Submitted:
31 July 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
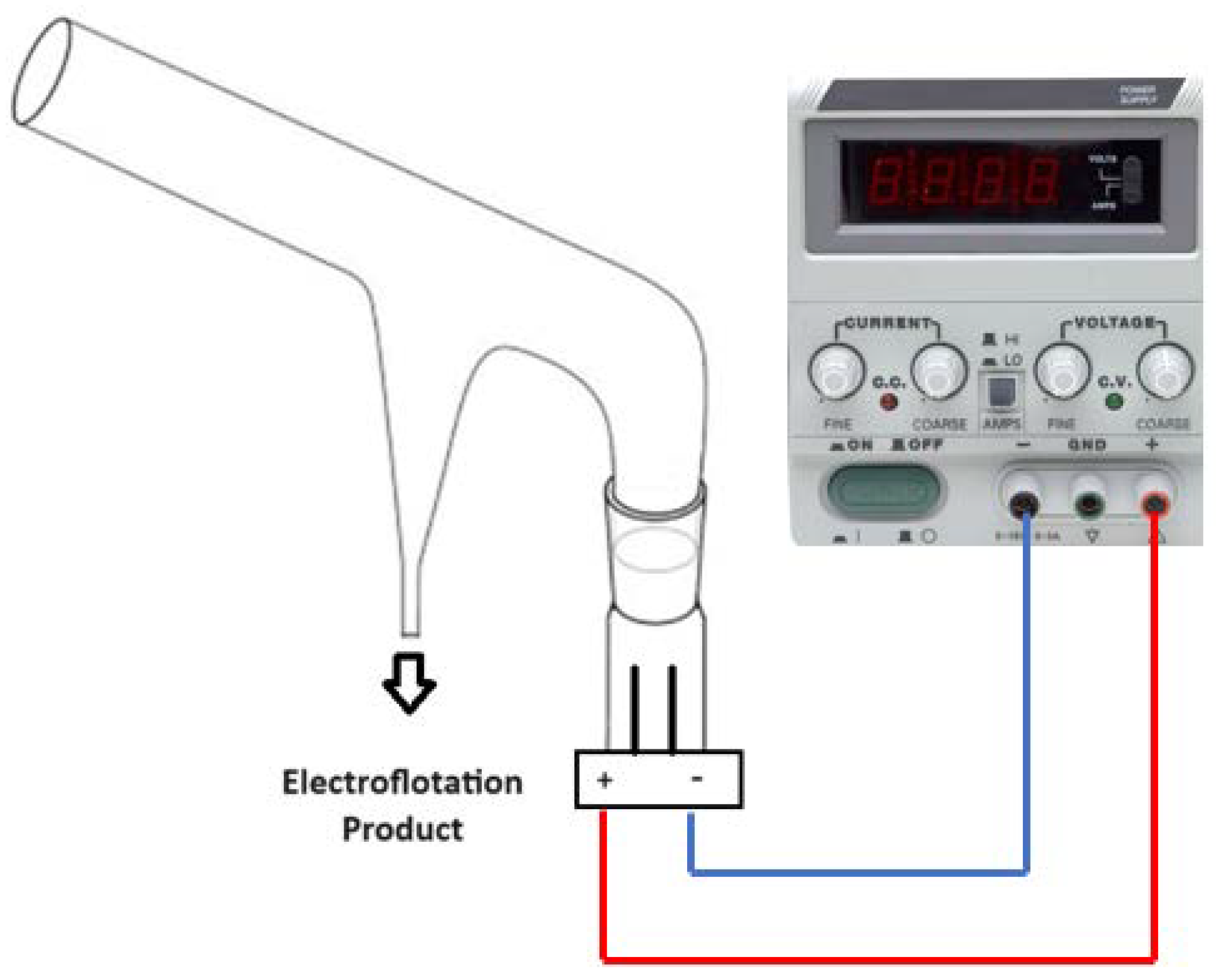
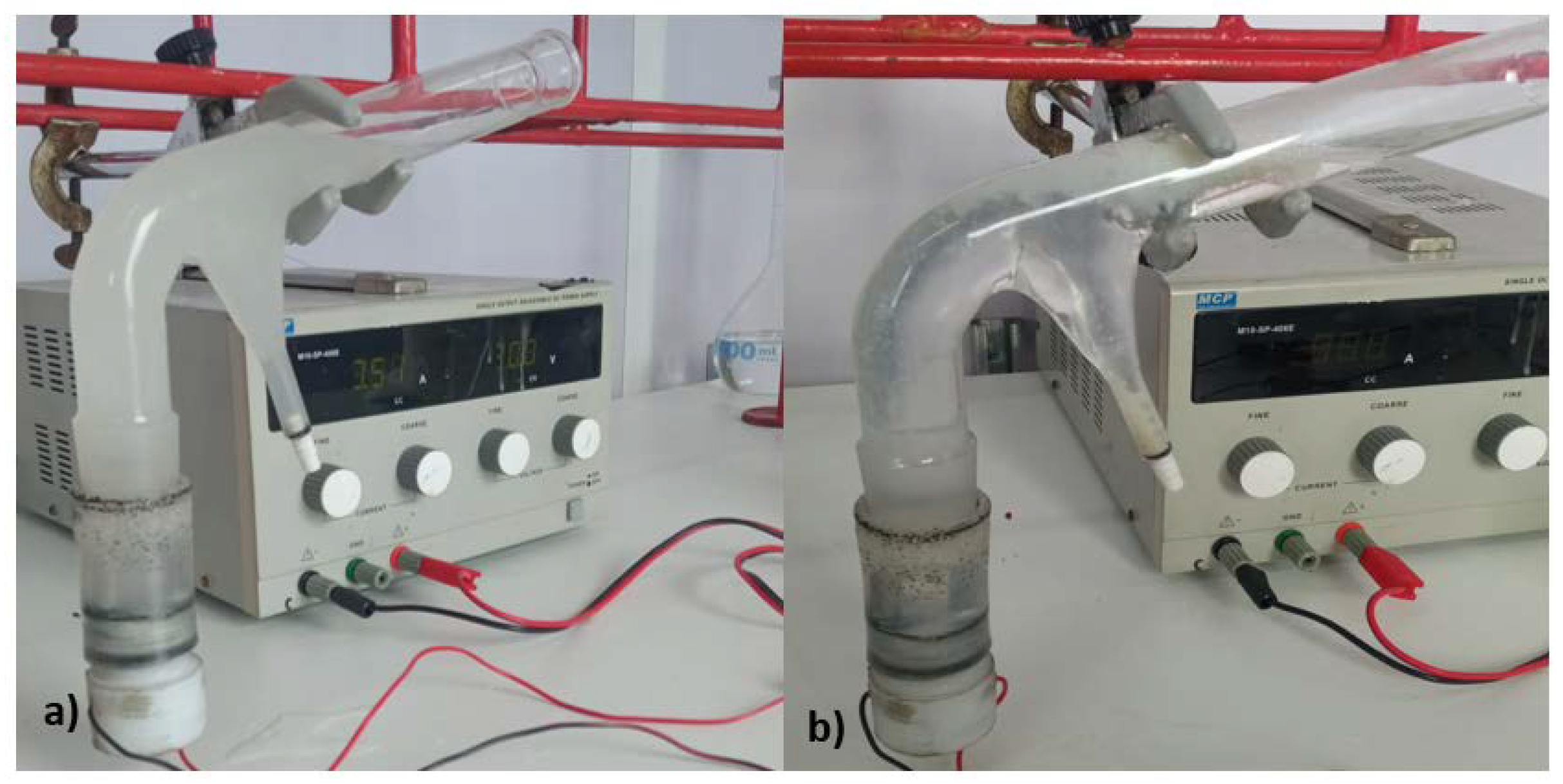
2.1. Electroflotation System
2.2. Materials and Solutions
2.3. Electroflotation Tests
2.4. Corrosion Studies for Ti Gr. 2
2.5. Kinetic Corrosion Analysis
3. Results
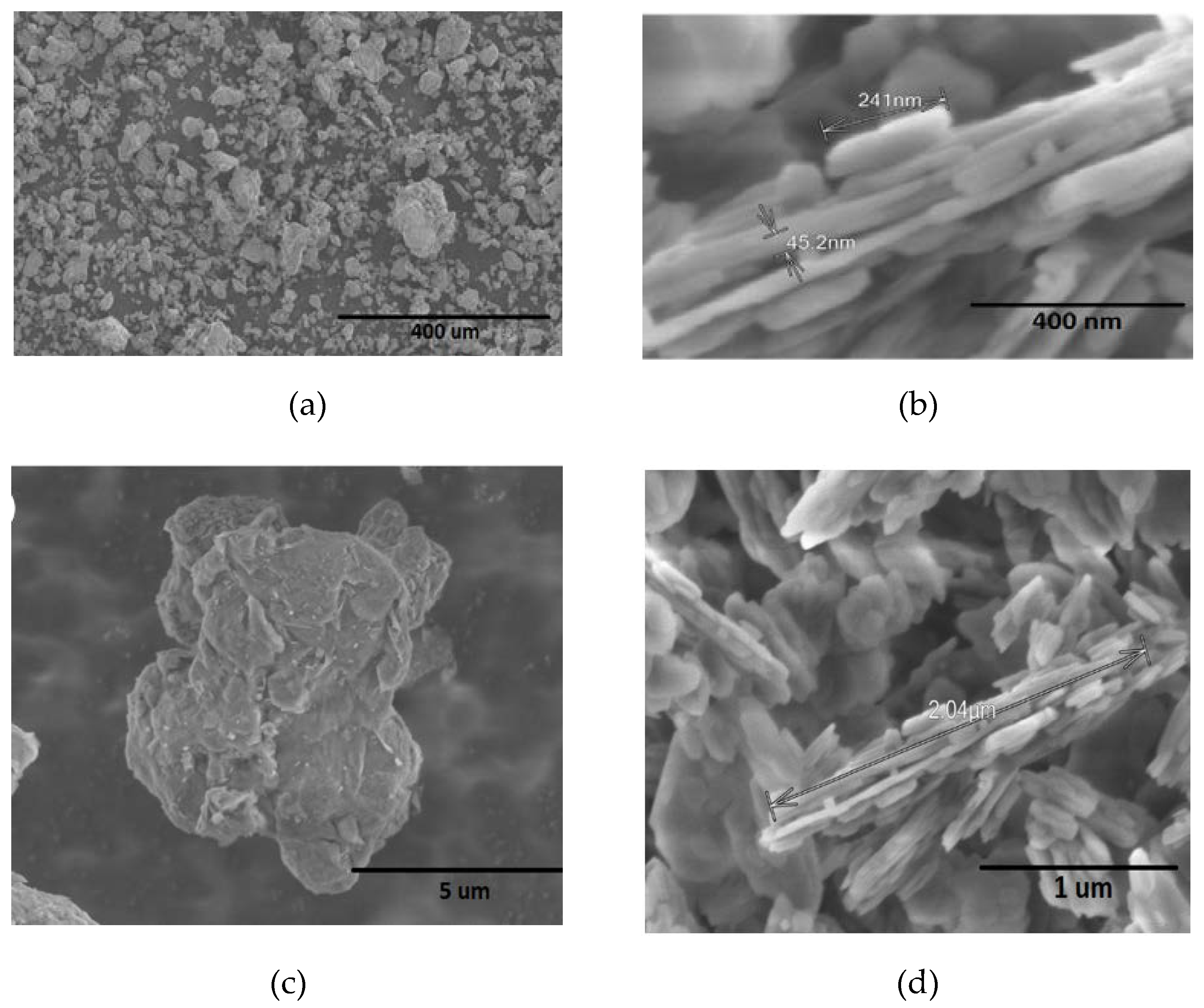
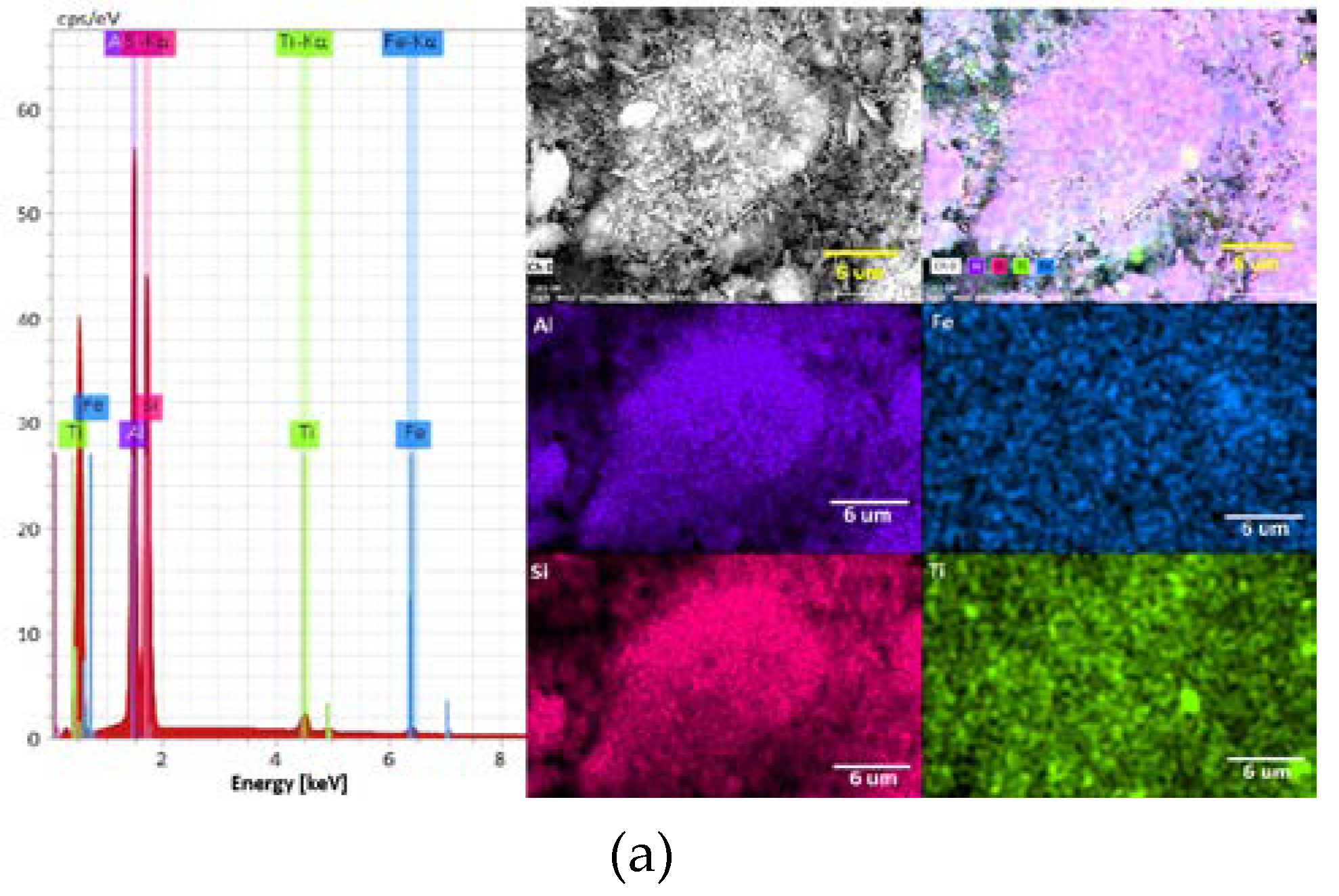
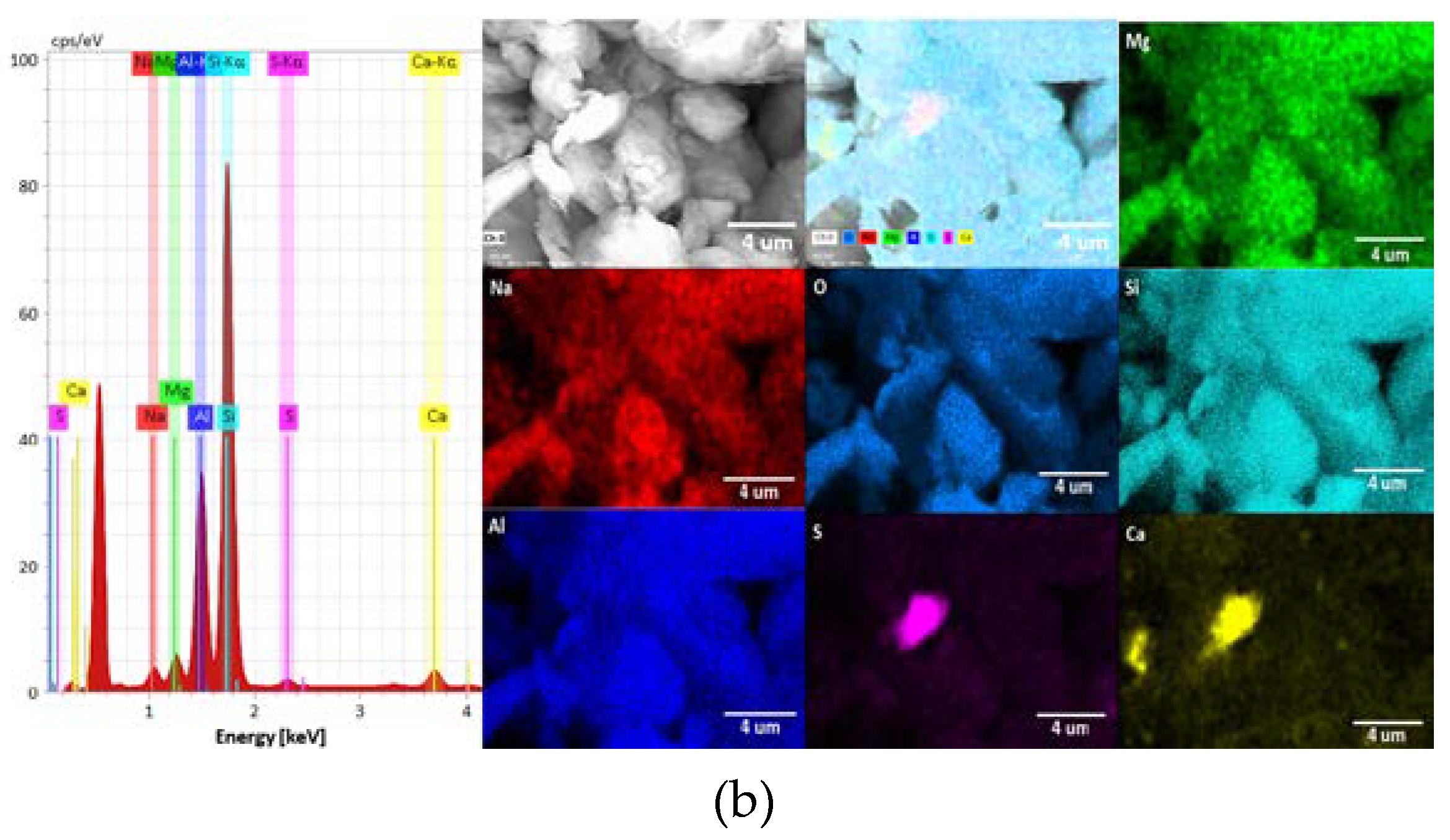
3.1. Mineralogical Characterization of Clays
3.2. Electroflotation of Clays in NaCl Solutions
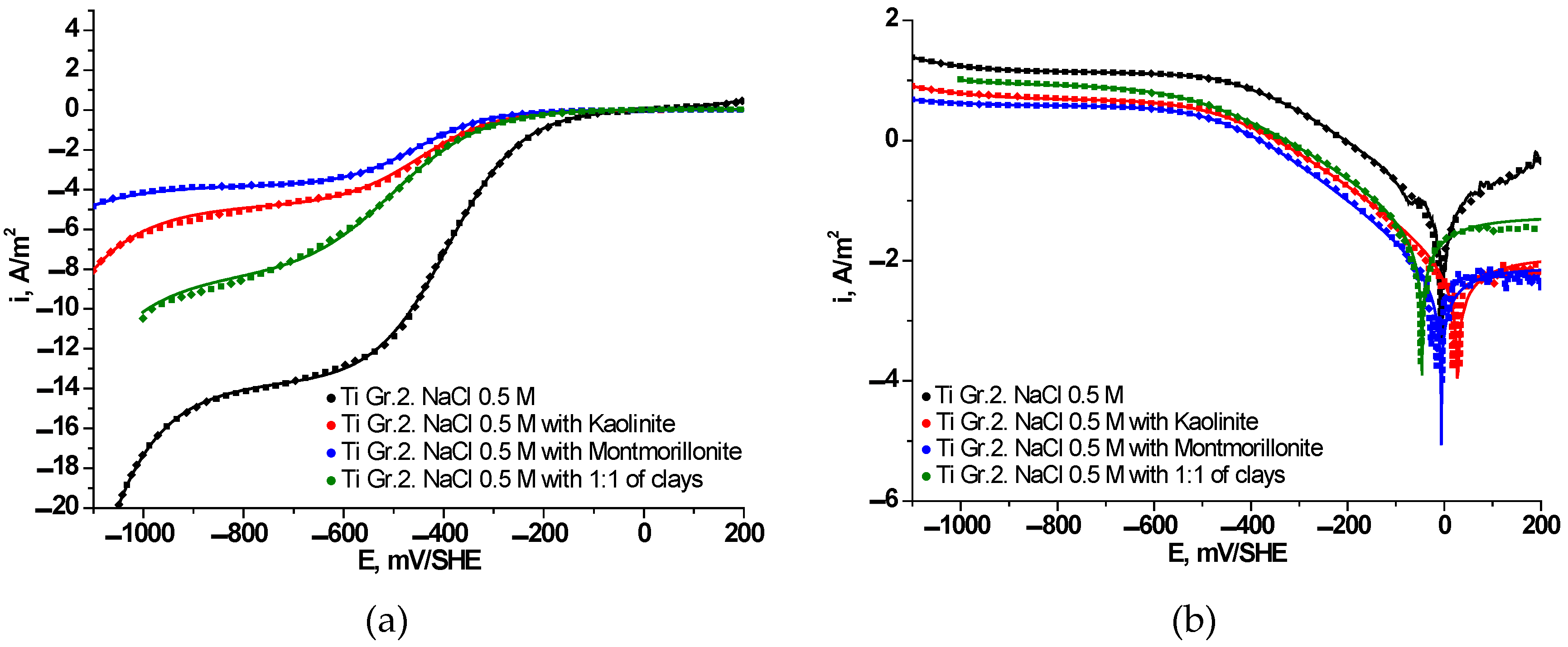
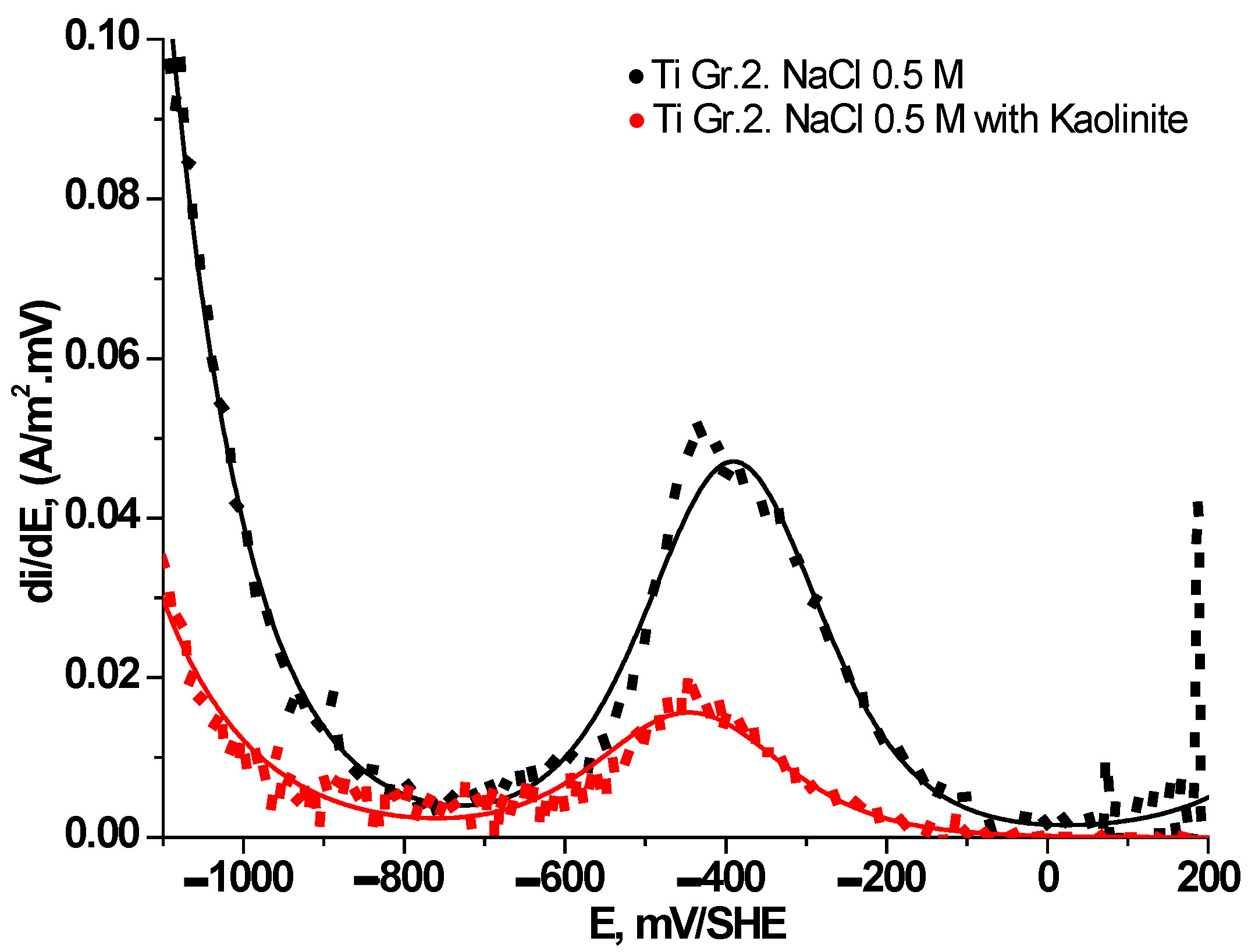
3.3. Electrochemical Behavior of Ti Gr. 2 in Presence of Clays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, D.; Li, X.; Gu, W. A Method for Temporary Water Scarcity Analysis in Humid Region Under Droughts Condition. Water Resour. Manage. 2015, 29, 3823–3839. [Google Scholar] [CrossRef]
- Fernandez-Scagliusi, M.Á. Herramientas para lograr un uso sostenible del agua en la minería: la huella hídrica y la huella de agua. Rev. Catalana Dret. Ambient. 2021, 12, 1–37. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Min. Proc. Ext. Met. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- COCHILCO, 2022, Yearbook: Copper and Other Mineral statistics 2003–2022. Available online: https://www.cochilco.cl/Lists/Anuario/Attachments/27/ANUARIO_ESTADISTICO_COCHILCO%20A%C3%91O%202022.pdf (accessed on 24 May 2024).
- Witecki, K.; Polowczyk, I.; Kowalczuk, P. Chemistry of wastewater circuits in mineral processing industry-a review. J. Water Process, 2022, 45, 102509. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Peng, Y. The interaction between kaolinite and saline water in affecting the microstructure, rheology and settling of coal flotation products. Powder Technol. 2020, 372, 76–83. [Google Scholar] [CrossRef]
- Gorakhki, M.H.; Bareither, C.A. Salinity effects on sedimentation behavior of kaolin, bentonite, and soda ash mine tailings. Appl. Clay Sci. 2015, 114, 593–602. [Google Scholar] [CrossRef]
- Rand, B.; Melton, I.E. Particle interactions in aqueous kaolinite suspensions. I. Effect of pH and electrolyte upon the mode of particle interaction in homoionic sodium kaolinite suspensions. J. Colloid Interface Sci. 1977, 60, 308–320. [Google Scholar] [CrossRef]
- Yaghmaeiyan, N.; Mirzaei, M.; Delghavi, R. Montmorillonite clay: Introduction and evaluation of its applications in different organic syntheses as catalyst: A review. Results Chem. 2022, 4, 100549. [Google Scholar] [CrossRef]
- Romanov, A.M. Electroflotation in Waste Water Treatment: Results and Perspectives. In Mineral Processing and the Environment; Springer: Berlin, Germany, 1998; pp. 335–360. [Google Scholar]
- Srinivasan, V.; Subbaiyan, M. Electroflotation Studies on Cu, Ni, Zn, and Cd with Ammonium Dodecyl Dithiocarbamate. Sep. Sci. Technol. 1989, 24, 145–150. [Google Scholar] [CrossRef]
- Alexandrova, L.; Nedialkova, T.; Nishkov, I. Electroflotation of Metal Ions in Waste Water. Int. J. Miner. Process. 1994, 41, 285–294. [Google Scholar] [CrossRef]
- Oussedik, S.M.; Khelifa, A. Reduction of Copper Ions Concentration in Wastewaters of Galvanoplastic Industry by Electroflotation. Desalination 2001, 139, 383. [Google Scholar] [CrossRef]
- Khelifa, A.; Moulay, S.; Naceur, A.W. Treatment of Metal Finishing Effluents by the Electroflotation Technique. Desalination 2005, 181, 27–33. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Sekki, A.; Madani, K.; Chibane, M. Removal Turbidity and Separation of Heavy Metals Using Electrocoagulation-Electroflotation Technique. A Case Study. J. Hazard Mater. 2009, 164, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.I.; Matis, K.A. Cadmium Ion Removal by Electroflotation onto Sewage Sludge Biomass. Int. J. Environ. Waste Manag. 2012, 9, 245–256. [Google Scholar] [CrossRef]
- Madrid, F.M.G.; Arancibia-Bravo, M.P.; Sepúlveda, F.D.; Lucay, F.A.; Soliz, A.; Cáceres, L. Ultrafine Kaolinite Removal in Recycled Water from the Overflow of Thickener Using Electroflotation: A Novel Application of Saline Water Splitting in Mineral Processing. Molecules 2023, 28, 3954. [Google Scholar] [CrossRef] [PubMed]
- Madrid, F.M.G.; Arancibia-Bravo, M.; Cisterna, J.; Soliz, A.; Salazar-Avalos, S.; Guevara, B.; Sepúlveda, F.; Cáceres, L. Corrosion of Titanium Electrode Used for Solar Saline Electroflotation. Materials 2023, 16, 3514. [Google Scholar] [CrossRef]
- Kydros, K.A.; Gallios, G.P.; Matis, K.A. Electrolytic Flotation of Pyrite. J. Chem. Technol. Biotechnol. 1994, 59, 223–232. [Google Scholar] [CrossRef]
- Bhaskar Raju, G.; Khangaonkar, P.R. Electro-Flotation of Chalcopyrite Fines. Int. J. Miner. Process. 1982, 9, 133–143. [Google Scholar] [CrossRef]
- Makuei, F.; Tadesse, B.; Albijanic, B.; Browner, R. Electroflotation of Ultrafine Chalcopyrite Particles with Sodium Oleate Collector. Miner. Eng. 2018, 120, 44–46. [Google Scholar] [CrossRef]
- Hacha, R.R.; LeonardoTorem, M.; Gutiérrez Merma, A.; da Silva Coelho, V.F. Electroflotation of Fine Hematite Particles with Rhodococcus Opacus as a Biocollector in a Modified Partridge–Smith Cell. Miner. Eng. 2018, 126, 105–115. [Google Scholar] [CrossRef]
- Llerena, C.; Ho, J.C.K.; Piron, D.L. Effects of pH on Electroflotation of Sphalerite. Chem. Eng. Commun. 1996, 155, 217–228. [Google Scholar] [CrossRef]
- Liu, A.; Fan, P.; Han, F.; Han, H.; Li, Z.; Wang, H.; Fan, M. Effect of Electroflotation on Quartz and Magnetite and Its Utilization on the Reverse Flotation of Magnetic Separation Concentrate. Miner. Eng. 2022, 175, 107292. [Google Scholar] [CrossRef]
- Tadesse, B.; Albijanic, B.; Makuei, F.; Browner, R. Recovery of Fine and Ultrafine Mineral Particles by Electroflotation—A Review. Miner. Process. Extr. Metall. Rev. 2019, 40, 108–122. [Google Scholar] [CrossRef]
- Hernlem, B.J.; Tsai, L.-S. Chlorine Generation and Disinfection by Electroflotation. J. Food Sci. 2000, 65, 837–837. [Google Scholar] [CrossRef]
- Chen, R.; Trieu, V.; Natter, H.; Kintrup, J.; Bulan, A.; Hempelmann, R. Wavelet analysis of chlorine bubble evolution on electrodes with different surface morphologies. Electrochem. Commun. 2012, 22, 16–20. [Google Scholar] [CrossRef]
- Mraz, R.; Krysa, J. Dimensionally Stables Anodes with a Long Lifetime for Electroflotation. In Precision Process Technology; Springer: Berlin/Heidelberg, Germany, 1993; pp. 681–688. [Google Scholar]
- Tadesse, B.; Albijanic, B.; Makuei, F.; Browner, R. Recovery of Fine and Ultrafine Mineral Particles by Electroflotation—A Review. Miner. Process. Extr. Metall. Rev. 2019, 40, 108–122. [Google Scholar] [CrossRef]
- Soliz, A.; Cáceres, L. Corrosion behavior of carbon steel in LiBr in comparison to NaCl solutions under controlled hydrodynamic conditions. Int. J. Electrochem. Sci. 2015, 10, 5673–5693. [Google Scholar] [CrossRef]
- Soliz, A.; Cáceres, L.; Pineda, F.; Galleguillos, F. Erosion–Corrosion of AISI 304L Stainless Steel Affected by Industrial Copper Tailings. Metals 2020, 10, 1005. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Q.; Li, X.; Yao, F.; Xie, L.; Zhao, J.; Chen, F.; Xie, T.; Zeng, G. Electrochemically Induced Pitting Corrosion of Ti Anode: Application to the Indirect Reduction of Bromate. Chem. Eng. J. 2016, 289, 114–122. [Google Scholar] [CrossRef]
- El-Ghenymy, A.; Alsheyab, M.; Khodary, A.; Sirés, I.; Abdel-Wahab, A. Corrosion Behavior of Pure Titanium Anodes in Saline Medium and Their Performance for Humic Acid Removal by Electrocoagulation. Chemosphere 2020, 246, 125674. [Google Scholar] [CrossRef]
- Hatch, C.D.; Wiese, J.S.; Crane, C.C.; Harris, K.J.; Kloss, H.G.; Baltrusaitis, J. Water Adsorption on Clay Minerals as a Function of Relative Humidity: Application of BET and Freundlich Adsorption Models. Langmuir 2012, 28, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Bai, H.; Ai, Z.; Chen, P.; Hu, Y.; Song, S.; Komarneni, S. Role of Montmorillonite, Kaolinite, or Illite in Pyrite Flotation: Differences in Clay Behavior Based on Their Structures. Langmuir 2020, 36, 10860–10867. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhang, P.; Wei, D.; Hu, H.; Li, F.; Yang, K. Corrosion and Tribocorrosion Behaviors for TA3 in Ringer’s Solution after Implantation of Nb Ions. Appl. Sci. 2020, 10, 8329. [Google Scholar] [CrossRef]
- Lazić, M.M.; Mitić, D.; Radović, K.; Đorđević, I.; Majerič, P.; Rudolf, R.; Grgur, B.N. Corrosion Behavior of Nickel–Titanium Continuous-Casted Alloys. Metals 2024, 14, 88. [Google Scholar] [CrossRef]
- Kostelac, L.; Pezzato, L.; Colusso, E.; Natile, M.M.; Brunelli, K.; Dabalà, M. Black PEO Coatings on Titanium and Titanium Alloys Produced at Low Current Densities. Appl. Sci. 2023, 13, 12280. [Google Scholar] [CrossRef]
- Ganor, J.; Mogollón, J.L.; Lasaga, A.C. The effect of pH on kaolinite dissolution rates and on activation energy. Geochim. Et. Cosmochim. Acta 1995, 59, 1037–1052. [Google Scholar] [CrossRef]
- Rozalén, M.L.; Huertas, F.J.; Brady, P.V.; Cama, J.; García-Palma, S.; Linares, J. Experimental study of the effect of pH on the kinetics of montmorillonite dissolution at 25 °C. Geochim. Cosmochim. Acta 2008, 72, 4224–4253. [Google Scholar] [CrossRef]
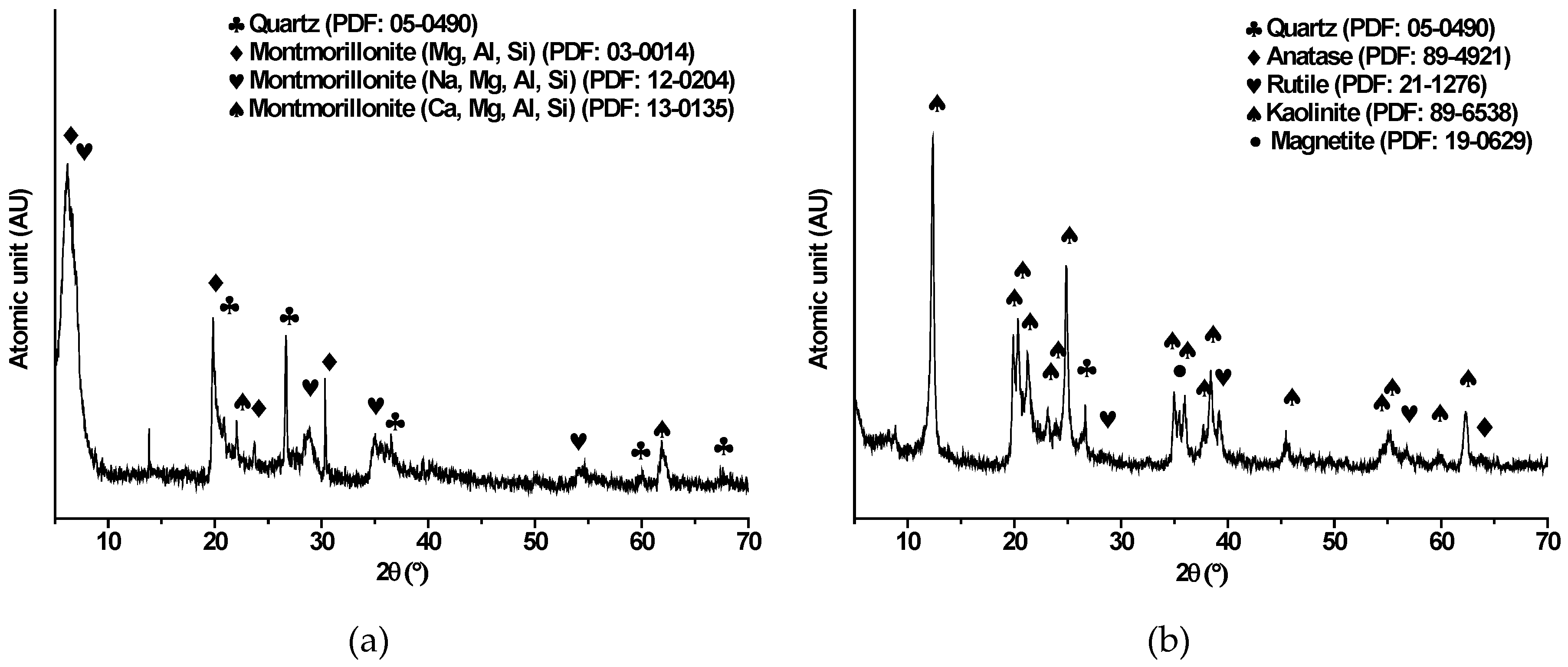
| Elements | Kaolinite, wt% | Montmorillonite, wt% |
| Fe | 2.47 | - |
| Al | 44.53 | 21.71 |
| Si | 48.57 | 67.48 |
| Ti | 4.44 | - |
| Na | - | 0.83 |
| Mg | - | 1.81 |
| S | - | 1.91 |
| Ca | - | 6.25 |
| Test | Clay type | NaCl, M | tEF, min |
Ecell, V |
Icell, A (t=0 min) |
Icell, A (t=tEF) |
%RMF |
| 1 | Montmorillonite | 0.1 | 10 | 10 | 0.14 | 0.05 | 1.49 |
| 2 | 0.3 | 15 | 15 | 0.17 | 0.05 | 43.52 | |
| 3 | 0.5 | 20 | 20 | 0.40 | 0.03 | 72.68 | |
| 4 | Kaolinite | 0.1 | 10 | 10 | 0.15 | 0.03 | 2.37 |
| 5 | 0.3 | 15 | 15 | 0.23 | 0.04 | 45.72 | |
| 6 | 0.5 | 20 | 20 | 0.80 | 0.03 | 88.44 | |
| 7 | Mix (1:1 wt%) Kao/Mont | 0.1 | 10 | 10 | 0.40 | 0.04 | 2.33 |
| 8 | 0.3 | 15 | 15 | 0.56 | 0.04 | 31.36 | |
| 9 | 0.5 | 20 | 20 | 0.71 | 0.05 | 67.36 |
| Parameters | 0.5M NaCl | 0.5 M NaCl + (1:1) Kao./Mont. | 0.5 M NaCl + 1000 ppm Kao. | 0.5 M NaCl + 1000 ppm Mont. |
| , A/m2 | 1.99×10-9 | 5.13×10-2 | 9.60×10-3 | 7.20×10-3 |
| , mV/dec | 238 | 231629 | 159369 | 243684 |
| , A/m2 | –2.03×10-6 | –7.15×10-6 | –7.57×10-7 | –1.83×10-7 |
| , mV/dec | –168 | –207 | –171 | –165 |
| , A/m2 | –13.68 | –8.38 | –4.76 | –3.79 |
| , A/m2 | –1.46×10-2 | –1.44×10-2 | –1.23×10-2 | –3.54×10-3 |
| , mV/dec | –217 | –247 | –256 | –253 |
| , mV/SHE | –7 | –48 | 27 | -6 |
| , A/m2 | 0.0693 | 0.0522 | 0.0098 | 0.0073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





