Wang (2018) 'Hierarchical porous PANI/MIL-101 nanocomposites based solid-state flexible supercapacitor', Electrochem. Acta, Vol.281, p 582-593. [CrossRef]
- Liangjing Zhang, Yiyue Zhang, Shaolong Huang, Yuliang Yuan, Hui Li, Zhengyuan Jin, Jiahao Wu, Qiufan Liao, Liang Hu, Jiamguo Lu, Shuangyuan Ruan and Yu-Jia Zeng (2018) 'Co3O4/Ni-based MOFs on carbon cloth for flexible alkaline battery supercapacitor hybrid devices and near-infrared photocatalytic hydrogen evolution', Electrochem. Acta, Vol. 281, p 189-197. [CrossRef]
- Chunmei Zhu, Ying He, Yijun Liu, Natalia Kazantseva, Petr Saha and Qilin Cheng (2019) 'ZnO@MOF@PANI core-shell nanoarrays on carbon cloth for high-performance supercapacitor electrodes', J Ener. Chem., Vol. 35, p 124-131. [CrossRef]
- Zheng, F., Zhang, Z., Xiang, D., Li, P., Du, C., Zhuang, Z., Li, X. and Chen, W., 2019. Fe/Ni bimetal organic framework as efficient oxygen evolution catalyst with low overpotential. Journal of colloid and interface science, 555, pp.541-547. [CrossRef]
- Dai, L., Xie, M., Liu, J. and Peng, H., 2023. A Bimetallic Ni–Fe MOF Nanofiber as High Performance Anode for Enhancing Lithium Storage. ACS Applied Energy Materials, 6(23), pp.12114-12119. [CrossRef]
- Yaqoob, L., Noor, T., Iqbal, N., Nasir, H., Zaman, N. and Talha, K., 2021. Electrochemical synergies of Fe–Ni bimetallic MOF CNTs catalyst for OER in water splitting. Journal of Alloys and Compounds, 850, p.156583. [CrossRef]
- Zheng, F., Xiang, D., Li, P., Zhang, Z., Du, C., Zhuang, Z., Li, X. and Chen, W., 2019. Highly conductive bimetallic Ni–Fe metal organic framework as a novel electrocatalyst for water oxidation. ACS sustainable chemistry & engineering, 7(11), pp.9743-9749. [CrossRef]
- Taffa, D.H., Balkenhohl, D., Amiri, M. and Wark, M., 2023. Minireview: Ni–Fe and Ni–Co Metal–Organic Frameworks for Electrocatalytic Water-Splitting Reactions. Small Structures, 4(6), p.2200263. [CrossRef]
- You, S.M., El Rouby, W.M., Thamilselvan, A., Tsai, C.K., Darmanto, W., Doong, R.A. and Millet, P., 2020. Fe/Ni bimetallic organic framework deposited on TiO2 nanotube array for enhancing higher and stable photoelectrochemical activity of oxygen evaluation reaction. Nanomaterials, 10(9), p.1688. [CrossRef]
- Li, Q., Wang, Q., Li, Y., Zhang, X. and Huang, Y., 2021. 2D bimetallic Ni/Fe MOF nanosheet composites as a peroxidase-like nanozyme for colorimetric assay of multiple targets. Analytical Methods, 13(17), pp.2066-2074. [CrossRef]
- Zhao, T., Cheng, C., Wang, D., Zhong, D., Hao, G., Liu, G., Li, J. and Zhao, Q., 2021. Preparation of a Bimetallic NiFe-MOF on Nickel Foam as a Highly Efficient Electrocatalyst for Oxygen Evolution Reaction. ChemistrySelect, 6(6), pp.1320-1327. [CrossRef]
- Wang, D., Le, F., Lv, J., Yang, X., Chen, X., Yao, H. and Jia, W., 2023. Fe-Incorporated Nickel-Based Bimetallic Metal–Organic Frameworks for Enhanced Electrochemical Oxygen Evolution. Molecules, 28(11), p.4366. [CrossRef]
- Acharya, D., Pathak, I., Dahal, B., Lohani, P.C., Bhattarai, R.M., Muthurasu, A., Kim, T., Ko, T.H., Chhetri, K. and Kim, H.Y., 2023. Immoderate nanoarchitectures of bimetallic MOF derived Ni–Fe–O/NPC on porous carbon nanofibers as freestanding electrode for asymmetric supercapacitors. Carbon, 201, pp.12-23. [CrossRef]
- Qin, Z., Xu, Y., Liu, L., Liu, M., Zhou, H., Xiao, L., Cao, Y. and Chen, C., 2022. Ni-MOF composite polypyrrole applied to supercapacitor energy storage. RSC advances, 12(45), pp.29177-29186. [CrossRef]
- Iftikhar Hussain, Sumanta Sahoo, Muhammad Sufyan Javed, Jian Lu, Kaili Zhang, Flexible 2D MXenes for wearable next-generation energy storage applications, Materials Science and Engineering: R: Reports, Volume 160, 2024,100814, ISSN 0927-796X. [CrossRef]
- Wang, L., Wang, X., Xiao, X., Xu, F., Sun, Y., & Li, Z. (2013). Reduced graphene oxide/nickel cobaltite nanoflake composites for high specific capacitance supercapacitors. Electrochimica acta, 111, 937-945. [CrossRef]
- Jamil, S., Khan, S. R., Bibi, S., Jahan, N., Mushtaq, N., Rafaqat, F., ... & Janjua, M. R. S. A. (2023). Recent advances in synthesis and characterization of iron–nickel bimetallic nanoparticles and their applications as photo-catalyst and fuel additive. RSC advances, 13(42), 29632-29644. [CrossRef]
- Dikio, E. D., & Farah, A. M. (2013). Synthesis, characterization and comparative study of copper and zinc metal organic frameworks. Chemical Science Transactions, 2(4), 1386-1394.
- Aziz, S. B., Dannoun, E. M., Murad, A. R., Mahmoud, K. H., Brza, M. A., Nofal, M. M., ... & Kadir, M. F. Z. (2022). Influence of scan rate on CV Pattern: Electrical and electrochemical properties of plasticized Methylcellulose: Dextran (MC: Dex) proton conducting polymer electrolytes. Alexandria Engineering Journal, 61(8), 5919-5937. [CrossRef]
- ABID, M. A. A. M., Radzi, M. I., Mupit, M., Osman, H., Munawar, R. F., Samat, K. F., ... & Islam, M. R. (2020). Cyclic voltammetry and galvanostatic charge-discharge analyses of polyaniline/graphene oxide nanocomposite based supercapacitor. Malaysian Journal on Composites Science and Manufacturing, 3(1), 14-26. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).